Seaman previews work from the Jia laboratory describing how MAPK signaling regulates endosomal protein sorting during stress.
Abstract
Endosome-to–cell surface recycling is mediated by retromer and Snx27. In this issue, Mao et al. (2021. J. Cell Biol. https://doi.org/10.1083/jcb.202010048) detail how endosomal protein sorting responds to external stimuli and reveal that phosphorylation of Snx27 regulates its cargo-binding function resulting in reduced endosome-to–cell surface recycling.
Endosomes serve as critical sorting stations and a point of convergence in the endocytic and secretory pathways. The processes that govern sorting at endosomes modulate the retrieval of membrane proteins to the TGN, the recycling of proteins to the cell surface, and the delivery of soluble and membrane proteins to lysosomes (1). Endosomal protein sorting is mediated by conserved machinery and is generally regarded as occurring in a constitutive fashion. However, it has long been understood that post-translational modification of membrane proteins can profoundly influence their fate at endosomes. For example, the ubiquitylation of receptors such as the epidermal growth factor receptor targets them into intralumenal vesicles. But what about the machinery involved in sorting membrane proteins at endosomes? Can that be regulated to alter how proteins are sorted at endosomes? In this issue, Mao et al. describe how Snx27, a key protein in endosomal protein sorting, is regulated through phosphorylation and the impact this has on the endosome-to–cell surface recycling of many membrane proteins (2).
Starting with a hypothesis that endosomal protein sorting might be regulated through external stimuli, Mao et al. found that several membrane proteins, including the glucose transporter Glut-1, redistributed to endosomes and lysosomes upon starvation. Using CD8-reporter protein constructs, other membrane proteins were similarly affected. The researchers determined that localization to endosomes and lysosomes was not due to increased uptake from the cell surface but instead was caused by decreased recycling back to the cell surface. The change in localization required initiation of autophagy as cells lacking a component of the autophagy machinery did not exhibit an increase in intracellular Glut-1. A candidate protein that might be regulating the endosome-to–cell surface recycling pathway is TBC1D5, a GTPase-activating protein for Rab7a that associates with the retromer complex (3, 4). In this instance, however, no involvement of TBC1D5 in the starvation-induced redistribution of Glut-1 to endosomes was observed. Rather, the authors revealed that phosphorylation of Snx27, another retromer-associated protein, was markedly increased when cells were starved.
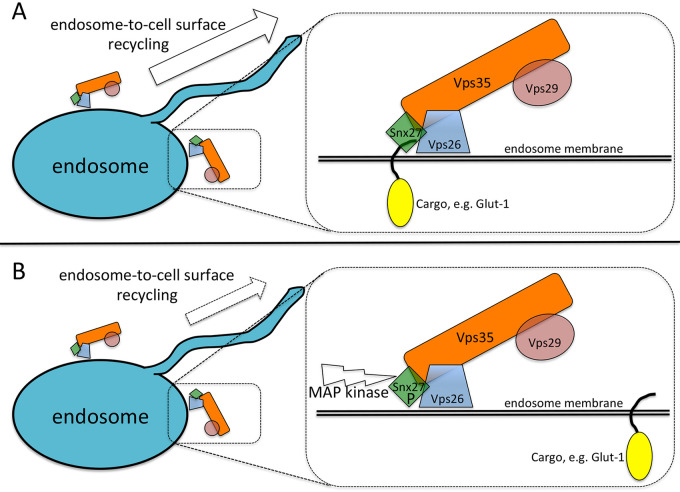
Most intriguingly, the phosphorylation of Snx27 occurred at serine 51, a conserved residue within a PDZ domain that binds specific cargo proteins harboring PDZ-binding motifs (PDZbm)—cargo such as Glut-1. Computer modeling suggested that addition of a phosphate at position 51 altered binding to PDZbms and therefore impacted the sorting of cargoes. In vitro studies confirmed that phospho-Snx27 bound to cargo with reduced affinity compared with WT. Importantly, immunoprecipitation experiments demonstrated that phospho-Snx27 remained able to associate with its known interacting proteins: Vps26 from retromer and the Fam21 subunit of the Wiskott Aldrich Syndrome protein and scar homologue complex (5, 6). Thus, endosome-to–cell surface recycling could still occur for cargoes sorted independently of the Snx27 PDZ domain. The addition of the phosphate to S51 was shown to be mediated by kinases of the MAPK pathway (Fig. 1).
Figure 1.
Phosphorylation of Snx27 impairs cargo recognition. (A) Snx27, in association with retromer (the complex formed by Vps35, Vps29, and Vps26), sorts cargo proteins such as Glut-1 into tubules for recycling to the cell surface. (B) MAPK-mediated phosphorylation of the Snx27 PDZ domain impairs the affinity of Snx27 for binding some cargoes, thereby reducing endosome-to–cell surface recycling. Other machinery (e.g., retromer) is unaffected by Snx27 phosphorylation, and thus recycling can still occur, albeit less efficiently.
Mao et al. observed that Snx27 KO cells exhibited a profound redistribution of Glut-1 from the cell surface to endosomes and lysosomes. These cells were rescued by the transient expression of WT Snx27, an S51A mutant, but not the S51D mutant that mimics the addition of phosphate at serine 51. Other stimuli including EGF, lipopolysaccharide, and the cytokine IL6 caused phosphorylation of Snx27 and redistribution of Glut-1, but chemical treatments that induce mitochondrial or ER stress did not. Together, the data reported reveals a novel “switch” that can attenuate the cargo-binding activity of Snx27, thereby resulting in reduced endosome-to–cell surface recycling of membrane proteins including Glut-1.
To my knowledge, this is the first evidence that the machinery of endosomal protein sorting can be modified through post-translational modification to directly impinge on the primary function of that machinery, namely recognizing and sorting membrane proteins into an exit pathway from the endosome. Previous reports have revealed that the Vps35 component of retromer can be ubiquitylated by the Parkin Ub-ligase and that this modification can affect the sorting of ATG9a, but it is currently not known how ubiquitylation alters Vps35 function, or whether this modification impacts the cargo-recognition activity of retromer (7). Other studies conducted some years ago indicated that Vps35 (also known as MEM3) and Vps5p, the yeast Snx1 homologue, can be phosphorylated, but the role of these modifications is unknown (8, 9).
Possibly one question that remains unanswered is why limiting the activity of Snx27 in endosomal protein sorting might be a useful response to the various extracellular stimuli tested. Reducing endosome-to–cell surface recycling will alter the population of membrane proteins at the cell surface, possibly reducing the sensitivity to further stimulation and hence might serve as a negative feedback response. Conversely however, increasing the endosomal localization of membrane proteins could result in their accelerated degradation, which might be disadvantageous. It is also presently not known which phosphatase dephosphorylates Snx27 and how rapid the removal of the phosphate is. Given the importance of endosomal protein sorting in different physiological processes, could the modulation of Snx27 activity through the targeting of the relevant kinases or phosphatases be a viable potential therapeutic avenue? This is an open question, but the role of Snx27 in the endosome-to–cell surface transport of metalloproteases required for tumor metastasis makes this is an intriguing possibility (10).
This may be the first report of a role for phosphorylation in modulating the machinery of endosomal protein sorting, but I predict it will not be the last. Many of the proteins known to function in endosomal protein sorting are phosphoproteins, and understanding how phosphorylation alters function is one of the few remaining great unknowns.
Acknowledgments
The author declares no competing financial interests.
References
- 1.Seaman, M.N.J. 2012. J. Cell Sci. 10.1242/jcs.103440 [DOI] [Google Scholar]
- 2.Mao, L., et al. 2021. J. Cell Biol. 10.1083/jcb.202010048 [DOI] [Google Scholar]
- 3.Seaman, M.N.J., et al. 2009. J. Cell Sci. 10.1242/jcs.048686 [DOI] [Google Scholar]
- 4.Roy, S., et al. 2017. Mol. Cell. 10.1016/j.molcel.2017.05.020 [DOI] [Google Scholar]
- 5.Gallon, M., et al. 2014. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.1410552111 [DOI] [Google Scholar]
- 6.Temkin, P., et al. 2011. Nat. Cell Biol. 10.1038/ncb2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams, E.T., et al. 2018. Hum. Mol. Genet. 10.1093/hmg/ddy224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lock, P., et al. 1998. EMBO J. 10.1093/emboj/17.15.4346 [DOI] [Google Scholar]
- 9.Horazdovsky, B.F., et al. 1997. Mol. Biol. Cell. 10.1091/mbc.8.8.1529 [DOI] [Google Scholar]
- 10.Sharma, P., et al. 2020. J. Cell Biol. 10.1083/jcb.201812098 [DOI] [Google Scholar]