Abstract
Objectives:
Biologic therapies are emerging as an option to treat a subset of patients with severe asthma, however no direct comparison between these agents has been conducted. Furthermore, heterogeneity of outcomes in clinical trials makes it difficult to compare these agents and traditional therapies. The extent to which this heterogeneity exists has major implications for evidence-based decisions and is yet to be fully reported. We conducted a literature search to examine outcomes currently being used in clinical trials for asthma.
Data Sources:
The Cochrane Library and Clinicaltrials.gov were searched for clinical trials of asthma interventions.
Study Selections:
We limited our search to phase 2 through 4 clinical trials in adults, as early-phase trials tend to have pharmacodynamic and pharmacokinetic endpoints as primary outcomes. Interventions for acute exacerbations were excluded.
Results:
We identified 117 studies and subsequently identified 111 outcomes. The most prevalent outcomes were asthma control and symptom severity, FEV1, and change in ACQ scale. Twenty patient-reported outcomes instruments were identified and de-facto standard asthma outcomes and PROs were under-reported in examined literature. Existing quality of life tools did not capture the day-to-day experience or the unique treatment burden from oral corticosteroids for patient with severe asthma. Compounding the absence of trials directly comparing therapies, the significant variation we identified in outcome definitions and measurement create hurdles to effectively compare traditional and biologic therapies.
Conclusion:
With the growing number of clinical trials evaluating advanced therapies such as biologics, a wide range of primary and secondary outcomes are evaluated. A core outcome set created by relevant stakeholders is needed to collectively evaluate pooled outcomes in order to allow more meaningful comparisons of asthma therapies and to incorporate the patient experience.
Keywords: Reviews, Quality of Life
Introduction
Asthma is characterized by variable levels of chronic airway inflammation and episodes of cough, wheeze, and shortness of breath. With conventional inhaled or anti-inflammatory treatments for asthma, most patients can achieve adequate symptom control. However, a subset of 3-10% of patients fail to respond to available options.1,2 Joint guidelines published by the European Respiratory Society and American Thoracic Society (ERS/ATS) define severe asthma as asthma that requires treatment with high dose inhaled corticosteroids (ICS) plus a second controller to prevent it from becoming “uncontrolled” or which remains “uncontrolled” despite this therapy.2 People with severe asthma suffer multiple symptoms, stress, and more frequent exacerbations that often require hospitalization. In addition to ICS, about 30% of patients with severe asthma are also treated with oral corticosteroids (OCS).2 OCS and high dose ICS come with their own unique burden of side effects.3,4 Severe asthma impacts many facets of daily life, including school or career, social relationships, and family life.5,6 Notably, the 20-year direct costs associated with uncontrolled asthma are estimated to be $300.6 billion, increasing to $963.5 billion when indirect costs are added.7
In recent years, new biologic therapies – e.g. omalizumab – have been developed to treat moderate to severe asthma.8 Targeting different immunologic pathways, asthma biologics offer the potential for a paradigm shift in the standard of asthma care, however at considerably higher price. There are currently five FDA-approved biologic agents. For all five agents, Cochrane meta-analyses showed an approximate a 50% reduction in exacerbations, compared to placebo. While these agents also demonstrated statistically significant improvements in quality of life (QoL) measures, all fell short of attaining minimally clinically important differences (MCID) in QoL except for the St. George’s Respiratory Questionnaire (SGRQ) in a single study of mepolizumab.9-12 No direct comparison between these agents has been conducted with none proposed, likely due to lack of impetus for pharmaceutical companies to do so. In addition, it is difficult to assess the added value of these agents for patients, given the heterogeneity of outcomes in these trials at multiple levels: outcomes measured, outcomes definitions used, and which instruments are used to measure outcomes (e.g. a standard assessment for QoL). There is an awareness of the need and thus a growing effort toward collaboration and establishing a more standardized reporting, monitoring and capture of standardized data for clinicians, scientific research, and patient outcomes.13 The current state of heterogeneity of outcomes is predictable as clinical outcomes (subjective), lung function (objective), and medication reduction (patient preference) are all potentially desired however may be different across patient populations. The extent of heterogeneity of outcomes in clinical trials in the current and emerging evidence has major implications for evidence-based decisions from clinical practice guidelines to coverage and pricing. Our vantage point is that to collectively evaluate various modalities of therapy, an agreed upon core set of outcomes should be considered and included in as many future intervention trials as possible, thereby allowing comparisons across the potential therapies available and allowing for better counseling to patients related to their desired outcomes. In order to understand the extent of current state of asthma outcome heterogeneity, we conducted a targeted literature review to examine outcomes currently being used in clinical trials for asthma.
Methods
Data Sources and Search Strategy
The objective of the search was to systematically identify outcomes reported in recent or ongoing clinical trials for moderate-to-severe asthma, in order to generate an initial list of outcomes to be reviewed and discussed by a multi-stakeholder panel. The list of outcomes was to be supplemented by peer-reviewed literature (for new patient-important outcomes, including PROs), key informant interviews, and suggestions from the panel. Given a goal to reach saturation on a list of relevant outcomes for discussion rather than synthesize evidence to assess treatment effectiveness, a targeted review was more appropriate than a meta-analysis. Thus, we focused efforts on two primary sources: Cochrane Library and clinicaltrials.gov.
Between the U.S.-focused registered trials in clinicaltrials.gov and the outcomes identified by international Cochrane systematic reviews, we felt confident in the representativeness of our search for the purposes of a core outcome set (COS) scoped for U.S., Canada, and Europe. Cochrane search methodology mandates searches of CENTRAL, MEDLINE, and Embase, and advises searches of clinicaltrials.gov, the WHO International Clinical Trials Registry Platform, and the Specialized Register of relevant Cochrane Review Groups. The MEDLINE and CENTRAL databases combined contain over 25 million references to biomedical journal articles and trial reports.14 Moreover, asthma systematic reviews include searches in the Cochrane Airways Trial Register, which source from CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, and abstracts from conferences such as the American Thoracic Society and European Respiratory Society.15 As such, the outcomes we extracted from these databases are likely to be comprehensive and appropriate in the health systems for which our COS is intended.
We limited our search to phase 2 through 4 clinical trials in those aged >17 years old, as early-phase trials tend to have pharmacodynamic and pharmacokinetic endpoints as primary outcomes. Our review was initially limited to publications within the recent three years (March 2016-June 2019) to encompass the period when a number of trials for biologic agents were conducted, reflecting the intent of the COS to be used prospectively. To check whether the initial outcomes list generated from our original search criteria had indeed reached saturation, we then extended our inclusion criteria for the Cochrane reviews to five years. This broader search identified an additional eight reviews without adding any new outcomes, suggesting we had reached saturation.
Selection Criteria
Interventions for acute exacerbations were excluded given a different set of interventions and outcomes compared to stable asthma. The target populations were adults and older adults. Given heterogeneous inclusion criteria with inconsistent study populations, we applied the search term “asthma” to ensure capture of relevant results. From included sources (trials and reviews), outcomes were abstracted and documented.
Data Extraction
Outcomes were classified into different domains based on the taxonomy suggested by Core Outcome Measures in Effectiveness Trials (COMET) Initiative.16 The classification was initially performed by one individual (H.C.) and then with consensus review by all other co-authors. All harmful occurrences were labelled as “adverse event” and categorized into an adverse event domain. To further understand the outcomes collected in recent clinical trials for biologics, we conducted a parallel search on Clinicaltrials.gov of the phase 3 trials supporting the FDA approval of the following drugs: dupilumab, reslizumab, benralizumab, and mepolizumab. We selected the more recently approved four agents and excluded omalizumab to select for trials more recently performed. Primary outcomes and definitions in these trials were extracted and reviewed.
Results
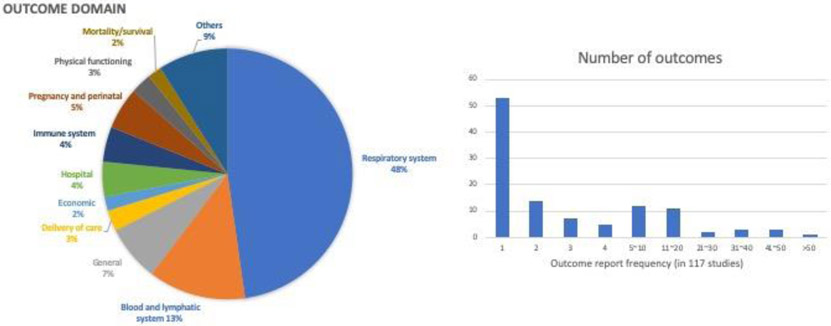
Thirteen Cochrane reviews and 104 clinical trials met our inclusion and exclusion criteria (Figure 1). Thirty-six Cochrane reviews and 74 clinical trials were excluded due to following reasons: non-pharmacotherapy interventions (e.g. behavioral intervention, food supplement, traditional Chinese medicine), not measuring drug effectiveness (e.g. comparing device performance), inclusion of other respiratory diseases, pharmacodynamic/pharmacokinetic studies, and inclusion of pediatric population. At second-round screening, 8 Cochrane reviews and 3 clinical trials assessing therapies for acute exacerbations were excluded. Among 104 clinical trials reviewed, 54 are single country and 38 are multinational trials (12 don’t have geographic information available). In total, 111 outcomes were identified in 117 references. Using the COMET taxonomy outcome classification,16 53 (48%) fell into the respiratory domain, 14 (13%) the blood and lymphatic system domain, 6 (5%) the pregnancy and perinatal domain, and 5 (5%) into the hospital domain and immune system domain (Figure 2). Besides adverse events and safety endpoints, the most prevalent outcomes were: asthma control and symptom severity (46%), pre-bronchodilator FEV1 (37%), forced expiratory volume-one second (FEV1) (35%), change in Asthma Control Questionnaire (ACQ) scale (34%), use of rescue medication (27%), Peak expiratory flow (26%), health-related quality of life (HRQoL) (26%), frequency of exacerbations (25%), level of blood eosinophils (17%), fractional exhaled nitric oxide (15%) and change in forced vital capacity (15%) (Table 1). Definitions varied within prevalent outcomes. Within exacerbations, more than ten definitions were documented, including for example: unscheduled hospital visits or emergency department visit, hospitalizations, use of OCS, an increase in maintenance systemic corticosteroid therapy dose or use for at least three days, increased bronchodilator use for at least two days, or worsening lung function (Table 2).17,18 Similarly, also shown in Table 2, different criteria were used to define asthma worsening such as reduction in peak expiratory flow or FEV1, increas in rescue medication use or ACQ score, occurrence of a severe asthma exacerbation, nocturnal awakenings due to asthma requiring short-acting beta agonist use. Among twenty patient-reported outcome (PRO) instruments identified in examined literature, ACQ and Asthma Quality of Life Questionnaire (AQLQ) were most frequently reported (Table 3). Nine different instruments were used to measure asthma control and symptom severity (Table 3). For HRQoL, the following instruments were used (Table 3): Asthma Quality of Life Questionnaire (AQLQ), SGRQ, and the 5-level EQ-5D (EQ-5D-5L).
Figure 1.
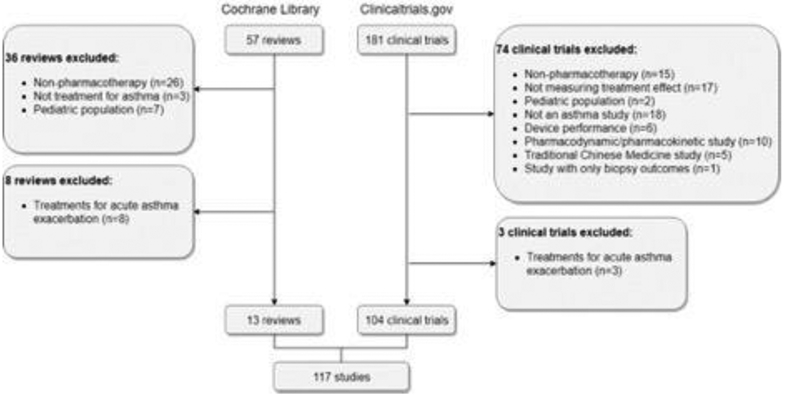
Flow diagram for study selection
Figure 2.
The outcome domains of outcomes (2A) and distribution of the number of times outcomes were reported (2B) in trials or Cochrane reviews
Table 1.
Report frequency of outcomes
| Outcomes | Counts | Frequency among 117 studies |
|---|---|---|
| Asthma symptom severity/ asthma control | 54 | 46% |
| Adverse events | 44 | 38% |
| Change in Pre-bronchodilator FEV1 | 43 | 37% |
| Forced expiratory volume-one second, FEV1 | 41 | 35% |
| Change in ACQ scale | 40 | 34% |
| Rescue medication use | 32 | 27% |
| Change in Peak expiratory flow (PEF) (lung function) | 31 | 26% |
| Health-related quality of life (HRQoL) | 30 | 26% |
| Rate of (severe) exacerbations | 29 | 25% |
| Blood eosinophil levels | 20 | 17% |
| Fractional exhaled nitric oxide (FeNO) | 18 | 15% |
| Change in Forced vital capacity (FVC) | 17 | 15% |
Table 2.
Definitions of exacerbation and worsening from examined studies
| Outcome: Exacerbation | |
|---|---|
|
|
| Outcome: Moderate Exacerbation | |
|
|
| Outcome: Severe Exacerbation | |
|
|
| Outcome: Asthma Worsening | |
|
|
ED: Emergency Department, SCS: Systemic corticosteroids, OCS: Oral corticosteroids, PEF: Peak expiratory flow, FEV1: Forced expiratory volume in 1 second, ACQ: Asthma Control Questionnaire, SABA: Short-acting beta agonist
Table 3.
PRO Instruments Report Frequency
| PRO instrument | Report Frequency |
|---|---|
| Asthma Control and Symptom Severity | |
| Asthma Control Questionnaire (ACQ) | 50 |
| Asthma Control Test (ACT) | 19 |
| Asthma symptom diary (ADSD, ASD…, etc.) | 14 |
| CompEx | 2 |
| Sino-Nasal Outcome Test (SNOT) questionnaire | 2 |
| Evaluating Respiratory Symptoms (E-RS) | 1 |
| Asthma Symptom Utility Index (ASUI) | 1 |
| Nasal Congestion Score questionnaire | 1 |
| Visual asthma symptom scores | 1 |
| HRQoL | |
| Asthma Quality of Life Questionnaire (AQLQ) | 31 |
| St. George’s Respiratory Questionnaire (SGRQ) | 9 |
| EQ-5D-5L | 4 |
| Illness Perception | |
| Brief Illness Perception Questionnaire (BIPQ) | 1 |
| Patient Global Impression of Severity (PGI-S) | 1 |
| Treatment Experience | |
| Patients' beliefs Brief about Medication Questionnaire (BMQ) | 1 |
| Patient Global Impression of Change (PGI-C) | 1 |
| Global Evaluation of Treatment Effectiveness (GETE) | 1 |
| Work Productivity | |
| Work Productivity and Activity Impairment (WPAI) Questionnaire plus Classroom Impairment Questions (CIQ) | 2 |
| Mental Health | 1 |
| Patient Health Questionnaire (PHQ) |
For phase 3 studies of FDA-approved biologics, we identified 11 studies with ten multinational clinical trials and one multicenter trial based in the US. Similar primary outcomes were collected in these trials with a primary focus on asthma exacerbation rate, change in FEV1, and reduction in OCS dose (Table 4). While the definitions of outcomes are very much identical within trials of the same products, various terms and definitions were used across studies for different drugs. For example, the terms used to measure the frequency of asthma exacerbation with definitions each to their own include: “severe exacerbation events”, “asthma exacerbation”, “clinical asthma exacerbations (CAEs)”, and “clinically significant exacerbations of asthma.”
Table 4.
Outcomes in Phase 3 Trials for FDA-approved biologic agents
| Primary Outcome |
Frequency | Definition(s) | Trials (Time Frame) |
|---|---|---|---|
| Annualized Rate of Severe Exacerbation Events | 1 | A severe exacerbation was defined as a deterioration of asthma requiring: use of systemic corticosteroids for >=3 days; or hospitalization or emergency room visit because of asthma, requiring systemic corticosteroids. Annualized event rate was the total number of exacerbations that occurred during the treatment period divided by the total number of participant-years treated. | NCT02414854 (baseline to week 52) |
| Annual Asthma Exacerbation Rate | 2 | The annual exacerbation rate is based on unadjudicated annual exacerbation rate reported by the investigator in the eCRF. |
NCT01914757 (baseline to week 56) NCT01928771 (baseline to week 48) |
| Frequency of Clinical Asthma Exacerbations (CAEs) | 2 | An exacerbation event was considered a CAE if the patient met either or both of the criteria listed below and this was corroborated with at least 1 other measurement to indicate the worsening of clinical signs and symptoms of asthma: Use of systemic, or an increase in the use of inhaled, corticosteroid treatment for 3 or more days; or an increased 2 or more fold for at least 3 or more days for patient's already on corticosteroids. asthma-related emergency treatment, such as an unscheduled visit to the physician's office or emergency room for nebulizer treatment or other urgent treatment to prevent worsening of asthma symptoms, or an asthma-related hospitalization. |
NCT01285323 (baseline to month 12) NCT01287039 (baseline to week 52) |
| Number of Clinically Significant Exacerbations of Asthma Per Year | 1 | Clinically significant exacerbations of asthma are defined as worsening of asthma which required use of systemic corticosteroids (IV or oral steroid like prednisone, for at least 3 days or a single intramuscular (IM) corticosteroid (CS) dose is required. For maintenance of systemic corticosteroids, at least double the existing maintenance dose for at least 3 days was required) and/or hospitalization and/or emergency department (ED) visits. | NCT01691521 (baseline to week 32) |
| Frequency of Each of the Two Criteria for CAEs | 2 | An exacerbation event was considered a CAE if the patient met either or both of the criteria listed below and this was corroborated with at least 1 other measurement to indicate the worsening of clinical signs and symptoms of asthma: Use of systemic, or an increase in the use of inhaled, corticosteroid treatment for 3 or more days; or an increased 2 or more fold for at least 3 or more days for patient's already on corticosteroids. asthma-related emergency treatment, such as an unscheduled visit to the physician's office or emergency room for nebulizer treatment or other urgent treatment to prevent worsening of asthma symptoms, or an asthma-related hospitalization CAEs were adjudicated by committee to assure consistency. |
NCT01285323 (baseline to month 12) NCT01287039 (baseline to week 52) |
| Absolute Change from Baseline in Pre-Bronchodilator FEV1 | 1 | FEV1 was the volume of air exhaled in the first second of a forced expiration as measured by spirometer. | NCT02414854 (baseline, week 12) |
| Change from Baseline in FEV1 | 2 | FEV1 is a standard measurement of air movement in the lungs of patients with asthma. It is the volume of air expired in the first second of a forced expiration. Improvement in FEV1 is a measure in the reduction of bronchospasm, the reduction of airway inflammation, or both. FEV1 was measured using forced expiratory air spirometry. | NCT01270464 (baseline, week 4, 8, 12 and 16) |
| Percentage Reduction from Baseline in OCS Dose While Maintaining Asthma Control | 1 | Percentage reduction of OCS dose was calculated as (optimized OCS dose [mg/day] at baseline - final OCS dose at Week 24)/optimized OCS dose at baseline x 100. | NCT02528214 (baseline, week 24) |
| Percentage Reduction from Baseline in Final OCS Dose While Maintaining Asthma Control | 1 | Baseline OCS dose is the dose upon which the patient is stabilised at randomisation (Week 0). Final OCS dose is the dose at Week 28. The percentage reduction from baseline is defined as: {(Baseline dose-final dose)/baseline dose}*100%. If a patient discontinues from the study during a given dose reduction period, or the patient experiences an exacerbation between Weeks 24 and 28 or immediately before discontinuation, then the final OCS dose will be 1 dose level higher than that which directly preceded the event. | NCT02075255 (baseline, week 28) |
| Median Percentage Reduction from Baseline in OCS Dose While Maintaining Asthma Control | 1 | A supplementary presentation of the Primary Outcome Measure data; result is presented as median (inter-quartile range). Percentage reduction of OCS dose was calculated as (optimized OCS dose [mg/day] at baseline - final OCS dose at Week 24)/optimized OCS dose at baseline x 100. | NCT02528214 (baseline, week 24) |
| Number of Participants with the indicated Percent Reduction from Baseline in OCS Dose While Maintaining Asthma Control | 1 | Baseline (BL) dose was the prescribed optimized prednisone/prednisolone dose following the OCS Optimization Phase. Maintenance (MN) dose was the mean of all daily prednisone/prednisolone doses during the MN Phase (weeks 20 to 24). The percent reduction of OCS dose during weeks 20 to 24 compared to BL dose was calculated as: 100 x (BL dose minus MN dose)/BL dose. Asthma control between weeks 20 and 24 was defined as no clinically significant exacerbation (worsening of asthma that required use of systemic corticosteroids or hospitalization and/or emergency department visits) during this period. The percent reduction of OCS was categorized as: 90 to 100%; 75 to <90%; 50 to <75%; >0 to <50%; no decrease in prednisone dose, or lack of asthma control, or withdrawal (WD) from treatment. |
NCT01691508 (baseline, week 20-24) |
Discussion
Despite an assumption that certain outcomes, such as lung function and frequency of exacerbations, are de-facto standard asthma outcomes, they are unreported in a significant proportion (approximately 20% and 60%, respectively). Multiple definitions were encountered across studies for the same outcomes, including asthma control. Among the 46% of outcomes classified as asthma control and symptom severity, variation was significant, whether reported by clinicians or patients, or captured by physiologic tests. These studies reported ACQ scores, ACT scores, and other symptom scores (e.g., Asthma Symptom Utility Index, Asthma Daily Symptom Diary [ADSD], clinical severity scores, symptom scores, daytime/nighttime symptom scores). While change in ACQ scale is explicitly reported in 34% of trials, there is no MCID – thus any small numerical change is reported as an outcome. As seen in the phase 3 trials for FDA-approved biologics where similar primary outcomes were reported across studies, they were in fact using different terminologies and criteria for the outcomes. This observed heterogeneity in clinical trial outcomes is commensurate with a recent report by Gliklich et al. on outcomes in asthma registries.13 This body of work highlights the challenges of cross-interpretation across various registries as well as clinical trials and therefore creating linkage across these efforts is essential.
Context also plays an important role in understanding the relevance or reproducibility of different definitions. For example, exacerbations including use of systemic corticosteroids may be easy to collect in efficacy trials, but they may be under-reported in effectiveness studies using claims data: patients are sometimes able to use a single fill to cover multiple short bursts. Without clarity and agreement around a consistent minimal set of clearly defined outcomes to measure across studies, efforts to compare interventions and aggregate studies to inform decision-making could remain challenging at best and uninformed at worst.
The development of a core outcome set in asthma would create a more consistent and transparent body of evidence. Core outcome sets (COS) are agreed upon minimum sets of outcomes to be collected across studies for a given therapeutic area.19 Patient input and multi-stakeholder consensus-building are central tenets of robust, high-quality COS development. The multi-stakeholder approach to COS development also enables greater understanding and transparency across regulatory, value assessment and market access institutions. Consensus between multi-stakeholder workgroups can be obtained through discussion, as recently performed for outcomes in asthma registries.13 However, a robust structured consensus process utilizing both modified Delphi surveys and in person discussion, and representing a large, multi-stakeholder panel including patients payers, regulators, health technology assessors, clinicians and industry will more likely be adopted. A COS for clinical trials also streamlines clinical development in a patient-relevant manner.
The value of new biologics
Another reason to develop an asthma COS is the growing number of asthma biologics.20 Pavord et al. describe a change moment occurring in our understanding of the pathogenesis of asthma.21 Allowing individualized therapy is paramount in the heterogeneous spectrum of asthma and its personalized pathobiology and resultant symptomatology. Considering cost effective care, there is an opportunity to re-consider existing outcomes, prioritize those most likely to help define the true and relative value of these therapies, and the optimal approach for incorporating them into clinical practice.20 The Institute for Clinical and Economic Review (ICER) report concluded that there was a reduction in the number of exacerbations compared with placebo.22 Although QoL measures across all products were statistically improved, the majority of studies revealed that the degree of improvement did not meet the MCID. In the era of precision medicine, contemporary tools may be too blunt to provide information that can differentiate available biologics in an actionable way. This is particularly concerning in severe or uncontrolled asthma populations, where there is some debate regarding the applicability of existing asthma PRO measures for severe patients, who have unique burden of disease.
PRO assessment in asthma clinical trials
In this age of patient-centered research and patient-focused drug development, there is an opportunity to engage the patient community at a critical pivot point in asthma care. As indicated by Gliklich et al., further work is needed to determine how best to incorporate asthma-specific PROs into asthma research and practice.13 Besides aggregate measures of asthma control and symptom severity, we observed only occasional reporting on other PROs that would help to characterize the real-life experiences of asthma patients and inform shared patient-clinician treatment decisions. Physiological measures do not usually reflect the subjective perception of disease impact,23 and although asthma exacerbations are stressful, they do not describe the day-to-day experience for patients suffering from chronic asthma. Given PROs play an important role in capturing the asthma patient experience, there is increasing interest in using PROs for patient assessment in respiratory medicine, including to guide treatment decisions.24,25 Regulatory bodies are also recognizing PRO measurement as a component in the drug approval process. The FDA has proposed a systematic approach to patient-focused drug development and this mandate has been further exemplified by their recent qualification of the ADSD.26 Yet the status quo suggests an unrealized opportunity to leverage PRO data for asthma decision-making. In a review by Braido et al., authors counted only 20 out of 300 asthma clinical trials published over 18 months with a PRO evaluation.27 Table 2 lists the type of PRO instruments and frequency of reporting among the studies we examined.
There is ongoing debate as to whether current asthma PROs sufficiently capture HRQoL, which is an important predictor of clinical outcomes for patients. Severe asthma in particular is associated with a significant HRQoL burden due to excessive symptoms, frequent and life-threatening attacks, increased comorbidity burden, and burden of side effects from treatment.28 In a commentary from the Asthma and Allergy Foundation of America, the ICER report on asthma biologic therapies was criticized for not sufficiently incorporating patients’ experiences in their analysis and underestimating actual disease burden, especially for severe, uncontrolled asthma.29 Current knowledge suggests the severe asthma patient experience may be unique from milder forms. One aspect of this experience, unmeasured by existing asthma QoL, is the impact of oral corticosteroids.30 It is especially important to have PROs relevant to severe asthma, weighted according to patient preferences, and sensitive enough to detect meaningful changes, in order to provide patient-centered assessment of therapeutic benefits.
Conclusion
There has been a renaissance in biologic therapies for the treatment of moderate to severe asthma. However, given heterogeneity in the outcomes of the various studies and the absence of head-to-head trials, there are no clear conclusions regarding the comparative effectiveness of one agent to another. This heterogeneity also creates hurdles to robust indirect comparisons. Having a core set of agreed-upon outcomes included in the various clinical trials, regardless of whether additional heterogeneous primary or secondary outcomes are included, will allow comparability between studies. This, in fact, would be the only feasible manner in which various therapeutic modalities may be compared, given the current practices in industry intervention trials.9-11 Using other diseases with rapid development of novel therapies as a model (e.g. hemophilia31), establishment of a multi-stakeholder consensus-based COS for pivotal clinical trials – will allow more meaningful comparisons in clinical trials of the effect of asthma therapies on symptoms, lung function, costs, and the daily lives of patients.
Acknowledgments
Disclosures
V.Tejwani has nothing to disclose. He is supported by a National Institutes of Health (NIH) F32 (NHLBI 1F32HL149258-01) grant.
H. Chang has nothing to disclose.
A. Tran has nothing to disclose.
R. Moloney has nothing to disclose.
S. Khatri has been an investigator in clinical trials for mepolizumab in severe asthma (GlaxoSmithKline).
References
- 1.Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 2.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 3.Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018. doi: 10.1016/j.jaci.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Pandya D, Puttanna A, Balagopal V. Systemic Effects of Inhaled Corticosteroids: An Overview. Open Respir Med J. 2015. doi: 10.2174/1874306401408010059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee LK, Obi E, Paknis B, Kavati A, Chipps B. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018. doi: 10.1080/02770903.2017.1316394 [DOI] [PubMed] [Google Scholar]
- 6.Foster JM, McDonald VM, Guo M, Reddel HK. “i have lost in every facet of my life”: The hidden burden of severe asthma. Eur Respir J. 2017. doi: 10.1183/13993003.00765-2017 [DOI] [PubMed] [Google Scholar]
- 7.Yaghoubi M, Adibi A, Safari A, FitzGerald JM, Sadatsafavi M. The Projected Economic and Health Burden of Uncontrolled Asthma in the United States. Am J Respir Crit Care Med. 2019:rccm.201901-0016OC. doi: 10.1164/rccm.201901-0016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy, Asthma Immunol. 2014. doi: 10.1016/j.anai.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 9.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014. doi: 10.1002/14651858.CD003559.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017. doi: 10.1002/14651858.CD010834.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro M, Corren J, Pavord ID, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018. doi: 10.1056/nejmoa1804092 [DOI] [PubMed] [Google Scholar]
- 12.Rabe KF, Nair P, Brusselle G, et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N Engl J Med. 2018. doi: 10.1056/nejmoa1804093 [DOI] [PubMed] [Google Scholar]
- 13.Gliklich RE, Castro M, Leavy MB, et al. Harmonized outcome measures for use in asthma patient registries and clinical practice. J Allergy Clin Immunol. 2019;144(3):671–681.e1.doi: 10.1016/j.jaci.2019.02.025 [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre C, Glanville J, Briscoe S, et al. Cochrane Handbook for Systematic Reviews of Interventions.; 2019. [Google Scholar]
- 15.Cochrane Airways. Sources and search methods for the Cochrane Airways Trials Register.
- 16.Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018. doi: 10.1016/j.jclinepi.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. doi: 10.1164/rccm.201602-0419OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuhlbrigge A, Peden D, Apter AJ, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129(3 Suppl):S34–48. doi: 10.1016/j.jaci.2011.12.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: Issues to consider. Trials. 2012. doi: 10.1186/1745-6215-13-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutsen RM. Drugmakers target severe asthma patients with biologics, but payers raise pricing issue. [Google Scholar]
- 21.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 22.Biologic Therapies for Treatment of Asthma Associated with Type 2 Inflammation: Effectiveness, Value, and Value-Based Price Benchmarks. Institute for Clinical and Economic Review. https://icer-review.org/wp-content/uploads/2018/04/ICER_Asthma_Draft_Report_092418v1.pdf. Published 2018. Accessed August 9, 2019. [Google Scholar]
- 23.Worth A, Hammersley V, Knibb R, et al. Patient-reported outcome measures for asthma: a systematic review. NPJ Prim care Respir Med. 2014;24:14020. doi: 10.1038/npjpcrm.2014.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocks JWH, Seys SF, van Duin TS, Diamant Z, Tsiligianni IG. Assessing patient-reported outcomes in asthma and COPD patients: which can be recommended in clinical practice? Curr Opin Pulm Med. 2018;24(1):18–23. doi: 10.1097/MCP.0000000000000447 [DOI] [PubMed] [Google Scholar]
- 25.Pocket guide for asthma management and prevention. https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf. Accessed October 8, 2019.
- 26.Qualification of Asthma Daytime Symptom Diary and Asthma Nighttime Symptom Diary: Patient-Reported Outcome Instruments for Measurement of Symptoms of Asthma.
- 27.Braido F, Baiardini I, Canonica GW. Patient-reported outcomes in asthma clinical trials. Curr Opin Pulm Med. 2018;24(1):70–77. doi: 10.1097/MCP.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 28.McDonald VM, Hiles SA, Jones KA, Clark VL, Yorke J. Health-related quality of life burden in severe asthma. Med J Aust. 2018;209(S2):S28–S33. http://www.ncbi.nlm.nih.gov/pubmed/30453870. [DOI] [PubMed] [Google Scholar]
- 29.Mendez K ICER Can Do Better for Patients. J Manag care Spec Pharm. 2019;25(5):514–516. doi: 10.18553/jmcp.2019.25.5.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyland ME, Lanario JW, Pooler J, Masoli M, Jones RC. How patient participation was used to develop a questionnaire that is fit for purpose for assessing quality of life in severe asthma. Health Qual Life Outcomes. 2018;16(1):24. doi: 10.1186/s12955-018-0851-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.coreHEM: Developing Comparative Effectiveness Outcomes for Gene Therapy in Hemophilia. http://www.cmtpnet.org/docs/resources/coreHEM_Final_Report_21_MAY_2018.pdf. Published 2018. Accessed August 9, 2019.