Abstract
In the adult mammalian hippocampus, new neurons arise from stem and progenitor cell division, a process known as adult neurogenesis. Adult-generated neurons are sensitive to experience and may participate in hippocampal functions, including cognition, anxiety/stress regulation, and social behavior. Increasing evidence emphasizes the importance of new neuron connectivity within the hippocampal circuitry for understanding the impact of adult neurogenesis on brain function. In this Review, we discuss how the functional consequences of new neurons arise from the collective interactions of presynaptic and postsynaptic neurons, glial cells, and the extracellular matrix, which together form the “tetrapartite synapse”.
Introduction
It is now generally accepted that stem cells reside in the adult mammalian brain, and in certain regions, these cells divide in situ and give rise to new neurons, a phenomenon referred to as adult neurogenesis. In the hippocampus, adult neurogenesis produces one type of neuron – the dentate gyrus granule cell. The process begins with the largely asymmetric division of radial glial stem cells residing in the subgranular zone (sgz), a region just between the granule cell layer (gcl) and the hilus (Seri et al., 2001). These cells have the capacity to self-renew and to generate either neurons or astrocytes (Bonaguidi et al., 2011; Suh et al., 2007). The daughter cells of radial glial stem cells are highly proliferative amplifying stem cells, which in turn generate neuroblasts (Gonçalves et al., 2016a; Suh et al., 2007) (Figure 1A). The cell bodies of these new neurons then migrate the short distance from the sgz into the gcl where they begin their differentiation into granule neurons (Gonçalves et al., 2016a) (Figure 1A). These granule neurons then can integrate into the circuitry of the hippocampus, where they subsequently can impact behavioral function.
Figure 1. Overview of adult neurogenesis in the hippocampus, the circuitry into which the new cells are incorporated, and their involvement in behavior.
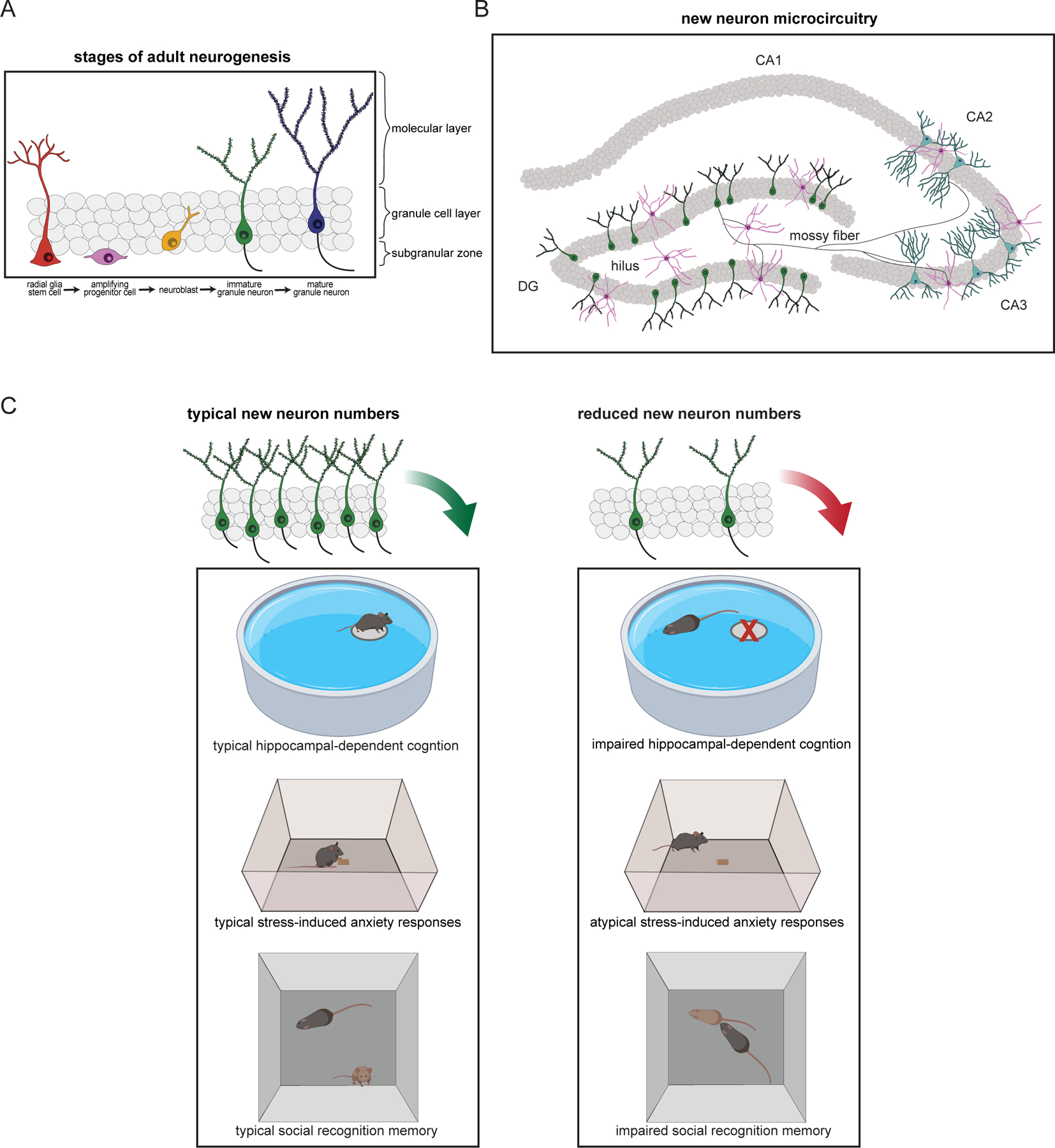
(A) Different stages of adult neurogenesis in the dentate gyrus (DG) of the hippocampus. New granule neurons originate from radial glial stem cells residing in the subgranular zone. These stem cells give rise to amplifying progenitor cells that in turn produce neuroblasts. The neuroblasts migrate into the granule cell layer and develop into immature granule neurons with a single dendritic tree extending toward the molecular layer and an axon (mossy fiber, mf) extending through the hilus toward the CA3 and CA2 subfields. (B) Schematic diagram of new neuron projections, showing the different hippocampal subfields (DG, CA3, CA2, and CA1) which serve as new neuron targets. Mossy fibers from new granule neurons form connections with hilar mossy cells, pyramidal cells in the CA3 and CA2, as well as with inhibitory interneurons in the DG, hilus, and CA3. (C) New granule neurons have been linked to behaviors associated with the hippocampus, such as spatial navigation learning, anxiety/stress regulation, and social cognition. Compared to rodents with typical numbers of new neurons (left), rodents with reduced adult neurogenesis (right) are impaired on some cognitive tests (for example, unable to locate the location of a hidden platform in the Morris water maze), have atypical responses in anxiety tests (for example, different latencies to approach food in the novelty suppressed feeding task), and impaired social cognition (for example, inability to recognize previously encountered mice in a social interaction test).
In order to understand the functional consequences of adult neurogenesis, it is necessary to identify the circumstances that modulate the connectivity of newly generated neurons. The majority of research on this topic has focused exclusively on connectivity between neurons, without consideration of the influence of nonneuronal cells or the extracellular milieu in which new connections are formed. A growing literature suggests that understanding synaptic function and plasticity requires consideration of both glial cells as well as the extracellular matrix (ECM), which together with the presynaptic and postsynaptic neurons form the “tetrapartite synapse” (Dityatev and Rusakov, 2011; Smith et al., 2015a). In this review, we consider the formation of new neurons from stem cells in the adult hippocampus, with specific relevance to the integration of new neurons into the circuitry and their impact on brain function. To review this material in a comprehensive and functionally meaningful way, we consider the production and integration of new neurons in the context of the tetrapartite synapse with specific reference to the influence of glia and the ECM on adult neurogenesis and hippocampal function.
Evidence of adult neurogenesis and the integration of new neurons into the hippocampal circuitry
Although adult neurogenesis is robust in the hippocampus of rodents, the extent to which it exists in humans has been a matter of continued debate. Evidence that adult neurogenesis exists for a large number of species, ranging from rodents (Jessberger and Gage, 2014; Snyder et al., 2009a) to monkeys (Gould et al., 1998, 1999), strongly suggests that this phenomenon is common to most mammalian species. In the rodent brain, the number of new neurons is substantial, with as many as 9,000 new granule cells being added to the young adult rat dentate gyrus daily (Cameron and McKay, 2001). Relatively high numbers of new neurons have also been reported in the adult human hippocampus (Boldrini et al., 2018; Eriksson et al., 1998; Knoth et al., 2010; Spalding et al., 2013). A few studies contradict these findings (Cipriani et al., 2018; Sorrells et al., 2018), but the majority of reports provide positive evidence for adult neurogenesis in the hippocampus of humans, suggesting that studying this process in rodents is likely relevant to understanding human brain plasticity. For this review, evidence from rodent studies will be considered except in cases otherwise stated.
Once stem cell division produces immature neurons, these cells undergo an extensive maturation process that includes the growth of axons and dendrites and the formation of synaptic connections. Within 2 weeks of their generation, new neurons begin to take on the morphological characteristics of mature granule cells with a single dendritic tree extending toward the molecular layer and the formation of dendritic spines, primary sites of excitatory synapses (Zhao et al., 2006) (Figure 1A). Beginning less than 1 week after their generation, new granule cells extend axons, known as mossy fibers, through the hilus toward the CA3 and CA2 subregions of the hippocampus (Llorens-Martín et al., 2015; Zhao et al., 2006). Mossy fibers from new granule cells begin to form synapses with excitatory pyramidal cells in the CA3 and CA2 subregions (Gu et al., 2012; Llorens-Martín et al., 2015; Toni et al., 2008) as well as with hilar mossy cells (Toni et al., 2008) by approximately 2–3 weeks post-mitosis (Figure 1B).
Much research has focused on understanding how and when new granule cells integrate into the circuitry of the hippocampus. Mature granule cells are known to receive synaptic input from local GABAergic inhibitory interneurons, local and cortical glutamatergic inputs, and basal forebrain/midbrain/hindbrain neuromodulatory (dopaminergic, cholinergic, noradrenergic) nuclei (Deshpande et al., 2013; Vivar et al., 2012). The formation of synapses onto new neurons is known to develop within a week or two of their generation, with the first inputs coming from local GABAergic inhibitory interneurons (Espósito et al., 2005; Ge et al., 2006; Markwardt et al., 2009) followed by glutamatergic input from mossy cells in the hilus (Chancey et al., 2014) and then from the entorhinal cortex via the perforant path a few weeks later (Espósito et al., 2005; Ge et al., 2006). Neuromodulatory input to adult-generated neurons occurs later (Deshpande et al., 2013; Vivar et al., 2012), as does the formation of mature inhibitory inputs from local inhibitory interneurons (Espósito et al., 2005), all of which strongly resemble those of developmentally-generated granule cells within 4–6 weeks post-mitosis (Laplagne et al., 2006) On the output side, adult-generated granule cells form synapses with several populations of neurons within the hippocampus, including mossy cells in the hilus (Toni et al., 2008), pyramidal cells of the CA2 and CA3 regions (Llorens-Martín et al., 2015; Toni et al., 2008), as well as inhibitory interneurons in the dentate gyrus itself and the CA3 region (Drew et al., 2016; Restivo et al., 2015) (Figure 1B).
Function of adult-generated neurons
One of the defining characteristics of a neuron is its ability to generate action potentials. Electrophysiological studies of new cells produced in the adult dentate gyrus have shown that this capability emerges relatively quickly (within a week) post-mitosis. For some time thereafter, new granule cells exhibit excitatory instead of inhibitory responses to typically inhibitory neurotransmitter GABA (Chancey et al., 2013), suggesting that these cells are more likely to fire than mature granule cells, which are strongly inhibited (Amaral et al., 2007; Pelkey et al., 2017). Adult-generated granule neurons also display other unusual electrophysiological properties that disappear as they reach full maturity, including enhanced synaptic plasticity with a lower threshold for LTP induction (Ge et al., 2007; Schmidt-Hieber et al., 2004). The heightened plasticity of adult-generated neurons make them uniquely positioned to play a role in hippocampal-related functions, of which there are many candidates.
Although the hippocampus was originally recognized for its role in certain types of learning and memory (Eichenbaum, 2017), more recent evidence suggests it plays an important role in anxiety and stress regulation (Kheirbek et al., 2013), as well as certain aspects of social behavior (Opendak and Gould, 2015). Studies have shown that different parts of the hippocampus serve these different functions, with the dorsal hippocampus supporting spatial navigation learning and memory, and the ventral hippocampus as important for anxiety and stress regulation (Fanselow and Dong, 2010; Kheirbek et al., 2013). Since new neurons are generated throughout the dorsoventral extent of the dentate gyrus, the possibility that they might participate in more than one hippocampal function seems high. Indeed, adult-born neurons have been causally linked to many of the known functions and behaviors associated with the hippocampus. Much of this evidence comes from studies that knockdown or eliminate new neurons in the adult brain using transgenic, pharmacological, or focal x-irradiation approaches. Reports have shown that reduction of new neurons impairs cognitive performance on hippocampal-dependent tests, including object memory, contextual fear conditioning, as well as spatial learning and memory in the Morris water maze, Barnes maze, and radial arm maze (reviewed in Cameron and Glover, 2015; Gonçalves et al., 2016a) (Figure 1C). On the other hand, contradictory studies have found either no effect of reduced neurogenesis on hippocampal cognition (Groves et al., 2013; Saxe et al., 2006; Shors et al., 2002) or only on certain types of hippocampal cognitive tests (Shors et al., 2002). One study has even found that the absence of new neurons improves cognitive function (Saxe et al., 2007). Furthermore, new neurons, presumably through their unique electrophysiological properties and sparse connections to CA3 neurons (Amaral et al., 2007), have been found to participate in pattern separation (Clelland et al., 2009; Danielson et al., 2016; Sahay et al., 2011), the process of making similar neural inputs distinct. Elimination or optogenetic silencing of adult-born neurons impairs pattern separation-like behaviors (Clelland et al., 2009; Danielson et al., 2016), while transgenic increases in adult neurogenesis enhance the ability of mice to distinguish between highly similar contexts (Sahay et al., 2011). One study, however, reported that pattern separation-like behaviors did not differ between transgenic rats with reduced neurogenesis and controls with typical new neuron numbers (Groves et al., 2013). While reports from direct manipulations of adult neurogenesis often lack consistent results, the majority of studies suggest that new neurons are involved in some aspect of hippocampal-related cognition.
Reduced numbers of new neurons have also been associated with excessive anxiety (Revest et al., 2009), but most studies suggest that the role of new neurons in anxiety regulation only comes into play under conditions of stress (Figure 1C). Adult neurogenesis in the hippocampus seems to provide resilience to stress-induced anxiety (Anacker et al., 2018; Hill et al., 2015; Snyder et al., 2011). Studies have shown impairments in social behavior tests in rodents lacking new neurons. Adult neurogenesis in the hippocampus has been shown to be necessary for stress-induced social avoidance (Lagace et al., 2010), as well as in social cognition (Garrett et al., 2015) (Figure 1C). Mice with fewer new neurons were unable to discriminate between novel and previously encountered mice (Garrett et al., 2015). Taken together, this evidence suggests that increased numbers of new neurons are associated with improved hippocampal function, while decreased numbers of new neurons are associated with deficient hippocampal function.
Paradoxical effects of adult neurogenesis on hippocampal function
Some results from brain injury models suggest that a simple interpretation that more new neurons improve hippocampal function is not always the case. Brain injury models, such as stroke, seizure, and blunt trauma, all increase the rate of adult neurogenesis (Table 1), yet each of these conditions is associated with reduced cognitive function (Cuartero et al., 2019; Parent and Lowenstein, 2002; Sun et al., 2015). While damage-induced neurogenesis could be an adaptive mechanism to replace dead cells, the stroke and seizure literature does not support this hypothesis. In fact, inhibiting adult neurogenesis reduces the frequency of chronic seizures along with seizure-related cognitive decline (Cho et al., 2015) as well as stroke-induced cognitive decline (Cuartero et al., 2019). Since integration of new granule neurons into the dentate gyrus unavoidably modifies the existing synaptic circuitry, one possible interpretation of these paradoxical findings is that damage-induced adult neurogenesis causes abnormal synaptic connectivity. In fact, seizures induce faster maturation of new granule neurons including early and more extensive mossy fiber sprouting and dendritic outgrowth as well as aberrant basal dendritic trees extending into the hilus, which receive recurrent excitatory drive from mossy fibers (Overstreet-Wadiche et al., 2006; Shapiro and Ribak, 2006) (Table 1). Moreover, accelerated and atypical excitatory and inhibitory synapse formation alters the excitability of the DG (Jakubs et al., 2006; Overstreet-Wadiche et al., 2006), which may be further exaggerated by the loss of inhibitory interneurons that often co-occurs with seizures (Kuruba et al., 2011). Some new granule neurons born after seizures abnormally migrate into the hilus/CA3 region where they atypically synchronize their firing activity with CA3 pyramidal cells (Scharfman et al., 2000).
Table 1.
Effects of Experience and Pathological Conditions on Production, Survival, and Connectivity of New Neurons
Refers to studies with differing effects.
Additional work examining an animal model of depression also produced results that raise questions about whether increased numbers of new neurons necessarily promote healthy hippocampal function. Treatment with the antidepressant fluoxetine resulted in an overproduction of new neurons in the adult hippocampus (Alves et al., 2017) (Table 1). This effect, which at first glance appeared to be beneficial, was surprisingly associated with cognitive decline, as well as increased susceptibility to stress-induced depression and excessive anxiety (Alves et al., 2017). Pharmacological blockade of adult neurogenesis rescued the deleterious behavioral effects induced by antidepressant treatment strongly suggesting that in this case, the new neurons impaired hippocampal function (Alves et al., 2017). Closer examination of the new neurons in this case revealed that when overproduced, their dendritic trees were stunted and this likely impaired, instead of promoted, function. It is worth mentioning that these effects of antidepressants may not be limited to new granule neurons. One study showed that fluoxetine treatment causes dematuration of adult granule neurons such that their functional characteristics resemble immature granule neurons (Kobayashi et al., 2010). Taken together with the findings from seizure and stroke studies, these data suggest the effect that new neurons have on behavior is not simply a direct linear relationship where more necessarily leads to improved function. Instead, the findings emphasize the importance of new neuron connectivity in determining their influence over hippocampal circuitry. As introduced above, functionally relevant aspects of new neuron connectivity likely involve not only connections with other neurons, but also associations with glial cells and the ECM.
Rewarding experiences alter adult neurogenesis
Adult neurogenesis is highly sensitive to environmental stimuli, suggesting that new neurons may serve as an important substrate for experience-dependent change. Several experiences that are rewarding for rodents, such as voluntary physical exercise, environmental enrichment, and sexual experience, robustly enhance the number of new neurons (reviewed in Opendak and Gould, 2015). In some of these cases, e.g., physical exercise, evidence suggests that the number of new neurons is increased by actions at multiple stages in the adult neurogenesis process, including by stimulating the proliferation of radial stem cells and amplifying progenitor cells, leading to an increase in the production of neuroblasts (Bednarczyk et al., 2010; Kronenberg et al., 2006; Lugert et al., 2010; Nokia et al., 2016) (Table 1). After these new cells are produced and start down the path toward neuronal differentiation, physical exercise enhances their survival, as it increases the development of their dendrites, and the complexity of both their afferent and efferent connections (Ambrogini et al., 2013; Gonçalves et al., 2016b; Llorens-Martín et al., 2015; Snyder et al., 2009b; Zhao et al., 2015) (Table 1). Given the positive influence physical exercise has over virtually all aspects of adult neurogenesis, it is not surprising that this experience also enhances function of the hippocampus. Indeed, numerous studies have shown that physical exercise improves performance on hippocampus-dependent cognitive tasks (reviewed in Vivar et al., 2013) and reduces anxiety-like behaviors (Schoenfeld et al., 2013). Studies have shown similar findings with enriched environment living with enhanced stem cell production, progenitor cell proliferation, immature neuron production, cell survival, and dendritic differentiation (Ambrogini et al., 2013; Bednarczyk et al., 2010; Kempermann et al., 1997; Nilsson et al., 1999; Vega-Rivera et al., 2016) (Table 1), although no studies yet have investigated how enriched environment living alters the formation of new connections by the axons of adult-generated neurons. Also along the lines of research on the effects of physical exercise, enriched environment living is also associated with improved hippocampal function (reviewed in Simpson and Kelly, 2011). Studies have also investigated whether improved function after physical exercise or enriched environment living requires the increase in adult neurogenesis and those findings for the most part establish causality with regard to cognitive function with more equivocal results in studies attempting to causally link new neurons with anxiety reduction (Clark et al., 2008; Meshi et al., 2006; Onksen et al., 2012). These results are generally consistent with what is known about the effects of sexual experience in male rodents where increases in the numbers of adult-generated neurons have been reported (Glasper and Gould, 2013; Kim et al., 2013; Leuner et al., 2010) (Table 1). However, this phenomenon has been explored much less thoroughly than physical exercise and enriched environment living, so its effects on stem cells and connectivity (afferent or efferent) remain uninvestigated (Table 1).
Aversive experiences alter adult neurogenesis
While rewarding experiences have been shown to enhance several aspects of adult neurogenesis, experiences that are aversive seem to have the opposite effect. Many different types of stress (social and physical) diminish the number of new neurons in the hippocampus (Dagytė et al., 2009; De Miguel et al., 2018; Llorens-Martín et al., 2016; Mirescu et al., 2004; Opendak et al., 2016). This effect can even be observed by taking an otherwise rewarding stimulus such as running and making it aversive (by using forced running or running in social isolation) (Inoue et al., 2015; Leasure and Decker, 2009; Stranahan et al., 2006). Although not to the extent of forced running, along these lines, the positive effects of physical exercise on adult neurogenesis are not further enhanced under conditions of rewarded running, which increases running wheel performance compared to voluntary running (Klaus et al., 2009). Stress seems to inhibit adult neurogenesis by having a suppressive action on the proliferation of radial stem cells and amplifying progenitors, leading to a reduction in neuroblasts (Dagytė et al., 2009; Mirescu et al., 2004; Opendak et al., 2016) (Table 1). The survival of immature neurons can also be adversely affected by stress, as can their dendritic differentiation and formation of appropriate afferent and efferent connections (De Miguel et al., 2018; Llorens-Martín et al., 2016; Opendak et al., 2016) (Table 1). The general suppressive effects of stress on adult neurogenesis can be largely attributed to the catabolic actions of glucocorticoids and the reduction in new neuron formation has been associated with impaired cognitive function (Bondi et al., 2008; Burgado et al., 2014). However, it should be noted that some studies have demonstrated no effects of stress on either anxiety-like behavior or cognitive function (Opendak et al., 2016), raising the possibility that reductions in new neuron number and connectivity may be compensated for by other mechanisms in some cases.
A link between declining adult neurogenesis and reduced hippocampal function has also been observed with aging where reduced cell proliferation and diminished adult neurogenesis in general are associated with age-related cognitive decline (Klempin and Kempermann, 2007) (Table 1). However, it is important to point out that reduced adult neurogenesis occurs considerably before age-related cognitive decline, again suggesting the possibility of compensatory mechanisms, perhaps with regard to the strengthening of synapses that were formed earlier.
Social conditions that have both rewarding and aversive components also exert mixed effects on both adult neurogenesis and behavior. A good example of this is parenting, which is highly hedonic for rodents, has enriching effects on the brain, and is, at the same time, stressful, producing elevations in glucocorticoid levels. In both mothers and fathers, parenting has suppressive effects on adult neurogenesis (Darnaudéry et al., 2007; Glasper et al., 2011; Hyer et al., 2016; Leuner et al., 2007; Pawluski and Galea, 2007; Mahmoud et al., 2016). Behavioral studies have yielded mixed results for the effects of parenting on hippocampal-dependent cognition. In some cases, mothering has been shown to improve cognition (Darnaudéry et al., 2007; Gatewood et al., 2005; Kinsley et al., 1999) and in others, reduce cognition (Darnaudéry et al., 2007); whereas, fathering appears to have no effect (Glasper et al., 2011).
Experiential regulation of new neuron connectivity
One way that experience may alter hippocampal function is by altering connectivity patterns of the new neurons. Using viral-based methods, running has been found to increase the ratio of inhibitory interneuron versus excitatory mossy cell innervation onto new granule neurons (Sah et al., 2017; Vivar et al., 2016) as well as to enhance the number of new axon fibers projecting to the CA3 and CA2 subregions (Llorens-Martín et al., 2015). Afferent projections to immature neurons are also altered in mice housed in enriched conditions such that they have increased innervation by local inhibitory interneurons and long-distance cortical neurons compared to control mice (Bergami et al., 2015). Experiences that either increase neurogenesis, such as an environmental enrichment, or decrease adult neurogenesis, such as aging, also affect local inhibition levels (Drew et al., 2016). Physical exercise engages local inhibitory circuits that suppress new neurons in the ventral hippocampus and ultimately reduce stress-induced anxiety (Schoenfeld et al., 2013). In addition to its suppressive effects on adult neurogenesis, stress causes dendritic retraction on CA3 pyramidal neurons (Schoenfeld et al., 2017; reviewed in McEwen, 2018), one of the main targets of new granule neurons. Transgenic reduction of adult neurogenesis also reduces dendritic morphology on CA3 pyramidal neurons (Schoenfeld et al., 2017). With regard to the function of new neurons, these data suggest that the complex effects of experience on brain function cannot be explained by just knowing the numbers of new neurons, but instead must incorporate the effects of experience on the synaptic connectivity of new neurons.
Regulation of synaptic connectivity involves at least four main cellular/molecular components: the presynaptic neuron, postsynaptic neuron, glia, and the ECM, which together make up the “tetrapartite synapse” (Dityatev and Rusakov, 2011). It is likely that the functional influence new neurons have on the hippocampus arises from differences in their connectivity at the circuit level, as well as differences in the nonneuronal components that regulate their connections. Through their direct contact and/or secretion of factors, glia and the ECM participate in adult neurogenesis and the integration of new neurons into the hippocampal circuitry. At the presynaptic and postsynaptic level, new granule cells interact with glial cells as well as with the ECM itself. Clearly, the relationship between adult neurogenesis and hippocampal function is complex, and understanding how these components function together at the tetrapartite synapse may provide clarity in understanding the mechanisms that best support new neuron integration into existing circuitry.
Glia regulate new neuron number and connectivity
Considerable evidence suggests that glia modulate adult neurogenesis in the hippocampus. Astrocytes within the adult hippocampus influence stem cell proliferation, their commitment to a neuronal phenotype, and their survival (Song et al., 2002), raising the possibility that there are distinct properties of astrocytes in the hippocampus that promote adult neurogenesis (Figure 2A). Mature astrocytes also aid in the maturation and integration of adult-born neurons into the hippocampal circuitry. Blockade of vesicular release from astrocytes has been shown to impair new neuron survival and dendritic maturation by reducing dendritic branching and numbers of dendritic spines on new neurons (Sultan et al., 2015), suggesting that astrocytes are important regulators of adult neurogenesis at many stages in the process. Studies have shown that microglia have both beneficial and detrimental effects on adult neurogenesis in the hippocampus, depending on the circumstances. Microglia participate in stem cell proliferation and survival through their release of important trophic factors and anti-inflammatory cytokines (Battista et al., 2006; Borsini et al., 2015). Immune-deficient mice have impaired adult neurogenesis, even when housed in environmentally enriched conditions which typically increase stem cell proliferation, suggesting that immune molecules, potentially originating from microglia, are necessary for adult neurogenesis (Ziv et al., 2006). Microglia also participate in the adult neurogenesis process by engulfing stem/progenitor cells as well as immature neurons once they have died (Sierra et al., 2010) (Figure 2A). In response to damage and disease, microglia transition to a proinflammatory state where they release cytokines, such as IL-1β, 1L-6, and TNF-α, which can impair new neuron production and cell survival (reviewed in Borsini et al., 2015) (Figure 2A). One mechanism by which new neurons regulate the neurotoxic effects of microglia is through the binding of the neuronal chemokine fractalkine to its microglial receptor Cx3cr1 (Cardona et al., 2006). Genetic disruption of Cx3cr1 decreases the number of new neurons (Bachstetter et al., 2011) supporting the importance of microglia-neuron communication in regulating adult neurogenesis.
Figure 2. Glia influence adult neurogenesis and synaptic connections formed by new neurons.
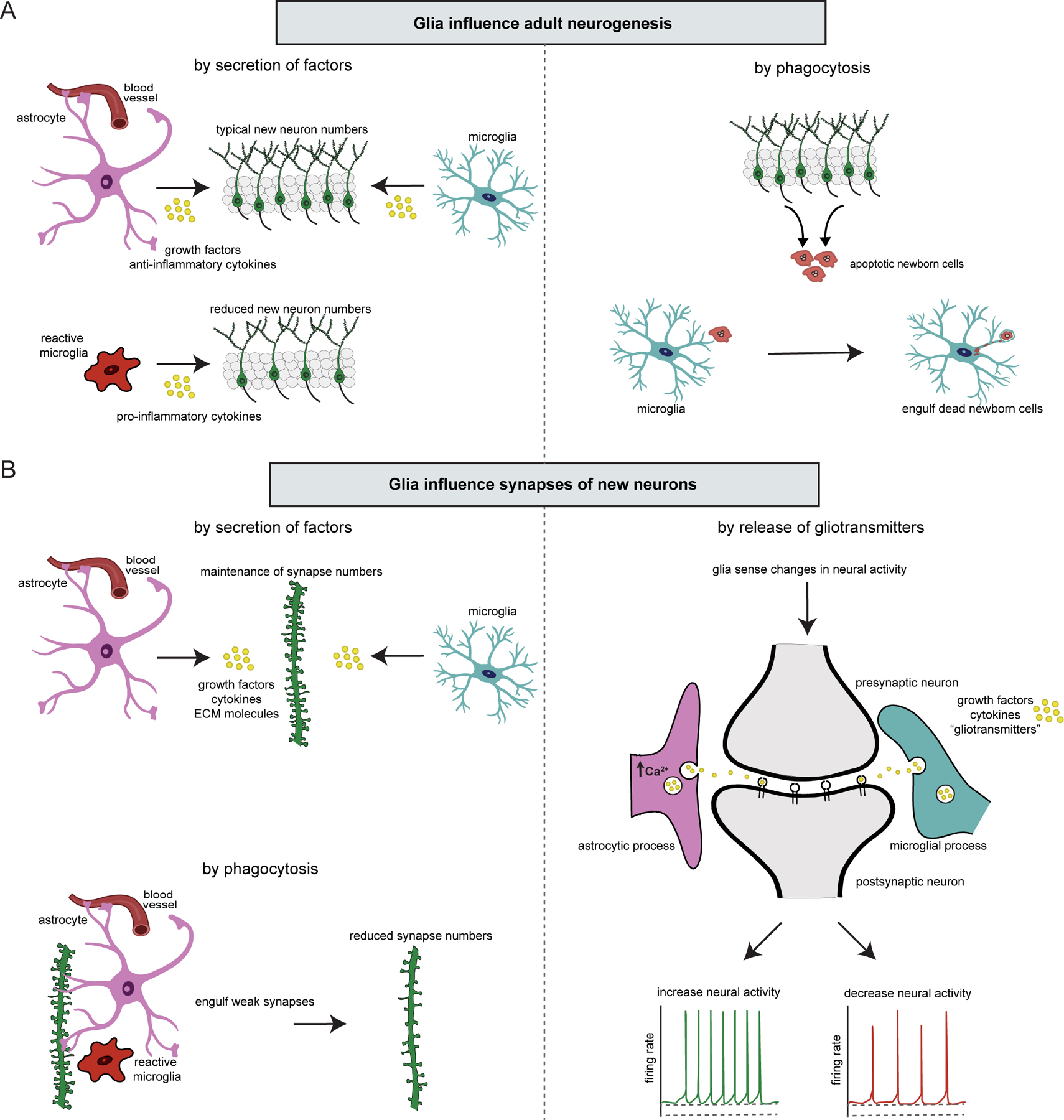
(A) Mechanisms of glial influences on adult neurogenesis. Through their secretion of growth factors and anti-inflammatory cytokines, astrocytes and microglia promote adult neurogenesis. Under pathological conditions, reactive microglia produce inflammatory cytokines that impair adult neurogenesis. Microglia also participate in adult neurogenesis by phagocytosing new neurons after they have died. (B) Mechanisms of glial influence on new neuron synapses. Astrocytes and microglia participate in regulating synapse number by secreting growth factors, cytokines, and ECM molecules. Astrocytes and microglia also shape neural circuits by engulfing weak synapses through phagocytosis. Glia modulate synaptic transmission by sensing changes in synaptic activity and releasing growth factors, cytokines, and/or gliotransmitters which, in turn, increase or decrease neural activity.
Glia are also involved in synapse formation and elimination during development and although their involvement in this process has been less thoroughly investigated in the adult, it seems likely that they continue to do so throughout life. In the developing brain, peak synaptogenesis coincides with the differentiation and maturation of astrocytes (reviewed in Chung et al., 2015). Astrocytes secrete several regulatory factors that affect presynaptic and postsynaptic maturation (Christopherson et al., 2005; Kucukdereli et al., 2011) (Figure 2B). Microglia have also been shown to play a role in synapse formation and maintenance through their direct contact with synapses as well as from their secretion of growth factors and cytokines (Figure 2B). Time-lapse imaging of microglia has shown that microglial contacts with dendrites induce spine formation in hippocampal cultures (Weinhard et al., 2018), suggesting that microglia may aid in reorganization of postsynaptic sites to produce more efficient synapses. Addition of microglia to hippocampal cultures increases the number of both excitatory and inhibitory synapses through their release of IL-10 (Lim et al., 2013). In addition to their stimulatory effects on synapses, microglia play an important role in synapse elimination by phagocytosis (Paolicelli et al., 2011l; Schafer et al., 2012) after the recognition of immune complement proteins on weak synapses (Schafer et al., 2012) (Figure 2B). Recent work has provided direct evidence for partial phagocytosis of presynaptic sites via time-lapse light sheet imaging of microglia-synaptic interactions in organotypic hippocampal cultures (Weinhard et al., 2018). Astrocytes also participate in circuit refinement by synaptic engulfment of both inhibitory and excitatory synapses through MEGF10 and MERTK pathways during development and in adulthood (Chung et al., 2013) (Figure 2B). Astrocytic secretion of TGF-β regulates complement expression to initiate microglia to begin synaptic elimination (Bialas and Stevens, 2013).
Examination of afferent and efferent synapses of adult-born neurons has shown that astrocytic processes ensheath synapses from adult-born neurons (Krzisch et al., 2015), suggesting that astrocytes likely regulate synaptic signaling between new hippocampal neurons and their connections. In vivo imaging studies of astrocytes have shown that astrocytes are sensitive to changes in neural activity and respond with increases in intracellular calcium levels (Wang et al., 2006; Winship et al., 2007). Through their release of signaling molecules such as glutamate, ATP, d-serine, or GABA, as well as trophic factors, astrocytes influence synaptic activity at certain synapses, a process known as gliotransmission (reviewed in Harada et al., 2015; Parpura and Zorec, 2010) (Figure 2B). Microglia also closely monitor neural activity and regulate synaptic plasticity. The highly motile processes of microglia are constantly surveying the brain (Nimmerjahn et al., 2005) and make frequent contacts with synapses (Tremblay et al., 2010). Similar to astrocytes, microglia express many neurotransmitter receptors such as glutamate and purinergic receptors that sense changes in neural activity (York et al., 2018). Microglia respond to increases in neural activity in the hippocampus by increasing their process numbers and prolonging their contact with synapses (Pfeiffer et al., 2016), and releasing factors such as TNF-α or IL-1β, both of which influence synaptic plasticity (Prieto et al., 2018; Stellwagen and Malenka, 2006) (Figure 2B). Available evidence indicates that astrocytes and microglia play a role in adult neurogenesis, but the extent to which these cells influence functional connectivity of new neurons remains incompletely explored.
Extracellular matrix regulation of new neuron number and connectivity
Accumulating evidence suggests a role for the ECM in adult neurogenesis, as well as synaptogenesis, and synaptic signaling. Approximately 20% of the brain’s volume is composed of the ECM (Nicholson et al., 2011). ECM molecules are expressed in a diffuse manner throughout the brain in areas adjacent to and between synapses. The ECM aids in the communication between neurons and glial cells by incorporating their secreted signaling molecules into the extracellular space where they interact with other ECM molecules and/or cell surface receptors (Dityatev and Rusakov, 2011). In addition to the loose ECM molecules, the ECM can form distinct organized structures called perineuronal nets (PNNs). PNNs are lattice-like structures that form around the cell body and proximal dendrites of certain types of neurons (van ‘t Spijker and Kwok, 2017) and have been implicated in synaptic stabilization in the adult brain (Lensjø et al., 2017a).
Chondroitin sulfate proteoglycans, structural ECM components, are known to surround new neurons in the hippocampus, and recent work has shown that this molecule is important for the production and differentiation of new neurons in adulthood (Yamada et al., 2018). Depletion of chondroitin sulfate proteoglycans in the hippocampus decreases new neuron formation and dendritic tree maturation, and produces cognitive impairment (Figure 3A). Environmental enrichment-induced increases in adult neurogenesis are associated with increased synthesis of chondroitin sulfate proteoglycans, which is prevented by chondroitin sulfate proteoglycan synthesis disruption (Yamada et al., 2018). Chondroitin sulfate proteoglycans bind neurotrophic factors such as FGF-2 and BDNF (Karumbaiah et al., 2015), which may facilitate the local potency of growth factors on adult-born neurons.
Figure 3. The extracellular matrix influences adult neurogenesis and the synaptic connections formed by new neurons.
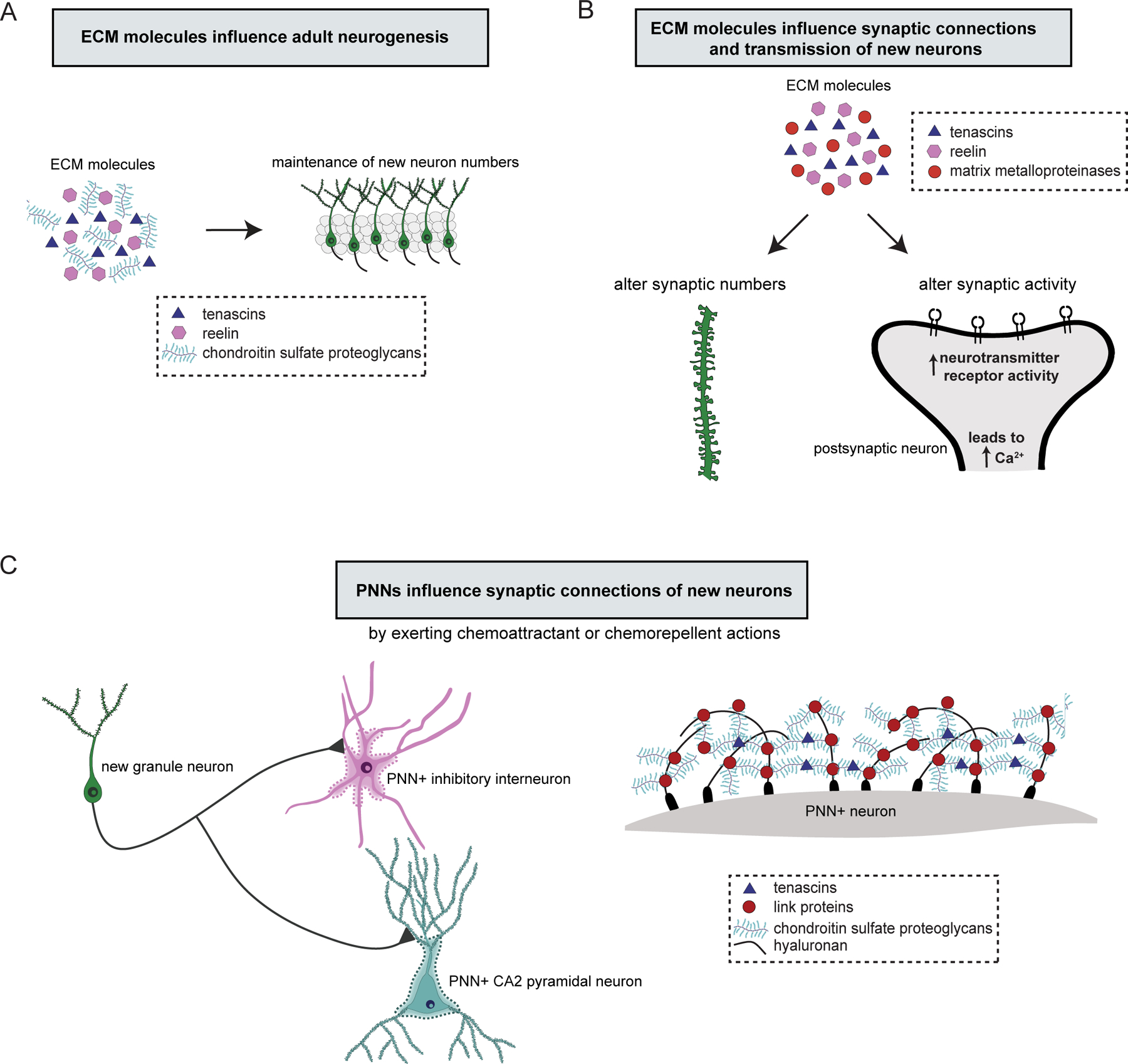
(A) Extracellular matrix (ECM) molecules such as tenascins, reelin, and chondroitin sulfate proteoglycans promote adult neurogenesis. (B) ECM influences new neuron connections and synaptic transmission. ECM molecules participate in the formation and stability of synapses, and through their interactions with cell surface receptors, influence synaptic activity by increasing neurotransmitter receptor activity, which increases intracellular calcium levels and enhances neural activity. (C) Specialized ECM structures, perineuronal nets (PNNs), which enwrap the cell body and proximal dendrites of hippocampal inhibitory interneurons and CA2 pyramidal cells, may attract or repel mossy fibers from new neurons.
Reelin, an ECM glycoprotein secreted by GABAergic interneurons in the adult brain (Pesold et al., 1998), has been shown to play a critical role in neuronal migration and synaptic plasticity (reviewed in Lee and D’Arcangelo, 2016). In the adult mouse hippocampus, reelin deficiency reduces neurogenesis (Won et al., 2006), while reelin overexpression increases neurogenesis (Pujadas et al., 2010) (Figure 3A). Using hippocampal slice culture and live imaging from wild-type and reelin-deficient mice, reelin has been shown to act as an attractant to control the migration direction of new granule neurons toward the molecular layer (Wang et al., 2018). Disruption of reelin signaling in hippocampal stem cells causes aberrant migration such that new granule neurons are scattered throughout the dentate gyrus area, where they form aberrant dendrites (Teixeira et al., 2012). In the developing and adult brain, evidence suggests that reelin is an important mediator of circuit formation by regulating dendritic tree maturation, spine formation and stability, glutamatergic neurotransmission, and synaptic plasticity (Pujadas et al., 2010) (Figure 3B). Overexpression of reelin accelerates dendritic development of adult-born neurons and increases the number of synaptic contacts in the dentate gyrus (Pejadas et al., 2010; Teixeira et al., 2012), whereas the absence of reelin signaling reduces dendritic tree complexity on new granule neurons (Teixeira et al., 2012). The reelin pathway also participates in the integration of adult-born neurons into the hippocampal circuitry by regulating spine morphology, spine type, and the number of synaptic connections on new granule neurons as well as the degree of perisynaptic astroglial ensheathment of new granule neuron synapses (Bosch et al., 2016). Reelin is also known to enhance hippocampal synaptic plasticity (Pujadas et al., 2010; Weeber et al., 2002) by altering the composition of NMDA receptor subunits and promoting surface clustering of AMPA receptors (Qiu et al., 2006) (Figure 3B).
Active extracellular proteases, such as matrix metalloproteinases (MMPs), play key roles in driving plasticity in response to changes in neural activity by degrading components of the ECM (Ferrer-Ferrer and Dityatev, 2018). MMPs can modulate structural plasticity by loosening the ECM structure allowing for a more permissive environment. For instance, the ECM may spatially restrict microglial and astrocytic processes from invading the perisynaptic space, thus ECM remodeling may prompt invasion of glial cells, which in turn may regulate synaptic signaling. One of the most widely studied MMPs in the adult hippocampus, MMP9, is involved in hippocampal synaptic plasticity. Enhanced neural activity in the hippocampus increases the active form of MMP9 into the perisynaptic space (Bozdagi et al., 2007; Nagy et al., 2006; Wiera et al., 2013). Mice deficient in MMP9 have impaired hippocampal LTP, including at mossy fiber-CA3 synapses, which is restored by adding recombinant active MMP9 (Nagy et al., 2006; Weira et al., 2013). Although the role of MMPs in adult neurogenesis has only been explored in damage models (Wójcik-Stanaszek et al., 2011), it seems plausible that these molecules play similar roles under intact conditions.
Tenascin-R, an ECM glycoprotein, is also important for new neuron production and synaptic plasticity in the adult hippocampus. The functional activity of tenascin stems from its interaction with other ECM proteins and receptors (Jones and Jones, 2000). In neural stem cultures, tenascin-R has been shown to modulate proliferation and differentiation of neural stem cells through interactions with the cell-surface receptor β1 integrin (Liao et al., 2008). In the developing and adult hippocampus, tenascin-R deficiency reduces the number of proliferating stem cells, while increasing the number of cells committed to a neuronal phenotype (Xu et al., 2014). Several studies have shown that tenascin-R knockout mice have altered synaptic plasticity in the hippocampus, including impaired LTP and increased basal excitatory transmission (Bukalo et al., 2001; Gurevicius et al., 2004; Saghatelyan et al., 2001). Studies suggest that tenascins are a critical component of PNNs (Suttkus et al., 2014), which surround many of the neurons that are postsynaptic to adult-generated granule cells.
Potential role for perineuronal nets in new neuron connectivity
PNNs are specialized extracellular structures composed of tenascin-R, hyaluronan, chondroitin sulfate proteoglycans, and link proteins (Figure 3C), which play important roles in brain plasticity during development and in adulthood (Sorg et al., 2016). In the hippocampus, PNNs primarily surround inhibitory interneurons, many of which are fast-spiking PV+ interneurons (Lensjø et al., 2017b; Yamada et al., 2015). In the CA2 subregion, however, PNNs also enwrap excitatory pyramidal cells (Carstens et al., 2016). In general, PNNs restrain plasticity and promote synaptic stabilization in the adult brain. The maturation of PNNs in the developing cortex coincides with the closure of the critical periods of synaptogenesis and circuit refinement (Beurdeley et al., 2012; Ye and Miao, 2013). Accumulating evidence suggests that PNN formation is driven by neuronal activity (Dityatev et al., 2007) and one possibility is that changes in the number of new neurons, which alter the overall excitability of the hippocampus (Ikrar et al., 2013), may then regulate the expression of PNNs at postsynaptic sites. In addition to glutamatergic input, new granule neurons also receive local inhibitory input (Espósito et al., 2005; Ge et al., 2006), and inhibitory control of new neurons may be indirectly altered through the expression of PNNs on PV+ interneurons. Since activation of PV+ interneurons has been shown to promote new neuron cell survival while suppression of PV+ interneuron activity reduces new neuron cell survival (Song et al., 2013), changes in PNNs surrounding these cells may have an important influence on adult neurogenesis. However, despite their compelling association with cells in the adult neurogenesis hippocampal circuitry, as well as their identified functional roles in other neural systems, PNNs have been relatively uninvestigated in the context of adult neurogenesis.
Proposed models and remaining questions
It has become increasingly clear that the connectivity of new neurons in the adult hippocampus is highly relevant to their functional influence. All components of the synapse, including glia, the ECM, and the presynaptic and postsynaptic neurons must be considered in an effort to elucidate the diverse effects new neurons have on brain function. In addition to adult-generated neurons themselves, associated glial cells and ECM are differentially sensitive to experience and pathological conditions (reviewed in Ruddy and Morshead, 2018). Glial cells play important roles in the production and elimination of synaptic connections, while PNNs may attract or repel axons of new neurons. Experience-dependent or pathological disruptions in glial cells or PNNs are likely to produce abnormal connectivity of new neurons and therefore impaired hippocampal function.
Many studies have shown that glial cells as well as PNNs are influenced by experience. Physical exercise, which increases new neuron production and improves behaviors associated with the hippocampus, also stimulates astrocyte growth (Brockett et al., 2015; Fahimi et al., 2017) and reduced microglial reactivity (Kohman et al., 2012, 2013). Rats given access to running wheels also have decreased PNN expression in the hippocampus (Smith et al., 2015b). Stress, on the other hand, which diminishes adult neurogenesis and impairs hippocampal function, has been shown to reduce the size and number of astrocytes (Czéh et al., 2006; Tynan et al., 2013) as well as trigger microglial reactivity (Kreisel et al., 2014; Llorens-Martín et al., 2016). Early life stress in mice has been shown to increase PNNs surrounding PV+ inhibitory interneurons in the hippocampus (Murthy et al., 2019). Since physical exercise and stress have for the most part opposite effects on adult neurogenesis, synaptic connectivity, and behavior, it is perhaps not surprising that these experiences also have different effects on glia and PNN structures. For example, under optimal function conditions as observed with physical exercise, healthy glial cells and reduced PNNs may enhance new neuron connections with their targets cells (Figure 4A), while under suboptimal function conditions as observed with stress, changes in glial cells along with increased PNNs may prevent these connections from forming appropriately with their target cells (Figure 4B).
Figure 4. Models of new neuron connectivity under optimal, suboptimal, and pathologically dysfunctional states.
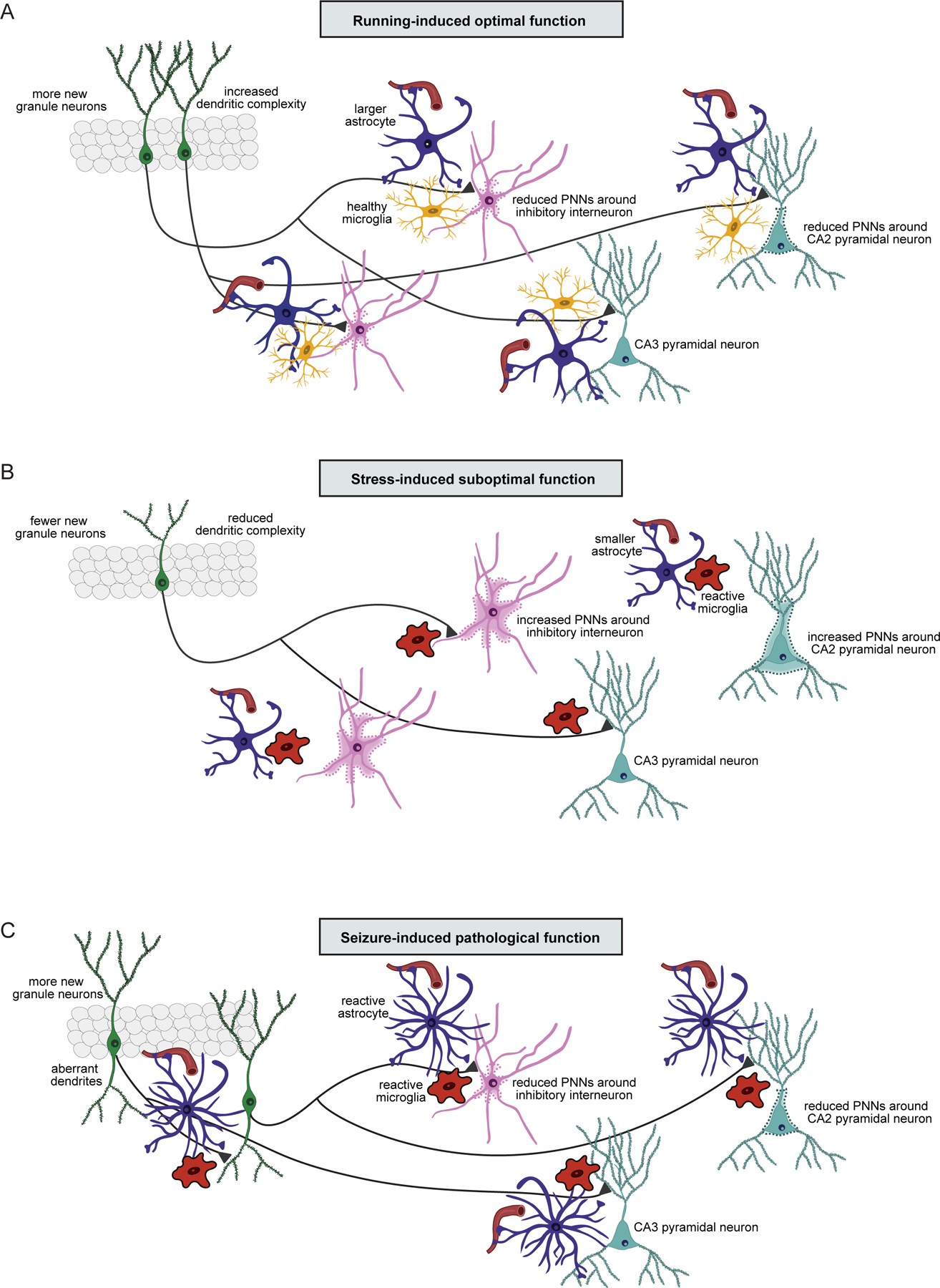
(A) Long-term physical exercise improves cognitive function and reduces anxiety. Optimization of hippocampus function may be achieved through the stimulation of adult neurogenesis, with the production of more new granule cells, each with more extensive dendritic trees and increased numbers of dendritic spines. New granule neurons form more connections with their targets, including inhibitory interneurons and CA2/CA3 pyramidal cells. Reduced PNNs along with healthy glial cells surrounding new synapses promote healthy new granule neuron connections to their target cells. (B) Chronic stress diminishes cognitive function and increases anxiety. Suboptimal function of the hippocampus occurs as a result of suppressed adult neurogenesis, with the production of fewer new granule cells, each with stunted dendritic trees and reduced numbers of dendritic spines. Stress-induced increases in PNNs along with alterations in glial cells (fewer astrocytes and reactive microglia) prevent healthy new granule neuron connections to their target cells. (C) Seizures significantly impair cognitive function and increase anxiety. Pathological function of the hippocampus results from the paradoxical production of more new granule neurons, each with with more extensive dendritic branching and increased numbers of dendritic spines. These new neurons are in ectopic locations and often have an aberrant basal dendritic tree extending into the hilus. These abnormalities impair integration by causing recurrent excitatory connections with other new granule neurons. Reduced PNNs along with reactive astrocytes and reactive microglia prevent the formation of healthy connections between new neurons and their target cells.
Examination of disease models adds further complexity about how alterations in glia, ECM and specifically PNNs, lead to dysfunctional circuits associated with adult neurogenesis. For example, seizures stimulate adult neurogenesis and impair hippocampal function. Seizure-induced reactive astrocytes (Shapiro et al., 2008; Yang et al., 2010), reactive microglia (Shapiro et al., 2008; Yang et al., 2010), and reduced the expression of PNNs (McRae and Porter, 2012; Rankin-Gee et al., 2015) around postsynaptic neurons of adult-generated cells likely contribute to the functional impairments. The combination of increased adult neurogenesis along with glial reactivity and reduced PNNs may set the stage for improper new neuron connections with their targets and ultimately, impaired hippocampal function (Figure 4C). Clearly, additional research is needed to understand how adult-generated neurons in the hippocampus participate in optimal, suboptimal, and pathological functional outcomes and considering their connections in the tetrapartite synapse is likely a step in the right direction.
In this Review, Cope and Gould discuss how the functional consequences of new neurons generated during adult neurogenesis arise from the collective interactions of presynaptic and postsynaptic neurons, glial cells, and the extracellular matrix, which together form the “tetrapartite synapse”.
Acknowledgments
We thank Jessica Verpeut and Biorender for assistance with the figures. We also thank Adam T. Brockett, Blake J. Laham, and Sahana Murthy for their helpful comments and advice on the manuscript. This work was supported by NIH grant MH117459-01 (EG) and NIH training fellowship T32MH065214 (ECC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves ND, Correia JS, Patrício P, Mateus-Pinheiro A, Machado-Santos AR, Loureiro-Campos E, Morais M, Bessa JM, Sousa N, and Pinto L (2017). Adult hippocampal neuroplasticity triggers susceptibility to recurrent depression. Transl. Psychiatry 7, e1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, and Lavenex P (2007). The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res 163, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogini P, Lattanzi D, Ciuffoli S, Betti M, Fanelli M, and Cuppini R (2013). Physical exercise and environment exploration affect synaptogenesis in adult-generated neurons in the rat dentate gyrus: possible role of BDNF. Brain Res 1534, 1–12. [DOI] [PubMed] [Google Scholar]
- Åmellem I, Suresh S, Chang CC, Tok SSL, and Tashiro A (2017). A critical period for antidepressant-induced acceleration of neuronal maturation in adult dentate gyrus. Transl. Psychiatry 7, e1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, and Hen R (2018). Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, et al. (2011). Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol. Aging 32, 2030–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, and Pitossi FJ (2006). Neurogenic niche modulation by activated microglia: transforming growth factor β increases neurogenesis in the adult dentate gyrus. Eur. J. Neurosci 23, 83–93. [DOI] [PubMed] [Google Scholar]
- Bednarczyk MR, Hacker LC, Fortin-Nunez S, Aumont A, Bergeron R, and Fernandes KJL (2010). Distinct stages of adult hippocampal neurogenesis are regulated by running and the running environment. Hippocampus 21, 1334–1347. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NM-B, Slomianka L, Vyssotski AL, and Lipp H-P (2010). Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging 31, 151–161. [DOI] [PubMed] [Google Scholar]
- Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann K-K, Schinder AF, Götz M, et al. (2015). A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron 85, 710–717. [DOI] [PubMed] [Google Scholar]
- Beurdeley M, Spatazza J, Lee HHC, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, and Prochiantz A (2012). Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J. Neurosci 32, 9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas AR, and Stevens B (2013). TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci 16, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, et al. (2018). Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22, 589–599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolós M, Terreros-Roncal J, Perea JR, Pallas-Bazarra N, Ávila J, and Llorens-Martín M (2019). Maturation Dynamics of the Axon Initial Segment (AIS) of Newborn Dentate Granule Cells in Young Adult C57BL/6J Mice. J. Neurosci 39, 1605–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming G-L, and Song H (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, and Morilak DA (2008). Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33, 320–331. [DOI] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, and Pariante CM (2015). The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38, 145–157. [DOI] [PubMed] [Google Scholar]
- Bosch C, Masachs N, Exposito-Alonso D, Martínez A, Teixeira CM, Fernaud I, Pujadas L, Ulloa F, Comella JX, DeFelipe J, et al. (2016). Reelin Regulates the Maturation of Dendritic Spines, Synaptogenesis and Glial Ensheathment of Newborn Granule Cells. Cereb. Cortex 26, 4282–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Nagy V, Kwei KT, and Huntley GW (2007). In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol 98, 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockett AT, LaMarca EA, and Gould E (2015). Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex. PLoS One 10, e0124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, and Dityatev A (2001). Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104, 359–369. [DOI] [PubMed] [Google Scholar]
- Burgado J, Harrell CS, Eacret D, Reddy R, Barnum CJ, Tansey MG, Miller AH, Wang H, and Neigh GN (2014). Two weeks of predatory stress induces anxiety-like behavior with co-morbid depressive-like behavior in adult male mice. Behav. Brain Res 275, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, and Glover LR (2015). Adult neurogenesis: beyond learning and memory. Annu. Rev. Psychol 66, 53–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, and McKay RD (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol 435, 406–417. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. (2006). Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci 9, 917–924. [DOI] [PubMed] [Google Scholar]
- Carstens KE, Phillips ML, Pozzo-Miller L, Weinberg RJ, and Dudek SM (2016). Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons. J. Neurosci 36, 6312–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, and Overstreet-Wadiche LS (2013). GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J. Neurosci 33, 6614–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancey JH, Poulsen DJ, Wadiche JI, and Overstreet-Wadiche L (2014). Hilar mossy cells provide the first glutamatergic synapses to adult-born dentate granule cells. J. Neurosci 34, 2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K-O, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, et al. (2015). Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun 6, 6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CCA, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, and Barres BA (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433. [DOI] [PubMed] [Google Scholar]
- Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W-S, Allen NJ, and Eroglu C (2015). Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol 7, a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Ferrer I, Aronica E, Kovacs GG, Verney C, Nardelli J, Khung S, Delezoide A-L, Milenkovic I, Rasika S, et al. (2018). Hippocampal Radial Glial Subtypes and Their Neurogenic Potential in Human Fetuses and Healthy and Alzheimer’s Disease Adults. Cereb. Cortex 28, 2458–2478. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, and Rhodes JS (2008). Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 155, 1048–1058. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope EC, Morris DR, Gower-Winter SD, Brownstein NC, and Levenson CW (2016) Effect of zinc supplementation on neuronal precursor proliferation in the rat hippocampus after traumatic brain injury. Exp Neurol 279, 96–103. [DOI] [PubMed] [Google Scholar]
- Cuartero MI, de la Parra J, Pérez-Ruiz A, Bravo-Ferrer I, Durán-Laforet V, García-Culebras A, García-Segura JM, Dhaliwal J, Frankland PW, Lizasoain I, et al. (2019). Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J. Clin. Invest [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Simon M, Schmelting B, Hiemke C, and Fuchs E (2006). Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31, 1616–1626. [DOI] [PubMed] [Google Scholar]
- Dagytė G, Van der Zee EA, Postema F, Luiten PGM, Den Boer JA, Trentani A, and Meerlo P (2009). Chronic but not acute foot-shock stress leads to temporary suppression of cell proliferation in rat hippocampus. Neuroscience 162, 904–913. [DOI] [PubMed] [Google Scholar]
- Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, et al. (2016). Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron 90, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudéry M, Perez-Martin M, Del Favero F, Gomez-Roldan C, Garcia-Segura LM, and Maccari S (2007). Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology 32, 803–812. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, and Moore AN (2001). Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. Journal of Neuroscience Research 63, 313–319. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux J-P, et al. (2009). Neurogenesis-Dependent and -Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron 62, 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel Z, Haditsch U, Palmer TD, Azpiroz A, and Sapolsky RM (2018). Adult-generated neurons born during chronic social stress are uniquely adapted to respond to subsequent chronic social stress. Mol. Psychiatry [DOI] [PubMed]
- Deshpande A, Bergami M, Ghanem A, Conzelmann KK, Lepier A, Götz M, and Berninger B (2013). Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb. Proc. Natl. Acad. Sci. U. S. A 110, 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, and Rusakov DA (2011). Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol 21, 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Brückner G, Dityateva G, Grosche J, Kleene R, and Schachner M (2007). Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev. Neurobiol 67, 570–588. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Kheirbek MA, Luna VM, Denny CA, Cloidt MA, Wu MV, Jain S, Scharfman HE, and Hen R (2016). Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus 26, 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). On the Integration of Space, Time, and Memory. Neuron 95, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, and Enikolopov G (2006). Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. U. S. A 103, 8233–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park J-H, Tordo J, Peterson DA, Fishell G, Koulakov A, and Enikolopov G (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, and Gage FH (1998). Neurogenesis in the adult human hippocampus. Nat. Med 4, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, and Schinder AF (2005). Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci 25, 10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahimi A, Baktir MA, Moghadam S, Mojabi FS, Sumanth K, McNerney MW, Ponnusamy R, and Salehi A (2017). Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct. Funct 222, 1797–1808. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, and Dong H-W (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Ferrer M, and Dityatev A (2018). Shaping Synapses by the Neural Extracellular Matrix. Front. Neuroanat 12, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett L, Zhang J, Zimprich A, Niedermeier KM, Fuchs H, Gailus-Durner V, Hrabě de Angelis M, Vogt Weisenhorn D, Wurst W, and Hölter SM (2015). Conditional Reduction of Adult Born Doublecortin-Positive Neurons Reversibly Impairs Selective Behaviors. Front. Behav. Neurosci 9, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EAA, Lomas LM, Kozub FJ, Lambert KG, et al. (2005). Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res. Bull 66, 91–98. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming G-L, and Song H (2006). GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang C-H, Hsu K-S, Ming G-L, and Song H (2007). A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasper ER, and Gould E (2013). Sexual experience restores age-related decline in adult neurogenesis and hippocampal function. Hippocampus 23, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasper ER, Kozorovitskiy Y, Pavlic A, and Gould E (2011). Paternal experience suppresses adult neurogenesis without altering hippocampal function in Peromyscus californicus. J. Comp. Neurol 519, 2271–2281. [DOI] [PubMed] [Google Scholar]
- Gonçalves JT, Schafer ST, and Gage FH (2016a). Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167, 897–914. [DOI] [PubMed] [Google Scholar]
- Gonçalves JT, Tiago Gonçalves J, Bloyd CW, Shtrahman M, Johnston ST, Schafer ST, Parylak SL, Tran T, Chang T, and Gage FH (2016b). In vivo imaging of dendritic pruning in dentate granule cells. Nat. Neurosci 19, 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, and Fuchs E (1998). Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. U. S. A 95, 3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, and Fuchs E (1999). Hippocampal neurogenesis in adult Old World primates. Proc. Natl. Acad. Sci. U. S. A 96, 5263–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JO, Leslie I, Huang GJ, McHugh SB, Taylor A, Mott R, Munafò M, Bannerman DM, and Flint J (2013). Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genet 9, e1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, and Ge S (2012). Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat. Neurosci 15, 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Bao Y, Chen J, Huang C, Zhang X, Jiang R, Liu Q, Liu Y, Xu X, and Shi W (2016). The Expression of NP847 and Sox2 after TBI and Its Influence on NSCs. Front. Cell. Neurosci 10, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevicius K, Gureviciene I, Valjakka A, Schachner M, and Tanila H (2004). Enhanced cortical and hippocampal neuronal excitability in mice deficient in the extracellular matrix glycoprotein tenascin-R. Mol. Cell. Neurosci 25, 515–523. [DOI] [PubMed] [Google Scholar]
- Harada K, Kamiya T, and Tsuboi T (2015). Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front. Neurosci 9, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Sahay A, and Hen R (2015). Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology 40, 2368–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhäuser C, and Gray WP (2003). Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur. J. Neurosci 18, 2769–2778. [DOI] [PubMed] [Google Scholar]
- Hyer MM, Hunter TJ, Katakam J, Wolz T, and Glasper ER (2016). Neurogenesis and anxiety-like behavior in male California mice during the mate’s postpartum period. Eur. J. Neurosci 43, 703–709. [DOI] [PubMed] [Google Scholar]
- Ikrar T, Guo N, He K, Besnard A, Levinson S, Hill A, Lee H-K, Hen R, Xu X, and Sahay A (2013). Adult neurogenesis modifies excitability of the dentate gyrus. Front. Neural Circuits 7, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T, Rakwal R, and Soya H (2015). Long-Term Mild, rather than Intense, Exercise Enhances Adult Hippocampal Neurogenesis and Greatly Changes the Transcriptomic Profile of the Hippocampus. PLoS One 10, e0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, and Lindvall O (2006). Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron 52, 1047–1059. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Römer B, Babu H, and Kempermann G (2005) Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol 196, 342–351. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD Jr, Li Y, and Gage FH (2007). Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci 27, 9400–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, and Gage FH (2014). Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol 24, 558–563. [DOI] [PubMed] [Google Scholar]
- Jones PL, and Jones FS (2000). Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol 19, 581–596. [DOI] [PubMed] [Google Scholar]
- Karumbaiah L, Enam SF, Brown AC, Saxena T, Betancur MI, Barker TH, and Bellamkonda RV (2015). Chondroitin Sulfate Glycosaminoglycan Hydrogels Create Endogenous Niches for Neural Stem Cells. Bioconjug. Chem 26, 2336–2349. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Georg Kuhn H, and Gage FH (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, and Hen R (2013). Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-I, Lee JW, Lee YA, Lee D-H, Han NS, Choi Y-K, Hwang BR, Kim HJ, and Han JS (2013). Sexual activity counteracts the suppressive effects of chronic stress on adult hippocampal neurogenesis and recognition memory. Brain Res 1538, 26–40. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, and Lambert KG (1999). Motherhood improves learning and memory. Nature 402, 137–138. [DOI] [PubMed] [Google Scholar]
- Klaus F, Hauser T, Slomianka L, Lipp HP, and Amrein I (2009). A reward increases running-wheel performance without changing cell proliferation, neuronal differentiation or cell death in the dentate gyrus of C57BL/6 mice. Behav Brain Res 204, 175–181. [DOI] [PubMed] [Google Scholar]
- Klempin F, and Kempermann G (2007). Adult hippocampal neurogenesis and aging. Eur. Arch. Psychiatry Clin. Neurosci 257, 271–280. [DOI] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, and Kempermann G (2010). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One 5, e8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayash K., Iked Y., Saka A., Yamasak N., Haned E., Miyakaw T., and Suzuk H. (2010). Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc. Natl. Acad. Sci. U. S. A 107, 8434–8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, and Rhodes JS (2012). Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav. Immun 26, 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Wojcik E, and Rhodes JS (2013). Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J. Neuroinflammation 10, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, and Yirmiya R (2014). Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry 19, 699–709. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, and Kempermann G (2006). Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging 27, 1505–1513. [DOI] [PubMed] [Google Scholar]
- Krzisch M, Temprana SG, Mongiat LA, Armida J, Schmutz V, Virtanen MA, Kocher-Braissant J, Kraftsik R, Vutskits L, Conzelmann K-K, et al. (2015). Pre-existing astrocytes form functional perisynaptic processes on neurons generated in the adult hippocampus. Brain Struct. Funct 220, 2027–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, et al. (2011). Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. U. S. A 108, E440–E449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruba R, Hattiangady B, Parihar VK, Shuai B, and Shetty AK (2011). Differential susceptibility of interneurons expressing neuropeptide Y or parvalbumin in the aged hippocampus to acute seizure activity. PLoS One 6, e24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, and Gage FH (1996). Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci 16, 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, and Eisch AJ (2010). Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci. U. S. A 107, 4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Espósito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, and Schinder AF (2006). Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol 4, e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, and Decker L (2009) Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus 19, 907–912. [DOI] [PubMed] [Google Scholar]
- Lee GH, and D’Arcangelo G (2016). New Insights into Reelin-Mediated Signaling Pathways. Front. Cell. Neurosci 10, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjø KK, Lepperød ME, Dick G, Hafting T, and Fyhn M (2017a). Removal of Perineuronal Nets Unlocks Juvenile Plasticity Through Network Mechanisms of Decreased Inhibition and Increased Gamma Activity. J. Neurosci 37, 1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjø KK, Christensen AC, Tennøe S, Fyhn M, and Hafting T (2017b). Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mirescu C, Noiman L, and Gould E (2007). Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus 17, 434–442. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, and Gould E (2010). Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One 5, e11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Huang W, Schachner M, Guan Y, Guo J, Yan J, Qin J, Bai X, and Zhang L (2008). Beta 1 integrin-mediated effects of tenascin-R domains EGFL and FN6–8 on neural stem/progenitor cell proliferation and differentiation in vitro. J. Biol. Chem 283, 27927–27936. [DOI] [PubMed] [Google Scholar]
- Lim S-H, Park E, You B, Jung Y, Park A-R, Park SG, and Lee J-R (2013). Neuronal synapse formation induced by microglia and interleukin 10. PLoS One 8, e81218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martín M, Jurado-Arjona J, Avila J, and Hernández F (2015). Novel connection between newborn granule neurons and the hippocampal CA2 field. Exp. Neurol 263, 285–292. [DOI] [PubMed] [Google Scholar]
- Llorens-Martín M, Jurado-Arjona J, Bolós M, Pallas-Bazarra N, and Ávila J (2016). Forced swimming sabotages the morphological and synaptic maturation of newborn granule neurons and triggers a unique pro-inflammatory milieu in the hippocampus. Brain Behav. Immun 53, 242–254. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, Haas CA, Kempermann G, Taylor V, and Giachino C (2010). Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6, 445–456. [DOI] [PubMed] [Google Scholar]
- Mahmoud R, Wainwright SR, Galea LA.(2016) Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front Neuroendocrinol 41, 129–52. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, and Duman RS (2000). Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. The Journal of Neuroscience 20, 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt SJ, Wadiche JI, and Overstreet-Wadiche LS (2009). Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J. Neurosci 29, 15063–15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2018) Redefining neuroendocrinology: Epigenetics of brain-body communication over the life course. Front Neuroendocrinol 49, 8–30. [DOI] [PubMed] [Google Scholar]
- McRae PA, and Porter BE (2012). The perineuronal net component of the extracellular matrix in plasticity and epilepsy. Neurochem. Int 61, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, and Hen R (2006). Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat. Neurosci 9, 729–731. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, and Gould E (2004). Early life experience alters response of adult neurogenesis to stress. Nat. Neurosci 7, 841–846. [DOI] [PubMed] [Google Scholar]
- Murthy S, Kane GA, Katchur KJ, Lara Mejia PS, Obiofuma G, Buschman TJ, McEwen BS, and Gould E (2019). Perineuronal nets, inhibitory interneurons and anxiety-related ventral hippocampal neuronal oscillations are altered by early life adversity. Biol Psychiatry, in press [DOI] [PMC free article] [PubMed]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, and Huntley GW (2006). Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci 26, 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Kamali-Zare P, and Tao L (2011). Brain extracellular space as a diffusion barrier. Comput. Vis. Sci 14, 309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, and Eriksson PS (1999). Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol 39, 569–578. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, and Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, and Kainulainen H (2016). Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol 594, 1855–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onksen JL, Briand LA, Galante RJ, Pack AI, and Blendy JA (2012). Running-induced anxiety is dependent on increases in hippocampal neurogenesis. Genes Brain Behav 11, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M, and Gould E (2015). Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn. Sci 19, 151–161. [DOI] [PubMed] [Google Scholar]
- Opendak M, Offit L, Monari P, Schoenfeld TJ, Sonti AN, Cameron HA, and Gould E (2016). Lasting Adaptations in Social Behavior Produced by Social Disruption and Inhibition of Adult Neurogenesis. J. Neurosci 36, 7027–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, and Westbrook GL (2006). Seizures accelerate functional integration of adult-generated granule cells. J. Neurosci 26, 4095–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Parent JM, and Lowenstein DH (2002). Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog. Brain Res 135, 121–131. [DOI] [PubMed] [Google Scholar]
- Parpura V, and Zorec R (2010). Gliotransmission: Exocytotic release from astrocytes. Brain Res. Rev 63, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, and Galea LAM (2007). Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience 149, 53–67. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, and McBain CJ (2017). Hippocampal GABAergic Inhibitory Interneurons. Physiol. Rev 97, 1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, and Caruncho HJ (1998). Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc. Natl. Acad. Sci. U. S. A 95, 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T, Avignone E, and Nägerl UV (2016). Induction of hippocampal long-term potentiation increases the morphological dynamics of microglial processes and prolongs their contacts with dendritic spines. Sci. Rep 6, 32422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto GA, Aleph Prieto G, Tong L, Smith ED, and Cotman CW (2018). TNFα and IL-1β but not IL-18 Suppresses Hippocampal Long-Term Potentiation Directly at the Synapse. Neurochem. Res 44, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, de Lecea L, Martínez A, Delgado-García JM, and Soriano E (2010). Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J. Neurosci 30, 4636–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, and Weeber EJ (2006). Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J. Neurosci 26, 12943–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin-Gee EK, McRae PA, Baranov E, Rogers S, Wandrey L, and Porter BE (2015). Perineuronal net degradation in epilepsy. Epilepsia 56, 1124–1133. [DOI] [PubMed] [Google Scholar]
- Restivo L, Niibori Y, Mercaldo V, Josselyn SA, and Frankland PW (2015). Development of Adult-Generated Cell Connectivity with Excitatory and Inhibitory Cell Populations in the Hippocampus. J. Neurosci 35, 10600–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest J-M, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza P-V, and Abrous DN (2009). Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry 14, 959–967. [DOI] [PubMed] [Google Scholar]
- Ruddy RM, Morshead CM (2018). Home sweet home: the neural stem cell niche throughout development and after injury. Cell Tissue Res 371, 125–141. [DOI] [PubMed] [Google Scholar]
- Saghatelyan AK, Dityatev A, Schmidt S, Schuster T, Bartsch U, and Schachner M (2001). Reduced perisomatic inhibition, increased excitatory transmission, and impaired long-term potentiation in mice deficient for the extracellular matrix glycoprotein tenascin-R. Mol. Cell. Neurosci 17, 226–240. [DOI] [PubMed] [Google Scholar]
- Sah N, Peterson BD, Lubejko ST, Vivar C, and van Praag H (2017). Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci. Rep 7, 10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, and Hen R (2011). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, and Drew MR (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U. S. A 103, 17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, and Hen R (2007) Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl. Acad. Sci. U. S. A 104, 4642–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, and Stevens B (2012). Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, and Sollas AL (2000). Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J. Neurosci 20, 6144–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, and Bischofberger J (2004). Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, and Gould E (2013). Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J. Neurosci 33, 7770–7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, and Cameron HA (2017). Stress and Loss of Adult Neurogenesis Differentially Reduce Hippocampal Volume. Biol. Psychiatry 82, 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, McEwen BS, and Alvarez-Buylla A (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci 21, 7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, and Ribak CE (2006). Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res 69, 53–66. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Wang L, and Ribak CE (2008). Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia 49 Suppl 2, 33–41. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, and Gould E (2002). Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, and Maletic-Savatic M (2010). Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 7, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, and Kelly JP (2011). The impact of environmental enrichment in laboratory rats— Behavioural and neurochemical aspects. Behav. Brain Res 222, 246–264. [DOI] [PubMed] [Google Scholar]
- Smith ACW, Scofield MD, and Kalivas PW (2015a). The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res 1628, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Mauricio R, Nobre L, Marsh B, Wüst RCI, Rossiter HB, and Ichiyama RM (2015b). Differential regulation of perineuronal nets in the brain and spinal cord with exercise training. Brain Res. Bull 111, 20–26. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, and Cameron HA (2009a). Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J. Neurosci 29, 14484–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, and Cameron HA (2009b). The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus 19, 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, and Cameron HA (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, and Gage FH (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39–44. [DOI] [PubMed] [Google Scholar]
- Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, et al. (2013). Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat. Neurosci 16, 1728–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JCF, and Miquel M (2016). Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci 36, 11459–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ‘t Spijker HM, and Kwok JCF (2017). A Sweet Talk: The Molecular Systems of Perineuronal Nets in Controlling Neuronal Communication. Front. Integr. Neurosci 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, and Malenka RC (2006). Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, and Gould E (2006). Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci 9, 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, and Gage FH (2007). In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1, 515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan S, Li L, Moss J, Petrelli F, Cassé F, Gebara E, Lopatar J, Pfrieger FW, Bezzi P, Bischofberger J, et al. (2015). Synaptic Integration of Adult-Born Hippocampal Neurons Is Locally Controlled by Astrocytes. Neuron 88, 957–972. [DOI] [PubMed] [Google Scholar]
- Sun D, Daniels TE, Rolfe A, Waters M, and Hamm R (2015). Inhibition of injury-induced cell proliferation in the dentate gyrus of the hippocampus impairs spontaneous cognitive recovery after traumatic brain injury. J. Neurotrauma 32, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttkus A, Rohn S, Weigel S, Glöckner P, Arendt T, and Morawski M (2014). Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis 5, e1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CM, Kron MM, Masachs N, Zhang H, Lagace DC, Martinez A, Reillo I, Duan X, Bosch C, Pujadas L, et al. (2012). Cell-autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. J. Neurosci 32, 12051–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, and Schinder AF (2008). Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci 11, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M-È, Lowery RL, and Majewska AK (2010). Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchero MF, Buttner KA, Sulkes Cuevas JN, Temprana SG, Fontanet PA, Monzón-Salinas MC, Ledda F, Paratcha G, and Schinder AF (2017). High Plasticity of New Granule Cells in the Aging Hippocampus. Cell Rep 21, 1129–1139. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, and Walker FR (2013). Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol 126, 75–91. [DOI] [PubMed] [Google Scholar]
- Vega-Rivera NM, Ortiz-López L, Gómez-Sánchez A, Oikawa-Sala J, Estrada-Camarena EM, and Ramírez-Rodríguez GB (2016). The neurogenic effects of an enriched environment and its protection against the behavioral consequences of chronic mild stress persistent after enrichment cessation in six-month-old female Balb/C mice. Behav. Brain Res 301, 72–83. [DOI] [PubMed] [Google Scholar]
- Villasana LE, Kim KN, Westbrook GL, and Schnell E (2015). Functional Integration of Adult-Born Hippocampal Neurons after Traumatic Brain Injury(1,2,3). eNeuro 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee J-Y, Stringer TP, Callaway EM, Gage FH, Suh H, and van Praag H (2012). Monosynaptic inputs to new neurons in the dentate gyrus. Nat. Commun 3, 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, and van Praag H (2013). All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top. Behav. Neurosci 15, 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Peterson BD, and van Praag H (2016). Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage 131, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Keiner S, Witte OW, and Redecker C (2010). Differential stroke-induced proliferative response of distinct precursor cell subpopulations in the young and aged dentate gyrus. Neuroscience 169, 1279–1286. [DOI] [PubMed] [Google Scholar]
- Wang S, Brunne B, Zhao S, Chai X, Li J, Lau J, Failla AV, Zobiak B, Sibbe M, Westbrook GL, et al. (2018). Trajectory Analysis Unveils Reelin’s Role in the Directed Migration of Granule Cells in the Dentate Gyrus. J. Neurosci 38, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian G-F, Peng WG, Han X, Kang J, Takano T, and Nedergaard M (2006). Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci 9, 816–823. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, and Herz J (2002). Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem 277, 39944–39952. [DOI] [PubMed] [Google Scholar]
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, et al. (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun 9, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiera G, Wozniak G, Bajor M, Kaczmarek L, and Mozrzymas JW (2013). Maintenance of long-term potentiation in hippocampal mossy fiber-CA3 pathway requires fine-tuned MMP-9 proteolytic activity. Hippocampus 23, 529–543. [DOI] [PubMed] [Google Scholar]
- Winship IR, Plaa N, and Murphy TH (2007). Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J. Neurosci 27, 6268–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcik-Stanaszek L, Sypecka J, Szymczak P, Ziemka-Nalecz M, Khrestchatisky M, Rivera S, and Zalewska T (2011). The potential role of metalloproteinases in neurogenesis in the gerbil hippocampus following global forebrain ischemia. PLoS One 6, e22465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won SJ, Kim SH, Xie L, Wang Y, Mao XO, Jin K, and Greenberg DA (2006). Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exp. Neurol 198, 250–259. [DOI] [PubMed] [Google Scholar]
- Xu J-C, Xiao M-F, Jakovcevski I, Sivukhina E, Hargus G, Cui Y-F, Irintchev A, Schachner M, and Bernreuther C (2014). The extracellular matrix glycoprotein tenascin-R regulates neurogenesis during development and in the adult dentate gyrus of mice. J. Cell Sci 127, 641–652. [DOI] [PubMed] [Google Scholar]
- Yamada J, Ohgomori T, and Jinno S (2015). Perineuronal nets affect parvalbumin expression in GABAergic neurons of the mouse hippocampus. Eur. J. Neurosci 41, 368–378. [DOI] [PubMed] [Google Scholar]
- Yamada J, Nadanaka S, Kitagawa H, Takeuchi K, and Jinno S (2018). Increased Synthesis of Chondroitin Sulfate Proteoglycan Promotes Adult Hippocampal Neurogenesis in Response to Enriched Environment. J. Neurosci 38, 8496–8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Liu Z-R, Chen J, Zhang S-J, Quan Q-Y, Huang Y-G, and Jiang W (2010). Roles of astrocytes and microglia in seizure-induced aberrant neurogenesis in the hippocampus of adult rats. J. Neurosci. Res 88, 519–529. [DOI] [PubMed] [Google Scholar]
- Ye Q, and Miao Q-L (2013). Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol 32, 352–363. [DOI] [PubMed] [Google Scholar]
- York EM, Bernier L-P, and MacVicar BA (2018). Microglial modulation of neuronal activity in the healthy brain. Dev. Neurobiol 78, 593–603. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG Jr, Ming G-L, and Gage FH (2006). Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci 26, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Jou J, Wolff LJ, Sun H, and Gage FH (2015). Spine morphogenesis in newborn granule cells is differentially regulated in the outer and middle molecular layers. J. Comp. Neurol 523, 1588. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, and Schwartz M (2006). Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci 9, 268–275. [DOI] [PubMed] [Google Scholar]


