Abstract
The preparation of a new non-natural gabosine is reported, in which the chirality is transferred from the toluene’s biotransformed metabolite (1R,2S)-3-methylcyclohexa-3.5-diene-1,2-diol. Further chemical transformations to introduce additional functionality and chirality to the molecule were also accomplished.
Keywords: gabosine, chemoenzymatic, enantioselective synthesis
1. Introduction
Monosubstituted benzenes can be enzymatically transformed into chiral diols [1,2,3] that are valuable synthons for enantioselective synthesis. They have been widely and successfully used for the preparation of natural products and analogs with biological properties of interest [4,5].
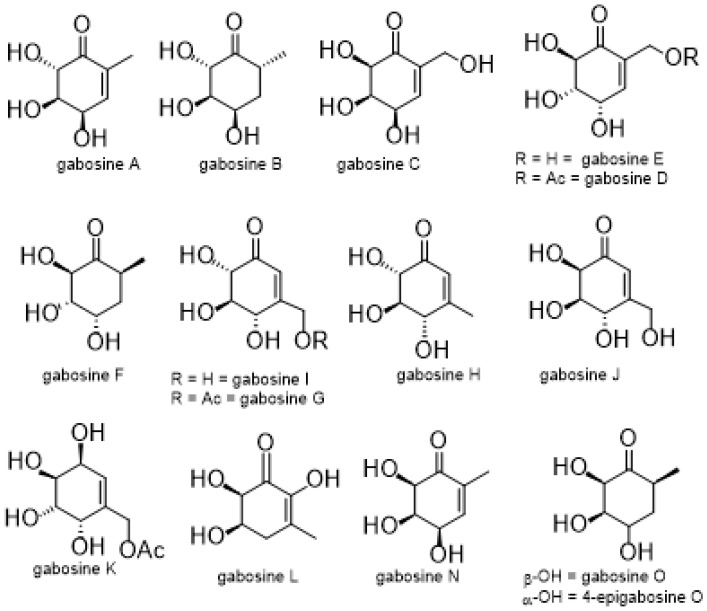
In this field, we have focused on gabosines and anhydrogabosines. Gabosines (Figure 1) are keto-carbasugars bearing a methyl or hydroxymethyl side chain, originally described in 1974 [6,7]. They show promising pharmacological activities as antibiotic, anticancer, enzyme inhibition, and DNA-binding properties [8,9,10,11,12,13,14].
Figure 1.
The gabosine family.
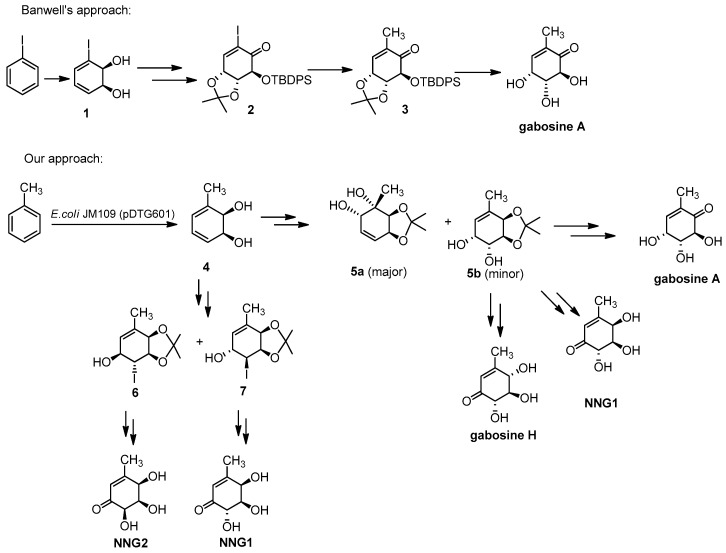
This wide range of biological properties turned the gabosine family into a group of coveted synthetic targets. During the last 30 years, several strategies have been developed to prepare gabosines [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] and related derivatives [31,32,33,34,35,36,37,38]. Among these and other synthetic efforts, only Banwell’s group and ours have applied biotransformations to introduce chirality into the process. Although toluene is the most suitable aromatic substrate, as it bears the methyl side chain of some gabosines, in 2001, Banwell and co-workers failed in their attempt to use it, and reported the preparation of gabosine A from iodobenzene derived diol 1 [39], introducing the methyl group in one of the lasts steps, as shown in Figure 2. In 2011, we were able to prepare the same target from toluene [40] and in 2017 we reported the synthesis of gabosine H and two non-natural methylgabosines [41] from the same starting material (Figure 2).
Figure 2.
Gabosine’s syntheses from biotransformation of monosubstituted benzenes.
A key intermediate in our syntheses of gabosines A and H was compound 5b. This compound is the minor product obtained during osmilation of toluene diol acetonide, which is a technique extensively used in our lab to functionalize the diene. Toluene derived diols reacts preferentially on the more substituted olefine, leading to 5a [42] and consequently 5b, the usually unwanted minor regioisomer, is readily available to us. We have already reported the synthesis of a non-natural gabosine (NNG1) from compound 5b [41].
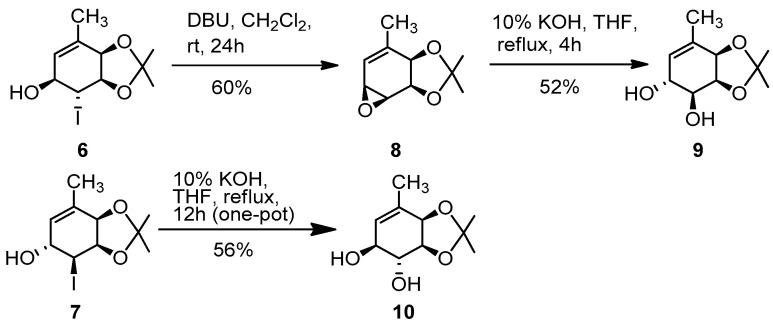
To prepare NNG2, along with an alternative route for NNG1, previously, we took advantage of another methodology extensively studied and used in our lab: the hydroxyhalogenation of the cyclohexadiendiols [43]. Using both the major (6) and the minor (7) products, we reported the preparation of NNG1 and NNG2. Major iodohydrin 6 was converted into NNG2 and minor iodohydrin 7 was converted into NNG1. In both cases, the first step was the substitution of the iodine atom by a hydroxy group, through a sequence of epoxide formation and opening [44]. In that previous report, we essayed a stepwise sequence, as well as a one-pot reaction, as shown in Figure 3.
Figure 3.
Reported conversion of iodohydrins into diols.
Herein, we focused on the challenge of synthesizing a new non-natural gabosine, NNG3, an epimer of gabosine A, as shown in Figure 4.
Figure 4.
Proposed strategy for the synthesis of NNG3.
The preparation of a new gabosine, NNG3, confirms the efficiency and usefulness of our methodology, using toluene as starting material.
2. Results
First, we decided to broad and optimize the use of the hydroxyhalogenation to prepare non-natural gabosines, starting from major iodohydrin 6 (prepared from diol 4 in 2 steps, 77% overall yield) [43]. The first goal was to improve the yield of our own previous work: the transformation of iodohydrins 6 and 7 into diols 9 and 10 respectively, as shown in Figure 3 (31% for 9 in two steps from 6, 56% for 10 in one step from 7) [41]. Table 1 displays the results achieved for the conversion of 6 into 9 in one step performed in a 3:1 THF:H2O mixture.
Table 1.
Conditions assayed for the preparation of diol 9 in one step.
| Entry | Base | Equivalents | [6] (M) | T (°C) | Time (h) | Products (Yield) * |
|---|---|---|---|---|---|---|
| 1 | NaOH | 4.0 | 0.18 | R.T.-reflux | 24 h R.T.+ 4 h reflux | 9 (55%) + 8 (10%) |
| 2 | NaOH | 4.0 | 0.20 | reflux | 6 | 9 (42%) |
| 3 | NaOH | 4.0 | 0.15 | reflux | 48 | 9 (54%) |
| 4 | NaOH | 5.0 | 0.18 | reflux | 6 | 9 (45%) |
| 5 | NaOH | 6.0 | 0.15 | reflux | 8 | 9 (58%) + 8 (8%) |
| 6 | KOH | 4.0 | 0.15 | reflux | 9 | 9 (61%) |
| 7 | KOH | 6.6 | 0.15 | reflux | 10 | 9 (55%) |
| 8 | KOH | 8.0 | 0.15 | reflux | 8 | 9 (65%) |
R.T: room temperature * The yields correspond to chromatographically purified products.
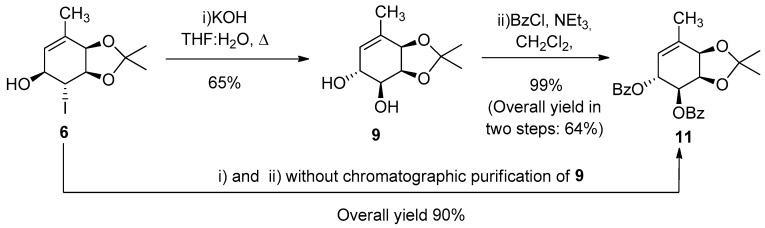
During these experiments, we observed that diol 9 was not stable under chromatographic purification conditions. Therefore, we first assayed the protection of purified 9, and then a “tandem” procedure was also explored. At this stage, we were pleased to verify an increase of the yield from 64% to 90% taking advantage of the “tandem” strategy.
These results are summarized in Figure 5.
Figure 5.
Optimization of key intermediate 11’s preparation.
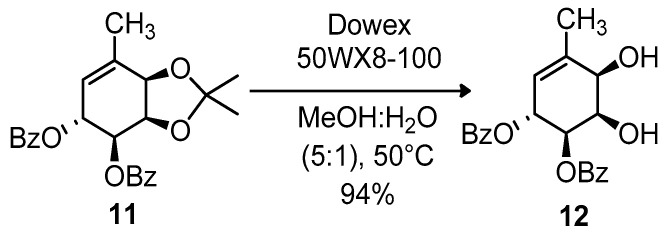
Next, the acetonide group was selectively removed from compound 11 by means of an acidic resin (Figure 6).
Figure 6.
Selective deprotection of 11.
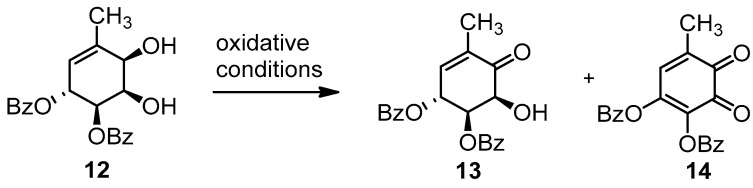
In the following step, the oxidation of the allylic alcohol, was unexpectedly laborious, mainly due to the formation of an over-oxidized product (Figure 7).
Figure 7.
Selective oxidation of diol 12.
We assayed a series of oxidizing agents, with poor-to-moderate results, which are summarized in Table 2.
Table 2.
Conditions for the selective oxidation of 12.
| Entry | Oxidizing Agent (Equivalents) |
Solvent | [12] (M) | T (°C) | Time (h) | Products (Yield) * |
|---|---|---|---|---|---|---|
| 1 | IBX (2.0) | DMF | 0.10 | 0–R.T. | 48 | 13 (45%) + 14 |
| 2 | PCC/SiO2 (2.0) | CH2Cl2 | 0.15 | R.T. | 0.75 | 13 (51%) |
| 3 | PCC/SiO2 (1.5) | CH2Cl2 | 0.05 | R.T. | 0.75 | 13 (26%) |
| 4 | PCC/molecular sieves 4Å (2.0) | CH2Cl2 | 0.22 | R.T. | 1.5 | 14 + D.P. |
| 5 | SO3.Py (3.1), NEt3 (6.2) | CH2Cl2:DMSO (3:1) |
0.15 | 0–R.T. | 3 | 13 (36%) +14 |
| 6 | MnO2 (10% m-m) | CHCl2 | 0,05 | 0–R.T. | 0.25 | 14 + D.P. |
| 7 | MnO2 (14% m-m) | CHCl2 | 0.05 | −20 | 24 | 13 (29%) + 14 |
| 8 | NBS (3.0), β-cyclodextrin (1.0) |
H2O | 0.07 | R.T. | 48 | 14 + D.P. |
| 9 | IBX (1.1), β-cyclodextrin (0.1) | H2O:acetone (86:14) |
0.07 | R.T. | 24 | 12 |
| 10 | 2-IBA (0.6), Oxone (5.0) | MeCN:H2O (2:1) | 0.08 | R.T. | 96 | 12 |
| 11 | 2-IBA (0.6), Oxone (5.0) | MeCN:H2O (2:1) | 0.08 | 70 | 16 | 13 (34%) |
* The yields correspond to chromatographically purified products. The yields of secondary product 14 could not be determined. D.P. = decomposition products.
Initially, we used IBX, a reagent routinely employed by our group for these transformations [40] (Table 2, entry 1). However, we were surprised to observe the formation of the diene-dione 14 along with the target product 13. This fact led us to switch the oxidizing agent to PCC with acceptable results (Table 2, entry 2). We attempted to improve this yield, based on preventing the adsorption of the products on SiO2. We decreased the equivalents of PCC, diluted the reaction mixture and replaced SiO2 with molecular sieves (Table 2, entries 3 and 4) [19] with poor results. Attempts to improve on these results using SO3.Py [45] (Table 2, entry 5), MnO2 (Table 2, entries 6 and 7) [46], NBS and IBX in the presence of cyclodextrin [47,48](Table 2, entries 8 and 9) and in situ generation of IBX with Oxone [49] (Table 2, entries 10 and 11) led to lower yields. Consequently, we reverted to the use of IBX and PCC. Since IBX is a safer reagent and the formation of by-products is minimized compared to PCC, we decided to focus on this oxidizing agent. Following methodology on the influence of solvents in oxidations using IBX [50], we improved the yield in the preparation of 13. These results are shown in Table 3.
Table 3.
Conditions for the selective oxidation of 12, using IBX prepared according to Frigerio et al. [51].
| Entry | IBX (eq.) | Solvent | [12] (M) | T (°C) | Time (h) | Products (Yield) * |
|---|---|---|---|---|---|---|
| 1 | 2.0 | DMF | 0.10 | 0–R.T. | 48 | 13(45%) + 14 |
| 2 | 3.0 | DMF | 0.10 | 0–R.T. | 24 | 13(22%) + 14 |
| 3 | 3.0 | DMF | 0.25 | 0–R.T. | 1.0 | 14 + D.P |
| 4 | 1.5 | DMSO | 0.35 | 0–R.T. | 0.33 | 14 + D.P. |
| 5 | 3.0 | AcOEt | 0.10 | 40 | 23 | 13(10%) + 14 |
| 6 | 3.0 | AcOEt | 0.03 | R.T. | 72 | 14 + D.P. |
| 7 | 3.0 | AcOEt | 0.03 | reflux | 4 | 14 + D.P. |
| 8 | 3.0 | Acetone | 0.02 | reflux | 2 | 13(52%) + 12 |
| 9 | 3.0 | Acetone | 0.03 | reflux | 0.75 | 13(56%) + 12 |
| 10 | 3.0 | Acetone | 0.03 | reflux | 1.5 | 13(36%) + 14 |
| 11 | 3.0 | Acetone | 0.03 | R.T. | 48 | 14 + D.P. |
| 12 | 2.0 | Acetone | 0.03 | reflux | 0.83 | 13(38%) + 14 |
| 13 | 1.5 | Acetone | 0.03 | reflux | 24 | 13(24%) + 12 |
eq.: equivalents * The yields correspond to chromatographically purified products. The yields of secondary product 14 could not be determined. D.P. = decomposition products.
Although the use of polar solvents in which IBX is more soluble (Table 3, entries 1–4) allowed working at lower temperatures, this did not avoid the formation of over-oxidized compound 14, thereby not increasing the yield of 13 significantly. The use of AcOEt was not useful either (Table 3, entries 5–7). The best results were achieved in acetone (Table 3, entries 8–13) with strict chromatographic monitoring of unwanted 14 to stop the reaction (Table 3, entry 8). These efforts permitted to enhance yields and reduce reaction times, as well as to prevent the formation of decomposition products and thus facilitate the further purification steps. The final step was the benzoyl groups removal. We were aware of previous reports concerning the instability of gabosines in basic medium, including the first gabosine’s isolation [6]. In Banwell´s chemoenzymatic approach [39], the authors describe gabosines as “base-sensitive compounds”. Despite this, we first decided to perform the classic basic methanolysis, noticing evident decomposition of the starting material 13, low yield for the final product NNG3 and partial deprotection (Figure 8).
Figure 8.
Basic deprotection of 13.
However, none of the attempts to deprotect the diol in acid medium succeeded. Most of them (5% HCl in methanol, catalytic BF3.Me2S in CH2Cl2 and Dowex 50W8 strongly acid in HCl 1M) led to 100% decomposition, and when treated with trifluoroacetic acid in the presence of water, we only recovered the starting material. Deprotection was finally successful by means of KCN [52], as shown in Figure 9.
Figure 9.
Synthesis of NNG3.
The experiments concerning the structural elucidation of all new compounds can be consulted in the Supplementary Materials section.
3. Materials and Methods
3.1. Chemicals and Materials
Commercially available reagents were purchased and used without further purification. Solvents were distilled prior to use. Non-hydrolytic reactions were carried out in nitrogen atmosphere with standard techniques for the exclusion of air. Reactions were monitored by thin-layer chromatography (TLC) performed on plates pre-coated with silica gel 60F 254 and visualized with UV light (254 nm) and/or p-anisaldehyde in acidic ethanolic solution and/or vanillin in acidic ethanolic solution. Flash column chromatography was performed using silica gel (Kieselgel 60, EM reagent, 230–400 mesh (Macherey-Nagel, Düren, Germany) The ratio of the eluents is informed in volume in all cases. Yields refer to chromatographically and spectroscopically homogeneous materials.
3.2. Instrumentation
Melting points were recorded in Fisher-Johns melting point apparatus serial 4446 Fisher-Scientific company (Fisher-Scientific international Inc., Pittsbugh, PA, USA) and are uncorrected. Mass spectra were recorded, using the electron impact mode (70 or 20 eV) in a GCMS QP2010 Shimadzu HP 5971 spectrometer (Shimadzu Corporation, Kyoto, Japan). Elementary analyses were performed in a microanalyzer Fisons EA 1108 CHNS-O (Fison Instruments Ltd., Glasglow, UK) Infrared spectra were recorded on neat samples (NaCl disks), using a Shimadzu FT/IR-8101 Type A spectrometer (Shimadzu Corporation, Kyoto, Japan). NMR spectra were obtained in a Bruker Advance DPX-400 instrument at 400 MHz for 1H NMR and 100 MHz for 13C NMR in CDCl3 or MeOH-D4 or (CD3)2CO (Bruker Corporation, Billerica, MA, USA). Proton chemical shifts are reported in ppm downfield from TMS as an internal reference, and carbon chemical shifts are reported in ppm relative to the center line of the CDCl3 triplet (77.0 ppm). Optical rotations were measured in a Krüss Optronic P8000 polarimeter (A. Krüss Optronic GmbH, Hamburg, Germany), using a 0.5 dm cell. Moreover, [α]D values are given in units of deg cm2 g−1, and concentration values are expressed in g/100 mL.
3.3. Experimental Procedures
3.3.1. Synthesis of (1R,2R,5R,6S)-1,2-O-isopropyliden-3-methylciclohex-3-en-1,2,5,6-tetraol (9)
Compound 6 (1.3 g, 3.9 mmol) was dissolved in a THF:H2O mixture (3:1, 29 mL), and KOH (1.8 g, 32 mmol) was added. The mixture was heated under reflux for 8 h, cooled to room temperature, and poured on H2O (80 mL). The aqueous phase was extracted with AcOEt (1 × 50 mL, 4 × 25 mL), and the combined organic layers were first washed with saturated NH4Cl (1 × 25 mL) and then with saturated NaCl (1 × 25 mL). After drying over Na2SO4 and evaporating the solvent under vacuum, the product was purified by flash column chromatography, in SiO2, using 7:3 AcOEt:hexanes, to afford 9 (508 mg, 65%) as a white solid. All spectroscopic and physical properties are in concordance with the reported data.
3.3.2. Synthesis of (1R,2R,5R,6S)-5,6-dibenzoyl-1,2-O-isopropyliden-3-methylciclohex-3- en-1,2,5,6-tetraol (11)
(a) One step from 9:
Compound 9 (508 mg, 2.58 mmol) was dissolved in CH2Cl2 (25 mL), under nitrogen, and cooled 0 °C. NEt3 (1.4 mL, 10.3 mmol) was added and after a few minutes, and then BzCl (0.75 mL, 6.45 mmol) was slowly dropped into the mixture. The system was allowed to reach room temperature and stirred for 24 h; the mixture was then poured on saturated NaHCO3 (50 mL) and extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were first washed with saturated NH4Cl (1 × 20 mL) and then with saturated NaCl (1 × 20 mL). After drying over Na2SO4 and evaporating the solvent under vacuum, the product was purified by flash column chromatography, in SiO2, using 4:6 CH2Cl2:hexanes, to afford 11 (1.04 g, 99%) as a white foamy solid.
(b) “Tandem” synthesis from 6:
The procedures described in Section 3.3.1 and Section 3.3.2. (a) are followed one after the other, with no chromatographic purification of 9, starting from 6 (600 mg, 1.94 mmol). 11 obtained as a foamy white solid (788 mg, 90% overall)
1H-NMR (400 MHz, CDCl3) δ(ppm): 8.07–7.97 (m, 4H), 7.50–7.41 (m, 2H), 7.40–7.36 (m, 4H), 6.12–6.08 (m, 1H), 5.55–5.54 (m, 2H), 4.69 (dd, J1 = 5.0, J2 = 2.5 Hz, 1H), 4.59 (dd, J1 = 5.1, J2 = 1.8 Hz, 1H), 1.86 (s, 3H), 1.44 (s, 3H), 1.38 (s, 3H); 13C- NMR(101 MHz, CDCl3) δ(ppm):166.27,166.10, 136.49, 133.27, 133.11, 130.00, 129.85, 129.70, 129.56, 128.38, 121.61, 110.65, 76.83, 74.36, 72.45, 69.70, 27.59, 26.82, 19.12; EA: 70.6189% C (calculated 70.5882%, |δ= 0.0307%), 5.7118% H (calculated 5.8824%, |δ= 0.1706%); M.P. = (50–52) °C; [α]D19 = −183.1 (c 0.855, acetone); IR (NaCl)/cm−1: 3062, 2985, 1712, 1602, 1585, 1490, 1315, 1236, 1178,1161, 1111, 995, 886.
3.3.3. Synthesis of (1R,2R,5R,6R)-5,6-dibenzoyl-3-methylciclohex-3-en-1,2,5,6-tetraol (12)
Compound 11 (277 mg, 0.68 mmol) was dissolved in a mixture of MeOH:H2O (5:1, 4.3 mL), acid resin Dowex 50wx8-100 (1.1 g) was added, and the system was stirred at 50 °C for 24 h. Once all the starting material was consumed, the resin was vacuum filtered and washed several times with methanol, until the product was no longer detectable in the filtrate by TLC. Methanol was evaporated under vacuum and water was also eliminated by vacuum distillation in the presence of a few drops of AcOEt (azeotropic distillation). The product was purified by flash column chromatography in SiO2, using 7:3 AcOEt:hexanes, to afford 12 (233 mg, 94%) as a foamy white solid.
1H-NMR (400 MHz, CDCl3) δ(ppm): 8.02–7.97 (m, 4H), 7.52–7.50 (m, 2H), 7.40–7.37 (m, 4H), 6.08–6.05 (m, 1H), 5.60 (t, J = 1.2 Hz, 1H), 5.46 (dd, J1 = 7.5, J2 = 2.0 Hz, 1H), 4.46 (dd, J1 = 3.9, J2 = 2.0 Hz, 1H), 4.34 (d, J = 3.7 Hz, 1H), 3.14 (s, 1H), 2.74 (s, 1H), 1.89 (s, 3H); 13C-NMR (101 MHz, CDCl3) δ(ppm): 166.17, 166.05, 138.53, 133.44, 133.21, 129.81, 129.73, 129.36, 128.52, 128.40, 120.71, 74.28, 70.45, 70.18, 69.99, 19.32; EA: 68.2454% C (calculated 68.4783%, |δ = 0.2329%), 5.6997% H (calculated 5.4348%, |δ = 0.2649%); M.P. = (70–72) °C; [α]D19 = −222.6 (c 0.540, acetone); IR (NaCl)/cm−1:3396, 3091, 3070, 2953, 2976, 2918, 1693, 1645, 1633, 1602, 1585, 1452, 1276, 1178, 1111, 1026, 985, 706.
3.3.4. Synthesis of (4R,5R,6S)-4,5-dibenzoyl-6-hydroxy-2-methylciclohex-2-enone (13)
(a) Procedure using PCC/SiO2:
In a mortar, PCC (60 mg) and SiO2 (60 mg) were grinded until a homogeneous fine orange mixture was obtained. This solid was placed in a round-bottom flask, CH2Cl2 (28 mL) was added, and the mixture was stirred for a few minutes. Then 12 (40 mg) was added, the solution got darker, and after 45 min, the reaction was stopped. Et2O (15 mL) was added, and then it was vacuum filtered through a double celite and SiO2 layer. The filter aid was washed with Et2O, until the product was no longer detectable in the filtrate by TLC. The solvent was removed by vacuum distillation, and the crude product was re-dissolved in Et2O (15 mL). The organic phase was washed with NaHCO3 (1 × 5 mL) y NaCl (1 × 5 mL) and then dried over Na2SO4. The solvent was vacuum distilled, and the product was purified by flash column chromatography, in SiO2, using 1:9 AcOEt:hexanes, to afford 13 (20.4 mg, 51%) as a yellowish solid.
(b) Procedure using SO3Py:
Compound 12 (64 mg, 0.17 mmol) was dissolved in a mixture of CH2Cl2:DMSO (3:1, 1.3 mL), under nitrogen. The mixture was cooled to 0 °C, and then NEt3 (0.15 mL, 1.054 mol) and the complex SO3Py (145 mg, 0.561 mmol) were added. The mixture was allowed to reach room temperature, until no more starting material was detected (3 h), and then it was diluted with CH2Cl2 (20 mL) and washed with H2O (20 mL). The aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried over Na2SO4. The solvent was vacuum distilled and the product was purified by flash column chromatography in SiO2 using 1:9 AcOEt:hexanes to afford 13 (23 mg, 36%) as a yellowish solid.
(c) Procedure using MnO2:
Compound 12 (50.0 mg, 0.13 mmol) was dissolved in CH2Cl2 (3.0 mL) and cooled to −20 °C. MnO2* (700 mg, 8.06 mmol) was added in portions and the mixture was stirred for 24 h. The reaction mixture was vacuum filtered through celite, washing with CH2Cl2. The solvent was vacuum distilled, and the product was purified by flash column chromatography in SiO2, using 1:9 AcOEt:hexanes, to afford 13 (12 mg, 29%) as a yellowish solid.
Preparation of MnO2: KMnO4 (4.7 g) was dissolved in H2O (30 mL), and a solution of MnSO4.H2O (5 g) in H2O (30 mL) was slowly added, drop by drop. After an hour, the precipitate was filtered; washed with H2O, at 0 °C; and dried in an oven, at 120 °C, for 24 h (81%).
(d) Procedure using 2-IBA-Oxone
Compound 12 (89.0 mg, 0.24 mmol) was dissolved in a mixture MeCN:H2O (2:1, 3.1 mL). The, 2-Iodobenzoic acid (36.0 mg, 0.072 mmol) and Oxone (738 mg, 1.2 mmol) were added. The mixture was heated to 70 °C and stirred for 16 h; it was then cooled to 0 °C and filtered. The precipitate was washed twice with H2O and with CH2Cl2, until the product was no longer detectable in the filtrate by TLC. The aqueous phase was extracted with CH2Cl2 (3 × 5mL). The combined organic layers were dried over Na2SO4. The solvent was vacuum distilled, and the product was purified by flash column chromatography in SiO2, using a gradient of AcOEt:hexanes (from 1:99 to 2:8), to afford 13 (30 mg, 34%) as a yellowish solid.
(e) Optimized procedure using IBX
Compound 12 (59.4 mg, 0.16 mmol) was dissolved in acetone (5.4 mL), IBX* (136 mg, 0.49 mmol) was added, and the mixture was heated under reflux. The appearance of a yellow-to-orange color in 45 min indicates the formation of over-oxidized product 14. Thus, after 45 min, the reaction was allowed to cool to room temperature, and AcOEt (10 mL) was added. The mixture was filtered, and the solid was washed with AcOEt, until the product was no longer detectable in the filtrate by TLC. The organic phase was washed with saturated NaHSO3 (1 × 10 mL) and saturated NaCl (1 × 5 mL). The combined organic layers were dried over Na2SO4. The solvent was vacuum distilled, and the product was purified by flash column chromatography, in SiO2, using a gradient of AcOEt:hexanes (from 1:99 to 2:8), to afford 13 (33 mg, 56%) as a yellowish solid.
1H-NMR (400 MHz, CDCl3) δ(ppm): 8.08–8.06 (m, 2H), 7.92–7.90 (m, 2H), 7.62–7.55 (m, 2H), 7.46 (t, J = 7.7 Hz, 2H), 7.44 (t, J = 7.7 Hz, 2H), 6.79 (dq, J1 = 5.0, J2 = J3 = J4 = 1.6 Hz, 1H), 5.91–5.90 (m, 1H), 5.80–5.79 (m, 1H), 4.98 (t, J = 2.9 Hz, 1H), 3.57 (d, J = 3.2 Hz, 1H), 2.01 (t, J = 1.3 Hz, 3H); 13C- NMR (101 MHz, CDCl3) δ(ppm):197.47, 165.07, 137.75, 135.96, 133.75, 133.57, 129.92, 129.86, 129.07, 128.98, 128.63, 128.50, 73.36, 71.25, 67.11, 15.61; E.A.: 68.7589% C (calculated 68.8852%, |δ = 0.1263%), 5.2039%H (calculated 4.9180%, |δ = 0.2859%): M.P. = (120–124) °C (decomposes); [α]D19 = −168.6 (c 0.535, acetone); IR (NaCl)/cm−1:3446, 3093, 3070, 2959, 1720, 1697, 1647, 1601, 1585, 1491, 1452, 1315, 1261, 1178, 1095, 1070, 710.
Preparation of IBX: [51] 2-iodobenzoic acid (2.5 g, 0.050 mol) was added to a solution of Oxone in distilled water (18.6 g, 0.031 mol, 3 eq/0.2 L of H2O). The suspension was heated to 70 °C and allowed to react until turbidity disappeared, or for 3 h. Then, the mixture was cooled to 0–5 °C, and the precipitate was vacuum filtered. The white solid was washed with H2O (6 × 5 mL) and then with acetone (2 × 5 mL); allowed to dry for 2 h, at room temperature; and stored at −20 °C, protected from light and under nitrogen (1.78 g, 64%).
3.3.5. Synthesis of (4R,5S,6S)-4,5,6-trihidroxy-2-methylcyclohex-2-enone (NNG3)
(a) Procedure using K2CO3
Compound 13 (45 mg, 0.12 mmol) was dissolved in dry MeOH (4.7 mL), under nitrogen, cooled to 0 °C, and a catalytic amount of K2CO3 (2–4 mg), previously dried in an oven, at 120 °C, was added. The mixture was allowed to reach room temperature and stirred for 5 days. It progressively darkened until it turned black, thus indicating the decomposition of the product. The crude mixture was filtered through dry SiO2, which was washed with AcOEt:MeOH (7:3), until the product was no longer detectable in the filtrate by TLC. The solvent was vacuum distilled, and the product was purified by flash column chromatography, in SiO2, using a gradient of AcOEt:hexanes (from 8:2 to AcOEt 100%), to afford NNG3 (5 mg, 25%) as white solid.
(b) Procedure using KCN
Compound 13 (50.4 mg, 0.14 mmol) was dissolved in dry MeOH (1.4 mL), under nitrogen, cooled to 0 °C, and then KCN (18.2 mg, 0.28 mmol) was added. The mixture was allowed to reach room temperature and stirred for 4 h. The appearance of a reddish color was observed. Then, Ion exchange resin “Ion exchanger IV” (36.0 mg) was added to the reaction mixture and stirred for several minutes. The resin was filtered and washed with MeOH (3 × 5 mL). The crude mixture was filtered through dry SiO2, which was washed with AcOEt:MeOH (7:3), until the product was no longer detectable in the filtrate by TLC. The solvent was vacuum distilled, and the product was purified by flash column chromatography, in SiO2, using AcOEt to afford NNG3 (15.1 mg, 70%) as a white solid.
1H-NMR (400 MHz, (C3D3)2O) δ(ppm):6.44 (dt, J1 = 4.7, J2 = 1.6 Hz, 1H), 4.44 (d, J = 5.5 Hz, 1H), 4.39–4,37 (m, 1H), 4.26–4.22 (md, 1H), 4.12 (d, J = 3.7 Hz, 1H), 4.01–3.99 (m, 1H), 3.91 (d, J = 4.0 Hz, 1H), 1.64 (t, J = 1.4 Hz, 3H); 13C-NMR(101 MHz, (C3D3)2O) δ(ppm): 199.01, 141.11, 133.67, 75.24, 73.02, 68.13, 14.49; EA: 51.2868% C (calculated 53.1617%, |δ = 1.8749%), 6.6664% H (calculated 6.3727%, |δ = 0.2937%) M.P. = (118–120) °C; [α]D20 = −34.5 (c 0.18, acetone); IR (NaCl)/cm−1: 3431, 2955, 2922, 2851, 1640, 1632.
4. Conclusions
We succeeded in preparing a new non-natural gabosine, with potential bioactivity, in an enantiomerically pure form. This was possible starting from a biotransformation of toluene that introduces chirality to the process, in seven steps, from diol 4, with 25% overall yield. Exhaustive methodological work was conducted, especially for the optimization of the selective oxidation step, along with the iodine-substitution reaction and the final deprotection. Further application of this methodology in the synthesis of new gabosines and analogs, bioactivity assays, and crystallographic analyses will be reported in due course.
Acknowledgments
PEDECIBA (PNUD/URU/06/004) for financial support. Gabriel Cavalli for text proofreading. Gonzalo Hernández and Horacio Pezaroglo for NMR experiments. Lucía Otero and Santiago Rostán for Elemental Analysis. Agustina Vila for biotransformations. Juan Carlos Ramos for preparative TLC supplies.
Abbreviations
| NNG | Non-natural gabosine |
| TBDPS | Tert-butyl-diphenylsilane |
| DBU | 1,8-Diazabicycle [5.4.0]-undec-7ene |
| THF | Tetrahydrofuran |
| IBX | Iodoxybenzoic acid |
| PCC | Pyridinium chlorochromate |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| IBA | Iodobenzoic acid |
Supplementary Materials
Figure S1: 1H-NMR spectrum of 11. Figure S2: Extended 1H-NMR spectrum of 11 (I). Figure S3: Extended 1H-NMR spectrum of 11 (II). Figure S4: Extended 1H-NMR spectrum of 11 (III). Figure S5: Extended 1H-NMR spectrum of 11 (IV). Figure S6: Extended 1H-NMR spectrum of 11 (V). Figure S7: Extended 1H-NMR spectrum of 11 (VI). Figure S8: 13C-NMR spectrum of 11. Figure S9: IR spectrum of 11. Figure S10: 1H-NMR spectrum of 12. Figure S11: Extended 1H-NMR spectrum of 12 (I). Figure S12: Extended 1H-NMR spectrum of 12 (II). Figure S13: Extended 1H-NMR spectrum of 12 (III). Figure S14: Extended 1H-NMR spectrum of 12 (IV). Figure S15: Extended 1H-NMR spectrum of 12 (V). Figure S16: Extended 1H-NMR spectrum of 12 (VI). Figure S17: 13C-NMR spectrum of 12. Figure S18: IR spectrum of 12. Figure S19: 1H-NMR spectrum of 13. Figure S20: Extended 1H-NMR spectrum of 13 (I), Figure S21: Extended 1H-NMR spectrum of 13 (II). Figure S22: Extended 1H-NMR spectrum of 13 (III). Figure S23: Extended 1H-NMR spectrum of 13 (IV). Figure S24: Extended 1H-NMR spectrum of 13 (V), Figure S25: Extended 1H-NMR spectrum of 13 (VI). Figure S26: 13C-NMR of 13. Figure S27: IR spectrum of 13. Figure S28 1H-NMR spectrum of 14. Figure S29: COSY experiment of 14. Figure S30: 1H-NMR spectrum of NNG3. Figure S31: Extended 1H-NMR spectrum of NNG3 (I). Figure S32: Extended 1H-NMR spectrum of NNG3 (II). Figure S33: Extended 1H-NMR spectrum of NNG3 (III). Figure S34: Extended 1H-NMR spectrum of NNG3 (IV). Figure S35: COSY experiment of NNG3. Figure S36: 13C-NMR spectrum of NNG3. Figure S37: HSQC experiment of NNG3. Figure S38: HMBC experiment of NNG3. Figure S39: IR spectrum of NNG3. Figure S40: 1H-NMR spectrum of 15. Figure S41: COSY experiment of 15.
Author Contributions
Conceptualization, V.S. and E.P.; methodology, M.C.; research, all authors.; writing—original draft preparation, V.S.; writing—review and editing, V.S. and E.P.; supervision, V.S. and E.P.; project administration, V.S.; funding acquisition, V.S. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and in the Supplementary Materials section.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gibson D.T., Koch J., Kallio R. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968;7:2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- 2.Gibson D.T., Hensley M., Yoshioka H., Mabry T.J. Oxidative degradation of aromatic hydrocarbons by microorganisms. III. Formation of (+)-cis-2,3-dihydroxy-1-methyl-4,6-cyclohexadiene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 3.Vila M.A., Brovetto M., Gamenara D., Bracco P., Zinola G., Seoane G., Rodríguez S., Carrera I. Production of cis-1,2-dihydrocatechols of high synthetic value by whole-cell fermentation using Escherichia coli JM109 (pDTG601): A detailed study. J. Mol. Catal. B Enzym. 2013;96:14–20. doi: 10.1016/j.molcatb.2013.06.003. [DOI] [Google Scholar]
- 4.Hudlicky T., González D., Gibson D.T. Enzymatic dihydroxylation of aromatics in enantioselective synthesis: Expanding asymmetric methodology. Aldrichim. Acta. 1999;32:35–62. [Google Scholar]
- 5.Hudlicky T., Reed J. Celebrating 20 Years of SYNLETT—Special Account On the Merits of Biocatalysis and the Impact of Arene cis -Dihydrodiols on Enantioselective Synthesis. Synlett. 2009;2009:685–703. doi: 10.1055/s-0028-1087946. [DOI] [Google Scholar]
- 6.Tatsuta K., Tsuchiya T., Mikami N., Umezawa S., Umezawa H., Naganawa H. KD16-U1, a new metabolite of Streptomyces: Isolation and structural studies. J. Antibiot. 1974;22:579–586. doi: 10.7164/antibiotics.27.579. [DOI] [PubMed] [Google Scholar]
- 7.Bayón P., Figueredo M. The Gabosine and Anhydrogabosine Family of Secondary Metabolites. Chem. Rev. 2013;113:4680–4707. doi: 10.1021/cr300150w. [DOI] [PubMed] [Google Scholar]
- 8.Huntley C.F.M., Hamilton D.S., Creighton D.J., Ganem B. Reaction of COTC with Glutathione: Structure of the Putative Glyoxalase I Inhibitor. Org. Lett. 2000;2:3143–3144. doi: 10.1021/ol006341z. [DOI] [PubMed] [Google Scholar]
- 9.Bach G., Breiding-Mack S., Grabley S., Hammann P., Hütter K., Thiericke R., Uhr H., Wink J., Zeeck A. Secondary Metabolites by Chemical Screening, 22. Gabosines, New Carba-sugars From Streptomyces. Liebigs Ann. der Chem. 1993;1993:241–250. doi: 10.1002/jlac.199319930143. [DOI] [Google Scholar]
- 10.Kumar V., Das P., Ghosal P., Shaw A.K. Syntheses of (−)-gabosine A, (+)-4-epi-gabosine A, (−)-gabosine E, and (+)-4-epi-gabosine E. Tetrahedron. 2011;67:4539–4546. doi: 10.1016/j.tet.2011.04.082. [DOI] [Google Scholar]
- 11.Tang Y.-Q., Maul C., Höfs R., Sattler I., Grabley S., Feng X.-Z., Zeeck A., Thiericke R. Gabosines L, N and O: New Carba-Sugars from Streptomyces with DNA-Binding Properties. Eur. J. Org. Chem. 2000;2000:149–153. doi: 10.1002/(SICI)1099-0690(200001)2000:1<149::AID-EJOC149>3.0.CO;2-S. [DOI] [Google Scholar]
- 12.Kamiya D., Uchihata Y., Ichikawa E., Kato K., Umezawa K. Reversal of anticancer drug resistance by COTC based on intracellular glutathione and glyoxalase I. Bioorg. Med. Chem. Lett. 2005;15:1111–1114. doi: 10.1016/j.bmcl.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Das M., Manna K. Bioactive Cyclohexenones: A Mini Review. Curr. Bioact. Compd. 2015;11:239–248. doi: 10.2174/157340721104151230104138. [DOI] [Google Scholar]
- 14.Douglas K.T., Shinkai S. Chemical Basis of the Action of Glyoxalase I, an Anticancer Target Enzyme. Angew. Chem. Int. Ed. Engl. 1985;24:31–44. doi: 10.1002/anie.198500311. [DOI] [Google Scholar]
- 15.Mac D.H., Chandrasekhar S., Grée R. Total Synthesis of Gabosines. Eur. J. Org. Chem. 2012;2012:5881–5895. doi: 10.1002/ejoc.201200477. [DOI] [Google Scholar]
- 16.Mirza S., Molleyres L.-P., Vasella A. Synthesis of a Glyoxalase I Inhibitor fromStreptomyces griseosporeus. Helv. Chim. Acta. 1986;68:988–996. doi: 10.1002/hlca.19850680425. [DOI] [Google Scholar]
- 17.Fresneda M.Á., Alibés R., Font J., Bayón P., Figueredo M. How a diversity-oriented approach has inspired a new hypothesis for the gabosine biosynthetic pathway. A new synthesis of (+)-gabosine C. Org. Biomol. Chem. 2013;11:6562. doi: 10.1039/c3ob41183d. [DOI] [PubMed] [Google Scholar]
- 18.Fresneda M.Á., Alibés R., Font J., Bayón P., Figueredo M. Stereoselective Synthesis and Relative Configuration Assignment of Gabosine J. J. Org. Chem. 2012;77:5030–5035. doi: 10.1021/jo300456q. [DOI] [PubMed] [Google Scholar]
- 19.Sivakrishna B., Pal S. Asymmetric total synthesis of (+)-gabosine C and (+)-4-epi-gabosine J using acetate migration and RCM reaction. Tetrahedron. 2019;75:3046–3052. doi: 10.1016/j.tet.2019.04.048. [DOI] [Google Scholar]
- 20.Bihelovic F., Vulovic B., Saicic R.N. Synthesis of Natural Products and the Development of Synthetic Methodology: The Case Study of (–)-Atrop-abyssomicin C. Nat. Prod. Commun. 2017;12:1209–1214. doi: 10.1177/1934578X1701200816. [DOI] [Google Scholar]
- 21.Yang X., Yuan P., Shui F., Zhou Y., Chen X. A divergent strategy to synthesize gabosines featuring a switchable two-way aldol cyclization. Org. Biomol. Chem. 2019;17:4061–4072. doi: 10.1039/C9OB00469F. [DOI] [PubMed] [Google Scholar]
- 22.Babu D.C., Rao C.B., Venkatesham K., Selvam J.J.P., Venkateswarlu Y. Toward synthesis of carbasugars (+)-gabosine C, (+)-COTC, (+)-pericosine B, and (+)-pericosine C. Carbohydr. Res. 2014;388:130–137. doi: 10.1016/j.carres.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Lygo B., Swiatyj M., Trabsa H., Voyle M. Synthesis of (+)-Gabosines C and E from D-ribose. Tetrahedron Lett. 1994;35:4197–4200. doi: 10.1016/S0040-4039(00)73150-9. [DOI] [Google Scholar]
- 24.Tatsuta K., Yasuda S., Araki N., Takahashi M., Kamiya Y. Total synthesis of a glyoxalase I inhibitor and its precursor, (−)-KD16-U1. Tetrahedron Lett. 1998;39:401–402. doi: 10.1016/S0040-4039(97)10559-7. [DOI] [Google Scholar]
- 25.Shinada T., Fuji T., Ohtani Y., Yoshida Y., Ohfune Y. Syntheses of Gabosine A, B, D, and E from Allyl Sulfide Derived from(−)-Quinic Acid. Synlett. 2002:1341–1343. doi: 10.1055/s-2002-32985. [DOI] [Google Scholar]
- 26.Ramana G.V., Rao B.V. Stereoselective synthesis of (−)-gabosine C using a Nozaki–Hiyama–Kishi reaction and RCM. Tetrahedron Lett. 2005;46:3049–3051. doi: 10.1016/j.tetlet.2005.03.018. [DOI] [Google Scholar]
- 27.Shing T.K.M., So K.H., Kwok W.S. Carbocyclization of Carbohydrates: Diastereoselective Synthesis of (+)-Gabosine F, (−)-Gabosine O, and (+)-4- epi -Gabosine O. Org. Lett. 2009;11:5070–5073. doi: 10.1021/ol902071e. [DOI] [PubMed] [Google Scholar]
- 28.Shing T.K.M., Chen Y., Ng W.-L. Carbocyclization of d-glucose: Syntheses of gabosine I and streptol. Tetrahedron. 2011;67:6001–6005. doi: 10.1016/j.tet.2011.06.028. [DOI] [Google Scholar]
- 29.Shing T.K.M., Cheng H.M. Facile syntheses of (+)-gabosines A, D, and E. Org. Biomol. Chem. 2009;7:5098–5102. doi: 10.1039/b911810a. [DOI] [PubMed] [Google Scholar]
- 30.Shing T.K.M., Cheng H.M. Short Syntheses of Gabosine I and Gabosine G from δ-D -Gluconolactone. J. Org. Chem. 2007;72:6610–6613. doi: 10.1021/jo0709697. [DOI] [PubMed] [Google Scholar]
- 31.Fresneda M.Á., Alibés R., Bayón P., Figueredo M. Filling Some Blanks in a Divergent Approach to Gabosines: Enantioselective Synthesis of (−)-Epiepoxydon, (+)-Phyllostine, (−)-Gabosine D, and (−)-Gabosine E. Eur. J. Org. Chem. 2016;2016:3568–3574. doi: 10.1002/ejoc.201600492. [DOI] [Google Scholar]
- 32.Raju G., Rao M.V., Rao B.V. Concise stereoselective synthesis of 5-hydroxy carba-β-D-rhamnose, carba-β-D-rhamnose, (−)-gabosine O, and carba-α-D-rhamnose. J. Carbohydr. Chem. 2016;35:150–160. doi: 10.1080/07328303.2016.1170138. [DOI] [Google Scholar]
- 33.Stathakis C.I., Athanatou M.N., Gallos J.K. Synthesis of ent-gabosine E from d-mannose by intramolecular nitrone–olefin cycloaddition. Tetrahedron Lett. 2009;50:6916–6918. doi: 10.1016/j.tetlet.2009.09.154. [DOI] [Google Scholar]
- 34.Rao J.P., Rao B.V. A common strategy for the synthesis of (+)-gabosine N, (+)-gabosine O, and some carbapyranoses using a Nozaki–Hiyama–Kishi reaction and RCM. Tetrahedron Asymmetry. 2010;21:930–935. doi: 10.1016/j.tetasy.2010.05.041. [DOI] [Google Scholar]
- 35.Shan M., O’Doherty G.A. Synthesis of SL0101 Carbasugar Analogues: Carbasugars via Pd-Catalyzed Cyclitolization and Post-Cyclitolization Transformations. Org. Lett. 2010;12:2986–2989. doi: 10.1021/ol101009q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan M., O’Doherty G.A. Synthesis of Cyclitols via Cyclopropanation/Palladium-Catalyzed Ring Opening. Synthesis. 2008;19:3171–3179. doi: 10.1055/s-2008-1067262. [DOI] [Google Scholar]
- 37.Shan M., O’Doherty G.A. Synthesis of Carbasugar C-1 Phosphates via Pd-Catalyzed Cyclopropanol Ring Opening. Org. Lett. 2008;10:3381–3384. doi: 10.1021/ol801106r. [DOI] [PubMed] [Google Scholar]
- 38.Biduś N., Banachowicz P., Buda S. Application of a tandem seleno-michael/aldol reaction in the total syntheses of (+)-Pericosine B, (+)-Pericosine C, (+)-COTC and 7-chloro-analogue of (+)-Gabosine C. Tetrahedron. 2020;76:131397. doi: 10.1016/j.tet.2020.131397. [DOI] [Google Scholar]
- 39.Banwell M.G., Bray A.M., Wong D.J. A concise and chemoenzymatic synthesis of (−)-gabosine A, a carba-sugar enone from Streptomycetes. New J. Chem. 2001;25:1351–1354. doi: 10.1039/b106419c. [DOI] [Google Scholar]
- 40.Labora M., Schapiro V., Pandolfi E. Concise chemoenzymatic synthesis of gabosine A, ent-epoformin and ent-epiepoformin. Tetrahedron Asymmetry. 2011;22:1705–1707. doi: 10.1016/j.tetasy.2011.10.004. [DOI] [Google Scholar]
- 41.Tibhe G.D., Macias M.A., Pandolfi E., Suescun L., Schapiro V. Total Synthesis of Gabosine H and Two Non-natural Gabosines. Synthesis. 2017;49:565–570. doi: 10.1055/s-0036-1588111. [DOI] [Google Scholar]
- 42.Brovetto M., Schapiro V., Cavalli G., Padilla P., Sierra A., Seoane G., Suescun L., Mariezcurrena R. Osmylation of chiral cis-cyclohexadienediols. New J. Chem. 1999;23:549–555. doi: 10.1039/a900732f. [DOI] [Google Scholar]
- 43.Carrera I., Brovetto M.C., Seoane G. Selectivity in the halohydroxylation of cyclohexadienediols. Tetrahedron. 2007;63:4095–4107. doi: 10.1016/j.tet.2007.02.109. [DOI] [Google Scholar]
- 44.Macías M.A., Suescun L., Pandolfi E., Schapiro V., Tibhe G.D., Mombrú Á.W. Crystal structure and absolute configuration of (3aS,4S,5R,7aR)-2,2,7-trimethyl-3a,4,5,7a-tetrahydro-1,3-benzodioxole-4,5-diol. Acta Crystallogr. Sect. E Crystallogr. Commun. 2015;71:1013–1016. doi: 10.1107/S2056989015014590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh J.R., Doering W. Sulfur trioxide in the oxidation of alcohols by dimethyl sulfoxide. J. Am. Chem. Soc. 1967;89:5505–5507. doi: 10.1021/ja00997a067. [DOI] [Google Scholar]
- 46.Gritter R.J., Wallace T.J. The Manganese Dioxide Oxidation of Allylic Alcohols. J. Org. Chem. 1959;24:1051–1056. doi: 10.1021/jo01090a006. [DOI] [Google Scholar]
- 47.Surendra K., Krishnaveni N., Reddy M., Nageswar Y., Rao K.R. Mild Oxidation of Alcohols with o-Iodoxybenzoic Acid (IBX) in Water/Acetone Mixture in the Presence of β-Cyclodextrin. J.Org.Chem. 2003;68:2058–2059. doi: 10.1021/jo026751w. [DOI] [PubMed] [Google Scholar]
- 48.Krishnaveni N., Surendra K., Rama Rao K. A Simple and Highly Selective Biomimetic Oxidation of Alcohols and Epoxides with N-Bromosuccinimide in the Presence of β-Cyclodextrin in Water. Adv. Synth. Catal. 2004;346:379–382. doi: 10.1002/adsc.200303164. [DOI] [Google Scholar]
- 49.Thottumkara A.P., Bowsher M.S., Vinod T. In Situ Generation of o-Iodoxybenzoic Acid (IBX) and the Catalytic Use of It in Oxidation Reactions in the Presence of Oxone as a Co-oxidant. Org. Lett. 2005;7:2933–2936. doi: 10.1021/ol050875o. [DOI] [PubMed] [Google Scholar]
- 50.Hudlicky T., Reed J.W. Applications of biotransformations and biocatalysis to complexity generation in organic synthesis. Chem. Soc. Rev. 2009;38:3117–3132. doi: 10.1039/b901172m. [DOI] [PubMed] [Google Scholar]
- 51.Frigerio M., Santagostino M., Sputore S. A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX) J. Org. Chem. 1999;64:4537–4538. doi: 10.1021/jo9824596. [DOI] [Google Scholar]
- 52.Herzig J., Nudelman A., Gottlieb H.E., Fischer B. Studies in sugar chemistry. 2. A simple method for O-deacylation of polyacylated sugars. J. Org. Chem. 1986;51:727–730. doi: 10.1021/jo00355a026. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available within the article and in the Supplementary Materials section.