Abstract
Rationale:
Obsessive-compulsive disorder (OCD) is characterized by repetitive behaviors exacerbated by stress. Many OCD patients do not respond to available pharmacotherapies, but neurosurgical ablation of the anterior cingulate cortex (ACC) can provide symptomatic relief. Although the ACC receives noradrenergic innervation and expresses adrenergic receptors (ARs), the involvement of norepinephrine (NE) in OCD has not been investigated.
Objective:
To determine the effects of genetic or pharmacological disruption of NE neurotransmission on marble burying (MB) and nestlet shredding (NS) in two animal models of OCD.
Methods:
We assessed NE-deficient (Dbh −/−) mice and NE-competent (Dbh +/−) controls in MB and NS tasks. We also measured the effects of anti-adrenergic drugs on NS and MB in control mice and the effects of pharmacological restoration of central NE in Dbh −/− mice. Finally, we compared c-fos induction in the locus coeruleus (LC) and ACC of Dbh −/− and control mice following both tasks.
Results:
Dbh −/− mice virtually lacked MB and NS behaviors seen in control mice but did not differ in the elevated zero maze (EZM) model of general anxiety-like behavior. Pharmacological restoration of central NE synthesis in Dbh −/− mice completely rescued NS behavior, while NS and MB were suppressed in control mice by anti-adrenergic drugs. Expression of c-fos in the ACC was attenuated in Dbh −/− mice after MB and NS.
Conclusion:
These findings support a role for NE transmission to the ACC in the expression of stress-induced compulsive behaviors and suggest further evaluation of anti-adrenergic drugs for OCD is warranted.
Keywords: norepinephrine, dopamine β-hydroxylase, obsessive-compulsive disorder, nestlet shredding, marble burying, guanfacine, adrenergic receptor, c-fos, locus coeruleus, anterior cingulate cortex, anxiety
Introduction
In patients with obsessive-compulsive disorder (OCD), intrusive thoughts drive repetitive and compulsive behaviors, among them checking, counting, and excessive hand washing. These obsessions are emotionally aversive and disproportionately command attentional resources until the ritual that interrupts the obsession is completed (Graybiel and Rauch 2000; Pigott et al. 1994). In OCD and other compulsive disorders, symptom severity increases during periods of psychological stress (Adams et al. 2018; Lin et al. 2007; Rosso et al. 2012). Currently, the only drugs approved for the management of OCD are serotonergic antidepressants (tricyclics and selective serotonin reuptake inhibitors; SSRIs), which are ineffective for about half of patients and unfortunately associated with adverse side effects that limit compliance (Dominguez 1992; Mavissakalian and Michelson 1983; Pizarro et al. 2014; van Balkom et al. 1994).
The neural circuitry and neurochemistry that drive compulsive behaviors have not been clearly delineated (Micallef and Blin 2001; Stein 2000), but converging evidence from neuroimaging, neurosurgical, and neurobiological studies implicates a corticolimbic interface structure called the anterior cingulate cortex (ACC) in the pathophysiology of OCD (Alarcon et al. 1994; Brennan et al. 2013; Fitzgerald et al. 2005; McGovern and Sheth 2017). Data from human functional imaging studies have frequently identified ACC hyperactivity in OCD patients (De Ridder et al. 2017; Fitzgerald et al. 2005; Riffkin et al. 2005; Van Laere et al. 2006). Both functional and structural abnormalities in the ACC of patients with OCD are correlated with disturbances in emotional reactivity, attention, and cognitive control (Brennan et al. 2015; McGovern and Sheth 2017; Pauls et al. 2014). The ACC receives glutamatergic and neuromodulatory input from a distributed network of forebrain and hindbrain structures, and is well positioned to regulate attention, emotions, motivation, and action under conditions of uncertainty or change (Margulies et al. 2007; Stevens et al. 2011; Yücel et al. 2003).
Evidence from the neurosurgical literature suggests that ~40% of antidepressant-resistant OCD patients can achieve lasting remission of symptoms by undergoing a surgical procedure called a cingulotomy (Brown et al. 2016; Kim et al. 2003; McGovern and Sheth 2017), in which the ACC is lesioned bilaterally with gamma radiation (Cosgrove and Rauch 2003; Fodstad et al. 1982). Despite the efficacy of this intervention for improving the quality of life in some treatment-resistant OCD patients, invasive surgical ablation of an important brain region is an aggressive and irreversible treatment strategy that should only be considered as a last resort after pharmacological interventions have failed. Indeed, cingulotomy is associated with significant long-term cognitive and emotional side effects, including emotional apathy and deficits in attention (Cohen et al. 1999a; Cohen et al. 1999b; Janer and Pardo 1991).
Because of the modest efficacy of selecting serotonin reuptake inhibitors (SSRIs) in alleviating OCD symptoms, most preclinical research on OCD pathophysiology has focused on the serotonin (5-HT) system and, to a lesser extent, the dopaminergic and glutamatergic systems (Billett et al. 1998; Goodman et al. 1990; Pittenger et al. 2011; Pittenger et al. 2006; Zohar et al. 2000). The development of more effective and tolerable pharmacotherapies has been impeded by an incomplete understanding of the neurobiological basis of OCD and other compulsive disorders (Langen et al. 2011; Micallef and Blin 2001; Ting and Feng 2011). Thus, there is an urgent need to develop and characterize better preclinical models of OCD to unravel the neurochemistry and neurocircuitry that drive compulsive behaviors.
Some advances in understanding the neurobiology of OCD have come from genetic and behavioral models of the disease, which have various degrees of face, construct, and predictive validity (Ahmari and Dougherty 2015; Fineberg et al. 2011; Joel 2006; Ting and Feng 2011; Witkin 2008). The nestlet shredding (NS) and marble burying (MB) tasks are two rodent models of repetitive, compulsive behaviors that may be useful for testing novel pharmacotherapies for OCD (Angoa-Pérez et al. 2013; Li et al. 2006; Wolmarans et al. 2016). In these tasks, repetitive behaviors (digging for MB; shredding for NS) are elicited by cage-change stress and do not habituate even after repeated daily exposures to marbles or nestlets (Thomas et al. 2009; Witkin 2008).
The central norepinephrine (NE) system mediates stress responses, attention, arousal, emotional state, and behavioral flexibility (Aston-Jones et al. 2007; Sara 2009), all of which are disrupted in patients with OCD (Adams et al. 2018; Cocchi et al. 2012; Gehring et al. 2000; Kalanthroff et al. 2016; Spitznagel and Suhr 2002). The 5-HT and NE neuromodulatory systems regulate one another (Kim et al. 2004; O’Leary et al. 2007; Pasquier et al. 1977; Pudovkina et al. 2002; Segal 1979) and have overlapping terminal fields in forebrain regions, such as the ACC, where 5-HT exerts an inhibitory influence (Czyrak et al. 2003; Hajós et al. 2003; Tanaka and North 1993) and NE exerts an excitatory influence (Berridge et al. 1993; Gompf et al. 2010; Marzo et al. 2014; Stone et al. 2006). Moreover, reciprocal excitatory communication between the noradrenergic locus coeruleus (LC) and the ACC is required to support sustained arousal during exposure to unfamiliar environments (Gompf et al. 2010), suggesting that the LC-ACC axis is a critical substrate of arousal and attention in response to contextual change (Aston-Jones et al. 1999; Vankov et al. 1995).
Although studies on the subject are limited, there is some compelling evidence for abnormalities in central NE function and adrenergic receptor (AR) sensitivity in patients with compulsive disorders. Elevated plasma levels of NE metabolites (Siever et al. 1983), altered neuroendocrine responses to adrenergic drug challenges (Brambilla et al. 1997; Hollander et al. 1991; Siever et al. 1983), and genetic polymorphisms in the NE-metabolizing enzyme COMT (Karayiorgou et al. 1999; Pooley et al. 2007; Schindler et al. 2000) have been reported in OCD patients, but the role of the central NE system in OCD pathophysiology in humans has not been investigated thoroughly. Similarly, the effects of serotonergic drugs on NS and MB behavior have been extensively documented, but the effects of drugs targeting the NE system have only been described in a handful of studies (Li et al. 2006; Millan et al. 2000; Sugimoto et al. 2007; Young et al. 2006).
Here we determined the effects of genetic or pharmacological disruption of central NE signaling on OCD-like behaviors in the NS and MB tasks using NE-deficient (Dbh −/−) mice and their NE-competent (Dbh +/−) counterparts (Thomas et al. 1995). To provide a contrast to canonical anxiety-like behavior, we also tested performance in the elevated zero maze (EZM). Finally, we assessed the effects of genetic NE deficiency on c-fos induction in the LC and ACC as a measure of task-specific neuronal activity during NS and MB tasks.
Materials and methods
Subjects
Dbh −/− mice were maintained on a mixed 129/SvEv and C57BL/6J background, as previously described (Thomas et al. 1998; Thomas et al. 1995). Pregnant Dbh +/− dams were given drinking water that contained the βAR agonist isoproterenol and α1AR agonist phenylephrine (20 μg/ml each) + vitamin C (2 mg/ml) from E9.5–E14.5, and L-3,4-dihydroxyphenylserine (DOPS; 2 mg/ml + vitamin C 2 mg/ml) from E14.5-parturition to prevent the embryonic lethality associated with the homozygous Dbh deficiency (Mitchell et al. 2008; Thomas et al. 1995). Dbh −/− mice are readily identified by their ptosis phenotype, and genotypes were subsequently confirmed by PCR. Dbh +/− littermates were used as controls because their behavior and catecholamine levels are indistinguishable from wild-type (Dbh +/+) mice (Marino et al. 2005; Mitchell et al. 2006; Thomas et al. 1998; Thomas et al. 1995).
Male and female mice 3–8 months old were used in all experiments. Because no sex differences were reported in the literature or observed in pilot experiments, male and female mice of the same Dbh genotype were pooled. All animal procedures and protocols were approved by the Emory University Animal Care and Use Committee in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. Mice were maintained on a 12 h light/12 h dark cycle with ad libitum access to food and water. Behavioral testing was conducted during the light cycle in a quiet room in which the animals were housed to minimize the stress of cage transport on test days.
Drugs
The following drugs were used for behavioral pharmacology experiments: the non-selective β-adrenergic receptor (βAR) antagonist DL-propranolol hydrochloride (Sigma-Aldrich, St. Louis, MO), the α1AR antagonist prazosin hydrochloride (Sigma-Aldrich), the α2AR agonists guanfacine hydrochloride (Sigma-Aldrich) and dexmedetomidine (Patterson Veterinary Supply, Greeley, CO), the peripheral non-selective βAR antagonist nadolol (Sigma-Aldrich), the DBH inhibitor nepicastat (Synosia Therapeutics, Basel, Switzerland), the β1AR antagonist betaxolol (Sigma-Aldrich), the β2AR antagonist ICI 118,551 (Sigma-Aldrich), the α2AR antagonist atipamezole (Patterson Veterinary Supply), the peripheral aromatic acid decarboxylase inhibitor benserazide (Sigma-Aldrich), and the synthetic NE precursor 1-3,4-dihydroxyphenylserine (DOPS; Lundbeck, Deerfield, IL). All drugs were dissolved in sterile saline (0.9% NaCl) except for prazosin, which was dissolved in saline containing 1.5% DMSO + 1.5% Cremophor EL, and DOPS, which was dissolved in distilled water with 2% HCl, 2% NaOH, and 2 mg/kg vitamin C.
All compounds except DOPS were administered by i.p. injections at a volume of 10 ml/kg. Sterile saline vehicle was injected to control for any confounding effect of injection stress on behavior, and vehicle-treated animals were used for statistical comparison with anti-adrenergic compounds. For the DOPS rescue experiment, Dbh −/− mice were injected with DOPS (1 g/kg, s.c.) + benserazide (250 mg/kg; Sigma-Aldrich), then tested 5 h later once NE levels peaked. The vehicle control for the DOPS experiment was also administered 5 h before testing (Rommelfanger et al. 2007; Schank et al. 2008; Thomas et al. 1998). The doses for all compounds tested were based on previous studies (Durcan et al. 1989; Ji et al. 2014; Kauppila et al. 1991; Luttinger et al. 1985; Millan et al. 2000; Murchison et al. 2004; Rudoy and Van Bockstaele 2007; Schank et al. 2008; Schroeder et al. 2013; Stemmelin et al. 2008; Van Der Laan et al. 1985) and optimized in pilot experiments to ensure that behavioral effects were not due to sedation.
Elevated zero maze
Mice were exposed to the EZM (2” wide track, 20” diameter) under low light for 5 min (Shepherd et al. 1994; Tillage et al. 2020). Time spent in the open and closed segments of the maze, entries into the open segments, distance traveled, and velocity were recorded on an overhead camera and measured using TopScan software (Clever Sys Inc., Reston, VA).
Nestlet shredding
Individual mice were removed from their home cages and placed into a new standard mouse cage (13” x 7” x 6”) with clean bedding and a standard cotton nestlet square (5 cm x 5 cm, roughly 3g). The nestlets were pre-weighed before the start of testing to calculate the % shredded at the end of the task (Angoa-Pérez et al. 2013; Angoa-Pérez et al. 2012; Li et al. 2006). Mice were left undisturbed for 30, 60, or 90 min, after which they were returned to their home cages. The weights of the remaining non-shredded nestlet material were recorded, as previously described (Angoa-Pérez et al. 2013; Li et al. 2006). In one experiment, mice were singly housed with nestlets for 24 h. In another set of experiments Dbh +/− and −/− mice were observed in the NS task for 90 min and latencies to begin shredding and to sleep were recorded. Shredding was operationalized as 2 min of uninterrupted NS behavior and sleeping was operationalized as 2 min of uninterrupted sleep behavior (immobility, even breathing, sleep posture). Animals that did not shred or fall asleep within the 90 min task window were assigned a score of 90 min for each parameter.
For pharmacological experiments, all drugs except DOPS and nepicastat were administered 15 min before testing, and the task duration was set at 60 min. Nepicastat was administered 2 h prior to testing to allow for maximal NE depletion (Schroeder et al. 2013), and DOPS was administered 5 h before testing (Schank et al. 2008; Thomas et al. 1998). To determine the effect of test cage habituation on NS behavior, individual Dbh −/− and control mice were moved into new, clean mouse cages without a nestlet. After 3 h of habituation, a nestlet was introduced to the test cage, and NS within 60 min was measured. The NS behavior of habituated mice was compared with NS behavior in mice tested without habituation.
Marble burying
Individual mice were removed from their home cages and placed into a large novel cage (10” x 18” x 10”) with 20 black glass marbles arranged in a 4 x 5 grid pattern on top of 2” of normal bedding substrate. The test cage had no lid, and the room was brightly lit. Mice were left undisturbed in the test cage for 30 min. At the end of the test, mice were returned to their home cages. Digital photographs of each test cage were captured at uniform angles and distances. The number of marbles buried was determined by counting the marbles that remained unburied and subtracting this number from 20. A marble was counted as “buried” if at least 2/3 of it was submerged by bedding (Angoa-Pérez et al. 2013; Tillage et al. 2020). For pharmacological experiments, all drugs were administered by i.p. injection 30 min prior to testing, as described (Jimenez-Gomez et al. 2011; Sugimoto et al. 2007).
c-fos immunohistochemistry
Dbh −/− and control mice were exposed to MB or NS tasks, after which they were left undisturbed in the test cage. 90 min following the start of the task, mice were euthanized with an overdose of sodium pentobarbital (Fatal Plus, 150 mg/kg, i.p.; Med-Vet International, Mettawa, IL) and transcardially perfused with cold 4% paraformaldehyde in 0.01 M PBS. After extraction, brains were postfixed for 24 h in 4% paraformaldehyde at 4°C, and then transferred to cryoprotectant 30% sucrose/PBS solution for 72 h at 4°C. Brains were then embedded in OCT medium (Tissue-Tek) and sectioned by cryostat into 40-um coronal slices at the level of the ACC and LC. Another group of Dbh −/− and control mice was selected for baseline comparisons of c-fos induction in the LC and ACC; these animals were naive to the tasks and were removed from their home cages and immediately euthanized and perfused.
Brain sections were blocked for 1 h at room temperature in 5% normal goat serum (NGS) in 0.01 M PBS/0.1% Triton-X permeabilization buffer. Sections were then incubated for 48 h at 4°C in NGS blocking/permeabilization buffer, including primary antibodies raised against c-fos (rabbit anti-c-fos, Millipore, Danvers, MA, ABE457; 1:5000) and NET (mouse anti-NET; MAb Technologies, Neenah, WI, NET05-2; 1:1000). After washing in 0.01 M PBS, sections were incubated for 2 h in blocking buffer, including goat anti-rabbit AlexaFluor 568 and goat anti-mouse AlexaFluor 488 (Invitrogen, Carlsbad, CA; 1:500). After washing, the sections were mounted onto Superfrost Plus slides and coverslipped with Fluoromount-G plus DAPI (Southern Biotech, Birmingham, AL).
Fluorescent imaging and c-fos quantification
Fluorescent micrographs of immunostained sections were acquired on a Leica DM6000B epifluorescent upright microscope at 20x magnification with uniform exposure parameters. One representative atlas-matched section of the LC and the ACC was selected from each animal for c-fos quantification. An identically sized region of interest was drawn for all images to delineate the borders of both structures in all animals after both behaviors. Image processing and analysis were performed using ImageJ software. Our analysis pipeline includes background subtraction, intensity thresholding (Otsu method), and automated quantification within defined regions of interest, guided by size and shape criteria for c-fos+ cells (size: 50–100 mm2, circularity: 0.6–1.0). The NET antibody was used to define LC cell bodies and identify axon terminals in apposition to ACC neurons.
Statistical analysis
For NS experiments, the effects of time on shredding in Dbh −/− and Dbh +/− mice were compared using a two-way repeated measures ANOVA (genotype x time), with post hoc Sidak’s test for multiple comparisons. The effects of pre-habituation to the test cage on shredding were also compared by two-way repeated measures ANOVA for each genotype (genotype x habituation time), with post hoc Sidak’s test for multiple comparisons. NS behavior between genotypes after 24 h, NS behavior in DOPS- and vehicle-treated Dbh −/− mice, and between-genotype NS and sleep latencies were compared using an unpaired t-test. NS behavior in DOPS- and vehicle-treated Dbh −/− mice was also assessed by unpaired t-test. The effects of anti-adrenergic drugs on NS compared to vehicle were assessed using a one-way ANOVA, with post hoc Dunnett’s test for multiple comparisons. For the MB experiments, genotype differences in burying were assessed by unpaired t-test. The effects of anti-adrenergic drugs on MB compared to vehicle were assessed by one-way repeated-measures ANOVA, with post hoc Dunnett’s test for multiple comparisons.
For c-fos quantification, genotype differences were compared in the LC and ACC at baseline and after MB or NS. Comparisons were made within behavioral tasks and between genotypes by multiple t-tests using the Holm-Sidak correction for multiple comparisons. The threshold for adjusted significance was set at p < 0.05, and two-tailed variants of tests were used throughout. Graphical data are presented as group mean ± SEM. Statistical analyses were conducted and graphs were made using Prism Version 7 (GraphPad Software, San Diego, CA).
Results
Central norepinephrine is necessary and sufficient for stress-induced nestlet shredding behavior
A cohort of age- and sex-matched Dbh −/− and Dbh +/− control mice were compared in the NS task, with three different task durations on three different test days: 30 min on Day 1, 60 min on Day 2, and 90 min on Day 3. A two-way repeated measures ANOVA (genotype x task duration) showed a main effect of time (F(2,24) = 20.93, p < 0.0001), a main effect of genotype (F(1,12) = 171.2, p < 0.0001), and a time x genotype interaction (F(2,24) = 15.81, p < 0.001). Post hoc analyses revealed that control mice shredded more nestlets at 60 min (75.44%) and 90 min (96.16%) than at 30 min (22.82%) [p < 0.0001], while shredding was minimal in Dbh −/− mice at all time points (p > 0.05 for all Dbh −/− time point comparisons) (Fig. 1a). After animals were individually housed with nestlets for 24 h, all mice of both genotypes shredded 100% of their nestlets (t( 14) = 0, p > 0.99) (Fig. 1b). Acute restoration of central NE synthesis in Dbh −/− mice with DOPS + benserazide increased shredding to control levels at the 60-min time point (81.68% vs 6.88%; t(9) = 10.97, p < 0.0001) (Fig. 1c).
Fig. 1.
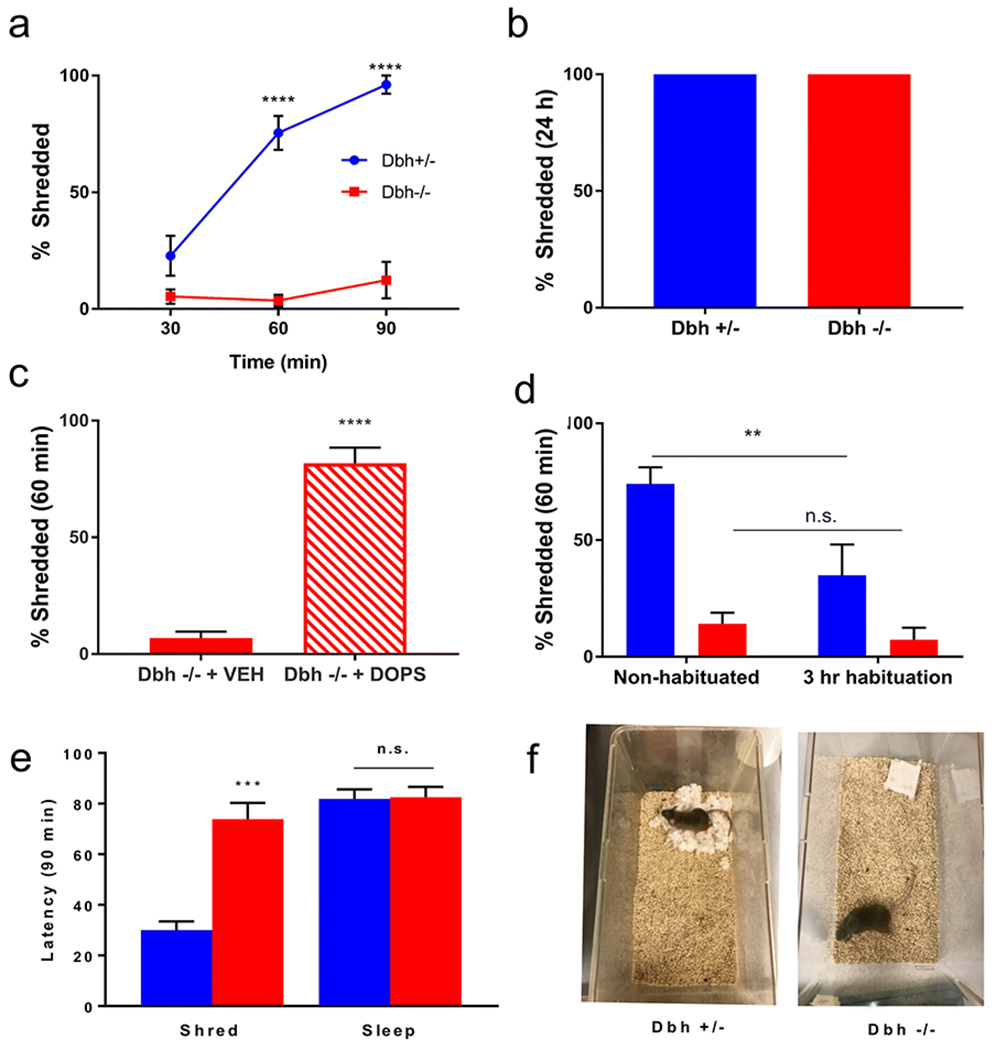
Assessment of nestlet shredding behavior in Dbh −/− and Dbh +/− mice, a Regardless of task duration, NS behavior was profoundly reduced in Dbh −/− mice (n = 7) compared to controls (n = 7). In control mice, NS increased when task duration was extended to 60 or 90 min compared to 30 min, but NS behavior in Dbh−/− mice (n = 7) did not increase as a function of task duration. b When mice were singly housed with nestlets overnight, NS reached 100% and did not differ between Dbh −/− (n = 8) and control mice (n = 8). c Restoring central NE levels in Dbh −/− mice by treating them with DOPS (1 g/kg) + benserazide (250 mg/kg) 5 h prior to testing (n = 5) increased NS compared to vehicle (VEH; n = 6). d 3-h habituation to the test cage significantly reduced NS in control mice (n = 6), indicating that shredding was mediated by acute cage-change stress. Habituation did not affect NS in Dbh −/− mice (n = 6). e During a 90 min test period, Dbh −/− mice (n = 6) exhibited a longer latency to shred but not to sleep compared to Dbh +/− controls (n = 6). f Representative nests after 60 min for Dbh +/− and Dbh −/− mice. ****p < 0.0001, ***p < 0.001, **p < 0.01, n.s. = not significant.
The effect of a 3-h habituation to the test cage on NS (60-min time point) was compared between genotypes. A repeated measures two-way ANOVA (genotype x habituation status) showed a main effect of habituation status (F(1,10) = 9.47, p = 0.01), a main effect of genotype (F(1,10) = 23.62, p < 0.001), and a strong trend for a genotype x habituation status interaction [F(1,10) = 4.71, p = 0.06], Post hoc comparisons revealed that habituation reduced NS in control mice compared to no habituation (74.1% vs 34.84%; p < 0.01), but NS in Dbh −/− mice did not differ significantly after habituation (14.19% vs 7.41%; p > 0.05) (Fig. 1d).
To characterize genotype differences in behavioral repertoires during NS, Dbh −/− and Dbh +/− mice were compared for their latencies to shred and to sleep during a 90 min NS test. Though there was no significant difference between genotypes for latency to sleep (82.6±4.1 vs 81.9±3.7 min; t(10) = 0.12, p > 0.05), Dbh −/− mice demonstrated much longer latencies to shred than Dbh +/− mice (73.9±6.4 vs 30.1±3.4 min; t(10) = 6.04, p < .001). Thus, the absence of NS behavior in Dbh −/− cannot be explained by reduced wakefulness during the NS task. Instead of shredding, Dbh −/− mice engaged in qualitatively unusual behaviors such as frequent bouts of grooming and unsupported rearing. Interestingly, Dbh −/− mice also exhibited two behaviors never observed in Dbh +/− controls. The first of these behaviors were episodes of brief shredding lasting only a few seconds that fell below the 2 min criteria for assessing latency to shred, and which resulted in minimal dispersal of nestlet. By contrast, once Dbh +/− mice began to shred, they continued to do so vigorously until the entire nest was completed. The second behavior unique to the Dbh −/− mice was a tendency to fall asleep outside of nests or on top of unshredded nestlet squares; roughly half of all animals fell asleep within the 90 min task window, but 100% of Dbh +/− fell asleep in fully shredded nests compared to 0% of Dbh −/− mice.
Nestlet shredding can be suppressed in control mice by anti-adrenergic drugs
Because Dbh +/− mice demonstrated robust but not maximal NS behavior at 60 min (Fig. 1a,d), we used this task duration for pharmacological experiments. Control mice were administered either saline vehicle or experimental compounds in their home cages 15 min prior to testing. The test period began once the mouse was placed into a new clean cage with a pre-weighed cotton nestlet square. The experimental compounds used here were the following anti-adrenergic drugs and their mechanisms of action: the α1AR antagonist prazosin (0.5 mg/kg), the βAR antagonist propranolol (10 mg/kg), the α2AR antagonist atipamezole (0.5 mg/kg), the α2AR agonists guanfacine (0.3 mg/kg) and dexmedetomidine (0.02 mg/kg), and the DBH inhibitor nepicastat (100 mg/kg). A one-way ANOVA showed a main effect of treatment (F(6,41) = 126.3, p < 0.0001). Post hoc comparisons revealed that propranolol (p < 0.0001), guanfacine (p < 0.0001), dexmedetomidine (p < 0.0001), and nepicastat (p < 0.0001) reduced NS compared to vehicle. There was no significant difference in NS in the prazosin group compared to saline (p > 0.05). By contrast, atipamezole significantly increased NS behavior compared to vehicle (p = 0.03) (Fig. 2a).
Fig. 2.
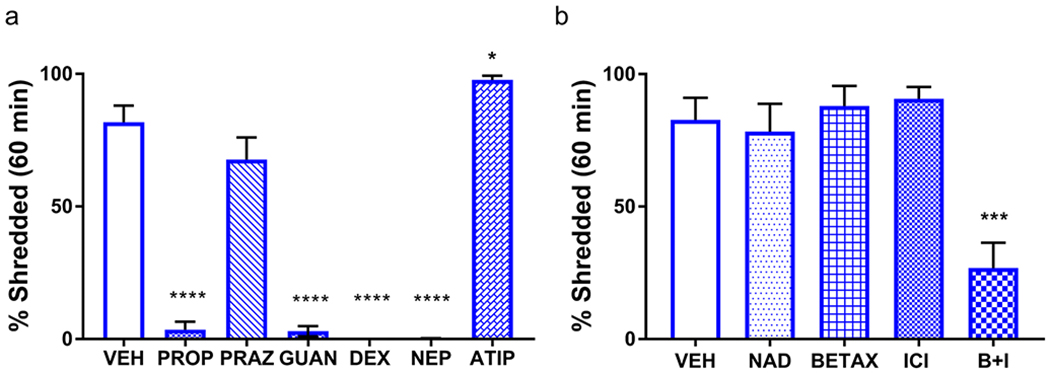
Anti-adrenergic drugs suppress nestlet shredding in control mice. a Compared to vehicle-treated mice (VEH; n = 7), NS was reduced by propranolol (10 mg/kg; PROP; n = 7), guanfacine (0.3 mg/kg; GUAN; n = 7), dexmedetomidine (0.02 mg/kg; DEX; n = 7), and nepicastat (100 mg/kg, NEP; n = 7). NS was enhanced by atipamezole (0.5 mg/kg; ATIP; n = 7) and unaffected by prazosin (0.5 mg/kg; PRAZ; n = 6). b Compared to VEH-treated mice (n = 6), nadolol (10 mg/kg; NAD; n = 6), betaxolol (5 mg/kg; BETAX; n = 7), and ICI-118,551 (1 mg/kg; ICI; n = 7) were ineffective at reducing NS, but the combination of BETAX and ICI (B+I; n = 7) reduced NS compared to VEH. ****p < 0.0001, ***p < 0.001, *p < 0.05.
To rule out the contribution of peripheral βARs and determine whether different subtypes of central βAR regulate the expression of NS, another group of Dbh +/− mice was tested in a separate series of experiments. A one-way ANOVA of treatment efficacy showed a main effect of treatment (F(4, 28) = 10.91, p < 0.0001). Post hoc analyses revealed that the peripheral βAR antagonist nadolol (10 mg/kg) was ineffective at reducing NS compared to vehicle (p > 0.05), as was the selective β1 antagonist betaxolol (5 mg/kg) (p > 0.05) and the selective β2 antagonist ICI 118,551 (1 mg/kg) (p > 0.05). However, a cocktail of the β1 and β2AR antagonists was sufficient to reduce NS compared to vehicle (p < 0.001), consistent with a partially redundant role for β1 and β2 ARs in NS behavior (Fig. 2b).
Genetic or pharmacological suppression of norepinephrine transmission attenuates marble burying
Dbh −/− and Dbh +/− control mice were assessed in the MB task. Compared to control mice, Dbh −/− mice buried fewer marbles (t(26) = 2.37, p = 0.03) (Fig. 3a). Because NE is important for arousal and wakefulness, a subset of mice was filmed to determine whether Dbh −/− mice were not burying marbles because they were falling asleep. Neither control nor Dbh −/− mice fell asleep during the MB task, and mice of both genotypes engaged in typical behaviors, including grooming, rearing, and sniffing. We did notice that some Dbh −/− mice occasionally exhibited qualitatively unusual behaviors not observed in control mice, such as sitting on top of the marbles (data not shown).
Fig. 3.
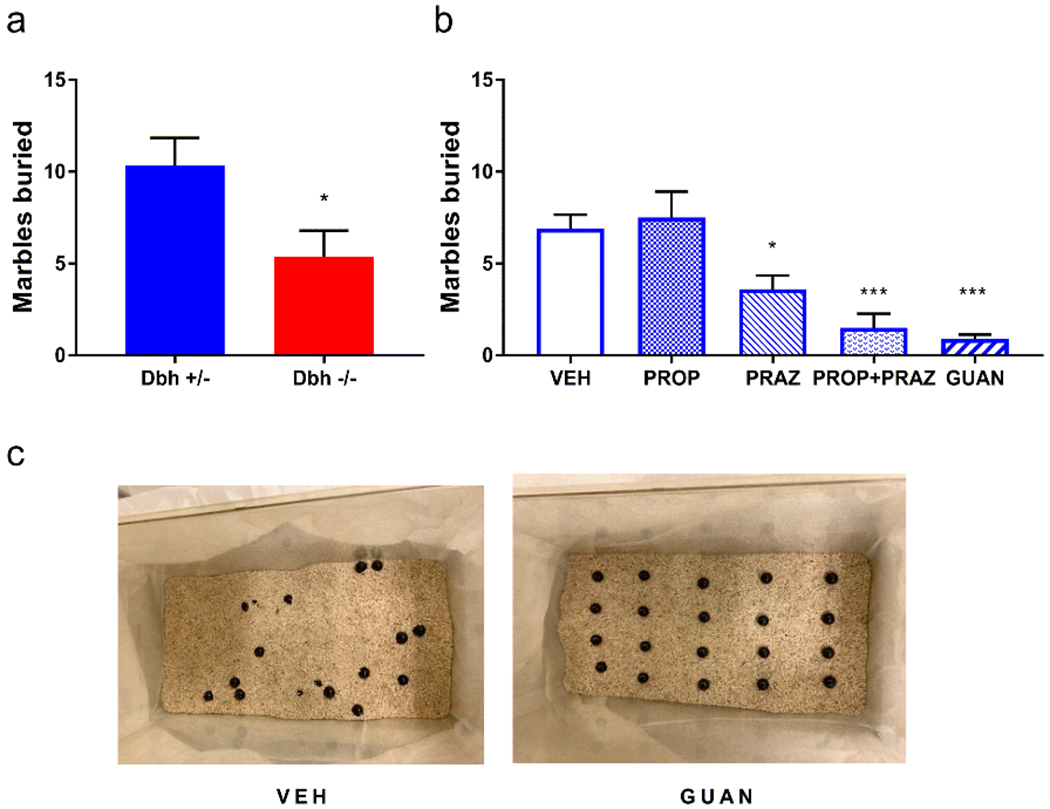
Consequences of genetic and pharmacological disruption of norepinephrine transmission on marble burying. a Compared to Dbh +/− controls (n = 15), Dbh −/− (n = 13) mice buried fewer marbles. b In control mice, PROP (10 mg/kg) was ineffective, but PRAZ (0.5 mg/kg), co-administration of PRAZ + PROP, or GUAN (0.3 mg/kg) reduced MB compared to VEH (n = 10, repeated measures). c Representative images of MB cages from Dbh +/− mice treated with VEH compared to GUAN. ***p < 0.001, *p < 0.05.
Dbh +/− control mice were used for the pharmacological characterization of anti-adrenergic drug effects on MB. The experimental compounds used here were prazosin (0.5 mg/kg), propranolol (10 mg/kg), a cocktail of prazosin and propranolol at the same doses, and guanfacine (0.3 mg/kg). A one-way repeated measures ANOVA of treatment efficacy revealed a main effect of treatment (F(2,18) = 12.11, p < 0.001). Post hoc comparisons demonstrated that, compared to vehicle, prazosin (p < 0.05), prazosin + propranolol (p < 0.001), and guanfacine (p < 0.001) potently suppressed MB compared to vehicle, while propranolol alone was not effective (p > 0.05) (Fig. 3b).
Dbh −/− mice exhibit normal anxiety-like behavior in the elevated zero maze
Dbh −/− and Dbh +/− mice have previously been compared in conflict-based anxiety tasks, including the elevated plus maze (EPM), open field test, and light/dark box, and no knockout phenotypes have been observed (Marino et al. 2005; Schank et al. 2008). To confirm that their MB and NS phenotypes were unique to stress-induced repetitive behaviors and unrelated to general anxiety, we evaluated the performance of knockouts and controls in the EZM, another canonical anxiety assay. There were no statistically significant genotype differences on anxiety-like measures in this task, including the percent of time spent in the open segment (t(17) = 0.01, p > 0.05) (Fig. 4a) or open segment entries (t(17) = 1.10, p > 0.05) (Fig. 4b). Dbh −/− mice did exhibit diminished locomotor activity during the EZM task; they traveled at a reduced average velocity compared to controls (t(17)=2.34, p = 0.03) (Fig. 4c) and tended to travel shorter distances, although the latter did not reach statistical significance (t(17) = 1.92, p = 0.07) (Fig. 4d). Attenuation of these locomotor measures of arousal in Dbh −/− mice is consistent with previous reports that these animals exhibit attenuated locomotor responses to novel environments (Porter-Stransky et al. 2019; Weinshenker et al. 2002).
Fig. 4.
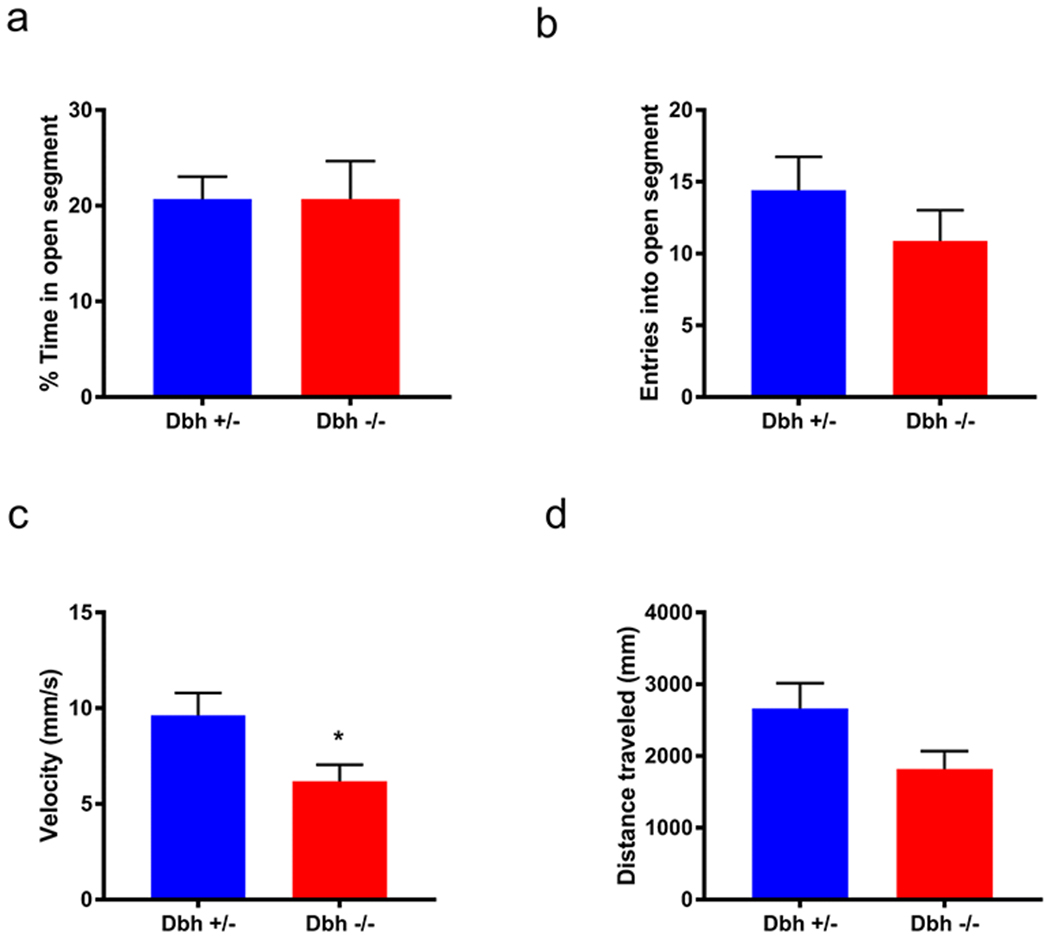
Assessment of Dbh −/− and Dbh +/− mice in the elevated zero maze. Dbh −/− mice did not differ from controls on anxiety-like measures, including a % time in the open (anxiogenic) segment and b entries into the open segment. There was a significant genotype difference in c average velocity and a trend for d total distance traveled. N = 9-10 per group, *p < 0.05.
NE-deficient mice show diminished marble burying- and nestlet shredding-induced c-fos expression in anterior cingulate cortex
Dbh −/− and Dbh +/− control mice were euthanized for quantification of c-fos+ cells in the LC and ACC at baseline and following either NS or MB. At baseline, c-fos expression was minimal and there were no significant genotype differences in c-fos+ cells in either the LC (t(4) = 0.76, p > 0.05) or the ACC (t(4)=0.39 , p > 0.05) (Fig. 5a). Both MB and NS induced robust c-fos expression in both regions, but genotype differences emerged. Following MB, Dbh −/− mice had significantly fewer c-fos+ cells in the ACC (t(7) = 6.38, p < 0.001), but normal c-fos+ induction in the LC (t(7) = 0.07, p > 0.05) (Fig. 5b). Following NS, Dbh −/− mice had significantly fewer c-fos+ cells in the ACC (t(10) = 5.77, p < 0.001) and the LC (t(10) = 5.66, p < 0.001) compared to control mice (Fig. 5c).
Fig. 5.
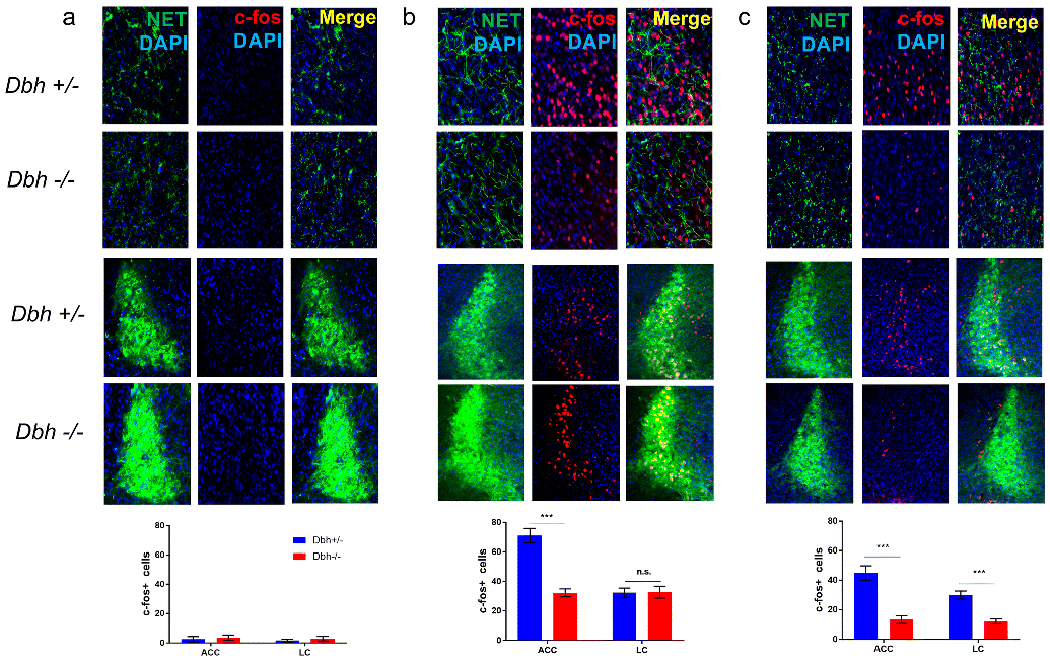
Comparison of nestlet shredding- and mable burying-induced c-fos expression in anterior cingulate cortex and locus coeruleus between Dbh −/− and Dbh +/− mice. a In experimentally naive mice at baseline, c-fos induction was minimal in the ACC (top rows) and LC (bottom rows), and there were no differences in c-fos+ cells between Dbh −/− (n = 3) and control mice (n = 3) in either region. b After MB, fewer c-fos+ cells were detected in the ACC of Dbh −/− mice (n = 4) compared to control mice (n = 5), but no genotype differences were found in the LC. c After NS, fewer c-fos+ cells were detected in both the ACC and LC of Dbh −/− mice (n = 6) compared to control mice (n = 6). ***p < 0.001, n.s. = not significant.
Discussion
Central norepinephrine is necessary and sufficient for stress-induced nestlet shredding behavior
In this study, we compared NS behavior induced by cage-change stress in NE-deficient and control mice. We found that over the course of 90 min, NE-deficient mice demonstrated virtually no NS, whereas their NE-competent littermates vigorously shredded nearly 100% of their nestlets. Importantly, we observed no genotype differences in NS behavior when mice were given 24 h to shred, indicating that the NE-deficient mice are capable of shredding but simply do not do so with any urgency when placed in a new cage. This finding provides evidence that NE is necessary for rapid NS behavior following cage change, but not for typical nest-building behavior in general. When we acutely restored central NE to Dbh −/− mice using DOPS + benserazide, rapid NS behavior was rescued to control levels. This key finding demonstrates that transient pharmacological restoration of central NE synthesis and transmission is sufficient to support stress-induced NS behavior in NE-deficient animals.
Norepinephrine-deficient mice bury fewer marbles in the marble burying task
Although MB is often interpreted as an anxiety-like behavior, performance in the MB task does not correlate with behavioral measures in other models of anxiety, such as the EPM or open field test (Jimenez-Gomez et al. 2011; Thomas et al. 2009). However, MB behavior is highly correlated with NS behavior and does not habituate after repeated exposures, indicating that it better reflects compulsive behavior (Angoa-Pérez et al. 2013; Li et al. 2006; Witkin 2008). Indeed, NE-deficient mice exhibited normal anxiety-like behavior in the EZM but buried fewer marbles than controls in the MB task. This dissociation in behavioral phenotypes is consistent with previously published reports of normal Dbh −/− behavior in canonical conflict-based anxiety paradigms (Marino et al. 2005; Schank et al. 2008) and suggests that NE may play a more important role in the expression of stress-induced compulsive behaviors than in innate anxiety (Brady 1994; Kedia and Chattarji 2014; Mantsch et al. 2010; McCall et al. 2015; Valentino et al. 1993).
Nestlet shredding and marble burying can be suppressed in control mice by anti-adrenergic drugs
NE acts primarily through α1 and βARs to exert neuromodulatory effects on target cells, and NE transmission can be decreased by activation of α2 inhibitory autoreceptors. To determine which ARs are involved in the expression of stress-induced NS behavior, we treated control mice with a battery of anti-adrenergic drugs that block ARs on target cells (α1 and βAR antagonists), activate inhibitory autoreceptors (α2AR agonists), or prevent NE biosynthesis (DBH inhibitors). Prazosin, an α1AR antagonist, did not significantly affect NS compared to saline vehicle. However, disruption of NE synthesis with nepicastat, reduction of NE release with the α2AR agonists guanfacine and dexmedetomidine, or antagonism of βARs with propranolol profoundly suppressed NS behavior in control mice. In a separate set of experiments to determine the requirement of central β1 and β2 AR activation for NS behavior, we found that blockade of βARs outside the brain with the peripherally restricted antagonist nadolol did not suppress NS. We also found that selective antagonism of either β1ARs or β2ARs alone was not sufficient to suppress NS, but NS could be reduced by co-administration of β1- and β2AR-selective antagonists. Thus, the effect of propranolol on NS behavior is likely to be mediated by central β1 and β2ARs, which have overlapping distributions in some brain regions like the ACC, amygdala, and hippocampus, and may serve partially redundant signaling functions (Abraham et al. 2008; Qu et al. 2008; Rainbow et al. 1984; Zheng et al. 2015; Zhou et al. 2013).
NS behavior was increased when NE transmission was enhanced via the α2AR antagonist atipamezole, which blocks α2 inhibitory autoreceptors. In addition, we found that anti-adrenergic drugs potently suppressed MB behavior in control mice. Guanfacine and prazosin, but not propranolol, dramatically reduced MB. These results are consistent with the literature implicating α1AR activation in behavioral reactivity to novelty (Stone et al. 2006), maintenance of generalized arousal (Broese et al. 2012; Porter-Stransky et al. 2019; Stone et al. 2003), and stress-induced anxiety (Rasmussen et al. 2016; Skelly and Weiner 2014).
NE-deficient mice show diminished c-fos induction in anterior cingulate cortex following nestlet shredding and marble burying
The ACC is implicated in the pathophysiology of OCD (Brennan et al. 2015; McGovern and Sheth 2017), and surgical ablation of the ACC can relieve OCD symptoms (Jung et al. 2006; Kim et al. 2003). Furthermore, the ACC expresses all subtypes of ARs (Crino et al. 1993; Rainbow et al. 1984) and is bidirectionally connected to the LC (Gompf et al. 2010; Loughlin et al. 1982). Baseline genotype differences in c-fos induction in the LC and ACC were not seen when animals were euthanized immediately after removal from their home cages. However, c-fos induction was dramatically reduced in the ACC of NE-deficient mice after NS and MB compared to controls. After NS, but not MB, c-fos induction was reduced in the LC of NE-deficient mice compared to controls.
The reason for the emergence of genotype differences in LC activity following NS but not MB is unclear but could be related to task duration and complexity, or possibly to engagement of other structures and neurotransmitter systems that were not considered in our analysis. Given that hyperactivity of the ACC (Fitzgerald et al. 2005; Mavrogiorgou et al. 2002), reduced endocrine response to clonidine (Hollander et al. 1991; Siever et al. 1983), and abnormal catecholamine metabolism (Benkelfat et al. 1991; Schindler et al. 2000) have all been reported in patients with OCD, we propose that excessive NE transmission to the ACC may account for some of the cognitive and affective symptoms of OCD (De Geus et al. 2007).
Clinical implications
Although clinical studies examining the effects of α1AR blockade on OCD symptoms are scarce (Feenstra et al. 2016), our findings suggest that further clinical trials with prazosin for OCD patients are justified. The ability of guanfacine to suppress MB is consistent with evidence from the limited number of studies showing that other α2AR agonists like clonidine and dexmedetomidine can reduce MB (Millan et al. 2000; Young et al. 2006). However, clonidine and dexmedetomidine have potent sedative properties, which can hamper the interpretation of their behavioral effects in rodents and constrains their tolerability for psychiatric patients. At the dose used in this study, guanfacine had no sedative or motor-impairing effects; in fact, the sedative and motor-impairing ED50 for guanfacine in rodents is roughly five-fold higher than the dose used in the present study (Luttinger et al. 1985; Scholtysik 1980; Van Der Laan et al. 1985). Clinically, guanfacine is preferred over clonidine for treatment of pediatric ADHD due to its lack of sedative effects and superior tolerability (Arnsten et al. 1988; Posey and McDougle 2007; Sallee and Eaton 2010; Sallee et al. 2009). Propranolol and guanfacine are well tolerated by patients and have been used both on- and off-label for the treatment of other psychiatric disorders (Chappell et al. 1995; Cummings et al. 2002; Lederman 1999; Steenen et al. 2016). Nepicastat and guanfacine are effective for attenuating compulsive drug-seeking behavior in animal models of addiction (Colombo et al. 2014; Le et al. 2011; Schroeder et al. 2013), as well as relapse in patients with substance abuse disorders (De La Garza II et al. 2015). In scattered psychiatric case studies, the α2AR agonists guanfacine and clonidine have substantially improved OCD symptoms in certain patients (Knesevich 1982; Lipsedge and Prothero 1987; Taormina et al. 2016).
Limitations and future directions
It is noteworthy that mice with genetic 5-HT depletion (Tph2−/−) display exaggerated MB and NS behaviors (Angoa-Pérez et al. 2013; Angoa-Pérez et al. 2012; Kane et al. 2012). MB and NS also show predictive validity in pharmacological experiments; SSRIs and tricyclic antidepressants reduce these repetitive behaviors (Jimenez-Gomez et al. 2011; Li et al. 2006).
Disentangling the role of the NE and 5-HT neuromodulatory systems in the expression of stress-induced compulsive behaviors will be a challenging but worthwhile endeavor. From a circuitry standpoint, it is generally agreed that serotonergic neurons in the raphe nuclei exert a tonic inhibitory influence on the LC, while the LC exerts a tonic excitatory influence on serotonergic neurons in the dorsal raphe (Kim et al. 2004; Pudovkina et al. 2002; Segal 1979; Szabo and Blier 2001). Thus, a possible explanation for the excessive MB and NS behavior observed in 5-HT-deficient mice is that the LC may be hyperactive in the absence of serotonergic modulation. This hypothesis could be tested by administering anti-adrenergic agents, such as nepicastat or guanfacine, to 5-HT-deficient mice and measuring NS and MB behavior when NE transmission is reduced.
A limitation of our study is that we cannot infer a causal role for LC-NE transmission to the ACC in the expression of NS and MB behaviors. More sophisticated studies using tools to manipulate distinct circuits will be required to functionally dissect the NE-dependent network that governs OCD-like behavior. Furthermore, our finding that βARs mediate NS, while α1ARs mediated MB, suggests that different cell types and/or circuits may support these correlated but distinct behaviors. The requirement of α1AR activation for MB but not NS may be related to the size and complexity of the test cage for each task. For MB, testing occurred in large cages “enriched” with marbles, whereas NS was performed in a normal mouse cage with clean bedding and a standard nestlet square. The relative complexity of the MB test environment might activate arousal-promoting α1ARs that are not engaged in the simpler NS environment (Stone et al. 1999; Stone et al. 2005; Stone et al. 2004; Stone et al. 2006). The requirement of βARs for NS but not MB may be related to the longer duration of the task (60 min for NS vs 30 min for MB), which could allow for synergistic actions of corticosterone on βAR signaling in stress-responsive brain regions (Gorman et al. 1993; Roozendaal et al. 2004; Roozendaal et al. 2006).
Finally, this study focused on neuronal activity in two brain regions, the LC and ACC, following MB and NS. The central NE system is anatomically and functionally complex (Robertson et al. 2013; Robertson et al. 2016; Uematsu et al. 2015), and discrete manipulations of genetically defined subpopulations of NE neurons can produce unique or even opposite effects on behavior (Aston-Jones and Waterhouse 2016; Chen et al. 2019; Flavin and Winder 2013; McCall et al. 2017). Moving forward, it will be important to consider the contribution of noradrenergic brainstem groups besides the LC, such as the A2 neurons residing in the nucleus of the solitary tract, to the expression of these behaviors (Itoi and Sugimoto 2010; Rinaman 2010).
Conclusions
In summary, these findings support an expanded model of OCD pathophysiology that incorporates dysregulation of central NE signaling, possibly between the LC and ACC. In addition, we propose that anti-adrenergic agents should be assessed for clinical efficacy in treating OCD, since many of these drugs are already used both on- and off-label for the treatment of related psychiatric diseases. Given the high cost and failure rate of psychiatric drug testing, the possibility of repurposing an approved drug such as guanfacine to treat OCD represents an attractive alternative to new drug development (Lee and Kim 2016).
Acknowledgments:
We thank Lundbeck for providing the DOPS, Synosia Therapeutics for providing the nepicastat, and C. Strauss for helpful editing of the manuscript. This work was supported by the National Institutes of Health (AG061175, NS102306, and DA038453 to DW; GM8602-22 to DL; MH116622 to RPT).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: DW is co-inventor on a patent concerning the use of selective dopamine β-hydroxylase inhibitors for the treatment of cocaine dependence (US-2010-0105748-A1; “Methods and Compositions for Treatment of Drug Addiction”). The other authors declare no conflicts of interest.
References
- Abraham PA, Xing G, Zhang L, Eric ZY, Post R, Gamble EH, Li H. (2008). β1-and β2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain research 1209:65–73 [DOI] [PubMed] [Google Scholar]
- Adams TG, Kelmendi B, Brake CA, Gruner P, Badour CL, Pittenger C. (2018). The role of stress in the pathogenesis and maintenance of obsessive-compulsive disorder. Chronic Stress 2:2470547018758043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari SE, Dougherty DD. (2015). Dissecting OCD circuits: from animal models to targeted treatments. Depression and anxiety 32:550–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon RD, Libb J, Boll T. (1994). Neuropsychological testing in obsessive-compulsive disorder: A clinical review. Journal of neuropsychiatry clinical neurosciences 6:217–217 [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. (2013). Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. JoVE:e50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Briggs DI, Sykes CE, Shah MM, Francescutti DM, Rosenberg DR, Thomas DM, Kuhn DM. (2012). Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. Journal of neurochemistry 121:974–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A, Cai JX, Goldman-Rakic PS. (1988). The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. Journal of Neuroscience 8:4287–4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Iba M, Clayton E, Rajkowski J, Cohen J. (2007). The locus coeruleus and regulation of behavioral flexibility and attention: Clinical implications. Brain norepinephrine: Neurobiology therapeutics:196–235 [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. (1999). Role of locus coeruleus in attention and behavioral flexibility. Biological psychiatry 46:1309–1320 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Waterhouse B. (2016). Locus coeruleus: from global projection system to adaptive regulation of behavior. Brain research 1645:75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkelfat C, Mefford IN, Masters CF, Nordahl TE, King AC, Cohen RM, Murphy DL. (1991). Plasma catecholamines and their metabolites in obsessive-compulsive disorder. Psychiatry research 37:321–331 [DOI] [PubMed] [Google Scholar]
- Berridge C, Arnsten A, Foote S. (1993). Noradrenergic modulation of cognitive function: clinical implications of anatomical, electrophysiological and behavioural studies in animal models. Psychological medicine 23:557–564 [DOI] [PubMed] [Google Scholar]
- Billett E, Richter M, Sam F, Swinson R, Dai X-Y, King N, Badri F, Sasaki T, Buchanan J, Kennedy J. (1998). Investigation of dopamine system genes in obsessive–compulsive disorder. Psychiatric genetics [DOI] [PubMed] [Google Scholar]
- Brady LS. (1994). Stress, antidepressant drugs, and the locus coeruleus. Brain research bulletin 35:545–556 [DOI] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G, Arancio C, Bertani A, Perini G, Carraro C, Gava F. (1997). Noradrenergic receptor sensitivity in obsessive compulsive disorder: II. Cortisol response to acute clonidine administration. Psychiatry research 69:163–168 [DOI] [PubMed] [Google Scholar]
- Brennan BP, Rauch SL, Jensen JE, Pope HG Jr. (2013). A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Biological psychiatry 73:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan BP, Tkachenko O, Schwab ZJ, Juelich RJ, Ryan EM, Athey AJ, Pope HG, Jenike MA, Baker JT, Killgore WD. (2015). An examination of rostral anterior cingulate cortex function and neurochemistry in obsessive–compulsive disorder. Neuropsychopharmacology 40:1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broese M, Riemann D, Hein L, Nissen C. (2012). α-Adrenergic receptor function, arousal and sleep: mechanisms and therapeutic implications. Pharmacopsychiatry 45:209–216 [DOI] [PubMed] [Google Scholar]
- Brown LT, Mikell CB, Youngerman BE, Zhang Y, McKhann GM, Sheth SA. (2016). Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: a systematic review of observational studies. Journal of neurosurgery 124:77–89 [DOI] [PubMed] [Google Scholar]
- Chappell PB, Riddle MA, Scahill L, Lynch KA, Schultz R, Arnsten A, Leckman JF, Cohen DJ. (1995). Guanfacine treatment of comorbid attention-deficit hyperactivity disorder and Tourette’s syndrome: preliminary clinical experience. Journal of the American Academy of Child Adolescent Psychiatry 34:1140–1146 [DOI] [PubMed] [Google Scholar]
- Chen Y-W, Das M, Oyarzabal EA, Cheng Q, Plummer NW, Smith KG, Jones GK, Malawsky D, Yakel JL, Shih Y-YI, Jensen P. (2019). Genetic identification of a population of noradrenergic neurons implicated in attenuation of stress-related responses. Molecular Psychiatry 24:710–725 doi: 10.1038/s41380-018-0245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Harrison BJ, Pujol J, Harding IH, Fornito A, Pantelis C, Yücel M. (2012). Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Human brain mapping 33:1089–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R, Kaplan R, Moser D, Jenkins M, Wilkinson H. (1999a). Impairments of attention after cingulotomy. Neurology 53:819–819 [DOI] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Zuffante P, Moser DJ, Jenkins MA, Salloway S, Wilkinson H. (1999b). Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. The Journal of neuropsychiatry and clinical neurosciences 11:444–453 [DOI] [PubMed] [Google Scholar]
- Colombo G, Maccioni P, Vargiolu D, Loi B, Lobina C, Zaru A, Carai MA, Gessa GL. (2014). The dopamine β-hydroxylase inhibitor, nepicastat, reduces different alcohol-related behaviors in rats. Alcoholism: Clinical Experimental Research 38:2345–2353 [DOI] [PubMed] [Google Scholar]
- Cosgrove GR, Rauch SL. (2003). Stereotactic cingulotomy. Neurosurgery Clinics of North America 14:225–235 [DOI] [PubMed] [Google Scholar]
- Crino PB, Morrison JH, Hof PR (1993) Monoaminergic innervation of cingulate cortex. In: Neurobiology of cingulate cortex and limbic thalamus. Springer, pp 285–310 [Google Scholar]
- Cummings DD, Singer HS, Krieger M, Miller TL, Mahone EM. (2002). Neuropsychiatric effects of guanfacine in children with mild tourette syndrome: a pilot study. Clinical neuropharmacology 25:325–332 [DOI] [PubMed] [Google Scholar]
- Czyrak A, Czepiel K, Maćkowiak M, Chocyk A, Wędzony K. (2003). Serotonin 5-HT1A receptors might control the output of cortical glutamatergic neurons in rat cingulate cortex. Brain research 989:42–51 [DOI] [PubMed] [Google Scholar]
- De Geus F, Denys DA, Sitskoorn MM, Westenberg HG. (2007). Attention and cognition in patients with obsessive–compulsive disorder. Psychiatry clinical neurosciences 61:45–53 [DOI] [PubMed] [Google Scholar]
- De La Garza R II, Bubar MJ, Carbone CL, Moeller FG, Newton TF, Anastasio NC, Harper TA, Ware DL, Fuller MA, Holstein GJJPiN-P, Psychiatry B. (2015). Evaluation of the dopamine β-hydroxylase (DβH) inhibitor nepicastat in participants who meet criteria for cocaine use disorder. 59:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Leong SL, Manning P, Vanneste S, Glue P. (2017). Anterior cingulate implant for obsessive-compulsive disorder. World neurosurgery 97:754. e757–754. e716 [DOI] [PubMed] [Google Scholar]
- Dominguez RA. (1992). Serotonergic antidepressants and their efficacy in obsessive compulsive disorder. The Journal of clinical psychiatry [PubMed] [Google Scholar]
- Durcan MJ, Lister RG, Linnoila M. (1989). Behavioral effects of alpha 2 adrenoceptor antagonists and their interactions with ethanol in tests of locomotion, exploration and anxiety in mice. Psychopharmacology 97:189–193 [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Klompmakers A, Figee M, Fluitman S, Vulink N, Westenberg HG, Denys D. (2016). Prazosin addition to fluvoxamine: A preclinical study and open clinical trial in OCD. European Neuropsychopharmacology 26:310–319 [DOI] [PubMed] [Google Scholar]
- Fineberg N, Chamberlain S, Hollander E, Boulougouris V, Robbins T. (2011). Translational approaches to obsessive-compulsive disorder: from animal models to clinical treatment. British journal of pharmacology 164:1044–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. (2005). Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological psychiatry 57:287–294 [DOI] [PubMed] [Google Scholar]
- Flavin SA, Winder DG. (2013). Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology 70:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodstad H, Strandman E, Karlsson B, West K. (1982). Treatment of chronic obsessive compulsive states with stereotactic anterior capsulotomy or cingulotomy. Acta neurochirurgica 62:1–23 [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. (2000). Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological science 11:1–6 [DOI] [PubMed] [Google Scholar]
- Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB,Lu J. (2010). Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. Journal of Neuroscience 30:14543–14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, McDougle CJ, Price LH, Riddle MA, Pauls D, Leckman J. (1990). Beyond the serotonin hypothesis: a role for dopamine in some forms of obsessive compulsive disorder? The Journal of clinical psychiatry [PubMed] [Google Scholar]
- Gorman AL, Dunn AJJPB, Behavior. (1993). β-Adrenergic receptors are involved in stress-related behavioral changes. Pharmacology Biochemistry Behavior 45:1–7 [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. (2000). Toward a neurobiology of obsessive-compulsive disorder. Neuron 28:343–347 [DOI] [PubMed] [Google Scholar]
- Hajós M, Gartside SE, Varga V, Sharp T. (2003). In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology 45:72–81 [DOI] [PubMed] [Google Scholar]
- Hollander E, DeCaria C, Nitescu A, Cooper T, Stover B, Gully R, Klein DF, Liebowitz MR. (1991). Noradrenergic function in obsessive-compulsive disorder: behavioral and neuroendocrine responses to clonidine and comparison to healthy controls. Psychiatry research 37:161–177 [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. (2010). The brainstem noradrenergic systems in stress, anxiety and depression. Journal of neuroendocrinology 22:355–361 [DOI] [PubMed] [Google Scholar]
- Janer KW, Pardo JV. (1991). Deficits in selective attention following bilateral anterior cingulotomy. Journal of Cognitive Neuroscience 3:231–241 [DOI] [PubMed] [Google Scholar]
- Ji M-H, Jia M, Zhang M-Q, Liu W-X, Xie Z-C, Wang Z-Y, Yang J-J. (2014). Dexmedetomidine alleviates anxiety-like behaviors and cognitive impairments in a rat model of post-traumatic stress disorder. Progress in Neuro-Psychopharmacology Biological Psychiatry 54:284–288 [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez C, Osentoski A, Woods JH. (2011). Pharmacological evaluation of the adequacy of marble burying as an animal model of compulsion and/or anxiety. Behavioural pharmacology 22:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D (2006). Current animal models of obsessive compulsive disorder: a critical review. Progress in Nenro-Psychopharmacology and Biological Psychiatry 30:374–388 [DOI] [PubMed] [Google Scholar]
- Jung HH, Kim C-H, Chang JH, Park YG, Chung SS, Chang JW. (2006). Bilateral anterior cingulotomy for refractory obsessive-compulsive disorder: Long-term follow-up results. Stereotactic functional neurosurgery 84:184–189 [DOI] [PubMed] [Google Scholar]
- Kalanthroff E, Linkovski O, Weinbach N, Pascucci O, Anholt GE, Simpson HB. (2016). What underlies the effect of sleep disruption? The role of alertness in obsessive-compulsive disorder (OCD). Journal of behavior therapy experimental psychiatry 57:212–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Peréz M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, Kuhn DM. (2012). Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PloS one 7:e48975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P, Ott J, Gogos JA. (1999). Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biological psychiatry 45:1178–1189 [DOI] [PubMed] [Google Scholar]
- Kauppila T, Tanila H, Carlson S, Taira T. (1991). Effects of atipamezole, a novel α2-adrenoceptor antagonist, in open-field, plus-maze, two compartment exploratory, and forced swimming tests in the rat. European journal of pharmacology 205:177–182 [DOI] [PubMed] [Google Scholar]
- Kedia S, Chattarji S. (2014). Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. Journal of neuroscience methods 233:150–154 [DOI] [PubMed] [Google Scholar]
- Kim CH, Chang J, Koo MS, Kim J, Suh H, Park I, Lee H. (2003). Anterior cingulotomy for refractory obsessive-compulsive disorder. Acta Psychiatrica Scandinavica 107:283–290 [DOI] [PubMed] [Google Scholar]
- Kim M-A, Lee HS, Lee BY, Waterhouse BD. (2004). Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain research 1026:56–67 [DOI] [PubMed] [Google Scholar]
- Knesevich JW. (1982). Successful treatment of obsessive-compulsive disorder with clonidine hydrochloride. The American journal of psychiatry [DOI] [PubMed] [Google Scholar]
- Langen M, Durston S, Kas MJ, van Engeland H, Staal WG. (2011). The neurobiology of repetitive behavior:… and men. Neuroscience Biobehavioral Reviews 35:356–365 [DOI] [PubMed] [Google Scholar]
- Le A, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. (2011). Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology 218:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman RJ. (1999). Medical treatment of performance anxiety. Anxiety [Google Scholar]
- Lee H-M, Kim Y. (2016). Drug repurposing is a new opportunity for developing drugs against neuropsychiatric disorders. Schizophrenia research treatment 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Morrow D, Witkin JM. (2006). Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life sciences 78:1933–1939 [DOI] [PubMed] [Google Scholar]
- Lin H, Katsovich L, Ghebremichael M, Findley DB, Grantz H, Lombroso PJ, King RA, Zhang H, Leckman JF. (2007). Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive-compulsive disorder. Journal of Child Psychology Psychiatry 48:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsedge M, Prothero W. (1987). Clonidine and clomipramine in obsessive-compulsive disorder. The American journal of psychiatry [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Fallon JH. (1982). Locus coeruleus projections to cortex: topography, morphology and collateralization. Brain research bulletin 9:287–294 [DOI] [PubMed] [Google Scholar]
- Luttinger D, Ferrari R, Perrone M, Haubrich D. (1985). Pharmacological analysis of alpha-2 adrenergic mechanisms in nociception and ataxia. Journal of Pharmacology Experimental Therapeutics 232:883–889 [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. (2010). Involvement of noradrenergic neurotransmission in the stress-but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology 35:2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. (2007). Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37:579–588 [DOI] [PubMed] [Google Scholar]
- Marino MD, Bourdélat-Parks BN, Liles LC, Weinshenker D. (2005). Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behavioural brain research 161:197–203 [DOI] [PubMed] [Google Scholar]
- Marzo A, Totah NK, Neves RM, Logothetis NK, Eschenko O. (2014). Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. Journal of Neurophysiology 111:2570–2588 [DOI] [PubMed] [Google Scholar]
- Mavissakalian M, Michelson L. (1983). Tricyclic antidepressants in obsessive-compulsive disorder: Anti obsessional or antidepressant agents? Journal of Nervous Mental Disease [DOI] [PubMed] [Google Scholar]
- Mavrogiorgou P, Juckel G, Frodl T, Gallinat J, Hauke W, Zaudig M, Dammann G, Möller H-J, Hegerl U. (2002). P300 subcomponents in obsessive-compulsive disorder. Journal of Psychiatric Research 36:399–406 [DOI] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. (2015). CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87:605–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR. (2017). Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife 6:e18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern RA, Sheth SA. (2017). Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery. Journal of neurosurgery 126:132–147 [DOI] [PubMed] [Google Scholar]
- Micallef J, Blin O. (2001). Neurobiology and clinical pharmacology of obsessive-compulsive di sorder. Clinical neuropharmacology 24:191–207 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Lejeune F, Gobert A, Brocco M, Auclair A, Bosc C, Rivet J-M, Lacoste J-M, Cordi A, Dekeyne A. (2000). S18616, a highly potent spiroimidazoline agonist at α2-adrenoceptors: II. Influence on monoaminergic transmission, motor function, and anxiety in comparison with dexmedetomidine and clonidine. Journal of Pharmacology Experimental Therapeutics 295:1206–1222 [PubMed] [Google Scholar]
- Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D. (2006). The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biological psychiatry 60:1046–1052 [DOI] [PubMed] [Google Scholar]
- Mitchell HA, Bogenpohl JW, Liles LC, Epstein MP, Bozyczko-Coyne D, Williams M, Weinshenker D. (2008). Behavioral responses of dopamine β-hydroxylase knockout mice to modafinil suggest a dual noradrenergic–dopaminergic mechanism of action. Pharmacology Biochemistry Behavior 91:217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison CF, Zhang X-Y, Zhang W-P, Ouyang M, Lee A, Thomas SA. (2004). A distinct role for norepinephrine in memory retrieval. Cell 117:131–143 [DOI] [PubMed] [Google Scholar]
- O’Leary OF, Bechtholt AJ, Crowley JJ, Valentino RJ, Lucki I. (2007). The role of noradrenergic tone in the dorsal raphe nucleus of the mouse in the acute behavioral effects of antidepressant drugs. European Nearopsychopharmacology 17:215–226 [DOI] [PubMed] [Google Scholar]
- Pasquier DA, Kemper TL, Forbes WB, Morgane PJ. (1977). Dorsal raphe, substantia nigra and locus coeruleus: interconnections with each other and the neostriatum. Brain research bulletin 2:323–339 [DOI] [PubMed] [Google Scholar]
- Pauls DL, Abramovitch A, Rauch SL, Geller DA. (2014). Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nature Reviews Neuroscience 15:410–424 [DOI] [PubMed] [Google Scholar]
- Pigott TA, L’Heureux F, Dubbert B, Bernstein S, Murphy DL. (1994). Obsessive compulsive disorder: comorbid conditions. Journal of clinical psychiatry 55:15–27; discussion 28-32 [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Williams K. (2011). Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacology therapeutics 132:314–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Krystal JH, Coric V. (2006). Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRx 3:69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro M, Fontenelle LF, Paravidino DC, Yücel M, Miguel EC, de Menezes GB. (2014). An updated review of antidepressants with marked serotonergic effects in obsessive–compulsive disorder. Expert opinion on pharmacotherapy 15:1391–1401 [DOI] [PubMed] [Google Scholar]
- Pooley E, Fineberg N, Harrison P. (2007). The met 158 allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: case–control study and meta-analysis. Molecular Psychiatry 12:556. [DOI] [PubMed] [Google Scholar]
- Porter-Stransky KA, Centanni SW, Karne SL, Odil LM, Fekir S, Wong JC, Jerome C, Mitchell HA, Escayg A, Pedersen NP. (2019). Noradrenergic Transmission at Alpha1-Adrenergic Receptors in the Ventral Periaqueductal Gray Modulates Arousal. Biological psychiatry 85:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey DJ, McDougle CJ. (2007). Guanfacine and guanfacine extended release: treatment for ADHD and related disorders. CNS drug reviews 13:465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudovkina OL, Cremers TI, Westerink BH. (2002). The interaction between the locus coeruleus and dorsal raphe nucleus studied with dual-probe microdialysis. European journal of pharmacology 445:37–42 [DOI] [PubMed] [Google Scholar]
- Qu LL, Guo NN, Li BM. (2008). β1-and β2-Adrenoceptors in basolateral nucleus of amygdala and their roles in consolidation of fear memory in rats. Hippocampus 18:1131–1139 [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. (1984). Quantitative autoradiography of beta 1-and beta 2-adrenergic receptors in rat brain. Proceedings of the National Academy of Sciences 81:1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Kincaid CL, Froehlich JC. (2016). Prazosin prevents increased anxiety behavior that occurs in response to stress during alcohol deprivations. Alcohol and Alcoholism:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffkin J, Yücel M, Maruff P, Wood SJ, Soulsby B, Olver J, Kyrios M, Velakoulis D, Pantelis C. (2005). A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Research: Neuroimaging 138:99–113 [DOI] [PubMed] [Google Scholar]
- Rinaman L (2010). Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 300:R222–R235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, De Marchena J, Jensen P. (2013). Developmental origins of central norepinephrine neuron diversity. Nature neuroscience 16:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, Jensen P. (2016). Uncovering diversity in the development of central noradrenergic neurons and their efferents. Brain research 1641:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger K, Edwards G, Freeman K, Liles L, Miller G, Weinshenker D. (2007). Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proceedings of the National Academy of Sciences 104:13804–13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, Dominique J-F, McGaugh JL. (2004). Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. Journal of Neuroscience 24:8161–8169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JLJ. (2006). Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences 103:6741–6746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso G, Albert U, Asinari GF, Bogetto F, Maina G. (2012). Stressful life events and obsessive–compulsive disorder: clinical features and symptom dimensions. Psychiatry research 197:259–264 [DOI] [PubMed] [Google Scholar]
- Rudoy C, Van Bockstaele E. (2007). Betaxolol, a selective β1-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Progress in Neuro-Psychopharmacology Biological Psychiatry 31:1119–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, Eaton K. (2010). Guanfacine extended-release for attention-deficit/hyperactivity disorder (ADHD). Expert opinion on pharmacotherapy 11:2549–2556 [DOI] [PubMed] [Google Scholar]
- Sallee FR, Mcgough J, Wigal T, Donahue J, Lyne A, Biederman J. (2009). Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. Journal of the American Academy of Child Adolescent Psychiatry 48:155–165 [DOI] [PubMed] [Google Scholar]
- Sara SJJNrn. (2009). The locus coeruleus and noradrenergic modulation of cognition. 10:211. [DOI] [PubMed] [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. (2008). Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biological psychiatry 63:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler KM, Richter M, Kennedy JL, Pato MT, Pato CN. (2000). Association between homozygosity at the COMT gene locus and obsessive compulsive disorder. American journal of medical genetics 96:721–724 [DOI] [PubMed] [Google Scholar]
- Scholtysik G (1980). Pharmacology of guanfacine. British journal of clinical pharmacology 10:21S–24S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Epps SA, Grice TW, Weinshenker D. (2013). The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology 38:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. (1979). Serotonergic innervation of the locus coeruleus from the dorsal raphe and its action on responses to noxious stimuli. The Journal of physiology 286:401–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. (1994). Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology 116:56–64 [DOI] [PubMed] [Google Scholar]
- Siever LJ, Insel TR, Jimerson DC, Lake CR, Uhde TW, Aloi J, Murphy DL. (1983). Growth hormone response to clonidine in obsessive-compulsive patients. The British Journal of Psychiatry 142:184–187 [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Weiner JL. (2014). Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain and behavior 4:468–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel MB, Suhr JA. (2002). Executive function deficits associated with symptoms of schizotypy and obsessive–compulsive disorder. Psychiatry research 110:151–163 [DOI] [PubMed] [Google Scholar]
- Steenen SA, van Wijk AJ, Van Der Heijden GJ, van Westrhenen R, de Lange J, de Jongh A. (2016). Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. Journal of Psychopharmacology 30:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ. (2000). Neurobiology of the obsessive–compulsive spectrum disorders. Biological psychiatry 47:296–304 [DOI] [PubMed] [Google Scholar]
- Stemmelin J, Cohen C, Terranova J-P, Lopez-Grancha M, Pichat P, Bergis O, Decobert M, Santucci V, Françon D, Alonso R. (2008). Stimulation of the β 3-adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology 33:574. [DOI] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, Taber KH. (2011). Anterior cingulate cortex: unique role in cognition and emotion. Journal of neuropsychiatry clinical neurosciences 23:121–125 [DOI] [PubMed] [Google Scholar]
- Stone E, Zhang Y, Rosengarten H, Yeretsian J, Quartermain D. (1999). Brain alpha 1-adrenergic neurotransmission is necessary for behavioral activation to environmental change in mice. Neuroscience 94:1245–1252 [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Ahsan MR, Quartermain D. (2005). α 1-Adrenergic and α 2-adrenergic balance in the dorsal pons and gross behavioral activity of mice in a novel environment. Psychopharmacology 183:127. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Ahsan R, Quartermain D. (2004). Gross mapping of α 1-adrenoceptors that regulate behavioral activation in the mouse brain. Behavioural brain research 152:167–175 [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Rosengarten H, Kramer HK, Quartermain D. (2003). Emerging evidence for a central epinephrine-innervated α 1-adrenergic system that regulates behavioral activation and is impaired in depression. Neuropsychopharmacology 28:1387. [DOI] [PubMed] [Google Scholar]
- Stone EA, Yan L, Ahsan MR, Lehmann ML, Yeretsian J, Quartermain D. (2006). Role of CNS α1-adrenoceptor activity in central fos responses to novelty. Synapse 59:299–307 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Tagawa N, Kobayashi Y, Hotta Y, Yamada J. (2007). Effects of the serotonin and noradrenaline reuptake inhibitor (SNRI) milnacipran on marble burying behavior in mice. Biological Pharmaceutical Bulletin 30:2399–2401 [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. (2001). Functional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neurons. Brain research 922:9–20 [DOI] [PubMed] [Google Scholar]
- Tanaka E, North RA. (1993). Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. Journal of Neurophysiology 69:1749–1757 [DOI] [PubMed] [Google Scholar]
- Taormina SP, Galloway MP, Rosenberg DR. (2016). Treatment Efficacy of Combined Sertraline and Guanfacine in Comorbid OCD and ADHD: Two Case Studies. Journal of developmental behavioral pediatrics 37:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. (2009). Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 204:361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. (1998). Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine β-hydroxylase. Journal of neurochemistry 70:2468–2476 [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. (1995). Noradrenaline is essential for mouse fetal development. Nature 374:643. [DOI] [PubMed] [Google Scholar]
- Tillage RP, Sciolino NR, Plummer NW, Lustberg D, Liles LC, Hsiang M, Powell JM, Smith KG, Jensen P, Weinshenker D. (2020). Elimination of galanin synthesis in noradrenergic neurons reduces galanin in select brain areas and promotes active coping behaviors. Brain Structure Function:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Feng G. (2011). Neurobiology of obsessive–compulsive disorder: insights into neural circuitry dysfunction through mouse genetics. Current opinion in neurobiology 21:842–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Johansen JP. (2015). Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learning and memory 22:444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Page ME. (1993). The Locus Coeruleus as a Site for Integrating Corticotropin-Releasing Factor and Noradrenergic Mediation of Stress Responses a. Annals of the New York Academy of Sciences 697:173–188 [DOI] [PubMed] [Google Scholar]
- van Balkom AJ, van Oppen P, Vermeulen AW, van Dyck R, Nauta MC, Vorst HC. (1994). A meta-analysis on the treatment of obsessive compulsive disorder: A comparison of antidepressants, behavior, and cognitive therapy. Clinical psychology review 14:359–381 [Google Scholar]
- Van Der Laan JW, Van Veenendaal W, Voorthuis P, Weick G, Hillen FC. (1985). The effects of centrally acting adrenergic agonists on temperature and on explorative and motor behaviour. Relation with effects on quasi-morphine withdrawal behaviour. European journal of pharmacology 107:367–373 [DOI] [PubMed] [Google Scholar]
- Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, Cosyns P. (2006). Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. Journal of Nuclear Medicine 47:740–747 [PubMed] [Google Scholar]
- Vankov A, Hervé-Minvielle A, Sara SJ. (1995). Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. European Journal of Neuroscience 7:1180–1187 [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. (2002). Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proceedings of the National Academy of Sciences 99:13873–13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM. (2008). Animal models of obsessive-compulsive disorder. Current protocols in neuroscience 45:9.30. 31–39.30. 39 [DOI] [PubMed] [Google Scholar]
- Wolmarans DW, Stein DJ, Harvey BH. (2016). Of mice and marbles: Novel perspectives on burying behavior as a screening test for psychiatric illness. Cognitive, Affective, Behavioral Neuroscience 16:551–560 [DOI] [PubMed] [Google Scholar]
- Young R, Batkai S, Dukat M, Glennon RA. (2006). TDIQ (5, 6, 7, 8-tetrahydro-1, 3-dioxolo [4, 5-g] isoquinoline) exhibits anxiolytic-like activity in a marble-burying assay in mice. Pharmacology Biochemistry Behavior 84:62–73 [DOI] [PubMed] [Google Scholar]
- Yücel M, Wood SJ, Fornito A, Riffkin J, Velakoulis D, Pantelis C. (2003). Anterior cingulate dysfunction: implications for psychiatric disorders? Journal of Psychiatry and Neuroscience 28:350. [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Luo F, Guo N-n, Cheng Z-y, Li B-m. (2015). β1-and β2-adrenoceptors in hippocampal CA3 region are required for long-term memory consolidation in rats. Brain research 1627:109–118 [DOI] [PubMed] [Google Scholar]
- Zhou H-C, Sun Y-Y, Cai W, He X-T, Yi F, Li B-M, Zhang X-H. (2013). Activation of β2-adrenoceptor enhances synaptic potentiation and behavioral memory via cAMP-PKA signaling in the medial prefrontal cortex of rats. Learning and memory 20:274–284 [DOI] [PubMed] [Google Scholar]
- Zohar J, Chopra M, Sasson Y, Amiaz R, Amital D. (2000). Obsessive compulsive disorder: serotonin and beyond. The World Journal of Biological Psychiatry 1:92–100 [DOI] [PubMed] [Google Scholar]


