Abstract
Objectives
The no-reflow phenomenon occurs in 25% of patients with ST elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI), and may be associated with adverse outcomes. The aim of our study was to detect novel predictors of no-reflow phenomenon and the resulting adverse long term outcomes.
Methods
We enrolled 400 STEMI patients undergoing primary PCI; 228 patients had TIMI flow 3 after PCI (57%) and the remaining 172 patients had TIMI flow <3 (43%). Fibrinogen to albumin ratio (FAR), high sensitive C-reactive protein to albumin ratio (CAR), and atherogenic index of plasma (AIP) were calculated. Long term mortality and morbidity during 6 months follow up were recorded. These data were compared among both groups.
Results
In multivariate regression analysis, old age (OR = 1.115, 95% CI: 1.032–1.205, P = 0.006), higher troponin level >5.6 ng/mL (OR = 1.040, 95% CI: 1.001–1.080, P = 0.04), diabetes mellitus (OR = 4.401, 95% CI: 1.081–17.923, P = 0.04) and heavy thrombus burden (OR = 16.915, 95% CI: 5.055–56.602, P < 0.001) could be considered as predictors for the development of no-reflow. Interestingly, CAR >0.21, FAR >11.56, and AIP >0.52 could be considered as novel powerful independent predictors (OR = 3.357, 95% CI: 2.288–4.927, P < 0.001, OR = 4.187, 95% CI: 2.761–6.349, P < 0.001, OR = 16.794, 95% CI: 1.018–277.01, P = 0.04, respectively). Higher long term mortality (P < 0.001) and heart failure (P < 0.001) was also strongly related to incidence of no-reflow.
Conclusion
No-reflow could be attributed to novel predictors as CAR, FAR, and AIP. This phenomenon was associated with long term adverse events as higher mortality and pump failure.
Keywords: Myocardial infarction, No-reflow, Novel predictors, Outcomes
1. Introduction
Acute treatment of ST elevation myocardial infarction (STEMI) is restoration of myocardial perfusion by recanalization of the occluded vessel. Early recanalization is associated with better outcome. Therefore, primary percutaneous coronary intervention (PCI) has become the treatment of choice for acute STEMI.1
The no-reflow phenomenon is defined as inadequate myocardial perfusion passing through a given segment of coronary circulation with no angiographic evidence of mechanical vessel obstruction. No-reflow occurs in 25% of STEMI patients undergoing primary PCI.2 Microvascular obstruction due to thrombosis, distal embolization, and microvascular spasm are the suggested mechanisms for no-reflow.3 No-reflow is associated with larger infarct size, lower left ventricular ejection fraction (LVEF), adverse left ventricular remodelling in the late phase of myocardial infarction (MI), and increased risk of heart failure, risk of cardiac rupture, and risk of death.4 Both short term and long term prognoses of no-reflow are poor in humans. Malignant arrhythmias, pump failure, cardiac rupture and re-infarction are potential complications of no-reflow during the immediate in-hospital course.5
The inflammatory biomarkers; as high sensitive C-reactive protein (hs-CRP), fibrinogen, and albumin levels; have a positive correlation with no-reflow and platelets aggregation in STEMI patients who underwent primary PCI.6 Furthermore, atherogenic index of plasma (AIP) has been reported to be a strong independent predictor of all-cause mortality in acute coronary syndromes (ACS) after coronary revascularization.7 Thus, the aim of this study was to detect novel predictors of no-reflow as CRP to albumin ratio (CAR), fibrinogen to albumin ratio (FAR) and AIP. Also, the long term adverse events (mortality, malignant arrhythmias, heart failure, and recurrent myocardial infarction) associated with no-reflow phenomenon were investigated in this study.
2. Methods
2.1. Study population
Between 2017 and 2019, a total of 400 patients with acute STEMI who underwent primary PCI at our institution were considered for inclusion in this cross-sectional observational study. Acute STEMI was defined, according to the “Fourth Universal Definition of Myocardial Infarction.8 The exclusion criteria were: patients with atrial fibrillation, thrombolytic therapy or glycoprotein inhibitors (GPI) prior to PCI, unsuitable lesion for PCI, coronary spasm and prior or emergent coronary artery bypass grafting (CABG). Ethical approval was obtained from the Ethics and Research Committee of our institution. All patients gave written informed consent prior to enrolment in the study. The research was conducted in accordance with the principles of the Declaration of Helsinki.
The patients were classified into two main groups: Group I with normal coronary blood flow post PCI (TIMI = 3, n = 228), and Group II with no or slow coronary blood reflow post PCI (TIMI<3, n = 172).
2.2. Clinical data collection
The traditional risk factors were collected9 with calculation of the following parameters: time-to-door, door-to-balloon time, and time-to-reperfusion.10 The CHA2DS2VASc score was also calculated.11 Though patients with atrial fibrillation were excluded from this study, we did the scoring in all patients.12 In the long term follow up, mortality and other adverse outcomes as malignant arrhythmias, heart failure, and recurrent myocardial infarction (MI) were recorded within 6 months.
2.3. Laboratory analysis and echocardiography
Venous blood samples were obtained from peripheral veins after the provision of emergency service. Novel parameters were measured as AIP was also calculated using the formula log (TG/HDL-C).13 Transthoracic echocardiography (TTE) was done to assess left ventricular functions.
2.4. Coronary angiography protocol
Coronary angiography and revascularization options were related to the standard guidelines and the discretion of the treating physician.14 In heavy thrombus burden lesion, thrombus aspiration was performed manually using aspiration catheters. Intravenous anti-platelet drug that was used in the setting of primary PCI is GPI IIb/IIIa Tirofiban (0.4 mic/kg/min for 30 min, then 0.1 mic/kg/min for 18 h post PCI). Bare metal stents (BMS) were preferred over drug eluting stents (DES) in 170 patients (42.5%) due to financial issues (64 patients, 37.7%) in addition to concerns of a high bleeding risk (n = 34, 20%), poor compliance (n = 39, 22.9%) or the need for urgent/semi-urgent surgery (n = 33, 19.4%).
2.5. Angiographic analysis
Angiographic analysis was done by two independent expert operators. Infarct related artery (IRA) was identified and its severity was considered as: total and subtotal. Thrombolysis in Myocardial Infarction (TIMI) flow grading system is used to evaluate myocardial perfusion in IRA before and after PCI.15 Angiographic analysis was done according to standard guidelines.16
3. Statistical analyses
Data distribution was assessed according to the Kolgormonov–Smirnov test. Categorical data were compared using the chi-square test or Fisher exact test. Continuous variables were compared using an unpaired Student’s t-test or Mann–Whitney U-test. Data were expressed as mean ± standard deviation. The no-reflow independent predictions were found using the multivariate model, which contained all significantly associated variables based on the P-value of the univariate conditional regression analyses (P < 0.05). Receiver operating characteristic curve (ROC) was generated to determine different cut off values. The Kaplan Meier method was used to create long term survival curves. All tests were two-sided, and a p-value of ˂ 0.05 represented statistically significant differences. All analyses were performed using SPSS version 20 (SPSS Inc., USA).
4. Results
A total of 400 consecutive STEMI patients who presented to the emergency department and underwent primary PCI was included in this study. Out of the 400 patients, 228 patients had TIMI flow 3 after PCI (57%) and the remaining 172 patients had TIMI flow <3 (43%); 28 patients with TIMI flow 0 (7%), 40 patients with TIMI flow 1(10%), and 104 patients with TIMI flow 2(26%).
4.1. Demographic characteristics
The study population included 284 males (71%) and 116 females (29%). In normal flow group, 68.4% of the patients were males compared to 74.4% in the no-reflow group (P = 0.19). The mean age of the no-reflow group was significantly higher than that of the normal flow one (65.21 ± 11.89 vs. 56.61 ± 10.39 years, P < 0.001). Baseline demographic characteristics are shown in (Table 1).
Table 1.
Demographic, clinical and laboratory characteristics of the studied groups.
| Normal flow (TIMI = 3) (n = 228) | No reflow (TIMI <3) (n = 172) | P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Male sex, n(%) | 156 (68.4%) | 128 (74.4%) | 0.19 |
| Age (years) | 56.61 ± 10.39 | 65.21 ± 11.89 | <0.001∗∗ |
| BMI (kg/m2) | 26.58 ± 3.94 | 28 ± 5.43 | 0.004∗ |
| Cardiovascular risk factors | |||
| Diabetes Mellitus | 104 (45.6%) | 120 (69.8%) | <0.001∗∗ |
| Hypertension | 100 (43.9%) | 120(69.8%) | <0.001∗∗ |
| Family history of CADs | 24 (10.5%) | 16 (9.4%) | 0.72 |
| Smoker | 140 (61.4%) | 104 (60.5%) | 0.85 |
| Dyslipidemia | 64 (28.1%) | 52 (30.2%) | 0.64 |
| CHA2DS2VASc score | 1.74 ± 1.19 | 2.77 ± 1.48 | <0.001∗∗ |
| Clinical characteristics | |||
| Symptoms to door (hours) | 7.81 ± 5.66 | 11.63 ± 5.42 | <0.001∗∗ |
| Door to balloon (hours) | 1.3 ± 0.41 | 1.4 ± 0.68 | 0.09 |
| Time to reperfusion (hours) | 9.11 ± 5.66 | 13.02 ± 5.4 | <0.001∗∗ |
| Blood pressure (mmHg) | |||
| SBP | 124.77 ± 30.87 | 140.72 ± 29.11 | <0.001∗∗ |
| DBP | 68.16 ± 14.93 | 78.37 ± 19.35 | <0.001∗∗ |
| KILLIP class ≥ II | 40 (17.5%) | 68 (39.5%) | <0.001∗∗ |
| ECG | |||
| Inferior MI | 108 (47.4%) | 80 (46.5%) | 0.87 |
| Anterior MI | 120 (52.6%) | 92 (53.5%) | |
| LVEF (%) | 46.37 ± 10.65 | 42.26 ± 9.19 | <0.001∗∗ |
| LV diastolic dysfunction | |||
| Grade 1 | 120 (52.6%) | 68 (39.5%) | 0.02∗ |
| Grade 2 | 80 (35.1%) | 84 (48.8%) | |
| Grade 3 | 28 (12.3%) | 20 (11.6%) | |
| Laboratory characteristics | |||
| CK-MB (U/L) | 194.29 ± 145.75 | 255.07 ± 172.25 | <0.001∗∗ |
| Troponin (ng/ml) | 13.4 ± 16.12 | 22.28 ± 16.54 | <0.001∗∗ |
| Creatinine (mg/dl) | 0.98 ± 0.37 | 1.09 ± 0.57 | 0.03∗ |
| GFR (%) | |||
| Pre PCI | 93.53 ± 36.68 | 96 ± 49.09 | 0.58 |
| Post PCI | 95.93 ± 44.17 | 95.61 ± 49.64 | 0.95 |
| TGs (mg/ml) | 135.91 ± 48.92 | 179.33 ± 58.42 | <0.001∗∗ |
| LDL (mg/dl) | 123.56 ± 24.21 | 125.83 ± 23.27 | 0.35 |
| HDL (mg/dl) | 38.36 ± 7.69 | 39.74 ± 7.59 | 0.08 |
| Hemoglobin (gm/dl) | 13.59 ± 1.91 | 13.97 ± 2.08 | 0.06 |
| Leukocytes (×103/μL) | 9.72 ± 1.59 | 12.14 ± 1.99 | <0.001∗∗ |
| Platelet count (×103/μL) | 237.6 ± 75.08 | 212.72 ± 67.422 | 0.001∗ |
| Neutrophils (×103/μL) | 7.47 ± 9.76 | 9.76 ± 3.17 | <0.001∗∗ |
| Lymphocytes (×103/μL) | 1.76 ± 0.88 | 1.38 ± 0.68 | 0.03∗ |
| Serum albumin (g/L) | 36.37 ± 3.5 | 35.39 ± 3.37 | 0.005∗ |
| hs-CRP (mg/dL) | 7.34 ± 1.89 | 9.14 ± 2.03 | <0.001∗∗ |
| Fibrinogen (mg/dL) | 359.98 ± 42.24 | 457.62 ± 36.19 | <0.001∗∗ |
| CAR | 0.2 ± 0.05 | 0.25 ± 0.07 | <0.001∗∗ |
| FAR | 9.95 ± 1.27 | 13.02 ± 0.55 | <0.001∗∗ |
| AIP | 0.53 ± 0.18 | 0.64 ± 0.14 | <0.001∗∗ |
AIP: atherogenic index of plasma; BMI: body mass index; CADs: coronary artery diseases; CAR: C-reactive protein to albumin ratio; CK-MB: creatinine kinase-MB; DBP: diastolic blood pressure; ECG: electrocardiogram; FAR: fibrinogen to albumin ratio; GFR: glomerular filtration rate; LDL-C: low density lipoprotein cholesterol; LVEF: Left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; PVD: peripheral vascular disease; SBP: systolic blood pressure; TGs: triglycerides; TIMI: thrombolysis in myocardial infarction.
∗Statistically significant difference between two groups (p < 0.05).
∗∗Highly statistically significant difference between two groups (p < 0.001).
4.2. Clinical characteristics
Clinical parameters that were significantly higher in the no-reflow group were: symptoms-to-door (P < 0.001), time to reperfusion (P < 0.001), systolic and diastolic blood pressure (SBP and DBP) (P < 0.001 for each), and KILLIP classification (P < 0.001). LVEF was significantly lower in no-reflow group compared to normal flow group (P < 0.001). Baseline clinical characteristics are shown in (Table 1).
4.3. Laboratory characteristics
Baseline laboratory characteristics are shown in (Table 1). There was a highly significant increase in the values of CK-MB level (P < 0.001) and troponin I level (P < 0.001) in the no-reflow group, compared to normal flow group. Inflammatory parameters were more frequent in no-reflow group, where both leukocytes and neutrophil counts (P < 0.001), hs-CRP (P < 0.001) and fibrinogen levels were significantly higher (P < 0.001), compared to normal flow group.
Interestingly, in this study, the following novel parameters were significantly higher in no-reflow group: CAR (P < 0.001), FAR (P < 0.001), and AIP (P < 0.001), compared to normal flow group.
4.4. Angiographic and procedural characteristics
It was noted that, compared to normal flow group, the percentage of the totally occluded arteries, long and proximal lesions were significantly higher in the no-reflow group (P < 0.001, P = 0.001, P = 0.004, respectively). Moreover, heavy thrombus burden was more frequent in no-reflow group (P < 0.001). Also, compared to normal flow group, thrombus aspiration was needed in 81.4% of patients in no-reflow group (P < 0.001), with the use of GPI in 65.1% patients (P < 0.001). The amount of contrast used during PCI was significantly more in the no-reflow group (P < 0.001). Myocardial Blush Grade (MBG) after the procedure could be correlated to post-PCI TIMI flow (P < 0.001). Of note, the deployed stents were significantly longer in no-reflow group compared to normal flow group (30.18 ± 11.48 vs. 25.7 ± 10.04 mm, P < 0.001). However, there was no significant difference between both groups regarding the site of vascular access (P = 0.89), the need of balloon pre-dilatation (P = 0.15), and the number (P = 0.07) and diameter of used stents (P = 0.6) (Table 2).
Table 2.
Angiographic and procedural characteristics of the studied groups.
| Normal flow (TIMI = 3) (n = 228) | No reflow (TIMI <3) (n = 172) | P-value | |
|---|---|---|---|
| Angiographic characteristics | |||
| Culprit artery | 0.71 | ||
| LAD | 124 (54.4%) | 92 (53.5%) | |
| LCX | 26 (11.4%) | 16 (9.3%) | |
| RCA | 78 (34.2%) | 64 (37.2%) | |
| Type of occlusion | <0.001∗∗ | ||
| Total | 172 (75.4%) | 156 (90.7%) | |
| Subtotal | 56 (24.6%) | 16 (9.3%) | |
| Site of occlusion | 0.004∗ | ||
| Proximal | 108 (47.4%) | 100 (58.1%) | |
| Distal | 120 (52.6%) | 68 (39.5%) | |
| Mid segment | 0 (0%) | 4 (2.3%) | |
| Lesion length (mm) | 23.12 ± 9.21 | 27.83 ± 10.27 | 0.001∗ |
| Number of vessels | 0.56 | ||
| 1 | 124 (54.4%) | 96 (55.8%) | |
| 2 | 52 (22.8%) | 32 (18.6%) | |
| 3 | 52 (22.8%) | 44 (25.6%) | |
| Thrombus burden (heavy) | 80 (35.1%) | 140 (81.4%) | <0.001∗∗ |
| Procedural Characteristics | |||
| Vascular access | |||
| Radial | 36 (15.8%) | 28 (16.3%) | 0.89 |
| Femoral | 192 (84.2%) | 144 (83.7%) | |
| Balloon pre-dilatation | 116 (50.9%) | 100 (58.1%) | 0.15 |
| Stent Type | |||
| DES | 116 (50.9%) | 114 (66.3%) | 0.002∗ |
| BMS | 112 (49.1%) | 58 (33.7%) | |
| Number | |||
| 1 | 168 (73.3%) | 122 (70.9%) | 0.07 |
| 2 | 60 (26.3%) | 46 (26.7%) | |
| 3 | 0 (0%) | 4 (2.3%) | |
| Diameter (mm) | 3.15 ± 0.43 | 3.12 ± 0.55 | 0.6 |
| Length (mm) | 25.7 ± 10.04 | 30.18 ± 11.48 | <0.001∗∗ |
| Thrombus aspiration | 80 (35.1%) | 140 (81.4%) | <0.001∗∗ |
| GPI | 60 (26.3%) | 112 (65.1%) | <0.001∗∗ |
| Amount of contrast (ml) | 199.12 ± 68.47 | 228.02 ± 89.27 | <0.001∗∗ |
| MBG (Post -PCI) | |||
| 0 | 0 (0%) | 52 (30.2%) | <0.001∗∗ |
| 1 | 0 (0%) | 68 (39.5%) | |
| 2 | 96 (42.1%) | 52 (30.2%) | |
| 3 | 132 (57.9%) | 0 (0%) | |
BMS: bare-metal stent; DES: drug-eluting stent; GPI: glycoprotein inhibitor; LAD: left anterior descending artery; LCX: left circumflex artery; MBG: myocardial blush grade; PCI: percutaneous coronary intervention; RCA: right coronary artery; TIMI: thrombolysis in myocardial infarction.
∗Statistically significant difference between two groups (p < 0.05).
∗∗Highly statistically significant difference between two groups (p < 0.001).
4.5. Long term outcomes
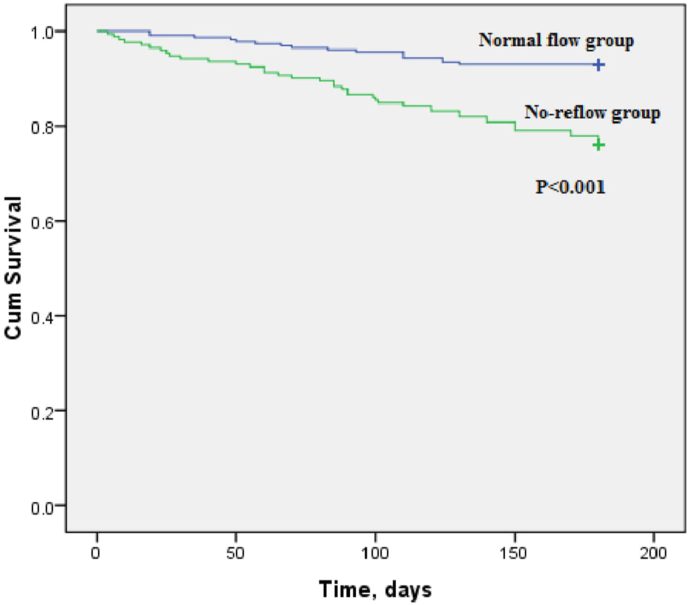
Kaplan–Meier curves between patients with no-reflow and those with normal flow for 6 months mortality revealed worse outcomes with higher mortality in no-reflow group (P < 0.001) (Fig. 1). Among other long term outcome parameters that were recorded in both groups within 6 months follow up, pump failure was found to be significantly higher in the no-reflow group than the normal flow group (37.2% vs. 17.5%, P < 0.001). However, there was no significant difference between both groups regarding other adverse outcomes as recurrent MI and malignant arrhythmia (Table 3).
Fig. 1.
Kaplan–Meier curve between normal flow group and no-reflow group for 6-months mortality. There is a highly significant difference between both group (P < 0.001) regarding 6-months mortality rate.
Table 3.
Long term outcomes of the studied groups.
| Normal flow (TIMI = 3) |
No reflow (TIMI <3) |
P-value |
|
|---|---|---|---|
| (n = 228) | (n = 172) | ||
| Mortality | 16 (7%) | 41 (23.8%) | <0.001∗∗ |
| Heart failure | 40 (17.5%) | 64 (37.2%) | <0.001∗∗ |
| Recurrent MI | 16 (7%) | 9 (5.2%) | 0.5 |
| Malignant arrhythmia | 48 (21.1%) | 24 (14%) | 0.07 |
MI: myocardial infarction; TIMI: thrombolysis in myocardial infarction; VF: ventricular fibrillation; VT: ventricular tachyarrhythmia.
∗Statistically significant difference between two groups (p < 0.05).
∗∗Highly statistically significant difference between two groups (p < 0.001).
4.6. Independent predictors of no-reflow phenomenon
Independent predictors of no-reflow phenomenon were summarized in (Table 4). In univariate logistic regression analysis, there were significant relations of age, symptom-to-door, time-to-reperfusion, KILLIP class ≥ II, CHA2DS2VASc score, CK-MB, troponin I level, heavy thrombus burden, hypertension, diabetes mellitus, total occlusion, CAR, FAR, and AIP with incidence of no-reflow phenomenon (P < 0.001). In multivariate logistic regression analysis, it was found that age (P < 0006), diabetes mellitus (P < 0.04), troponin I level (P < 0.04), heavy thrombus burden (P < 0.001), CAR (P < 0.001), FAR (P < 0.001), and AIP (P = 0.04) were independent predictors of no-reflow phenomenon.
Table 4.
Univariate and multivariate regression analysis for the parameters affecting no reflow cases (TIMI <3).
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| P-value | OR (95% C.I) | P-value | OR (95% C.I) | |
| Age (years) | <0.001∗∗ | 1.072∗ | 0.006∗ | 1.115∗ |
| (1.050–1.093) | (1.032–1.205) | |||
| Diabetes mellitus | <0.001∗∗ | 2.751∗ | 0.04∗ | 4.401∗ |
| (1.813–4.175) | (1.081–17.923) | |||
| Hypertension | <0.001∗∗ | 2.954∗ | 0.16 | 2.518 |
| (1.946–4.484) | (0.701–9.045) | |||
| CHA2DS2VASc score | <0.001∗∗ | 1.759∗ | 0.06 | 0.460 |
| (1.496–2.067) | (0.205–1.030) | |||
| Symptoms-to-door | <0.001∗∗ | 1.129∗ | 0.16 | 0.335 |
| (1.085–1.174) | (0.073–1.543) | |||
| Time to reperfusion | <0.001∗∗ | 1.132∗ | 0.12 | 3.412 |
| (1.088–1.177) | (0.739–15.756) | |||
| KILLIP class | <0.001∗∗ | 3.073∗ | 0.79 | 0.845 |
| (class I vs.class ≥ II) | (1.944–4.859) | (0.245–2.908) | ||
| CK-MB | <0.001∗∗ | 1.002∗ | 0.25 | 0.998 |
| (1.001–1.004) | (0.994–1.002) | |||
| Troponin (ng/ml) | <0.001∗∗ | 1.033∗ | 0.04∗ | 1.040∗ |
| (1.020–1.046) | (1.001–1.080) | |||
| CAR | <0.001∗∗ | 6.094∗ | < 0.001∗∗ | 3.357∗ |
| (4.284–8.668) | (2.288–4.927) | |||
| FAR | <0.001∗∗ | 5.099∗ | < 0.001∗∗ | 4.187∗ |
| (3.761–6.912) | (2.761–6.349) | |||
| AIP | <0.001∗∗ | 97.070∗ | 0.04∗ | 16.794∗ |
| 23.312–404.196 | (1.018–277.01) | |||
| Type of occlusion | <0.001∗∗ | 3.174∗ | 0.66 | 0.722 |
| (total vs. subtotal occlusion) | (1.749–5.763) | (0.170–3.060) | ||
| Thrombus burden (heavy) | <0.001∗∗ | 8.094∗ | <0.001∗∗ | 16.915∗ |
| (5.056–12.958) | (5.055–56.602) | |||
AIP: atherogenic index of plasma; CAR: C-reactive protein to albumin ratio; C.I: confidence interval; CK-MB: creatinine kinase-MB; FAR: fibrinogen to albumin ratio; OR: odds ratio.
∗Statistically significant difference between two groups (p < 0.05).
∗∗Highly statistically significant difference between two groups (p < 0.001).
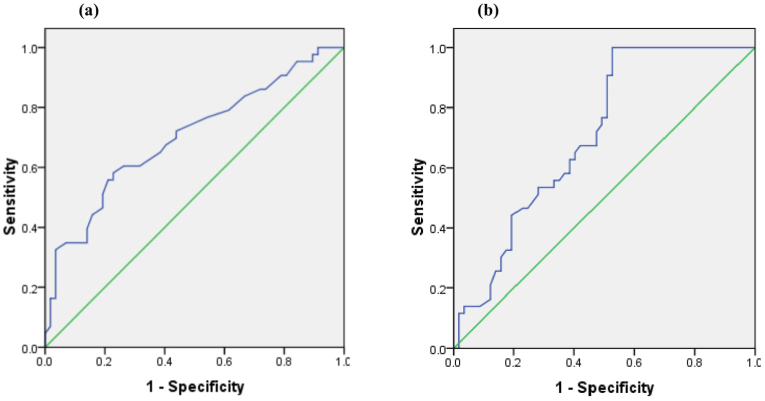
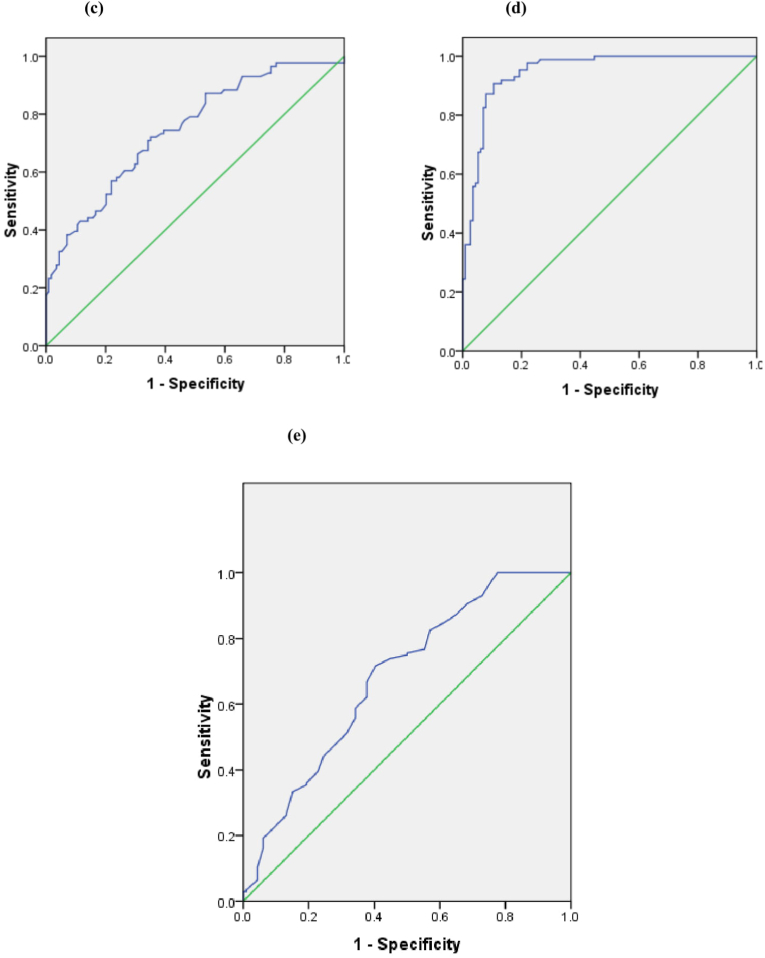
ROC statistical analyses showed that age >56 years (sensitivity 72.1%, specificity 56.1%), troponin I > 5 ng/mL (sensitivity 76.7%, specificity 50.9%), CAR > 0.21 (sensitivity 74.4%, specificity 55.3%), FAR > 11.56 (sensitivity 87.2%, specificity 89.5%), and AIP > 0.52 (sensitivity 75.6%, specificity 50%) were the best cut-off values for predicting no-reflow phenomenon (Fig. 2).
Fig. 2.
Receiver operating characteristic (ROC) curve (a) the cut-off age value on the ROC curve was >56 years old with sensitivity of 72.1% and specificity of 56.1%, (b) the cut-off serum troponin value on the ROC curve was >5 ng/mL with sensitivity of 76.7% and specificity of 50.9%, (c) the cut-off CAR value on the ROC curve was >0.21 with sensitivity 74.4% and specificity 55.3%, (d) the cut-off FAR value on the ROC curve was >11.56 with sensitivity 87.2%, specificity 89.5%, and (e) the cut-off AIP value on the ROC curve was >0.52 with sensitivity 75.6%, specificity 50%.
5. Discussion
The main findings of this study are summarized as follows: 1) Old age, history of diabetes mellitus, high troponin levels, and heavy thrombus burden, all were found to be independent predictors of incidence of no-reflow in STEMI patients treated with primary PCI.; 2) CAR, FAR, and AIP could be considered as novel predictors for the development of no-reflow phenomenon; 3) Adverse long term outcomes as higher mortality and pump failure were more frequent in patients with no-reflow.
Previous studies documented that increased incidence of no-reflow phenomenon was related to higher CHA2DS2VASc score11 in addition to other individual traditional risk factors as old age, hypertension, higher KILLIP class,17 higher BMI,18,19 diabetes mellitus.20 The present study confirmed these findings, and this may be attributed to neuro-hormonal activation17 and the fact that hypertension may induce interstitial fibrosis and remodelling of the small intra-myocardial vessels,17 in addition to coronary micro-vascular dysfunction induced by diabetes mellitus.20
It was previously stated that no-reflow may be related to some clinical predictors.21 In this study, patients with no-reflow had higher troponin I and creatine kinase-MB (CK-MB) (P < 0.001) with more prolonged symptoms to door and time to reperfusion (P < 0.001) times, compared to those with normal flow. Also, the incidence of no-reflow was associated with decreased LVEF on admission (P < 0.001) with no significant superiority of anterior MI over inferior MI regarding the incidence of no-reflow (P = 0.87). These data are concordant with Mazhar et al,2 Sabin et al,3 and Fajar et al.17 However, Ipek et al11 and Tanaka et al22 found no significant difference in incidence of no-reflow in relation to time-to reperfusion.
Regarding the angiographic predictors, total and proximal occlusions were associated with increased incidence of no-reflow.23 This study confirmed this hazardous correlation which may result in larger areas of myocardial infarction with more significant hemodynamic instability.20 In addition, no-reflow in this study was associated with lower MBG, Longer lesion, thrombus aspiration, lower pre-procedural TIMI flow and more amount of used contrast (P < 0.001). This is concordant with Sabin et al,3 Jeong et al,24 and the data obtained from the meta-analysis conducted by Fajar et al.17
This study confirmed also the role of inflammatory parameters in no-reflow development with significantly higher leukocyte and neutrophil counts (P < 0.001) and lower lymphocyte count (P < 0.001), compared to normal reflow group. This finding is supported by the retrospective study of Kosuge et al25 and Takahashi et al26 who stated that a higher leucocytic count was an independent predictor of no reflow in patients with a first anterior AMI. The underlying mechanism is complex. Ischemic injury damages myocardiocytes with subsequent neutrophil plugging and release of oxygen-free radicals leading to endothelial injury.20,27
The effect of inflammatory biomarkers; as hs-CRP, fibrinogen, and albumin levels; on no-reflow development has been more clearly investigated in this study. Serum albumin is the major serum protein that participates in inflammatory response. Fibrinogen is also a biomarker of chronic inflammation, which is known to be a precursor to fibrin and accelerates platelet aggregation. CRP is one of the most commonly used biomarkers with a positive correlation with no-reflow in STEMI patients who underwent primary PCI.6
The present study confirmed this link between these inflammatory mediators and no-reflow development. It was noted that CRP and fibrinogen levels were significantly higher (P < 0.001), and the albumin level was significantly lower (P = 0.005) in no-reflow group than in normal flow group.
Previous studies have demonstrated that AIP is strongly associated with CAD. Furthermore, AIP has been reported to be a strong independent predictor of all-cause mortality in ACS after coronary revascularization.7 In concordance with these studies, our study revealed that AIP was significantly higher in no-reflow group than normal flow group (P < 0.001).
Of note, multivariate regression analysis in our study stated that CAR, FAR, AIP could be considered as novel powerful independent predictors for the development of no-reflow phenomenon (OR = 3.357, 95% CI: 2.288–4.927, P < 0.001, OR = 4.187, 95% CI: 2.761–6.349, P < 0.001, OR = 16.794, 95% CI: 1.018–277.01, P = 0.04, respectively). The best cut-off values were >0.21 for CAR (sensitivity 76.7%, specificity 50.9%), >11.56 for FAR (sensitivity 87.2%, specificity 89.5%), and >0.52 for AIP (sensitivity 75.6%, specificity 50%).
The exact underlying pathophysiology between these novel predictors and no-reflow phenomenon remained controversial. Serum albumin is an important inhibitor of platelet activation and aggregation and is an important mediator of platelet induction.28 Raised CAR can be linked to the diminished coronary microvascular reactions to both independent vasodilators and endothelium-dependent stimuli.29 The underlying mechanism between higher levels of FAR and no-reflow development could be attributed to the fact that low albumin levels promotes fibrinolysis, thereby inhibiting the physiological fbrinolytic system and reducing the spontaneous dissolution of the thrombus.30 The association of AIP with no-reflow may be a consequence of that inflammatory process, where the remnant particles formed by lipolysis of TG-rich lipoproteins may participate in vascular wall inflammation.31
Other independent predictors for incidence of no-reflow phenomenon were old age (OR = 1.115, 95% CI: 1.032–1.205, P = 0.006) with cut off value > 56 years (sensitivity 72.1%, specificity 56.1%), higher troponin level (OR = 1.040, 95% CI: 1.001–1.080, P = 0.04) with cut-off value > 5.6 ng/mL (sensitivity 76.7%, specificity 50.9%), and heavy thrombus burden (OR = 16.915, 95% CI: 5.055–56.602, P < 0.001). Also, diabetes mellitus was considered as important independent clinical predictors of no-reflow phenomenon (OR = 4.401, 95% CI: 1.081–17.923, P = 0.04), and this may be attributed to the effects of diabetes mellitus on both micro-vasculature and macro-vasculature.20
Regarding long term outcome of no-reflow phenomenon in our study, Kaplan–Meier curve revealed a significant difference between no-reflow and normal groups for 6 months mortality (P < 0.001). Also, incidence of pump failure (P < 0.001) was strongly related to incidence of no-reflow. This data is concordant with that obtained from Uyarel et al32 and Acet et al.33 However, there were no significant differences regarding recurrent MI and malignant arrhythmias between both groups.
6. Limitations
The present study has some limitations. First, this study included relatively small numbers of patients as the results were obtained from only one centre. Therefore, a larger study population with no-reflow phenomenon is needed in future studies. Second, the present study was a cross-sectional observational one. However, data processing, and statistical analyses were conducted by independent research personnel. Finally, duration of follow-up was relatively short. So, further studies with longer follow-up periods are needed.
7. Conclusions
The no-reflow phenomenon may be related to variable clinical, angiographic and inflammatory predictors, with subsequent development of adverse long term outcomes as high mortality and pump failure. This study confirmed the valuable role of CAR, FAR, and AIP as novel predictors for the development of no-reflow phenomenon.
What is already known?
-
•
No-reflow occurs in 25% of STEMI patients undergoing primary angioplasty.
-
•
Micro-vascular obstruction due to thrombosis, distal embolization, and microvascular spasm are the suggested mechanisms for no-reflow.
-
•
Prediction of patients at risk for no-reflow before primary angioplasty may be beneficial from the perspective of prevention.
What this study adds?
-
•
This study confirmed the valuable role of some novel predictors; as CAR, FAR and AIP, for the development of no-reflow phenomenon with subsequent development of adverse long term outcomes as high mortality and pump failure.
Authorship
All authors have made substantial contributions to all of the following: (1) the conception and study design, data acquisition, and data analysis and interpretation, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Declaration of competing interest
There is no conflict of interests.
Acknowledgement
None.
References
- 1.Choudhary S. Association of syntax score with short-term outcomes among acute ST-elevation myocardial infarction patients undergoing primary PCI. Indian Heart J. 2017;69(1):S20–S23. doi: 10.1016/j.ihj.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazhar J., Mashicharan M., Farshid A. Predictors and outcome of no-reflow post primary percutaneous coronary intervention for ST elevation myocardial infarction. Int J Cardiol Heart Vasc. 2016;10:8–12. doi: 10.1016/j.ijcha.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabin P., Koshy A.G., Gupta P.N. Predictors of no-reflow during primary angioplasty for acute myocardial infarction, from Medical College Hospital, Trivandrum. Indian Heart J. 2017;69(1):S34–S45. doi: 10.1016/j.ihj.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezkalla S.H., Stankowski R.V., Hanna J. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. 2017;10(3):215–223. doi: 10.1016/j.jcin.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S., Gupta M.M. No reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J. 2016;68(4):539–551. doi: 10.1016/j.ihj.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian J., Liu Y., Liu Y. Prognostic association of circulating neutrophil count with no-reflow in patients with ST-segment elevation myocardial infarction following successful primary percutaneous intervention. Dis Markers. 2017 doi: 10.1155/2017/8458492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan K., Zhao J., Huang H. The association between triglyceride/high-density lipoprotein cholesterol ratio and all-cause mortality in acute coronary syndrome after coronary revascularization. PloS One. 2015;10(4) doi: 10.1371/journal.pone.0123521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 9.Refaat H., Niccoli G., Gramegna M. Optical coherence tomography features of angiographic complex and smooth lesions in acute coronary syndromes. Int J Cardiovasc Imag. 2015;31(5):927–934. doi: 10.1007/s10554-015-0632-z. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi M., Ako J., Morimoto T. Modifiable factors associated with prolonged door to balloon time in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Ves. 2018;33(10):1139–1148. doi: 10.1007/s00380-018-1164-y. [DOI] [PubMed] [Google Scholar]
- 11.Ipek G., Onuk T., Karatas M.B. CHA2DS2-VASc score is a predictor of no-reflow in patients with ST-segment elevation myocardial infarction who underwent primary percutaneous intervention. Angiology. 2016;67(9):840–845. doi: 10.1177/0003319715622844. [DOI] [PubMed] [Google Scholar]
- 12.Barman H.A., Kahyaoglu S., Durmaz E. The CHADS-VASc score is a predictor of no-reflow in patients with non-ST-segment elevation myocardial infarction. Coron Artery Dis. 2020;31(1):7–12. doi: 10.1097/MCA.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 13.Dobiasova M., Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterifcation rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34(7):583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 14.Ibanez B., James S., Agewall S. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2017;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 15.Chesebro J.H., Knatterud G., Roberts R. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76(1):142–154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 16.Windecker S., Kolh P., Alfonso F. ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS) developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI) Eur Heart J. 2014;35(37):2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 17.Fajar J.K., Heriansyah T., Rohman M.S. The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: a meta-analysis. Indian Heart J. 2018;70(3):S406–S418. doi: 10.1016/j.ihj.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison R.W., Aggarwal A., Ou F.S. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol. 2013;111(2):178–184. doi: 10.1016/j.amjcard.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Katayama T., Kubo N., Takagi Y. Relation of atherothrombosis burden and volume detected by intravascular ultrasound to angiographic no-reflow phenomenon during stent implantation in patients with acute myocardial infarction. Am J Cardiol. 2006;97(3):301–304. doi: 10.1016/j.amjcard.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Durante A., Camici P.G. Novel insights into an “old” phenomenon: the no reflow. Int J Cardiol. 2015;187:273–280. doi: 10.1016/j.ijcard.2015.03.359. [DOI] [PubMed] [Google Scholar]
- 21.Ndrepepa G., Tiroch K., Keta D. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv. 2010;3(1):27–33. doi: 10.1161/CIRCINTERVENTIONS.109.896225. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka A., Kawarabayashi T., Nishibori Y. No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation. 2002;105(18):2148–2152. doi: 10.1161/01.cir.0000015697.59592.07. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H., He X.Y., Zhuang S.W. Clinical and procedural predictors of no-reflow in patients with acute myocardial infarction after primary percutaneous coronary intervention. World J Emerg Med. 2014;5(2):96–102. doi: 10.5847/wjem.j.issn.1920-8642.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong Y.H., Kim W.J., Park D.W. Serum B-type natriuretic peptide on admission can predict the ‘no-reflow’ phenomenon after primary drug-eluting stent implantation for ST-segment elevation myocardial infarction. Int J Cardiol. 2010;141(2):175–181. doi: 10.1016/j.ijcard.2008.11.189. [DOI] [PubMed] [Google Scholar]
- 25.Kosuge M., Kimura K., Ishikawa T. Relation between white blood cell counts and myocardial reperfusion in patients with recanalized anterior acute myocardial infarction. Circ J. 2004;68(6):526–531. doi: 10.1253/circj.68.526. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T., Hiasa Y., Ohara Y. Relation between neutrophil counts on admission, microvascular injury, and left ventricular functional recovery in patients with an anterior wall first acute myocardial infarction treated with primary coronary angioplasty. Am J Cardiol. 2007;100(1):35–40. doi: 10.1016/j.amjcard.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 27.Wong D.T., Puri R., Richardson J.D. Myocardial ‘no-reflow’-diagnosis, pathophysiology and treatment. Int J Cardiol. 2013;167(5):1798–1806. doi: 10.1016/j.ijcard.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 28.Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005;39(4):S143–S146. doi: 10.1097/01.mcg.0000155514.17715.39. [DOI] [PubMed] [Google Scholar]
- 29.Karabağ Y., Çağdaş M., Rencuzogullari I. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Invest. 2018;48(6) doi: 10.1111/eci.12928. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y., Yang J., Ji Y. Usefulness of fibrinogen-to-albumin ratio to predict no-reflow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Ves. 2019;34(10):1600–1607. doi: 10.1007/s00380-019-01399-w. [DOI] [PubMed] [Google Scholar]
- 31.Süleymanoğlu M., Rencüzoğullari I., Karabağ Y. The relationship between atherogenic index of plasma and no-reflow in patients with acute ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention. Int J cardiovasc Imaging. 2020;36(5):789–796. doi: 10.1007/s10554-019-01766-8. [DOI] [PubMed] [Google Scholar]
- 32.Uyarel H., Ayhan E., Cicek G. Suboptimal coronary blood flow after primary percutaneous coronary intervention for acute myocardial infarction: incidence, a simple risk score, and prognosis. Coron Artery Dis. 2012;23(2):98–104. doi: 10.1097/MCA.0b013e32834f1b8a. [DOI] [PubMed] [Google Scholar]
- 33.Acet H., Ertaş F., Akil M.A. The utility of the TIMI risk index on admission for predicting angiographic no-reflow after primary percutaneous coronary intervention in patients with STEMI. Turk J Med Sci. 2016;46(3):604–613. doi: 10.3906/sag-1411-157. [DOI] [PubMed] [Google Scholar]