Patients with heart failure with reduced ejection fraction (HFrEF) face high risks of deterioration in health status, hospitalization, or death. Diabetes mellitus (DM) is a frequent comorbidity which adds therapeutic complexity and further contributes to adverse health outlook. The Food and Drug Administration recently reinforced that therapies safely improving patient-reported outcomes, such as HRQOL, may meet standards for regulatory approval in HF(1). Despite its broad recognition as an important, patient-centered outcome, few real-world data are available tracking the expected history of HRQOL over time. We explore longitudinal changes in HRQOL among ambulatory patients with comorbid DM and HFrEF in a contemporary US registry.
From December 2015 to October 2017, across 152 US sites, CHAMP-HF enrolled 4,983 adults with chronic HFrEF(≤40%) receiving ≥1 oral HF therapy. Key exclusion criteria included enrollment in an interventional study, planned or history of heart transplantation, left ventricular assist system, or dialysis, hospice care, or life expectancy<1-year. We further excluded patients who were missing all demographic data (n=13). Follow-up was available through October 2018.
The 12-item Kansas City Cardiomyopathy Questionnaire Short Form (KCCQ) is a validated multi-domain HF-specific tool with scores ranging from 0(worst) to 100(best). The EuroQol-5 Dimension (EQ-5D) is a standardized generic instrument that is summarized as a single index (0[dead] to 1[best health]) across 5 domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.
Among patients alive with paired baseline and 12-month KCCQ and/or EQ5D scores (n=3,093), we assessed the association between DM and HRQOL using linear regression models, adjusted for baseline HRQOL and 11 pre-specified covariates (age, sex, current smoking, chronic obstructive pulmonary disease, EF, systolic blood pressure, body mass index, serum creatinine, first diagnosis of HF<18 months, β-blocker, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker). Participation required written informed consent. The study was approved by site institutional review boards or ethics committees.
Of 4,970 CHAMP-HF participants analyzed, 2,085 (42%) had a history of DM. Patients with DM were older (68[60–75] vs. 67[57–76] years), less likely to be white (70.4% vs. 75.8%) or have private insurance (23.6% vs. 27.4%), and more frequently had cardiovascular comorbidities and histories of HF hospitalization within 12 months (40.6% vs. 36%); all P<0.02.
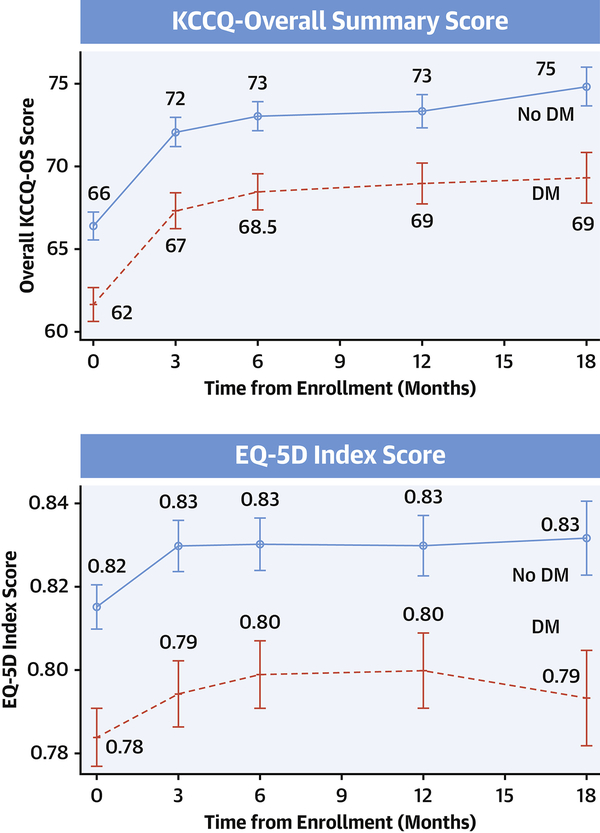
Compared with those without DM, patients with DM had lower baseline KCCQ-OS (61.7±24.1 vs. 66.4±23.2; P<0.001) and EQ-5D scores (0.78±0.16 vs. 0.82±0.15; P<0.001). Absolute differences across KCCQ domains ranged from ~3 to 6 (quality of life 3.2; social limitations 4.4; symptom frequency 5.1; physical limitations 6.2); P<0.001 for all. After accounting for covariates, patients with DM had 2.8 (95% CI 1.2–4.4, P<0.001) lower adjusted KCCQ-OS scores.
Over 12-months, patients with DM (62.9±23.4 to 69.0±22.4; difference +6.1±21.0) and without DM (67.7±22.6 to 73.3±21.8; difference +5.7±19.7) experienced longitudinal improvements KCCQ-OS scores; most increases were observed within 3-months (Figure). Similarly, EQ5D scores increased in patients with DM (0.79±0.16 to 0.80±0.16; difference 0.01±0.16) and without DM (0.82±0.14 to 0.83±0.16; difference 0.01±0.14). Irrespective of DM status, 49%, 37%, and 20% experienced longitudinal increases in KCCQ-OS by 5, 10, and 20 points, respectively. At 12-months, differences in adjusted KCCQ-OS scores persisted between patients with and without DM: difference 2.4 (95% CI 0.9–3.9, P=0.001).
Figure 1. Measures of Health-Related Quality of Life at Baseline and Through 18 Months in Chronic Heart Failure with Reduced Ejection Fraction by Diabetes Mellitus (DM) Status.
The Kansas City Cardiomyopathy Questionnaire (KCCQ) interrogates heart failure symptoms, physical and social limitations, and quality of life, which is summarized as the KCCQ overall summary score (KCCQ-OS). KCCQ scores range from 0 (worst) to 100 (best) health status with a difference of 5-points considered clinically meaningful. EuroQol-5 Dimension (EQ-5D) is summarized as a single index (0 [dead] to 1 [best health]) across 5 domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Patients with comorbid DM have worse health-related quality of life, determined by these 2 validated questionnaires, at baseline and through 18 months follow-up.
Patients with comorbid DM have worse HRQOL throughout their longitudinal experience as ascertained across key domains of 2 validated questionnaires. Regardless of DM status, half of patients experience clinically meaningful 5-point improvements in KCCQ-OS by 1-year, despite CHAMP-HF being a non-interventional study with limited changes to dosing of guideline-directed medical therapies(2). This finding may reflect favorable perceptions of health under study observation (Hawthorne effect), selective retention of a healthier cohort (as patients with worse HRQOL may be more likely to die), and/or regression to the mean (due to a tendency of worse health status at study enrollment). These short-term improvements, coupled with wide confidence intervals and variation in trajectories of patient-reported outcomes, may explain seemingly modest average between-arm treatment effects on KCCQ with effective recent HFrEF therapies: dapagliflozin (+2.8 in KCCQ total symptoms score over 8-months)(3) and sacubitril/valsartan (+1.3 in KCCQ-OS over 8-months)(4). In the evaluation of HRQOL changes in a HF trial, statistical approaches such as the win-ratio should be used to account for competing risks of death. These CHAMP-HF data corroborate the broad health consequences of DM adversely impacting patient experience and inform designs of studies employing HRQOL as an endpoint.
Acknowledgments
FUNDING SOURCES
The CHAMP-HF registry is sponsored by Novartis Pharmaceuticals Corporation (East Hanover, NJ). Data were managed by United BioSource Corporation (Blue Bell, PA). The Duke Clinical Research Institute (Durham, NC) served as the data analytic center.
DISCLOSURES
Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, and Relypsa, and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH. Dr Fonarow reports research funding from the NIH and serving as a consultant for Abbott, Amgen, Bayer, CHF Solutions, Janssen, Medtronic, and Novartis. Dr. Greene is supported by a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis, has received research support from Amgen, Bristol-Myers Squibb and Novartis, and serves on an advisory board for Amgen. Dr. DeVore reports receiving research funding from Akros Medical, the American Heart Association, Amgen, Bayer, Intra-Cellular Therapies, Luitpold Pharmaceuticals, the NHLBI, Novartis, and PCORI and serving as a consultant for Novartis. Dr. Albert reports serving as a consultant for Novartis and Boston Scientific. Ds. Duffy is an employee of Novartis. Dr. Spertus reports that, relevant to this work, he serves as a consultant for Novartis and owns the copyright to the Kansas City Cardiomyopathy Questionnaire. He also serves as a consultant for Bayer, AstraZeneca, United Healthcare and Janssen; has equity in Health Outcomes Sciences and serves on the Board of Directors for Blue Cross-Blue Shield of Kansas City. Dr. Butler has received research support from the National Institutes of Health, PCORI and the European Union; and serves as a consultant for Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmaceutical, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, SC Pharma, Vifor, and ZS Pharma. All other authors report no disclosures.
ABBREVIATIONS
- DM
Diabetes mellitus
- EQ-5D
European Quality of Life Five Dimension
- HFrEF
Heart failure with reduced ejection fraction
- HRQOL
Health-related quality of life
- KCCQ
Kansas City Cardiomyopathy Questionnaire
Footnotes
Tweet: New #CHAMPHF registry data track longitudinal changes in health-related QOL in ambulatory patients with HFrEF with and without comorbid DM
References
- 1.US Food and Drug Administration. Treatment for Heart Failure: Endpoints for Drug Development. Available at: https://www.fda.gov/media/128372/download. Accessed on September 27th, 2019.
- 2.Greene SJ, Fonarow GC, DeVore AD et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol 2019;73:2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019. (in press). [DOI] [PubMed] [Google Scholar]
- 4.Lewis EF, Claggett BL, McMurray JJV et al. Health-Related Quality of Life Outcomes in PARADIGM-HF. Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]