Abstract
Obesity has become a pandemic. It is one of the strongest risk-factors of new-onset chronic kidney disease (CKD). However, the effects of obesity and abdominal obesity on the risk of developing CKD in young adults has not been elucidated. From a nationwide health screening database, we included 3,030,884 young adults aged 20–39 years without CKD during a baseline examination in 2009–2010, who could follow up during 2013–2016. Patients were stratified into five levels based on their baseline body mass index (BMI) and six levels based on their waist circumference (WC; 5-cm increments). The primary outcome was the development of CKD. During the follow up, until 2016, 5853 (0.19%) participants developed CKD. Both BMI and WC showed a U-shaped relationship with CKD risk, identifying the cut-off values as a BMI of 21 and WC of 72 cm in young adults. The obesity group (odd ratio [OR] = 1.320, 95% confidence interval [CI]: 1.247–1.397) and abdominal obesity group (male WC ≥ 90, female WC ≥ 85) (OR = 1.208, 95%CI: 1.332–1.290) showed a higher CKD risk than the non-obesity or non-abdominal obesity groups after adjusting for covariates. In the CKD risk by obesity composite, the obesity displayed by the abdominal obesity group showed the highest CKD risk (OR = 1.502, 95%CI: 1.190–1.895), especially in those under 30 years old. During subgroup analysis, the diabetes mellitus (DM) group with obesity or abdominal obesity paradoxically showed a lower CKD risk compared with the non-obesity or non-abdominal obesity group. Obesity and abdominal obesity are associated with increased risk of developing CKD in young adults but a decreased risk in young adults with diabetes.
Keywords: obesity, abdominal obesity, chronic kidney disease, body mass index, waist circumference, diabetes mellitus, korean
1. Introduction
The prevalence of obesity has been steadily and significantly increasing worldwide [1,2]. Its impact on diabetes mellitus (DM), cardiovascular disease, and chronic kidney disease (CKD) has rendered obesity one of the most serious global health issues [3,4]. A high body mass index (BMI) is one of the strongest risk factors for new-onset CKD. Obesity is associated with incident and prevalent microalbuminuria and higher glomerular filtration rate (GFR) or hyperfiltration. Together with albuminuria, it may herald progressive kidney dysfunction and glomerulosclerosis [3,5,6]. In people with obesity, a compensatory hyperfiltration occurs to sustain the heightened metabolic demands of increased body weight. The increase in intraglomerular pressure can damage the kidneys and increase the risk of developing CKD in the long term. The incidence of obesity-related glomerulopathy has increased tenfold in recent years. Today’s young adult population is at a high risk of developing obesity-related kidney disease in the future, providing opportunities for preventive interventions [7].
Obesity is a risk factor for diabetes. However, a recent report revealed that diabetic patients who are underweight are at a higher risk of CKD [8]. This predicament suggests a need for different standards for diabetic and non-diabetic people with obesity. In addition, waist circumference (WC) appears to be a better marker of obesity-related health risks than other surrogates for obesity [5,9]. WC indicates abdominal obesity. In the era of personalized medicine, there is evidence that abdominal obesity might have a different association with CKD risk factors compared with obesity in young adults.
Therefore, we conducted this study to evaluate the relationship between obesity or abdominal obesity and CKD risk in young adults using the National Health Insurance Service’s (NHIS) health checkup data.
2. Experimental Section
Due to the confidentiality of the data used in this study and the strict privacy policy from the data holder indicating that the data should be kept among designated research personnel only, the data cannot be provided to others, regardless of whether or not the data are made anonymous.
2.1. Study Design and Database
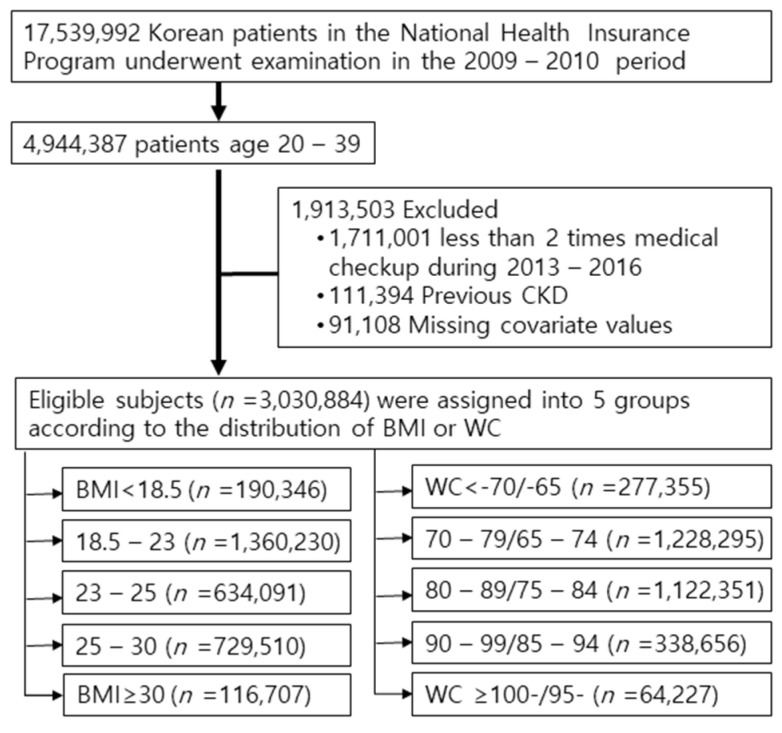
The Korean National Health Insurance Service (NHIS) comprises a complete set of health information pertaining to 50 million Koreans, which includes an eligibility database, a medical treatment database, a health examination database, and a medical care institution database [10,11,12]. The National Health Insurance Corporation is the single insurer, managed by the Korean government, to which approximately 97% of the Korean population subscribes. Enrollees in the NHIC are recommended to undergo a standardized medical examination at least every 2 years. Among 4,944,387 young adults aged 20–39 in 2009–2010 (index year), 3,233,386 participants were selected who could follow up during 2013–2016. To avoid confounders by pre-existing diseases and minimize the possible effects of reverse causality, those who had a history of CKD before the index year were also excluded (n = 111,394). Ultimately, retrospective analysis was performed on 3,030,884 subjects (Figure 1).
Figure 1.
Flow diagram of the study. Abbreviations: CKD, chronic kidney disease; BMI, body mass index; WC, waist circumference.
This study was approved by the Chonnam National University Hospital (study approval number: CNUH-EXP-2020-284) and NHIS (study approval number:NHIS-2019-1-594), and it was conducted according to the principles of the Declaration of Helsinki. The need for written informed consent was waived by our review board.
2.2. Definitions of BMI and WC
For each participant, BMI was calculated by dividing the weight (in kg) by the square of the height (in m2). We defined obesity as a BMI ≥ 25 kg/m2. The participants were then categorized by the definition of obesity as follows: underweight (BMI < 18.5 kg/m2), normal (≥ 18.5 to <23 kg/m2), overweight (≥23 to <25 kg/m2), stage 1 obesity (≥25 to 30 kg/m2), and stage 2 obesity (≥ 30 kg/m2) according to the World Health Organization’s recommendations for Asian populations [13].
The WC of each participant was also measured at the midpoint between the rib cage and iliac crest by a trained examiner. The patients were divided into 6 categories based on 5-cm WC increments: < 80 cm in men and < 75 cm in women, 80–85 cm in men and 75–80 cm in women, 85–90 cm in men and 80–85 cm in women (reference group), 90–95 cm in men and 85–90 cm in women, 95–100 cm in men and 90–95 cm in women, and ≥ 100 cm in men and ≥ 95 cm in women. Abdominal obesity was defined as a WC ≥90 cm in men and ≥ 85 cm in women according to the definition of the Korean Society for the Study of Obesity [14].
2.3. Definition of Chronic Disease and Covariates
Type 2 DM was defined as a fasting plasma glucose level ≥ 126 mg/dL or at least one claim per year for the prescription of hypoglycemic drugs under ICD-10 codes E11–14 [15]. Patients with type 1 DM who had claims under ICD-10 code E10 were excluded from this study [16,17]. Comorbidities were identified using information gathered in the 1 year before the index date. Hypertension was defined as a previous hypertension diagnosis ICD-10 codes (I10–13, I15) and a history of taking at least 1 antihypertensive drug, or a recorded systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg in the health examination database. Dyslipidemia was identified using the appropriate diagnostic code (E78) and a history of lipid-lowering drug use, or a total serum cholesterol concentration of ≥ 240 mg/dL in the health examination database. CKD was defined as an estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2, calculated using the CKD epidemiology collaboration (CKD-EPI) equation, and as a combination of ICD-10 codes (N18–19). The participants’ fasting blood glucose (mg/dL), total cholesterol (mg/dL), triglyceride (mg/dL), high-density lipoprotein cholesterol (mg/dL), and low-density lipoprotein cholesterol (mg/dL) concentrations were measured in a fasting state. The quality of the laboratory tests has been warranted by the Korean Association for Laboratory Medicine, and the hospitals participating in the NHI health checkup programs are certified by the NHIS.
2.4. Study Outcomes and Follow Up
The study population was followed from baseline to the date of CKD diagnosis or until 31 December 2016. The primary end point was incident CKD, which was defined using a combination of ICD-10 codes (N18–19) and an estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2, calculated using the CKD epidemiology collaboration equation, more than twice during 2013–2016 medical checkup.
2.5. General Health Behaviors and Sociodemographic Variables
Smoking history was categorized as nonsmokers, former smokers, and current smokers. Alcohol drinking was categorized into 0, 1 to ~2, or ≥ 3 times/week by frequency (none, mild, and heavy, respectively), and regular exercise, defined as vigorous physical activity for at least 20 min/day, was categorized into 0, 1 to ~4, and ≥ 5 times/week by frequency. Income level was divided by quartile: Q1 (the lowest), Q2, Q3, and Q4 (the highest).
2.6. Statistical Analysis
We report the mean ± SD with intervals for continuous variables and the numbers (with percentages) for categorical variables. Hazard ratios (HRs) with 95% confidence intervals (CIs) for End-stage renal disease (ESRD) in the BMI and WC categories were obtained using multivariable Cox proportional hazard models using normal BMI (BMI 18.5–23 kg/m2) and normal WC (85–90/80–85 cm) as references after adjustment using 3 models. Model 1 was the crude model. Model 2 was adjusted from model 1 with the addition of age, sex, income, DM, dyslipidemia, and hypertension. Model 3 was adjusted from model 2 with the addition of smoking, alcohol drinking, physical activity, and eGFR. Cumulative ESRD incidence was estimated by constructing Kaplan-Meier curves for the mean 5.4-year follow-up period, and we used the log-rank test to examine differences in ESRD development by the level of BMI and WC. We also performed subgroup analysis for DM status. A p-value < 0.05 was considered to reflect statistical significance. SAS version 9.3 software and SAS survey procedures (SAS Institute, Inc., Cary, NC, USA) were used for all statistical analyses.
3. Results
3.1. Baseline Characteristics of the Study Population
Table 1 shows the baseline characteristics of participants regarding the development of CKD. Among the participants, 5853 (0.19%) developed CKD. The mean age was higher among individuals who developed CKD than among those who did not (31.82 ± 4.81 vs. 35.02 ± 3.82, p < 0.001). The proportions of males (72.29%), people with obesity (BMI ≥ 25), and abdominal obesity (WC ≥ 90 in male, ≥ 85 in female) were higher in the incident CKD group than in the non-CKD group. Comorbidities such as diabetes, hypertension, dyslipidemia, and proteinuria were more prevalent in the CKD group than in the non-CKD group. eGFR was lower, and blood pressure (BP), total cholesterol, and glucose levels were higher in the CKD group than in the non-CKD group (Table 1). Medication rate for DM, hypertension and dyslipidemia was increased according to increasing BMI and WC (Supplementary Table S1).
Table 1.
Baseline characteristics of subjects according to the incident chronic kidney disease.
| Group | Total (n = 3,030,884) |
None CKD (n = 3,025,031) |
CKD (n = 5853) |
p Value |
|---|---|---|---|---|
| Age | 31.83 ± 4.81 | 31.82 ± 4.81 | 35.02 ± 3.82 | <0.0001 |
| Sex, male(%) | 2,030,479 (66.99) | 2,026,248 (66.98) | 4231 (72.29) | <0.0001 |
| Current smoker | 1,118,316 (36.9) | 1,116,411 (36.91) | 1905 (32.55) | <0.0001 |
| Heavy drinker * | 275,476 (9.09) | 275,023 (9.09) | 453 (7.74) | 0.0003 |
| Physical activity-regular | 431,623 (14.24) | 430,539 (14.23) | 1084 (18.52) | <0.0001 |
| Income-low ** | 450,211 (14.85) | 449,356 (14.85) | 855 (14.61) | 0.596 |
| BMI | 23.22 ± 3.45 | 23.22 ± 3.45 | 24.63 ± 3.69 | <0.0001 |
| Obesity(BMI ≥ 25) | 846,217 (27.92) | 843,632 (27.89) | 2585 (44.17) | <0.0001 |
| BMI 5 level | <0.0001 | |||
| <18.5 | 190,346 (6.28) | 190,167 (6.29) | 179 (3.06) | |
| 18.5–23 | 1,360,230 (44.88) | 1,358,376 (44.9) | 1854 (31.68) | |
| 23–25 | 634,091 (20.92) | 632,856 (20.92) | 1235 (21.1) | |
| 25–30 | 729,510 (24.07) | 727,403 (24.05) | 2107 (36) | |
| ≥30 | 116,707 (3.85) | 116,229 (3.84) | 478 (8.17) | |
| Waist circumference (WC) | 78.42 ± 9.56 | 78.41 ± 9.56 | 81.62 ± 9.91 | <0.0001 |
| Abdominal obesity | 402,883 (13.29) | 401,559 (13.27) | 1324 (22.62) | <0.0001 |
| WC 5 level | <0.0001 | |||
| M: <70/F: <65 | 277,355 (9.15) | 277,050 (9.16) | 305 (5.21) | |
| M:70–79/F:65–74 | 1,228,295 (40.53) | 1,226,510 (40.55) | 1785 (30.5) | |
| M:80–89/F:75–84 | 1,122,351 (37.03) | 1,119,912 (37.02) | 2439 (41.67) | |
| M:90–99/F:85–94 | 338,656 (11.17) | 337,567 (11.16) | 1089 (18.61) | |
| M: ≥100/F: ≥95 | 64,227 (2.12) | 63,992 (2.12) | 235 (4.02) | |
| Diabetes mellitus | 58,312 (1.92) | 57,867 (1.91) | 445 (7.6) | <0.0001 |
| Diabetes mellitus, vintage | 0.89 ± 1.93 | 0.88 ± 1.91 | 2.86 ± 3.05 | <0.0001 |
| Hypertension | 268,318 (8.85) | 266,654 (8.81) | 1664 (28.43) | <0.0001 |
| Dyslipidemia | 220,966 (7.29) | 219,937 (7.27) | 1029 (17.58) | <0.0001 |
| CCI score | 0.32 ± 0.67 | 0.32 ± 0.66 | 0.64 ± 1.1 | <0.0001 |
| CCI grade | <0.0001 | |||
| 0 | 2,303,416 (76) | 2,299,590 (76.02) | 3826 (65.37) | |
| 1 | 561,901 (18.54) | 560,852 (18.54) | 1049 (17.92) | |
| 2 | 120,860 (3.99) | 120,310 (3.98) | 550 (9.4) | |
| ≥3 | 44,707 (1.48) | 44,279 (1.46) | 428 (7.31) | |
| Proteinuria | <0.0001 | |||
| Negative | 2,922,684 (96.67) | 2,917,819 (96.7) | 4865 (83.3) | |
| Trace | 56,833 (1.88) | 56,642 (1.88) | 191 (3.27) | |
| 1+ | 31,663 (1.05) | 31,342 (1.04) | 321 (5.5) | |
| 2+ | 9894 (0.33) | 9589 (0.32) | 305 (5.22) | |
| 3+ | 1872 (0.06) | 1738 (0.06) | 134 (2.29) | |
| 4+ | 308 (0.01) | 284 (0.01) | 24 (0.41) | |
| Height (cm) | 169.13 ± 8.17 | 169.13 ± 8.17 | 169.63 ± 7.86 | <0.0001 |
| Weight (cm) | 66.82 ± 13.06 | 66.82 ± 13.06 | 71.3 ± 13.85 | <0.0001 |
| Fasting blood glucose (mg/dL) | 91.07 ± 15.49 | 91.05 ± 15.43 | 97.71 ± 33.39 | <0.0001 |
| Systolic blood pressure (mmHg) | 118.51 ± 12.88 | 118.5 ± 12.87 | 123.85 ± 15.68 | <0.0001 |
| Diastolic blood pressure (mmHg) | 74.39 ± 9.27 | 74.38 ± 9.27 | 78.29 ± 11.13 | <0.0001 |
| Pulse pressure (mmHg) | 44.12 ± 8.23 | 44.12 ± 8.23 | 45.55 ± 9.16 | <0.0001 |
| Total cholesterol (mg/dL) | 186.42 ± 33.75 | 186.4 ± 33.74 | 197.74 ± 37.97 | <0.0001 |
| Estimated GFR (mL/min/1.73 m2) | 96.28 ± 51.37 | 96.31 ± 51.36 | 80.26 ± 51.93 | <0.0001 |
Abbreviations. CKD, chronic kidney disease; BMI, body mass index; WC, waist circumference; CCI, Chalson Comorbidity Index; GFR, glomerular filtration rate. * Alcohol consumptions ≥30 g/day, ** low income 25%, abdominal obesity: WC ≥ 90 in males, ≥85 in females.
Characteristics of participants classified by BMI levels and WC are presented in Table 2 and Table 3, respectively. Subjects in the underweight group (BMI <18.5) were younger, exercised less, and had a lower prevalence of DM, hypertension, and dyslipidemia (Table 2). BP, fasting glucose, and total cholesterol were also lower in the underweight group (Table 2).
Table 2.
Baseline characteristics of participants by level of body mass index.
| Variable | Distribution of Body Mass Index | |||||
|---|---|---|---|---|---|---|
| <18.5 (n = 190,346) |
18.5~23 (n = 1,360,230) |
23~25 (n = 634,091) |
25~30 (n = 729,510) |
30~ (n = 116,707) |
p | |
| Age | 29.62 ± 4.93 | 31.29 ± 4.94 | 32.41 ± 4.62 | 32.83 ± 4.39 | 32.24 ± 4.46 | <0.0001 |
| Age grade | <0.0001 | |||||
| 20 s | 101,396 (53.27) | 529,544 (38.93) | 184,564 (29.11) | 181,446 (24.87) | 33,714 (28.89) | |
| 30 s | 88,950 (46.73) | 830,686 (61.07) | 449,527 (70.89) | 548,064 (75.13) | 82,993 (71.11) | |
| Sex(male) | 49,380 (25.94) | 735,330 (54.06) | 512,593 (80.84) | 636,587 (87.26) | 96,589 (82.76) | |
| Smoking | <0.0001 | |||||
| None | 144,387 (75.86) | 815,922 (59.98) | 270,571 (42.67) | 261,484 (35.84) | 42,818 (36.69) | |
| Ex- | 8700 (4.57) | 130,974 (9.63) | 100,467 (15.84) | 121,109 (16.6) | 16,136 (13.83) | |
| Current | 37,259 (19.57) | 413,334 (30.39) | 263,053 (41.49) | 346,917 (47.55) | 57,753 (49.49) | |
| Drinking | <0.0001 | |||||
| None | 982,92 (51.64) | 556,799 (40.93) | 195,454 (30.82) | 203,834 (27.94) | 36,666 (31.42) | |
| Mild | 85,661 (45) | 715,325 (52.59) | 371,661 (58.61) | 428,251 (58.7) | 63,465 (54.38) | |
| Heavy * | 6393 (3.36) | 88,106 (6.48) | 66,976 (10.56) | 97,425 (13.35) | 16,576 (14.2) | |
| Exercise ** | 12,970 (6.81) | 170,635 (12.54) | 105,304 (16.61) | 123,655 (16.95) | 19,059 (16.33) | <0.0001 |
| Income *** | 30,047 (15.79) | 212,947 (15.66) | 89,929 (14.18) | 99,164 (13.59) | 18,124 (15.53) | <0.0001 |
| DM | 1270 (0.67) | 13,856 (1.02) | 11,239 (1.77) | 23,600 (3.24) | 8347 (7.15) | <0.0001 |
| DM vintage | 0.62 ± 1.8 | 0.86 ± 2.01 | 0.91 ± 1.97 | 0.91 ± 1.9 | 0.91 ± 1.82 | <0.0001 |
| HTN | 4911 (2.58) | 62,781 (4.62) | 54,608 (8.61) | 110,487 (15.15) | 35,531 (30.44) | <0.0001 |
| Dyslipid | 3279 (1.72) | 49,198 (3.62) | 50,609 (7.98) | 95 982 (13.16) | 21,898 (18.76) | <0.0001 |
| Weight (kg) | 47.82 ± 4.88 | 59.05 ± 7.41 | 69.96 ± 6.5 | 79.18 ± 7.75 | 94.21 ± 9.71 | <0.0001 |
| Height(cm) | 164.54 ± 7.51 | 167.52 ± 8.34 | 170.69 ± 7.67 | 171.63 ± 7.26 | 171.33 ± 7.69 | <0.0001 |
| WC (cm) | 65.05 ± 5.07 | 72.97 ± 6.1 | 80.7 ± 5.14 | 87.04 ± 5.81 | 97.38 ± 7.35 | <0.0001 |
| BMI | 17.62 ± 0.73 | 20.96 ± 1.25 | 23.96 ± 0.57 | 26.83 ± 1.32 | 32.05 ± 2 | <0.0001 |
| SBP(mmHg) | 110.26 ± 11.23 | 115.02 ± 11.83 | 120.11 ± 11.83 | 123.98 ± 12.28 | 129.77 ± 13.7 | <0.0001 |
| DBP(mmHg) | 69.5 ± 8.2 | 72.1 ± 8.58 | 75.27 ± 8.67 | 77.96 ± 9.06 | 82.01 ± 10.05 | <0.0001 |
| PP(mmHg) | 40.76 ± 7.53 | 42.92 ± 7.88 | 44.85 ± 8.11 | 46.01 ± 8.32 | 47.76 ± 8.97 | <0.0001 |
| Glu(mg/dL) | 87.29 ± 11.5 | 89.02 ± 12.78 | 91.58 ± 15.1 | 94.21 ± 18.18 | 98.68 ± 24.84 | <0.0001 |
| TC (mg/dL) | 171.49 ± 28.02 | 178.74 ± 30.52 | 189.91 ± 33.27 | 198.62 ± 35.09 | 205.16 ± 36.52 | <0.0001 |
| CCI | 0.33 ± 0.65 | 0.3 ± 0.64 | 0.31 ± 0.65 | 0.33 ± 0.7 | 0.41 ± 0.81 | <0.0001 |
| CCI grade | <0.0001 | |||||
| 0 | 142,556 (74.89) | 1,040,172 (76.47) | 486,174 (76.67) | 550,174 (75.42) | 84,340 (72.27) | |
| 1 | 37,173 (19.53) | 251,251 (18.47) | 114,780 (18.1) | 135,796 (18.61) | 22 901 (19.62) | |
| 2 | 8 170 (4.29) | 51,861 (3.81) | 24,345 (3.84) | 30,488 (4.18) | 5996 (5.14) | |
| 3 | 2447 (1.29) | 16,946 (1.25) | 8 792 (1.39) | 13,052 (1.79) | 3 470 (2.97) | |
| **** GFR | 99.86 ± 49.19 | 97.33 ± 49.08 | 95.34 ± 53.58 | 94.43 ± 54.21 | 94.85 ± 49.75 | <0.0001 |
| CKD event | 179 (0.09) | 1 854 (0.14) | 1235 (0.19) | 2107 (0.29) | 478 (0.41) | <0.0001 |
Abbreviations. DM, diabetes mellitus; HTN, hypertension; WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP diastolic blood pressure; PP, pulse pressure; TC, total cholesterol; GFR, glomerular filtration rate (mL/min/1.73 m2); CCI, Charlson Comorbidity Index; CKD, chronic kidney disease. * Alcohol consumptions ≥30 g/day, ** Regular exercise: mid-term exercise ≥5 days or vigorous exercise ≥3 days in a week, *** low income 25%, **** GFR: mL/min/1.73 m2.
Table 3.
Baseline characteristics of participants by level of waist circumference.
| Variable | Distribution of Waist Circumference | |||||
|---|---|---|---|---|---|---|
| <70/<65 (n = 227,355) |
70~79/65~74 (n = 1,228,295) |
80~89/75~84 (n = 1,122,351) |
90~99/85~94 (n = 338,656) |
>100/>95 (n = 64,227) |
p | |
| Age | 29.57 ± 5.03 | 31.23 ± 4.94 | 32.65 ± 4.48 | 33.02 ± 4.29 | 32.33 ± 4.39 | <0.0001 |
| Age grade | <0.0001 | |||||
| 20 s | 149,795 (54.01) | 484,035 (39.41) | 300,697 (26.79) | 78,107 (23.06) | 18,030 (28.07) | |
| 30 s | 127,560 (45.99) | 744,260 (60.59) | 821,654 (73.21) | 260,549 (76.94) | 46,197 (71.93) | |
| Sex (male) | 77,960 (28.11) | 683,983 (55.69) | 924,402 (82.36) | 292,422 (86.35) | 51,712 (80.51) | |
| Smoking | <0.0001 | |||||
| None | 210,802 (76) | 729,199 (59.37) | 452,825 (40.35) | 118,138 (34.88) | 24,218 (37.71) | |
| Ex- | 13,630 (4.91) | 120,810 (9.84) | 179,006 (15.95) | 55,228 (16.31) | 8712 (13.56) | |
| Current | 52,923 (19.08) | 378,286 (30.8) | 490,520 (43.7) | 165,290 (48.81) | 31,297 (48.73) | |
| Drinking | <0.0001 | |||||
| None | 138,917 (50.09) | 498,087 (40.55) | 337,093 (30.03) | 95,677 (28.25) | 21,271 (33.12) | |
| Mild | 129,203 (46.58) | 649,434 (52.87) | 658,175 (58.64) | 194,013 (57.29) | 33,538 (52.22) | |
| Heavy * | 9235 (3.33) | 80,774 (6.58) | 127,083 (11.32) | 48,966 (14.46) | 9418 (14.66) | |
| Exercise ** | 25,947 (9.36) | 169,998 (13.84) | 176,370 (15.71) | 50,108 (14.8) | 9200 (14.32) | <0.0001 |
| Income *** | 46,573 (16.79) | 202,423 (16.48) | 149,534 (13.32) | 42,585 (12.57) | 9096 (14.16) | <0.0001 |
| DM | 1597 (0.58) | 12,569 (1.02) | 24,439 (2.18) | 14,423 (4.26) | 5284 (8.23) | <0.0001 |
| DM vintage | 0.71 ± 1.86 | 0.83 ± 1.96 | 0.9 ± 1.95 | 0.92 ± 1.89 | 0.97 ± 1.88 | <0.0001 |
| HTN | 7325 (2.64) | 58822 (4.79) | 115602 (10.3) | 65,827 (19.44) | 20,742 (32.29) | <0.0001 |
| Dyslipid | 5595 (2.02) | 46082 (3.75) | 103735 (9.24) | 52,556 (15.52) | 12,998 (20.24) | <0.0001 |
| Weight (Kg) | 50.24 ± 5.99 | 59.73 ± 7.85 | 72.03 ± 8.35 | 83.39 ± 9.21 | 95.81 ± 11.66 | <0.0001 |
| Height (cm) | 162.88 ± 7.21 | 167.15 ± 7.9 | 171.42 ± 7.36 | 173.09 ± 7.34 | 173.33 ± 7.98 | <0.0001 |
| WC (cm) | 63.13 ± 3.24 | 72.59 ± 4.07 | 82.91 ± 3.48 | 92.47 ± 3.15 | 103.16 ± 4.56 | <0.0001 |
| BMI | 18.9 ± 1.54 | 21.32 ± 1.9 | 24.48 ± 2.14 | 27.81 ± 2.37 | 31.85 ± 3.06 | <0.0001 |
| SBP (mmHg) | 110.9 ± 11.31 | 115.41 ± 11.87 | 121.03 ± 12.15 | 125.46 ± 12.85 | 129.82 ± 14.25 | <0.0001 |
| DBP (mmHg) | 69.82 ± 8.22 | 72.34 ± 8.59 | 75.94 ± 8.9 | 79.02 ± 9.51 | 82.05 ± 10.49 | <0.0001 |
| PP (mmHg) | 41.08 ± 7.59 | 43.08 ± 7.91 | 45.1 ± 8.18 | 46.44 ± 8.5 | 47.77 ± 9.12 | <0.0001 |
| Gluco (mg/dL) | 87.34 ± 11.1 | 89.11 ± 12.72 | 92.29 ± 15.98 | 95.56 ± 20.34 | 99.48 ± 26.86 | <0.0001 |
| TC (mg/dL) | 173.4 ± 28.44 | 179.07 ± 30.68 | 191.92 ± 33.91 | 201.7 ± 35.95 | 206.67 ± 37.45 | <0.0001 |
| CCI | 0.31 ± 0.64 | 0.3 ± 0.63 | 0.32 ± 0.67 | 0.36 ± 0.74 | 0.44 ± 0.85 | <0.0001 |
| CCI grade | <0.0001 | |||||
| 0 | 210,942 (76.05) | 942,618 (76.74) | 854,069 (76.1) | 250,141 (73.86) | 45,646 (71.07) | |
| 1 | 51,946 (18.73) | 224,591 (18.28) | 206,867 (18.43) | 65,576 (19.36) | 12,921 (20.12) | |
| 2 | 11,063 (3.99) | 46,202 (3.76) | 44,407 (3.96) | 15,722 (4.64) | 3466 (5.4) | |
| 3 | 3404 (1.23) | 14,884 (1.21) | 17,008 (1.52) | 7217 (2.13) | 2194 (3.42) | |
| GFR **** | 98.19 ± 46.4 | 96.96 ± 48.24 | 95.26 ± 53.52 | 95.44 ± 57.64 | 97.19 ± 55.7 | <0.0001 |
| CKD event | 305 (0.11) | 1785 (0.15) | 2439 (0.22) | 1089 (0.32) | 235 (0.37) | <0.0001 |
Abbreviations. DM, diabetes mellitus; HTN, hypertension; WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP diastolic blood pressure; PP, pulse pressure; TC, total cholesterol; GFR, glomerular filtration rate (mL/min/1.73 m2); CCI, Charlson Comorbidity Index; CKD, chronic kidney disease. * Alcohol consumptions ≥ 30 g/day, ** Regular exercise: mid-term exercise ≥5 days or vigorous exercise ≥3 days in a week, *** low income 25%, **** GFR:mL/min/1.73 m2.
CKD events were highest in those with stage 2 obesity (BMI ≥ 30). Table 3 shows that patients in the abdominal obesity group were older, mostly male, exercised less, and had a higher prevalence of DM, hypertension, and dyslipidemia (Table 3). Apart from eGFR, BP, fasting glucose, and lipid level were also higher in the abdominal obesity group (Table 3).
3.2. Association of BMI and WC with the Risk of CKD
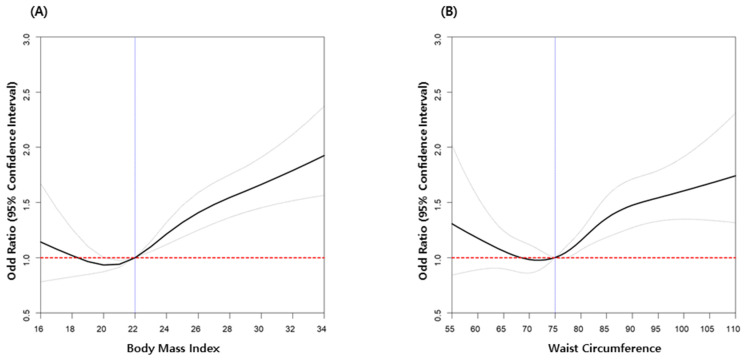
CKD risk increased proportional to BMI in all participants after adjusting for age, sex, income, presence of DM, dyslipidemia, hypertension, smoking, alcohol drinking, physical activity, and GFR (Figure 2A and Table 4). The obesity group (BMI ≥ 25) showed a higher CKD risk (OR: 1.323, 95%CI: 1.250–1.400) compared with the non-obesity group (BMI < 25) (Table 4).
Figure 2.
Odd ratios for chronic kidney disease according to body mass index (A) and waist circumference (B) in young adults. Adjusted for age, sex, low income (20%), diabetes mellitus, hypertension, dyslipidemia, current smoker, alcohol consumption, regular exercise, and eGFR.
Table 4.
Multivariate cox analysis for incident chronic kidney disease (CKD) by level of body mass index in young adults.
| Group | Total (n) | CKD (n) |
% | HR (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||||
| Body mass index 5 level | |||||||
| <18.5 | 190,346 | 179 | 0.09 | 0.690 (0.592–0.804) |
0.863 (0.740–1.007) |
0.876 (0.751–1.023) |
0.901 (0.771–1.052) |
| 18.5~23 | 1,360,230 | 1854 | 0.14 | 1(Ref.) | 1(Ref.) | 1(Ref.) | 1(Ref.) |
| 23~25 | 634,091 | 1235 | 0.19 | 1.43 (1.330–1.537) |
1.278 (1.187–1.376) |
1.269 (1.179–1.367) |
1.138 (1.057–1.227) |
| 25~30 | 729,510 | 2107 | 0.29 | 2.122 (1.994–2.259) |
1.830 (1.713–1.955) |
1.84 (1.722–1.965) |
1.368 (1.277–1.465) |
| 30~ | 116,707 | 478 | 0.41 | 3.013 (2.724–3.332) |
2.816 (2.540–3.120) |
2.855 (2.576–3.163) |
1.550 (1.391–1.727) |
| p for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Obesity (Body mass index ≥25) | |||||||
| <25 | 2,184,667 | 3268 | 0.15 | 1(Ref.) | 1(Ref.) | 1(Ref.) | 1(Ref.) |
| ≥25 | 846,217 | 2585 | 0.31 | 2.045 (1.942–2.154) |
1.789 (1.695–1.888) |
1.802 (1.707–1.902) |
1.323 (1.25,0–1.400) |
| p for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
Abbreviations. CKD, chronic kidney disease; HR, hazard ratio. Model 1: non-adjusted. Model 2: adjusted for age, sex. Model 3: adjusted for model 2 plus smoking, alcohol drinking, regular exercise, income. Model 3: adjusted for model 3 plus glomerular filtration rate, hypertension, diabetes mellitus, dyslipidemia.
According to increasing WC, the risk of CKD also increased in all participants after adjusting for age, sex, income, presence of DM, dyslipidemia, hypertension, smoking, alcohol drinking, physical activity, and GFR (Figure 2B and Table 5). The abdominal obesity group (male WC ≥ 90/female WC ≥ 85) showed a higher CKD risk (OR: 1.208, 95%CI: 1.132–1.290) compared with the non-abdominal obesity group (Table 5).
Table 5.
Multivariate cox analysis for incident CKD by level of waist circumference in young adults.
| Group | Total (n) | CKD (n) |
% | HR (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||||
| Waist circumference 5 level | |||||||
| <70/<65 | 277,355 | 305 | 0.11 | 0.756 (0.67–0.854) |
0.95 (0.841–1.074) |
0.959 (0.848–1.084) |
0.959 (0.848–1.085) |
| 70~79/65~74 | 1,228,295 | 1785 | 0.15 | 1(Ref.) | 1(Ref.) | 1(Ref.) | 1(Ref.) |
| 70~79/65~74 | 1,122,351 | 2439 | 0.22 | 1.496 (1.408–1.591) |
1.272 (1.194–1.355) |
1.281 (1.203–1.365) |
1.117 (1.047–1.191) |
| 70~79/65~74 | 338,656 | 1089 | 0.32 | 2.217 (2.056–2.391) |
1.810 (1.674–1.957) |
1.858 (1.718–2.009) |
1.297 (1.195–1.407) |
| >100/>95 | 64,227 | 235 | 0.37 | 2.524 (2.203–2.893) |
2.268 (1.977–2.602) |
2.334 (2.034–2.677) |
1.277 (1.107–1.472) |
| p for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Abdominal obesity (male WC ≥ 90, female WC ≥ 85) | |||||||
| No | 2,628,001 | 4529 | 0.17 | 1(Ref.) | 1(Ref.) | 1(Ref.) | 1(Ref.) |
| Yes | 402,883 | 1324 | 0.33 | 1.910 (1.796–2.031) |
1.642 (1.543–1.748) |
1.679 (1.577–1.787) |
1.208 (1.132–1.290) |
| p for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
Abbreviations. CKD, chronic kidney disease; HR, hazard ratio. Model 1: non-adjusted. Model 2: adjusted for age and sex. Model 3: adjusted for model 2 plus smoking, alcohol drinking, regular exercise, income. Model 3: adjusted for model 3 plus glomerular filtration rate, hypertension, diabetes mellitus, dyslipidemia.
Analysis for CKD risk by obesity composite showed the highest CKD risk in obesity within the abdominal obesity group (OR: 1.339, 95%CI: 1.247–1.438) compared with the only obesity (OR: 1.318, 95%CI: 1.232–1.409) or only abdominal obesity groups (OR: 1.227, 95%CI: 0.979–1.538). In addition, the abdominal-obesity-only group (OR: 1.478, 95%CI: 0.698–3.129) had higher risk of CKD compared with the obesity-only group (OR: 1.217, 95%CI: 0.968–1.531) in those under age 30 (Table 6). The correlation value between BMI and WC was 0.81441 (p < 0.0001)
Table 6.
Multivariate cox analysis for chronic kidney disease risk by obesity composite.
| Obesity | Abdominal Obesity | Total | Male | Female | Age 20–29 years | Age 30–39 years |
|---|---|---|---|---|---|---|
| No | No (n = 2,148,970; 70.9%) |
1(Ref.) |
1(Ref.) |
1(Ref.) |
1(Ref.) |
1(Ref.) |
| Yes (n = 35,697; 1.2%) |
1.227 (0.979–1.538) |
1.204 (0.915–1.584) |
1.163 (0.781–1.731) |
1.478 (0.698–3.129) |
1.203 (0.949–1.524) |
|
| Yes | No (n = 479,031; 15.8%) |
1.318 (1.232–1.409) |
1.355 (1.258–1.459) |
1.062 (0.895–1.261) |
1.217 (0.968–1.531) |
1.324 (1.234–1.420) |
| Yes (n = 367,186; 12.1%) |
1.339 (1.247–1.438) |
1.356 (1.254–1.467) |
1.196 (0.996–1.436) |
1.502 (1.190–1.895) |
1.320 (1.225–1.423) |
Abbreviations. Adjusted for age and sex. Income, diabetes mellitus, dyslipidemia, hypertension, smoking, alcohol drinking, regular exercise, glomerular filtration rate.
3.3. Subgroup Analyses
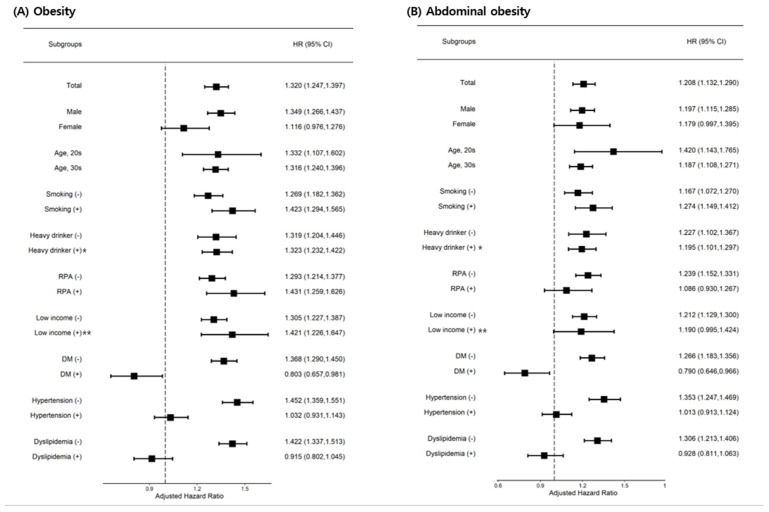
Subgroup analyses were performed. Female sex was not affected by obesity or abdominal obesity in the development of CKD. Abdominal obesity was not a risk factor for CKD in the regular exercise group. In the DM group, obesity and abdominal obesity had decreased risk for developing CKD. In the hypertension or dyslipidemia subgroup, CKD risk was not affected by obesity or abdominal obesity (Figure 3).
Figure 3.
Subgroup analysis for chronic kidney disease risk. Abbreviations: * Alcohol consumptions ≥30 g/day, ** low income 20%. RPA; regular physical activity; DM, diabetes mellitus.
4. Discussion
In this large, nationwide cohort study, we demonstrated that obesity or abdominal obesity were associated with a higher risk of developing CKD in the young adult population. In addition, obesity within the abdominal obesity group showed the highest CKD risk after adjusting for potential confounders. Paradoxically, the DM group with obesity or abdominal obesity showed a lower CKD risk compared with the non-obesity or non-abdominal obesity groups in young adults.
In research settings, BMI, an internationally accepted standard anthropomorphic measurement, is generally used to define obesity [18]. Several studies have examined the association between BMI and the future risk of CKD [7,19]. Despite conflicting results, most epidemiologic studies showed that higher BMI was associated with increased risk of CKD. Recent evidence from 2015 has suggested that CKD is the second leading cause of BMI-related disability-adjusted life-years [20]. According to the Global Burden of Diseases data, mortality attributed to CKD among young adults (< 50 years) has continuously increased proportional to adolescent obesity [21]. In the United States, an annual increase of 1.6% in CKD-related mortality in young adults was recorded between 1995 and 2015. In contrast, some reports have suggested that obesity does not confer a higher risk of CKD [22] and may even play a protective role in certain settings (a phenomenon also referred to as the “obesity paradox”) [23]. In either case, most investigations into the association between obesity and CKD have been limited to obesity in young adults. Therefore, our data support a positive correlation between obesity and future CKD risk in young adults. Likewise, we provide supporting evidence for the obesity paradox occurring in the DM group in young adults.
The exact mechanism of the relationship between being underweight and increased risk of CKD remains unclear. One possible reason for this is ethnic differences. Asian people are likely to develop type 2 diabetes at a lower BMI at younger ages and suffer longer with complications [2]. Lower BMI may not necessarily guarantee health in Asian populations. The incidence of obesity-related glomerulopathy has increased tenfold in recent years [24]. Although we did not directly evaluate insulin resistance or inflammatory markers, prior studies noted that visceral adipose tissue contributes to higher circulating levels of free fatty acids and inflammatory molecules that may promote the development of insulin resistance, endothelial dysfunction, and kidney disease [25,26,27].
Measures of central or abdominal obesity, defined by WC and the waist–hip ratio, have been used recently as more important predictors for assessing mortality risk than BMI [28,29]. WC, a representative marker of visceral body fat, was found to correlate with inflammation, whereas subcutaneous body fat may be an indicator of nutritional status [30]. However, to date, there is no consensus on an appropriate definition of WC to access CKD risk because of different cut-off points for different ethnicities. In a large cohort of Japanese workers aged 20–69 years, the optimal cut-off of WC to predict later development of DM was 85 cm for men [31]. A WC of 95 cm was independently associated with a decline in eGFR (≥ 3 mL/min/1.73 m2/year) in men, with eGFR ranging from 60 to 90 mL/min/1.73 m2 [32]. A WC of ≥85 cm was more closely associated with the prevalence of CKD than a BMI of 24.0 kg/m2 in urban men aged 18–60 years old [33]. Our findings determined that the practical cut-off point of a WC indicating increased CKD risk was appropriately 72 cm, which is lower than previous reports. An obvious difference from previous reports is that our population consisted of healthy young adults, not common dwelling men.
The exact mechanisms explaining the association of obesity or abdominal obesity with decreasing risk of developing CKD in the young adult DM group remain uncertain, requiring further studies.
Study Limitations
There are some limitations in this study. First, we did not collect relevant information on food habits or other comorbidities that might affect weight. Second, this study did not consider the use of medications, such as hypoglycemic agents or lipid lowing agents, and treatment adherences. Third, we were unable to obtain more information about the causes of CKD and urine protein. Fourth, we used data from the NHIS’s checkup program on a Korean population; therefore, we cannot generalize the results to other ethnic groups.
5. Conclusions
In conclusion, to the best of our knowledge, this is the first study on the relationship between BMI or WC and the future risk of CKD using a large general population of young adults from a well-established and validated longitudinal national database. Our study demonstrated that obesity and abdominal obesity are associated with increased risk of CKD in young adults but have a protective effect on young adults with DM.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/5/1065/s1, Table S1: Medication rate for diabetes mellitus, hypertension and dyslipidemia.
Author Contributions
Conceptualization: E.H.B., S.Y.L., H.S.C., C.S.K.; Data curation: J.-H.J., T.R.O., K.-D.H., H.S.C.; Formal analysis: J.-H.J., T.R.O., K.-D.H., E.H.B., S.Y.L., S.W.K.; Funding acquisition: E.H.B., S.W.K.; Methology: J.-H.J., T.R.O., K.-D.H., C.S.K.; Project administration, E.H.B., S.Y.L.; Supervision: S.K.M., S.W.K.; Writing—original draft, E.H.B., S.Y.L.; Writing—review and editing: C.S.K., H.S.C., S.K.M., S.W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Bio & Medical Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (2017M3A9E8023001), grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0331, HR20C0021), and by a grant (BCRI20025&20076) of Chonnam National University Hospital Biomedical Research Institute.
Institutional Review Board Statement
This study was approved by the Chonnam National University Hospital (study approval number: CNUH-EXP-2020-284) and NHIS (study approval number:NHIS-2019-1-594).
Informed Consent Statement
Patient consent was waived because the database used in this study did not include personal identifiers and the study was retrospective and observational in design.
Data Availability Statement
The data presented in this study are available with permission from the National Health Insurance Service review board.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patterson R.E., Frank L.L., Kristal A.R., White E. A comprehensive examination of health conditions associated with obesity in older adults. Am. J. Prev. Med. 2004;27:385–390. doi: 10.1016/j.amepre.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Yoon K.H., Lee J.H., Kim J.W., Cho J.H., Choi Y.H., Ko S.H., Zimmet P., Son H.Y. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 3.de Boer I.H., Sibley S.D., Kestenbaum B., Sampson J.N., Young B., Cleary P.A., Steffes M.W., Weiss N.S., Brunzell J.D., Diabetes C., et al. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J. Am. Soc. Nephrol. 2007;18:235–243. doi: 10.1681/ASN.2006040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An R., Ji M., Zhang S. Global warming and obesity: A systematic review. Obes. Rev. 2018;19:150–163. doi: 10.1111/obr.12624. [DOI] [PubMed] [Google Scholar]
- 5.Mendy V.L., Azevedo M.J., Sarpong D.F., Rosas S.E., Ekundayo O.T., Sung J.H., Bhuiyan A.R., Jenkins B.C., Addison C. The association between individual and combined components of metabolic syndrome and chronic kidney disease among African Americans: The Jackson Heart Study. PLoS ONE. 2014;9:e101610. doi: 10.1371/journal.pone.0101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong P.E., Verhave J.C., Pinto-Sietsma S.J., Hillege H.L. Obesity and target organ damage: The kidney. Int. J. Obes. Relat. Metab. Disord. 2002;26:S21–S24. doi: 10.1038/sj.ijo.0802213. [DOI] [PubMed] [Google Scholar]
- 7.Silverwood R.J., Pierce M., Thomas C., Hardy R., Ferro C., Sattar N., Whincup P., Savage C., Kuh D., Nitsch D., et al. Association between younger age when first overweight and increased risk for CKD. J. Am. Soc. Nephrol. 2013;24:813–821. doi: 10.1681/ASN.2012070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y.H., Kang J.G., Lee S.J., Han K.D., Ihm S.H., Cho K.H., Park Y.G. Underweight Increases the risk of end-stage renal diseases for type 2 diabetes in Korean population: Data from the national health insurance service health checkups 2009–2017. Diabetes Care. 2020;43:1118–1125. doi: 10.2337/dc19-2095. [DOI] [PubMed] [Google Scholar]
- 9.Janssen I., Katzmarzyk P.T., Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am. J. Clin. Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 10.Yang H.K., Han K., Kwon H.S., Park Y.M., Cho J.H., Yoon K.H., Kang M.I., Cha B.Y., Lee S.H. Obesity, metabolic health, and mortality in adults: A nationwide population-based study in Korea. Sci. Rep. 2016;6:30329. doi: 10.1038/srep30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y.H., Han K., Ko S.H., Ko K.S., Lee K.U. Data analytic process of a nationwide population-based study using national health information database established by national health insurance service. Diabetes Metab. J. 2016;40:79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., Lee J.S., Park S.H., Shin S.A., Kim K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; International Association for the Study of Obesity. Health Communications Australia; Sydney, Australia: 2000. [Google Scholar]
- 14.Lee S.Y., Park H.S., Kim D.J., Han J.H., Kim S.M., Cho G.J., Kim D.Y., Kwon H.S., Kim S.R., Lee C.B., et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Kim E.S., Jeong J.S., Han K., Kim M.K., Lee S.H., Park Y.M., Baek K.H., Moon S.D., Han J.H., Song K.H., et al. Impact of weight changes on the incidence of diabetes mellitus: A Korean nationwide cohort study. Sci. Rep. 2018;8:3735. doi: 10.1038/s41598-018-21550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo D.H., Han K.D., Park C.Y. The incremental risk of pancreatic cancer according to fasting glucose levels: Nationwide population-based cohort study. J. Clin. Endocrinol. Metab. 2019;104:4594–4599. doi: 10.1210/jc.2019-00033. [DOI] [PubMed] [Google Scholar]
- 17.Noh J., Han K.D., Ko S.H., Ko K.S., Park C.Y. Trends in the pervasiveness of type 2 diabetes, impaired fasting glucose and co-morbidities during an 8-year-follow-up of nationwide Korean population. Sci. Rep. 2017;7:46656. doi: 10.1038/srep46656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
- 19.Fouad M., Ismail M.I., Gaballah A., Reyad E., ELdeeb S. Prevalence of obesity and risk of chronic kidney disease among young adults in Egypt. Indian J. Nephrol. 2016;26:413–418. doi: 10.4103/0971-4065.172597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin S.Y., Kwon M.J., Park H., Woo H.Y. Comparison of chronic kidney disease prevalence examined by the chronic kidney disease epidemiology collaboration equation with that by the modification of diet in renal disease equation in Korean adult population. J. Clin. Lab. Anal. 2014;28:320–327. doi: 10.1002/jcla.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 22.Jin D.C., Yun S.R., Lee S.W., Han S.W., Kim W., Park J., Kim Y.K. Lessons from 30 years data of Korean end-stage renal disease registry, 1985–2015. Kidney Res. Clin. Pract. 2015;34:132–139. doi: 10.1016/j.krcp.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 24.Kovesdy C.P., Furth S., Zoccali C. Obesity and kidney disease: Hidden consequences of the epidemic. Physiol. Int. 2017;104:1–14. doi: 10.1556/2060.104.2017.1.9. [DOI] [PubMed] [Google Scholar]
- 25.Unger R.H. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg H.O., Chaker H., Leaming R., Johnson A., Brechtel G., Baron A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan E., O’Meara Y.M., Godson C. Lipid mediators of inflammation in obesity-related glomerulopathy. Nephrol. Dial. Transpl. 2013;28:iv22–iv29. doi: 10.1093/ndt/gft392. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadian S.M., Alaei H., Ghahremani P. An assessment between D1 receptor agonist and D2 receptor antagonist into the ventral tegmental area on conditioned place preference and locomotor activity. Adv. Biomed. Res. 2019;8:72. doi: 10.4103/abr.abr_82_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kittiskulnam P., Johansen K.L. The obesity paradox: A further consideration in dialysis patients. Semin. Dial. 2019;32:485–489. doi: 10.1111/sdi.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado C., Chertow G.M., Kaysen G.A., Dalrymple L.S., Kornak J., Grimes B., Johansen K.L. Associations of body mass index and body fat with markers of inflammation and nutrition among patients receiving hemodialysis. Am. J. Kidney Dis. 2017;70:817–825. doi: 10.1053/j.ajkd.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Hu H., Kurotani K., Sasaki N., Murakami T., Shimizu C., Shimizu M., Nakagawa T., Honda T., Yamamoto S., Okazaki H., et al. Optimal waist circumference cut-off points and ability of different metabolic syndrome criteria for predicting diabetes in Japanese men and women: Japan Epidemiology Collaboration on Occupational Health Study. BMC Public Health. 2016;16:220. doi: 10.1186/s12889-016-2856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guasch-Ferre M., Bullo M., Martinez-Gonzalez M.A., Corella D., Estruch R., Covas M.I., Aros F., Warnberg J., Fiol M., Lapetra J., et al. Waist-to-height ratio and cardiovascular risk factors in elderly individuals at high cardiovascular risk. PLoS ONE. 2012;7:e43275. doi: 10.1371/journal.pone.0043275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Y., Li F., Wang F., Ma X., Zhao X., Zeng Q. The association of chronic kidney disease and waist circumference and waist-to-height ratio in Chinese urban adults. Medicine. 2016;95:e3769. doi: 10.1097/MD.0000000000003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available with permission from the National Health Insurance Service review board.