As part of the JEM 125th Anniversary Insights, Ingber reviewed how Alexis Carrel's JEM study on the culture of whole organs inspired a series of scientific, engineering, and medical breakthroughs.
Abstract
In a 1937 issue of JEM, Carrel (1937. J. Exp. Med. https://doi.org/10.1084/jem.65.4.515) described a technique for culturing whole living organs outside the body. Here, Ingber reviews how this work led to a series of scientific, engineering, and medical breakthroughs that continue to this day.
In an early example of biologically inspired engineering published in JEM in 1937, Alexis Carrel, a surgeon who had already won a Nobel Prize for his work on organ transplantation, published his breakthrough article describing how to culture whole living organs ex vivo (Carrel, 1937). He accomplished this feat by teaming up with a luminary in the airplane industry, Charles Lindbergh, who had been the first to successfully pilot a solo, nonstop, transatlantic flight from New York to Paris in 1927. Upon learning of Carrel’s ongoing efforts attempting to maintain whole organs alive outside the body, Lindbergh was so surprised by the crudeness of the equipment being used that he offered to build a new apparatus that would be better designed to meet the task. This led to a separate JEM publication in 1935 with Lindbergh as the solo author (Lindbergh, 1935), which described the first functional perfusion pump designed to maintain a pulsating circulation of sterile oxygenated fluids through the vessels of whole explanted organs, essentially mimicking the function of the heart and lungs outside the body. Carrel’s 1937 JEM article describes the technique that leverages this device to maintain the viability of whole explanted organs for weeks in vitro; it also provides the first experimental results demonstrating the feasibility of this approach. Carrel and Lindbergh’s original vision was that the ultimate purposes for this technique would include manufacture of endocrine hormones, isolation of bioregulator substances, and the discovery of the “laws of the association of organs,” as well the development of treatments for vascular diseases (Carrel and Lindbergh, 1935). The impact of this work was, in fact, huge and broad; however, as is often the case, it influenced research directions in science and areas of clinical medicine that could have never been predicted or envisioned, even by these two world-renowned visionaries.

Insights from Donald E. Ingber.
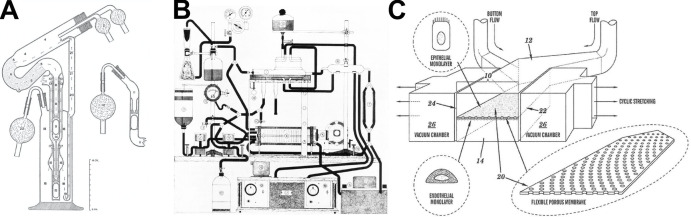
Carrel came at this problem with extensive experience in vascular surgery and organ transplantation, and he applied these skills to meet this challenge head-on. He would surgically remove an organ, such as a thyroid gland or ovary, with its attached tissues, arteries, veins, nerves, and lymphatics under sterile conditions as if for transplantation. Then it would be transplanted into Lindbergh’s pumping apparatus that contained three glass chambers (see panel A of figure) instead of another patient. The organ was placed in the top chamber with a cannula sutured within the lumen of its main artery; fluid in the lowest reservoir chamber was perfused through the artery driven by pulsatile gas pressure actuated by compressed air controlled by a rotating valve; and the middle chamber was used to ensure pressure equalization. Once the perfusion fluid passed through the vessels of the organ, it recirculated back to the lower reservoir. The gas composition of the fluid, flow rate, pulsation cycle, maximum and minimum pressures, and temperature all could be controlled, and the organ and perfusion fluid could be visualized at all times. To make this work, Carrel also had to develop a perfusion culture medium containing many of the same components that we still use today (e.g., glucose, bicarbonate, serum, vitamins, growth-activating factors, phenol red, etc.). Using this approach, he was able to culture whole thyroid glands from cats and rabbits outside of the body and maintain their morphology, growth, and viability for up to 3 wk in vitro (Carrel, 1937; Carrel and Lindbergh, 1935). When small fragments obtained from the organs after weeks in the perfusion apparatus were placed in culture flasks, they generated actively growing colonies of cells, confirming their viability ex vivo.
Perfusion apparatuses for supporting function of living vascularized organs in vitro. (A–C) Diagrams of Lindbergh’s perfusion pump used for whole organ culture by Carrel (A; Lindbergh, 1935); Folkman’s experimental setup used for his tumor studies in perfused whole organs (B; from Folkman et al., 1963, with permission of John Wiley and Sons); and a patent drawing of a microfluidic human organ-on-a-chip (C).
This breakthrough by Carrel and Lindbergh was the birth of perfusion culture, and modified methods are used to maintain the viability of whole organs for physiological and toxicological experiments ex vivo to this day. However, the work has had a much broader impact. For example, only 15 yr after Carrel’s JEM publication, another surgeon (Forest Dodrill) teamed up with the car industry (General Motors Research Laboratories) to develop a mechanical heart pump, which enabled the first open-heart surgery in a human patient in 1952 (Stephenson et al., 2002). There is no doubt that this heart pump, which not surprisingly looked like a 12-cylinder engine with six cylinder-like chambers, would not have been possible without building on the earlier work described in the JEM publications. The same can be said of the later development of the artificial kidney, hemodialysis, cardiopulmonary bypass, extracorporeal membrane oxygenation, and many other forms of extracorporeal therapy.
What is perhaps more surprising, however, is that Carrel’s work inspired an entirely new field of cancer biology as well as development of novel therapeutics. I know this firsthand because I trained with Judah Folkman, yet another visionary surgeon who was inspired by Carrel and Lindbergh. While working in the navy on development of hemoglobin solutions as potential blood substitutes, Folkman built a testbed for evaluating these materials that was an improved version of the Lindbergh’s glass-enclosed apparatus with significantly added complexity and control (see panel B of figure), and he too used this to perfuse whole explanted thyroid glands (Folkman et al., 1963).
Folkman decided to explore whether these perfused organs could support tumor growth outside the body by injecting melanoma cells into the thyroid. They grew, but he noticed that they would not expand beyond a couple of millimeters in diameter, even when cultured for weeks. Yet, when removed from the gland and reimplanted into living mice, the same tumor cells would grow uncontrollably and kill the animal. Folkman realized that the tumors in animals were highly vascularized, whereas they were not in the explanted glands. This led to the birth of the field of tumor angiogenesis, which has resulted in development of multiple FDA-approved drugs that inhibit uncontrolled capillary growth, both for cancers and for other conditions, such as blindness due to macular degeneration.
My last example comes from my own work on the development of human organ-on-a-chip microfluidic culture devices. We began with a very similar goal to that of Carrel; however, we attempted to engineer small functional parts of living organs from the bottom up. Carrel and Lindbergh borrowed manufacturing approaches from the airplane and car industries that were burgeoning in the first part of the last century. My team borrowed from the computer industry and used microchip manufacturing methods to create “microfluidic” culture devices. These organs-on-chips are composed of an optically clear, flexible polymer containing a tiny (<1-mm diameter) artificial “vascular” channel interfaced across a porous membrane with a second parallel “interstitial” channel (see panel C of figure), which we respectively lined with human microvascular endothelial cells and organ-specific epithelial cells (Huh et al., 2010). We also had to develop methods to perfuse appropriate nutrient fluids through the vascular endothelium-lined channels to feed and sustain the living cells at air–liquid (e.g., lung) and liquid–liquid (e.g., liver) interfaces within these devices for weeks to months in vitro. And we too could continuously visualize these living tissues functioning in an organ-level context within these optically clear devices, but now with high-resolution microscopic imaging.
Carrel had noted that complex mechanical procedures are required to enable organs to live outside the body, and we took this seriously as well. We incorporated hollow side chambers on either side of the cell-lined microchannels in our organs-on-chips, to which we apply cyclic suction; this drives rhythmic deformation of the porous membrane and attached tissue–tissue interface, thereby replicating physiological organ motions (e.g., breathing in lung, peristalsis in intestine). Importantly, both active fluid flow and mechanical cues are absolutely required for the highest level of physiological mimicry (Huh et al., 2010, 2012; Kim et al., 2016).
Most recently, organs-on-chips have been used to accomplish something that Carrel had envisioned: multiple perfused organs-on-chips have been linked fluidically to create what is effectively a human body-on-chips, which can be used, for example, to study organ–organ physiological coupling and to predict human drug pharmacokinetic parameters in vitro (Herland et al., 2020). These organ-on-a-chip devices, which would never have been possible without Carrel’s jump start of the field almost a century ago, are now at a point where they can model human patient responses to drugs, sometimes with higher fidelity than animal models (Ingber, 2020).
It is amazing to me to that so many of the challenges Carrel faced in his quest are the same ones we face today: isolating the best source of living tissue, minimizing infection, optimizing medium components, ensuring continuous flow, and outsmarting bubbles, which is the greatest source of system failure in both micro- and macro-fluidic systems. Carrel’s goal was to integrate immune responses, hormonal control, and other facets of multiorgan physiology into his system, as well as to model diseases ex vivo. These goals also remain prominent in the organ-on-a-chip field today. Newer manufacturing techniques are also emerging, such as 3D printing, which are being used to create even more complex vascularized organ-like structures outside the body (Homan et al., 2019). Moreover, by combining these bioinspired engineering approaches with patient-derived induced pluripotent stem cells and stem cell–containing organoids, entirely new paths are being opened that can be used to gain new mechanistic insights into rare genetic disorders, tailor therapies for specific genetic populations, and develop personalized medicines. Alexis Carrel might not have envisioned all of this, but I am confident that he would be pleased.
Acknowledgments
D.E. Ingber holds equity in Emulate Inc., chairs its scientific advisory board, and is a member of its board of directors.
References
- Carrel, A. 1937. J. Exp. Med. 10.1084/jem.65.4.515 [DOI] [Google Scholar]
- Carrel, A., and Lindbergh C.A.. 1935. Science. 10.1126/science.81.2112.621 [DOI] [PubMed] [Google Scholar]
- Folkman, J., et al. 1963. Cancer. [DOI] [PubMed] [Google Scholar]
- Herland, A., et al. 2020. Nat. Biomed. Eng. 10.1038/s41551-019-0498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan, K.A., et al. 2019. Nat. Methods. 10.1038/s41592-019-0325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, D., et al. 2010. Science. 10.1126/science.1188302 [DOI] [Google Scholar]
- Huh, D., et al. 2012. Sci. Transl. Med. 10.1126/scitranslmed.3004249 [DOI] [Google Scholar]
- Ingber, D.E. 2020. Adv. Sci. 10.1002/advs.202002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.J., et al. 2016. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.1522193112 [DOI] [Google Scholar]
- Lindbergh, C.A. 1935. J. Exp. Med. 10.1084/jem.62.3.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson, L.W., et al. 2002. J. Card. Surg. 10.1111/j.1540-8191.2002.tb01210.x [DOI] [Google Scholar]