Abstract
Amongst sulfur- and nitrogen-containing heterocyclic compounds, the 2-aminothiazole scaffold is one of the characteristic structures in drug development as this essential revelation has several biological activities abiding it to act as an anticancer, antioxidant, antimicrobial and anti-inflammatory agent, among other things. Additionally, various 2-aminothiazole-based derivatives as medical drugs have been broadly used to remedy different kinds of diseases with high therapeutic influence, which has led to their wide innovations. Owing to their wide scale of biological activities, their structural variations have produced attention amongst medicinal chemists. The present review highlights the recently synthesized 2-aminothiazole-containing compounds in the last thirteen years (2008–2020). The originality of this proposal is based on the synthetic strategies developed to access the novel 2-aminothiazole derivatives (N-substituted, 3-substituted, 4-substituted, multi-substituted, aryl/alkyl substituents or acyl/other substituents). The literature reports many synthetic pathways of these 2-aminothiazoles associated with four different biological activities (anticancer, antioxidant, antimicrobial and anti-inflammatory activities). It is wished that this review will be accommodating for new views in the expedition for rationalistic designs of 2-aminothiazole-based medical synthetic pathways.
Keywords: 2-aminothiazoles, antibacterial, anti-inflammatory
1. Introduction
Heterocyclic compounds are so important due to their versatile applications. A large number of heterocyclic compounds containing nitrogen and sulfur are used as medicine in different therapeutic targets. Thiazole is one of the important pharmacophores in drug discovery and development processes. There are many substituted thiazole-containing heterocycles covering a wide range of therapeutic targets including antimicrobial, anticancer, anti-inflammatory and anti-HIV. Aminothiazole scaffolds are important structural units in medicinal chemistry as they have shown antitumor [1,2,3], antiviral [4,5,6], antibacterial [7,8,9], anti-prion [10], psychotropic [11], anti-allergic [12], anti-hypertensive [13], anti-inflammatory [14,15], antifungal [16], antitubercular [17,18], anti-HIV [19], pesticidal [20], antiprotozoal [21], antipyretic [22], antioxidative [23] and analgesic activities [24]. Aminothiazole compounds act as ligands of estrogen receptors [25] and afford a new group of adenosine receptor antagonists [26]. They are also utilized as fungicides, inhibiting the in vivo growth of Xanthomonas, or as schistosomicidal and anthelmintic drugs [27].
2. Results
2.1. 2-Aminothiazoles as Anticancer Agents
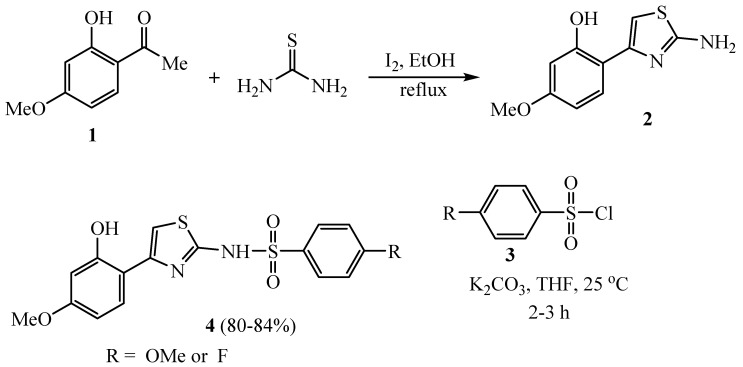
Clinical administration of high doses of anticancer drugs to defeat resistance leads to severe toxicities [28]. The literature survey revealed that heterocyclic thiazole derivatives were integrated with other moieties to evaluate their anticancer effect. The synthetic protocol of paeonol-2-aminothiazole-phenylsulfonyl derivatives 4 involved treating paeonol (1) with thiourea and iodine in refluxing ethyl alcohol to furnish the corresponding 2-aminothiazole scaffold 2, which was treated with phenylsulfonyl chloride 3 that had been substituted to produce the final wanted compound 4 (Scheme 1). The cytotoxic effects of various paeonol-2-aminothiazole-phenylsulfonyl derivatives 4 were assessed against fibroblast cells (BALB/3T3) and seven cancer cell lines. The F and OCH3 derivatives of the thiazole-paeonolphenylsulfonyl scaffold showed cytotoxic potent effects against the tested cancer cell lines [29].
Scheme 1.
Synthesis of various paeonol-2-aminothiazole-phenylsulfonyl derivatives 4.
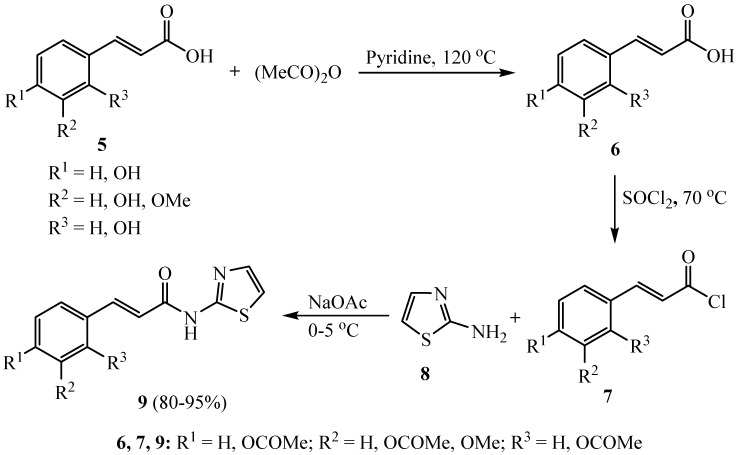
New cinnamic acid amide scaffolds 9 comprising thiazoles were designed and synthesized as outlined in Scheme 2. The results of anticancer activity of this work indicated that compound 9 (R1 = R2 = H, R3 = OCOMe) features potential characteristics for drug development combining both coagulant and platelet effects [30].
Scheme 2.
Synthesis of cinnamic acid amide scaffolds 9.
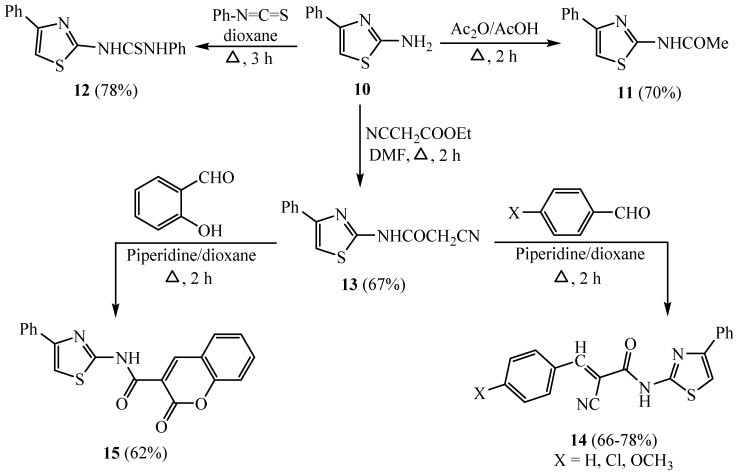
The thiazole derivative 10 was produced by heating phenacyl bromide with thiourea in ethyl alcohol, which acetylated by acetic anhydride to furnish the corresponding N-acetyl compound 11. The nucleophilic addition of 10 to phenyl isothiocyanate afforded the N-phenylthiourea derivative 12. In contrast, the reaction of 10 with ethyl cyanoacetate in dimethyl formamide produced N-cyanoacetamide derivative 13. Condensation of 13 with three types of substituted benzaldehydes (namely, benzaldehyde 4-chlorobenzaldehyde or 4-methoxybenzaldehyde) produced the corresponding benzylidene derivatives 14. In addition, when compound 13 reacted with salicylaldehyde, it gave the coumarin derivative 15 (Scheme 3) [31].
Scheme 3.
Synthesis of coumarin derivative 15.
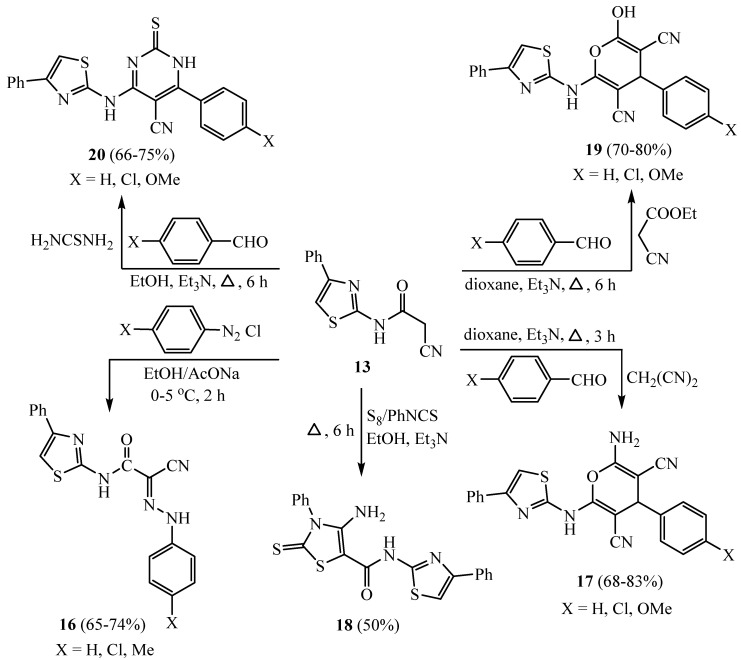
The reaction of acetamide derivative 13 with various aryl diazonium chlorides affords the aryl hydrazone compounds 16. In addition, the multi-component reaction of 13 with substituted benzaldehydes and malononitrile produced the pyran derivatives 17 (Scheme 4). Compound 13 was applied for thiazole synthesis, and as a result, compound 13 reacted with elemental sulfur and phenyl isothiocyanate to afford the thiazole derivative 18. Similarly, the multi-component reaction of 13 with substituted benzaldehydes and ethyl cyanoacetate produced the pyran derivatives 19a–c (Scheme 4). Furthermore, the multi-component reaction of 13 with substituted benzaldehydes and thiourea produced the pyrimidine scaffolds 20. 2-Amino-4-(4-chlorophenyl)-6-(4-phenylthiazol-2-yl)-4H-pyran-3,5-dicarbonitrile (17, X = Cl) indicated the maximum cytotoxicity among the synthesized compounds towards six cancer cell lines [31].
Scheme 4.
Synthesis of pyran derivatives 19a–c.
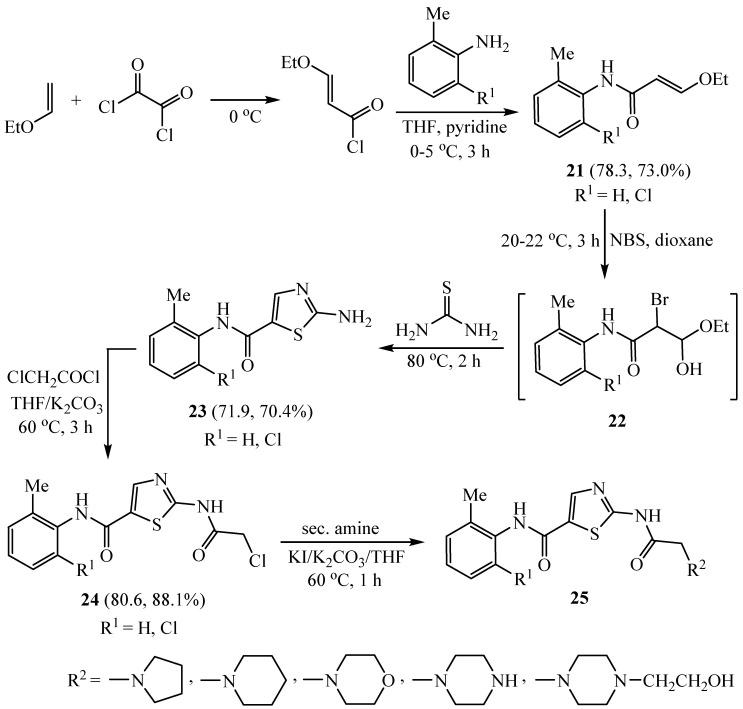
Treatment of 3-ethoxyacryloyl chloride with either 2-methylaniline or 2-chloro-6-methylaniline in tetrahydrofuran utilizing basic pyridine as a catalyst gave the substituted 3-ethoxyacrylamides 21, and then treatment of 21 with N-bromosuccinimide produced the crude α-formyl-α-bromoacetate hemiacetals 22. Addition of thiourea to hemiacetals 22 gave the 2-amino-thiazole-5-carboxylic acid phenylamides 23, which reacted with chloroacetyl chloride in the presence of K2CO3 as a base to afford the key intermediates 24. Finally, chloroacetamide derivatives 24 reacted with various secondary amine compounds to afford the final products 25 (Scheme 5). The synthesized series of 2-amino-thiazole-5-carboxylic acid phenylamide derivatives showed good anti-proliferative effects on human K563 leukemia cells [32].
Scheme 5.
Synthesis of 2-amino-thiazole-5-carboxylic acid phenylamide derivatives.
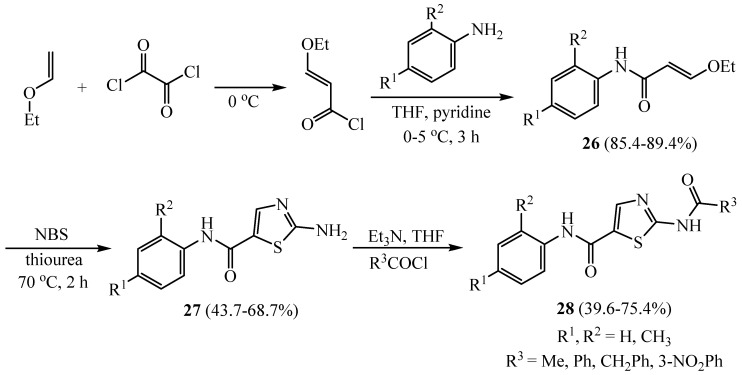
According to the significance of the carboxanilide side chain on the fifth position of the thiazole ring and the cytostatic impact on human chronic myeloid leukemia cell line K562, 2-aminothiazole-5-carbamides 28 were synthesized as outlined in Scheme 6. 3-Ethoxy-N-arylpropenamides 26 were synthesized by nucleophilic substitution of suitable aniline onto 3-ethoxyacryloyl chloride. Reaction of the advanced enones 26 with N-bromosuccinimide (NBS) followed by a coupling of thiourea installed thiazole ring 27. The last step was a nucleophilic displacement of acetic anhydride or various benzoyl chlorides to give the corresponding target 28 derivatives [33].
Scheme 6.
Synthesis of 2-aminothiazole-5-carbamides 28.
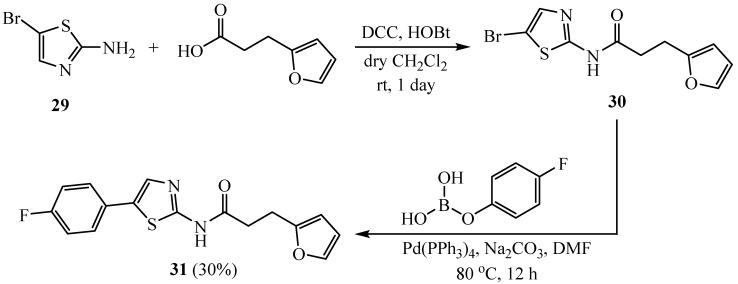
The synthesis of N-(5-(4-fluorophenyl)thiazol-2-yl)-3-(furan-2-yl)propanamide (31) as outlined in Scheme 7 involves the reaction of 2-amino-5-bromothiazole (29) with 3-(furan-2-yl)propanoic acid followed by the Suzuki reaction of the amide product 30 with 4-fluorophenylboric acid. Compound 31 affords the potent inhibitory effect on KPNB1 and anticancer activity in cell-based assays [34].
Scheme 7.
Synthesis of N-(5-(4-fluorophenyl)thiazol-2-yl)-3-(furan-2-yl) propanamide.
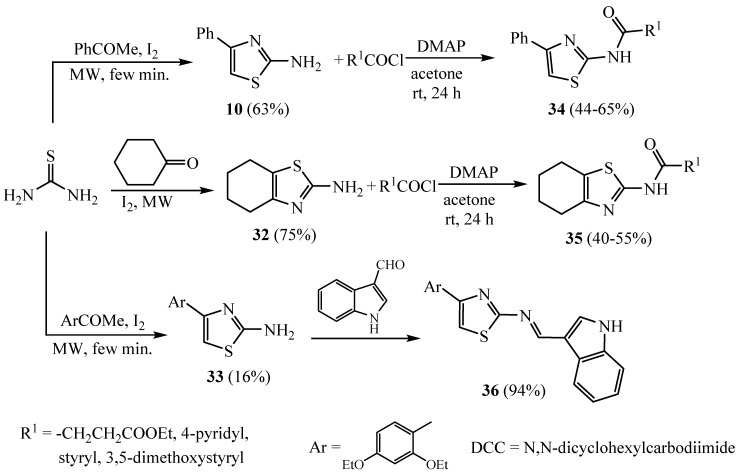
The synthesis of 4,5-substituted-2-aminothiazoles 10 and 32 (Scheme 8) has been achieved according to the literature by the reaction of acetophenone or cyclohexanone with thiourea in the presence of iodine. Compound 10 or 32 was stirred with acid and/or acyl chloride to afford the corresponding amide compounds 34 (R1 = -CH2CH2COOEt, 4-pyridyl, styryl and 3,5-dimethoxystyryl) and 35 (R1 = -CH2CH2COOEt and 3,5-dimethoxystyryl), respectively. Compound 4-(2,4-diethoxyphenyl)thiazol-2-amine (33) was reacted with 1H-indole-3- carboxaldehyde in ethyl alcohol to afford the Schiff base compound 36 [35]. Derivatives 32–36 were exhibited as potent Poly(ADP-Ribose) Polymerase-1 inhibitors.
Scheme 8.
Synthesis of 4,5-substituted-2-aminothiazoles 32–36.
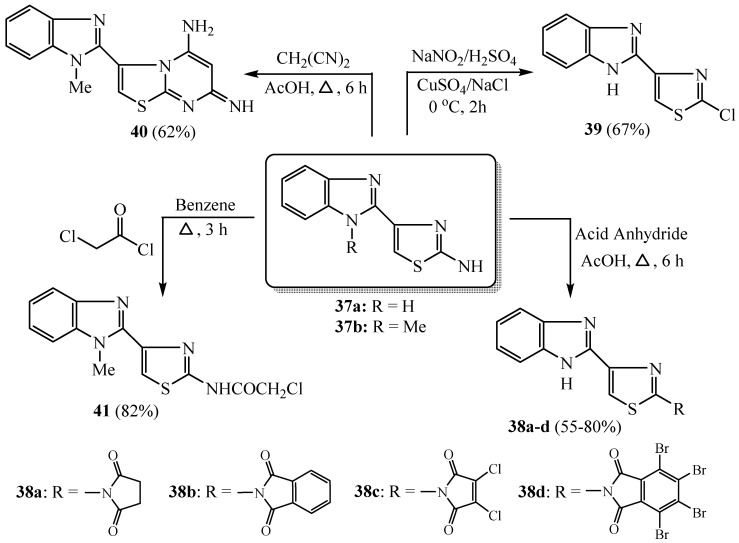
Benzimidazole-thiazole derivatives were prepared by heating a mixture of equimolar amounts of 2-acetylbenzimidazoles and thiourea in ethyl alcohol and an excess amount of iodine [36]. The acid anhydride effect on compound 37a was studied (Scheme 9) which was condensed with various acid anhydrides (namely, succinic anhydride, phthalic anhydride, dichloromalic anhydride and/or tetrabromophthalic anhydride) in acetic acid to produce the wanted anhydride compounds 38a–d [37]. It is known that an aromatic amino group substitution is workable by its diazonium salt preparation and subsequent replacement with a nucleophile via Sandmeyer reactions. Therefore, compound 37a was reacted with CuSO4, NaNO2 and NaCl to afford the corresponding 2-chlorothiazole scaffold 39. Meanwhile, 37a was condensed via different acid anhydrides to give the corresponding derivatives 38a–d. In contrast, compound 37b was reacted with malononitrile in acetic acid to produce 5-amino-pyrimidine derivative 40 [37]. The compound 37b was acylated with chloroacetyl chloride to give the corresponding chloroacetamide compound 41 (Scheme 9). All of these synthesized derivatives showed respectable anticancer activities toward HepG2 and PC12 cell lines.
Scheme 9.
Synthesis of derivatives 38–41.
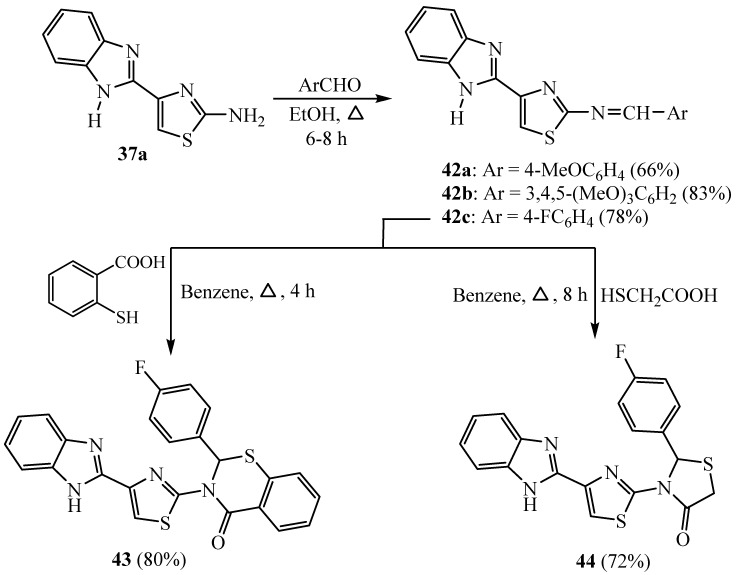
Meanwhile, Schiff’s bases are deemed significant intermediates for the synthesis of other heterocycles. Consequently, Schiff’s bases 42a–c were constructed by the reaction of 37a with different substituted benzaldehydes, namely, 4-methoxybenzaldehyde, 3,4,5-trimethoxybezaldehye and/or 4-fluorobenzaldehyde in ethanol to produce N-(substituted)-thiazol-2-amine 42a–c, respectively. A set of compounds comprising thiazolidinone and benzothiazine nuclei were accomplished by cyclizing Schiff’s base 42c by either thiosalicylic acid and/or thioglycolic acid to get thiazolidinone and benzothiazinone 43 and 44 derivatives, respectively [37] (Scheme 10). Meanwhile, compounds 43 showed promising anticancer activity against both of HepG2 and PC12 cell lines.
Scheme 10.
Synthesis of derivatives 42–44.
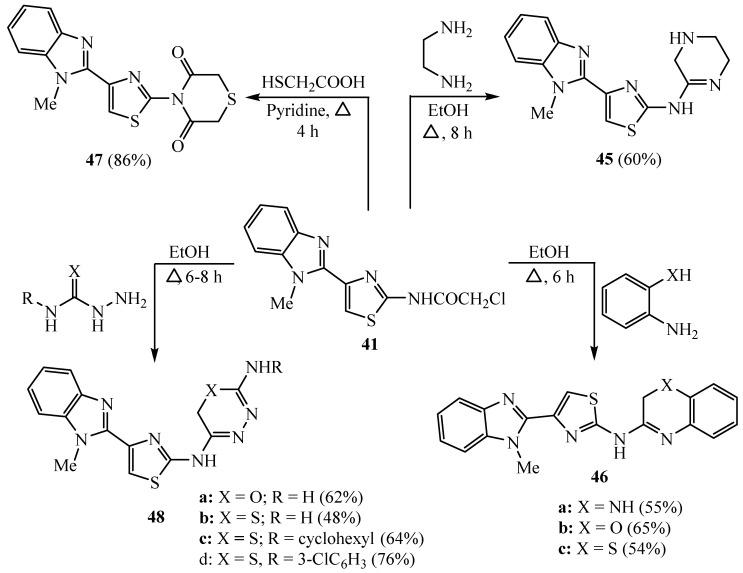
Additionally, aminothiazole derivative 41 was cyclized with 1,2-ethylenediamine and/or ortho-substituted aniline compounds to give the corresponding 2-amino-pyrazine 45 and 46a–c, respectively. In contrast, compound 41 was cyclized with HS-CH2-COOH to produce thiazinedione scaffold 47. Furthermore, compound 41 was cyclized with various semicarbazide or thiosemicarbazide derivatives to afford 1,3,4-oxadiazine 48a and 1,3,4-thiadiazine 48b–d compounds [37] (Scheme 11). Among these derivatives, 48c showed high activity against the PC12 anticancer cell line.
Scheme 11.
Synthesis of derivatives 45–48.
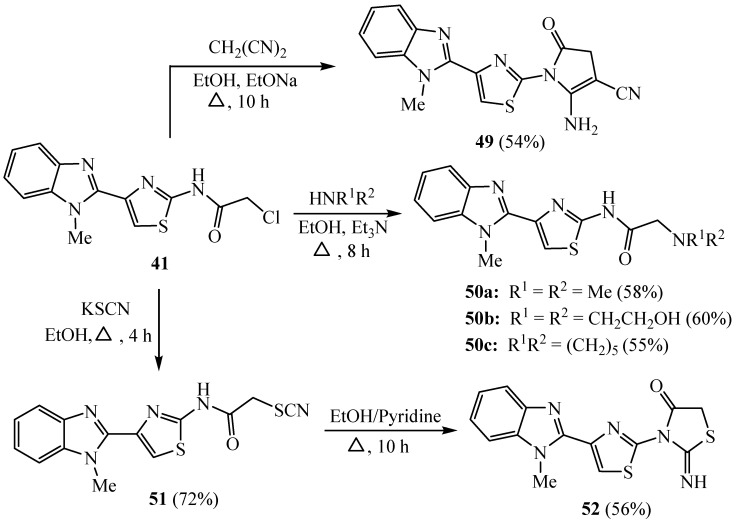
Compound 41 containing an aminothiazole moiety was heated with malononitrile in sodium ethoxide to give the corresponding 3-cyano-5-oxo-1H-pyrrole derivative 49. In addition, treatment of compound 41 with different secondary amines gave the acetamide derivatives 50a–c. Moreover, treatment of 41 with potassium thiocyanate produced the thiocyanate-acetamide scaffold 51, which was cyclized to yield the corresponding thiazolidinone 52 (Scheme 12). The prepared compounds have potent anticancer activity against PC12 and HepG2 cell lines [37].
Scheme 12.
Synthesis of derivatives 49–52.
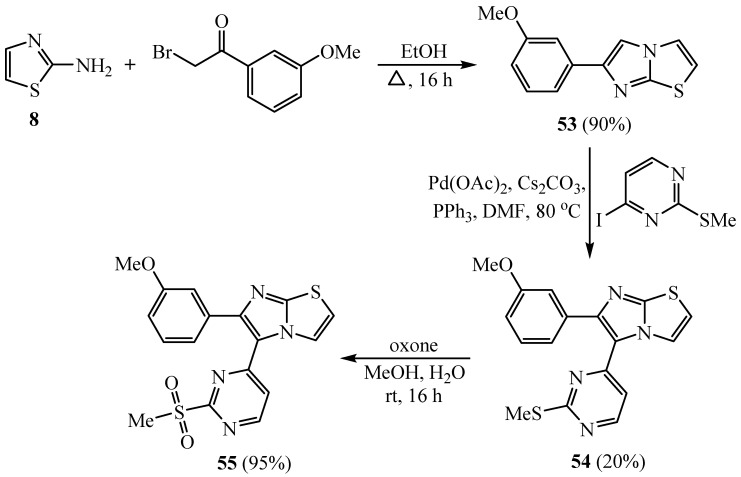
Heterocyclization of 2-aminothiazole 8 with α-bromo-3-methoxyacetophenone proceeded by heating in ethyl alcohol to yield 6-(3-methoxyphenyl)imidazo[2,1-b]thiazole 53, which underwent heating with 4-iodo-2-(methylthio)pyrimidine in the presence of palladium acetate, cesium carbonate and triphenyl phosphine to give the corresponding methyl thiopyrimidinyl compound 54 (Scheme 13). The sulfide moiety of 54 was oxidized by oxone to produce the corresponding sulfonyl compound 55 [38]. The derivatives 53–55 were utilized as precursors for the synthesis of compounds 58–61 that displayed a remarkable activity toward the A375P human melanoma cell line (HepG2).
Scheme 13.
Synthesis of derivatives 53–55.
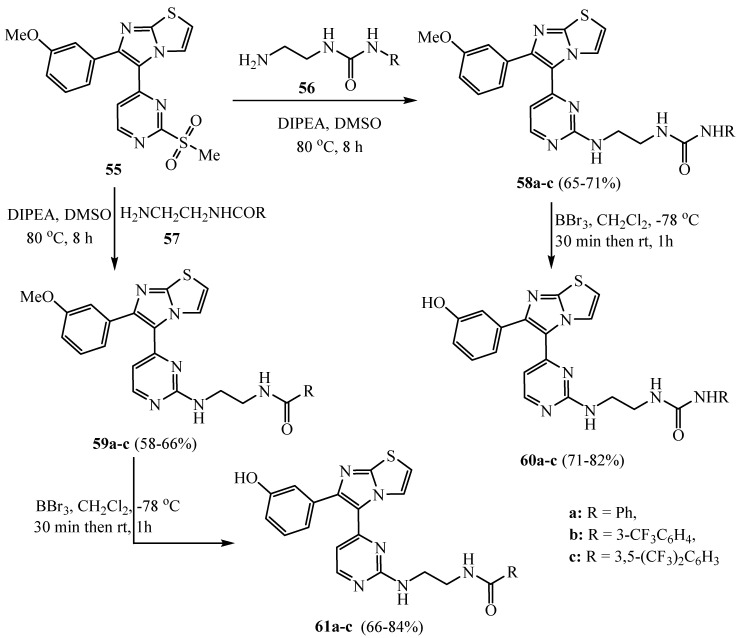
Heating of the sulfone-containing thiazolyl moiety compound 55 with the urea and/or amide reagents 56 and 57 in the presence of DIPEA (N,N-Diisopropyl ethyl amine) gave the target methoxy compounds 58 and 59, respectively. Demethylation of the methoxy group of 58 and 59 using boron tribromide produced the corresponding hydroxyl target compounds 60a–c and 61a–c, respectively (Scheme 14) [38]. The prepared derivatives 58b, 58c, 60b, 59b, 61a and 61b showed superior potency against the A375P “human melanoma cell line” than sorafenib. Moreover, derivatives 61a and 61b revealed the highest potency (IC50 = 0.5 and 2.1 µM, respectively). Derivatives with m-hydroxyphenyl on the imidazothiazole moiety such as 60b, 61a and 61b showed greater potency than the parallel methoxy hybrids 58b, 59a and 59b, which may due to the expected hydrogen bond with the hydroxyl group of the receptor site.
Scheme 14.
Synthesis of derivatives 58a–c–61a–c.
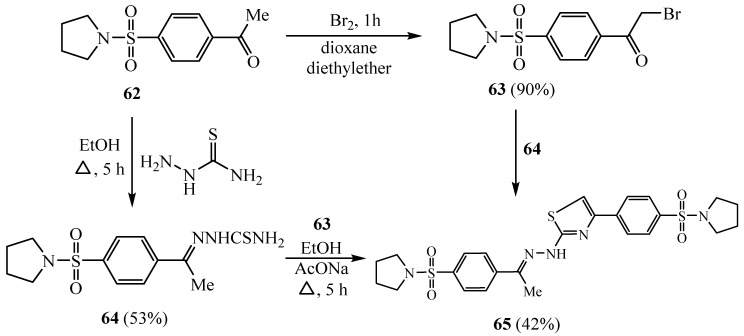
Bashandy reported on the preparation of benzenesulfonamide-based heterocycles with expected anticancer activity [39]. Thus, acetophenone derivative 62 was treated with bromine in a mixture of dioxane/diethyl ether to give the alpha bromoacetyl compound 63 (Scheme 15). Treatment of 62 with thiosemicarbazide yielded the thiosemicarbazone moiety 64, which, when heated with phenacyl bromide compound 63, produced the thiazole derivative 65.
Scheme 15.
Synthesis of derivatives 63–65.
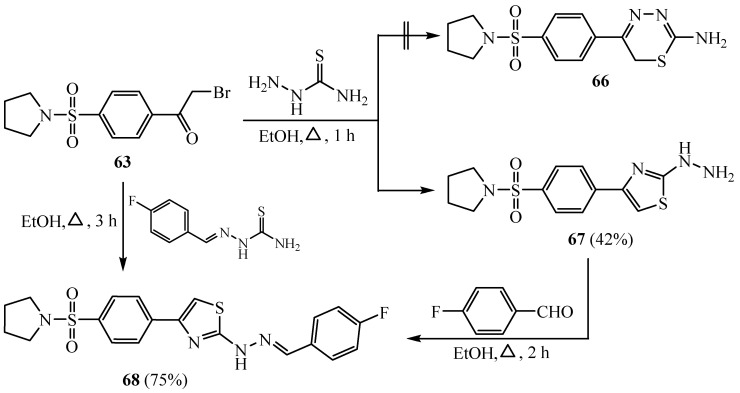
The interaction of phenacyl bromide compound 63 with thiosemicarbazide furnished the 2-hydrazinyl thiazole compound 67, instead of 2-aminothiadiazine derivative 66. Condensation of 67 with 4-fluorobenzyldehyde afforded the corresponding thiazolyl Schiff base 68 which displayed good activity towards hepatocellular carcinoma [39] (Scheme 16).
Scheme 16.
Synthesis of derivatives 67 and 68.
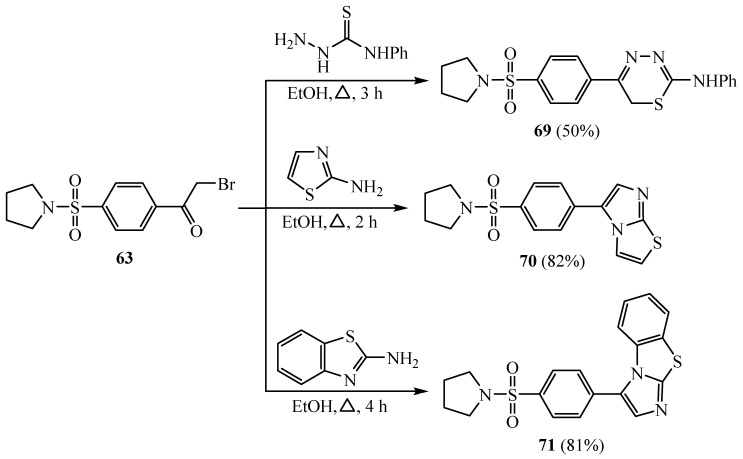
Cyclocondensation of 63 with phenyl thiosemicarbazide gave thiadiazine derivative 69. When compound 63 was treated with 2-aminothiazole and 2-aminobenzothiazole in hot ethyl alcohol, it gave the corresponding imidazo[2,1-b]thiazole derivatives 70 and 71, respectively (Scheme 17) [39]. Derivatives 69 and 71 presented a potent cytotoxicity against both the human liver hepatocellular carcinoma cell line (HepG2) and mammalian cells of the African green monkey kidney cell line (VERO).
Scheme 17.
Synthesis of derivatives 69–71.
Meanwhile, derivative 70 showed good results in relation to the selectivity index (SI), which is the ratio of the concentration that causes 50% death in the African green monkey kidney (VERO) (CC50) compared to the concentration that causes 50% death in the human liver hepatocellular carcinoma cell line (HepG2) (IC50).
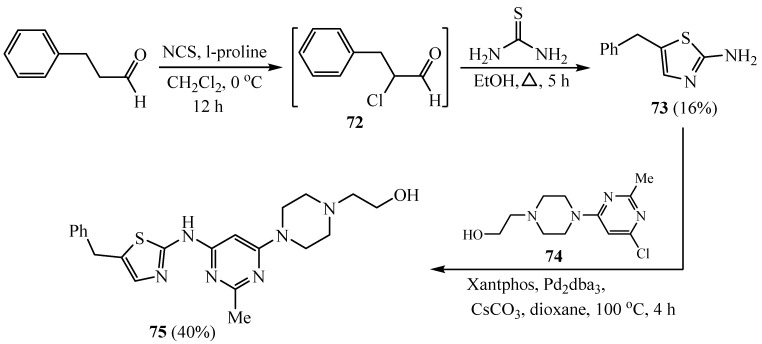
A potent inhibitor of Src family kinases (SFKs) with slow dissociation rates, aminothiazole derivative 75 of dasatinib, in which the methylene unit substitutes the amide linker between the moiety of thiazole and the aromatic ring of dasatinib, was prepared. The phenyl acetaldehyde was firstly converted to 2-amino5-benzylthiazole 73, which was combined with 74, a type of Buchwald reaction (Scheme 18) [40].
Scheme 18.
Synthesis of derivatives 73 and 75.
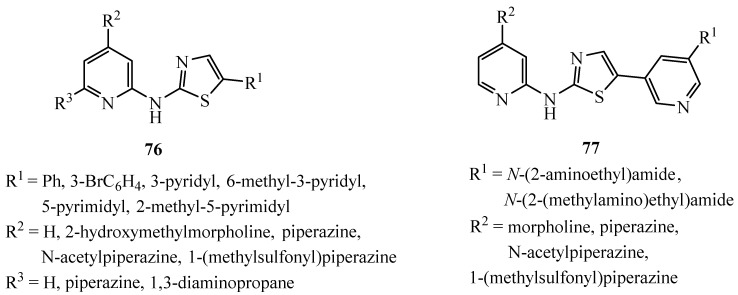
A series of 2-pyridylamino-thiazoles and 2-pyridylamino,5-pyridylthiazoles contains a novel category of ATP-competitive Chk1 inhibitors with outstanding inhibitory potential (Scheme 19) [41,42,43]. Modifications of the core with various amides accommodate compound 77 with picomolar potency and very high residence times.
Scheme 19.
Modification of derivatives 76 and 77.
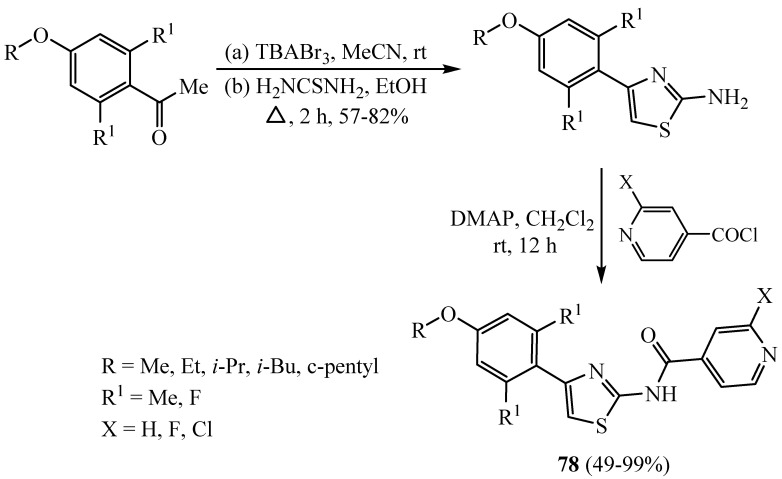
Some of acylated 4-aryl-N-arylcarbonyl-2-aminothiazole scaffolds 78 were designed and synthesized as highly active Hec1/Nek2 inhibitors. The fluoride derivative of 78 (Scheme 20) pointed to selectivity toward cancer cells over normal phenotype cells and was inactive in a [3H]astemizole rival binding assay for hERG liability screening. Thus, 2-aminothiazoles 78 (X = F, R = R1 = Me) are promising towards the discovery of a preclinical candidate targeting Hec1/Nek2 [44].
Scheme 20.
Synthesis of derivative 78.
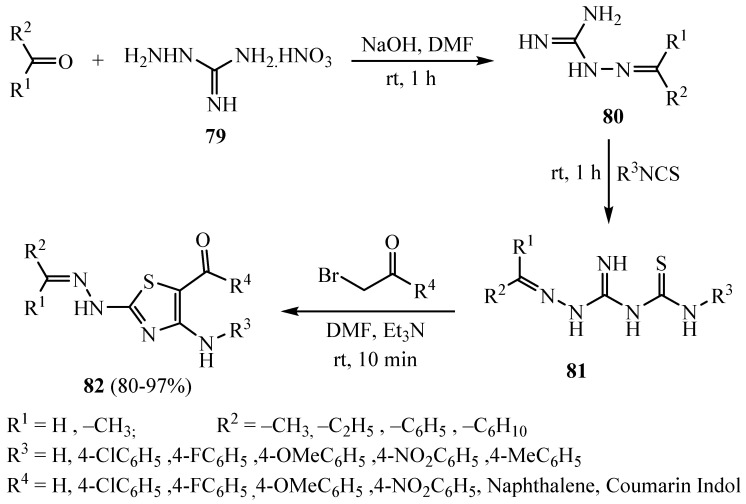
The variety elements were incorporated through azomethine linkage on the C4 hydrazine terminus in 2-arylaminothiazoles 82 using (CH3)2CH-, (CH3)2CHCH2-, cyclohexyl and C6H5CH2- fragments, and enrichment of the chemical space they were in was assessed (Scheme 21). Some of the prepared compounds were found to be a new type of Aurora kinase inhibitors [45].
Scheme 21.
Synthesis of derivative 82.
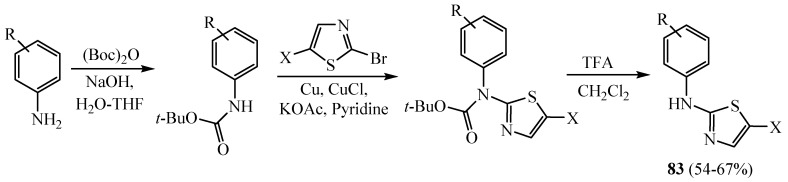
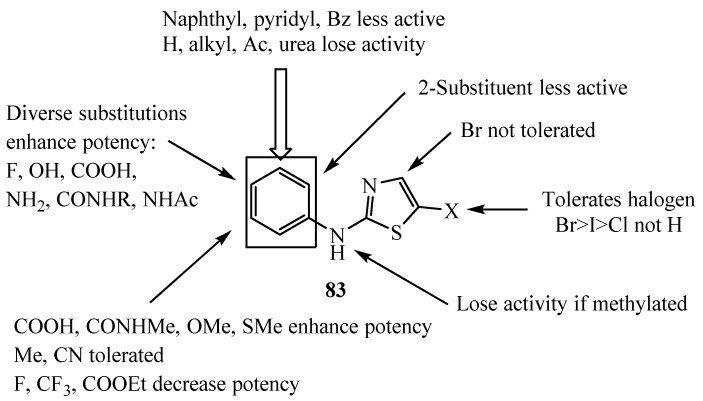
Regarding the important role of Aurora family kinases which regulate events during mitosis including centrosome maturation and chromosome segregation, the misregulation of Aurora kinases due to genetic amplification and protein overexpression results in aneuploidy and may contribute to tumorigenesis. A series of 2-aminophenyl-5-halothiazoles 83 was synthesized from the reaction of 2,5 substituted thiazoles with tert-butyl phenylcarbamate (Scheme 22) [46,47]. The synthesized derivatives displayed different activities on Aurora kinase inhibition, with decreased histone H3 serine 10 phosphorylation. To summarize SAR for aminothiazole Aurora inhibitors, arrows indicate the position and nature of each substitution tested in a biochemical Aurora A kinase assay (Figure 1).
Scheme 22.
Synthesis of derivative 83.
Figure 1.
SAR of aminothiazole 83.
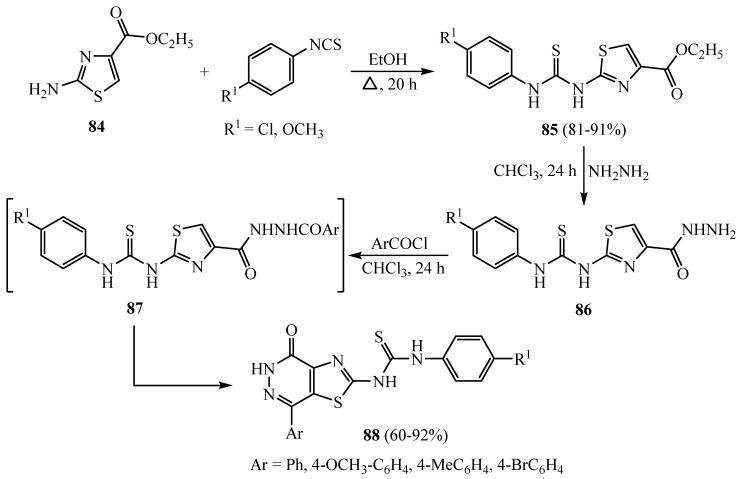
A series of thiazole and thiazolopyridazines, both containing the 2-thioureido function, were evaluated for in vitro antitumor activity against a cancer cell line collection. 1-(4-chloro-phenyl)-3-[4-oxo-7-(4-bromo-phenyl)-4,5-dihydrothiazolo[4,5-d]pyridazin-2-yl]thiourea derivative 88 (R1 = Cl, Ar = 4-BrC6H4) proved lethal to the HS 578T cancer breast cell line with an IC50 value of 0.8 µM. The title thiourea derivatives 85 were synthesized by reaction of ethyl 2-aminothiazole-4-carboxylate 84 with phenyl isothiocyanate derivatives. Then, the functional esters were reacted with NH2NH2 to give the acid hydrazides 86, which were treated with the benzoyl chlorides to afford the corresponding 3-phenylthioureas 87. An in situ cyclization was carried out on compound 87 to afford the thiazolo[4,5-d]pyridazin-2-yl]thiourea derivatives 88 (Scheme 23) [48]. The derivative 88 which contains (R1= Cl; Ar = 4-BrC6H4) proved to be the most active DHFR inhibitor with an IC50 of 0.06 µM and showed 31.4, 25.2, 37.7, 25.1 and 41.0 GI% against NCI-H522 non-small cell lung, HT29 colon, SK-OV-3 ovarian, MCF7 breast and T-47D breast cancers, respectively. Meanwhile, derivatives of compound 88 that contain (R1 = Cl, OCH3; Ar= OCH3, OCH3) were active with an IC50 of 0.1 and 2.5 µM, respectively. In addition, derivatives of compound 88 that contain (R1 = OCH3; Ar = Ph) showed antitumor activity against NCI-H522 non-small cell lung, HT29 colon and TK-10 renal with GI values of 31.7, 29.4 and 34.7%, respectively.
Scheme 23.
Synthesis of derivative 88.
2.2. 2-Aminothiazoles as Antioxidant Agents
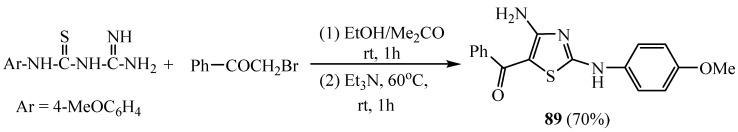
The energy production to fuel biological processes by oxidation is important to many living organisms. The outcomes indicated that, the synthesized derivative 4-amino-5-benzoyl-2-(4-methoxyphenyl-amino)thiazole (89) (Scheme 24) pointed to an important antioxidant potential in terms of scavenging free radicals. In addition, 4-aminothiazole hybrid 89 was an effective radio protector against radiation-induced damage in the liver of mice. In addition, 4-aminothiazole scaffold 89 can protect the mouse myocardium against damage and one of the possible reasons behind this protective effect can be attributed to its antioxidant property [49,50,51].
Scheme 24.
Synthesis of derivative 89.
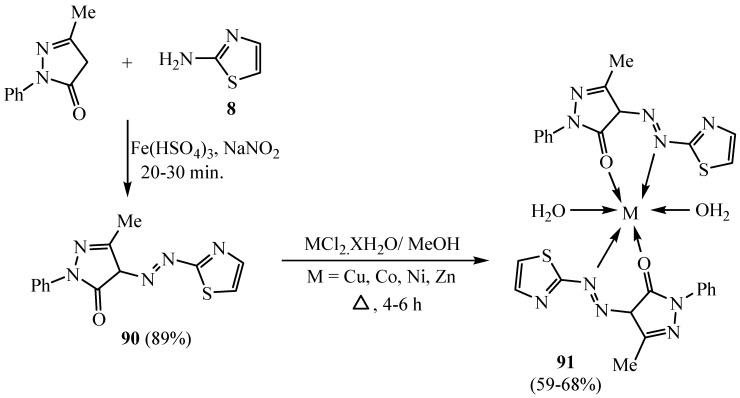
The 4-(thiazol-2-yl-azo)-2,4-dihydro-3H-pyrazol-3-one scaffold 91 was blended by a diazo-coupling reaction of 3-methyl-1-phenyl-5-pyrazolone with 2-aminothiazole (8) and ferric hydrogen sulfate (Scheme 25). The synthesized thiazole scaffolds 90 and 91 were assessed for an antioxidant effect, and among them, Cu(II) Co(II) and Ni(II) complexes indicated good activity in DPPH and nitric oxide scavenging [52].
Scheme 25.
Synthesis of derivative 91.
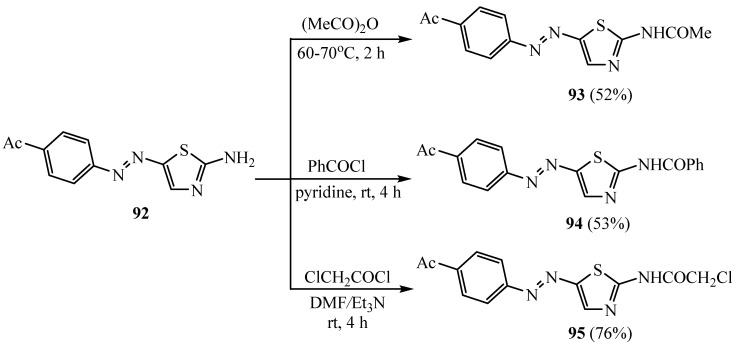
A reactivity study of both the aryl substituent and amino group of a novel synthesized 2-amino-5-(4-acetylphenylazo)-thiazole compound and its scaffolds via different electrophilic reagents was conducted. They were biologically evaluated in vitro and in vivo for their toxicity and antioxidant activity based on liver function enzymes. The new 2-amino-5-(4-acetylphenylazo)-thiazole (92) was reacted with various active carbonyl reagents (Scheme 26) [53]. A convenient acetylation reaction of 92 by solvent-free acetylation with acetic anhydride afforded the N-acetylated product 93. The electrophilic attack of the benzoyl cation towards 92 yielded the benzoyl amino derivative 94. A further reaction of the highly activated chloroacetyl chloride reagent with 92 under basic conditions was performed to produce the chloroacetyl amino derivative 95 [53].
Scheme 26.
Synthesis of derivatives 93–95.
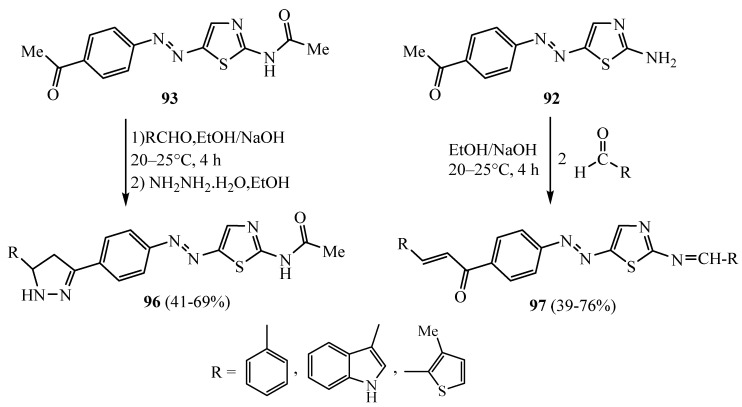
Claisen–Schmidt condensation of 2-acetylamino-5-(4-acetyl-phenylazo)-thiazole 93 with an equimolar ratio of aromatic and/or heterocyclic aldehydes in sodium hydroxide and water/ethanol medium led to the formation of chalcones which reacted with hydrazine hydrate in the presence of ethanol to afford 96 (Scheme 27). The reaction of 5-arylazo-2-aminothiazole 92 with the appropriate aldehydes (two moles) under the same reaction conditions led to the formation of the chalcone-imine derivatives 97 [53]. The synthesized derivatives 96 (R = 3-methylthiopnene) and 97 (R = indole) showed a significant increase in antioxidant enzyme activities in the treated rat groups at doses of 50 and 100 mg/kg.
Scheme 27.
Synthesis of derivatives 96 and 97.
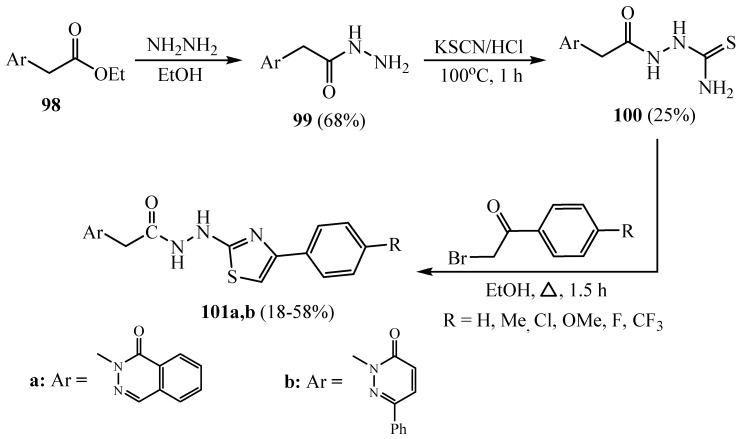
The treatment of phthalazin-1(2H)-one and 6-phenylpyridazin-3(2H)-one compounds with ethyl bromoacetate produced the ester derivatives 98. The reactions of compounds 98 with hydrazine hydrate afforded the hydrazide derivatives 99a and 99b. Then, compounds 100 hydrazine carbothioamide moieties were prepared by the reaction of compound 99 with potassium thiocyanate in the presence of hydrochloric acid (Scheme 28). Finally, hydrazinothiazoles 101 were blended by cyclization key intermediates 100 with suitable phenacyl bromides in ethanol [54]. Although the synthesized derivatives 101a,b demonstrated good antioxidant activity, particularly in the DPPH radical scavenging assay, their inhibition activity on cholinesterase enzymes suggested a structure-specific interaction.
Scheme 28.
Synthesis of derivatives 99–101.
2.3. 2-Aminothiazoles as Antimicrobial Agents
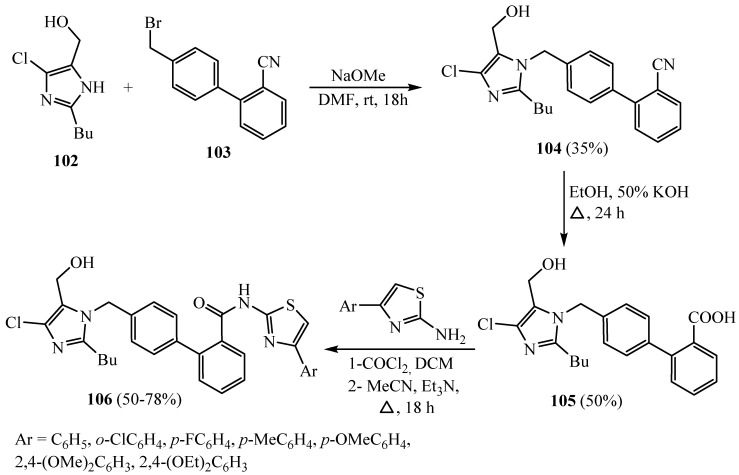
Thiazole and imidazole scaffolds are an essential kind of heterocyclic compound. They occupy a significant position in medicinal chemistry, showing a wide range of bioactivities. A series of 4-(2-N-butyl-4-chloro-5-hydroxymethyl-imidazol-1-yl-methyl-biphenyl-2 carboxylic acid-(substitutedphenyl-thiazole)-amides 106 was prepared as outlined in Scheme 29 by the conversion of the carboxylic acid derivative 105 into its acid chloride followed by acylation with many 2-aminothiazoles. The newly synthesized title compounds were screened for their in vitro antibacterial activity against S. Aureus and B. Subtilis and also for an in vitro antifungal effect against C. Albicans and Aspergillus niger. Some of the compounds exhibited encouraging outcomes [55].
Scheme 29.
Synthesis of derivative 106.
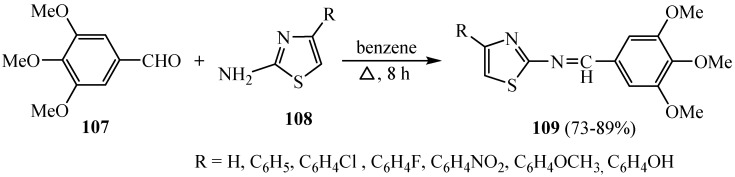
The condensation of 3,4,5-trimethoxybenzaldehyde 107 with 2-aminothiazole and/or 2-amino-4-(p-substituted/unsubstituted)-phenyl thiazole 108 (Scheme 30) was reported to furnish the corresponding Schiff bases 109. The effect of three methoxy groups in the carbon phenyl nucleus on the course of reactions with the substituted thiazole nucleus and the compounds containing a nitro group and fluoro at para positions exhibited very good activity against both the strains, i.e., the electron-withdrawing group showed maximum inhibition in both the strains on the antibacterial and antifungal activities of the synthesized products [56].
Scheme 30.
Synthesis of derivative 109.
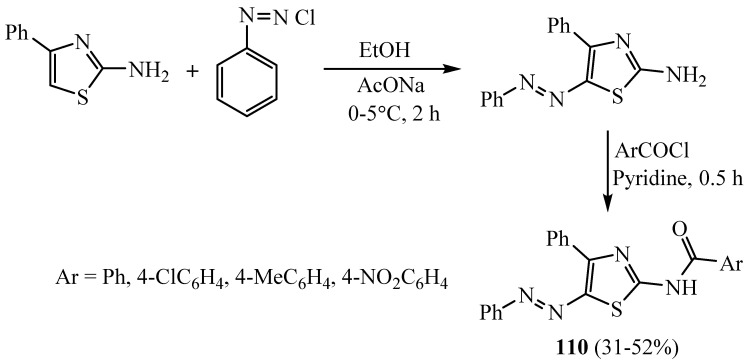
Some derivatives of 2-aminothiazole bearing arylazo moiety at the fifth position have been used for antimicrobial activities. The amide of 2-amino-4-phenyl-5-phenylazothiazoles derivatives 110 (Scheme 31) was obtained when 2-amino-4-phenyl-5-phenylazothiazole was acylated with appropriate substituted aromatic acid chlorides by employing the Schotten–Bauman synthesis protocol. All the synthesized compounds showed good antimicrobial activity against E. coli, S. aureus, A. niger and A. oryzaeto [57].
Scheme 31.
Synthesis of derivative 110.
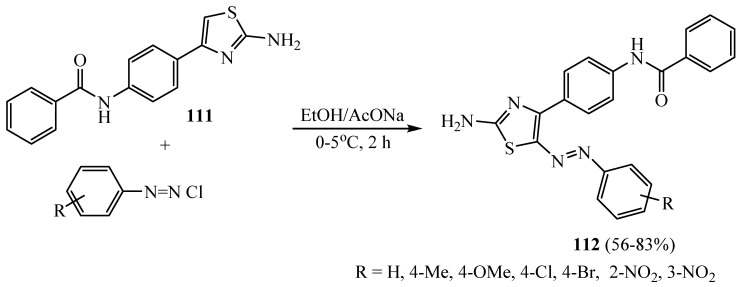
Coupling of diazonium salts with 2-aminothiazole derivative 111 provided the phenylazo-thiazole derivatives 112 in excellent yield (Scheme 32). The synthesized series of benzamide-linked 2-aminothiazole-based compounds showed excellent antibacterial activity and antifungal activity [58].
Scheme 32.
Synthesis of derivative 112.
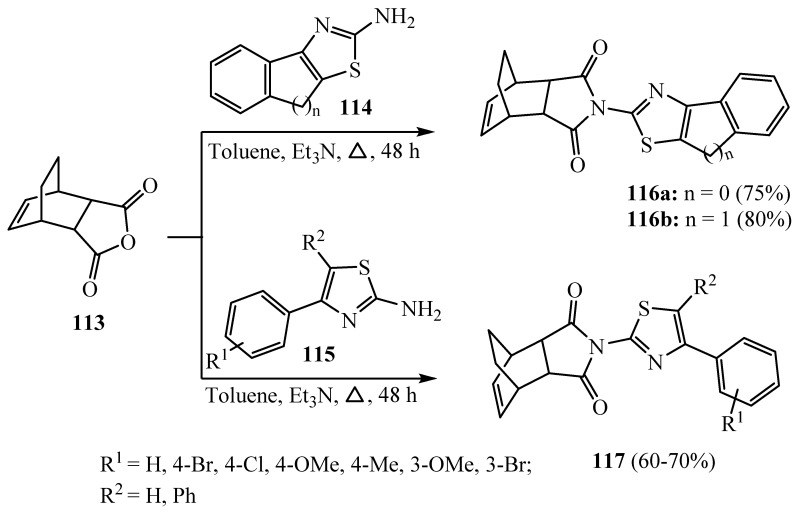
Ethanoisobenzofuran-1,3-dione (113) was obtained by the addition of maleic anhydride to cyclohexadiene. The reaction of 2-aminothiazole derivatives 114 and 115 with the anhydride derivative 113 gave a group of new 2-(4-arylthiazol-2-yl)-3a,4,7,7a-tetrahydro-1H-4,7- ethanoisoindole1,3(2H)-dione derivatives 116 and 117 (Scheme 33). According to MIC values, derivatives 117 (R1 = OCH3) and 117 (R1 = CH3) presented remarkable efficacy toward E. coli. Derivative 117 (R1 =H, R2 = Ph) showed significant efficacy toward P. Aeruginosa. Derivatives 117 (R1 = 4-Br), 116 (n = 0) and 117 (R1 = 4-Cl) displayed low activity, and 117 (R1 = OCH3) and 117 (R1 = CH3) showed remarkable efficacy toward S. marcescens. In summary, the utmost active derivatives are 117 (R1 = 4-Cl) (MIC: 0.039 μg/mL) toward C. perfringes and 117 (R1 = H) (MIC: 0.078 μg/mL) toward A. tumefacens. Regarding SAR, derivatives 117 containing 4-Br and 4-Cl groups were established to be the utmost active compounds according to the inhibition zone. They displayed particularly high efficacy toward the utmost utilized microorganisms [59].
Scheme 33.
Synthesis of derivatives 116 and 117.
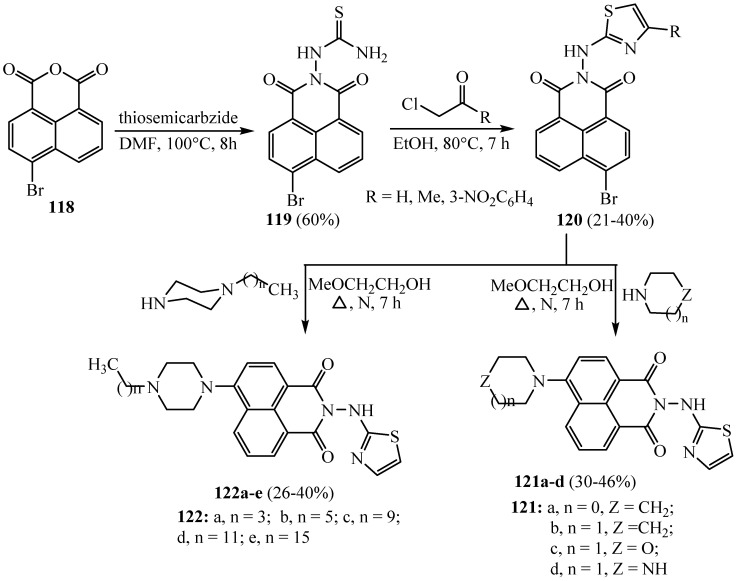
The target naphthalimide aminothiazoles 121a–d and 122a–e were synthesized through multi-step reactions beginning from 4-bromo-1,8-naphthalic anhydride 118 according to Scheme 34. Condensation of 118 and thiosemicarbazide gave compound 119, which was further cyclized with the α-halogenated carbonyl compounds to afford aminothiazole scaffolds 120. Compound 120 was further treated with alicyclic amines to give 121a-d. The N-alkylation of piperazine with alkylhalides generated mono-substituted alkyl piperazines 122a−e. Piperazinyl derivatives effectively prevent the growth of methicillin-resistant S. Aureus and E. coli with MIC values of 4 and 8 μg/mL, respectively. The utmost active derivative 121d with the NH free piperazine moiety (MIC. values from 2 to 128 micromolar) displayed the most toxicity toward Gram-positive bacteria such as S. aureus 29213 and aureus 25923 and was also effective in inhibiting Gram-negative bacteria such as E. coli, E. coli 25922, P. aeruginosa and P. aeruginosa 27853 at low concentrations. These designated 121d had massive potentiality to be more effective broad-spectrum antimicrobial agents. In addition, the extents of alkyl chains possess diverse effects on biological efficacy as in derivative 122b with the hexyl group, which provided enhanced antibacterial efficacy in contrast to further alkyl derivatives. Likewise, when the alkyl substituents were lengthy to decyl, dodecyl and hexadecyl groups, derivatives 122c–e showed weak activity in preventing the growth of the examined bacteria. This real idea presented that only an appropriate alkyl length chain in the piperazine ring was essential for a respectable antibacterial efficacy [8].
Scheme 34.
Synthesis of derivatives 119–117.
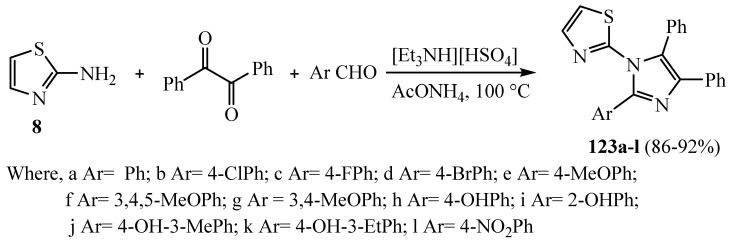
A group of imidazole-thiazole derivatives 123a–l were blended using the green protocol (Scheme 35). The synthesized derivatives 123a–l were assessed for their in vitro antifungal activity, and the compounds 123j and 123k inhibited ergosterol biosynthesis by inhibiting enzyme cytochrome P450 lanosterol 14α-demethylase of C. albicans. The obtained results suggest that these compounds might inhibit fungal lanosterol 14α-demethylase related to the accepted mechanism of fluconazole [60].
Scheme 35.
Synthesis of derivative 123a–l.
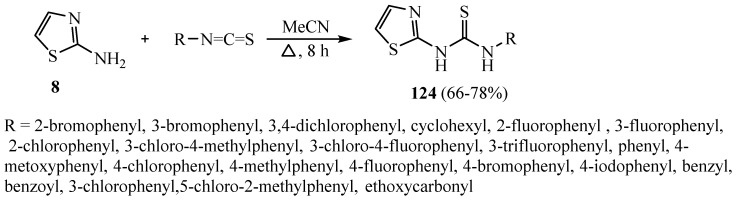
A series of thiazolyl-thiourea derivatives 124 was synthesized by the addition reaction of 2-aminothiazole to isothiocyanate (Scheme 36). The obtained thioureas were examined in vitro against a number of microorganisms. Initial antibacterial investigations found that halogen derivative of thiourea 124 has (3,4-dichlorophenyl) and 124 has (3-chloro-4-fluorophenyl), which reveals the supreme promising efficacy toward staphylococcal species. Generally, MIC results of S. aureus and S. epidermidis were displayed at 16 to 4 μg/mL. These thiourea analogues were investigated to explain their ability to prevent the formation of biofilms of eight methicillin-resistant strains of S. epidermidis (MRSE) [6].
Scheme 36.
Synthesis of derivative 124.
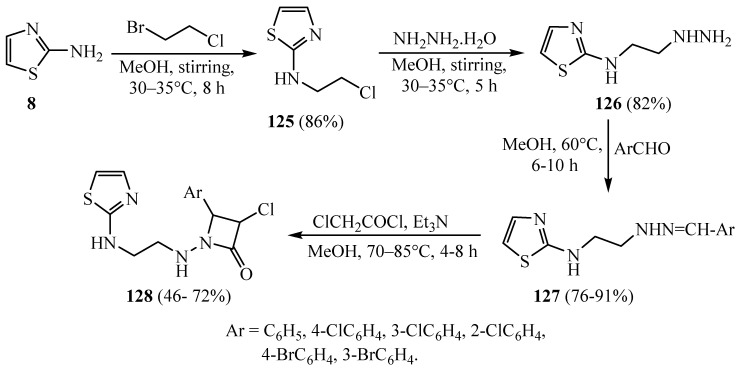
Chloro-4-(substitutedphenyl)-1--2-azetidinone compounds 128 (Scheme 37) were synthesized in four dissimilar steps. 2-Aminothiazole 8 on reaction with Cl(CH2)2Br at room temperature gave 2-[(2-chloroethyl) amino]thiazole 125. Compound 125 on reaction with hydrazine hydrate at room temperature produced N-(2-hydrazinylethyl)-2-thiazolamine 126. Compound 126 on further reaction with several chosen substituted aromatic aldehydes yielded substituted benzaldehyde, 2-[2-(thiazolylamino)–ethyl]–hydrazone compounds 127. Compounds 127 on treatment with ClCH2COCl in the presence of Et3N furnished compounds 128. The antimicrobial and antitubercular activity of the newly synthesized compounds bearing a 2-azetidinone moiety exposed that all the evaluated compounds showed moderate to good antibacterial, antifungal and antitubercular activities against the chosen microbial strains [61].
Scheme 37.
Synthesis of derivative 128.
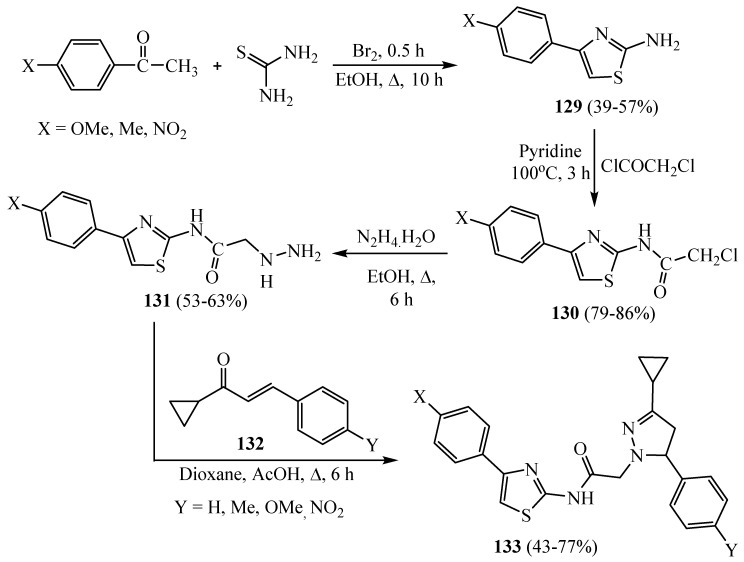
Some pyrazolone-linked thiazole derivatives 133 containing substituents at 1,3,5-positions were synthesized according to Scheme 38. The commencing chalcones 132 were made by conventional Claisen–Schmidt condensation by reacting suitably substituted benzaldehydes and cyclopropyl-methyl ketone. 2-Aminothiazoles 129 were gained by cyclocondensation of suitably substituted acetophenones with thiourea in the presence of bromine. Chloroacetamides 130 was obtained by reacting 2-aminothiazoles 129 with chloroacetyl chloride in the presence of pyridine. When chloroacetamides 130 were heated with hydrazine hydrate in ethanol, hydrazines 131 were obtained. When chalcones 132 were heated with hydrazines 131 in dioxane containing a few drops of acetic acid, pyrazoline derivatives 133 were gained. The target compounds 133 indicated more significant antimicrobial activity than some known standard drugs, and most compounds pointed out a moderate degree of potent antimicrobial activity [62].
Scheme 38.
Synthesis of derivative 133.
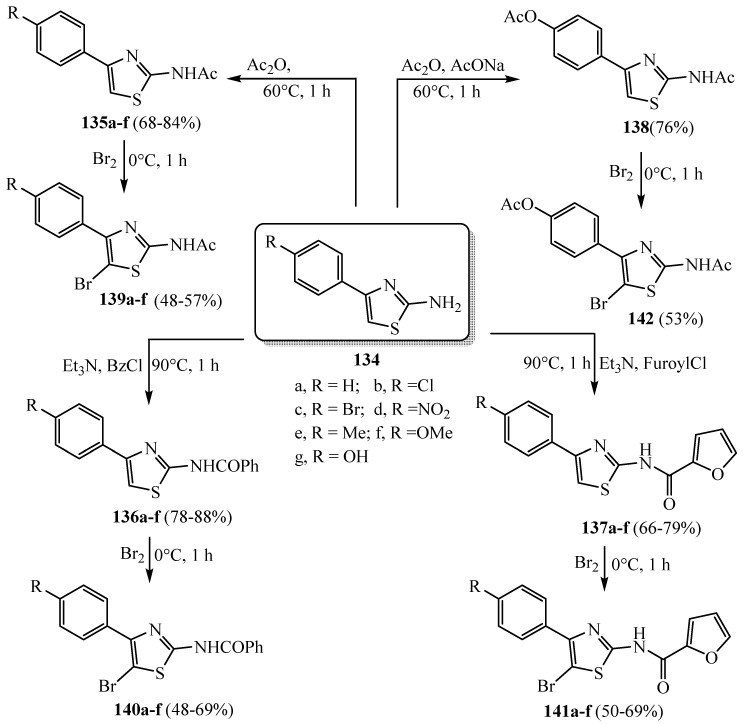
Seven 2-amino-4-arylthiazole scaffolds 134a–g were synthesized under microwave irradiation. Compounds 134a–f were reacted with (CH3CO)2O, C6H5COCl and 2-furoyl chloride, respectively, to furnish thiazoles 135a–f, 136a–f and 137a–f (Scheme 39). The reaction of 134g with (CH3CO)2O led to the diacetyl derivative 139. The bromine derivatives 139a–f, 140a–f, 141a–f and 142 were obtained by the reaction of 2-amino-4-arylthiazoles, N-(4-arylthiazol-2-yl)-acetamides, N-(4-arylthiazol-2-yl)- benzamide, furan-2-carboxylic acid (4-aryl-thiazol-2-yl)-amide and acetic acid 4-(2-acetylamino- thiazol-4-yl)-phenyl ester with molecular bromine under acid conditions [63]. The synthesized compounds displayed a remarkable anti-giardial activity.
Scheme 39.
Synthesis of derivatives 135–141.
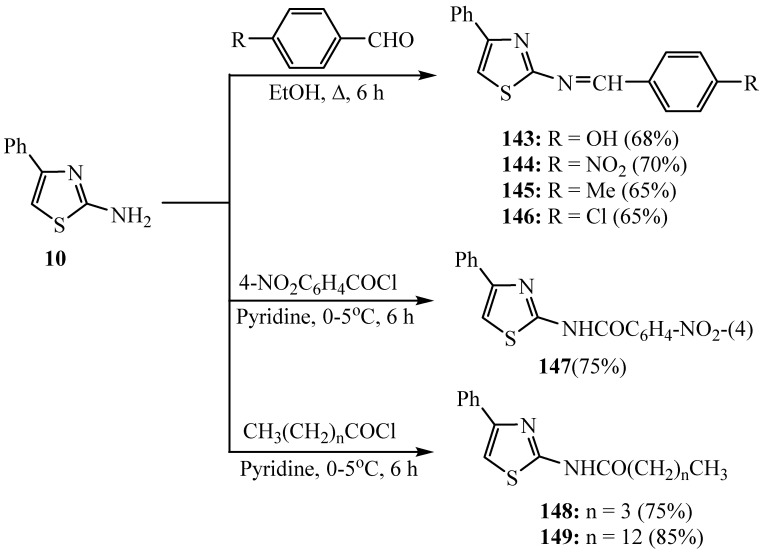
Refluxing of compound 2-amino-4-phenylthiazole with different aromatic aldehydes in ethanol produced the corresponding 2-arylideneamino-4-phenylthiazoles 143–146 (Scheme 40) in good yields. Acylation of 2-aminothiazole 10 with various acyl halides in dry pyridine produced the corresponding amides 147–149 (Scheme 40) in high yields. Amongst the synthesized compounds investigated for the antibacterial activity, compound 144 indicated the highest activity against B. cereus. Some of the compounds pointed out low antimicrobial activities and some were incompetent to demonstrate inhibition. For the antifungal activity, all compounds pointed to outstanding outcomes against C. Lunata [64].
Scheme 40.
Synthesis of 2-arylideneamino-4-phenylthiazoles 143–146.
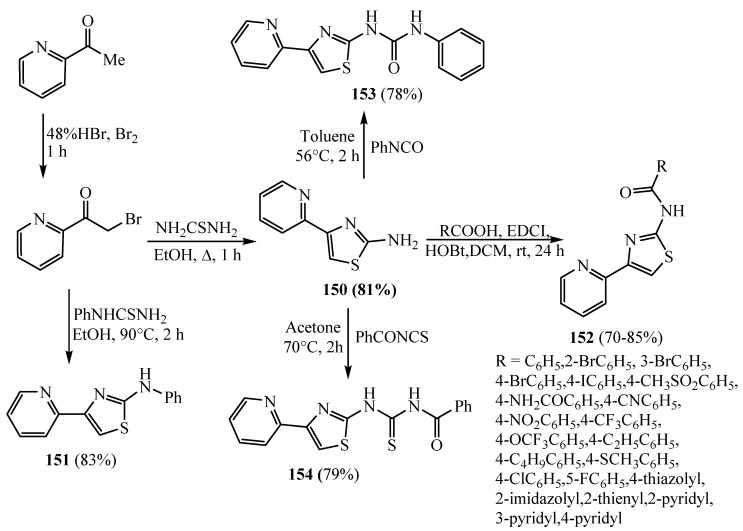
A set of compounds was prepared from the 2-amino-4-(2-pyridyl) thiazole derivative 150 which was been synthesized by α-bromination of 2-acetylpyridine followed by condensation with thiourea. In the presence of mono-substituted carboxylic acids, 2-aminothiazole 150 underwent an EDCI (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide)-mediated coupling to deliver the target amides 152. Compound 151 was gained via the reaction of 2-bromoacetylpyridine with phenylthiourea (Scheme 41). Compounds 153 and 154 were obtained from the reaction of compound 150 with phenyl isocyanate and benzoyl isothiocyanate, respectively [65]. The antimycobacterial efficacy results for the synthesized derivatives revealed that derivative 152 with a phenyl ring which had an amide linker at position 2 had superior antimycobacterial efficacy that matched derivatives 151, 153 and 154 which had amino, urea and acylthiourea linkers, respectively. However, derivatives 152 with a thiazole, imidazole and 2-pyridyl ring, respectively, displayed no activities toward Mycobacterium tuberculosis (M.tb). Meanwhile, analogues 152 with thiophene, 3-pyridyl, 4-pyridyl and the monosubstitution in the four position with 4-Br, 4-I, 4-CH3SO2, 4-NH2CO, 4-CN, 4-NO2 and 4-CF3, respectively, enhanced the activity like the unsubstituted phenyl derivative. The position of the substitution on the phenyl had an influence on activity as demonstrated by the bromo-substituted compounds with activity of the para > meta > ortho. Switching the 2-pyridyl substituent by a 3-pyridyl or 4-pyridyl resulted in loss of antimycobacterial activity.
Scheme 41.
Synthesis of derivatives 150–154.
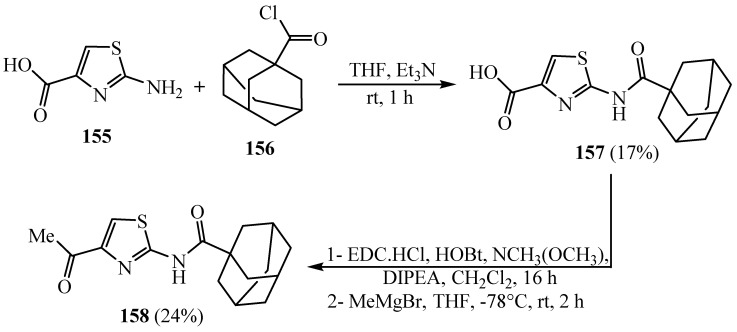
Treatment of the available compound 2-aminothiazole-4-carboxylate 155 with 1-adamantanoyl chloride 156 in hot tetrahydrofuran followed by conversion to Weinreb amide and Grignard in the existence of methyl magnesium bromide afforded 158 (Scheme 42) [66]. Meanwhile, the substituted amino group in C-2 position of the thiazole can accommodate a range of lipophilic substitutions, while the thiazole moiety is sensitive to modification. The synthesized derivative 158 showed respectable activity against (M.tb) growth with sub-micromolar minimum inhibitory concentrations being achieved. A demonstrative hybrid was selective for mycobacterial species over other bacteria and was rapidly bactericidal against replicating (M.tb). It was concluded that these derivatives have potential for additional progress as novel antitubercular agents.
Scheme 42.
Synthesis of derivative 158.
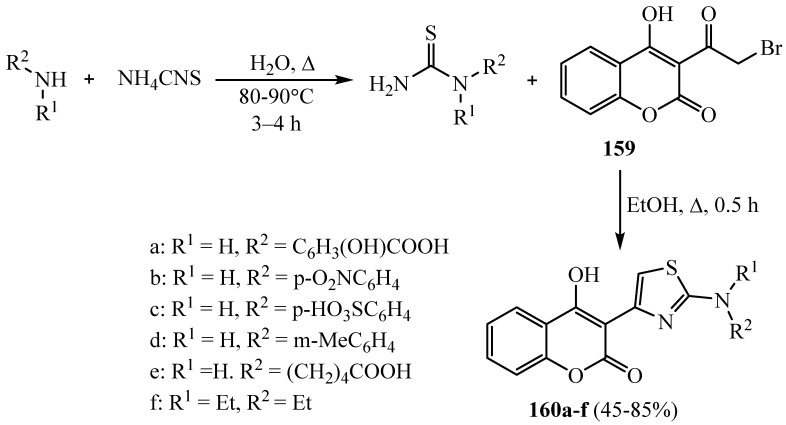
Synthesis of 2-aminothiazole derivatives 160a-f substituted with 4-hydroxy-chromene-2-one at the position number 4 was reported from cyclization of 3-(2-bromoacetyl)-4-hydroxy- chromene-2-one 159 with the corresponding thiourea derivatives (Scheme 43). All synthesized compounds exhibited antibacterial and antifungal activity [67].
Scheme 43.
Synthesis of derivative 160a–f.
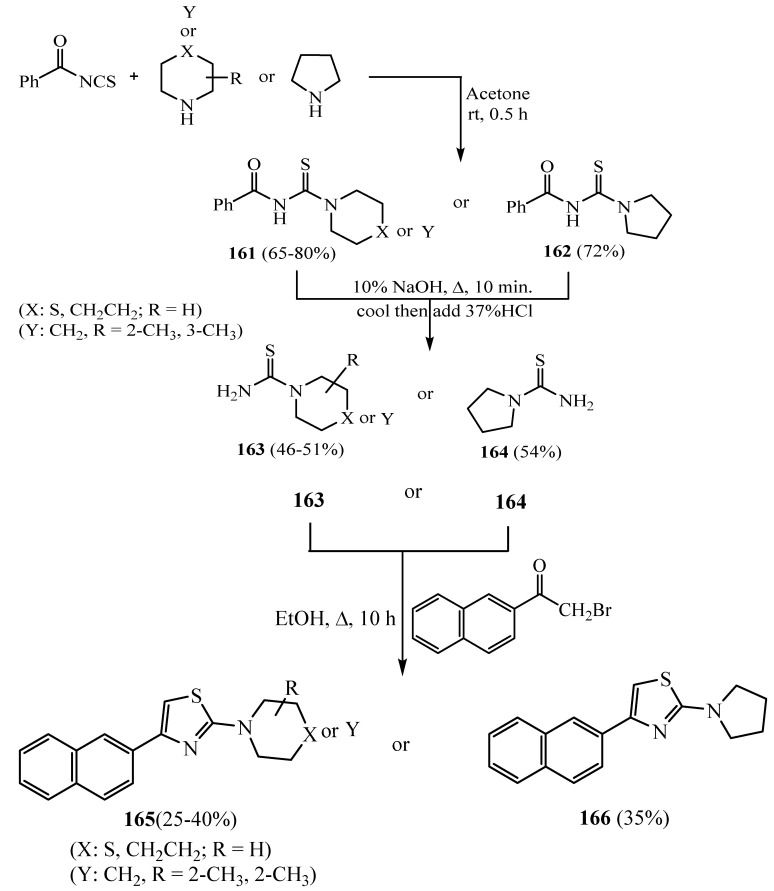
The synthesis of new thiazole derivatives 165 and 166 from dialkyl aminothiocarbamides 163 and 164 with 2-bromo-(naphthalene-2-yl)ethanone) (Scheme 44) was considered and their in vitro antimicrobial and anticancer activity was tested. The antimicrobial properties of these naphthylthiazolylamine compounds were evaluated against various selected bacterial and fungal strains using the minimum inhibitory concentration (MIC) method. In addition, cytotoxicity studies were also carried out in Hep-G2 and A549 cell lines to examine the ability of these compounds to inhibit cell growth [68].
Scheme 44.
Synthesis of derivatives 161–166.
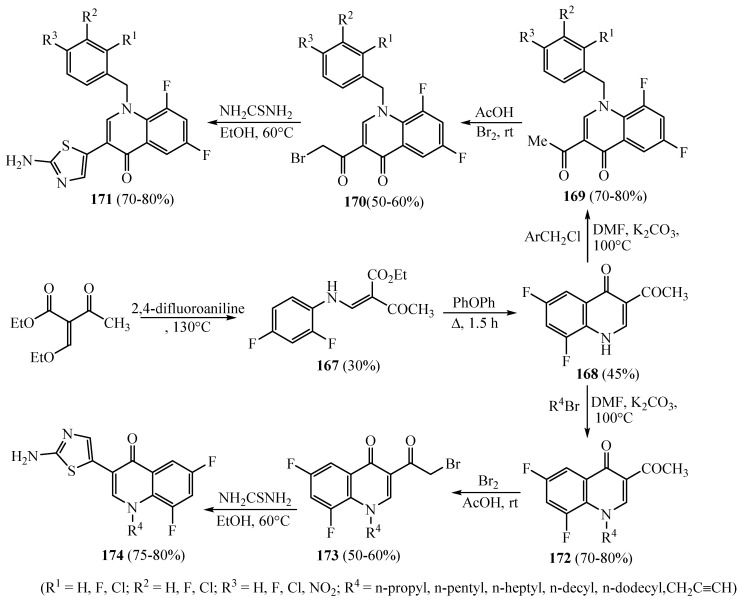
The 2-aminothiazolyl quinolones 171 and 174 were synthesized (Scheme 45) via multi-step reactions. Ethyl (ethoxymethylene)-3-oxobutanoate was treated with 2,4-difluoroaniline to furnish ethyl 2-((2,4-difluorophenylamino)methylene)-3-oxobutanoate (167), which was then further recyclized in hot phenoxy-benzene to afford the needed 3-acetyl quinolone 168. Compound 168 was N-aralkylated or alkylated to afford N-aralkyl quinolones 169 and alkyl derivatives 172, which were then brominated to produce the corresponding 3-(2-bromoacetyl)-quinolone derivatives 170 and 174. The cyclization of the bromoacetyl group at the C-3 position of 170 and 173 with thiourea in ethyl alcohol at 60 °C yielded aralkyl 2-aminothiazolyl quinolones 171 (R1 = H, F, Cl; R2 = H, F, Cl; R3 = H, F, Cl, NO2) and alkyl derivatives 174 (R4 = n-propyl, n-pentyl, n-heptyl, n-decyl, n-dodecyl, CH2 C≡CH). The new 2-aminothiazolyl quinolones’ in vitro antimicrobial activity could effectively restrain the growth of some tested strains [69]. Antibacterial screening of the synthesized hybrids exhibited that N-1 propargyl modified 2- aminothiazolyl quinolone 174, which presented high antibacterial activities in contrast to the rest of the derivatives against B. typhi. Further, this derivative exhibited equal or better activity in contrast to the two reference drugs toward S. dysenteriae and P. aeruginosa. Likewise, it was found that a shorter carbon chain such as the propyl derivative was more favorable in exerting antibacterial efficacy in comparison to norfloxacin and chloromycin as standard drugs. However, in the pentyl, octyl, decyl and dodecyl chains, a decrease in antibacterial efficacy was observed. Meanwhile, the monoflouroderivatives 171 were more active than the monochloro-derivatives 171. Mainly, the derivative 171 with the substituent para chloro on the phenyl ring could prevent the growth of S. dysenteriae (MIC = 4 mg/mL). Amazingly, the activities of derivative 171 with the electro-donating OCH3 group and 171 with the electro-withdrawing NO2 group were not greatly diverse alongside the utmost strains and both presented comparably weak bioactivity.
Scheme 45.
Synthesis of derivatives 167–174.
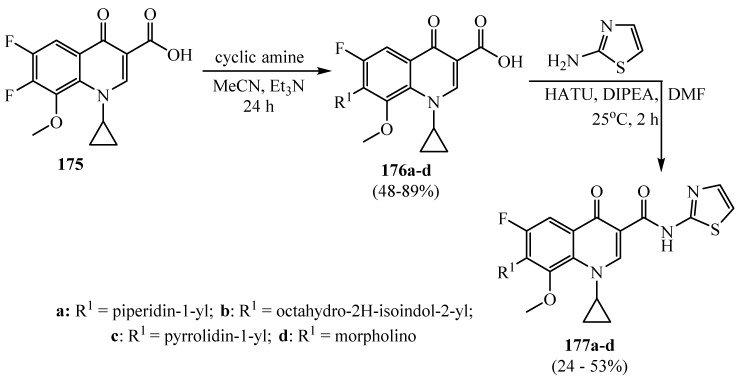
The synthesis of N-thiazolyl amide fluoroquinolone derivatives 177a-d involved the reaction sequence of nucleophilic aromatic substitution followed by acid derivatization to amides (Scheme 46). Amino-substituted fluoroquinolone compounds 176a-d were gained by heating 1,4-dihydroquinoline-3-carboxylic acids 175 with cyclic amine in acetonitrile and triethyl amine [70]. Further, the prepared derivatives were used to investigate non-carboxylic acid fluoroquinolones with an objective to enhance the anti-staphylococcal activity and improve their toxicity profile.
Scheme 46.
Synthesis of derivative 177a–d.
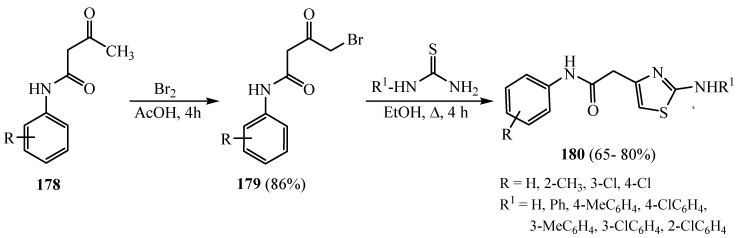
From ɷ-bromoacetoacetanilides 149 and thiourea/phenyl thioureas, a series of 4-arylacetamido-2-amino- and 2-arylamino-1,3-thiazoles 180 was synthesized (Scheme 47). The compounds were assessed for their in vitro antibacterial, antifungal and antioxidant activities [71].
Scheme 47.
Synthesis of derivative 180.
2.4. 2-Aminothiazoles as Anti-Inflammatory Agents
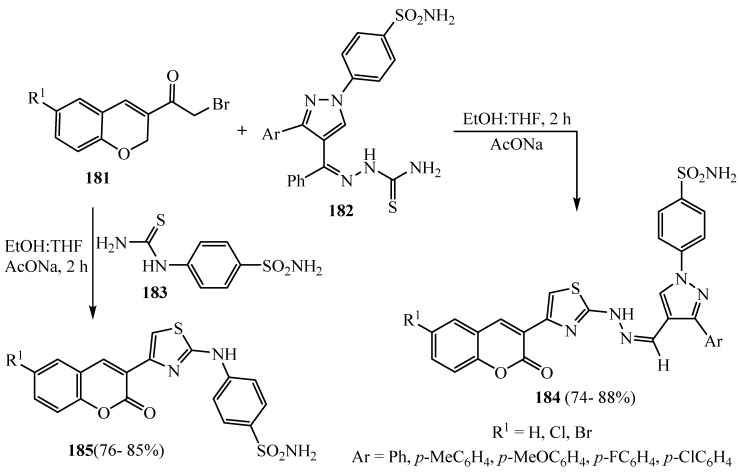
The synthetic pathway used to synthesize the target thiazolyl-hydrazinomethylidene pyrazoles 184 and N-substituted anilinothiazoles 185 are described in (Scheme 48). The present synthesis of thiazolyl-hydrazinomethylidene pyrazoles 184 makes up the condensation of appropriate 6-substituted-3-bromoacetylcoumarin 181 with suitable pyrazole-4-carbaldehyde thiosemicarbazone 182 in the presence of sodium acetate. 4-Thioureido-benzenesulfonamide 183 was treated with different 3-bromoacetylcoumarin compounds 182 in a hot mixture of ethyl alcohol and tetrahydrofuran in the presence of CH3COONa to give N-substituted anilinothiazole derivatives 185. All the synthesized thiazolyl-hydrazinomethylidene pyrazoles 184 and N-substituted anilinothiazoles 185 were assessed for there in vivo anti-inflammatory activity [72].
Scheme 48.
Synthesis of derivatives 184 and 185.
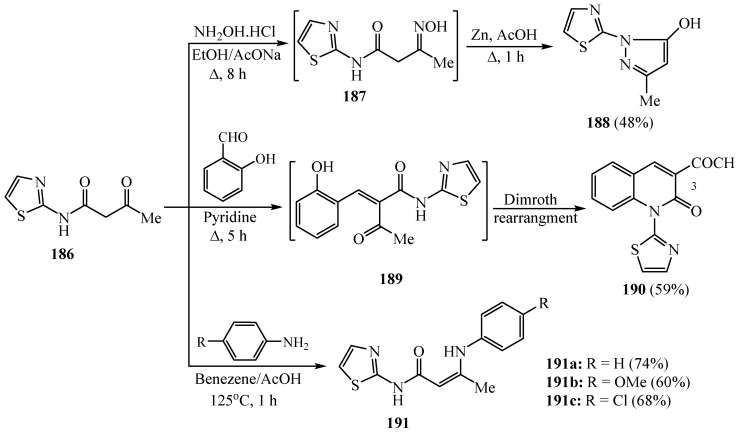
A series of thiazolyl derivatives 188, 190 and 191 was synthesized from the reactions of 3-oxo-N-(thiazol-2-yl)butanamide 186 with hydroxylamine, salicylaldehyde and aromatic aldehyde derivatives through the next synthetic pathway as shown in Scheme 49. The synthesized derivatives displayed inhibitory activities toward both the COX-1 isozyme (IC50 = 1.00–6.34 μM range) and the COX-2 isozyme (IC50 = 0.09–0.71 μM range), with COX-2 selectivity indexes in the range of 3.03 to 16 in comparison with the COX-2 selective standard drug celecoxib (COX-1, IC50 = 7.21 μM, COX-2, IC50 = 0.83 μM and S.I. = 8.68) [73].
Scheme 49.
Synthesis of derivatives 188–191.
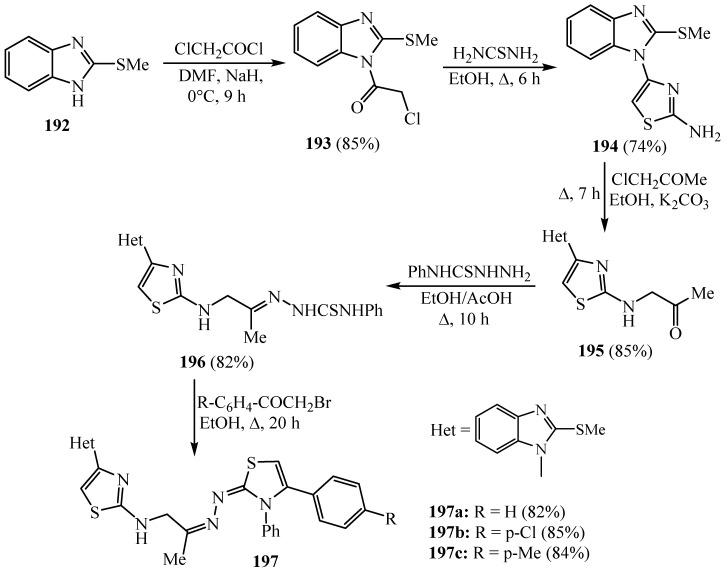
Recently, a new series of 2-aminothiazoles bonded with 2-methylthiobenzimidazole was prepared to investigate their anti-inflammatory properties on cyclooxygenase (COX) and lipoxygenase (15-LOX) enzymes’ inhibition Scheme 50. The synthesized hybrids containing the acetyl group 195, phenyl thiosemicarbazone 196 and 1,3-thiazolines 197a-c were demonstrated to be the most selective COX-2 as well as 15-LOX inhibitors, that is, due to the fact they provided a collaboration of not only molecular volume advantages but also steric, electronic, hydrogen bonding and hydrophobic advantages that are essential to confirm the optimal molecular interactions with the specific biological boards and to inhibit their biological responses. Currently, the importance of these derivatives connected to diverse aromatic and heterocyclic rings for evolving innovative anti-inflammatory agents with dual COX-2 /15-LOX enzyme inhibitory efficacy is avowed [74].
Scheme 50.
Synthesis of derivative 197a–c.
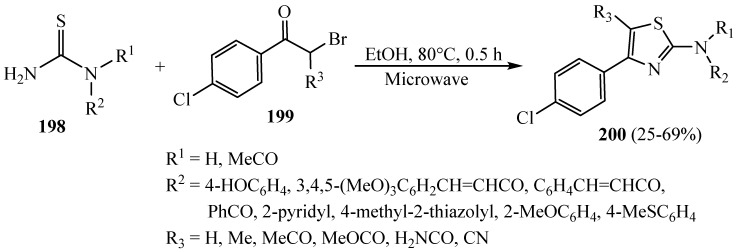
Finally, some new 4-(4-chlorophenyl)thiazol-2-amines 200 were prepared via cyclic condensation of an α-bromoketone 199 and N-substituted thiourea 198 in anhydrous ethanol, stirring under microwave irradiation at 80 °C for 30 min. The synthesized hybrids were examined to evaluate their inhibitory effectiveness against bovine pancreatic DNase I (Scheme 51). The in vitro evaluation of DNase I inhibition was based on spectrophotometric measurement of acid-soluble nucleotide formation at 260 nm. Inhibition of 5-LO activity was determined both in an intact cell system using freshly isolated polymorphonuclear leukocytes (PMNL) and in a cell-free assay using partially purified recombinant 5-LO, and 5- LO product formation was determined by HPLC. The synthesized hybrids reserved DNase I with IC50 values under 100 µM, and the derivative with (R1 = H, R2 = phenol and R3 = amide group) displayed a potent IC50 = 79.79 µM, where the crystal violet, used as a positive control in the absence of a “golden standard”, exhibited almost 5-fold weaker DNase I inhibition [75].
Scheme 51.
Synthesis of derivative 200.
3. Conclusions
The heterocycles of 2-aminothiazole scaffolds occupy a dominant part in organic/medicinal chemistry in relation to their reactivity and biological activity and mostly act as pharmacophores. The present review summarizes the literature reports of the various synthetic routes for 2-aminothiazole-containing molecules with four different biological activities (namely, anticancer, antioxidant, antimicrobial and anti-inflammatory activities). The presented information in this review is valuable for future innovation. The simple synthesis of 2-aminothiazole hybrids bids the structure–activity revisions of several substitutions of this multilateral pharmacophore. Further, several 2-aminothiazoles and their derivatives were generally utilized as drugs in the treatment of various diseases, which has led to their extensive improvements. Attributable to their broad scale of biological activities, their skeleton variants have attracted the attention of many biologists. This review highlighted the recently synthesized 2-aminothiazole-containing compounds within the last thirteen years ago. Further, the synthetic strategies developed for the admission of the recent 2-aminothiazole derivatives (N-substituted, 3-substituted, 4-substituted, multi-substituted, aryl/alkyl substituents or acyl/other substituents) were presented. The reported literature revealed several synthetic pathways of those 2-aminothiazoles related to four different biological activities (anticancer, antioxidant, antimicrobial and anti-inflammatory activities). It is hoped that this review will be useful in displaying the rationalistic designs of 2-aminothiazole-based medical synthetic pathways.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-23.
Author Contributions
All authors have equal contributions in software collections, writing—original draft preparation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia through Project no. (IFKSURP-23).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Suresh G., Nadh R.V., Srinivasu N., Yennity D. Synthesis and Antitumor Activity Evaluation of 2-Aminothiazoles Appended 5-methylisoxazoline and Pyridine-piperazine Hybrid Molecules. Lett. Org. Chem. 2018;15:1070–1077. doi: 10.2174/1570178615666180430122641. [DOI] [Google Scholar]
- 2.Das D., Sikdar P., Bairagi M. Recent developments of 2-aminothiazoles in medicinal chemistry. Eur. J. Med. Chem. 2016;109:89–98. doi: 10.1016/j.ejmech.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Alexandru M.-G., Velickovic T.C., Jitaru I., Grguric-Sipka S., Draghici C. Synthesis, characterization and antitumor activity of Cu (II), Co (II), Zn (II) and Mn (II) complex compounds with aminothiazole acetate derivative. Cent. Eur. J. Chem. 2010;8:639–645. doi: 10.2478/s11532-010-0022-2. [DOI] [Google Scholar]
- 4.Décor A., Grand-Maître C., Hucke O., O’Meara J., Kuhn C., Constantineau-Forget L., Brochu C., Malenfant E., Bertrand-Laperle M., Bordeleau J. Design, synthesis and biological evaluation of novel aminothiazoles as antiviral compounds acting against human rhinovirus. Bioorg. Med. Chem. Lett. 2013;23:3841–3847. doi: 10.1016/j.bmcl.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 5.Jalani H.B., Pandya A.N., Pandya D.H., Sharma J.A., Sudarsanam V., Vasu K.K. An efficient one-pot synthesis of functionally diverse 2-aminothiazoles from isothiocyanates, amidines/guanidines and halomethylenes. Tetrahedron Lett. 2013;54:5403–5406. doi: 10.1016/j.tetlet.2013.07.122. [DOI] [Google Scholar]
- 6.Stefanska J., Nowicka G., Struga M., Szulczyk D., Koziol A.E., Augustynowicz-Kopec E., Napiorkowska A., Bielenica A., Filipowski W., Filipowska A. Antimicrobial and anti-biofilm activity of thiourea derivatives incorporating a 2-aminothiazole scaffold. Chem. Pharm. Bull. 2015;63:225–236. doi: 10.1248/cpb.c14-00837. [DOI] [PubMed] [Google Scholar]
- 7.Elshaarawy R.F., Mustafa F.H., Sofy A.R., Hmed A.A., Janiak C. A new synthetic antifouling coatings integrated novel aminothiazole-functionalized ionic liquids motifs with enhanced antibacterial performance. J. Environ. Chem. Eng. 2019;7:102800. doi: 10.1016/j.jece.2018.11.044. [DOI] [Google Scholar]
- 8.Chen Y.-Y., Gopala L., Bheemanaboina R.R.Y., Liu H.-B., Cheng Y., Geng R.-X., Zhou C.-H. Novel naphthalimide aminothiazoles as potential multitargeting antimicrobial agents. ACS Med. Chem. Lett. 2017;8:1331–1335. doi: 10.1021/acsmedchemlett.7b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash A., Malhotra R. Co (II), Ni (II), Cu (II) and Zn (II) complexes of aminothiazole-derived Schiff base ligands: Synthesis, characterization, antibacterial and cytotoxicity evaluation, bovine serum albumin binding and density functional theory studies. Appl. Organomet. Chem. 2018;32:e4098. doi: 10.1002/aoc.4098. [DOI] [Google Scholar]
- 10.Cordeiro Y., Ferreira N.C. New approaches for the selection and evaluation of anti-prion organic compounds. Mini Rev. Med. Chem. 2015;15:84–92. doi: 10.2174/1389557515666150227111629. [DOI] [PubMed] [Google Scholar]
- 11.Patil R., Chavan J., Beldar A. Synthesis of aminothiazoles: Polymer-supported approaches. RSC Adv. 2017;7:23765–23778. doi: 10.1039/C7RA00790F. [DOI] [Google Scholar]
- 12.Alaraidh I.A., Okla M.K., Alamri S.A., Abdullah A., Soufan W.H., Allam A.A., Fouda M.M., Gaffer H.E. Synthesis of Bis-(2-thiazolyl) amine Analogues and Evaluation of Their Antibacterial, Antioxidant and Cytotoxic Activities. ChemistrySelect. 2019;4:11726–11734. doi: 10.1002/slct.201902272. [DOI] [Google Scholar]
- 13.Gaffer H.E., Fouda M.M., Khalifa M.E. Synthesis of some novel 2-amino-5-arylazothiazole disperse dyes for dyeing polyester fabrics and their antimicrobial activity. Molecules. 2016;21:122. doi: 10.3390/molecules21010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha S., Doble M., Manju S. Design, synthesis and identification of novel substituted 2-amino thiazole analogues as potential anti-inflammatory agents targeting 5-lipoxygenase. Eur. J. Med. Chem. 2018;158:34–50. doi: 10.1016/j.ejmech.2018.08.098. [DOI] [PubMed] [Google Scholar]
- 15.Vogt D., Weber J., Ihlefeld K., Brüggerhoff A., Proschak E., Stark H. Design, synthesis and evaluation of 2-aminothiazole derivatives as sphingosine kinase inhibitors. Bioorg. Med. Chem. 2014;22:5354–5367. doi: 10.1016/j.bmc.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 16.De Logu A., Saddi M., Cardia M.C., Borgna R., Sanna C., Saddi B., Maccioni E. In vitro activity of 2-cyclohexylidenhydrazo-4-phenyl-thiazole compared with those of amphotericin B and fluconazole against clinical isolates of Candida spp. and fluconazole-resistant Candida albicans. J. Antimicrob. Chemother. 2005;55:692–698. doi: 10.1093/jac/dki084. [DOI] [PubMed] [Google Scholar]
- 17.Ran K., Gao C., Deng H., Lei Q., You X., Wang N., Shi Y., Liu Z., Wei W., Peng C. Identification of novel 2-aminothiazole conjugated nitrofuran as antitubercular and antibacterial agents. Bioorg. Med. Chem. Lett. 2016;26:3669–3674. doi: 10.1016/j.bmcl.2016.05.088. [DOI] [PubMed] [Google Scholar]
- 18.Al-Balas Q., Anthony N.G., Al-Jaidi B., Alnimr A., Abbott G., Brown A.K., Taylor R.C., Besra G.S., McHugh T.D., Gillespie S.H. Identification of 2-Aminothiazole-4-Carboxylate Derivatives Active against Mycobacterium tuberculosis H37Rv and the β-Ketoacyl-ACP Synthase mtFabH. PLoS ONE. 2009;4:e5617. doi: 10.1371/journal.pone.0005617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridhar S., Pandeya S., De Clercq E. Synthesis and anti-HIV activity of some isatin derivatives. Boll. Chim. Farm. 2001;140:302. [PubMed] [Google Scholar]
- 20.Kabra V., Mitharwal S., Singh S. Synthesis and insecticidal activity of novel dithiophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2009;184:2431–2442. doi: 10.1080/10426500802487748. [DOI] [Google Scholar]
- 21.Jadav S.S., Badavath V.N., Ganesan R., Ganta N.M., Besson D., Jayaprakash V. Biological evaluation of 2-aminothiazole hybrid as antimalarial and antitrypanosomal agents: Design and synthesis. Anti-Infect. Agents. 2020;18:101–108. doi: 10.2174/2211352516666181016122537. [DOI] [Google Scholar]
- 22.Panico A.M., Geronikaki A., Mgonzo R., Cardile V., Gentile B., Doytchinova I. Aminothiazole derivatives with antidegenerative activity on cartilage. Bioorg. Med. Chem. 2003;11:2983–2989. doi: 10.1016/S0968-0896(03)00149-4. [DOI] [PubMed] [Google Scholar]
- 23.Khan E., Khan A., Gul Z., Ullah F., Tahir M.N., Khalid M., Asif H.M., Asim S., Braga A.A.C. Molecular salts of terephthalic acids with 2-aminopyridine and 2-aminothiazole derivatives as potential antioxidant agents; Base-Acid-Base type architectures. J. Mol. Struct. 2020;1200:127126. doi: 10.1016/j.molstruc.2019.127126. [DOI] [Google Scholar]
- 24.Gouda M.A., Sherif Y.E.-S., Elsherbini M.S. Synthesis, Anti-Inflammatory, and Analgesic Evaluation of Some 2-Amino-5-Selenothiazoles. Phosphorus Sulfur Silicon Relat. Elem. 2014;189:1633–1643. doi: 10.1080/10426507.2014.884091. [DOI] [Google Scholar]
- 25.Narender M., Reddy M.S., Sridhar R., Nageswar Y., Rao K.R. Aqueous phase synthesis of thiazoles and aminothiazoles in the presence of β-cyclodextrin. Tetrahedron Lett. 2005;46:5953–5955. doi: 10.1016/j.tetlet.2005.06.130. [DOI] [Google Scholar]
- 26.Pandya D.H., Sharma J.A., Jalani H.B., Pandya A.N., Sudarsanam V., Kachler S., Klotz K.N., Vasu K.K. Novel thiazole–thiophene conjugates as adenosine receptor antagonists: Synthesis, biological evaluation and docking studies. Bioorg. Med. Chem. Lett. 2015;25:1306–1309. doi: 10.1016/j.bmcl.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Raut D.G., Kadu V.D., Sonawane V.D., Bhosale R.B. Synthesis of Thiazole Scaffolds by Novel Method and Their In Vitro Anthelmintic Activity against Indian Adult Earthworm. Eur. J. Biomed. Pharm. Sci. 2015;2:922–931. [Google Scholar]
- 28.Vijayaraghavalu S., Peetla C., Lu S., Labhasetwar V. Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol. Pharm. 2012;9:2730–2742. doi: 10.1021/mp300281t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai C.-Y., Kapoor M., Huang Y.-P., Lin H.-H., Liang Y.-C., Lin Y.-L., Huang S.-C., Liao W.-N., Chen J.-K., Huang J.-S. Synthesis and evaluation of aminothiazole-paeonol derivatives as potential anticancer agents. Molecules. 2016;21:145. doi: 10.3390/molecules21020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nong W., Zhao A., Wei J., Lin X., Wang L., Lin C. Synthesis and biological evaluation of a new series of cinnamic acid amide derivatives as potent haemostatic agents containing a 2-aminothiazole substructure. Bioorg. Med. Chem. Lett. 2017;27:4506–4511. doi: 10.1016/j.bmcl.2017.07.058. [DOI] [PubMed] [Google Scholar]
- 31.Hamed F.I., Mohamed A.A., Abouzied A.S. The Uses of 2-Amino-4-Phenylthiazole in the Synthesis of Coumarin, Pyran, Pyridine and Thiazole Derivatives with Antitumor Activities. Open Access Libr. J. 2017;4:e3526. doi: 10.4236/oalib.1103526. [DOI] [Google Scholar]
- 32.Liu W., Zhou J., Qi F., Bensdorf K., Li Z., Zhang H., Qian H., Huang W., Cai X., Cao P. Synthesis and biological activities of 2-amino-thiazole-5-carboxylic acid phenylamide derivatives. Arch. Pharm. 2011;344:451–458. doi: 10.1002/ardp.201000281. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y., He X., Xiong Y., Chai X., Chen H. Synthesis of 2-aminoxazole-5-carbamides and 2-aminothiazole-5-carbamides as potent inhibitors of CML. Monatsh. Chem. Chem. Mon. 2015;146:997–1003. doi: 10.1007/s00706-014-1403-6. [DOI] [Google Scholar]
- 34.Ha S., Oh J., Jang J.M., Kim D.K., Ham S.W. Synthesis and Biological Evaluation of 2-Aminothiazole Derivative Having Anticancer Activity as a KPNB1 Inhibitor. Bull. Korean Chem. Soc. 2016;37:1743–1744. doi: 10.1002/bkcs.10968. [DOI] [Google Scholar]
- 35.Zhang W.-T., Ruan J.-L., Wu P.-F., Jiang F.-C., Zhang L.N., Fang W., Chen X.-L., Wang Y., Cao B.-S., Chen G.-Y. Design, synthesis, and cytoprotective effect of 2-aminothiazole analogues as potent poly (ADP-ribose) polymerase-1 inhibitors. J. Med. Chem. 2009;52:718–725. doi: 10.1021/jm800902t. [DOI] [PubMed] [Google Scholar]
- 36.Reddy V.M., Reddy K.R. Synthesis and antimicrobial activity of some novel 4-(1H-benz [d] imidazol-2yl)-1,3-thiazol-2-amines. Chem. Pharm. Bull. 2010;58:953–956. doi: 10.1248/cpb.58.953. [DOI] [PubMed] [Google Scholar]
- 37.Nofal Z.M., Soliman E.A., Abd El-Karim S.S., El-Zahar M.I., Srour A.M., Sethumadhavan S., Maher T.J. Synthesis of some new benzimidazole–thiazole derivatives as anticancer agents. J. Heterocycl. Chem. 2014;51:1797–1806. doi: 10.1002/jhet.1886. [DOI] [Google Scholar]
- 38.Park J.-H., El-Gamal M.I., Lee Y.S., Oh C.-H. New imidazo[2,1-b]thiazole derivatives: Synthesis, in vitro anticancer evaluation, and in silico studies. Eur. J. Med. Chem. 2011;46:5769–5777. doi: 10.1016/j.ejmech.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Bashandy M.S. 1-(4-(Pyrrolidin-1-ylsulfonyl)phenyl) ethanone in Heterocyclic Synthesis: Synthesis, Molecular Docking and Anti-Human Liver Cancer Evaluation of Novel Sulfonamides Incorporating Thiazole, Imidazo[1,2-a]pyridine, Imidazo[2,1-c] [1,2,4]triazole, Imidazo[2,1-b]thiazole, 1,3,4-Thiadiazine and 1,4-Thiazine Moieties. Int. J. Org. Chem. 2015;5:166–190. [Google Scholar]
- 40.Francini C.M., Fallacara A.L., Artusi R., Mennuni L., Calgani A., Angelucci A., Schenone S., Botta M. Identification of aminoimidazole and aminothiazole derivatives as Src family kinase inhibitors. ChemMedChem. 2015;10:2027–2041. doi: 10.1002/cmdc.201500428. [DOI] [PubMed] [Google Scholar]
- 41.Dudkin V.Y., Rickert K., Kreatsoulas C., Wang C., Arrington K.L., Fraley M.E., Hartman G.D., Yan Y., Ikuta M., Stirdivant S.M. Pyridyl aminothiazoles as potent inhibitors of Chk1 with slow dissociation rates. Bioorg. Med. Chem. Lett. 2012;22:2609–2612. doi: 10.1016/j.bmcl.2012.01.110. [DOI] [PubMed] [Google Scholar]
- 42.Konyar D., Erdas O., Alpaslan F.N., Buyukbingol E. An application of CIFAP for predicting the binding affinity of Chk1 inhibitors derived from 2-aminothiazole-4-carboxamide. J. Mol. Recognit. 2017;30:e2642. doi: 10.1002/jmr.2642. [DOI] [PubMed] [Google Scholar]
- 43.Balupuri A., Balasubramanian P., Gadhe C., Cho S. Docking-based 3D-QSAR study of pyridyl aminothiazole derivatives as checkpoint kinase 1 inhibitors. SAR QSAR Environ. Res. 2014;25:651–671. doi: 10.1080/1062936X.2014.923040. [DOI] [PubMed] [Google Scholar]
- 44.Lee Y.-S.E., Chuang S.-H., Huang L.Y., Lai C.-L., Lin Y.-H., Yang J.-Y., Liu C.-W., Yang S.-C., Lin H.-S., Chang C.-C. Discovery of 4-aryl-N-arylcarbonyl-2-aminothiazoles as Hec1/Nek2 inhibitors. Part I: Optimization of in vitro potencies and pharmacokinetic properties. J. Med. Chem. 2014;57:4098–4110. doi: 10.1021/jm401990s. [DOI] [PubMed] [Google Scholar]
- 45.Titus S., Sreejalekshmi K.G. Enriching biologically relevant chemical space around 2-aminothiazole template for anticancer drug development. Med. Chem. Res. 2018;27:23–36. doi: 10.1007/s00044-017-2039-y. [DOI] [Google Scholar]
- 46.Andersen C.B., Wan Y., Chang J.W., Riggs B., Lee C., Liu Y., Sessa F., Villa F., Kwiatkowski N., Suzuki M. Discovery of selective aminothiazole aurora kinase inhibitors. ACS Chem. Biol. 2008;3:180–192. doi: 10.1021/cb700200w. [DOI] [PubMed] [Google Scholar]
- 47.Qin J., Xi L., Du J., Liu H., Yao X. QSAR studies on aminothiazole derivatives as aurora A kinase inhibitors. Chem. Biol. Drug Des. 2010;76:527–537. doi: 10.1111/j.1747-0285.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 48.Ewida M.A., Abou El Ella D.A., Lasheen D.S., Ewida H.A., El-Gazzar Y.I., El-Subbagh H.I. Thiazolo[4,5-d]pyridazine analogues as a new class of dihydrofolate reductase (DHFR) inhibitors: Synthesis, biological evaluation and molecular modeling study. Bioorg. Chem. 2017;74:228–237. doi: 10.1016/j.bioorg.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Kalpana K., Srinivasan M., Menon V.P. Antioxidant potential of aminothiazole derivative and its protective effect on H2O2-induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol. Cell. Biochem. 2008;314:95–103. doi: 10.1007/s11010-008-9769-6. [DOI] [PubMed] [Google Scholar]
- 50.Kalpana K., Vishwanathan P., Thayalan K., Menon V.P. Protective effect of dendrodoine analog, an aminothiazole derivative against X-radiation induced hepatocellular damage in mice. Environ. Toxicol. Pharmacol. 2012;34:832–840. doi: 10.1016/j.etap.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 51.De S., Adhikari S., Devasagayam T.P. Cardioprotective effects of an aminothiazole compound on isoproterenol-induced myocardial injury in mice. Cell Biochem. Biophys. 2013;67:287–295. doi: 10.1007/s12013-011-9296-z. [DOI] [PubMed] [Google Scholar]
- 52.Shafeeulla M., Krishnamurthy G., Bhojynaik H.S., Manjuraj T. Synthesis, cytotoxicity and molecular docking study of complexes containing thiazole moiety. J. Turk. Chem. Soc. Sect. A Chem. 2017;4:787–810. [Google Scholar]
- 53.Khalifa M.E., Mohamed M.A.-H., Alshehri N.H. Synthesis of novel 2-amino-5-arylazothiazol derivatives and their biological impacts: Assessment of toxicity and antioxidant enzymes activities. Maced. J. Chem. Chem. Eng. 2015;34:309–319. doi: 10.20450/mjcce.2015.689. [DOI] [Google Scholar]
- 54.Yamalı C., Gülcan H.O., Kahya B., Çobanoğlu S., Şüküroğlu M.K., Doğruer D.S. Synthesis of some 3(2H)-pyridazinone and 1(2H)-phthalazinone derivatives incorporating aminothiazole moiety and investigation of their antioxidant, acetylcholinesterase, and butyrylcholinesterase inhibitory activities. Med. Chem. Res. 2015;24:1210–1217. doi: 10.1007/s00044-014-1205-8. [DOI] [Google Scholar]
- 55.Shreenivas M., Swamy B.K., Srinivasa G., Sherigara B. Synthesis and antibacterial evaluation of some novel aminothiazole derivatives. Pharma Chem. 2011;3:156–161. [Google Scholar]
- 56.Rawat B.S., Shukla S.K. Synthesis and evaluation of some new thiazole/oxazole derivatives for their biological activities. World J. Pharm. Pharm. Sci. 2016;5:1473–1482. [Google Scholar]
- 57.Prajapati A., Modi V.P. Synthesis and biological evaluation of some substituted amino thiazole derivatives. J. Chil. Chem. Soc. 2010;55:240–243. doi: 10.4067/S0717-97072010000200021. [DOI] [Google Scholar]
- 58.Yadlapalli R.K., Chourasia O., Jogi M.P., Podile A.R., Perali R.S. Design, synthesis and in vitro antimicrobial activity of novel phenylbenzamido-aminothiazole-based azasterol mimics. Med. Chem. Res. 2013;22:2975–2983. doi: 10.1007/s00044-012-0314-5. [DOI] [Google Scholar]
- 59.Özbek O., Usta N.C., Gürdere M.B., Aslan O.N., Budak Y., Ceylan M. Synthesis and antibacterial screening of novel 2-(4-(aryl) thiazol-2-yl)-3a,4,7,7a-tetrahydro-1H-4,7-ethanoisoindole-1,3(2H)-dione derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2017;192:1153–1157. doi: 10.1080/10426507.2017.1354209. [DOI] [Google Scholar]
- 60.Nikalje A.P.G., Tiwari S.V., Sarkate A.P., Karnik K.S. Imidazole-thiazole coupled derivatives as novel lanosterol 14-α demethylase inhibitors: Ionic liquid mediated synthesis, biological evaluation and molecular docking study. Med. Chem. Res. 2018;27:592–606. doi: 10.1007/s00044-017-2085-5. [DOI] [Google Scholar]
- 61.Samadhiya P., Sharma R., Srivastava S.K., Srivastava S.D. Synthesis of 2-oxo-azetidine derivatives of 2-amino thiazole and their biological activity. J. Serb. Chem. Soc. 2012;77:599–605. doi: 10.2298/JSC110616002S. [DOI] [Google Scholar]
- 62.Sharshira E., Hamada N. Synthesis, characterization and antimicrobial activities of some thiazole derivatives. Am. J. Org. Chem. 2012;2:69–73. doi: 10.5923/j.ajoc.20120203.06. [DOI] [Google Scholar]
- 63.Mocelo-Castell R., Villanueva-Novelo C., Cáceres-Castillo D., Carballo R.M., Quijano-Quiñones R.F., Quesadas-Rojas M., Cantillo-Ciau Z., Cedillo-Rivera R., Moo-Puc R.E., Moujir L.M. 2-Amino-4-arylthiazole derivatives as anti-giardial agents: Synthesis, biological evaluation and QSAR studies. Open Chem. 2015;13:1127–1136. doi: 10.1515/chem-2015-0127. [DOI] [Google Scholar]
- 64.Bhuiyan M., Rahman A. Synthesis and antimicrobial evaluation of some thiazole derivatives. J. Sci. Res. 2011;3:111. doi: 10.3329/jsr.v3i1.5419. [DOI] [Google Scholar]
- 65.Mjambili F., Njoroge M., Naran K., De Kock C., Smith P.J., Mizrahi V., Warner D., Chibale K. Synthesis and biological evaluation of 2-aminothiazole derivatives as antimycobacterial and antiplasmodial agents. Bioorg. Med. Chem. Lett. 2014;24:560–564. doi: 10.1016/j.bmcl.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 66.Kesicki E.A., Bailey M.A., Ovechkina Y., Early J.V., Alling T., Bowman J., Zuniga E.S., Dalai S., Kumar N., Masquelin T. Synthesis and evaluation of the 2-aminothiazoles as anti-tubercular agents. PLoS ONE. 2016;11:e0155209. doi: 10.1371/journal.pone.0155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vukovic N., Sukdolak S., Solujic S., Milosevic T. Synthesis and Antimicrobial Evaluation of Some Novel 2-Aminothiazole Derivatives of 4-Hydroxy-chromene-2-one. Arch. Pharm. Int. J. Pharm. Med. Chem. 2008;341:491–496. doi: 10.1002/ardp.200700215. [DOI] [PubMed] [Google Scholar]
- 68.Tay F., Erkan C., Sariozlu N.Y., Ergene E., Demirayak S. Synthesis, antimicrobial and anticancer activities of some naphthylthiazolylamine derivatives. Biomed. Res. 2017;28:2696–2703. [Google Scholar]
- 69.Cheng Y., Avula S.R., Gao W.-W., Addla D., Tangadanchu V.K.R., Zhang L., Lin J.-M., Zhou C.-H. Multi-targeting exploration of new 2-aminothiazolyl quinolones: Synthesis, antimicrobial evaluation, interaction with DNA, combination with topoisomerase IV and penetrability into cells. Eur. J. Med. Chem. 2016;124:935–945. doi: 10.1016/j.ejmech.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 70.Garza I., Wallace M.J., Fernando D., Singh A., Lee R.E., Gerding J.S., Franklin C., Yendapally R. Synthesis and Evaluation of Thiazolidine Amide and N-Thiazolyl Amide Fluoroquinolone Derivatives. Arch. Pharm. 2017;350:e201700029. doi: 10.1002/ardp.201700029. [DOI] [PubMed] [Google Scholar]
- 71.Madhura V., Revankar H.M., Kulkarni M.V. A new route for the synthesis of 4-arylacetamido-2-aminothiazoles and their biological evaluation. Z. Naturforsch. B. 2015;70:483–489. [Google Scholar]
- 72.Chandak N., Kumar P., Kaushik P., Varshney P., Sharma C., Kaushik D., Jain S., Aneja K.R., Sharma P.K. Dual evaluation of some novel 2-amino-substituted coumarinylthiazoles as anti-inflammatory–antimicrobial agents and their docking studies with COX-1/COX-2 active sites. J. Enzym. Inhib. Med. Chem. 2014;29:476–484. doi: 10.3109/14756366.2013.805755. [DOI] [PubMed] [Google Scholar]
- 73.Hussein A.H.M., Khames A.A., El-Adasy A.-B.A., Atalla A.A., Abdel-Rady M., Hassan M.I., Nemr M.T., Elshaier Y.A. Design, synthesis and biological evaluation of new 2-aminothiazole scaffolds as phosphodiesterase type 5 regulators and COX-1/COX-2 inhibitors. RSC Adv. 2020;10:29723–29736. doi: 10.1039/D0RA05561A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maghraby M.T.-E., Abou-Ghadir O.M., Abdel-Moty S.G., Ali A.Y., Salem O.I. Novel class of benzimidazole-thiazole hybrids: The privileged scaffolds of potent anti-inflammatory activity with dual inhibition of cyclooxygenase and 15-lipoxygenase enzymes. Bioorg. Med. Chem. 2020:115403. doi: 10.1016/j.bmc.2020.115403. [DOI] [PubMed] [Google Scholar]
- 75.Smelcerovic A., Zivkovic A., Ilic B.S., Kolarevic A., Hofmann B., Steinhilber D., Stark H. 4-(4-Chlorophenyl) thiazol-2-amines as pioneers of potential neurodegenerative therapeutics with anti-inflammatory properties based on dual DNase I and 5-LO inhibition. Bioorg. Chem. 2020;95:103528. doi: 10.1016/j.bioorg.2019.103528. [DOI] [PubMed] [Google Scholar]