Abstract
Aim:
The use of recombinant human platelet-derived growth factor–BB (rhPDGF) has received Food and Drug Administration approval for the treatment of periodontal and orthopedic bone defects and dermal wound healing. Many studies have investigated its regenerative potential in a variety of other oral clinical indications. The aim of this systematic review was to assess the efficacy, safety, and clinical benefit of recombinant human platelet-derived growth factor (rhPDGF) use for alveolar bone and/or soft tissue regeneration.
Material and Methods:
Comprehensive electronic and manual literature searches according to the PRISMA guidelines were performed to identify interventional and observational studies evaluating the regenerative applications of rhPDGF-BB. The primary outcomes were the safety, efficacy, and overall clinical benefit of rhPDGF use in oral regenerative procedures.
Results:
Sixty-three human clinical studies (mean ± SD follow-up period of 10.7 ± 3.3 mo) were included in the qualitative analysis. No serious adverse effects were reported in any of the 63 studies, aside from the postoperative complications routinely associated with surgical therapy. Use of rhPDGF was shown to be beneficial when combined with allografts, xenografts, and alloplasts (the latter tricalcium phosphate [β-TCP]) for the treatment of periodontal defects and gingival recession. The use of rhPDGF also led to favorable clinical outcomes when combined with allografts or xenografts for guided bone regeneration (GBR) and alveolar ridge preservation. While favorable clinical results support the use of the combination of rhPDGF plus allograft or xenograft for GBR, ARP, and sinus floor augmentation, current data support the use of rhPDGF and alloplasts (e.g., β-TCP) only in periodontal defects and gingival recession.
Conclusions:
Based on the clinical evidence, rhPDGF is safe and provides clinical benefits when used in combination with bone allografts, xenograft, or β-TCP for the treatment of intrabony and furcation periodontal defects and gingival recession or when used with allografts or xenograft for GBR and ARP (PROSPERO CRD42020142446).
Knowledge Transfer Statement:
Clinicians should be aware that rhPDGF is a safe and effective approach for the treatment of intrabony and furcation periodontal defects and gingival recession or when used with allografts or xenograft for bone regeneration and alveolar ridge preservation. With consideration of cost and patient preference, this result could lead to more appropriate therapeutic decisions.
Keywords: regenerative medicine, gingival recession, biologics, periodontal regeneration, bone regeneration
Introduction
Tissue regeneration currently requires 3 main components: cells, scaffolds (matrices), and signaling molecules such as growth factors. These components, with sufficient vascularization, wound stability, and time, each play an important role in regeneration. The introduction of growth factors has represented a new era in wound healing and periodontal and bone regeneration in medicine and dentistry (Pilipchuk et al. 2018; Vaquette et al. 2018). The rationale behind the use of these natural biological mediators is to regulate crucial cellular events involved in tissue repair, including DNA synthesis, cell replication, chemotaxis, differentiation, matrix synthesis, and tissue vascularization (Giannobile and Somerman 2003). Among them, recombinant human platelet-derived growth factor–BB (rhPDGF-BB) is the most extensively investigated growth factor for the promotion of wound repair (Khoshkam et al. 2015). The role of PDGF-AA, PDGF-AB, and PDGF-BB signaling has been well characterized in the development of anatomic structures, such as cranial and cardiac neural crest, lung, intestine, and skeleton, and in vasculogenesis (Andrae et al. 2008). In a dog periodontal defect model, platelet-derived growth factor (PDGF) was first shown to promote bone regeneration by Lynch and coworkers (Lynch et al. 1989; Lynch et al. 1991). Thereafter, multiple studies have evaluated the efficacy of recombinant human platelet-derived growth factor (rhPDGF) in periodontal and peri-implant bone regeneration (Camelo et al. 2003; Nevins et al. 2005). Mechanistic studies have shown that periodontal ligament (PDL) fibroblasts, osteoblasts, bone marrow–derived stem cells, and pericytes express multiple receptors (α, β, χ, δ) for PDGFs, which enhances the proliferation and chemotaxis of these cells (Cho et al. 1995; Park et al. 1995). Boyan et al. (1994) investigated the effect of the various isoforms of PDGF (PDGF-AA, PDGF-AB, and PDGF-BB) on the mitogenic and chemotactic responses of PDL fibroblasts, concluding that rhPDGF-BB was the most potent one. In 2005, the use of purified rhPDGF with an osteoconductive matrix, tricalcium phosphate (β-TCP), received Food and Drug Administration approval for its use in the treatment of osseous periodontal defects and associated gingival recession (GR). This combination of rhPDGF with a bone substitute appeared to act physically as an osteoconductive 3-dimensional matrix or scaffold, which was enhanced by the chemotactic, mitogenic, and angiogenic properties of rhPDGF, leading to improved wound healing, osteogenesis, and defect resolution (Nevins et al. 2005). Indeed, in a large multicenter randomized clinical trial, rhPDGF + β-TCP was able to significantly increase the rate of clinical attachment level (CAL) gain, reduce GR, and improve bone fill (BF) and defect resolution as compared with the positive control (TCP matrix alone), without eliciting any serious adverse events (Nevins et al. 2005). The clinical outcomes were found to be stable over the long term (Nevins et al. 2013). Additional clinical and human histologic studies evaluated the response to rhPDGF plus allograft or xenograft at the cellular and tissue level and showed histologic evidence of the new bone, cementum, and PDL following the use of rhPDGF + mineralized or demineralized freeze-dried bone allograft, rhPDGF + anorganic bone xenograft, or rhPDGF + β-TCP in infrabony and furcation defects (Camelo et al. 2003; Ridgway et al. 2008; Thakare and Deo 2012).
These studies demonstrated for the first time that rhPDGF can be safely and effectively used with different bone grafts to treat periodontal osseous defects. Due to its demonstrated regenerative potential, rhPDGF has been studied not only in intrabony or furcation defects but also in alveolar ridge preservation (ARP; Wallace et al. 2013), maxillary sinus augmentation (Kubota et al. 2017), guided bone regeneration (GBR; Urban et al. 2013; Lee 2017), and soft tissue augmentation and root coverage in periodontal plastic surgery (McGuire et al. 2009; Zadeh 2011). rhPDGF has also been studied in combination with a variety of bone graft materials in different clinical indications, including autogenous bone, xenogeneic bone, and allogeneic bone. Given the abundance of the literature evaluating rhPDGF in combination with various matrices/scaffolds and in multiple indications, a systematic review of the literature is appropriate. Therefore, the aim of this systematic review was to assess the safety, efficacy, and clinical benefits of rhPDGF in orofacial bone and soft tissue regeneration, by answering the following focused questions: 1) Is rhPDGF safe when used with autograft, allograft, xenograft, or synthetic grafts? 2) What are the outcomes of rhPDGF in combination with different graft materials for periodontal regeneration, soft tissue augmentation and root coverage, GBR, ARP, and/or sinus floor augmentation?
Material and Methods
Study Registration and Reporting Format
The protocol of the present review was registered and allocated the identification number CRD42020142446 in the PROSPERO International Prospective Register of Systematic Reviews database, hosted by the National Institute for Health Research, University of York, Center for Reviews and Dissemination (www.crd.york.ac.uk/PROSPERO). This systematic review and meta-analysis was prepared in consideration of the 27-item PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines (Moher et al. 2009) and the Cochrane Handbook (Higgins et al. 2011; Higgins et al. 2019).
Details regarding the PICO question (patient, intervention, comparison, outcome) are reported in the Appendix.
Type of Studies and Participants
Owing to the amplitude of procedures and potential regenerative applications of rhPDGF, interventional studies (i.e., randomized controlled trials [RCTs]) and observational studies (i.e., cohort and case-control studies and case series) were considered eligible for inclusion. Articles were included if they specifically involved the following: 1) treatment of systemically healthy patients who underwent oral bone, soft tissue, or periodontal regeneration therapy with rhPDGF; 2) adult patients (≥18 y old); and 3) at least 3 mo of follow-up for periodontal plastic surgery and GBR, at least 2 mo for ARP, at least 5 mo for sinus floor augmentation, and at least 6 mo for infrabony and furcation defects. Exclusion criteria included animal studies, in vitro studies, and reviews.
Outcome Measures
The following parameters were evaluated for each surgical procedure as available:
Clinical applicability: whether rhPDGF was used alone or in combination with other scaffold materials
Efficacy: which treatment group achieved the greatest or lowest clinical outcomes
Safety: whether minor or major complications were reported following the treatment with rhPDGF. Normal expected sequelae related to the surgical procedure were considered minor adverse effects (e.g., pain/discomfort, bleeding, swelling), while complications not likely to be due to the procedure but to the growth factor or the graft material were recorded as major adverse events (e.g., headache, fatigue, nausea, malaise, rashes, muscle tremor, allergic reactions; Nevins et al. 2005).
Histologic outcomes: if reported, in terms of healing by regeneration or by repair
Site- and patient-centered outcome measures were evaluated in the review. Site-centered outcomes included change in clinical parameters—specifically, clinical (tissue) attachment level, probing depth (PD), recession depth, keratinized tissue width, linear radiographic BF (linear or volumetric), and bone core histology. Patient-centered outcomes included parameters such as adverse effects (i.e., pain, bleeding, swelling, graft failure, dehiscence, and other reported adverse effects), patient preferences, and aesthetics.
Information Sources and Screening Process
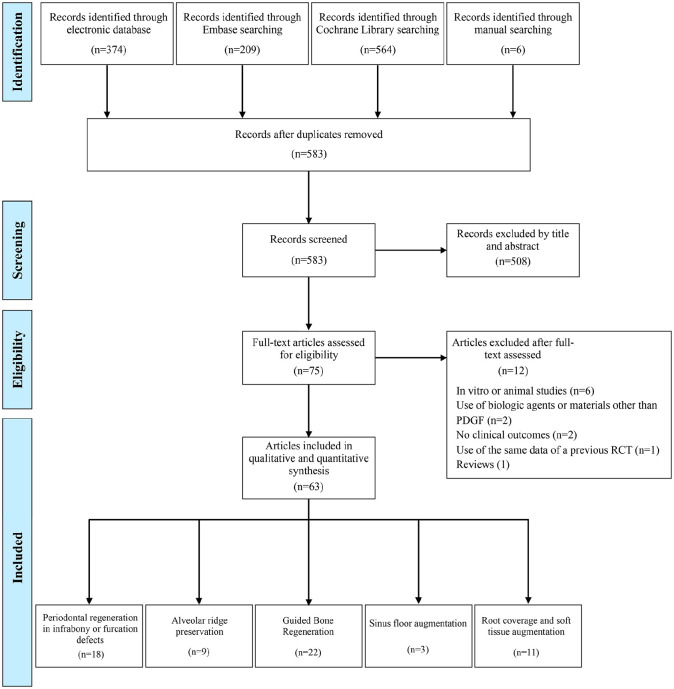
Electronic and manual literature searches were conducted by 2 independent reviewers (L.T. and A.R.). These studies included peer-reviewed publications up to and including June 2019 across the National Library of Medicine (MEDLINE by PubMed), Embase, the Cochrane Oral Health Group Trials Register, and the gray literature (Figure). The complete search strategy and data extraction are detailed in the Appendix. Moreover, reference lists of the included studies and previous reviews were screened in an attempt to identify potentially relevant articles not identified by electronic searching.
Figure.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flowchart. PDGF, platelet-derived growth factor; RCT, randomized controlled trial.
Data extraction and quality and risk-of-bias assessment are described in detail in the Appendix.
Data Analysis
Descriptive statistics were used to present the outcomes, safety, and efficacy of rhPDGF in each of the investigated approaches among the selected studies. When possible, the retrieved data from the original articles were entered into prefabricated spreadsheets, grouped by the method and approach of utilization of rhPDGF, and checked for consistency. Weighted means (based on the treated sample size) with standard deviations were calculated for each category based on the intent of rhPDGF utilization.
Results
Search Results and Study Selection
The Figure displays the search process for the selection of the included studies. Throughout the electronic systematic search, 583 potentially eligible records were found. Subsequent to duplicate removal and screening by titles and abstract, 75 records eventually remained, which were subjected to the full-text assessment. Following the evaluation of the individual texts and deep read of the full articles, 12 studies were excluded due to study design (in vitro or animal study), use of biologic agents or materials other than rhPDGF, no reporting of clinical outcomes, or being noncompatible with the study protocol. Full details on the excluded references are presented in Appendix Table 1. Therefore, 63 studies were included in the present review and reserved for data extraction (Appendix). Authors of missing data were contacted; however, no responses to the queries were received. Interexaminer agreement throughout the initial screening and inclusion by full-text assessment was excellent (kappa scores of 0.93, and 0.97, respectively).
Characteristics of the included articles are discussed in the Appendix.
Treatment Outcomes of Infrabony Defects with rhPDGF
Clinical Applicability
Sixteen studies (564 patients and 642 defects) were identified that reported on the regenerative outcomes of rhPDGF–treated infrabony defects: 12 RCTs, 3 prospective case series, and 1 retrospective study (Appendix Table 2). The use of rhPDGF has been investigated alone (without any scaffold) and combined with β-tricalcium phosphate (β-TCP); demineralized freeze-dried bone allograft (DFDBA); mineralized freeze-dried bone allograft; bovine-, porcine-, or equine-derived xenograft; hydroxyapatite (HA) stem cells; and β-TCP or recombinant human insulin-like growth factor–I (rhIGF-I). The amount of evaluated rhPDGF varied from 50 µg/mL to 5 mg/mL. Population, study characteristics, and the interventions are depicted in Appendix Table 2.
Efficacy
Among the reported outcomes from the studies, treatment with a combination of rhPDGF + allogeneic bone graft (AlBG) or rhPDGF + xenogeneic bone graft (XBG) showed the greatest CAL gain (mean ± SD: 5.9 ± 1.8 mm and 5.5 ± 1.7 mm, respectively), while treatment with open flap surgery alone showed a CAL gain of only 2.6 ± 0.4 mm. Similarly, the greatest PD reductions were observed with rhPDGF + AlBG and rhPDGF + XBG. In all trials, the control groups (open flap debridement surgery alone [i.e., without graft] or bone grafts without rhPDGF) had the poorest results in terms of PD reduction. A slight increase in GR was observed for all groups (ranging from 0.3 to 0.6 mm), except for rhPDGF + AlBG, which showed a mean improvement of 0.1 mm. The greatest linear bone gain was observed with rhPDGF + XBG (5.9 ± 3.6 mm), while rhPDGF and open flap surgery alone achieved 1.9 ± 1 mm and 1.8 ± 0.4 mm, respectively. rhPDGF + β-TCP was the group with the highest BF at 71.7% ± 18.1%. rhPDGF alone, HA + β-TCP, and β-TCP showed comparable BF, ranging from 48.5% to 54.1%, while open flap surgery alone showed the least BF (35% ± 11.3%). Table 1 illustrates the clinical outcomes for each treatment group.
Table 1.
Outcomes of Infrabony Defects Treated with rhPDGF Combined with a Scaffold Matrix.
| Mean ± SD | |||||
|---|---|---|---|---|---|
| Treatment Group (No. of Arms) | PD Reduction, mm | REC Increase, % | CAL Gain, mm | Linear Bone Growth, mm | Bone Fill, % |
| β-TCP (4) | 3.7 ± 0.7 | 0.5 ± 0 | 3.2 ± 0.6 | 2.2 ± 0.9 | 48.5 ± 9 |
| Flap (2) | 3.7 ± 0.2 | 0.6 ± 0 | 2.6 ± 0.4 | 1.8 ± 0.4 | 35 ± 11.3 |
| HA + β-TCP (1) | 3.7 ± 0.3 | 0.3 ± 0 | 2.1 ± 0.6 | 2.3 ± 1.1 | 54.1 ± 8.5 |
| rhPDGF-BB (1) | 4.1 ± 0.6 | 0.8 ± 0.6 | 3 ± 0.9 | 1.9 ± 1 | 50 ± 6.7 |
| + AlBG (3) | 6.1 ± 1.1 | −0.1 ± 0.5 | 5.9 ± 1.8 | 2.1 ± 0.8 | NA |
| + β-TCP (13) | 4.8 ± 1.7 | 0.4 ± 0.5 | 3.6 ± 1.3 | 3.5 ± 1.6 | 71.7 ± 18.1 |
| + XBG (1) | 5 ± 1.7 | NA | 5.5 ± 1.7 | 5.9 ± 3.6 | NA |
| + β-TCP + stem cells (1) | 4.5 ± 1.1 | NA | NA | 3.6 ± 1.1 | NA |
AlBG, allogeneic bone graft; β-TCP, β-tricalcium phosphate; CAL, clinical attachment level; HA, hydroxyapatite; NA, not available/applicable; PD, probing depth; REC, gingival recession; rhPDGF-BB, recombinant human platelet-derived growth factor–BB; XBG, xenogeneic bone graft.
Safety
No serious adverse effects were reported in the included studies when rhPDGF was used alone or in combination with any grafts/scaffolds. One study described minor adverse effects, such as fever, pain, swelling, and tooth mobility, for the test group (rhPDGF + β-TCP) and control group (β-TCP), without statistically significant differences (Jayakumar et al. 2011). Pain and headache were also reported as possible postoperative complications (Nevins et al. 2005).
Histologic Outcomes
Two clinical studies also reported on the histologic outcomes of rhPDGF for the treatment of infrabony defects, showing complete regeneration of the periodontium, including new cementum, PDL, and alveolar bone in 4 cases out of 6 (Nevins et al. 2003) and in 13 of 16 specimens (Ridgway et al. 2008). Ridgway et al. (2008) observed healing with new attachment or with long junctional epithelium in the 3 cases in which periodontal regeneration was not achieved.
Patient-Reported Outcomes
Patient-reported outcome measures were reported by only 1 study. This study showed that when rhPDGF and β-TCP were used with the single-flap surgical approach, less patient morbidity in terms of self-reported pain and consumption of analgesics was encountered as compared with the double-flap surgical approach (Schincaglia et al. 2015).
Treatment Outcomes of Furcation Defects with rhPDGF-BB
Clinical Applicability
Four studies (53 patients and 88 defects) investigated the regenerative outcomes of furcation defects: 3 case series and 1 RCT. Three studies investigated class II furcations, while 1 was on class III furcation defects. The tested treatment options were rhPDGF + DFDBA, a combination of rhPDGF (at different concentrations) with rhIGF-I, and rhPDGF + β-TCP + collagen membrane. Population, study characteristics, and interventions are depicted in Appendix Table 3.
Efficacy
rhPDGF + DFDBA and rhPDGF + β-TCP + collagen matrix (CM) showed a similar weighted average vertical PD reduction (4.1 ± 1.1 mm and 4.03 ± 1.9 mm, respectively), while the greatest reduction in the horizontal PD was observed with rhPDGF + DFDBA (3.5 ± 0.6 mm).
rhPDGF + DFDBA was associated with an increase in GR of 0.6 ± 1.6 mm and a CAL gain of 3.4 ± 1.6 mm, while rhPDGF + β-TCP + CM showed an increased in GR of 0.97 ± 1 mm and a CAL gain of 3.3 ± 1.9 mm. When rhPDGF + rhIGF-I was compared with open flap debridement surgery alone (with or without placebo), the concentration of 50-µg/mL rhPDGF + rhIGF-I showed the highest linear bone gain (2 ± 0.5 mm) and BF (42.3% ± 9%) as compared with 150-µg/mL rhPDGF + rhIGF-I and open flap surgery alone.
Safety
No serious adverse effects were reported in the included studies when rhPDGF was used alone or in combination with other graft/scaffold materials.
Histologic Outcomes
Regeneration of new bone, cementum, and PDL coronal to the reference notch was noted histologically in all rhPDGF-treated sites (Camelo et al. 2003), which replicated findings in another study (Nevins et al. 2003). One study observed periodontal regeneration in 3 cases, new attachment in 3 sites, and healing with long junctional epithelium in 1 site (Mellonig et al. 2009). Table 2 presents the clinical and histologic outcomes of rhPDGF in the treatment of furcation defects.
Table 2.
Outcomes of Furcation Defects Treated with rhPDGF and a Scaffold Matrix.
| Mean ± SD | |||||||
|---|---|---|---|---|---|---|---|
| Treatment Group (No. of Arms) | PD Reduction Vertical, mm | PD Reduction Horizontal, mm | REC Increase, % | CAL Gain, mm | Linear Bone Growth, mm | Bone Fill, % | Periodontal Regeneration, %a |
| rhPDGF + DFDBA (2) | 4.1 ± 1.1 | 3.5 ± 0.6 | 0.6 ± 1.6 | 3.4 ± 1.6 | NA | NA | 100 |
| rhPDGF (50 µg/mL) + rhIGF-I (1) | NA | 0.7 ± 1.2 | NA | NA | 2 ± 0.5 | 42.3 ± 9 | NA |
| rhPDGF (150 µg/mL) + rhIGF-I (1) | NA | 2 ± 1.1 | NA | NA | 0.8 ± 1.5 | 25 ± 7 | NA |
| rhPDGF + β-TCP + CM (1) | 4.03 ± 1.9 | NA | 0.97 ± 1 | 3.3 ± 1.9 | NA | NA | 42.9 |
| OFD (1) | NA | 1.8 ± 1.4 | NA | NA | 0.9 ± 1.3 | 18.5 ± 7 | NA |
β-TCP, β-tricalcium phosphate; CAL, clinical attachment level; CM, collagen membrane; DFDBA, demineralized freeze-dried bone allograft; NA, not available/applicable; OFD, open flap debridement; PD, probing depth; REC, gingival recession; rhIGF-I, recombinant human insulin-like growth factor–I; rhPDGF, recombinant human platelet-derived growth factor.
Histologic evidence of periodontal regeneration.
Treatment Outcomes of ARP with rhPDGF-BB
Clinical Applicability
The clinical applicability of rhPDGF in extraction site defects for ARP was evaluated in 9 clinical studies, including case series and RCTs (158 patients and 163 sites). The rhPDGF solution and the bone graft materials were consistently formulated chair-side with a ratio of 0.5 or 1.0 mL of 0.3-mg/mL rhPDGF per 1 g of bone matrix and allowed to sit for 10 to 15 min after mixing before implantation into the patients. Population, study characteristics, and the interventions of the referenced studies are presented in Appendix Table 4.
Efficacy
Clinical efficacy (volumetric changes) of the application of rhPDGF in ridge preservation has rarely been reported, as the main criterion of regeneration of the published articles has been the histologic evaluation of new vital bone based on bone core specimens prior to implant installation.
Safety
None of the included articles reported any complications associated with the use of rhPDGF.
Histologic Outcomes
In an RCT including 8 patients, histomorphometric analysis showed nonsignificant trends for greater new bone formation in the rhPDGF group (Nevins et al. 2014). A case series showed greater bone formation when rhPDGF was added to mineralized allograft as compared with the use of mineralized allograft alone (41.8 vs 32.5%; Wallace et al. 2013). McAllister and coworkers (2010) performed a histologic evaluation of 12 premolar extraction sockets treated with rhPDGF combined with either xenograft (collagen containing anorganic deproteinized bovine bone) or alloplast (TCP). Similar percentages of vital bone (21% and 24%) were found in both groups 3 mo after the socket augmentation procedure, suggesting that rhPDGF in combination with the xenograft or the alloplast performed similarly. Last, an RCT comparing the healing of grafted or nongrafted sockets and the effect of platelet-rich plasma and rhPDGF showed new bone to be consistently greater in the control group (Geurs et al. 2014).
Treatment Outcomes of GBR with rhPDGF
Clinical Applicability
Twenty-two clinical studies (281 patients and 425 sites) evaluated the use of rhPDGF in combination with a variety of bone graft material for horizontal and vertical GBR, including β-TCP, anorganic bovine bone matrix (ABBM), xenograft, and autogenous bone. In addition, some authors utilized resorbable membrane, nonresorbable membrane, or titanium mesh. Most of the included articles were case reports and case series, while 3 were RCTs. Details regarding the population, study characteristics, and interventions are depicted in Appendix Table 5.
Efficacy
A study comparing rhPDGF + TCP with the autogenous bone graft (gold standard) found that the 2 treatment groups resulted in similar outcomes in all the investigated parameters (Santana and Santana 2015). A wide range of results is reported regarding the use of rhPDGF in GBR procedures. Interstudy differences include the use of rhPDGF combined with different biomaterials, bone augmentation techniques, or study methodologies, which make comparisons of efficacy among studies challenging. In particular, the effect of rhPDGF on bone regeneration cannot be demonstrated by case series, and 1 RCT of 3 used rhPDGF in the test and control groups (Amorfini et al. 2014). An RCT aimed at evaluating the efficacy of GBR with rhPDGF + β-TCP versus autogenous bone grafting demonstrated similar clinical outcomes for both groups (Santana and Santana 2015), while another RCT from the same group showed that the use of rhPDGF + β-TCP and GBR in immediate implants in molars was as successful as conventional implant therapy in fully healed extraction sites (Santana et al. 2015).
Safety
No complications related with the use of rhPDGF were found. Postsurgical complications were minor and related to the bone augmentation procedures.
Histologic Outcomes
Limited information is available on histologic outcomes of the use of rhPDGF in bone augmentation procedures. Examination of a biopsy specimen from a case report by Simion and coworkers (2007) demonstrated newly formed bone surrounding bovine xenograft particles. The augmented area showed areas of ongoing bone remodeling with alternately occurring demineralization and remineralization. Bone remodeling with replacement of graft particles with newly formed vital bone was seen with confocal laser scanning microscopy 3 mo after the bone augmentation procedure with rhPDGF in combination with an equine xenograft bone block (De Angelis and Scivetti 2011).
Treatment Outcomes of Sinus Floor Augmentation with rhPDGF
Clinical Applicability
All 3 included articles investigated the use of rhPDGF in addition to ABBM xenograft. A variety of formulation amounts and times were used (Nevins et al. 2009; Froum et al. 2013; Kubota et al. 2017; Appendix Table 6).
Efficacy
More rapid formation of vital bone in patients treated with ABBM plus rhPDGF may allow for earlier implant placement (Froum et al. 2013). Kubota et al. (2017) reported that rhPDGF + ABBM reduced the healing period to 4 mo and resulted in a vertical bone height of 13.03 ± 1.22 mm. Nevins and coworkers (2009) also showed an efficient replacement of the ABBM particles with newly formed bone at 6 to 8 mo.
Safety
Among the included articles, only 1 study reported minor complications. No complications related with the use of rhPDGF were found (Kubota et al. 2017).
Histologic Outcomes
An RCT reported that the mean vital bone was 11.8% in ABBM alone and 21.1% for ABBM + rhPDGF (P < 0.05) after 4 to 5 mo, while the differences in vital bone were not more significant after 7 to 9 mo from the surgery (Froum et al. 2013). A study involving 10 patients treated with ABBM xenograft plus rhPDGF showed that 7 specimens demonstrated robust histologic and micro–computed tomography (micro-CT) evidence of new bone formation and resorption of ABBM, while the other specimens exhibited significant bone regeneration but with unresorbed matrix (Nevins et al. 2009).
Treatment Outcomes of Root Coverage Procedures and Soft Tissue Augmentation with rhPDGF
Clinical Applicability
rhPDGF was evaluated in 11 studies for root coverage purposes in combination with a CM and a small amount of β-TCP, a CM and β-TCP, an acellular dermal matrix (ADM), and the connective tissue graft (CTG). One article reported the use of rhPDGF + CM for soft tissue augmentation. The surgical techniques performed in combination with rhPDGF include the coronally advanced flap and vestibular incision subperiosteal tunnel access technique. RCTs and non-RCTs used ADM, CTG, CM, or open flap surgery alone as the control group. Single and multiple GRs were investigated (330 recession defects in 166 patients). rhPDGF was used at a concentration of 0.3 or 0.5 mg/mL. Most of the available studies reported data ≤12 mo, while 1 study showed the long-term outcomes (5 y) of rhPDGF + β-TCP (McGuire et al. 2014). Details are presented in Appendix Table 7.
Efficacy
The greatest calculated improvement in GR among the studies was obtained with rhPDGF + CM (3.6 ± 0.54 mm), followed by rhPDGF + CTG (2.7 ± 0.14 mm), rhPDGF + ADM (2.33 ± 0.28 mm), rhPDGF + CM + β-TCP (2.29 ± 0.22 mm), and rhPDGF + β-TCP (1.92 ± 0.48 mm). The CTG showed a mean recession reduction (Rec Red) of 2.48 ± 0.63 mm, as compared with 2.28 ± 0.37 mm for ADM. The lowest Rec Red was obtained with CM alone (1.29 ± 0.46 mm). Similarly, rhPDGF + CM showed a mean root coverage (mRC) of 87.4% ± 10.8%, while rhPDGF + CTG and CTG achieved an mRC of 88.7% ± 12.8% and 86.7% ± 13.4%, respectively. The greatest keratinized tissue gain was achieved by rhPDGF + CM and rhPDGF + CTG (3.6 ± 0.4 mm and 2.4 ± 0.3 mm, respectively). rhPDGF + CM also showed the greatest CAL gain (3.75 ± 0.4 mm). Table 3 summarizes the mean Rec Red, mRC, keratinized tissue gain, and CAL gain for each treatment group.
Table 3.
Root Coverage Outcomes with rhPDGF Combined with a Scaffold Matrix, Soft Tissue Graft, and/or a Barrier Membrane.
| Mean ± SD | ||||
|---|---|---|---|---|
| Treatment Group (No. of Arms) | REC Reduction, mm | mRC, % | KT Gain, mm | CAL Gain, mm |
| rhPDGF | ||||
| + CM (1) | 3.6 ± 0.5 | 87.4 ± 10.8 | 3.6 ± 0.4 | 3.75 ± 0.4 |
| + CM + β-TCP (2) | 2.3 ± 0.2 | 70.2 ± 13.1 | NA | NA |
| + β-TCP (4) | 1.9 ± 0.5 | 78.8 ± 10.5 | 0.85 ± 0.2 | 2.9 ± 0.3 |
| + ADM (1) | 2.3 ± 0.3 | 69 ± 18.7 | NA | 2.06 ± 0.4 |
| + CTG (2) | 2.7 ± 0.1 | 88.7 ± 12.8 | 2.4 ± 0.3 | 2.6 ± 0.3 |
| CM (1) | 1.3 ± 0.5 | NA | NA | NA |
| ADM (1) | 2.3 ± 0.4 | 76.7 ± 16 | −0.1 ± 0.1 | 2.4 ± 0.3 |
| CTG (4) | 2.5 ± 0.6 | 86.7 ± 13.4 | 1.4 ± 0.1 | 2.9 ± 0.5 |
ADM, acellular dermal matrix; β-TCP, β-tricalcium phosphate; CAL, clinical attachment level; CM, collagen matrix; CTG, connective tissue graft; KT, keratinized tissue; mRC, mean root coverage; NA, not available/applicable; REC, gingival recession; rhPDGF, recombinant human platelet-derived growth factor.
Safety
No serious adverse effects were reported in the included studies when rhPDGF was used alone or in combination with other graft/scaffold materials.
Histologic Outcomes
McGuire and coworkers (2009) compared the 9-mo histologic and micro-CT outcomes of CTG alone (gold standard) and rhPDGF + TCP + bioabsorbable collagen wound dressing and CTG. While the CTG group showed scarring by long junctional epithelium and connective tissue fibers running parallel to the root surface, the rhPDGF-treated sites showed evidence of true periodontal regeneration. Regenerated bone was visualized coronal to the notch by micro-CT evaluation, while histologic analysis showed osteocytes and cementocytes entombed in newly formed bone and cementum. The newly regenerated PDL exhibited Sharpey fibers obliquely inserting into the newly formed cementum and bone (McGuire et al. 2014). Simion et al. (2012) harvested a soft tissue biopsy 4 mo after soft tissue augmentation with CM + rhPDGF. They observed complete resorption of the CM and that the architecture of the regenerated soft tissue resembled the healthy gingival mucosa.
Aesthetics
Five studies evaluated the root coverage aesthetic outcomes with rhPDGF (Appendix Table 7). Comparable aesthetic results to the CTG gold standard were reported by McGuire et al. (McGuire et al. 2009; McGuire et al. 2014), while Rubins et al. (2013, 2014) described a color match with the adjacent tissues following rhPDGF + CTG. One study showed superior aesthetic outcomes with rhPDGF + CM as compared with the periosteal pedicle graft (Dandu and Murthy 2016).
Patient-Reported Outcomes
Patient-reported outcome measures were evaluated by 1 group, which showed no difference in terms of patient self-reported pain/discomfort and aesthetic results between rhPDGF + β-TCP and CTG. In cases where rhPDGF was used without the CTG, the patients were spared the pain and morbidity of the donor site from which the CTG was harvested (Appendix Table 7).
Qualitative Assessment
Most RCTs and non-RCTs were considered to have a low risk of bias. Regarding the case series, 8 were considered to have a low risk of bias, 10 moderate, and 3 showed a high risk of bias.
Details in this regard are reported in Appendix Tables 8 to 10.
Discussion
The 2015 American Academy of Periodontology Regeneration Workshop concluded that the use of biologics, such as enamel matrix derivative and rhPDGF, resulted in significantly improved BF, improved CAL, and reduced PD as compared with open flap debridement surgery alone for the treatment of intrabony defects, with similar outcomes to GTR, ABBM, and DFDBA (Kao et al. 2015; Reynolds et al. 2015). Additionally, given its positive effect on the proliferation and chemotaxis of PDL and alveolar bone cells, rhPDGF has been used not only for the treatment of osseous defects but also in ARP, GBR, sinus floor elevation, and soft tissue regeneration. In fact, 63 peer-reviewed publications were identified out of >500 screened articles that reported on the clinical application, safety, effectiveness, and overall clinical benefit of rhPDGF in combination with bone allografts, xenografts, and alloplasts (TCP) when used in at least 5 oral regenerative indications. With this volume of clinical literature (beyond a plethora of preclinical and cellular mechanistic studies), it is understood that clinicians may have difficulty in evaluating the risks and clinical benefits of any given combination of rhPDGF and graft materials or their risk:benefit ratio in a particular indication.
Safety of rhPDGF
Safety analyses for biologic-based treatments are based on the comparison between the rate of subjects who experienced adverse events in the test and control groups (usually a placebo or the matrix without the biologic agent). The safety of rhPDGF has been extensively shown for periodontal regeneration in intrabony and furcation defects (Nevins et al. 2003; Nevins et al. 2005), and its use in combination with β-TCP was approved by the US Food and Drug Administration for the treatment of periodontal osseous defects as well as for orthopedic applications (Lee et al. 2017; Daniels et al. 2019). The primary outcome of the present review was to assess the safety of rhPDGF when used in several clinical indications, in combination with several graft materials. Our review results show that rhPDGF is well tolerated and safe when used for periodontal regeneration, ARP, GBR, sinus floor elevation, root coverage, and soft tissue augmentation procedures. No serious adverse effects were reported, aside from the postoperative complications usually observed after surgical therapy, following use of rhPDGF in any of these indications.
It is interesting to highlight that rhPDGF has been combined with a variety of bone replacement grafts other than β-TCP, such as autogenous bone, DFDBA, freeze-dried bone allograft, and xenogeneic bone. In addition, rhPDGF has been used with collagen and dPTFE membranes, titanium mesh, ADM, CTG, stem cells, and rhIGF-I. Here again, no serious adverse effects were reported from these combined approaches. Based on an extensive volume of data, rhPDGF in combination with many types of bone matrices, with a dosage ranging from 0.05 to 5 mg/mL, is safe for use in osseous defects, ARP, GBR, sinus floor elevation, GRs, and soft tissue augmentation.
Clinical Applicability and Outcomes of rhPDGF
It has been shown that rhPDGF is a potent wound-healing biologic with mitogenic and chemotactic properties on periodontal and alveolar bone cells (Lynch et al. 1991). Its beneficial role in promoting regeneration of periodontal and other alveolar bone defects has been well demonstrated in preclinical and clinical studies (Nevins et al. 2005; Jayakumar et al. 2011; Nevins et al. 2013). In particular, it has been suggested that rhPDGF stimulates the adhesion of PDL cells to tooth roots previously affected by periodontal disease (Jayakumar et al. 2011). In addition, rhPDGF promotes the release of vascular endothelial growth factor stimulating neovascularization at the treated site (Cooke et al. 2006; Sarment et al. 2006).
Bearing in mind that it was not possible to perform a meta-analysis due to the heterogeneity of the included studies, our results from the systematic review showed higher regenerative outcomes in the treatment of intrabony defects for rhPDGF + AlBG and rhPDGF + XBG. Our findings seem to suggest that rhPDGF benefits from a scaffold material and that AlBG and XBG may be a better carrier than β-TCP. This difference, however, was not observed in furcation defects, where rhPDGF + DFDBA and rhPDGF + β-TCP + CM showed comparable results in terms of PD reduction and CAL gain. It can be speculated that DFDBA may also contribute to bone formation by the release of growth factors, such as BMPs (Shigeyama et al. 1995), although some authors failed to identify BMPs in commercial DFDBA (Li et al. 2000). Nevertheless, the heterogeneity of studies available in the literature indicate that rhPDGF enhances the clinical outcomes when used with many types of established osteoconductive bone replacement materials. Last, it is important to mention that it has been suggested that rhPDGF has the property of enhancing wound healing in smokers (Nevins et al. 2005; Nevins et al. 2013). Although the mechanism has yet to be investigated, it has been speculated that this may be due to the activation of nicotine receptors via smoking that stimulates PDGF-β receptors (Nevins et al. 2005; Nevins et al. 2013).
Evidence is available from >20 clinical trials that met the criteria for inclusion in this review related to use of rhPDGF for GBR, ARP, and sinus floor elevation. These studies appear to present a compelling argument for the clinical benefits of using rhPDGF in oral regenerative procedures. One review, however, reported no significant differences in the percentage of vital bone when rhPDGF was used (Yao et al. 2018). However, only 3 studies were included in that meta-analysis, leading the authors to conclude that more clinical trials are needed to explore the effectiveness or rhPDGF in ARP (Yao et al. 2018). Similarly, it was not possible to explore the volumetric changes following ARP with rhPDGF in our systematic review, since the main outcomes of the included studies came from bone core samples and not from cone beam computed tomography, casts, or optical scans. Further clinical studies incorporating these outcomes, with longer follow-up periods, are recommended.
The RCT investigating rhPDGF for sinus floor augmentation showed that XBG + rhPDGF achieved significantly higher vital bone formation than the use of the xenograft alone at 4 to 5 mo and that similar histomorphometric outcomes were present between the groups at 7 to 9 mo (Froum et al. 2013), leading the authors to suggest that the growth factor can accelerate more rapid vital bone formation, which may allow for earlier implant placement (Froum et al. 2013). In line with this speculation, Nevins and coworkers (2009) suggested that rhPDGF was able to stimulate the turnover of the slowing resorbing anorganic bovine bone particles with more newly formed bone after 6 to 8 mo.
Several studies have evaluated the effect of rhPDGF on GBR with a range of graft materials, membranes, and surgical techniques. Numerous authors have proposed a positive role of rhPDGF in promoting bone regeneration (Urban et al. 2013; Amorfini et al. 2014), and a recent RCT in 30 patients found no significant differences in GBR between rhPDGF + TCP/HA and the gold standard, autogenous bone block grafting, as positive control (Santana and Santana 2015); however, the patients treated with rhPDGF/TCP/HA were spared the pain, morbidity, and extra surgical time incurred by harvesting the autogenous bone block. The authors concluded that a composite bone ceramic graft that incorporated rhPDGF appears to be a suitable substitute for autogenous bone block grafting when employed in conjunction with GBR in humans. Similarly, according to Scheines and coworkers (2018), rhPDGF in combination with allograft or xenograft bone may enhance the GBR outcomes and reduce the potential morbidity as compared with GBR with autogenous bone. Furthermore, when GBR is performed with rhPDGF, a barrier membrane does not appear required, since the material may reduce the chemotactic potential of the growth factor (Scheines et al. 2018). Elimination of the need to place a barrier membrane further reduces the potential for postoperative complications.
Several soft tissue graft materials have been used as alternatives to the gold standard CTG to reduce patient morbidity (Tavelli, Barootchi, Cairo, et al. 2019; Tavelli, Barootchi, Di Gianfilippo, et al. 2019; McGuire et al. 2020). In particular, achieving periodontal regeneration while treating GRs has largely been attempted with guided tissue regeneration or the use of enamel matrix derivative (Tavelli, Barootchi, Cairo, et al. 2019; Tavelli et al. 2020a). Therefore, it is not surprising that rhPDGF has been investigated as a CTG alternative for root coverage purposes (McGuire et al. 2009; McGuire et al. 2014). Our review showed similar mRC among CTG, rhPDGF + CTG, and rhPDGF + CM (86.7%, 88.7%, and 87.4%, respectively), with the greatest CAL gain observed for rhPDGF + CM (3.75 ± 0.4 mm). While no statistical comparison was performed in our systematic review, the RCTs directly comparing rhPDGF with CTG appear to show comparable results. McGuire et al. (2009) found higher Rec Red and mRC for CTG, while rhPDGF + β-TCP showed greater PD reduction. Similar keratinized tissue gain, aesthetic results, and patient satisfaction were reported between the groups. The study provides compelling histologic evidence of regeneration of new cementum, bone, and PDL fibers in teeth treated with rhPDGF, as compared with healing with scarring, resulting in long junctional epithelium and connective tissue fibers running parallel to the root surface in sites treated with CTG (McGuire et al. 2009). The same group reported the 5-y results of this previous study, showing a stability of the investigated parameters, with the exception of keratinized tissue width gained, which was significantly higher in the CTG-treated teeth (McGuire et al. 2014). Additionally, another study obtained comparable mRC between rhPDGF + β-TCP and CTG for the treatment of multiple GRs, with both groups significantly better than coronally advanced flap alone (Deshpande et al. 2014). However, a greater keratinized tissue width was found for CTG, supporting the speculation that the induction of keratinization of the overlying epithelium is a property of CTG only (Tavelli et al. 2020b; Zucchelli et al. 2020). While further studies are advocated to better evaluate all of the benefits of rhPDGF in root coverage procedures, the current evidence supports the use of rhPDGF when the aim is to treat GRs while promoting periodontal regeneration (Tavelli et al. 2020a).
Limitations of the present review are discussed in the Appendix.
Conclusions
Based on the available extensive human data from 63 clinical studies, the following conclusions can be drawn.
First, the utilization of rhPDGF is safe when used in combination with a variety of bone matrices, including allografts, xenografts, and alloplasts, for periodontal regeneration, GBR, ARP, sinus floor augmentation, and tooth root coverage procedures.
Second, there is strong evidence that rhPDGF is effective in the regeneration of intrabony defects when used in conjunction with a bone matrix. In particular, rhPDGF benefits from the delivery with an osteoconductive scaffold matrix, with an allogeneic, or when followed by XBG, showing the greatest regenerative outcomes. Clinical and histologic evidence showed that rhPDGF plus a scaffold was also effective in the treatment of furcation defects. While only a relatively small sample size is available in human histologic studies, due to the invasive procedures necessary to obtain the biopsies, these data provide supporting evidence at the tissue and cell level for the safety, efficacy, and clinical benefit of rhPDGF.
Third, the outcomes are consistently positive for rhPDGF for GBR and sinus augmentation. This evidence is based on RCTs, as well as case reports and case series. The outcomes are also positive for rhPDGF for ARP according to histologic outcomes for vital bone, although additional data based on cone beam computed tomography, casts, or optical scans would be valuable for evaluating volumetric changes following ARP with rhPDGF.
Fourth, rhPDGF + β-TCP + collagen appears to be a viable alternative to autogenous CTG when the aim is to achieve periodontal regeneration in treating GR defects.
Fifth, future randomized clinical trials should focus on the effects of PDGF in ARP, GBR, and sinus floor augmentation procedures. Moreover, it is important to determine whether GBR with rhPDGF should be used with a barrier or not, since the material may reduce the chemotactic potential of the growth factor. These data are required to help fully understand the clinical benefits of rhPDGF–based procedures.
Author Contributions
L. Tavelli, contributed to conception, design, data acquisition, and interpretation, drafted and critically revised the manuscript; A. Ravidà, contributed to conception, design, data acquisition, and analysis, drafted and critically revised the manuscript; S. Barootchi, contributed to conception and design, drafted and critically revised the manuscript; L. Chambrone, contributed to conception, design, data acquisition, and interpretation, critically revised the manuscript; W.V. Giannobile, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_2380084420921353 for Recombinant Human Platelet–Derived Growth Factor: A Systematic Review of Clinical Findings in Oral Regenerative Procedures by L. Tavelli, A. Ravidà, S. Barootchi, L. Chambrone and W.V. Giannobile in JDR Clinical & Translational Research
Acknowledgments
The authors appreciate Dr. Samuel E. Lynch for the helpful discussions and the critical reading of this manuscript.
Footnotes
A supplemental appendix to this article is available online.
This research was supported by the National Institutes of Health / National Institute of Dental and Craniofacial Research (U24 DE026915) and the University of Michigan Periodontal Graduate Student Research Fund.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: L. Tavelli  https://orcid.org/0000-0003-4864-3964
https://orcid.org/0000-0003-4864-3964
S. Barootchi  https://orcid.org/0000-0002-5347-6577
https://orcid.org/0000-0002-5347-6577
W.V. Giannobile  https://orcid.org/0000-0002-7102-9746
https://orcid.org/0000-0002-7102-9746
References
- Amorfini L, Migliorati M, Signori A, Silvestrini-Biavati A, Benedicenti S. 2014. Block allograft technique versus standard guided bone regeneration: a randomized clinical trial. Clin Implant Dent Relat Res. 16(5):655–667. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. 2008. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22(10):1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyan LA, Bhargava G, Nishimura F, Orman R, Price R, Terranova VP. 1994. Mitogenic and chemotactic responses of human periodontal ligament cells to the different isoforms of platelet-derived growth factor. J Dent Res. 73(10):1593–1600. [DOI] [PubMed] [Google Scholar]
- Camelo M, Nevins ML, Schenk RK, Lynch SE, Nevins M. 2003. Periodontal regeneration in human class ii furcations using purified recombinant human platelet-derived growth factor–BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 23(3):213–225. [PubMed] [Google Scholar]
- Cho MI, Lin WL, Genco RJ. 1995. Platelet-derived growth factor–modulated guided tissue regenerative therapy. J Periodontol. 66(6):522–530. [DOI] [PubMed] [Google Scholar]
- Cooke JW, Sarment DP, Whitesman LA, Miller SE, Jin Q, Lynch SE, Giannobile WV. 2006. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 12(6):1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandu SR, Murthy KR. 2016. Multiple gingival recession defects treated with coronally advanced flap and either the VISTA technique enhanced with GEM 21S or periosteal pedicle graft: a 9-month clinical study. Int J Periodontics Restorative Dent. 36(2):231–237. [DOI] [PubMed] [Google Scholar]
- Daniels TR, Anderson J, Swords MP, Maislin G, Donahue R, Pinsker E, Quiton JD. 2019. Recombinant human platelet-derived growth factor BB in combination with a beta-tricalcium phosphate (rhPDGF-BB/beta-TCP)–collagen matrix as an alternative to autograft. Foot Ankle Int. 40(9):1068–1078. [DOI] [PubMed] [Google Scholar]
- De Angelis N, Scivetti M. 2011. Lateral ridge augmentation using an equine flex bone block infused with recombinant human platelet-derived growth factor BB: a clinical and histologic study. Int J Periodontics Restorative Dent. 31(4):383–388. [PubMed] [Google Scholar]
- Deshpande A, Koudale SB, Bhongade ML. 2014. A comparative evaluation of rhPDGF-BB + beta-TCP and subepithelial connective tissue graft for the treatment of multiple gingival recession defects in humans. Int J Periodontics Restorative Dent. 34(2):241–249. [DOI] [PubMed] [Google Scholar]
- Froum SJ, Wallace S, Cho SC, Rosenburg E, Froum S, Schoor R, Mascarenhas P, Tarnow DP, Corby P, Elian N, et al. 2013. A histomorphometric comparison of Bio-Oss alone versus Bio-Oss and platelet-derived growth factor for sinus augmentation: a postsurgical assessment. Int J Periodontics Restorative Dent. 33(3):269–279. [DOI] [PubMed] [Google Scholar]
- Geurs N, Ntounis A, Vassilopoulos P, Van der Velden U, Loos BG, Reddy M. 2014. Using growth factors in human extraction sockets: a histologic and histomorphometric evaluation of short-term healing. Int J Oral Maxillofac Implants. 29(2):485–496. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Somerman MJ. 2003. Growth and amelogenin-like factors in periodontal wound healing: a systematic review. Ann Periodontol. 8(1):193–204. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. 2019. Cochrane handbook for systematic reviews of interventions version 6.0 (updated August 2019). 2nd ed. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Jayakumar A, Rajababu P, Rohini S, Butchibabu K, Naveen A, Reddy PK, Vidyasagar S, Satyanarayana D, Pavan Kumar S. 2011. Multi-centre, randomized clinical trial on the efficacy and safety of recombinant human platelet-derived growth factor with beta-tricalcium phosphate in human intra-osseous periodontal defects. J Clin Periodontol. 38(2):163–172. [DOI] [PubMed] [Google Scholar]
- Kao RT, Nares S, Reynolds MA. 2015. Periodontal regeneration–intrabony defects: a systematic review from the AAP Regeneration Workshop. J Periodontol. 86(2):S77–S104. [DOI] [PubMed] [Google Scholar]
- Khoshkam V, Chan HL, Lin GH, Mailoa J, Giannobile WV, Wang HL, Oh TJ. 2015. Outcomes of regenerative treatment with rhPDGF-BB and rhfgf-2 for periodontal intra-bony defects: a systematic review and meta-analysis. J Clin Periodontol. 42(3):272–280. [DOI] [PubMed] [Google Scholar]
- Kubota A, Sarmiento H, Alqahtani MS, Llobell A, Fiorellini JP. 2017. The use of recombinant human platelet-derived growth factor for maxillary sinus augmentation. Int J Periodontics Restorative Dent. 37(2):219–225. [DOI] [PubMed] [Google Scholar]
- Lee EA. 2017. Subperiosteal minimally invasive aesthetic ridge augmentation technique (SMART): a new standard for bone reconstruction of the jaws. Int J Periodontics Restorative Dent. 37(2):165–173. [DOI] [PubMed] [Google Scholar]
- Lee JY, Na HJ, Kim HM, Lee SC, Lee JY, Chung CP, Seol YJ, Park YJ. 2017. Comparative study of rhPDGF-BB plus equine-derived bone matrix versus rhPDGF-BB plus beta-TCP in the treatment of periodontal defects. Int J Periodontics Restorative Dent. 37(6):825–832. [DOI] [PubMed] [Google Scholar]
- Li H, Pujic Z, Xiao Y, Bartold PM. 2000. Identification of bone morphogenetic proteins 2 and 4 in commercial demineralized freeze-dried bone allograft preparations: pilot study. Clin Implant Dent Relat Res. 2(2):110–117. [DOI] [PubMed] [Google Scholar]
- Lynch SE, de Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, Antoniades HN. 1991. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol. 62(7):458–467. [DOI] [PubMed] [Google Scholar]
- Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, Zappa UE, Antoniades HN. 1989. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 16(8):545–548. [DOI] [PubMed] [Google Scholar]
- McAllister BS, Haghighat K, Prasad HS, Rohrer MD. 2010. Histologic evaluation of recombinant human platelet-derived growth factor–BB after use in extraction socket defects: a case series. Int J Periodontics Restorative Dent. 30(4):365–373. [PubMed] [Google Scholar]
- McGuire MK, Scheyer ET, Schupbach P. 2009. Growth factor–mediated treatment of recession defects: a randomized controlled trial and histologic and microcomputed tomography examination. J Periodontol. 80(4):550–564. [DOI] [PubMed] [Google Scholar]
- McGuire MK, Scheyer ET, Snyder MB. 2014. Evaluation of recession defects treated with coronally advanced flaps and either recombinant human platelet-derived growth factor–BB plus beta-tricalcium phosphate or connective tissue: comparison of clinical parameters at 5 years. J Periodontol. 85(10):1361–1370. [DOI] [PubMed] [Google Scholar]
- McGuire MK, Tavelli L, Feinberg SE, Rasperini G, Zucchelli G, Wang HL, Giannobile WV. 2020. Living cell-based regenerative medicine technologies for periodontal soft tissue augmentation. J Periodontol. 91(2):155–164. [DOI] [PubMed] [Google Scholar]
- Mellonig JT, Valderrama Mdel P, Cochran DL. 2009. Histological and clinical evaluation of recombinant human platelet-derived growth factor combined with beta tricalcium phosphate for the treatment of human class III furcation defects. Int J Periodontics Restorative Dent. 29(2):169–177. [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. 2003. Periodontal regeneration in humans using recombinant human platelet-derived growth factor–BB (rhPDGF-BB) and allogenic bone. J Periodontol. 74(9):1282–1292. [DOI] [PubMed] [Google Scholar]
- Nevins M, Garber D, Hanratty JJ, McAllister BS, Nevins ML, Salama M, Schupbach P, Wallace S, Bernstein SM, Kim DM. 2009. Human histologic evaluation of anorganic bovine bone mineral combined with recombinant human platelet-derived growth factor BB in maxillary sinus augmentation: case series study. Int J Periodontics Restorative Dent. 29(6):583–591. [PubMed] [Google Scholar]
- Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, McAllister BS, Murphy KS, McClain PK, Nevins ML, et al. 2005. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 76(12):2205–2215. [DOI] [PubMed] [Google Scholar]
- Nevins M, Kao RT, McGuire MK, McClain PK, Hinrichs JE, McAllister BS, Reddy MS, Nevins ML, Genco RJ, Lynch SE, et al. 2013. Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial. J Periodontol. 84(4):456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins ML, Reynolds MA, Camelo M, Schupbach P, Kim DM, Nevins M. 2014. Recombinant human platelet-derived growth factor BB for reconstruction of human large extraction site defects. Int J Periodontics Restorative Dent. 34(2):157–163. [DOI] [PubMed] [Google Scholar]
- Park JB, Matsuura M, Han KY, Norderyd O, Lin WL, Genco RJ, Cho MI. 1995. Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J Periodontol. 66(6):462–477. [DOI] [PubMed] [Google Scholar]
- Pilipchuk SP, Fretwurst T, Yu N, Larsson L, Kavanagh NM, Asa’ad F, Cheng KCK, Lahann J, Giannobile WV. 2018. Micropatterned scaffolds with immobilized growth factor genes regenerate bone and periodontal ligament-like tissues. Adv Healthc Mater. 7(22):e1800750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MA, Kao RT, Camargo PM, Caton JG, Clem DS, Fiorellini JP, Geisinger ML, Mills MP, Nares S, Nevins ML. 2015. Periodontal regeneration–intrabony defects: a consensus report from the AAP Regeneration Workshop. J Periodontol. 86(2):S105–S107. [DOI] [PubMed] [Google Scholar]
- Ridgway HK, Mellonig JT, Cochran DL. 2008. Human histologic and clinical evaluation of recombinant human platelet-derived growth factor and beta-tricalcium phosphate for the treatment of periodontal intraosseous defects. Int J Periodontics Restorative Dent. 28(2):171–179. [PubMed] [Google Scholar]
- Rubins RP, Tolmie PN, Corsig KT, Kerr EN, Kim DM. 2013. Subepithelial connective tissue graft with growth factor for the treatment of maxillary gingival recession defects. Int J Periodontics Restorative Dent. 33(1):43–50. [DOI] [PubMed] [Google Scholar]
- Rubins RP, Tolmie PN, Corsig KT, Kerr EN, Kim DM. 2014. Subepithelial connective tissue graft with purified rhPDGF-BB for the treatment of mandibular recession defects: a consecutive case series. Int J Periodontics Restorative Dent. 34(3):315–321. [DOI] [PubMed] [Google Scholar]
- Santana RB, Santana CM. 2015. A clinical comparison of guided bone regeneration with platelet-derived growth factor–enhanced bone ceramic versus autogenous bone block grafting. Int J Oral Maxillofac Implants. 30(3):700–706. [DOI] [PubMed] [Google Scholar]
- Santana RB, Santana CM, Dibart S. 2015. Platelet-derived growth factor–mediated guided bone regeneration in immediate implant placement in molar sites with buccal bone defects. Int J Periodontics Restorative Dent. 35(6):825–833. [DOI] [PubMed] [Google Scholar]
- Sarment DP, Cooke JW, Miller SE, Jin Q, McGuire MK, Kao RT, McClain PK, McAllister BS, Lynch SE, Giannobile WV. 2006. Effect of rhPDGF-BB on bone turnover during periodontal repair. J Clin Periodontol. 33(2):135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheines C, Hokett SD, Katancik JA. 2018. Recombinant human platelet-derived growth factor–BB in human alveolar ridge augmentation: a review of the literature. Int J Oral Maxillofac Implants. 33(5):1047–1056. [DOI] [PubMed] [Google Scholar]
- Schincaglia GP, Hebert E, Farina R, Simonelli A, Trombelli L. 2015. Single versus double flap approach in periodontal regenerative treatment. J Clin Periodontol. 42(6):557–566. [DOI] [PubMed] [Google Scholar]
- Shigeyama Y, D’Errico JA, Stone R, Somerman MJ. 1995. Commercially-prepared allograft material has biological activity in vitro.J Periodontol. 66(6):478–487. [DOI] [PubMed] [Google Scholar]
- Simion M, Rocchietta I, Dellavia C. 2007. Three-dimensional ridge augmentation with xenograft and recombinant human platelet-derived growth factor–BB in humans: report of two cases. Int J Periodontics Restorative Dent. 27(2):109–115. [PubMed] [Google Scholar]
- Simion M, Rocchietta I, Fontana F, Dellavia C. 2012. Evaluation of a resorbable collagen matrix infused with rhPDGF-BB in peri-implant soft tissue augmentation: a preliminary report with 3.5 years of observation. Int J Periodontics Restorative Dent. 32(3):273–282. [PubMed] [Google Scholar]
- Tavelli L, Barootchi S, Cairo F, Rasperini G, Shedden K, Wang HL. 2019. The effect of time on root coverage outcomes: a network meta-analysis. J Dent Res. 98(11):1195–1203. [DOI] [PubMed] [Google Scholar]
- Tavelli L, Barootchi S, Di Gianfilippo R, Modarressi M, Cairo F, Rasperini G, Wang HL. 2019. Acellular dermal matrix and coronally advanced flap or tunnel technique in the treatment of multiple adjacent gingival recessions: a 12-year follow-up from a randomized clinical trial. J Clin Periodontol. 46(9):937–948. [DOI] [PubMed] [Google Scholar]
- Tavelli L, McGuire MK, Zucchelli G, Rasperini G, Feinberg SE, Wang HL, Giannobile WV. 2020. a. Biologics-based regenerative technologies for periodontal soft tissue engineering. J Periodontol. 91(2):147–154. [DOI] [PubMed] [Google Scholar]
- Tavelli L, McGuire MK, Zucchelli G, Rasperini G, Feinberg SE, Wang HL, Giannobile WV. 2020. b. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration.J Periodontol. 91(1):17–25. [DOI] [PubMed] [Google Scholar]
- Thakare K, Deo V. 2012. Randomized controlled clinical study of rhPDGF-BB + beta-TCP versus HA + beta-TCP for the treatment of infrabony periodontal defects: clinical and radiographic results. Int J Periodontics Restorative Dent. 32(6):689–696. [PubMed] [Google Scholar]
- Urban IA, Lozada JL, Jovanovic SA, Nagy K. 2013. Horizontal guided bone regeneration in the posterior maxilla using recombinant human platelet-derived growth factor: a case report. Int J Periodontics Restorative Dent. 33(4):421–425. [DOI] [PubMed] [Google Scholar]
- Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV, Ivanovski S. 2018. Tissue engineered constructs for periodontal regeneration: current status and future perspectives. Adv Healthc Mater. 7(21):e1800457. [DOI] [PubMed] [Google Scholar]
- Wallace SC, Snyder MB, Prasad H. 2013. Postextraction ridge preservation and augmentation with mineralized allograft with or without recombinant human platelet-derived growth factor BB (rhPDGF-BB): a consecutive case series. Int J Periodontics Restorative Dent. 33(5):599–609. [DOI] [PubMed] [Google Scholar]
- Yao W, Shah B, Chan HL, Wang HL, Lin GH. 2018. Bone quality and quantity alterations after socket augmentation with rhPDGF-BB or BMPs: a systematic review. Int J Oral Maxillofac Implants. 33(6):1255–1265. [DOI] [PubMed] [Google Scholar]
- Zadeh HH. 2011. Minimally invasive treatment of maxillary anterior gingival recession defects by vestibular incision subperiosteal tunnel access and platelet-derived growth factor BB. Int J Periodontics Restorative Dent. 31(6):653–660. [PubMed] [Google Scholar]
- Zucchelli G, Tavelli L, McGuire MK, Rasperini G, Feinberg SE, Wang HL, Giannobile WV. 2020. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J Periodontol. 91(1):9–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_2380084420921353 for Recombinant Human Platelet–Derived Growth Factor: A Systematic Review of Clinical Findings in Oral Regenerative Procedures by L. Tavelli, A. Ravidà, S. Barootchi, L. Chambrone and W.V. Giannobile in JDR Clinical & Translational Research