Abstract
BACKGROUND:
In the United States in 2019, there was an outbreak of electronic cigarette, or vaping, product use–associated lung injury (EVALI). The manifestations of EVALI in adolescents are not well characterized. We describe the diagnosis, evaluation, and management of EVALI in adolescents hospitalized at a tertiary care, university-affiliated children’s hospital.
METHODS:
A multidisciplinary committee developed an EVALI algorithm on the basis of guidelines from the Centers for Disease Control and Prevention. A retrospective chart review was conducted on patients diagnosed with EVALI. Descriptive analyses included sociodemographic characteristics, clinical presentation, laboratory and imaging results, pulmonary function testing, oxygen requirements, and clinic follow-up.
RESULTS:
Thirteen hospitalized adolescents were diagnosed with confirmed or probable EVALI. The majority were female (54%) with a mean age of 15.9 years. Sixty-nine percent of patients presented with respiratory symptoms, whereas gastrointestinal symptoms were prominent in 85% of patients. Vaping Δ−9-tetrahydrocannabinol was reported in 92% of patients, and vaping nicotine was reported in 62% of patients. All had bilateral ground-glass opacities on the chest computed tomography (CT) scan. Treatment with glucocorticoids led to clinical improvement in 11 of 12 patients. Treatment with glucocorticoids led to improvement in both forced expiratory volume in 1 second and forced vital capacity (P < .05). Four patients required home oxygen on the basis of 6-minute walk test results.
CONCLUSIONS:
Diagnosis of EVALI should be suspected on the basis of vaping history and clinical presentation. Glucocorticoid treatment led to an improvement in symptoms and lung function. The 6-minute walk test may help determine oxygen needs at discharge.
Among school-aged children, there has been a rapid increase in the use of electronic cigarettes (e-cigarettes), colloquially known as vaping, in which a battery-powered coil heats a carrier fluid producing an aerosol for inhalation.1 Recent data from a national survey revealed that the prevalence of vaping has more than doubled among eighth-, 10th-, and 12th-graders from 2017 to 2019; 25.4% of 12th-graders in 2019 reported vaping in the previous 30 days.1 Additional data from 2019 revealed that 3.9% of eighth-graders, 12.6% of 10th-graders, and 14.0% of 12th-graders reported vaping marijuana in the previous 30 days.2 The impact of rising e-cigarette use in children merits special attention because neurodevelopmental changes during adolescence confer particular vulnerability to substance abuse. Inhalational injury is also a concern given case reports of adverse effects on the lung, including increased school absence due to asthma,3 hypersensitivity pneumonitis,4 eosinophilic pneumonia,5 lipoid pneumonia,6 and diffuse alveolar hemorrhage.7
As of February 18, 2020, the Centers for Disease Control and Prevention (CDC) described a total of 2807 hospitalized patients with electronic cigarette, or vaping, product use–associated lung injury (EVALI) across 50 states and 2 US territories, of whom 15% were <18 years of age.8 The CDC has postulated that the additive vitamin E acetate (VEA) is a causative factor.9 The severity of illnesses reported ranges from minimal or no respiratory support to invasive mechanical ventilation and ICU admission.1,10–14 To our knowledge, there are no published clinically focused case series of EVALI in children. Our objective for this case series is to describe our institutional experience of diagnosing, evaluating, and managing pediatric EVALI, with special attention to psychosocial risk, clinical presentation, laboratory and imaging findings, pulmonary function testing (PFT), and response to therapy.
METHODS
A retrospective chart review on all reported patients with EVALI from December 2018 to November 2019 presenting to our tertiary care, university-affiliated children’s hospital was conducted. Patients were identified on the basis of consultations received by the medical toxicology and/or pulmonology service for suspicion of EVALI. Cases meeting the confirmed or probable CDC case definitions were included in the analysis.12 After several adolescents were diagnosed with EVALI, an EVALI steering committee of key stakeholders was formed and included the divisions of pediatric respiratory medicine, medical toxicology, pediatric emergency medicine, pediatric critical care, pediatric hospital medicine, and adult pulmonology and critical care. The committee collectively reviewed the literature and devised a clinical algorithm to standardize the inpatient management of EVALI (Fig 1). This can be summarized as follows: A clinician suspects EVALI on the basis of clinical symptoms, vaping history within the 90 days before admission, and radiographic or imaging findings of bilateral ground-glass opacities. This prompts consultation of both pulmonology and medical toxicology followed by a laboratory workup to rule out infection and other potential causes. The results of the workup should guide the decision to start antimicrobial agents or move directly to treatment with steroids for EVALI.
FIGURE 1.
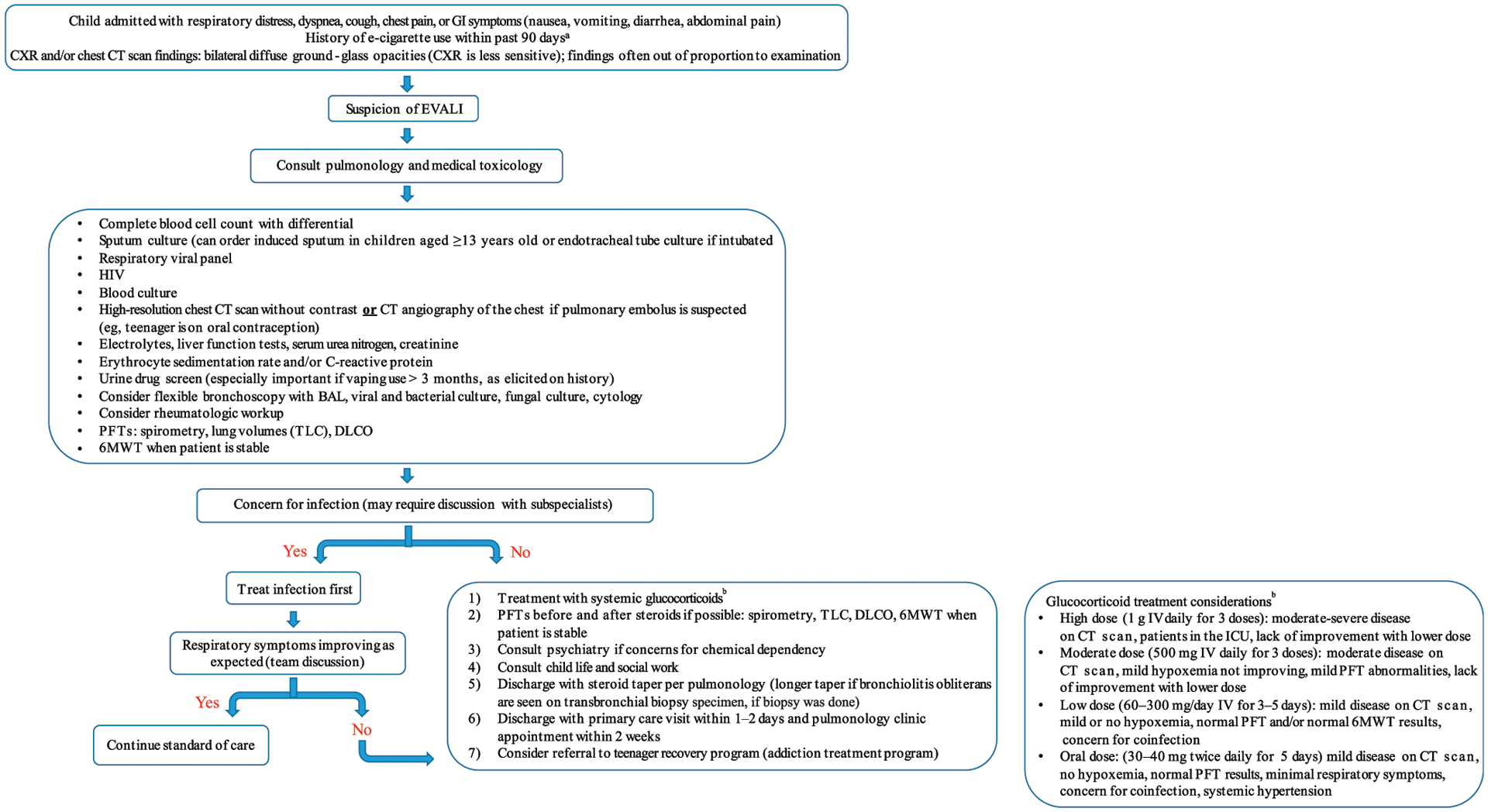
Clinical algorithm for pediatric patients hospitalized for suspected EVALI. The algorithm includes CDC-based diagnostic criteria, recommended consultations, laboratory workup and imaging, and recommended treatment and follow-up. a May need to speak with peers and family, especially for patients who are intubated. b glucocorticoid dosage considerations. CXR, chest radiograph; IV, intravenously; TLC, total lung capacity.
Data elements collected from an electronic medical record review are detailed in Table 1. Descriptive statistics were performed by using SAS version 7.12 (SAS Institute, Inc, Cary, NC). The Wilcoxon rank test was used to compare PFT results before and after corticosteroid treatment by using IBM SPSS version 24 (IBM SPSS Statistics, IBM Corporation). Some patients had PFTs obtained for the first time after steroid initiation because of inability to perform the forced maneuver while ill; these PFTs and subsequent PFTs for these patients were not included in the analysis of PFT data. PFTs were performed at an American Thoracic Society–accredited pulmonary function laboratory and reviewed by the first author for quality.15,16 All data were deidentified before statistical analysis. Institutional review board approval was obtained from the University of Texas Southwestern Medical Center.
TABLE 1.
Characteristics of Adolescent Patients Admitted With EVALI
| Characteristics | Results |
|---|---|
| No./total No. (%) | 13/13 (100)a |
| Mean age (range), y | 15.9 (13–18) |
| Female sex, No. (%) | 7 (54) |
| Race and/or ethnicity, No. (%) | |
| White | 7 (54) |
| Hispanic | 6 (46) |
| E-cigarette or vaping history, No. (%) | |
| E-cigarette or vaping use in previous 90 d | |
| Δ−9-tetrahydrocannabinol | 12 (92) |
| Nicotine | 8 (62) |
| Both nicotine and Δ−9-tetrahydrocannabinol | 7 (54) |
| Nicotine vaping history, No./total No. (%) | |
| Duration | |
| 3–6 mo | 1/8 (13) |
| >12 mo | 5/8 (63) |
| Unknown | 2/8 (25) |
| Frequency | |
| Multiple times a wk or less | 1/8 (13) |
| Daily | 1/8 (13) |
| Multiple times per d | 5/8 (63) |
| Unknown | 1/8 (13) |
| Cartridges used per wk | |
| <1 | 2/8 (25) |
| 2–7 | 2/8 (25) |
| Unknown | 4/8 (50) |
| Source of cartridge | |
| Friend | 3/8 (38) |
| Acquaintance or dealer | 1/8 (13) |
| Unknown | 4/8 (50) |
| Δ−9-tetrahydrocannabinol vaping history, No./total No. (%) | |
| Duration | |
| 3–6 mo | 3/12 (25) |
| 6–12 mo | 1/12 (8) |
| >12 mo | 8/12 (67) |
| Frequency | |
| Multiple times a wk or less | 5/12 (42) |
| Daily | 1/12 (8) |
| Multiple times per d | 6/12 (50) |
| Cartridges used per wk | |
| ≤1 | 6/12 (50) |
| 2–7 | 1/12 (8) |
| Unknown | 5/12 (42) |
| Source of cartridge | |
| Friend | 5/12 (42) |
| Acquaintance or dealer | 4/12 (33) |
| Black market | 1/12 (8) |
| Unknown | 2/12 (17) |
| Vaping brands reported, No./total No. (%)b | |
| Nicotine brands | |
| JUUL | 5/8 (63) |
| NJoy | 2/8 (25) |
| Suorin | 1/8 (13) |
| Delosi | 1/8 (13) |
| Δ−9-tetrahydrocannabinol brands | |
| Dank | 3/12 (25) |
| Rove | 2/12 (17) |
| Cali plug | 2/12 (17) |
| Chronic carts | 1/12 (8) |
| Runtz | 1/12 (8) |
| Stig | 1/12 (8) |
| EonSmoke | 1/12 (8) |
| Brass knuckles | 1/12 (8) |
| Dr Zodiac | 1/12 (8) |
| Reported length of e-cigarette use (range), d | 30–730 |
| Psychosocial history, No. (%) | |
| Stressors identified from psychosocial history | |
| Home environment | 6 (46) |
| Academic difficulty | 8 (62) |
| Behavior problems | 6 (46) |
| History of mood or anxiety disorder | 7 (54) |
| History of suicidal or homicidal ideation | 5 (38) |
| Nicotine use (other than e-cigarette or vaping) | 5 (38) |
| Substance use (other than e-cigarette or vaping) | 12 (92) |
| Symptoms reported at presentation | |
| Median duration of symptoms before presentation (range), d | 3 (2–21) |
| Constitutional symptoms, No. (%) | 13 (100) |
| Subjective fever | 13 (100) |
| Fatigue or malaise | 8 (62) |
| Weight loss | 6 (46) |
| Excessive sweating | 3 (23) |
| Respiratory symptoms, No. (%) | 11 (85) |
| Cough | 11 (85) |
| Shortness of breath | 10 (77) |
| Chest pain | 9 (69) |
| Dyspnea on exertion | 6 (46) |
| Wheezing | 3 (23) |
| GI symptoms, No. (%) | 11 (85) |
| Vomiting | 11 (85) |
| Nausea | 10 (77) |
| Diarrhea | 8 (62) |
| Abdominal pain | 6 (46) |
| Abnormal vital signs at presentation, No. (%) | |
| Respiratory rate ≥20 breaths per min | 11 (85) |
| Oxygen saturation ≤95% in ambient air | 11 (85) |
| Temperature ≥38°C | 10 (77) |
| Heart rate ≥120 beats per min | 9 (69) |
| Blood pressure ≥135/85 | 0 (0) |
| Initial laboratory evaluation | |
| White blood cell count >11 000 cells per mm3, No. (%) | 11 (85) |
| White blood cell count with >80% neutrophils, No./total No. (%) | 9/12 (75) |
| C-reactive protein level >1 mg/dL, No./total No. (%) | 12/12 (100) |
| Erythrocyte sedimentation rate >15 mm/h, No./total No. (%) | 10/11 (91) |
| Erythrocyte sedimentation rate >30 mm/h, No./total No. (%) | 8/11 (73) |
| Respiratory viral panel result, No./total No. (%) | |
| Negative | 10/12 (83) |
| Coronavirus | 1/12 (8) |
| Rhinovirus | 1/12 (8) |
| Urine drug screen positive for Δ−9-tetrahydrocannabinol, No./total No. (%) | 11/11 (100) |
| Imaging findings on chest CT scan, No. (%) | |
| Bilateral ground-glass opacities | 13 (100) |
| Lung bases greater than lung apices | 6 (46) |
| Interlobular septal thickening | 2 (15) |
| Pneumomediastinum | 2 (15) |
| Crazy paving | 1 (8) |
| Bronchoscopy findings | |
| Negative BAL bacterial culture result, No./total No. (%) | 6/6 (100) |
| Lymphocytes, mean (range), % | 13.3 (2–32) |
| Neutrophils, mean (range), % | 27.8 (2–81) |
| Macrophages, mean (range), % | 25.8 (15–69) |
| Eosinophils, mean (range), % | 14.0 (1–46) |
| Normal bronchial mucosa, No./total No. (%) | 1/6 (17) |
| Clinical course | |
| Hospitalization, No. (%) | 13 (100) |
| Admission to ICU | 4 (31) |
| Maximum respiratory support, No. (%) | |
| Room air | 1 (8) |
| Nasal cannula | 8 (62) |
| Bilevel positive airway pressure | 2 (15) |
| Intubation with invasive mechanical ventilation | 1 (8) |
| VV-ECMO | 1 (8) |
| Duration of hospitalization, median (range), d | 7 (2–120) |
| Treatment course | |
| Antibiotics for lower respiratory tract infection, No. (%) | |
| Oral antibiotics before hospitalization | 6 (46) |
| Intravenous antibiotics during hospitalization | 9 (69) |
| Systemic glucocorticoids, No./total No. (%) | |
| Oral and intravenous glucocorticoids | 10/12 (83) |
| Oral glucocorticoids alone | 1/12 (8) |
| Intravenous glucocorticoids alone | 1/12 (8) |
| Required supplemental oxygen at discharge, No. (%) | 4 (31) |
Denominator equals 13 unless otherwise specified.
Each patient may have reported multiple brands; 3 patients had unknown brands.
RESULTS
Thirteen patients were diagnosed with confirmed or probable EVALI. The mean age was15.9 years, 54% of patients were female, and 46% of patients were Hispanic. Twelve of 13 patients sought treatment from primary care clinics, urgent care, or outside emergency departments before diagnostic admission. One patient had 2 previous admissions to our institution before a urine toxicology positive for Δ−9-tetrahydrocannabinol prompted the team to consider the diagnosis of EVALI. EVALI admissions peaked in September 2019.
Vaping Δ−9-tetrahydrocannabinol was reported by 92% of patients, with 62% also vaping nicotine. Exclusive nicotine vaping was reported by one patient; however, caregivers had suspicion of additional substance use. A urine toxicology screening was not performed on this patient. Combustible cigarette use was reported by 38% of patients. Reported duration of Δ−9-tetrahydrocannabinol vaping ranged from 1 month to 2 years. Only one patient admitted knowingly obtaining products from an unregulated distributor. All others who disclosed their sources cited friends and acquaintances. Of note, Texas prohibits retail dispensaries of Δ−9-tetrahydrocannabinol–containing products.
A previous diagnosis of substance use disorder was present in 31% of patients. Presence of stressors in at least 3 of the 5 psychosocial risk domains was found in 54% of patients. Risk domains included home environment, academic difficulty, behavior problems, mental health, and substance use. Two patients had stressors in all 5 domains, whereas only 2 patients had no documented stressors. Six patients received psychiatry consultations, of whom5 received inpatient treatment recommendations for nicotine or Δ−9-tetrahydrocannabinol withdrawal. Symptoms concerning for Δ−9-tetrahydrocannabinol withdrawal were noted in 3 patients. Referral to an adolescent-specific addiction treatment program was provided for 5 patients.
All patients underwent evaluation for other conditions such as asthma, typical and atypical pneumonia, appendicitis, sepsis, and pulmonary embolism. Eighty-five percent of patients had respiratory symptoms at presentation ranging from mild to severe. Cough, shortness of breath, and chest pain were the most common complaints. Gastrointestinal (GI) distress with minimal or no respiratory symptoms characterized the presentation of 4 patients. GI symptoms occurred in 85% of patients, with vomiting and nausea predominating. All patients eventually developed respiratory symptoms.
The urine drug screen was positive for Δ−9-tetrahydrocannabinol metabolites in all 11 patients who received screening. Of 9 patients with a subsequent urine comprehensive toxicology panel analyzed by liquid chromatography and mass spectroscopy, all had Δ−9-tetrahydrocannabinol and/or Δ−9-tetrahydrocannabinol metabolites. A private laboratory analysis of 5 Δ−9-tetrahydrocannabinol cartridges provided by 1 patient confirmed the presence of Δ−9-tetrahydrocannabinol. Δ−9-tetrahydrocannabinol made up 31% of the e-liquid solution in the patient’s most recently used cartridge. In this same cartridge, VEA was detected and made up 38% of the solution. VEA was found in 3 of this patient’s cartridges. This patient reported obtaining cartridges from friends and acquaintances.
Chest computed tomography (CT) scans revealed diffuse bilateral ground-glass opacities in all patients (Figs 2–4). Notably, abnormal lung findings were first identified on abdominal CT scans in 23% of patients who underwent a primary workup for GI symptoms. Fifteen percent of patients with subtle chest radiograph findings had markedly abnormal lung findings on the chest CT scan.
FIGURE 2.
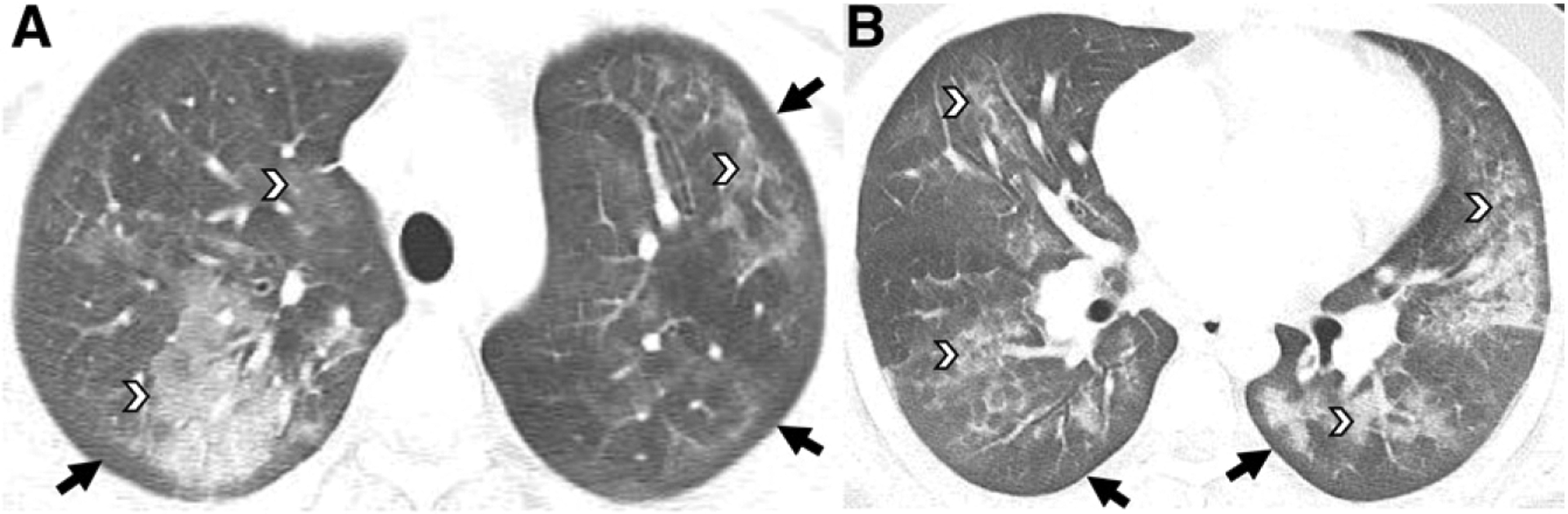
Fifteen-year-old boy with daily Δ−9-tetrahydrocannabinol vaping. A and B, Axial chest CT images reveal ground-glass opacities involving all lobes with a central, mid, and peripheral distribution (white arrowheads) and subpleural sparing throughout all lobes (black arrows).
FIGURE 4.
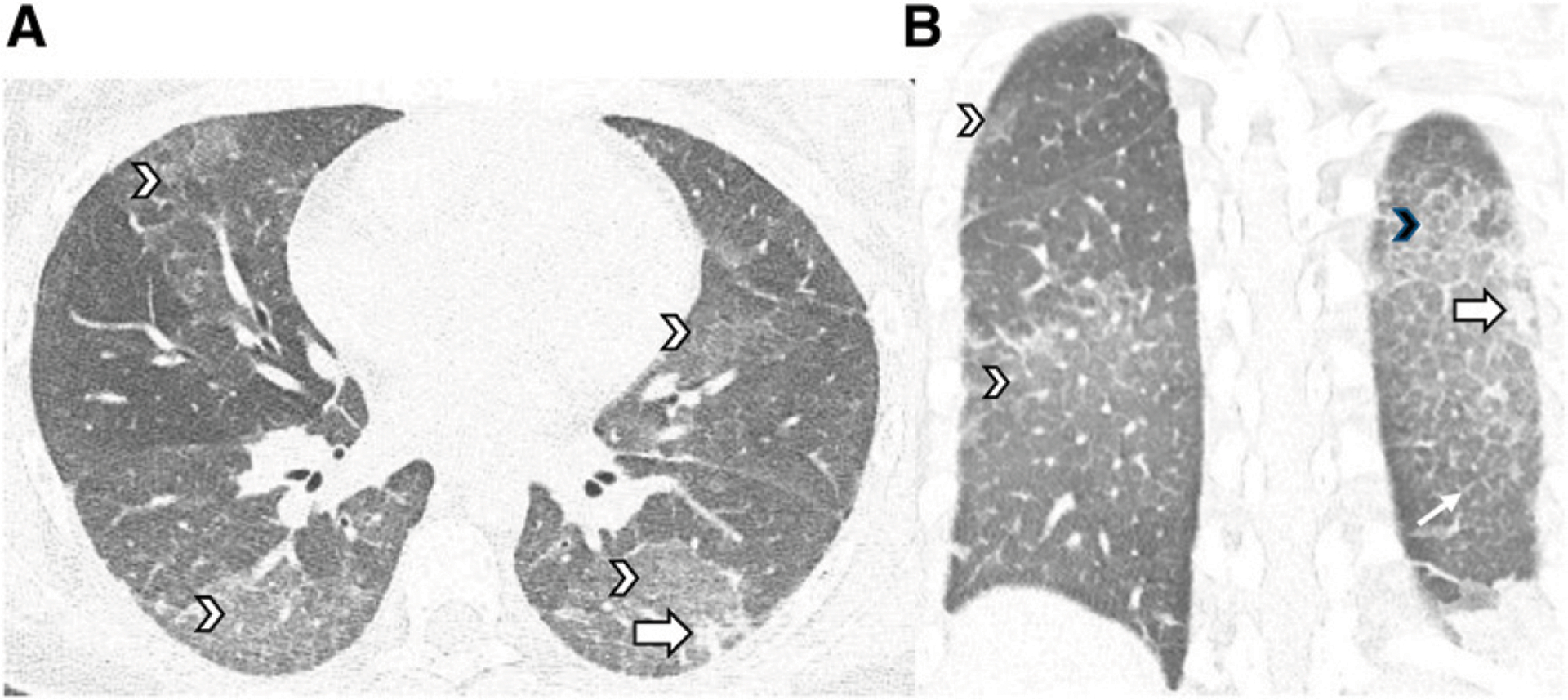
Sixteen-year-old girl with daily nicotine and Δ−9-tetrahydrocannabinol vaping. A and B, Chest CT images in the axial and coronal planes reveal diffuse ground-glass opacities (white arrowheads), a consolidative opacity (large white arrows), interlobular septal thickening (small white arrow), and crazy paving (black arrowhead) in the left lower lobe.
The degree of respiratory support among patients varied. Nasal cannula was required by most patients (62%), whereas 2 patients required bilevel positive airway pressure, 1 required intubation with invasive mechanical ventilation, and 1 required veno-venous extracorporeal membrane oxygenation (VV-ECMO). The patient who was the sickest required 2 courses of VV-ECMO, chest tube placement for persistent air leak syndrome, and an eventual tracheostomy.
Oral and/or intravenous glucocorticoid treatment was provided to 12 of 13 patients (Table 2). Of these, 11 demonstrated clinical improvement within 24 hours. One patient who remained on room air throughout admission had minimal abnormalities on the chest CT scan described as “subtle diffuse ground-glass pulmonary opacities” and improved with low-dose oral steroids. Eleven patients received intravenous steroids, of whom 7 received pulse dosing. The lone patient who did not demonstrate rapid improvement from glucocorticoid therapy was the patient who required VV-ECMO. Sixty-nine percent of patients received intravenous antibiotics but had no corresponding improvement.
TABLE 2.
Glucocorticoid Treatment Regimen by Patient
| Patient | IV Glucocorticoids | Pulse Dosinga | Oral Glucocorticoids | Taper Dosing |
|---|---|---|---|---|
| 1 | Yes | — | Yes | Yes |
| 2 | Yes | Yes | Yes | Yes |
| 3 | Yes | Yes | — | — |
| 4 | Yes | Yes | Yes | Yes |
| 5 | — | — | — | — |
| 6 | Yes | — | Yes | Yes |
| 7 | Yes | — | Yes | Yes |
| 8 | Yes | Yes | Yes | Yes |
| 9 | Yes | Yes | Yes | Yes |
| 10 | Yes | Yes | Yes | Yes |
| 11 | — | — | Yesb | Yes |
| 12 | Yes | — | Yes | Yes |
| 13 | Yes | Yes | Yes | Yes |
IV, intravenous; —, not received.
1000 mg of methylprednisolone every 20 h for 3 d.
40 mg of prednisone twice daily for 1 d followed by taper dosing.
All patients reported complete or near-complete resolution of their previous constitutional, respiratory, and GI symptoms before discharge; however, 31% were prescribed supplemental oxygen with activity at discharge, as indicated by 6-minute walk test (6MWT) results. The patient who required VV-ECMO and a tracheostomy was discharged to a rehabilitation facility with a home ventilator and eventually weaned from ventilator support 110 days after initial hospital admission. The patient is now decannulated.
Most patients performed PFTs (Table 3). Spirometry and 6MWT results were assessed at least once in 92% and 77% of patients, respectively. Two out of 13 patients had an obstructive pattern even after steroids were administered. Five patients completed the spirometry before and after initiation of steroids (Fig 5). Four total patients had plethysmography, diffusion capacity testing, and a 6MWT performed before and after initiation of steroids (Figs 5 and 6). All patients who had PFTs before and after initiation of steroids demonstrated improvement in forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), total lung capacity, and diffusion capacity of carbon monoxide (DLCO) (Table 4). The relative improvement in FEV1 ranged from 9% to 35%, with a mean of 23%, and was significant (P = .042).
TABLE 3.
PFT Completion by Patient
| Patient | Before Glucocorticoidsa | After Glucocorticoidsb | Clinic Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spirometry | LV | DLCO | 6MWT | Spirometry | LV | DLCO | 6MWT | Spirometry | LV | DLCO | 6MWT | |
| 1 | — | — | — | — | — | — | — | Yes | Yes | — | — | Yes |
| 2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3 | — | — | — | Yes | Yes | Yes | Yes | Yes | — | — | — | — |
| 4 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | — | — | — | — |
| 5 | — | — | — | — | — | — | — | — | Yes | Yes | Yes | — |
| 6 | — | — | — | — | Yes | Yes | — | Yes | — | — | — | — |
| 7 | — | — | — | — | Yes | Yes | Yes | Yes | — | — | — | Yes |
| 8 | — | — | — | — | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9 | — | — | — | — | Yes | Yes | Yes | Yesc | Yes | Yes | Yes | Yes |
| 10 | Yes | Yes | Yes | Yes | — | — | — | — | Yes | Yes | Yes | Yesd |
| 11 | Yes | Yes | Yes | Yes | — | — | — | — | Yes | Yes | Yes | — |
| 12 | — | — | — | — | — | — | — | — | — | — | — | — |
| 13 | Yes | — | — | — | Yes | — | — | — | — | — | — | — |
LV, lung volume; —, not performed.
Before or within 24 h of initiation of glucocorticoid treatment.
More than 24 h after initiation of glucocorticoid treatment and completed during hospitalization.
Tested with 1 L of oxygen via nasal cannula.
Questionable effort.
FIGURE 5.
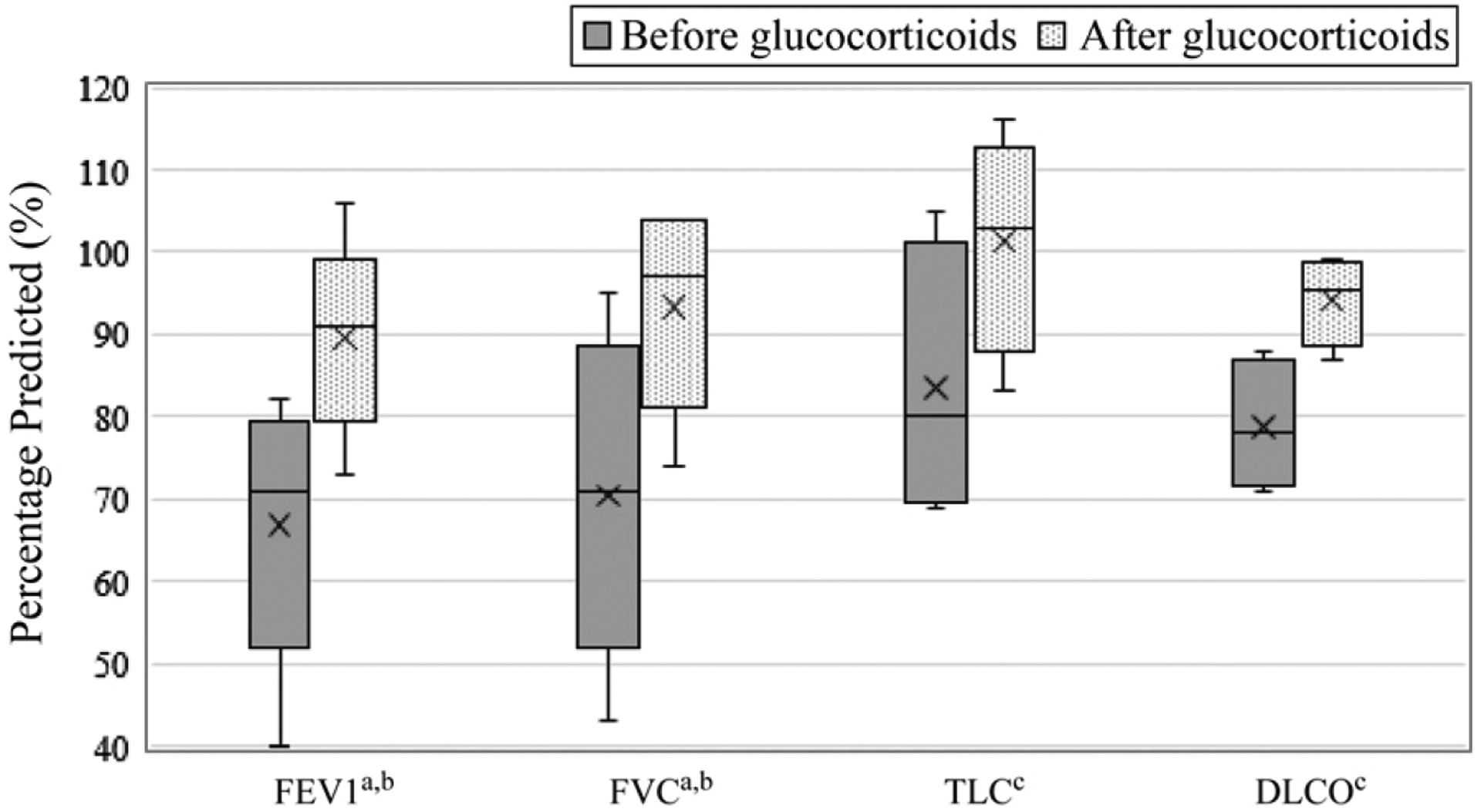
Pulmonary function response to glucocorticoid treatment. The figure reveals the change in PFT results in response to steroid treatment. Preglucocorticoid values were obtained within 24 hours of steroid initiation. Postglucocorticoid values were obtained at the first repeat testing during hospitalization or at the clinic follow-up. a n = 5. b P < .05. c n = 4. TLC, total lung capacity.
FIGURE 6.
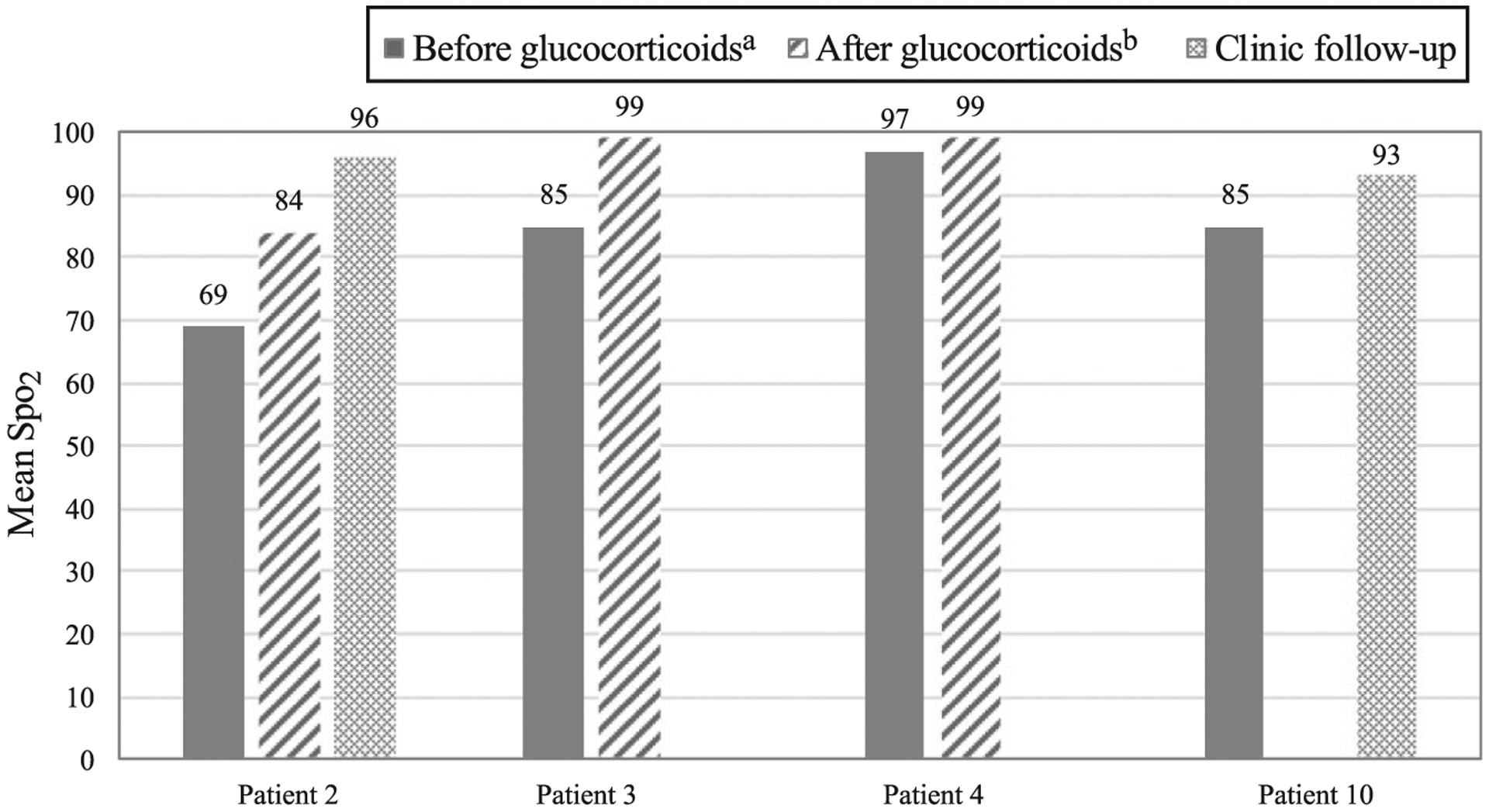
6MWT response to glucocorticoid treatment. The figure reveals the mean oxygen saturation during the 6MWT by patient, with testing completed both before and after initiation of glucocorticoid treatment. a Before or within 24 hours of initiation of glucocorticoid treatment. b More than 24 hours after initiation of glucocorticoid treatment and completed during hospitalization. Spo2, pulse oxygen saturation.
TABLE 4.
PFT Response to Glucocorticoid Treatment
| Before Glucocorticoids, Meana | After Glucocorticoids, Meanb | P | |
|---|---|---|---|
| FEV1, % predictedc | 66.8 | 89.6 | .042 |
| FVC, % predictedc | 70.4 | 93.4 | .043 |
| TLC, % predictedd | 83.5 | 101.3 | .066 |
| DLCO, % predictedd | 78.8 | 94.3 | .068 |
| 6MWD, % predictedd | 62.3 | 73.3 | .068 |
| 6MWT Spo2d | 84 | 93.8 | .068 |
Spo2, pulse oxygen saturation; TLC, total lung capacity; 6MWD, 6-min walk distance.
Before or within 24 h of initiation of glucocorticoid treatment.
First repeat testing after initiation of glucocorticoid treatment; completed during hospitalization or at clinic follow-up.
n = 5.
n = 4.
DISCUSSION
We describe a unique cohort of pediatric patients diagnosed with EVALI. As in other case series, the nature of the lung injury was not infectious.12,17 Rather, our patients demonstrated a systemic inflammatory process evidenced by elevated inflammatory markers and multisystem disease. The GI tract was affected in 85% of patients, similar to other EVALI case series in which authors reported GI symptoms in 77% to 92% of patients.12,17 Use of glucocorticoids was successful in abating this systemic inflammatory response, with clinical improvement noted in most cases, similar to other case series.12,17,18 However, our patient who was the sickest required 2 runs of VV-ECMO despite steroid therapy and underwent a tracheostomy for long-term mechanical ventilation. This patient’s extended course is likely due to prolonged air leak syndrome, and it is unclear what factors in this patient’s history led to such severe lung disease. Of note, this patient had several of the known EVALI risk factors reported by the CDC: use >5 times per day, exclusive vaping of Δ−9-tetrahydrocannabinol, and use of Dank vapes.19 In addition, the patient’s most recently used cartridges contained VEA.
The CDC has reported that the likely culprit for EVALI is VEA, although the exact mechanism of injury is unknown. In November 2019, the CDC described that residual bronchoalveolar lavage (BAL) fluid from 29 patients contained VEA,20 a thickening agent added to dilute Δ−9-tetrahydrocannabinol oil.21 More recently, a study revealed that of 51 BAL samples from patients with EVALI, 94% tested positive for VEA, compared with none of the BAL samples from 99 healthy adults.9 The authors suggested that the addition of VEA to Δ−9-tetrahydrocannabinol e-liquids became more popular in 2019, leading to the nationwide peak in EVALI cases in September 2019.
Our patients frequently reported buying Δ−9-tetrahydrocannabinol cartridges from dealers and acquaintances, a standard practice in this state without legal dispensaries. Products purchased from the underground economy are susceptible to adulteration. Adolescents preferentially use refillable vaping cartridges, putting them at higher risk for unintended exposures.22 Although the current outbreak appears to be subsiding,13 it is critical that clinicians note the lack of regulation on e-liquid formulations and that e-cigarette cartridges are easily modifiable with known and unknown additives.
The underlying mechanism of toxicity leading to EVALI remains unclear. On the basis of our findings, lung injury may be related to airways disease. We found an improvement in FEV1 and FVC after steroid treatment, which may reflect improved airflow from decreased airway inflammation. Airways disease as a result of nicotine-containing and nicotine-free e-cigarette use has been previously described. Airway hyperresponsiveness, defined as airway obstruction caused by nonspecific stimuli,23 has been shown in mice after exposure to both nicotine-free flavored e-cigarettes24 and nicotine-containing flavored e-cigarettes.25 In another study, adults vaped nicotine for 5 minutes, which resulted in airways disease in the form of increased airway resistance measured via forced oscillometry.26 A recent study of tobacco-using adults revealed that 25 puffs of a nicotine-free vape led to a decrease in the mean forced expiratory flow between 25% and 75% of the FVC, a measure of small airways.27 In addition to airways disease, we found evidence of parenchymal pulmonary inflammation from BAL results that revealed a predominance of lymphocytes and neutrophils as well as chest CT findings of diffuse bilateral ground-glass opacities.
Although the CDC has acknowledged that VEA in Δ−9-tetrahydrocannabinol oil is a leading suspect in the etiology of EVALI, other components of the e-cigarette aerosol may contribute to lung disease.20 Murine studies reveal that exposure to both nicotine-containing and nicotine-free aerosol results in an increase of neutrophils, macrophages, lymphocytes, and pro-inflammatory cytokines in bronchoalveolar fluid.25,28–30 Other e-cigarette aerosol components, such as vegetable glycerin, propylene glycol, and flavoring agents, can cause lung inflammation and cytotoxicity. One study of human tracheobronchial epithelial cells revealed that exposure to e-cigarette aerosol led to necrotic cell death.31 Another study revealed an increased level of the proinflammatory cytokine interleukin 6 when human bronchial epithelial cells were exposed to e-liquid without nicotine.32 Leigh et al33 found that exposing human bronchial epithelial cells to various e-cigarette flavorings led to a significant increase in proinflammatory cytokine levels and a decrease in cell viability. Bengalli et al34 demonstrated an increase in pro-inflammatory cytokine levels and cell death in human alveolar and lung endothelial cells exposed to e-liquid–generated aerosols containing cinnamon flavoring. Finally, metals found in e-liquids, including chromium, nickel, lead, manganese, aluminum, tin, iron, and cadmium, can also lead to lung injury; cadmium, specifically, is a known pulmonary toxin.35 Cartridges provided by 2 of our patients, including our sickest patient, were tested for cadmium, and results were negative.
Including 6MWT results in our EVALI algorithm helped identify residual lung disease in patients who had been weaned off oxygen because of clinical improvement after glucocorticoid treatment. Four of 8 patients tested after steroid initiation (including 5 patients who did not complete a presteroid 6MWT) had abnormal desaturations with the 6MWT and were prescribed oxygen for use with activity at discharge. These patients were otherwise clinically stable in the 24 to 48 hours before discharge and had normal oxygenation at rest, consistent with current CDC guidance for discharge planning.36 All 4 patients who were discharged on home oxygen received follow-up in the pulmonology clinic, where normalization of 6MWT results led to discontinuation of home oxygen. The 6MWT is an assessment of submaximal exercise capacity, akin to activities of daily living. It is a recommended test to assess treatment response in lung diseases16 such as pulmonary hypertension, sickle cell disease, and cystic fibrosis.37–39 Abnormal 6MWT oxygen saturation may reflect lung injury at the level of the alveolar epithelium, in which impaired gas exchange manifests during exercise. Impaired gas exchange has been demonstrated in adult tobacco smokers who vaped nicotine-free e-cigarettes and had a subsequent decrease in transcutaneous oxygen measurements after a short session of use.27
Our pediatric cohort demographic was more diverse than in previously described cohorts.12,17 Half of our cohort was female, and almost half were of Hispanic origin, in contrast to the mostly young, white, and male population described in the adult literature.12,17,40 A study in which adult e-cigarette users with EVALI were compared with e-cigarette users without EVALI showed that those with EVALI had higher odds of reporting ethnicity other than white. The reason for Hispanic ethnicity as a potential EVALI risk factor in our cohort is unknown. It may be related to underlying socioeconomic factors, but may also simply reflect regional population demographics.
Another unique finding among our patients was the large percentage of patients with a history of psychosocial stressors, including substance use and mood disorders. Data from the Canadian Community Health Survey have revealed adverse mental health to be associated with e-cigarette use in adolescents and adults.41 Additionally, a recent survey of students at 2 Midwestern universities suggested that e-cigarette users were more likely to engage in illicit drug use; to have a history of posttraumatic stress disorder, attention-deficit/hyperactivity disorder, or anxiety; to have poorer self-esteem; and to have depressive symptoms.42 A detailed psychosocial screening was an important part of the evaluation of EVALI and should be linked with appropriate outpatient referrals, including for chemical dependency management.
Our study highlights the severity of EVALI in adolescents and teenagers, but certain limitations should be noted. Our case series captured a single-center population. However, given that the underground product marketplace was likely not homogenous and potentially included out-of-state sources, our findings may still be generalizable to cases in other regions. The evolving nature of our understanding of this disease influenced our management and might have inherently impacted outcomes. We tended toward higher steroid dosages compared with other case series, and this decision was based on expert consensus at our institution. Variation in severity of illness impacted our ability to perform BAL studies; however, bronchoscopy and BAL analysis may not be useful tools and may increase respiratory morbidity post procedure.43
We lacked sufficient data to draw conclusions on brands or brand changes in correlation with symptom onset or severity. Samples of vaping devices were not consistently available for testing to determine potential chemical exposures. Patient effort during PFTs may have been suboptimal in some patients and may have led to an underestimation of lung function, including oxygen saturation during the 6MWT. To minimize this possibility, all PFT results were reviewed for adequate quality before inclusion in the data analysis. Finally, we have incomplete outpatient follow-up of our patients, including several who experienced financial or insurance barriers to return visits.
CONCLUSIONS
In our cohort of adolescents diagnosed with EVALI, we found that the clinical severity was variable, with some patients requiring no supplemental oxygen to one patient requiring invasive mechanical ventilation and extracorporeal membrane oxygenation. Glucocorticoids were a successful treatment modality, whereas antibiotics did not appear to result in improvement. Lung function improved after glucocorticoid administration, and the 6MWT was useful in identifying patients who could benefit from oxygen with activity at discharge. The mechanism of lung injury remains unclear, but exposure to Δ−9-tetrahydrocannabinol adulterated with VEA appears likely. All patients who followed-up in the pulmonary clinic reported cessation of e-cigarette use, so it remains to be seen whether the improvement in pulmonary function seen in our patients will also persist in adolescents who choose to resume e-cigarette use. The long-term effects of the other components of e-cigarette liquid on the lung remain unknown and is an area for future study.
FIGURE 3.
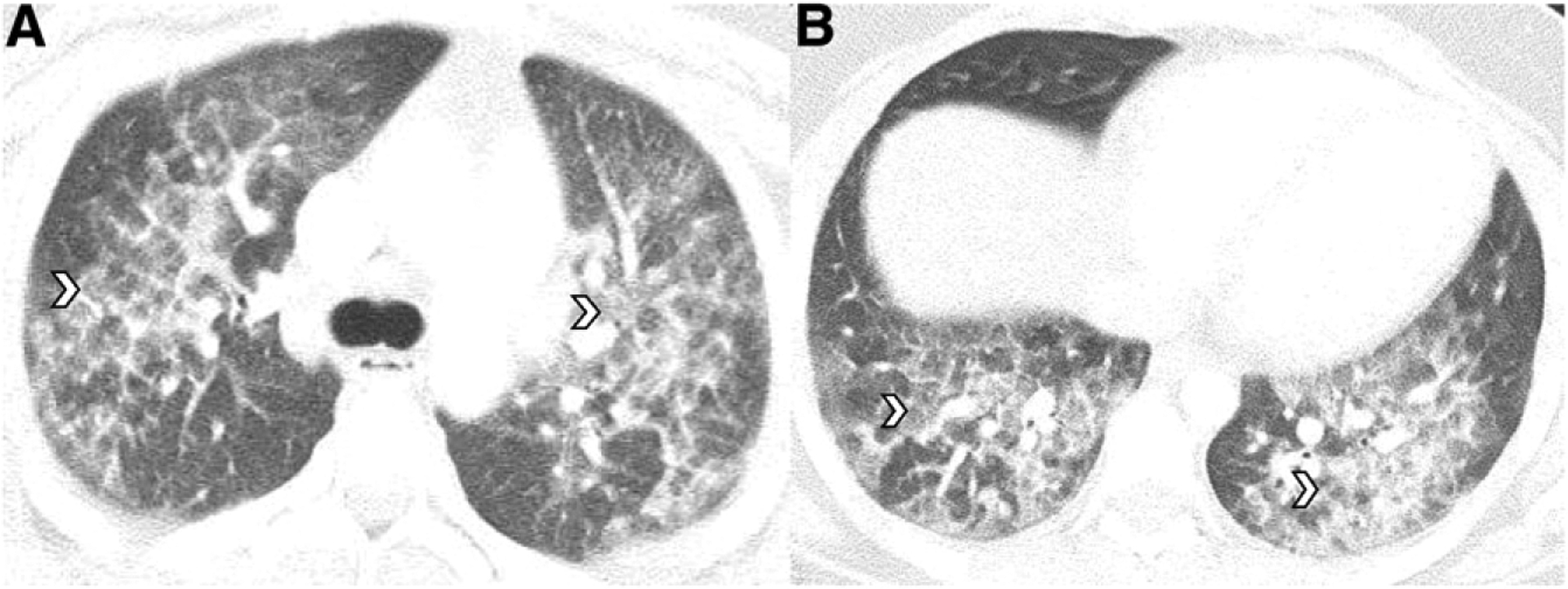
Seventeen-year-old boy with Δ−9-tetrahydrocannabinol vaping for one year who reported use of cartridges from an unregulated distributor. A and B, Axial chest CT images reveal ground-glass opacities throughout all lobes (white arrowheads).
WHAT’S KNOWN ON THIS SUBJECT:
The prevalence of electronic cigarette use has more than doubled among eighth- to 12th-graders over the past 2 years. An outbreak of electronic cigarette, or vaping, product use–associated lung injury (EVALI) linked to vaping Δ−9-tetrahydrocannabinol occurred this fall, and adolescents were among those affected.
WHAT THIS STUDY ADDS:
Many teenagers affected by EVALI were female, were Hispanic, and had multiple psychosocial stressors. Lung function deficits due to EVALI appear reversible with steroids. Six-minute walk test results may help identify adolescents who would benefit from home oxygen.
ACKNOWLEDGMENTS
We acknowledge the respiratory technicians of the PFT laboratory at Children’s Health Dallas: Dr Rosechelle Ruggiero from the Department of Internal Medicine; members of the Division of Respiratory Medicine, including Drs Andrew Gelfand, Tanya Martinez-Fernandez, Yadira Rivera-Sanchez, Preeti Sharma, Elisa Basora, Suja Nair, Michelle Caraballo, Kubra Melike Bozkanat, Fayruz Araji, and Bayan Abdallah, for their feedback regarding the clinical algorithm; and members of the Department of Emergency Medicine, including Drs Kurt Kleinschmidt, Mary Billington, Joshua McFalls, and Cherie Obilom, for their feedback regarding the clinical algorithm. We acknowledge Dr Guido Verbeck and Imesha De Silva of the University of North Texas Laboratory of Imaging Mass Spectrometry for their assistance in analyzing e-liquid samples.
FUNDING: No external funding.
ABBREVIATIONS
- 6MWT
6-minute walk test
- BAL
bronchoalveolar lavage
- CDC
Centers for Disease Control and Prevention
- CT
computed tomography
- DLCO
diffusion capacity of carbon monoxide
- E-cigarette
electronic cigarette
- EVALI
electronic cigarette, or vaping, product use–associated lung injury
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GI
gastrointestinal
- PFT
pulmonary function testing
- VEA
vitamin E acetate
- VV-ECMO
veno-venous extracorporeal membrane oxygenation
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2020-0902.
REFERENCES
- 1.Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME. Trends in adolescent vaping, 2017–2019. N Engl J Med. 2019;381(15):1490–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miech RA, Patrick ME, O’Malley PM, Johnston LD, Bachman JG. Trends in reported marijuana vaping among US adolescents, 2017–2019. JAMA. 2020;323(5):475–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho JH, Paik SY. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS One. 2016;11(3):e0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics. 2018;141(6):e20163927. [DOI] [PubMed] [Google Scholar]
- 5.Arter ZL, Wiggins A, Hudspath C, Kisling A, Hostler DC, Hostler JM. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019;27:100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dicpinigaitis PV, Trachuk P, Fakier F, Teka M, Suhrland MJ. Vaping-associated acute respiratory failure due to acute lipoid pneumonia. Lung. 2020;198(1):31–33 [DOI] [PubMed] [Google Scholar]
- 7.Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;2018:9724530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. 2020. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed March 18, 2020
- 9.Blount BC, Karwowski MP, Shields PG, et al. ; Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016 [Google Scholar]
- 11.Jatlaoui TC, Wiltz JL, Kabbani S, et al. ; Lung Injury Response Clinical Working Group. Update: interim guidance for health care providers for managing patients with suspected e-cigarette, or vaping, product use-associated lung injury - United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1081–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - final report. N Engl J Med. 2020;382(10):903–916 [DOI] [PubMed] [Google Scholar]
- 13.Lozier MJ, Wallace B, Anderson K, et al. ; Lung Injury Response Epidemiology/Surveillance Task Force. Update: demographic, product, and substance-use characteristics of hospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injuries - United States, December 2019. MMWR Morb Mortal Wkly Rep. 2019;68(49):1142–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171(8):788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culver BH, Graham BL, Coates AL, et al. ; ATS Committee on Proficiency Standards for Pulmonary Function Laboratories. Recommendations fora standardized pulmonary function report. An official American Thoracic Society technical statement. Am J Respir Crit Care Med. 2017;196(11):1463–1472 [DOI] [PubMed] [Google Scholar]
- 16.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test [published correction appears in Am J Respir Crit Care Med. 2016;193(10):1185]. Am J Respir Crit Care Med. 2002;166(1):111–117 [DOI] [PubMed] [Google Scholar]
- 17.Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triantafyllou GA, Tiberio PJ, Zou RH, et al. Vaping-associated acute lung injury: a case series. Am J Respir Crit Care Med. 2019;200(11):1430–1431 [DOI] [PubMed] [Google Scholar]
- 19.Navon L, Jones CM, Ghinai I, et al. Risk factors for e-cigarette, or vaping, product use-associated lung injury (EVALI) among adults who use e-cigarette, or vaping, products - Illinois, July-October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(45):1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blount BC, Karwowski MP, Morel-Espinosa M, et al. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of e-cigarette, or vaping, product use-associated lung injury - 10 states, August-October 2019 [published correction appears in MMWR Morb Mortal Wkly Rep. 2020;69(4):116]. MMWR Morb Mortal Wkly Rep. 2019;68(45):1040–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downs D Amid vape pen lung disease deaths: what exactly is vitamin E oil? 2019. Available at: https://www.leafly.com/news/health/vape-pen-lung-disease-vitamin-e-oil-explained. Accessed December 27, 2019
- 22.McMillen R, Tanski S, Wilson K, Klein JD, Winickoff JP. Adolescent use of different e-cigarette products. Pediatrics. 2018;142(4):e20180260. [DOI] [PubMed] [Google Scholar]
- 23.Postma DS, Meijer GG, Koppelman GH. Definition of asthma: possible approaches in genetic studies. Clin Exp Allergy. 1998;28(suppl 1):62–64–66 [DOI] [PubMed] [Google Scholar]
- 24.Chapman DG, Casey DT, Ather JL, et al. The effect of flavored e-cigarettes on murine allergic airways disease. Sci Rep. 2019;9(1):13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glynos C, Bibli SI, Katsaounou P, et al. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2018;315(5):L662–L672 [DOI] [PubMed] [Google Scholar]
- 26.Lappas AS, Tzortzi AS, Konstantinidi EM, et al. Short-term respiratory effects of e-cigarettes in healthy individuals and smokers with asthma. Respirology. 2018;23(3):291–297 [DOI] [PubMed] [Google Scholar]
- 27.Chaumont M, van de Borne P, Bernard A, et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L705–L719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Li G, Chan YL, et al. Maternal e-cigarette exposure in mice alters DNA methylation and lung cytokine expression in offspring. Am J Respir Cell Mol Biol. 2018;58(3):366–377 [DOI] [PubMed] [Google Scholar]
- 29.Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweitzer KS, Chen SX, Law S, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol. 2015;309(2):L175–L187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667–679 [DOI] [PubMed] [Google Scholar]
- 32.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9(9):e108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leigh NJ, Tran PL, O’Connor RJ, Goniewicz ML. Cytotoxic effects of heated tobacco products (HTP) on human bronchial epithelial cells. Tob Control. 2018;27(suppl 1):s26–s29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bengalli R, Ferri E, Labra M, Mantecca P. Lung toxicity of condensed aerosol from E-CIG liquids: influence of the flavor and the in vitro model used. Int J Environ Res Public Health. 2017;14(10):E1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Academies of Sciences Engineering and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. In: Eaton DL, Kwan LY, Stratton KR, eds. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018 [PubMed] [Google Scholar]
- 36.Evans ME, Twentyman E, Click ES, et al. ; Lung Injury Response Clinical Task Force; Lung Injury Response Clinical Working Group. Update: interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use-associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge - United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douwes JM, Humpl T, Bonnet D, Beghetti M, Ivy DD, Berger RM; TOPP Investigators. Acute vasodilator response in pediatric pulmonary arterial hypertension: current clinical practice from the TOPP registry. J Am Coll Cardiol. 2016;67(11):1312–1323 [DOI] [PubMed] [Google Scholar]
- 38.Hostyn SV, Carvalho WB, Johnston C, Braga JA. Evaluation of functional capacity for exercise in children and adolescents with sickle-cell disease through the six-minute walk test. J Pediatr (Rio J). 2013;89(6):588–594 [DOI] [PubMed] [Google Scholar]
- 39.Waltz X, Romana M, Hardy-Dessources MD, et al. Hematological and hemorheological determinants of the six-minute walk test performance in children with sickle cell anemia. PLoS One. 2013;8(10):e77830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis N, McCaffrey K, Sage K, et al. E-cigarette use, or vaping, practices and characteristics among persons with associated lung injury - Utah, April-October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(42):953–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pham T, Williams JVA, Bhattarai A, Dores AK, Isherwood LJ, Patten SB. Electronic cigarette use and mental health: a Canadian population-based study. J Affect Disord. 2020;260:646–652 [DOI] [PubMed] [Google Scholar]
- 42.Grant JE, Lust K, Fridberg DJ, King AC, Chamberlain SR. E-cigarette use (vaping) is associated with illicit drug use, mental health problems, and impulsivity in university students. Ann Clin Psychiatry. 2019;31(1):27–35 [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz CD, Carroll BJ, Hemyari A. Pulmonary illness related to e-cigarette use. N Engl J Med. 2020;382(4):384–385 [DOI] [PubMed] [Google Scholar]


