Abstract
The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing and approximately 25% of the global population may have NAFLD. NAFLD is associated with obesity and metabolic syndrome, but its pathophysiology is complex and only partly understood. The transsulfuration pathway (TSP) is a metabolic pathway regulating homocysteine and cysteine metabolism and is vital in controlling sulfur balance in the organism. Precise control of this pathway is critical for maintenance of optimal cellular function. The TSP is closely linked to other pathways such as the folate and methionine cycles, hydrogen sulfide (H2S) and glutathione (GSH) production. Impaired activity of the TSP will cause an increase in homocysteine and a decrease in cysteine levels. Homocysteine will also be increased due to impairment of the folate and methionine cycles. The key enzymes of the TSP, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), are highly expressed in the liver and deficient CBS and CSE expression causes hepatic steatosis, inflammation, and fibrosis in animal models. A causative link between the TSP and NAFLD has not been established. However, dysfunctions in the TSP and related pathways, in terms of enzyme expression and the plasma levels of the metabolites (e.g., homocysteine, cystathionine, and cysteine), have been reported in NAFLD and liver cirrhosis in both animal models and humans. Further investigation of the TSP in relation to NAFLD may reveal mechanisms involved in the development and progression of NAFLD.
Keywords: cystathionine β-synthase/cystathionine γ-lyase (CBS/CSE) system, glutathione, H2S production, liver fibrosis, non-alcoholic steatohepatitis, sulfur metabolism
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease and is present in 25% of the population worldwide [1]. The rapid increase in prevalence during recent years parallels the increasing occurrence of obesity and the metabolic syndrome. NAFLD is a disease continuum that ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), which is characterized by inflammation and hepatocyte damage. NASH can lead to fibrosis and eventually to cirrhosis, liver failure, and hepatocellular carcinoma. The primary characteristic of NAFLD is the hepatic accumulation of lipids, mainly triacylglycerols (TAGs), due to the increased influx of free fatty acids (FFA) [2]. Dietary overload is believed to be the main underlying driver, but the molecular mechanisms behind the lipotoxicity remain unclear [3,4,5].
The transsulfuration pathway (TSP) is coupled to the production of the antioxidant glutathione (GSH) and the signal molecule hydrogen sulfide (H2S), and both have been linked to the pathogenesis of NAFLD [6,7,8,9]. The large number of patients with NASH-related end-stage liver disease, and the emerging pharmacological treatment options, means that there is an urgent need for valid and reproducible biomarkers of disease development and progression.
Several scores have been developed to diagnose hepatic steatosis [10,11,12]. The scores combine different standard blood tests, such as liver enzymes and bilirubin, with markers of insulin resistance and body weight. The scores have been assessed in different populations using diagnostic imaging or histology as the gold standard and there are no studies showing the validity in larger unselected groups. There are no biomarkers which may be used to diagnose NASH but several which can indicate the degree of fibrosis, including the Fibrosis-4 Index and the NAFLD Fibrosis Score [12,13,14]. These scores include standard blood tests such as alanine amino transferase and aspartate amino transferase and are accurate in the exclusion of fibrosis and the identification of patients with cirrhosis but not in the grading of mild to moderate fibrosis. Other fibrosis biomarkers, including the Enhanced Liver Fibrosis test and procollagen III and IV, also accurately identify patients without fibrosis and patients with cirrhosis [12,15,16]. However, at present, a liver biopsy is required to diagnose NASH. Repeated biopsies in a large group of patients are resource-demanding and the invasive procedure carries a risk of complications. Inexpensive, valid, and reproducible non-invasive biomarkers are necessary for prognostication, monitoring, and evaluating the response in patients undergoing treatment.
Metabolites in the TSP have been shown to predict steatosis and fibrosis in alcoholic liver disease [17], and the TSP has been suggested as a potential drug target in NAFLD and portal hypertension [18,19]. Thus, TSP metabolites may have potential as NAFLD biomarkers, and as our understanding of its relation to NAFLD deepens, support for trials investigating pharmaceutical interventions targeting the TSP may gather strength. This review will focus on the TSP, its regulation and relation to other pathways, and what is known about its implications for NAFLD.
2. Description of the Transsulfuration Pathway
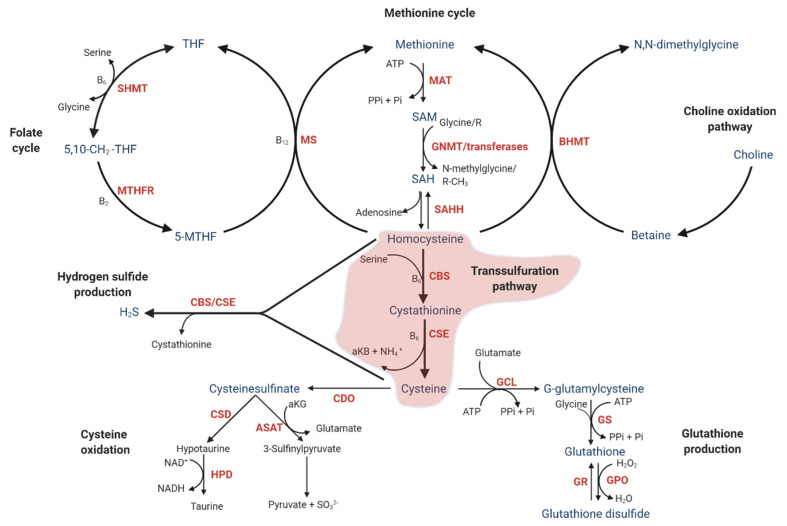
The TSP involves the conversion of homocysteine to cysteine via the intermediate cystathionine and plays a key role in sulfur metabolism and the redox environment of cells. The pathway is the only route for biosynthesis of cysteine. The first step is catalyzed by the vitamin B6-dependent enzyme cystathionine β-synthase (CBS), using homocysteine and serine as substrates to form cystathionine in a condensation reaction (Figure 1). The second step is a hydrolyzation reaction catalyzed by the vitamin B6-dependent cystathionine γ-Lyase (CSE), using cystathionine as substrate and producing cysteine and α-ketobuyrate (aKB).
Figure 1.
The transsulfuration pathway (TSP) and related pathways. Metabolites: 5-MTHF, 5-methyltetrahydrofolate; 5,10-CH2-THF. 5-10-methylenehydrofolate; aKG, α-ketoglutarate; aKB, α-ketobutyrate; ATP; adenosine triphosphate; B2, riboflavin; B6, pyridoxal 5′-phosphate; B12, cobalamin; H2S, hydrogen sulfide; NH4+, ammonia; Pi, inorganic phosphate; PPi, pyrophosphate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SO32−, sulfite; THF, tetrahydrofolate.
The figure shows the TSP (highlighted in red) and related pathways, including the methionine and folate cycles, glutathione production, cysteine oxidation, and choline oxidation pathway.
For enzyme abbreviations (in red text colour), please see Table 1.
Table 1.
An overview of the enzymes in the transsulfuration and related pathways.
| Enzyme Name | Gene Name | Cofactor | Structure | Tissue Expression | Cellular Location |
|---|---|---|---|---|---|
| Adenosylhomocysteinase (SAHH) | AHCY | NAD+ | Homotetramer 432 amino acids 47.7 kDa |
Low tissue specificity. Highly expressed in liver, pancreas and kidney, endocrine tissue, female and male tissue. |
Cytoplasma |
| Betaine-homocysteine S methyltransferase (BHMT)—Two genes |
BHMT
BHMT2 |
Zink | Homotetramer 406/363 amino acids 45.0/40.4 kDa |
High tissue specificity. Highly expressed in liver, kidney, and urinary tract. |
Cytoplasma |
| Cystathionine beta-synthase (CBS) | CBS | Pyridoxal 5′-phosphat (B6) |
Homotetramer 551 amino acids 61 kDA |
High tissue specificity. Highly expressed in liver and pancreas. Some expression in heart and brain. |
Nucleus/cytoplasma |
| Cystathionine gamma-lyase (CSE) | CTH | Pyridoxal 5′-phosphat (B6) |
Homotetramer 405 amino acids 44.5 kDa |
High tissue specificity. Highly expressed in liver, female tissue and endocrine tissue. Some in the pancreas, brain, and kidneys. |
Cytoplasma |
| Cysteine dioxygenase (CDO) |
CDO1 | Iron | Monomer 200 amino acids 30.0 kDA |
High tissue specificity. Highly expressed in liver and placenta. Some expression in heart, sdipose tissue, brain, and pancreas. |
Cytoplasma |
| Cysteine sulfinic acid decarboxylase (CSD) |
CSAD | Pyridoxal 5′-phosphat (B6) |
Homodimer 493 amino acids 55.0 kDA |
Low tissue specificity. Expressed in liver, gastrointestinal (GI) tract, brain female and male tissue, muscle tissue, and adipose tissue. |
Cytoplasma |
| Glutathione peroxidase (GPO)—several genes | GPX 1–8 | GPX2—Homotetramer 190 amino acids 22.0 kDa |
GPO has low tissue specificity. GPX 2 is highly expressed in the liver, gallbladder and GI tract. | Cytoplasma/mitochondrion | |
| Glutathione reductase (GR) |
GSR | FAD | Homodimer 552 amino acids 56.3 kDa |
Low tissue specificity. Highly expressed in liver, pancreas, GI tract, endocrine tissue, kidney, female and male tissue. |
Cytoplasma/mitochondrion |
| Glutathione synthetase (GS) |
GSS | Magnesium | Homodimer 474 amino acids 52.4 kDa |
Low tissue specificity. Highly expressed in brain, endocrine tissue, GI tract, kidney and liver. |
Cytoplasma |
| Glutamate-cysteine ligase (GCL) |
GCLM
GCLC |
Heterodimer 274 + 252 amino acids 33.7 + 28.1 kDa |
Methionine synthase Highly expressed in the liver. |
Cytoplasma | |
| Glycine N-methyltransferase (GNMT) |
GNMT | Homotetramer 295 amino acids 32.7 kDa |
High tissue specificity. Highly expressed in liver and pancreas. Some expression in brain, GI tract, and kidney. |
Cytoplasma | |
| Methionine adenosyltransferase (MAT) |
MAT1A | Potasium Magnesium |
Homodi- and tertramer 395 amino acids 43.6 kDA |
High tissue specificity. Highly expressed in liver, pancreas. Some expression lungs and female and male tissue. |
Cytoplasma |
| Methionine synthase (MS) |
MTR | Cobalamin (B12) Zink |
Monomer- and Dimer 1265 amino acids 140.5 kDa |
Low tissue specificity. Highly expressed in pancreas, heart, brain, skeletal muscle and placenta. Expressed at lower levels in lung, liver, and kidney. |
Cytoplasma |
| Methylenetetrahydrofolate reductase (MTHFR) |
MTHFR | FAD | Homodimer 656 amino acids 74.6 kDA |
Low tissue specificity. Highly expressed in female and male tissue, GI tract and kidney. Some expression in liver. |
Cytoplasma |
| Serine hydroxymethyltransferase (SHMT)—two genes | SHMT1 SHMT2 | Pyridoxal 5′-phosphat (B6) |
Homotetramer 486/504 amino acids 53.1/60.0 kDa |
High tissue specificity. Highly expressed in liver and kidney. Some expression in lungs, brain, pancreas, and GI tract. |
Cytoplasma/mitochondrion |
Data were retrieved from: www.proteinatlas.org; https://bgee.org/; https://www.ebi.ac.uk (accessed on 16 November 2020).
2.1. One-Carbon Metabolism
The TSP is part of the one-carbon metabolism (OCM), which is a universal metabolic process that serves to activate and transfer one-carbon (1C) units for the biosynthesis of multiple molecules including purine and thymidine and remethylation of homocysteine [20]. The methionine cycle, folate cycle, and choline oxidation pathway, which are all upstream of the TSP, are also part of OCM. Folate metabolism is central in OCM as many of the different molecules in the folate complex function as carriers of 1C units. The liver is a crucial organ for OCM, with a high capacity for the different processes in OCM [20].
The TSP is closely linked to methionine metabolism, which involves the conversion of methionine to homocysteine. The process is reversible as homocysteine can be converted back to methionine catalyzed by vitamin B12-dependent enzyme methionine synthase (MS) using 5-methyltetrahydrofolate as methyl-donor or by the enzyme betaine-homocysteine S-methyltransferase (BHMT) with betaine as methyl-donor. The latter process is folate-independent and the expression of BHMT is restricted to the kidneys and liver [21].
The remethylation of homocysteine is important because methionine is a substrate for methionine adenosyltransferase (MAT) that synthesizes S-adenosyl-methionine (SAM). SAM, a methyl carrier, is a common enzymatic cofactor and is involved in epigenetics and biosynthesis of phosphatidylcholine, creatinine, and polyamine [22,23,24].
2.2. Downstream of the Transsulfuration Pathway
The conversion of homocysteine to cysteine is irreversible. Due to the one-way direction of the TSP, all downstream pathways of the TSP are inherently part of cysteine metabolism. Cysteine, which is a non-essential amino acid, is central to sulfur metabolism and a precursor of important metabolites including GSH, H2S (Figure 2), sulfate, and taurine.
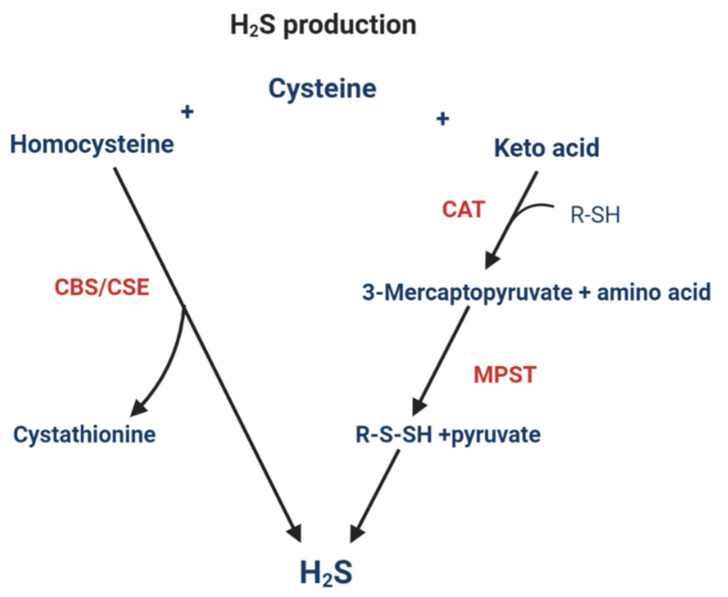
Figure 2.
Hydrogen sulfide production pathways. The biosynthesis of hydrogen sulfide (H2S) involves the three enzymes, cystathionine β-synthetase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (MPST). The process involving H2S production via CBS and CSE uses homocysteine and cysteine as substrates. The production of H2S via cysteine transaminase (CAT) and MPST uses cysteine and a keto-acid as substrates to form 3-mercaptopyruvate in the first step via CAT, followed by the synthesis of H2S from 3-mercaptopyruvate via MPST.
In healthy individuals, the steady-state metabolic condition involves a balance between the intake of sulfur from methionine (Figure 1) and the metabolism of sulfur from homocysteine through the TSP. Nearly all sulfur from methionine is transferred to cysteine [25]. Disruption of the TSP has been linked to several diseases including homocystinuria, Huntington’s chorea, and vascular dysfunction, as well as ageing-related changes [26].
2.3. Allosteric and Posttranslational Regulation of the Transsulfuration Pathway Flux
The pyridoxal 5-phosphate (PLP—the active form of vitamin B6)-dependent enzymes, CBS and CSE, regulate the metabolic flux through the TSP. Both are highly expressed in the liver and, to a lesser extent, in various other tissues (Table 1). In the liver, CBS and CSE are mainly expressed in the hepatocytes but can also be found in the hepatic vascular system [27,28,29]. CSE is also found in hepatic stellate cells and the terminal branches of the blood vessels in the intrahepatic portal triads [29,30]. The high expression of these enzymes affords the liver a high capacity for transsulfuration.
CBS is tightly regulated by S-adenosylmethionine (SAM), a methyl donor in most transmethylation reactions, through allosteric activation. SAM binds non-covalently to a heme group in CBS, controlling the redox sensitivity of CBS, and stabilizes the enzyme [31]. Decreasing concentration of SAM therefore leads to low CBS activity. The activity of CBS is also stimulated by S-glutathionylation under oxidative stress, thus creating a positive feedback loop [32]. Conversely, SAM inhibits the vitamin B2 (FAD)-dependent methylenetetrahydrofolate reductase (MTHFR) and BHMT, with the net effect being decreased remethylation of homocysteine to methionine and an increase in homocysteine metabolism through the TSP [33]. Thus, the TSP is important in controlling sulfur levels and the degradation of methionine, homocysteine, and cysteine. Other regulators of CBS activity include carbon monoxide (CO) and nitrogen oxide (NO), both by binding to the heme group in CBS, inhibiting its activity [34].
Unlike CBS, the activity of CSE is mainly regulated at the transcriptional level. However, posttranslational modifications such as phosphorylation and sulfhydration can also regulate CSE activity [35,36]. The enzymes in the TSP and related pathways are listed in Table 1.
2.4. Importance of Folate and Vitamin B6 and B12 Status in One-Carbon Metabolism and the Transsulfuration Pathway
As cofactor for CBS and CSE, adequate vitamin B6 status is important for the functioning of the TSP. Vitamin B6 deficiency causes reduced activity of CBS and CSE [37], with CSE activity being most affected [38,39,40]. In human studies evaluating moderate B6 insufficiency, no changes in the TSP flux were found [39]. However, increased cystathionine plasma concentration is observed, but with no changes in cysteine or GSH concentrations [41]. The TSP flux is largely maintained due to the increased cystathionine levels [39].
Extensive research has also demonstrated the importance of folate, vitamin 6, and vitamin B12 status in regulating OCM [20,42,43,44,45,46]. The classical symptom of folate and vitamin B12 deficiency is megaloblastic anemia caused by inhibition of DNA synthesis in erythropoietic cells in the bone marrow [46].
2.5. Transcriptional Regulation of Enzymes in the Transsulfuration Pathway
The expression of CSE is affected by stimuli such as oxidative stress, endoplasmic reticulum (ER) stress, Golgi stress, inflammation, and nutrient deprivation, whereas CBS is expressed at a less variable rate [26,47]. In the liver, the CSE expression is regulated through the farnesoid X receptor (FXR) and the G-protein-coupled bile acid receptor 1 (GPBAR-1) [35,48]. Upon activation, the FXR binds to an FXR-responsive element in the 5′ flanking region of the CSE promoter, while GPBAR-1 recruits CREB, a cyclic AMP responsive element (CRE) binding protein that binds to sites on the CSE promoter. [35,48].
The transcription factor specificity protein 1 (SP1) is also involved in the regulation of CSE expression. SP1 acts by binding to the CSE promoter, increasing CSE expression. The increased expression enhances H2S production by CSE, which causes sulfhydration of the p65 subunit of the transcription factor NF-κβ, which facilitates its recruitment to anti-apoptotic genes [49].
The protein nuclear factor erythroid 2-related factor 2 (NRF2), which regulates the expression of antioxidant proteins, increases the expression of CBS as well as CSE by binding to the promoters [50,51]. NRF2 activators also regulate GSH levels through de novo synthesis and recycling [52,53].
In cardiomyocytes, H2S and homocysteine affect CBS and CSE expression through feedback regulation [54]. A similar mechanism may exist in hepatocytes. The transcriptional regulation of the TSP appears to be a comprehensive multileveled network. The regulators (FXR, GPBAR-1, SP1, and NRF2) are also involved in lipid, glucose, and bile acid metabolism, inflammation, and oxidative stress [53,55,56,57], which in turn suggests that the expression of TSP enzymes is regulated in response to the same cellular and metabolic challenges.
2.6. Hydrogen Sulfide Production Is Inherently Coupled to the Transsulfuration Pathway
H2S is a colorless gas that has emerged as an important signaling molecule alongside NO and CO. It is an important mediator of cell functions and is involved in physiological processes such as inflammation, apoptosis, vasorelaxation, and neuromodulation [58]. It is also thought to increase the production of GSH [59]. One possible mechanism of H2S action is by posttranslational modification of the cysteine residues on target proteins, yielding a hydropersulfide moiety (-SSH) or polysulfide in a process known as S-sulfhydration or S-persulfidation [60]. An animal study found that a substantial number of proteins in the liver are S-sulfhydrated and that S-sulfhydration alters protein function [36]. In humans, H2S is mainly produced via CBS and CSE (Figure 2), but also by 3-mercaptopyruvate sulfurtransferase (MPST) as part of the cysteine catabolic pathway [61].
The extent to which CBS and CSE are active in the production of cysteine or H2S depends on several factors. Substrates binding to the heme group of CBS can direct the activity towards H2S production [62]. High CO levels inhibit CBS activity, causing an increase in homocysteine and subsequently H2S production [63]. Availability of substrates is also important since CBS has a higher affinity for serine, and CSE a higher affinity for cystathionine compared to cysteine. If serine levels are elevated, the production of cystathionine will increase, and if cystathionine is elevated, the production of cysteine will increase. A study using a NAFLD model and liver cells surprisingly found that the H2S-producing enzyme MPTS suppressed H2S production primarily through downregulation of CSE [64]. Partial knockout of MPST, either via adenovirus-mediated short hairpin RNA (shRNA) delivery or heterozygous deletion, significantly upregulated hepatic CSE expression and increased hepatic H2S levels in high-fat-diet (HFD)-fed mice and in FFA-treated L02 cells. This emphasizes the complexity of the metabolic processes involved in TSP regulation and H2S levels.
2.7. Pathways Involving the Conversion of Cysteine to Glutathione, Taurine, and Sulfate
Cysteine has several potential fates beyond protein synthesis and H2S production. The TSP is linked to the production of the major antioxidant GSH (Figure 1). Accordingly, TSP metabolic flux is important for the redox environment. GSH imbalance is linked to several diseases, including type 2 diabetes, cancer, and pulmonary fibrosis [65]. Nearly half of the intracellular GSH pool in human liver cells is derived from homocysteine via the TSP [66]. Oxidative stress increases the flux through the TSP, which subsequently leads to increased GSH production [67].
In addition to GSH, cysteine is converted to the sulfur-containing molecule taurine. The first reaction involves oxidation of the thiol in the cysteine molecule by cysteine dioxygenase (CDO) to form cysteinesulfinate, which is further degraded to taurine, sulfate, and pyruvate. Taurine is important for several biological functions, including muscle functioning, calcium homeostasis, and neuro- and immunomodulation [68]. Taurine also reduces the secretion of apolipoprotein B100 and lipids from liver cells, probably by inhibition of TAG and cholesterol ester synthesis [69]. CDO uses cysteine as substrate and can therefore modulate H2S and GSH production, and CDO levels are increased in response to high-intracellular cysteine through a block of ubiquitination (the bonding of ubiquitin to a substrate protein, marking the protein for degradation) [70]. CDO is highly expressed in the liver. Mice lacking CDO have elevated cysteine, GSH, and H2S levels, but the liver phenotype has not been described [71,72,73]. The expression of CDO in murine liver increases by up to 45-fold and the activity by up to 10-fold depending on cysteine availability [25].
3. Alterations in the Transsulfuration Pathway Linked to NAFLD in Experimental and Animal Models
NAFLD is a complex disease, with multiple molecular events and altered metabolic pathways being part of the pathogenesis. The TSP plays a role in many of these events and has been linked to steatosis, insulin resistance, oxidative stress, ER stress, inflammation, and portal hypertension [6,19,66,74,75,76,77,78]. The link between the TSP and oxidative stress is believed to be mediated through its regulation of GSH production. In CBS-deficient mice, plasma homocysteine levels are increased and steatosis, oxidative stress, and fibrosis develop in the liver [74,75,79]. The lack of CBS appears to upregulate the expression of genes involved in ER stress, hepatic lipid homeostasis, and genes associated with hepatic steatosis [79,80], while knockout of CSE causes reduced hepatic lipolysis [76].
In high-fat-diet models of NAFLD, the hepatic expression of CBS and CSE has provided conflicting results [81,82,83,84]. This could reflect the differing dietary compositions but also the timing of the assessment. It is likely that there is an early adaptive upregulation in the expression of CBS and CSE in response to the increased influx of FFA, and subsequently, the TSP is downregulated or dysfunctioning. Speculatively, enzyme expression could be upregulated with increased hepatic insulin signaling in the initial phase, followed by downregulation with the onset of insulin resistance; however, there are currently no data to support this. The increase in homocysteine caused by the disruption of the TSP also appears to have intrinsic pathophysiological effects. A study evaluating homocysteine-induced ER stress in human hepatocytes found activation of the unfolded protein response (UPR) and the sterol regulatory element-binding proteins sterol regulatory element-binding proteins (SREBPs) [85]. Mice with diet induced-homocysteinemia developed hepatic steatosis, suggesting that homocysteine plays a role in NAFLD development.
3.1. Reduced Production of H2S May Be Involved in NAFLD
The liver is a major organ for endogenous H2S production and clearance. H2S is involved in mitochondrial functioning, insulin sensitivity, lipoprotein synthesis, and glucose metabolism in the liver [6]. H2S also protects against ischemic liver injury [30,86,87]. An animal model of NAFLD showed that H2S production was reduced in a high-fat and methionine- and choline-deficient diet [88]. The reduced production could be explained be decreased expression of CSE and CBS, but also by the unavailability of methionine and choline [88]. It has been speculated that exogenous H2S prevents NASH development in mice by decreasing inflammation and oxidative stress [89]. The reason that H2S synthesis is impaired in NAFLD remains unclear but is probably due alterations in the TSP, since CBS and CSE are responsible for most H2S production.
H2S modulates hepatic steatosis through downregulation of the expression of SREBP-1c, the major transcriptional regulator of the enzymes involved in de novo lipogenesis [5], and the downstream lipogenic enzymes, fatty acid synthase (FAS), and acetyl-CoA carboxylase (ACC). The H2S mediated protection against oxidative stress and hepatocyte injury may be related to the suppression of the C-Jun N-terminal kinase (JNK) signaling pathway [64,90]. The FXR is involved in lipogenesis and is an activator of CSE expression and H2S production. The hepatic expression of SREBP-1c, FAS, and liver X receptor (LXR) was increased in patients with NAFLD, while the expression of FXR was decreased [91]. A phase 3 randomized trial investigating the FXR agonist obeticholic acid for the treatment of non-alcoholic steatohepatitis showed promising results [92]. FXR activation suppresses lipogenesis and decreases SREBP-1c expression [91]. SREBP-1c and its upstream regulators (H2S, CSE, and MPST) may therefore represent candidate targets in the treatment of NAFLD.
Dysregulation of TSP and H2S production may also play a role in the development of portal hypertension in NASH cirrhosis. Downregulation of the TSP leads to decreased levels of H2S, which is a potent vasodilator, and an impaired TSP contributes to increased intrahepatic vascular resistance in rodent models of liver cirrhosis [19]. In agreement with these findings, H2S administration leads to relaxation of the portal vein [93].
3.2. Taurine May Have Beneficial Effects in NAFLD Models
Taurine, the major end-product of cysteine oxidation, affects lipid metabolism and ameliorates the accumulation of lipids in the liver [94,95]. The capacity for de novo taurine biosynthesis is limited and hepatic taurine deficiency is primarily caused by an insufficient nutritional uptake rather than dysfunction of the endogenous biosynthesis [94]. Most studies performed on taurine and NAFLD have investigated the effect of taurine administration on cell cultures or animals. The anti-oxidative effect of taurine may reduce mitochondrial dysfunction and ER stress [95,96,97]. Furthermore, taurine administration leads to improved GSH production [95]. Knockout of the taurine transporter in mice is associated with hepatocyte apoptosis, inflammation, and liver fibrosis [98]. Administration of taurine in animal models of NAFLD results in decreased steatosis, inflammation, and oxidative stress [95,97].
3.3. Long-Term Lipotoxicity Leads to Glutathione Depletion and May Be Involved in NAFLD Development
In silico and in vitro analyses indicate that the initial cellular response to increased hepatic FFAs and hepatic steatosis is an increase in cellular GSH concentration [99,100]. Studies evaluating diet-induced NAFLD animal models have found increased as well as decreased GSH levels in plasma and the liver [7,77,101,102]. The ratio between GSH and glutathione disulfide (GSSG) (GSH/GSSG—an early marker of oxidative stress) was decreased in NAFLD models [7,102]. In addition, an increased influx of FFA for a prolonged period causes depletion of GSH in the livers of rats [77]. Thus, it appears that the biosynthesis of GSH and conversion of GSSG back to GSH is unable to keep pace with the demand to maintain redox homeostasis in the liver, whereby oxidative stress ensues, eventually leading to the development of NASH [7].
4. The Transsulfuration Pathway in Human NALFD
Studies investigating alterations in the TSP in humans with NAFLD are limited, but changes in the sulfur metabolism, especially methionine metabolism, in alcoholic liver disease and cirrhosis are well documented [103,104,105,106,107]. Recently, there has been a focus on the TSP. A study evaluating the relation between metabolites in the TSP and histopathology in alcoholic liver disease (ALD) found that cystathionine levels were positively associated with steatosis and fibrosis [17]. In fact, the levels of several of the metabolites in the TSP and OCM pathways differed between individuals with ALD, active drinkers without liver disease, and healthy controls. Most of the metabolites, including cysteine, were increased in ALD, but the downstream metabolite α-aminobutyrate (aABA) and the aABA/cystathionine ratio were decreased, suggesting an impairment of the TSP [17]. These findings concur with an earlier study measuring TSP metabolites in cirrhotic patients with mixed etiology compared to healthy controls [108]. Cysteine is also increased in NAFLD; the highest levels are seen in patients with NASH and/or fibrosis [8,109,110].
4.1. Increased Blood Levels of Homocysteine in Human NAFLD
Homocysteine is used in clinical practice in the assessment of folate (vitamin B9) and cobalamin (vitamin B12) deficiency. Some studies have found that circulating homocysteine is increased in NAFLD compared to controls [8,111,112,113,114,115,116,117,118], whereas other studies observe the opposite [119,120,121]. A meta-analysis combining the results of several studies concluded that homocysteine is increased in NAFLD [122]. However, the homocysteine levels are not higher in patients with simple steatosis compared to NASH [111,114,116]. When comparing homocysteine levels and hepatic histopathological findings, no consistent trends have been observed (Table 2).
Table 2.
Correlation between histological scores for non-alcoholic fatty liver disease (NAFLD) and homocysteine concentrations.
| Histology | |||||
|---|---|---|---|---|---|
| Study | Steatosis | Fibrosis | Inflammation | Ballooning | NAS |
| Brochado 2013 [111] | No | No | No | NA | NA |
| Gulsen 2005 [114] | NA | Positive | NA | NA | Positive |
| Hirsch 2005 [119] | NA | No | No | NA | No |
| Lai 2020 [116] | Positive | No | No | NA | Positive |
| Pylozos 2012 [120] | No | Negative | Negative (portal) | No | NA |
| Xu 2020 [123] | No | Negative | No | Negative | NA |
NA: not assessed. NAS: NAFLD activity score.
The variation in the results could reflect multiple factors. For instance, systemic homocysteine levels are regulated by hepatic efflux, renal clearance rates, and increase with age. Other possible confounders include nutritional factors (such as the intake of proteins, minerals, and B-vitamins) and body composition [124,125,126]. Given these considerations, and the association between elevated homocysteine and various inflammatory conditions and markers [127], homocysteine does not appear to be an adequate biomarker for NAFLD.
4.2. Genetic Association of the Transsulfuration Pathway with NAFLD
A study including 268 patients who underwent a liver biopsy during surgery found that single-nucleotide polymorphisms (SNPs) in the Glycine N-methyltransferase (GNMT) gene were associated with hepatic levels of the GNMT protein, while this was not the case for SNPs in the MAT1A gene and the MAT protein [128]. The T-allele of 677C>T polymorphism in the MTFHR gene (rs1801133) is associated with elevated tHcy levels [129]. A large cohort study from Sweden found that the T-allele was associated with elevated tHcy levels and cardiovascular multimorbidity [130]. A meta-analysis of the C677T-SNP and the variant A1298C (rs1801131) found that the homozygous TT genotype was associated with an increased susceptibility to NAFLD [131]. An SNP (variant c.1364G>T) in the CTH gene (CSE) has been associated with elevated homocysteine levels, but polymorphisms in the genes for CBS and CSE have not been linked to NAFLD in humans [132].
Only a few studies have reported on the genetic expression of the TSP and related pathways in human liver disease. In contrast, several NAFLD animal studies have reported reduced expression of the enzymes in the TSP and related pathways [39,60,114,115,116,117]. The expression of CBS and CSE is reduced [60,114], while CDO is found to be increased [115].
Reduced mRNA abundance was found for GNMT, MS, BHMT, MAT1A, and CBS in human cirrhotic livers compared to controls [133,134]. The hepatic expression of MPST correlated to the grade of steatosis in patients with NAFLD and was increased compared to healthy controls [64].
In liver biopsies of patients with NAFLD, advanced disease was associated with general DNA hypomethylation, suggesting less transcriptional control [135]. Some genes in the OCM, namely AHCY, MAT1A, and methylenetetrahydrofolate dehydrogenase (MTHFD2), involved in generating methyl groups for methylation, were hypermethylated, which correlated with low expression of the respective genes [135]. Theoretically, the decreased expression of the enzymes involved in homocysteine remethylation to methionine (MS, BHMT) may cause low hepatic concentration of the methyl donor SAM and subsequently hypomethylation of genes in the liver.
4.3. Reduced H2S Levels May Be Associated with Liver Disease
To date, there are limited studies investigating the association between H2S and liver disease. One study found decreased levels of H2S in cirrhotic patients of mixed etiologies compared to healthy controls [136], in line with the reduced expression of CBS observed in cirrhosis [133,134]. Likewise, the reduced H2S levels correlate with increased portal vein diameter and Child–Pugh Score [136]. Obesity and diabetes are also associated with reduced H2S levels [9,137,138], probably related to poor glycemic control [9].
4.4. Transsulfuration Pathway Metabolite Concentrations and Cofactor Status in Human NAFLD
The circulating and tissue concentrations of several metabolites related to TSP and OCM may be changed due to altered TSP flux. These metabolites include the amino acids serine, glutamate, and glycine, in addition to α-ketobutyrate, pyruvate, and sulfur-containing taurine, gamma-glutamylcysteine, and GSH (Figure 1). In analyses of amino acids, plasma glutamate levels were found to be increased while circulating concentrations of glycine and serine were decreased in patients with NAFLD [8,139]. Plasma glutamate and glycine were independent predictors of fibrosis, irrespective of insulin resistance [139]. In the setting of the dysregulated metabolism observed in hepatic steatosis, glycine appears to be the limiting factor in de novo GSH production [99]. Plasma GSH is low and the rate of hepatic glutathione turnover is high in patients with NAFLD compared to healthy controls [8,117]. The hepatic expression of several enzymes involved in the synthesis of GSH was reduced in individuals with obesity compared to healthy subjects [99]. Thus, the high GSH turnover is not accompanied by a corresponding increase in GSH production. Another study found a decrease in human liver GSH in steatotic compared to non-steatotic subjects [140]. The GSH/GSSG ratio in the steatotic group was also decreased, indicating impaired regeneration of GSH and/or increased oxidative stress [140]. The decreased levels of circulating GSH in NAFLD correspond to the findings in patients with type 2 diabetes, suggesting a potential link between GSH and metabolic disease [141]. A small, open-label, single-arm pilot study suggested that oral administration of GSH may improve liver enzymes in some individuals with NAFLD [142]. However, caution is needed when interpreting results from studies evaluating GSH and GSSH levels, because GSH and related thiols are notoriously unstable and sensitive to oxidation and or degradation during sample handling and analysis [143].
Circulating taurine levels are elevated in NAFLD compared to healthy controls [144]. However, when comparing NAFLD patients with and without advanced fibrosis, taurine levels were decreased in the former patient group [13]. Thus, systemic taurine may be a predictor of steatosis and inflammation rather than fibrosis. Hepatic taurine levels have also been found to be increased in human NASH liver tissue relative to samples from patients with simple steatosis or normal liver tissue, suggesting a hepatic origin for the changes seen in circulating taurine levels [145,146]. Elevated taurine levels could be due to increased biosynthesis in NASH patients, consistent with increased expression of CDO in an NAFLD animal model [147].
The role of vitamin B6 in human NAFLD has not been well examined. A study found that high vitamin B6 intake was associated with the occurrence of NAFLD as evaluated by controlled attenuation parameter (CAP) [148]. However, the circulating levels of vitamin B6 were not measured. In contrast, some animal models have shown that B6 deficiency causes hepatic lipid accumulation and is prevented by supplementation [149,150,151]. One study has reported reduced levels of circulating vitamin B6 in patients with NAFLD compared to controls [149]. Describing the mechanisms linking alteration in vitamin B6 status to NALFD is challenging as vitamin B6 is a cofactor for around 150 enzymatic activities, but vitamin B6 status has been coupled to type 2 DM and lipid metabolism [152].
The levels of circulating folate and vitamin B12 in human NAFLD have been studied more extensively. A meta-analysis found a tendency towards decreased levels of folate and increased levels of vitamin B12 in NAFLD compared to controls, but the results were not statistically different [122].
5. Summary
The TSP is involved in the synthesis and processing of several metabolites that are important for cellular function. These metabolites include homocysteine, cysteine, H2S, GSH, and taurine, among many others. Importantly, the TSP plays a central role in redox homeostasis and the modulation of cellular oxidative stress. The liver is a major organ in TSP and related OCM processes.
The TSP enzymes (CBS and CSE) along with the other enzymes involved in OCM are all highly expressed in the liver. Thus, changes in these processes may be involved in liver disease and metabolic alterations. A link between TSP and NAFLD may exist; see Figure 3.
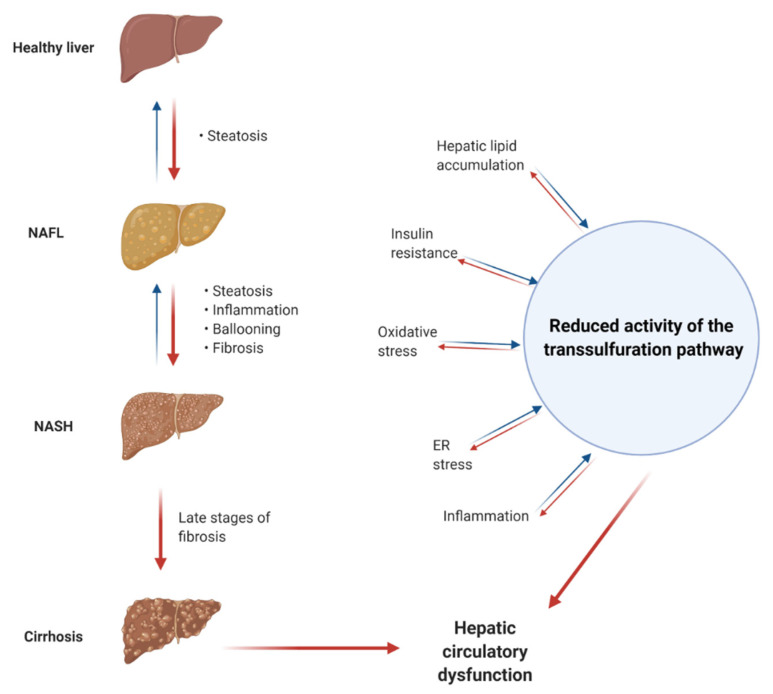
Figure 3.
Potential links between the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and reduced activity of the transsulfuration pathway (TSP) and related pathways. NAFL, non-alcoholic fatty liver; NASH, non-alcoholic steatohepatitis.
Known factors and comorbidities in the development of NAFLD, such as insulin resistance, type 2 DM, obesity, changed glucose and lipid metabolism, and inflammation, can be coupled to changes in the functioning of the TSP and related OCM processes. Knockout of CBS and CSE in animal models leads to liver steatosis, further strengthening the potential link between the TSP and NAFLD. Results from animal models and human liver disease suggest decreased expression of CBS and CSE. The decreased expression and potential changes in activity of the TSP and OCM enzymes will expectedly cause changes in several metabolites. Studies have found changed levels of methionine, SAM, SAH, homocysteine, cystathionine, cysteine, H2S, and GSH in human liver disease.
One of the most important challenges in NAFLD is to find new biomarkers of disease severity and development. The available evidence is not yet clear and additional studies are still needed to evaluate whether TSP or OCM metabolites may be used as biomarkers in NAFLD. Cystathionine could be a potential candidate as it has been shown to predict steatosis and fibrosis in ALD, and it is central in the TSP flux and will increase by reduced activity and low expression of CSE.
Because TSP and OCM are central in many metabolic processes, such as methylation, GSH production, amino acid metabolism, and H2S production, the enzymes and metabolites may represent potential candidates for therapeutic targets. Currently, it is difficult to nominate the most evident candidates. Modulation of CBS and CSE activity can potentially affect many processes by changing GSH and H2S production.
6. Conclusions
With the high expression of the CBS and CSE enzymes in the liver, it is not unexpected to find perturbation of the TSP in human liver diseases. To investigate the potential relationship of the TSP with NAFLD, experiments involving gene knockout and/or gain of function along with histological evaluation could provide more evidence. Despite increasing insights, it remains a challenge to fully comprehend the complex molecular mechanisms of the TSP and related OCM pathways and its relation to NAFLD pathophysiology. Additional knowledge may point to potential drug targets or biomarkers of disease severity and progression. However, it is important to remember that the TSP is only a small part of a large network of interconnected molecular pathways. Pharmacological modulation of the activity of the TSP enzymes could potentially lead to adverse effects that are difficult to predict due the complexity of the metabolic network involved.
TSP and OCM metabolites may emerge as predictors of disease development and progression in NAFLD. With the development of more complex multifactorial models using applying machine learning and deep learning algorithms, TSP and OCM metabolites can potentially be included in such models. Severity scores will likely require several factors/components, including multiple circulating biomarkers, as well as information concerning age, renal function, body composition, and nutritional status (e.g., measures of B-vitamin status).
Finally, we need additional studies in both cell lines, animals and humans, to further investigate the complex TSP and related pathways to unveil their potential as pharmacological targets and biomarkers.
Author Contributions
Writing—original draft preparation, M.P.W.; Writing—review and editing, M.P.W., A.M., E.D.G., D.H., A.B., N.J.W.A., and L.L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received support by Novo Nordisk, N.J.W.A, was supported by a grant from the Novo Nordisk Foundation (NNF19OC0055001).
Conflicts of Interest
The submitted work is supported by Novo Nordisk.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 4.Cusi K. Role of Obesity and Lipotoxicity in the Development of Nonalcoholic Steatohepatitis: Pathophysiology and Clinical Implications. Gastroenterol. 2012;142:711–725. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu D.-D., Wang D.-Y., Li H.-M., Guo J.-C., Duan S.-F., Ji X.-Y. Hydrogen Sulfide as a Novel Regulatory Factor in Liver Health and Disease. Oxidative Med. Cell. Longev. 2019;2019:3831713. doi: 10.1155/2019/3831713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L., Zhang G.-F., Lee K., Lopez R., Previs S.F., Willard B., McCullough A., Kasumov T. A Western diet induced NAFLD in LDLR(-/)(-) mice is associated with reduced hepatic glutathione synthesis. Free. Radic. Biol. Med. 2016;96:13–21. doi: 10.1016/j.freeradbiomed.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalhan S.C., Guo L., Edmison J., Dasarathy S., McCullough A.J., Hanson R.W., Milburn M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–413. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki K., Sagara M., Aoki C., Tanaka S., Aso Y. Clinical Implication of Plasma Hydrogen Sulfide Levels in Japanese Patients with Type 2 Diabetes. Intern. Med. 2017;56:17–21. doi: 10.2169/internalmedicine.56.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., Tiribelli C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabisch S., Bäther S., Dambeck U., Kemper M., Gerbracht C., Honsek C., Sachno A., Pfeiffer A.F.H. Liver Fat Scores Moderately Reflect Interventional Changes in Liver Fat Content by a Low-Fat Diet but Not by a Low-Carb Diet. Nutrients. 2018;10:157. doi: 10.3390/nu10020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne C.D., Targher G. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: Is universal screening appropriate? Diabetologia. 2016;59:1141–1144. doi: 10.1007/s00125-016-3910-y. [DOI] [PubMed] [Google Scholar]
- 13.Caussy C., Ajmera V.H., Puri P., Hsu C.L.-S., Bassirian S., Mgdsyan M., Singh S., Faulkner C., A Valasek M., Rizo E., et al. Serum metabolites detect the presence of advanced fibrosis in derivation and validation cohorts of patients with non-alcoholic fatty liver disease. Gut. 2019;68:1884–1892. doi: 10.1136/gutjnl-2018-317584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterling R.K., Lissen E., Clumeck N., Sola R., Correa M.C., Montaner J., Sulkowski M.S., Torriani F.J., Dieterich D.T., Thomas D.L., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava A., Gailer R., Tanwar S., Trembling P., Parkes J., Rodger A., Suri D., Thorburn D., Sennett K., Morgan S., et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2019;71:371–378. doi: 10.1016/j.jhep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Karsdal M.A., Henriksen K., Nielsen M.J., Byrjalsen I., Leeming D.J., Gardner S., Goodman Z., Patel K., Krag A., Christiansen C., et al. Fibrogenesis assessed by serological type III collagen formation identifies patients with progressive liver fibrosis and responders to a potential antifibrotic therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G1009–G1017. doi: 10.1152/ajpgi.00283.2016. [DOI] [PubMed] [Google Scholar]
- 17.Medici V., Peerson J.M., Stabler S.P., French S.W., Gregory J.F., Virata M.C., Albanese A., Bowlus C.L., Devaraj S., Panacek E.A., et al. Impaired homocysteine transsulfuration is an indicator of alcoholic liver disease. J. Hepatol. 2010;53:551–557. doi: 10.1016/j.jhep.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarna L.K., Siow Y.L., O K. The CBS/CSE system: A potential therapeutic target in NAFLD? Can. J. Physiol. Pharmacol. 2015;93:1–11. doi: 10.1139/cjpp-2014-0394. [DOI] [PubMed] [Google Scholar]
- 19.Fiorucci S., Distrutti E. Targeting the transsulfuration-H2S pathway by FXR and GPBAR1 ligands in the treatment of portal hypertension. Pharmacol. Res. 2016;111:749–756. doi: 10.1016/j.phrs.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Ducker G.S., Rabinowitz J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017;25:27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pajares M.A., Pérez-Sala D. Betaine homocysteine S-methyltransferase: Just a regulator of homocysteine metabolism? Cell. Mol. Life Sci. 2006;63:2792–2803. doi: 10.1007/s00018-006-6249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkelstein J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 23.Mudd S.H., Brosnan J.T., Brosnan M.E., Jacobs R.L., Stabler S.P., Allen R.H., Vance D.E., Wagner C. Methyl balance and transmethylation fluxes in humans. Am. J. Clin. Nutr. 2007;85:19–25. doi: 10.1093/ajcn/85.1.19. [DOI] [PubMed] [Google Scholar]
- 24.Su X., E Wellen K., Rabinowitz J.D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 2016;30:52–60. doi: 10.1016/j.cbpa.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stipanuk M.H., Ueki I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2010;34:17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sbodio J.I., Snyder S.H., Paul B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2018;176:583–593. doi: 10.1111/bph.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratnam S., MacLean K.N., Jacobs R.L., Brosnan M.E., Kraus J.P., Brosnan J.T. Hormonal Regulation of Cystathionine β-Synthase Expression in Liver. J. Biol. Chem. 2002;277:42912–42918. doi: 10.1074/jbc.M206588200. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L., Yang G., Untereiner A., Ju Y., Wu L., Wang R. Hydrogen Sulfide Impairs Glucose Utilization and Increases Gluconeogenesis in Hepatocytes. Endocrinology. 2013;154:114–126. doi: 10.1210/en.2012-1658. [DOI] [PubMed] [Google Scholar]
- 29.Siebert N., Cantré D., Eipel C., Vollmar B. H2S contributes to the hepatic arterial buffer response and mediates vasorelaxation of the hepatic artery via activation of KATP channels. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G1266–G1273. doi: 10.1152/ajpgi.90484.2008. [DOI] [PubMed] [Google Scholar]
- 30.Fiorucci S., Antonelli E., Mencarelli A., Orlandi S., Renga B., Rizzo G., Distrutti E., Shah V., Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 31.Prudova A., Bauman Z., Braun A., Vitvitsky V., Lu S.C., Banerjee R. S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc. Natl. Acad. Sci. USA. 2006;103:6489–6494. doi: 10.1073/pnas.0509531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu W.-N., Yadav P.K., Adamec J., Banerjee R. S-Glutathionylation Enhances Human Cystathionine β-Synthase Activity Under Oxidative Stress Conditions. Antioxid. Redox Signal. 2015;22:350–361. doi: 10.1089/ars.2014.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkelstein J.D. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin. Chem. Lab. Med. 2007;45:1694–1699. doi: 10.1515/CCLM.2007.341. [DOI] [PubMed] [Google Scholar]
- 34.Singh S., Madzelan P., Banerjee R. Properties of an unusual heme cofactor in PLP-dependent cystathionine β-synthase. Nat. Prod. Rep. 2007;24:631–639. doi: 10.1039/B604182P. [DOI] [PubMed] [Google Scholar]
- 35.Renga B., Bucci M., Cipriani S., Carino A., Monti M.C., Zampella A., Gargiulo A., Bianca R.D.D.V., Distrutti E., Fiorucci S. Cystathionine γ-lyase, a H2S-generating enzyme, is a GPBAR1-regulated gene and contributes to vasodilation caused by secondary bile acids. Am. J. Physiol. Hear. Circ. Physiol. 2015;309:H114–H126. doi: 10.1152/ajpheart.00087.2015. [DOI] [PubMed] [Google Scholar]
- 36.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. H2S Signals Through Protein S-Sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lima C.P., Davis S.R., Mackey A.D., Scheer J.B., Williamson J., Gregory J.F. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J. Nutr. 2006;136:2141–2147. doi: 10.1093/jn/136.8.2141. [DOI] [PubMed] [Google Scholar]
- 38.Stabler S.P., Sampson D.A., Wang L.-P., Allen R.H. Elevations of serum cystathionine and total homocysteine in pyridoxine-, folate-, and cobalamin-deficient rats. J. Nutr. Biochem. 1997;8:279–289. doi: 10.1016/S0955-2863(97)89666-0. [DOI] [Google Scholar]
- 39.Gregory J.F., DeRatt B.N., Rios-Avila L., Ralat M., Stacpoole P.W. Vitamin B6 nutritional status and cellular availability of pyridoxal 5′-phosphate govern the function of the transsulfuration pathway’s canonical reactions and hydrogen sulfide production via side reactions. Biochimie. 2016;126:21–26. doi: 10.1016/j.biochi.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez M., Cuskelly G.J., Williamson J., Toth J.P., Gregory I.J.F. Vitamin B-6 Deficiency in Rats Reduces Hepatic Serine Hydroxymethyltransferase and Cystathionine β-Synthase Activities and Rates of In Vivo Protein Turnover, Homocysteine Remethylation and Transsulfuration. J. Nutr. 2000;130:1115–1123. doi: 10.1093/jn/130.5.1115. [DOI] [PubMed] [Google Scholar]
- 41.Gregory J.F., Park Y., Lamers Y., Bandyopadhyay N., Chi Y.-Y., Lee K., Kim S., Da Silva V., Hove N., Ranka S., et al. Metabolomic Analysis Reveals Extended Metabolic Consequences of Marginal Vitamin B-6 Deficiency in Healthy Human Subjects. PLoS ONE. 2013;8:e63544. doi: 10.1371/journal.pone.0063544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rush E.C., Katre P., Yajnik C.S. Vitamin B12: One carbon metabolism, fetal growth and programming for chronic disease. Eur. J. Clin. Nutr. 2013;68:2–7. doi: 10.1038/ejcn.2013.232. [DOI] [PubMed] [Google Scholar]
- 43.Lyon P., Strippoli V., Fang B., Cimmino L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients. 2020;12:2867. doi: 10.3390/nu12092867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Froese D.S., Fowler B., Baumgartner M.R. Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019;42:673–685. doi: 10.1002/jimd.12009. [DOI] [PubMed] [Google Scholar]
- 45.Yamada K., Kawata T., Wada M., Isshiki T., Onoda J., Kawanishi T., Kunou A., Tadokoro T., Tobimatsu T., Maekawa A., et al. Extremely low activity of methionine synthase in vitamin B-12-deficient rats may be related to effects on coenzyme stabilization rather than to changes in coenzyme induction. J. Nutr. 2000;130:1894–1900. doi: 10.1093/jn/130.8.1894. [DOI] [PubMed] [Google Scholar]
- 46.Shane B. Folate and Vitamin B12Metabolism: Overview and Interaction with Riboflavin, Vitamin B6, and Polymorphisms. Food Nutr. Bull. 2008;29:S5–S16. doi: 10.1177/15648265080292S103. [DOI] [PubMed] [Google Scholar]
- 47.Sbodio J.I., Snyder S.H., Paul B.D. Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2018;115:780–785. doi: 10.1073/pnas.1717877115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renga B., Mencarelli A., Migliorati M., Distrutti E., Fiorucci S. Bile-acid-activated farnesoid X receptor regulates hydrogen sulfide production and hepatic microcirculation. World J. Gastroenterol. 2009;15:2097–2108. doi: 10.3748/wjg.15.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen N., Paul B.D., Gadalla M.M., Mustafa A.K., Sen T., Xu R., Kim S., Snyder S.H. Hydrogen Sulfide-Linked Sulfhydration of NF-κB Mediates Its Antiapoptotic Actions. Mol. Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassan M.I., Boosen M., Schaefer L., Kozlowska J., Eisel F., Von Knethen A., Beck M., Hemeida R.A.M., El-Moselhy M.A.M., Hamada F.M.A., et al. Platelet-derived growth factor-BB induces cystathionine γ-lyase expression in rat mesangial cells via a redox-dependent mechanism. Br. J. Pharmacol. 2012;166:2231–2242. doi: 10.1111/j.1476-5381.2012.01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hourihan J.M., Kenna J.G., Hayes J.D. The Gasotransmitter Hydrogen Sulfide Induces Nrf2-Target Genes by Inactivating the Keap1 Ubiquitin Ligase Substrate Adaptor Through Formation of a Disulfide Bond Between Cys-226 and Cys-613. Antioxid. Redox Signal. 2013;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 52.Steele M.L., Fuller S., Patel M., Kersaitis C., Ooi L., Münch G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 2013;1:441–445. doi: 10.1016/j.redox.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harvey C., Thimmulappa R., Singh A., Blake D., Ling G., Wakabayashi N., Fujii J., Myers A., Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free. Radic. Biol. Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nandi S.S., Mishra P.K. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep. 2017;7:3639. doi: 10.1038/s41598-017-03776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiao Y., Lu Y., Li X.-Y. Farnesoid X receptor: A master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin. 2015;36:44–50. doi: 10.1038/aps.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trauner M., Claudel T., Fickert P., Moustafa T., Wagner M. Bile Acids as Regulators of Hepatic Lipid and Glucose Metabolism. Dig. Dis. 2010;28:220–224. doi: 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- 57.Luedde T., Schwabe R.F. NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy B., Bhattacharya R., Mukherjee P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019;33:13098–13125. doi: 10.1096/fj.201901304R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimura Y., Goto Y.-I., Kimura H. Hydrogen Sulfide Increases Glutathione Production and Suppresses Oxidative Stress in Mitochondria. Antioxid. Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 60.Zhang D., Du J., Tang C., Huang Y., Jin H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017;8:608. doi: 10.3389/fphar.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C.W., Moore P.K. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide. Springer International Publishing; Cham, Switzerland: 2015. H2S synthesizing enzymes: Biochemistry and molecular aspects. Handbook of Experimental Pharmacology. [DOI] [PubMed] [Google Scholar]
- 62.Kabil O., Yadav V., Banerjee R. Heme-dependent Metabolite Switching Regulates H2S Synthesis in Response to Endoplasmic Reticulum (ER) Stress. J. Biol. Chem. 2016;291:16418–16423. doi: 10.1074/jbc.C116.742213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banerjee R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr. Opin. Chem. Biol. 2017;37:115–121. doi: 10.1016/j.cbpa.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li M., Xu C., Shi J., Ding J., Wan X., Chen D., Gao J., Li C., Zhang J., Lin Y., et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut. 2017;67:2169–2180. doi: 10.1136/gutjnl-2017-313778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teskey G., Abrahem R., Cao R., Gyurjian K., Islamoglu H., Lucero M., Martinez A., Paredes E., Salaiz O., Robinson B., et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018;87:141–159. doi: 10.1016/bs.acc.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Mosharov E., Cranford M.R., Banerjee R. The Quantitatively Important Relationship between Homocysteine Metabolism and Glutathione Synthesis by the Transsulfuration Pathway and Its Regulation by Redox Changes†. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- 67.Vitvitsky V., Mosharov E., Tritt M., Ataullakhanov F., Banerjee R. Redox regulation of homocysteine-dependent glutathione synthesis. Redox Rep. 2003;8:57–63. doi: 10.1179/135100003125001260. [DOI] [PubMed] [Google Scholar]
- 68.Schaffer S., Kim H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018;26:225–241. doi: 10.4062/biomolther.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanagita T., Han S.-Y., Hu Y., Nagao K., Kitajima H., Murakami S. Taurine reduces the secretion of apolipoprotein B100 and lipids in HepG2 cells. Lipids Health Dis. 2008;7:38. doi: 10.1186/1476-511X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stipanuk M.H., Hirschberger L.L., Londono M.P., Cresenzi C.L., Yu A.F. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am. J. Physiol. Endocrinol. Metab. 2004;286:E439–E448. doi: 10.1152/ajpendo.00336.2003. [DOI] [PubMed] [Google Scholar]
- 71.Jurkowska H., Roman H.B., Hirschberger L.L., Sasakura K., Nagano T., Hanaoka K., Krijt J., Stipanuk M.H. Primary hepatocytes from mice lacking cysteine dioxygenase show increased cysteine concentrations and higher rates of metabolism of cysteine to hydrogen sulfide and thiosulfate. Amino Acids. 2014;46:1353–1365. doi: 10.1007/s00726-014-1700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niewiadomski J., Zhou J.Q., Roman H.B., Liu X., Hirschberger L.L., Locasale J.W., Stipanuk M.H. Effects of a block in cysteine catabolism on energy balance and fat metabolism in mice. Ann. N. Y. Acad. Sci. 2016;1363:99–115. doi: 10.1111/nyas.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ueki I., Roman H.B., Valli A., Fieselmann K., Lam J., Peters R., Hirschberger L.L., Stipanuk M.H. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am. J. Physiol. Endocrinol. Metab. 2011;301:E668–E684. doi: 10.1152/ajpendo.00151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robert K., Nehmé J., Bourdon E., Pivert G., Friguet B., Delcayre C., Delabar J., Janel N. Cystathionine β Synthase Deficiency Promotes Oxidative Stress, Fibrosis, and Steatosis in Mice Liver. Gastroenterology. 2005;128:1405–1415. doi: 10.1053/j.gastro.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M.R., Maeda N. Mice deficient in cystathionine beta-synthase: Animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mani S., Li H., Yang G., Wu L., Wang R. Deficiency of cystathionine gamma-lyase and hepatic cholesterol accumulation during mouse fatty liver development. Sci. Bull. 2015;60:336–347. doi: 10.1007/s11434-014-0722-7. [DOI] [Google Scholar]
- 77.Grattagliano I., Caraceni P., Calamita G., Ferri D., Gargano I., Palasciano G., Portincasa P. Severe liver steatosis correlates with nitrosative and oxidative stress in rats. Eur. J. Clin. Investig. 2008;38:523–530. doi: 10.1111/j.1365-2362.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 78.Dickhout J.G., Carlisle R.E., Jerome D.E., Mohammed-Ali Z., Jiang H., Yang G., Mani S., Garg S.K., Banerjee R., Kaufman R.J., et al. Integrated Stress Response Modulates Cellular Redox State via Induction of Cystathionine γ-Lyase. J. Biol. Chem. 2012;287:7603–7614. doi: 10.1074/jbc.M111.304576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guillén N., Navarro M.A., Arnal C., Noone E., Arbonés-Mainar J.M., Acín S., Surra J.C., Muniesa P., Roche H.M., Osada J. Microarray analysis of hepatic gene expression identifies new genes involved in steatotic liver. Physiol. Genom. 2009;37:187–198. doi: 10.1152/physiolgenomics.90339.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamelet J., DeMuth K., Paul J.-L., Delabar J.-M., Janel N. Hyperhomocysteinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice. J. Hepatol. 2007;46:151–159. doi: 10.1016/j.jhep.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 81.Liu M., Deng M., Su J., Lin Y., Jia Z., Peng K., Wang F., Yang T. Specific downregulation of cystathionine β-synthase expression in the kidney during obesity. Physiol. Rep. 2018;6:e13630. doi: 10.14814/phy2.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang S.-Y., Sarna L.K., Siow Y.L., O K. High-fat diet stimulates hepatic cystathionine β-synthase and cystathionine γ-lyase expression. Can. J. Physiol. Pharmacol. 2013;91:913–919. doi: 10.1139/cjpp-2013-0106. [DOI] [PubMed] [Google Scholar]
- 83.Peh M.T., Anwar A.B., Ng D.S.W., Atan M.S.B.M., Kumar S.D., Moore P.K. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide. 2014;41:138–145. doi: 10.1016/j.niox.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 84.Bravo E., Palleschi S., Aspichueta P., Buqué X., Rossi B., Cano A., Napolitano M., Ochoa B., Botham K.M. High fat diet-induced non alcoholic fatty liver disease in rats is associated with hyperhomocysteinemia caused by down regulation of the transsulphuration pathway. Lipids Health Dis. 2011;10:60. doi: 10.1186/1476-511X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Werstuck G.H., Lentz S.R., Dayal S., Hossain G.S., Sood S.K., Shi Y.Y., Zhou J., Maeda N., Krisans S.K., Malinow M.R., et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Investig. 2001;107:1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang K., Zhao M., Jiang H., Tan G., Pan S., Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transplant. 2009;15:1306–1314. doi: 10.1002/lt.21810. [DOI] [PubMed] [Google Scholar]
- 87.Tan G., Pan S., Li J., Dong X., Kang K., Zhao M., Jiang X., Kanwar J.R., Qiao H., Jiang H., et al. Hydrogen Sulfide Attenuates Carbon Tetrachloride-Induced Hepatotoxicity, Liver Cirrhosis and Portal Hypertension in Rats. PLoS ONE. 2011;6:e25943. doi: 10.1371/journal.pone.0025943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luo Z.-L., Tang L.-J., Wang T., Dai R.-W., Ren J.-D., Cheng L., Xiang K., Tian F.-Z. Effects of treatment with hydrogen sulfide on methionine-choline deficient diet-induced non-alcoholic steatohepatitis in rats. J. Gastroenterol. Hepatol. 2013;29:215–222. doi: 10.1111/jgh.12389. [DOI] [PubMed] [Google Scholar]
- 89.Wu D., Zheng N., Ziqiang S., Cheng H., Sun Z., Gao B., Zhang Y., Pang W., Huangfu C., Ji S., et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med. Gas Res. 2015;5:1–8. doi: 10.1186/s13618-014-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng P., Wang F., Chen K., Shen M., Dai W., Xu L., Zhang Y., Wang C., Li J., Yang J., et al. Hydrogen Sulfide Ameliorates Ischemia/Reperfusion-Induced Hepatitis by Inhibiting Apoptosis and Autophagy Pathways. Mediat. Inflamm. 2014;2014:935251. doi: 10.1155/2014/935251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Z.-X., Shen W., Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol. Int. 2010;4:741–748. doi: 10.1007/s12072-010-9202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z., Bedossa P., Geier A., Beckebaum S., Newsome P.N., et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 93.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyazaki T., Matsuzaki Y. Taurine and liver diseases: A focus on the heterogeneous protective properties of taurine. Amino Acids. 2014;46:101–110. doi: 10.1007/s00726-012-1381-0. [DOI] [PubMed] [Google Scholar]
- 95.Murakami S., Ono A., Kawasaki A., Takenaga T., Ito T. Taurine attenuates the development of hepatic steatosis through the inhibition of oxidative stress in a model of nonalcoholic fatty liver disease in vivo and in vitro. Amino Acids. 2018;50:1279–1288. doi: 10.1007/s00726-018-2605-8. [DOI] [PubMed] [Google Scholar]
- 96.Jong C.J., Azuma J., Schaffer S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids. 2011;42:2223–2232. doi: 10.1007/s00726-011-0962-7. [DOI] [PubMed] [Google Scholar]
- 97.Gentile C.L., Nivala A.M., Gonzales J.C., Pfaffenbach K.T., Wang N., Wei Y., Jiang H., Orlicky D.J., Petersen D.R., Pagliassotti M.J., et al. Experimental evidence for therapeutic potential of taurine in the treatment of nonalcoholic fatty liver disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1710–R1722. doi: 10.1152/ajpregu.00677.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Warskulat U., Borsch E., Reinehr R., Heller-Stilb B., Mönnighoff I., Buchczyk D., Donner M., Flögel U., Kappert G., Soboll S., et al. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006;20:574–576. doi: 10.1096/fj.05-5016fje. [DOI] [PubMed] [Google Scholar]
- 99.Mardinoglu A., Bjornson E., Zhang C., Klevstig M., Söderlund S., Ståhlman M., Adiels M., Hakkarainen A., Lundbom N., Kilicarslan M., et al. Personal model-assisted identification of NAD + and glutathione metabolism as intervention target in NAFLD. Mol. Syst. Biol. 2017;13:916. doi: 10.15252/msb.20167422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garcia M.C., Amankwa-Sakyi M., Flynn T.J. Cellular glutathione in fatty liver in vitro models. Toxicol. Vitr. 2011;25:1501–1506. doi: 10.1016/j.tiv.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 101.Haque J.A., Mcmahan R.S., Campbell J.S., Shimizu-Albergine M., Wilson A.M., Botta D., Bammler T.K., Beyer R.P., Montine T.J., Yeh M.M., et al. Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice. Lab. Investig. 2010;90:1704–1717. doi: 10.1038/labinvest.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dou X., Li S., Hu L., Ding L., Ma Y., Ma W., Chai H., Song Z. Glutathione disulfide sensitizes hepatocytes to TNFα-mediated cytotoxicity via IKK-β S-glutathionylation: A potential mechanism underlying non-alcoholic fatty liver disease. Exp. Mol. Med. 2018;50:1–16. doi: 10.1038/s12276-017-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kinsell L.W., Harper H.A., Barton H.C., Michaels G.D., Weiss H.A. Rate of Disappearance From Plasma of Intravenously Administered Methionine in Patients With Liver Damage. Science. 1947;106:589–590. doi: 10.1126/science.106.2763.589. [DOI] [PubMed] [Google Scholar]
- 104.Horowitz J.H., Rypins E.B., Henderson J., Heymsfield S.B., Moffitt S.D., Bain R.P., Chawla R.K., Bleier J.C., Daniel R. Evidence for impairment of transsulfuration pathway in cirrhosis. Gastroenterology. 1981;81:668–675. doi: 10.1016/0016-5085(81)90489-3. [DOI] [PubMed] [Google Scholar]
- 105.Marchesini G., Bugianesi E., Bianchi G., Fabbri A., Marchi E., Zoli M., Pisi E. Defective methionine metabolism in cirrhosis: Relation to severity of liver disease. Hepatology. 1992;16:149–155. doi: 10.1002/hep.1840160125. [DOI] [PubMed] [Google Scholar]
- 106.Martensson J., Foberg U., Frydén A., Schwartz M.K., Sörbo B., Weiland O. Sulfur Amino Acid Metabolism in Hepatobiliary Disorders. Scand. J. Gastroenterol. 1992;27:405–411. doi: 10.3109/00365529209000096. [DOI] [PubMed] [Google Scholar]
- 107.Russmann S., Junker E., Lauterburg B.H. Remethylation and transsulfuration of methionine in cirrhosis: Studies with L -[2 H3 -methyl-1-13 C]methionine. Hepatology. 2002;36:1190–1196. doi: 10.1053/jhep.2002.36499. [DOI] [PubMed] [Google Scholar]
- 108.Look R.R.M.P. Is the Increase in Serum Cystathionine Levels in Patients with Liver Cirrhosis a Consequence of Impaired Homocysteine Transsulfuration at the Level of ?-Cystathionase? Scand. J. Gastroenterol. 2000;35:866–872. doi: 10.1080/003655200750023255. [DOI] [PubMed] [Google Scholar]
- 109.Hasegawa T., Iino C., Endo T., Mikami K., Kimura M., Sawada N., Nakaji S., Fukuda S. Changed Amino Acids in NAFLD and Liver Fibrosis: A Large Cross-Sectional Study without Influence of Insulin Resistance. Nutrients. 2020;12:1450. doi: 10.3390/nu12051450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ioannou G.N., Gowda G.A.N., Djukovic D., Raftery D. Distinguishing NASH Histological Severity Using a Multiplatform Metabolomics Approach. Metabolites. 2020;10:168. doi: 10.3390/metabo10040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brochado M.J.F., Domenici F.A., Martinelli A.D.L.C., Zucoloto S., Cunha S.F.D.C.D., Vannucchi H. Methylenetetrahydrofolate Reductase Gene Polymorphism and Serum Homocysteine Levels in Nonalcoholic Fatty Liver Disease. Ann. Nutr. Metab. 2013;63:193–199. doi: 10.1159/000353139. [DOI] [PubMed] [Google Scholar]
- 112.De Carvalho S.C.R., Muniz M.T.C., Siqueira M.D.V., Siqueira E.R.F., Gomes A.V., Silva K.A., Bezerra L.C.L., D’Almeida V., De Oliveira C.P.M.S., Pereira L.M.M.B. Plasmatic higher levels of homocysteine in Non-alcoholic fatty liver disease (NAFLD) Nutr. J. 2013;12:37. doi: 10.1186/1475-2891-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dai H., Wang W., Tang X., Chen R., Chen Z., Lu Y., Yuan H. Association between homocysteine and non-alcoholic fatty liver disease in Chinese adults: A cross-sectional study. Nutr. J. 2016;15:1–7. doi: 10.1186/s12937-016-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gulsen M., Yesilova Z., Bagci S., Uygun A., Ozcan A., Ercin C.N., Erdil A., Sanisoglu S.Y., Cakir E., Ates Y., et al. Elevated plasma homocysteine concentrations as a predictor of steatohepatitis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2005;20:1448–1455. doi: 10.1111/j.1440-1746.2005.03891.x. [DOI] [PubMed] [Google Scholar]
- 115.Jia G., Di F., Wang Q., Shao J., Gao L., Wang L., Li Q., Li N. Non-Alcoholic Fatty Liver Disease Is a Risk Factor for the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes Mellitus. PLoS ONE. 2015;10:e0142808. doi: 10.1371/journal.pone.0142808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lai Z., Chen J., Ding C., Wong K., Chen X., Pu L., Huang Q., Chen X., Cheng Z., Liu Y., et al. Association of Hepatic Global DNA Methylation and Serum One-Carbon Metabolites with Histological Severity in Patients with NAFLD. Obesity. 2019;28:197–205. doi: 10.1002/oby.22667. [DOI] [PubMed] [Google Scholar]
- 117.Leach N.V., Dronca E., Vesa S.C., Sampelean D.P., Craciun E.C., Lupsor M., Crisan D., Tarau R., Rusu R., Para I., et al. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur. J. Intern. Med. 2014;25:762–767. doi: 10.1016/j.ejim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 118.Pastore A., Alisi A., Di Giovamberardino G., Crudele A., Ceccarelli S., Panera N., Dionisi-Vici C., Nobili V. Plasma Levels of Homocysteine and Cysteine Increased in Pediatric NAFLD and Strongly Correlated with Severity of Liver Damage. Int. J. Mol. Sci. 2014;15:21202–21214. doi: 10.3390/ijms151121202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hirsch S., Poniachick J., Avendaño M., Csendes A., Burdiles P., Smok G., Díaz J.C., De La Maza M.P. Serum folate and homocysteine levels in obese females with non-alcoholic fatty liver. Nutrition. 2005;21:137–141. doi: 10.1016/j.nut.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 120.Polyzos S.A., Kountouras J., Patsiaoura K., Katsiki E., Zafeiriadou E., Deretzi G., Zavos C., Gavalas E., Katsinelos P., Mane V., et al. Serum homocysteine levels in patients with nonalcoholic fatty liver disease. Ann. Hepatol. 2012;11:68–76. doi: 10.1016/S1665-2681(19)31488-7. [DOI] [PubMed] [Google Scholar]
- 121.Wang X., Zhou Y., Zhang M., Wang Y., Qin B. The methylenetetrahydrofolate reductase genotype 677CT and non-alcoholic fatty liver disease have a synergistic effect on the increasing homocysteine levels in subjects from Chongqing, China. Genes Dis. 2019;6:88–95. doi: 10.1016/j.gendis.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dai Y., Zhu J., Meng D., Yu C., Li Y. Association of homocysteine level with biopsy-proven non-alcoholic fatty liver disease: A meta-analysis. J. Clin. Biochem. Nutr. 2016;58:76–83. doi: 10.3164/jcbn.15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu Y., Guan Y., Yang X., Xia Z., Wu J. Association of Serum Homocysteine Levels with Histological Severity of NAFLD. J. Gastrointest. Liver Dis. 2020;29:51–58. doi: 10.15403/jgld-529. [DOI] [PubMed] [Google Scholar]
- 124.Hortin G.L. Homocysteine: Clinical Significance and Laboratory Measurement. Lab. Med. 2006;37:551–553. doi: 10.1309/93G5JG1BF44N65BQ. [DOI] [Google Scholar]
- 125.Elshorbagy A.K., Nurk E., Gjesdal C.G., Tell G.S., Ueland P.M., Nygård O., Tverdal A., E Vollset S., Refsum H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: Does cysteine link amino acid and lipid metabolism? Am. J. Clin. Nutr. 2008;88:738–746. doi: 10.1093/ajcn/88.3.738. [DOI] [PubMed] [Google Scholar]
- 126.Satyanarayana A., Balakrishna N., Pitla S., Reddy P.Y., Mudili S., Lopamudra P., Suryanarayana P., Viswanath K., Ayyagari R., Reddy G.B. Status of B-Vitamins and Homocysteine in Diabetic Retinopathy: Association with Vitamin-B12 Deficiency and Hyperhomocysteinemia. PLoS ONE. 2011;6:e26747. doi: 10.1371/journal.pone.0026747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li T., Chen Y., Li J., Yang X., Zhang H., Qin X., Hu Y., Mo Z. Serum Homocysteine Concentration Is Significantly Associated with Inflammatory/Immune Factors. PLoS ONE. 2015;10:e0138099. doi: 10.1371/journal.pone.0138099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ji Y., Nordgren K.K.S., Chai Y., Hebbring S.J., Jenkins G.D., Abo R.P., Peng Y., Pelleymounter L.L., Moon I., Eckloff B.W., et al. Human Liver Methionine Cycle: MAT1A and GNMT Gene Resequencing, Functional Genomics, and Hepatic Genotype-Phenotype Correlation. Drug Metab. Dispos. 2012;40:1984–1992. doi: 10.1124/dmd.112.046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frosst P., Blom H., Milos R., Goyette P., Sheppard C., Matthews R., Boers G., Heijer M.D., Kluijtmans L., Heuve L.V.D., et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 130.Calderón-Larrañaga A., Saadeh M., Hooshmand B., Refsum H., Smith A.D., Marengoni A., Vetrano D.L. Association of Homocysteine, Methionine, and MTHFR 677C>T Polymorphism With Rate of Cardiovascular Multimorbidity Development in Older Adults in Sweden. JAMA Netw. Open. 2020;3:e205316. doi: 10.1001/jamanetworkopen.2020.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun M.-Y., Zhang L., Shi S.-L., Lin J.-N. Associations between Methylenetetrahydrofolate Reductase (MTHFR) Polymorphisms and Non-Alcoholic Fatty Liver Disease (NAFLD) Risk: A Meta-Analysis. PLoS ONE. 2016;11:e0154337. doi: 10.1371/journal.pone.0154337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J., Huff A., Spence J.D., Hegele R. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin. Genet. 2004;65:483–486. doi: 10.1111/j.1399-0004.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 133.Avila M.A., Berasain C., Torres L., Martín-Duce A., Corrales F.J., Yang H., Prieto J., Lu S.C., Caballería J., Rodés J., et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J. Hepatol. 2000;33:907–914. doi: 10.1016/S0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 134.Ruiz García-Tevijano E., Berasain C., Rodríguez J.A., Corrales F.J., Arias R., Martín-Duce A., Caballería J., Mato J.M., Avila M.A. Hyperhomocysteinemia in liver cirrhosis: Mechanisms and role in vascular and hepatic fibrosis. Hypertension. 2001;38:1217–1221. doi: 10.1161/hy1101.099499. [DOI] [PubMed] [Google Scholar]
- 135.Murphy S.K., Yang H., Moylan C.A., Pang H., Dellinger A., Abdelmalek M.F., Garrett M.E., Ashley–Koch A., Suzuki A., Tillmann H.L., et al. Relationship Between Methylome and Transcriptome in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2013;145:1076–1087. doi: 10.1053/j.gastro.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang C., Han J., Xiao L., Jin C.-E., Li D.-J., Yang Z. Role of hydrogen sulfide in portal hypertension and esophagogastric junction vascular disease. World J. Gastroenterol. 2014;20:1079–1087. doi: 10.3748/wjg.v20.i4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jain S.K., Bull R., Rains J.L., Bass P.F., Levine S.N., Reddy S., McVie R., Bocchini J.A. Low Levels of Hydrogen Sulfide in the Blood of Diabetes Patients and Streptozotocin-Treated Rats Causes Vascular Inflammation? Antioxid. Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Whiteman M., Gooding K.M., Whatmore J.L., Ball C.I., Mawson D., Skinner K., Tooke J.E., Shore A.C. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 139.Gaggini M., Carli F., Bugianesi E., Gastaldelli A., Rosso C., Buzzigoli E., Marietti M., Della Latta V., Ciociaro D., Abate M.L., et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology. 2018;67:145–158. doi: 10.1002/hep.29465. [DOI] [PubMed] [Google Scholar]
- 140.García-Cañaveras J.C., Donato M.T., Castell J.V., Lahoz A. A comprehensive untargeted metabonomic analysis of human steatotic liver tissue by RP and HILIC chromatography coupled to mass spectrometry reveals important metabolic alterations. J. Proteome Res. 2011;10:4825–4834. doi: 10.1021/pr200629p. [DOI] [PubMed] [Google Scholar]
- 141.Lutchmansingh F.K., Hsu J.W., Bennett F.I., Badaloo A.V., McFarlane-Anderson N., Gordon-Strachan G.M., Wright-Pascoe R.A., Jahoor F., Boyne M.S. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE. 2018;13:e0198626. doi: 10.1371/journal.pone.0198626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Honda Y., Kessoku T., Sumida Y., Kobayashi T., Kato T., Ogawa Y., Tomeno W., Imajo K., Fujita K., Yoneda M., et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017;17:96. doi: 10.1186/s12876-017-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Giustarini D., Tsikas D., Colombo G., Milzani A., Dalle-Donne I., Fanti P., Rossi R. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: An elephant in the room. J. Chromatogr. B. 2016;1019:21–28. doi: 10.1016/j.jchromb.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li H., Wang L., Yan X., Liu Q., Yu C., Wei H., Li Y., Zhang X., He F., Jiang Y. A Proton Nuclear Magnetic Resonance Metabonomics Approach for Biomarker Discovery in Nonalcoholic Fatty Liver Disease. J. Proteome Res. 2011;10:2797–2806. doi: 10.1021/pr200047c. [DOI] [PubMed] [Google Scholar]
- 145.Lake A.D., Novak P., Shipkova P., Aranibar N., Robertson D., Reily M.D., Lu Z., Lehman-McKeeman L.D., Cherrington N.J. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol. Appl. Pharmacol. 2013;268:132–140. doi: 10.1016/j.taap.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han J., Dzierlenga A.L., Lu Z., Billheimer D.D., Torabzadeh E., Lake A.D., Li H., Novak P., Shipkova P., Aranibar N., et al. Metabolomic profiling distinction of human nonalcoholic fatty liver disease progression from a common rat model. Obesity. 2017;25:1069–1076. doi: 10.1002/oby.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yun K.U., Ryu C.S., Oh J.M., Kim C.H., Lee K.S., Lee C.-H., Lee H.-S., Kim B.-H., Kim S.K. Plasma homocysteine level and hepatic sulfur amino acid metabolism in mice fed a high-fat diet. Eur. J. Nutr. 2012;52:127–134. doi: 10.1007/s00394-011-0294-0. [DOI] [PubMed] [Google Scholar]
- 148.Ferro Y., Carè I., Mazza E., Provenzano F., Colica C., Torti C., Romeo S., Pujia A., Montalcini T. Protein and vitamin B6 intake are associated with liver steatosis assessed by transient elastography, especially in obese individuals. Clin. Mol. Hepatol. 2017;23:249–259. doi: 10.3350/cmh.2017.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Z., Li P., Zhao Z.-H., Zhang Y., Ma Z.-M., Wang S.-X. Vitamin B6 Prevents Endothelial Dysfunction, Insulin Resistance, and Hepatic Lipid Accumulation in Apoe -/- Mice Fed with High-Fat Diet. J. Diabetes Res. 2016;2016:1748065. doi: 10.1155/2016/1748065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kaimoto T., Shibuya M., Nishikawa K., Maeda H. High incidence of lipid deposition in the liver of rats fed a diet supplemented with branched-chain amino acids under vitamin B6 deficiency. J. Nutr. Sci. Vitaminol. 2013;59:73–78. doi: 10.3177/jnsv.59.73. [DOI] [PubMed] [Google Scholar]
- 151.Mayengbam S., Raposo S., Aliani M., House J.D. Oral exposure to the anti-pyridoxine compound 1-amino d-proline further perturbs homocysteine metabolism through the transsulfuration pathway in moderately vitamin B6 deficient rats. J. Nutr. Biochem. 2015;26:241–249. doi: 10.1016/j.jnutbio.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 152.Mascolo E., Vernì F. Vitamin B6 and Diabetes: Relationship and Molecular Mechanisms. Int. J. Mol. Sci. 2020;21:3669. doi: 10.3390/ijms21103669. [DOI] [PMC free article] [PubMed] [Google Scholar]