Abstract
In the knee joint, articular cartilage injury can often lead to osteoarthritis of the knee (OAK). Currently, no point-of-care treatment can completely address OAK symptoms and regenerate articular cartilage to restore original functions. While various cell-based therapies are being developed to address OAK, exosomes containing various components derived from their cells of origin have attracted attention as a cell-free alternative. The potential for exosomes as a novel point-of-care treatment for OAK has been studied extensively, especially in the context of intra-articular treatments. Specific exosomal microRNAs have been identified as possibly effective in treating cartilage defects. Additionally, exosomes have been studied as biomarkers through their differences in body fluid composition between joint disease patients and healthy subjects. Exosomes themselves can be utilized as a drug delivery system through their manipulation and encapsulation of specific contents to be delivered to specific cells. Through the combination of exosomes with tissue engineering, novel sustained release drug delivery systems are being developed. On the other hand, many of the functions and activities of exosomes are unknown and challenges remain for clinical applications. In this review, the possibilities of intra-articular treatments utilizing exosomes and the challenges in using exosomes in therapy are discussed.
Keywords: exosome, point-of-care therapy, osteoarthritis of the knee, cartilage regeneration
1. Introduction
Cartilage has poor self-repair and regeneration ability once injured, as first reported in 1743 by William Hunter [1]. Thus, the regeneration of articular cartilage has been a long-standing challenge for physicians and researchers in the field of orthopedics. Articular cartilage consists of highly elastic hyaline cartilage, which is mainly composed of water and extracellular matrix (ECM), such as proteoglycans and collagen fibers. The cellular component of articular cartilage makes up only about 5% of its wet weight and <10% of its tissue volume. Articular cartilage has poor self-renewal ability due to the extremely low infiltration of oxygen, nutrients, and progenitor cells, as it is avascular [2,3].
Osteoarthritis of the knee (OAK) is one of the most common joint diseases. It is a chronic, progressive disease in which the articular cartilage gradually degenerates due to complex interactions between various factors including trauma due to accidents and sports, obesity, skeletal structure, heredity, and age-related overload on the joints. The global prevalence of OAK is estimated to be approximately 3.8%, and it is observed more in women (mean, 4.8%) than in men (mean, 2.8%) [4]. Initially, partial-thickness articular cartilage defects with wear and tear on the cartilage surface are observed. As the pathological condition progresses, the subchondral bone becomes exposed, leading to full-thickness articular cartilage defects. One of the most serious symptoms is pain during daily activities such as walking and climbing the stairs, leading to decreased physical activity and quality of life.
Non-surgical treatments for articular cartilage injury include non-pharmacological treatments such as exercise therapy, weight loss therapy, and orthotic therapy. Pharmacological treatments include utilization of analgesics or non-steroidal anti-inflammatory drugs (NSAID) and intra-articular injection of corticosteroids or hyaluronic acid. Currently, these are the limited options that are widely available for point-of-care treatment for OAK, and they fail to reverse the progress of cartilage degeneration. For patients who show no improvements with non-pharmacological and pharmacological treatments, surgical treatment options are considered.
Surgical options for injuries with small defects include subchondral drilling [5], microfracture [6,7], and mosaicplasty [8,9]. However, the cartilage regenerated through these methods is often fibrocartilage, which is mechanically inferior to the hyaline cartilage that constitutes the original articular cartilage. For larger cartilage defects, autologous chondrocyte implantation (ACI) [10] has been utilized. Currently, products such as JACC® [11,12], CaRes® [13,14], Hyalograft®C [15], and BioSeed®C [16] are on the market and used worldwide. However, the regenerated cartilage is reported to be either fibrocartilage or a composite of fibrocartilage and hyaline cartilage, and these methods are not yet applicable to OAK [17,18]. In Japan, cell sheet transplantation with high tibial osteotomy [19,20,21] is a cell therapy that has been approved for the treatment of OAK as an advanced medical care. However, the treatment is costly and time consuming. After other options are exhausted, total knee arthroplasty (TKA) is applied. Although the treatment outcomes of TKA are remarkable [22], artificial joints may need revision after 15 to 20 years. Thus TKA is often applied to people over the age of 65.
To bridge the gap between traditional pharmacological and surgical treatments, cell therapies such as mesenchymal stem cells (MSCs) [23,24] and platelet rich plasmas (PRPs) [25,26,27] have been in use as a point-of-care treatment for OAK. Patients can receive these treatments through intra-articular injections at relatively low costs in select places. However, therapeutic effects attributed to various modes of action including cytokines and growth factors can be variable [28,29,30,31]. In addition, risks of inappropriate transformation of the transplanted cells and inflammatory reactions exist [32,33,34,35,36]. Thus, a cell-free therapy that maximizes the therapeutic effect through the selected administration of identified factors is ideal.
To address such issues, extracellular vesicles (EVs), especially exosomes, are attracting attention as a novel tool that can be applied to treat cartilage and bone disease [37,38]. The therapeutic effects of MSCs have also been partially attributed to exosomes carrying specific cargos [39,40,41,42,43]. With recent advancements, exosomes are used as efficient drug delivery systems that can be manipulated to carry specific cargo such as micro ribonucleic acids (miRNAs). As such, exosomes may be used as a point-of-care treatment for joint disease, especially through intra-articular injection. In addition, exosomes may be useful as biomarkers to detect joint disease such as OAK at early onset. More recently, a combination of exosomes with tissue engineering methods has been investigated to prolong and improve the efficacy of exosomes. In this review, we present the recent concepts of exosomes that can be applied to the development of point-of-care treatment for joint disease such as OAK.
2. Extracellular Vesicles
Historically, membrane vesicles released by cells have been thought of as non-functional, inactive “debris” resulting from cell damage or turnover of the cellular membrane. However, in 1983, the existence of a 100-nm functional endoplasmic reticulum comprised of lipid bilayer membranes secreted from cells was confirmed [44]. Subsequent research identified membrane vesicles secreted by various cells, which were named according to their features, such as ectosomes, microvesicles, oncosomes, exosomes, and apoptotic bodies [45,46,47,48,49,50,51,52]. To resolve confusion, the International Society for Extracellular Vesicles (ISEV) suggested using the term “extracellular vesicles” (EVs) as “the generic term for particles naturally released from the cell that are delimited by a lipid bilayer membrane and cannot replicate, i.e., do not contain a functional nucleus” [53]. Various subtypes of EVs share similar characteristics, and a method to accurately classify EVs and their fundamental biological roles is unclear [54]. Therefore, the ISEV has suggested a classification of the subtypes of EVs according to the following characteristics: (a) physical characteristics, such as size (“small EVs” (sEVs, <100–200 nm) and “medium/large EVs” (m/lEVs, >200 nm)) or density (low, middle, high); (b) biochemical composition (cluster of differentiation (CD)63+/CD81+ EVs, Annexin A5-stained EVs, etc.); or (c) descriptions of conditions or cell of origin (podocyte EVs, hypoxic EVs, large oncosomes, apoptotic bodies) [53].
3. Biogenesis, Composition, and Isolation of Exosomes
The nomenclature of exosomes was suggested in 1987 [46]. Exosomes are a subtype of EVs that have a small diameter of 50–150 nm. They are secreted from most of the cells in the body and are observed in body fluids and cell culture media [47,52].
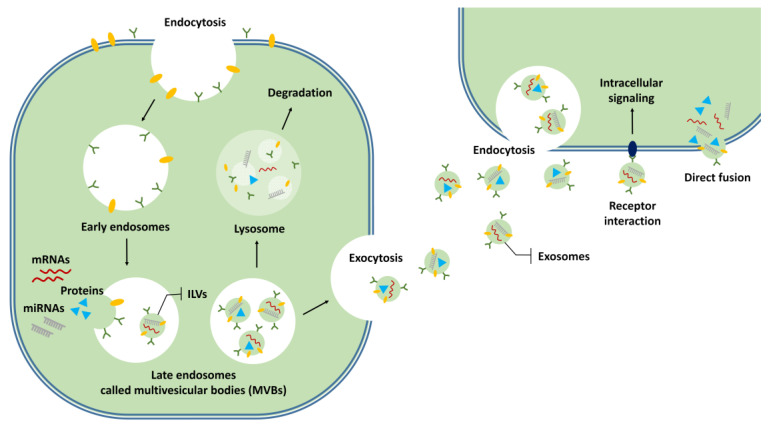
Exosomes are known to be formed by endocytosis (Figure 1). Early endosomes transition to late endosomes. Then a large number of intraluminal membrane vesicles (ILVs) are formed by internal budding of the endosome membrane. Late endosomes are called multivesicular bodies (MVBs). When the MVBs fuse with the cell membrane, the internal ILVs are released into the extracellular space as exosomes [55] (Figure 1). Endosomal sorting complexes required for transport (ESCRT) and tetraspanins are thought to be involved in the formation of endosomes [47,56,57]. The mechanisms for uptake of the exosomes into target cells and the delivery of the exosome contents are unclear. Many studies have suggested that endosomes are the putative location of EV-content delivery, and contents are released into the cytoplasm of the target cells by fusing with endosome membranes [47,55]. Other mechanisms of content delivery to target cells, such as interaction with the receptors present on the cell membrane and direct fusion with the cell membrane, have also been proposed [47,55].
Figure 1.
Formation of exosomes and uptake into target cells. Early endosomes are formed by endocytosis and they transition to late endosomes. These are called multivesicular bodies (MVBs), with intraluminal membrane vesicles (ILVs). ILVs that are released from MVBs into the extracellular space are called exosomes. Released exosomes are taken up by the target cells through endocytosis, interactions with receptors on the cell membrane, or direct fusion with the cell membrane.
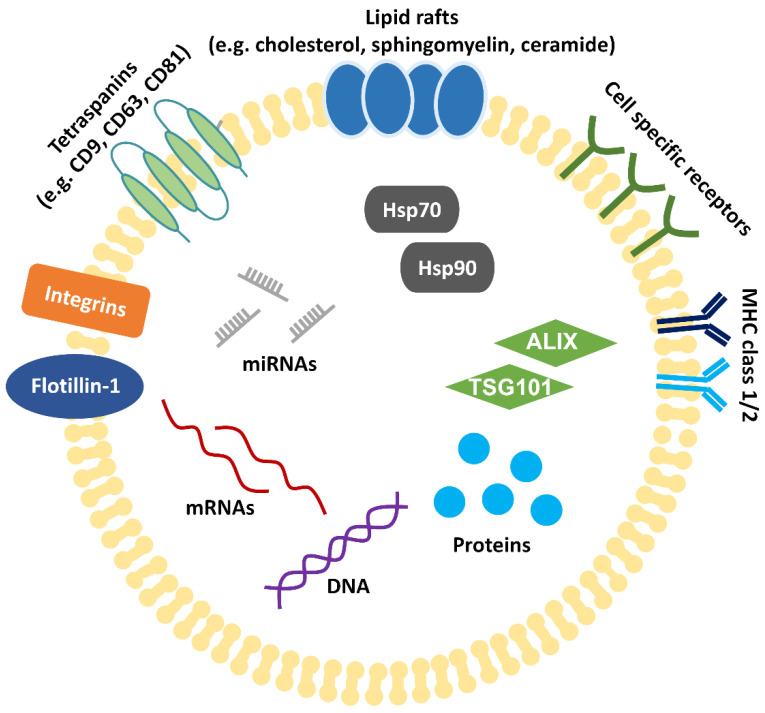
Exosomes are comprised of a lipid bilayer membrane containing lipid raft constituents, such as cholesterol, sphingomyelin, and ceramide, and contain messenger RNAs (mRNAs), miRNAs, deoxyribonucleic acid (DNA), and various proteins (Figure 2) [58,59,60,61]. As of January 2021, 9769 proteins, 1116 lipids, 3408 mRNAs, and 2838 miRNAs have been registered in the ExoCarta database (http://www.exocarta.org/, accessed on 2 January 2021), which catalogues exosome contents. Although various proteins are associated with the lipid membrane of exosomes, no clear biomarker has been identified. Proteins, such as major histocompatibility complex (MHC) class I and class II, heat shock proteins (e.g., heat shock protein (Hsp) 70, Hsp90), flottilin-1, and actin, have been used as “exosome markers” in the past and have been shown to be present in all types of EVs [62]. In addition, the co-expression of tetraspanins (e.g., CD9, CD63, CD81) and proteins associated with MVB formation (e.g., ALG-2-interacting protein X (ALIX), tumor susceptibility gene 101 (TSG101)) have been suggested (Figure 2) [62]. It is not yet clear whether exosomes have specific functions not found in other EVs or whether they can be reliably distinguished and separated from other EVs.
Figure 2.
Structure of exosomes. Exosomes have a lipid bilayer membrane composed of lipid raft constituents, tetraspanins (such as CD9, 63 and 81), and proteins (such as integrin, cell specific receptors, MHC class I and class II, and flottilin-1). Exosomes contain cellular components such as proteins, DNA, mRNAs, miRNAs, proteins associated with MVB formation (ALIX, TSG101), and heat shock proteins (Hsp70, Hsp90).
EVs are a heterogeneous population with a mixture of cell vesicles of various fractions. In order to use exosomes for research and treatment, a technique for isolating only “exosome fractions” in large quantities and with high purity is required. Various exosome isolation methods have been developed to this day [63,64,65]. The most classic method is ultracentrifugation. This method is still the most widely used [66] and has become the gold standard for exosome isolation. In addition, there are various methods such as ultrafiltration, polymer precipitation, size exclusion chromatography, immunoaffinity purification, and microfluidics-based techniques. Furthermore, various exosome isolation kits such as exoEasy Maxi kit (QIAGEN, Venlo, Nederland), ExoQuick (System Biosciences, Palo Alto, CA, USA), qEV size exclusion columns (Izon Science, Oxford, United Kingdom), and MagCapture™ Exosome Isolation Kit PS (Wako Pure Chemical corporation, Osaka, Japan) are on the market. Each isolation method has advantages and disadvantages, and the quality and amount of exosomes obtained vary greatly depending on the isolation method selected [63,64,65]. In order to isolate the exosome fraction with the desired activity, it is important to select the optimal exosome isolation method for each objective.
4. Potential of Exosomes for Diagnosis and Treatment of Joint Diseases
The potential for exosomes and their derivatives to be delivered through intra-articular injection opens up new possibilities for the treatment of joint diseases such as OAK. Exosomes contain specific information about the cells from which they are released, and may have the ability to deliver molecules to specific organs or tissues at a distance [61,67,68]. Studies have shown that exosomal miRNAs play an important role in joint homeostasis. Furthermore, the imbalances in exosomes created during joint disease can be utilized as diagnostic biomarkers. Studies have also shown the potential for exosomes to be utilized as a drug delivery system (DDS) to deliver specific cargo to localized areas. In combination with tissue engineering, studies have utilized exosomes in a sustained release drug delivery system (SRDDS) to further improve the efficacy. Here, we discuss such topics in regards to their applications to the diagnosis and treatment of joint diseases.
4.1. Exosomal miRNAs
MiRNAs are small non-coding RNAs that have approximately 22 nucleotides. These induce gene silencing by sequence-complementary binding to target sites located within the three prime untranslated regions (3’UTR) of the target mRNA and regulate multiple biochemical pathways [69]. Many miRNAs associated with chondrocyte function and homeostasis have been reported to contribute to cartilage repair [70,71]. For example, miR-140, a promising miRNA for OAK treatment, is expressed specifically in articular cartilage and plays an important role in cartilage development and metabolic balance of the cartilage matrix by inhibiting a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) and matrix metalloproteinase 13 (MMP13) [72,73]. Ukai et al. examined cartilage tissues from pediatric patients with polydactylism, young patients with anterior cruciate ligament injury, and elderly patients with OAK and identified miRNA-199a-3p, 193b, and 320c as markers that suppress age-related cartilage metabolism [74]. The discovery that mRNAs and miRNAs are packaged in exosomes that are transported between cells and function in target cells was a breakthrough [59,75,76,77]. Exosomal miRNAs are protected from endogenous RNase activity by the exosome membrane and can exist stably in body fluids and cell culture media. Therefore, exosomal miRNAs can be collected and administered through intra-articular injections. This section introduces the current research on intra-articular treatments with miRNAs (i.e., exosomal miRNAs) secreted extracellularly via exosomes (Table 1).
Table 1.
Studies on exosomal miRNAs regulating chondrocyte function and homeostasis for the purpose of intra-articular treatments.
| Exosome Origin Cell Source | Study Design | Animal Models | miRNA | Result(s) | Target(s) | Reference |
|---|---|---|---|---|---|---|
| Human BMSCs | in vitro | - | miR-320c | Upregulates SOX9 and downregulates MMP13 expression in OA chondrocyte. | Not mentioned | [78] |
| Human BMSCs | in vitro | - | miR-95-5p | Enhances histone H3 acetylation and maintains the function of articular chondrocytes. Promotes SOX9, COL2A1 and Aggrecan expression and enhances cartilage development. | HDAC2/8 | [79] |
| Human chondrocytes | in vitro | - | miR-8485 | Activates Wnt/β-catenin pathways. Promotes chondrogenic differentiation of hBMSCs. | GSK3B, DACT1 | [80] |
| Human BMSCs | in vitro and in vivo |
Mice | miR-92a-3p | Promotes cartilage proliferation. In both MSCs and PHCs, promotes matrix genes expression and inhibits WNT5A expression. | WNT5A | [81] |
| Human BMSCs | in vitro and in vivo |
Rats | miR-26a-5p | It was shown that the damage of synovial fibroblasts is suppressed in vitro, and the OA damage is alleviated in vivo. | PTGS2 | [82] |
| Human IPFP MSCs | in vitro and in vivo |
Mice | miR-100-5p | Protects cartilage from damage and ameliorate gait patterns of DMM-induced OA mice. | mTOR | [83] |
| Human SMSCs | in vitro and in vivo |
Rats | miR-140-5p | Enhances the proliferation and migration of ACs, and the progression of early OA and prevents severe damage to knee articular cartilage in the OA rats. | RalA | [84] |
hBMSC: human bone marrow-derived MSC, IPFP: infrapatellar fat pad, SMSC: synovium-derived MSC, MSC: mesenchymal stem cell, PHC: primary human chondrocytes, WNT5A: Wnt family member 5A, OA: osteoarthritis, SOX9: SRY-box transcription factor 9, MMP13: matrix metalloproteinase 13, COL2A1: Collagen type II alpha 1, DMM: destabilization of the medial meniscus, AC: articular cartilage, PTGS2: Prostaglandin-endoperoxide synthase 2, HDAC: histone deacetylase, mTOR: mammalian target of rapamycin, RalA: Ras-related protein, GSK-3β: glycogen synthase kinase 3 beta, DACT1: dishevelled binding antagonist of beta catenin 1.
Studies have shown that exosomal miRNAs may influence cartilage differentiation and homeostasis. Sun et al. performed a microarray analysis of exosomes secreted from both human bone marrow-derived MSCs (hBMSCs) undifferentiated and hBMSCs differentiated into cartilage [78]. Through the comparative analysis, they identified 35 miRNAs, including miR-1246, miR-1290, miR-193a-5p, miR-320c, and miR-92a, that were upregulated in exosomes derived from hBMSCs differentiated into cartilage. In addition, they demonstrated that exosomes derived from miR-320c-overexpressing hBMSCs upregulate SRY-box transcription factor 9 (SOX9) and downregulate MMP13 in OA chondrocytes, and enhance chondrogenesis of hBMSCs [78]. Mao et al. demonstrated that exosomes secreted by OA cartilage express significantly less miR-95-5p compared to normal cartilage [79]. In addition, they showed that exosomes derived from miR-95-5p-overexpressing primary chondrocytes promote cartilage development and cartilage matrix expression by hBMSCs and chondrocytes, and that miR-95-5p may be effective in the treatment of OA by directly targeting histone deacetylase (HDAC) 2/8. Li et al. showed that chondrocyte-derived exosomal miR-8485 promotes the chondrocyte differentiation of BMSCs [80]. The study suggested that miR-8485 may suppress the production of glycogen synthase kinase 3 beta (GSK-3β) by regulating the expression and activity of GSK-3β, activate the Wnt/β-catenin pathway through phosphorylation of GSK-3β by targeting disheveled binding antagonist of beta catenin 1 (DACT1), and promote chondrocyte differentiation.
Other studies have shown the potential of exosomal miRNAs in various animal models. Mao et al. showed that miR-92a-3p directly targets Wnt family member 5A (WNT5A) and promotes proliferation and migration of chondrocytes and the differentiation of MSCs into chondrocytes [81]. In addition, they showed that exosomes derived from miR-92a-3p-overexpressing hBMSCs inhibit the progression of early osteoarthritis (OA) and prevent the severe damage of cartilage in an OA model mouse induced with collagenase VII. Jin et al. found that the expression of miR-26a-5p decreases and the expression of prostaglandin-endoperoxide synthase 2 (PTGS2) increases in OA patients and in synovial fibroblasts treated with interleukin-1 beta (IL-1β) [82]. In addition, they showed that hBMSC-derived exosomes provide miR-26a-5p to synovial fibroblasts and alleviate the damage of OA synovial fibroblasts by controlling PTGS2 expression. Moreover, articular injection of exosomes derived from miR-26a-5p-overexpressing hBMSC into an OA rat model prevents OA damage by alleviating the inflammation of synovial tissues and decreasing apoptosis. Wu et al. demonstrated that infrapatellar fat pad (IPFP) MSC-derived exosomes (MSCIPFP-Exos) can deliver miR-100-5p to chondrocytes [83]. The miR-100-5p specifically targets the 3′UTR region of mammalian target of rapamycin (mTOR) and significantly enhances autophagy levels in chondrocytes, inhibits cell apoptosis, enhances anabolism, and represses catabolism in IL-1β-treated OA chondrocytes. In addition, MSCIPFP-Exos improved the pathological degree of severity and foot gait patterns in a destabilization of the medial meniscus (DMM)-induced OA mouse model. Tao et al. showed that exosomes derived from synovium-derived MSCs (SMSCs) activate Yes-associated protein (YAP) and promote the proliferation and migration of chondrocytes, but decrease ECM secretion [84]. Therefore, exosomes derived from miR-140-5p-overexpressing SMSCs (SMSC-140-Exos) affected proliferation and migration of chondrocytes without ECM secretion being influenced. Moreover, the administration of SMSC-140-Exos to an anterior cruciate ligament transection (ACLT) OA rat model demonstrated the inhibition of severe damage of the articular cartilage and the progression of OA.
In addition, exosomes are thought to be deeply involved in the development of various physiological phenomena and pathological conditions, and further research on exosomal miRNAs may lead to the elucidation of the mechanism of onset and progression of joint diseases such as OAK. Ni et al. showed that exosome-like vesicles from OA chondrocytes inhibit the expression of autophagy related 4B cysteine peptidase (ATG4B) via miR-449a-5p [85]. This result suggests that inflammation of the synovial membrane may be promoted by the inhibition of autophagy of macrophages and the increase in production of mature IL-1β, leading to progression of OA diseases.
4.2. Exosomes as Biomarkers
As OAK patients experience few symptoms during early onset and because of the difficulty in providing treatment as the disease progresses, early diagnostic methods using tools such as biomarkers are desired. Exosomes are anticipated to be useful as novel biomarkers as they contain specific information from the released cells and can stably carry encapsulated substances. In the diagnosis of lung cancer, Exo Dx™ Lung (ALK) (Exosome Diagnostics, Inc., Waltham, MA, USA) is the first Clinical Laboratory Improvement Amendments-validated exosome-based clinical liquid biopsy test that isolates exosomal mRNAs in blood to diagnose cancer [86,87]. For the diagnosis of OAK, exosomes isolated from blood or joint fluid have been identified as useful to date.
For instance, several studies have shown that the expression of specific exosomal miRNAs is altered in patients with joint disease. Kolhe et al. showed that synovial fluid exosomal miRNAs may be altered in OAK patients [88]. In particular, exosomal miRNAs expressed specifically in female OAK patients are responsive to estrogen and target the Toll-like receptor (TLR) signal pathway. Meng et al. confirmed that the expression of exosomal miRNA-193b-3p was decreased in the plasma of OAK patients compared to that of healthy subjects [89].
In addition, Zhao et al. mentioned the possibility that long noncoding RNAs (lncRNAs) can be used as biomarkers for OAK. They conducted an analysis of plasma-derived and synovial fluid-derived exosomal lncRNAs to assess the progression of OAK [90]. As a result, the expression of plasma-derived exosomal lncRNAs showed no significant difference; however, the expression of synovial fluid-derived exosomal lncRNAs was higher in early OA and late-stage OA than in healthy subjects. Specifically, exosomal lncRNA prostate-specific transcript 1 (PCGEM1) was much higher in late OA than in early OA and much higher in early OA than in healthy subjects.
Other studies have examined the use of exosomes as biomarkers to differentiate joint diseases. Chen et al. reported that specific plasma-derived exosomal miRNAs were connected to the common pathogenesis of psoriatic arthritis, psoriasis vulgaris, rheumatoid arthritis (RA), and gouty arthritis. An analysis of the potential target genes revealed that these miRNAs are related to immune disorders and bone injury [91]. In addition, Tsuno et al. demonstrated that serum exosomes of patients with active RA possess different protein profiles compared to patients with inactive RA, patients with OA, and healthy donors [92].
Together, these results indicate that differences in exosome profiles in patients with joint diseases and healthy subjects can be investigated using body fluids such as synovial fluid and plasma, which may lead to the development of various tests and diagnostic methods for OAK and other joint diseases in the future.
4.3. Exosomes as a Drug Delivery System (DDS)
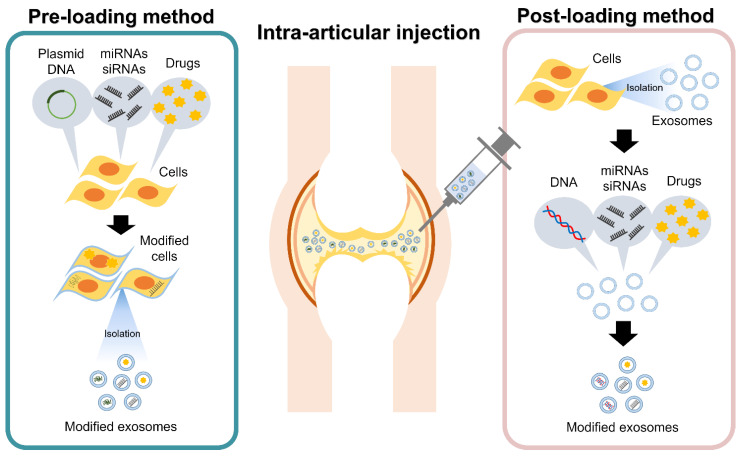
If specific miRNAs and other molecules are identified as having a therapeutic effect for the treatment of OAK, they can be packaged in exosomes that can act as a DDS to transport specific contents to target tissues or organs [68,93]. There are two types of methods to enclose the target substances in exosomes: pre-loading and post-loading methods [94] (Figure 3).
Figure 3.
Intra-articular treatments utilizing modified exosomes as a drug delivery system. The loading of specific components into exosomes can be conducted either before exosome isolation (pre-loading method) or after exosome isolation (post-loading method).
Pre-loading methods collect exosomes released by the modified cells after cell modification is performed by miRNA or small interfering RNA (siRNA) transfection or by the uptake of target substances from the culture media during cell culture (Figure 3). Many studies have confirmed the ability of cartilage repair by exosomes collected from cells overexpressing specific miRNAs [71,74,76]. In addition, Wang et al. showed that the expression of miR-135b within the released exosomes can be upregulated by stimulating MSCs using transforming growth factor beta 1 (TGF-β1) [95]. The pre-loading method is a relatively simple procedure that relies on cells to package exosomes. However, it can be difficult to control the loading of specific target molecules in exosomes. A further understanding of the mechanisms by which intracellular miRNAs, mRNAs, and proteins load exosomes efficiently with specific target molecules is necessary.
In contrast, post-loading methods extract exosomes from cells and body fluids, and the target molecules are encapsulated in the exosomes through disruption of the exosome membrane (Figure 3). Post-loading methods include physical induction by electroporation, freeze/thaw method, sonication, etc. and chemical induction by saponin treatment, transfection reagents, etc. In the post-loading method, the target molecules can be directly loaded into the exosomes, so the loading efficiency is expected to be higher than that of pre-loading methods. However, physical disruption may affect the size and shape of exosomes. Chemical induction has been shown to be more efficient in loading target molecules compared to physical induction methods [96]. However, chemicals used for the loading of molecules may be toxic, so removing them in the process is essential.
In terms of applications to joint therapy, post-loading methods may allow the targeting of specific tissues, such as cartilage, subchondral bone, or synovium, to treat the various symptoms. Liang et al. reported on the loading of specific miRNAs through electroporation into engineered exosomes [97]. They engineered chondrocyte-affinity peptide (CAP) exosomes by fusing CAP with lysosome-associated membrane glycoprotein 2b (Lamp2b) on the surface of exosomes. Furthermore, they loaded miR-140 into the CAP exosomes using electroporation (CAP exosome/miR-140). The CAP exosomes/miR-140 allowed for the specific delivery of chondrocytes. Moreover, in a DMM-induced OA rat model, they demonstrated that the progression of OA can be reduced by CAP-exosome/miR-140. Through modification of exosomes and post-loading methods, they were able to simultaneously achieve tissue-specific delivery and inclusion of target molecules into exosomes. These improvements in exosome modification technology could enhance the effectiveness of exosome-based intra-articular treatments.
4.4. Potential of Exosomes in a Sustained Release Drug Delivery System (SRDDS)
Exosomes are relatively stable in vivo, but multiple administrations may be required to overcome the natural elimination of exosomes in the joint. As such, to prolong and enhance the effect of joint treatment methods, studies have combined exosomes with various biomaterials to be used in a SRDDS [98,99,100,101]. Chen et al. produced a 3D-printed cartilage ECM/gelatin methacrylate (GelMA)/exosome scaffold with radially oriented channels through a desktop-stereolithography technology [102]. This 3D printed scaffold was shown to continuously release exosomes for 14 days in vitro. When the 3D printed scaffold was transplanted through an invasive procedure in a rabbit osteochondral model, the scaffold retained exosomes for at least 7 days in vivo and significantly promoted cartilage repair.
Other studies have combined exosomes with injectable materials that can be administered through intra-articular injection. Liu et al. utilized stem cell-derived exosomes in combination with photoinduced imine crosslinking hydrogel glue to produce a cell-free exosome tissue patch [103]. The tissue patch continuously released exosomes for 14 days. In vivo studies showed that the new cartilage tissue regenerated by the tissue patch was hyaline cartilage-like in a rabbit articular cartilage defect model. Alternatively, Hu et al. produced a Gelma/nanoclay hydrogel (Gel-nano-sEVs) with embedded human umbilical cord MSC-derived small extracellular vesicles (hUC-MSCs-sEVs) [104]. In this study, they showed that miR-23a-3p, which is highly expressed in hUC-MSCs-sEVs, activates the phosphatase and tensin homolog deleted from chromosome 10 (PTEN)/AKT serine/threonine kinase (AKT) signaling pathway and promotes cartilage regeneration. The Gel-nano-sEVs were demonstrated to release exosomes continuously for approximately 30 days and contribute to cartilage regeneration. Further studies are expected in this field.
5. Advantages and Challenges of Developing Treatments Utilizing Exosomes
Treatments utilizing exosomes have remarkable potential to target and affect specific tissues. With proper control, specific cargos such as miRNAs can be packaged to be delivered to various tissues affected by OAK, such as cartilage, synovium, and subchondral bone. Unlike cell-based therapies, exosomes have a lower immunogenicity and an inability to directly form tumors [105,106]. Cell-based therapies rely on a more complicated mode of action such as the production of humoral factors that may be influenced by local conditions [34,107]. While exosomes may also be influenced by local conditions as well, with proper manufacturing they can carry and deliver a consistent set of cargos at desired concentrations.
On the other hand, many challenges remain, such as the large number of cells required for the manufacturing of exosomes, the multiple steps and time required for collection, and the unclear risks in ensuring safety and efficacy [39,41,43,55]. In addition, when exosomes are used clinically, the possible transmission of infectious diseases, due to contamination with viruses, bacteria, and fungi, harmful effects of components other than exosomes that are simultaneously administered, and potential risks of inconsistent efficacy and quality must be considered.
Exosomes possess complicated characteristics such as their size, the cargos they hold, and their destinations, which must be properly characterized in addition to the origins of the exosomes. As such, setting quality standards in the manufacturing process will be a challenge. When manufacturing exosomes for treatments, the quality of cells used as raw materials and their constituents and the management of the manufacturing process will be important additions to the quality control of the exosomes themselves. The ISEV proposed Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines in 2014, which was updated in 2018, suggesting a comprehensive yet evolving guideline for studies using extracellular vehicles, including exosomes [53,108]. To secure the quality, efficacy, and safety of exosomes, strict quality control and manufacturing control must be implemented.
6. Conclusions
EVs, including exosomes, present new possibilities for treatment, diagnosis, and drug development for patients with joint diseases including OAK. Exosomes from body fluids such as joint fluid and plasma can provide important prognoses of disease to administer appropriate treatments and other preventive measures. The use of exosomes for treating OAK through intra-articular injection provides a novel treatment method to bridge the gap between pharmacological and surgical procedures. However, many unclear aspects of the functions and mechanisms of EVs, including exosomes, and their roles in treatments of joint diseases such as OAK, remain to be elucidated. For the realization of exosome therapy, it is essential to clarify the risks of the treatment methods, implement strict manufacturing controls, and prepare appropriate guidelines and laws.
Further research in this field and scientific evidence of safety and efficacy may greatly contribute to the point-of-care treatment and cell-free therapy of joint disease.
Abbreviation
| 3′UTR | three prime untranslated region |
| ACI | autologous chondrocyte implantation |
| ACLT | anterior cruciate ligament transection |
| ADAMTS5 | a disintegrin and metalloproteinase with thrombospondin motifs 5 |
| AKT | AKT serine/threonine kinase 1 |
| ALIX | ALG-2-interacting protein X |
| ATG4B | autophagy related 4B cysteine peptidase |
| CAP | chondrocyte-affinity peptide |
| CD | cluster of differentiation |
| DACT1 | dishevelled binding antagonist of beta catenin 1 |
| DDS | drug delivery system |
| DMM | destabilization of the medial meniscus |
| DMOAD | disease-modifying OA drugs |
| DNA | deoxyribonucleic acid |
| ECM | extracellular matrix |
| ESCRT | endosomal sorting complexes required for transport |
| EV | extracellular vesicle |
| GelMA | gelatin methacrylate |
| GSK-3β | glycogen synthase kinase 3 beta |
| hBMSC | human bone marrow-derived MSC |
| HDAC | histone deacetylase |
| Hsp | heat shock protein |
| hUC | human umbilical cord |
| IL-1β | interleukin-1 beta |
| ILV | intraluminal membrane vesicle |
| IPFP | infrapatellar fat pad |
| ISEV | International Society for Extracellular Vesicles |
| Lamp2b | lysosome-associated membrane glycoprotein 2b |
| lncRNA | long noncoding RNA |
| MCH | major histocompatibility complex |
| MCS | mesenchymal stem cell |
| miRNA | microRNA |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| MMP13 | matrix metalloproteinase 13 |
| mTOR | mammalian target of rapamycin |
| MVB | multivesicular body |
| NSAID | non-steroidal anti-inflammatory drugs |
| OAK | osteoarthritis of knee |
| PCGEM1 | prostate-specific transcript 1 |
| PHC | primary human chondrocytes |
| PTEN | phosphatase and tensin homolog deleted from chromosome 10 |
| PTGS2 | prostaglandin-endoperoxide synthase 2 |
| RA | rheumatoid arthritis |
| RNA | ribonucleic acid |
| siRNA | small interfering RNA |
| SMSC | synovium-derived MSC |
| SOX9 | SRY-box transcription factor 9 |
| SRDDS | sustained release drug delivery system |
| TGF-β1 | transforming growth factor beta 1 |
| TKA | total knee arthroplasty |
| TLR | Toll-like receptor |
| TSG101 | tumor susceptibility gene 101 |
| WNT5A | Wnt family member 5A |
| YAP | Yes-associated protein |
Author Contributions
Conceptualization, M.M. and M.S.; writing—original draft preparation, M.M., E.T., T.T. and M.S.; writing—review and editing, M.M. and T.T.; supervision, M.W. and M.S.; funding acquisition, M.M. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
Authors received financial support for the authorship and publication of this article by JSPS KAKENHI Grant Number JP19K18509 and TERUMO LIFE SCIENCE FOUNDATION.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunter W. Of the Structure and Diseases of Articu-lating Cartilages. Philos. Trans. R. Soc. Lond. 1743;42:514–521. doi: 10.1098/rstl.1742.0079. [DOI] [Google Scholar]
- 2.Ulrich-Vinther M., Maloney M.D., Schwarz E.M., Rosier R., O′Keefe R.J. Articular Cartilage Biology. JAAOS J. Am. Acad. Orthop. Surg. 2003;11:421–430. doi: 10.5435/00124635-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Aspden R., Saunders F. Osteoarthritis as an Organ Disease: From the Cradle to the Grave. Eur. Cells Mater. 2019;37:74–87. doi: 10.22203/eCM.v037a06. [DOI] [PubMed] [Google Scholar]
- 4.Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., Bridgett L., Williams S., Guillemin F., Hill C.L., et al. The Global Burden of Hip and Knee Osteoarthritis: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 5.Pridie K., Gordon-Strachan G. A Method of Resurfacing Osteoarthritis Knee Joints. J. Bone Jt. Surg. Br. 1959;41:618–619. [Google Scholar]
- 6.Steadman J.R., Rodkey W.G., Rodrigo J.J. Microfracture: Surgical Technique and Rehabilitation to Treat Chondral Defects. Clin. Orthop. 2001;391:S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 7.Mithoefer K., Williams R.J., Warren R.F., Potter H.G., Spock C.R., Jones E.C., Wickiewicz T.L., Marx R.G. Chondral Resurfacing of Articular Cartilage Defects in the Knee with the Microfracture Technique: Surgical Technique. JBJS Essent. Surg. Tech. 2006;88:294–304. doi: 10.2106/JBJS.F.00292. [DOI] [PubMed] [Google Scholar]
- 8.Hangody L., Vásárhelyi G., Hangody L.R., Sükösd Z., Tibay G., Bartha L., Bodó G. Autologous Osteochondral Grafting—Technique and Long-Term Results. Injury. 2008;39:32–39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Szerb I., Hangody L., Duska Z., Kaposi N.P. Mosaicplasty: Long-Term Follow-Up. Bull. Hosp. Jt. Dis. 2005;63:54–62. [PubMed] [Google Scholar]
- 10.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 11.Uchio Y., Ochi M., Matsusaki M., Kurioka H., Katsube K. Human Chondrocyte Proliferation and Matrix Synthesis Cultured in Atelocollagen Gel. J. Biomed. Mater. Res. 2000;50:138–143. doi: 10.1002/(SICI)1097-4636(200005)50:2<138::AID-JBM7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Ochi M., Uchio Y., Kawasaki K., Wakitani S., Iwasa J. Transplantation of Cartilage-like Tissue Made by Tissue Engineering in the Treatment of Cartilage Defects of the Knee. J. Bone Jt. Surg. Br. 2002;84:571–578. doi: 10.1302/0301-620X.84B4.0840571. [DOI] [PubMed] [Google Scholar]
- 13.Nehrer S., Halbwirth F., Luksch T. CaReS®, Cartilage Regeneration System: Autologous Chondrocyte Transplantation in a Collagen Gel. In: Shetty A.A., Kim S.-J., Nakamura N., Brittberg M., editors. Techniques in Cartilage Repair Surgery. Springer; Berlin/Heidelberg, Germany: 2014. pp. 245–250. [Google Scholar]
- 14.Schneider U., Rackwitz L., Andereya S., Siebenlist S., Fensky F., Reichert J., Löer I., Barthel T., Rudert M., Nöth U. A Prospective Multicenter Study on the Outcome of Type I Collagen Hydrogel-Based Autologous Chondrocyte Implantation (CaReS) for the Repair of Articular Cartilage Defects in the Knee. Am. J. Sports Med. 2011;39:2558–2565. doi: 10.1177/0363546511423369. [DOI] [PubMed] [Google Scholar]
- 15.Tognana E., Borrione A., De Luca C., Pavesio A. Hyalograft C: Hyaluronan-Based Scaffolds in Tissue-Engineered Cartilage. Cells Tissues Organs. 2007;186:97–103. doi: 10.1159/000102539. [DOI] [PubMed] [Google Scholar]
- 16.Kreuz P.C., Müller S., Ossendorf C., Kaps C., Erggelet C. Treatment of Focal Degenerative Cartilage Defects with Polymer-Based Autologous Chondrocyte Grafts: Four-Year Clinical Results. Arthritis Res. Ther. 2009;11:R33. doi: 10.1186/ar2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts S., McCall I.W., Darby A.J., Menage J., Evans H., Harrison P.E., Richardson J.B. Autologous Chondrocyte Implantation for Cartilage Repair: Monitoring Its Success by Magnetic Resonance Imaging and Histology. Arthritis Res. Ther. 2003;5:R60–R73. doi: 10.1186/ar613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett W., Skinner J.A., Gooding C.R., Carrington R.W.J., Flanagan A.M., Briggs T.W.R., Bentley G. Autologous Chondrocyte Implantation versus Matrix-Induced Autologous Chondrocyte Implantation for Osteochondral Defects of the Knee: A Prospective, Randomised Study. J. Bone Jt. Surg. Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 19.Maehara M., Sato M., Toyoda E., Takahashi T., Okada E., Kotoku T., Watanabe M. Characterization of Polydactyly-Derived Chondrocyte Sheets versus Adult Chondrocyte Sheets for Articular Cartilage Repair. Inflamm. Regen. 2017;37:22. doi: 10.1186/s41232-017-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato M., Yamato M., Mitani G., Takagaki T., Hamahashi K., Nakamura Y., Ishihara M., Matoba R., Kobayashi H., Okano T., et al. Combined Surgery and Chondrocyte Cell-Sheet Transplantation Improves Clinical and Structural Outcomes in Knee Osteoarthritis. NPJ Regen. Med. 2019;4:4. doi: 10.1038/s41536-019-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T., Sato M., Toyoda E., Maehara M., Takizawa D., Maruki H., Tominaga A., Okada E., Okazaki K., Watanabe M. Rabbit Xenogeneic Transplantation Model for Evaluating Human Chondrocyte Sheets Used in Articular Cartilage Repair. J. Tissue Eng. Regen. Med. 2018;12:2067–2076. doi: 10.1002/term.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varacallo M., Luo T.D., Johanson N.A. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Total Knee Arthroplasty (TKA) Techniques. [Google Scholar]
- 23.Colombini A., Perucca Orfei C., Kouroupis D., Ragni E., De Luca P., ViganÒ M., Correa D., de Girolamo L. Mesenchymal Stem Cells in the Treatment of Articular Cartilage Degeneration: New Biological Insights for an Old-Timer Cell. Cytotherapy. 2019;21:1179–1197. doi: 10.1016/j.jcyt.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso P., Raman S., Glynn A., Barry F., Murphy J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019;7:9. doi: 10.3389/fbioe.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascarenhas R., Saltzman B.M., Fortier L.A., Cole B.J. Role of Platelet-Rich Plasma in Articular Cartilage Injury and Disease. J. Knee Surg. 2015;28:3–10. doi: 10.1055/s-0034-1384672. [DOI] [PubMed] [Google Scholar]
- 26.Dhillon M.S., Patel S., John R. PRP in OA Knee—Update, Current Confusions and Future Options. SICOT J. 2017;3:27. doi: 10.1051/sicotj/2017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasai S., Sato M., Maehara M., Toyoda E., Uchiyama R., Takahashi T., Okada E., Iwasaki Y., Suzuki S., Watanabe M. Characteristics of Autologous Protein Solution and Leucocyte-Poor Platelet-Rich Plasma for the Treatment of Osteoarthritis of the Knee. Sci. Rep. 2020;10:10572. doi: 10.1038/s41598-020-67099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuffler D.P. Variables Affecting the Potential Efficacy of PRP in Providing Chronic Pain Relief. J. Pain Res. 2018;12:109–116. doi: 10.2147/JPR.S190065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kretlow J.D., Jin Y.-Q., Liu W., Zhang W.J., Hong T.-H., Zhou G., Baggett L.S., Mikos A.G., Cao Y. Donor Age and Cell Passage Affects Differentiation Potential of Murine Bone Marrow-Derived Stem Cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaim M., Karaman S., Cetin G., Isik S. Donor Age and Long-Term Culture Affect Differentiation and Proliferation of Human Bone Marrow Mesenchymal Stem Cells. Ann. Hematol. 2012;91:1175–1186. doi: 10.1007/s00277-012-1438-x. [DOI] [PubMed] [Google Scholar]
- 31.Mazzocca A.D., McCarthy M.B.R., Chowaniec D.M., Dugdale E.M., Hansen D., Cote M.P., Bradley J.P., Romeo A.A., Arciero R.A., Beitzel K. The Positive Effects of Different Platelet-Rich Plasma Methods on Human Muscle, Bone, and Tendon Cells. Am. J. Sports Med. 2012;40:1742–1749. doi: 10.1177/0363546512452713. [DOI] [PubMed] [Google Scholar]
- 32.Breitbach M., Bostani T., Roell W., Xia Y., Dewald O., Nygren J.M., Fries J.W.U., Tiemann K., Bohlen H., Hescheler J., et al. Potential Risks of Bone Marrow Cell Transplantation into Infarcted Hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 33.Lalu M.M., McIntyre L., Pugliese C., Fergusson D., Winston B.W., Marshall J.C., Granton J., Stewart D.J. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kan C., Chen L., Hu Y., Lu H., Li Y., Kessler J.A., Kan L. Microenvironmental Factors That Regulate Mesenchymal Stem Cells: Lessons Learned from the Study of Heterotopic Ossification. Histol. Histopathol. 2017;32:977–985. doi: 10.14670/HH-11-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caplan H., Olson S.D., Kumar A., George M., Prabhakara K.S., Wenzel P., Bedi S., Toledano-Furman N.E., Triolo F., Kamhieh-Milz J., et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drela K., Stanaszek L., Nowakowski A., Kuczynska Z., Lukomska B. Experimental Strategies of Mesenchymal Stem Cell Propagation: Adverse Events and Potential Risk of Functional Changes. [(accessed on 1 March 2021)]; doi: 10.1155/2019/7012692. Available online: https://www.hindawi.com/journals/sci/2019/7012692/ [DOI] [PMC free article] [PubMed]
- 37.Liu Y., Wang Y., Lv Q., Li X. Exosomes: From Garbage Bins to Translational Medicine. Int. J. Pharm. 2020;583:119333. doi: 10.1016/j.ijpharm.2020.119333. [DOI] [PubMed] [Google Scholar]
- 38.Pourakbari R., Khodadadi M., Aghebati-Maleki A., Aghebati-Maleki L., Yousefi M. The Potential of Exosomes in the Therapy of the Cartilage and Bone Complications; Emphasis on Osteoarthritis. Life Sci. 2019;236:116861. doi: 10.1016/j.lfs.2019.116861. [DOI] [PubMed] [Google Scholar]
- 39.Allan D., Tieu A., Lalu M., Burger D. Mesenchymal Stromal Cell-derived Extracellular Vesicles for Regenerative Therapy and Immune Modulation: Progress and Challenges toward Clinical Application. Stem Cells Transl. Med. 2020;9:39–46. doi: 10.1002/sctm.19-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witwer K.W., Balkom B.W.M.V., Bruno S., Choo A., Dominici M., Gimona M., Hill A.F., Kleijn D.D., Koh M., Lai R.C., et al. Defining Mesenchymal Stromal Cell (MSC)-Derived Small Extracellular Vesicles for Therapeutic Applications. J. Extracell. Vesicles. 2019;8:1609206. doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elahi F.M., Farawell D.G., Nolta J.A., Anderson J.D. Preclinical Translation of Exosomes Derived from Mesenchymal Stem/Stromal Cells. Stem Cells. 2020;38:15–21. doi: 10.1002/stem.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phinney D.G., Pittenger M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 43.Rohde E., Pachler K., Gimona M. Manufacturing and Characterization of Extracellular Vesicles from Umbilical Cord–Derived Mesenchymal Stromal Cells for Clinical Testing. Cytotherapy. 2019;21:581–592. doi: 10.1016/j.jcyt.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Pan B.T., Johnstone R.M. Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes in Vitro: Selective Externalization of the Receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 45.Stahl P.D., Raposo G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology. 2019;34:169–177. doi: 10.1152/physiol.00045.2018. [DOI] [PubMed] [Google Scholar]
- 46.Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes) J. Biol. Chem. 1987;262:9412–9420. doi: 10.1016/S0021-9258(18)48095-7. [DOI] [PubMed] [Google Scholar]
- 47.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 48.Stein J.M., Luzio J.P. Ectocytosis Caused by Sublytic Autologous Complement Attack on Human Neutrophils. The Sorting of Endogenous Plasma-Membrane Proteins and Lipids into Shed Vesicles. Biochem. J. 1991;274:381–386. doi: 10.1042/bj2740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cocucci E., Racchetti G., Meldolesi J. Shedding Microvesicles: Artefacts No More. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Di Vizio D., Kim J., Hager M.H., Morello M., Yang W., Lafargue C.J., True L., Rubin M.A., Adam R.M., Beroukhim R., et al. Oncosome Formation in Prostate Cancer: Association with a Region of Frequent Chromosomal Deletion in Metastatic Disease. Cancer Res. 2009;69:5601–5609. doi: 10.1158/0008-5472.CAN-08-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hristov M., Erl W., Linder S., Weber P.C. Apoptotic Bodies from Endothelial Cells Enhance the Number and Initiate the Differentiation of Human Endothelial Progenitor Cells in Vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 52.Van Niel G., D′Angelo G., Raposo G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 53.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tkach M., Kowal J., Théry C. Why the Need and How to Approach the Functional Diversity of Extracellular Vesicles. Philos. Trans. R. Soc. B Biol. Sci. 2018;373:20160479. doi: 10.1098/rstb.2016.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalluri R., LeBleu V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colombo M., Raposo G., Théry C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 57.Villarroya-Beltri C., Baixauli F., Gutiérrez-Vázquez C., Sánchez-Madrid F., Mittelbrunn M. SORTING IT OUT: REGULATION OF EXOSOME LOADING. Semin. Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guescini M., Genedani S., Stocchi V., Agnati L.F. Astrocytes and Glioblastoma Cells Release Exosomes Carrying MtDNA. J. Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 59.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 60.Turturici G., Tinnirello R., Sconzo G., Geraci F. Extracellular Membrane Vesicles as a Mechanism of Cell-to-Cell Communication: Advantages and Disadvantages. Am. J. Physiol. Cell Physiol. 2014;306:C621–C633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 61.Raposo G., Stoorvogel W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gurunathan S., Kang M.-H., Jeyaraj M., Qasim M., Kim J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019;8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konoshenko M.Y., Lekchnov E.A., Vlassov A.V., Laktionov P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed. Res. Int. 2018;2018:1–27. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gardiner C., Vizio D.D., Sahoo S., Théry C., Witwer K.W., Wauben M., Hill A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kosaka N., Yoshioka Y., Fujita Y., Ochiya T. Versatile Roles of Extracellular Vesicles in Cancer. J. Clin. Investig. 2016;126:1163–1172. doi: 10.1172/JCI81130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tominaga N., Yoshioka Y., Ochiya T. A Novel Platform for Cancer Therapy Using Extracellular Vesicles. Adv. Drug Deliv. Rev. 2015;1:50–55. doi: 10.1016/j.addr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 69.He L., Hannon G.J. MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 70.Malemud C.J. MicroRNAs and Osteoarthritis. Cells. 2018;7:92. doi: 10.3390/cells7080092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong E., Reddi A.H. MicroRNAs in Chondrogenesis, Articular Cartilage, and Osteoarthritis: Implications for Tissue Engineering. Tissue Eng. Part B Rev. 2012;18:445–453. doi: 10.1089/ten.teb.2012.0116. [DOI] [PubMed] [Google Scholar]
- 72.Grigelioniene G., Suzuki H.I., Taylan F., Mirzamohammadi F., Borochowitz Z.U., Ayturk U.M., Tzur S., Horemuzova E., Lindstrand A., Weis M.A., et al. Gain-of-Function Mutation of MicroRNA-140 in Human Skeletal Dysplasia. Nat. Med. 2019;25:583–590. doi: 10.1038/s41591-019-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyaki S., Nakasa T., Otsuki S., Grogan S.P., Higashiyama R., Inoue A., Kato Y., Sato T., Lotz M.K., Asahara H. MicroRNA-140 Is Expressed in Differentiated Human Articular Chondrocytes and Modulates Interleukin-1 Responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ukai T., Sato M., Akutsu H., Umezawa A., Mochida J. MicroRNA-199a-3p, MicroRNA-193b, and MicroRNA-320c Are Correlated to Aging and Regulate Human Cartilage Metabolism. J. Orthop. Res. 2012;30:1915–1922. doi: 10.1002/jor.22157. [DOI] [PubMed] [Google Scholar]
- 75.Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., Ochiya T. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J. Biol. Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., Van Eijndhoven M.A.J., Hopmans E.S., Lindenberg J.L., De Gruijl T.D., Würdinger T., Middeldorp J.M. Functional Delivery of Viral MiRNAs via Exosomes. Proc. Natl. Acad. Sci. USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J., Li S., Li L., Li M., Guo C., Yao J., Mi S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun H., Hu S., Zhang Z., Lun J., Liao W., Zhang Z. Expression of Exosomal MicroRNAs during Chondrogenic Differentiation of Human Bone Mesenchymal Stem Cells. J. Cell. Biochem. 2019;120:171–181. doi: 10.1002/jcb.27289. [DOI] [PubMed] [Google Scholar]
- 79.Mao G., Hu S., Zhang Z., Wu P., Zhao X., Lin R., Liao W., Kang Y. Exosomal MiR-95-5p Regulates Chondrogenesis and Cartilage Degradation via Histone Deacetylase 2/8. J. Cell. Mol. Med. 2018;22:5354–5366. doi: 10.1111/jcmm.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Z., Wang Y., Xiang S., Zheng Z., Bian Y., Feng B., Weng X. Chondrocytes-Derived Exosomal MiR-8485 Regulated the Wnt/β-Catenin Pathways to Promote Chondrogenic Differentiation of BMSCs. Biochem. Biophys. Res. Commun. 2020;523:506–513. doi: 10.1016/j.bbrc.2019.12.065. [DOI] [PubMed] [Google Scholar]
- 81.Mao G., Zhang Z., Hu S., Zhang Z., Chang Z., Huang Z., Liao W., Kang Y. Exosomes Derived from MiR-92a-3p-Overexpressing Human Mesenchymal Stem Cells Enhance Chondrogenesis and Suppress Cartilage Degradation via Targeting WNT5A. Stem Cell Res. Ther. 2018;9:247. doi: 10.1186/s13287-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin Z., Ren J., Qi S. Human Bone Mesenchymal Stem Cells-Derived Exosomes Overexpressing MicroRNA-26a-5p Alleviate Osteoarthritis via down-Regulation of PTGS2. Int. Immunopharmacol. 2020;78:105946. doi: 10.1016/j.intimp.2019.105946. [DOI] [PubMed] [Google Scholar]
- 83.Wu J., Kuang L., Chen C., Yang J., Zeng W.-N., Li T., Chen H., Huang S., Fu Z., Li J., et al. MiR-100-5p-Abundant Exosomes Derived from Infrapatellar Fat Pad MSCs Protect Articular Cartilage and Ameliorate Gait Abnormalities via Inhibition of MTOR in Osteoarthritis. Biomaterials. 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 84.Tao S.C., Yuan T., Zhang Y.-L., Yin W.-J., Guo S.-C., Zhang C.-Q. Exosomes Derived from MiR-140-5p-Overexpressing Human Synovial Mesenchymal Stem Cells Enhance Cartilage Tissue Regeneration and Prevent Osteoarthritis of the Knee in a Rat Model. Theranostics. 2017;7:180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ni Z., Kuang L., Chen H., Xie Y., Zhang B., Ouyang J., Wu J., Zhou S., Chen L., Nan S., et al. The Exosome-like Vesicles from Osteoarthritic Chondrocyte Enhanced Mature IL-1β Production of Macrophages and Aggravated Synovitis in Osteoarthritis. Cell Death Dis. 2019;10:522. doi: 10.1038/s41419-019-1739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sheridan C. Exosome Cancer Diagnostic Reaches Market. Nat. Biotechnol. 2016;34:359–360. doi: 10.1038/nbt0416-359. [DOI] [PubMed] [Google Scholar]
- 87.Brinkmann K., Enderle D., Flinspach C., Meyer L., Skog J., Noerholm M. Exosome Liquid Biopsies of NSCLC Patients for Longitudinal Monitoring of ALK Fusions and Resistance Mutations. J. Clin. Oncol. 2018;36:e24090. doi: 10.1200/JCO.2018.36.15_suppl.e24090. [DOI] [Google Scholar]
- 88.Kolhe R., Hunter M., Liu S., Jadeja R.N., Pundkar C., Mondal A.K., Mendhe B., Drewry M., Rojiani M.V., Liu Y., et al. Gender-Specific Differential Expression of Exosomal MiRNA in Synovial Fluid of Patients with Osteoarthritis. Sci. Rep. 2017;7:2029. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meng F., Li Z., Zhang Z., Yang Z., Kang Y., Zhao X., Long D., Hu S., Gu M., He S., et al. MicroRNA-193b-3p Regulates Chondrogenesis and Chondrocyte Metabolism by Targeting HDAC3. Theranostics. 2018;8:2862–2883. doi: 10.7150/thno.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Y., Xu J. Synovial Fluid-Derived Exosomal LncRNA PCGEM1 as Biomarker for the Different Stages of Osteoarthritis. Int. Orthop. 2018;42:2865–2872. doi: 10.1007/s00264-018-4093-6. [DOI] [PubMed] [Google Scholar]
- 91.Chen X.M., Zhao Y., Wu X.D., Wang M.J., Yu H., Lu J.J., Hu Y.J., Huang Q.C., Huang R.Y., Lu C.J. Novel Findings from Determination of Common Expressed Plasma Exosomal MicroRNAs in Patients with Psoriatic Arthritis, Psoriasis Vulgaris, Rheumatoid Arthritis, and Gouty Arthritis. Discov. Med. 2019;28:47–68. [PubMed] [Google Scholar]
- 92.Tsuno H., Arito M., Suematsu N., Sato T., Hashimoto A., Matsui T., Omoteyama K., Sato M., Okamoto K., Tohma S., et al. A Proteomic Analysis of Serum-Derived Exosomes in Rheumatoid Arthritis. BMC Rheumatol. 2018;2:35. doi: 10.1186/s41927-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Somiya M., Yoshioka Y., Ochiya T. Drug Delivery Application of Extracellular Vesicles; Insight into Production, Drug Loading, Targeting, and Pharmacokinetics. AIMS Bioeng. 2017;4:73. doi: 10.3934/bioeng.2017.1.73. [DOI] [Google Scholar]
- 94.Antimisiaris S.G., Mourtas S., Marazioti A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics. 2018;10:218. doi: 10.3390/pharmaceutics10040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang R., Xu B., Xu H. TGF-Β1 Promoted Chondrocyte Proliferation by Regulating Sp1 through MSC-Exosomes Derived MiR-135b. Cell Cycle. 2018;17:2756–2765. doi: 10.1080/15384101.2018.1556063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., Wang D., Xia J. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl. Mater. Interfaces. 2020;12:36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 98.Xie H., Wang Z., Zhang L., Lei Q., Zhao A., Wang H., Li Q., Cao Y., Zhang W.J., Chen Z. Extracellular Vesicle-Functionalized Decalcified Bone Matrix Scaffolds with Enhanced Pro-Angiogenic and Pro-Bone Regeneration Activities. Sci. Rep. 2017;7:45622. doi: 10.1038/srep45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi Q., Qian Z., Liu D., Sun J., Wang X., Liu H., Xu J., Guo X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017;8:904. doi: 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang J., Chen Z., Pan D., Li H., Shen J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020;11:5911–5926. doi: 10.2147/IJN.S249129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li L., Zhang Y., Mu J., Chen J., Zhang C., Cao H., Gao J. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett. 2020;20:4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
- 102.Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., Gu C., Chen J., Qiu P., Mei S., et al. Desktop-Stereolithography 3D Printing of a Radially Oriented Extracellular Matrix/Mesenchymal Stem Cell Exosome Bioink for Osteochondral Defect Regeneration. Theranostics. 2019;9:2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu X., Yang Y., Li Y., Niu X., Zhao B., Wang Y., Bao C., Xie Z., Lin Q., Zhu L. Integration of Stem Cell-Derived Exosomes with in Situ Hydrogel Glue as a Promising Tissue Patch for Articular Cartilage Regeneration. Nanoscale. 2017;9:4430–4438. doi: 10.1039/C7NR00352H. [DOI] [PubMed] [Google Scholar]
- 104.Hu H., Dong L., Bu Z., Shen Y., Luo J., Zhang H., Zhao S., Lv F., Liu Z. MiR-23a-3p-Abundant Small Extracellular Vesicles Released from Gelma/Nanoclay Hydrogel for Cartilage Regeneration. J. Extracell. Vesicles. 2020;9:1778883. doi: 10.1080/20013078.2020.1778883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liew L.C., Katsuda T., Gailhouste L., Nakagama H., Ochiya T. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Glimmer of Hope in Treating Alzheimer’s Disease. Int. Immunol. 2017;29:11–19. doi: 10.1093/intimm/dxx002. [DOI] [PubMed] [Google Scholar]
- 106.Mendt M., Rezvani K., Shpall E. Mesenchymal Stem Cell-Derived Exosomes for Clinical Use. Bone Marrow Transpl. 2019;54:789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 107.Murphy M.B., Moncivais K., Caplan A.I. Mesenchymal Stem Cells: Environmentally Responsive Therapeutics for Regenerative Medicine. Exp. Mol. Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lener T., Gimona M., Aigner L., Börger V., Buzas E., Camussi G., Chaput N., Chatterjee D., Court F.A., del Portillo H.A., et al. Applying Extracellular Vesicles Based Therapeutics in Clinical Trials—An ISEV Position Paper. J. Extracell. Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]