Abstract
While Lck has been widely recognized to play a pivotal role in the initiation of the T cell receptor (TCR) signaling pathway, an understanding of the precise regulation of Lck in T cells upon TCR activation remains elusive. Investigation of protein-protein interaction (PPI) using proximity labeling techniques such as TurboID has the potential to provide valuable molecular insights into Lck regulatory networks. By expressing Lck-TurboID in Jurkat T cells, we have uncovered a dynamic, short-range Lck protein interaction network upon 30 minutes of TCR stimulation. In this novel application of TurboID, we detected 27 early signaling-induced Lck-proximal interactors in living T cells, including known and novel Lck interactors, validating the discovery power of this tool. Our results revealed previously unappreciated Lck PPI which may be associated with cytoskeletal rearrangement, ubiquitination of TCR signaling proteins, activation of the MAPK cascade, coalescence of the LAT signalosome, and formation of the immunological synapse. In this study, we demonstrated for the first time in immune cells and for the kinase Lck that TurboID can be utilized to unveil PPI dynamics in living cells at a time scale consistent with early TCR signaling. Data are available via ProteomeXchange with identifier PXD020759.
Keywords: TurboID, Lck, proximity labeling, interactome, T cell receptor
Graphical Abstract

Introduction
The stimulation of TCR upon engagement with antigenic ligands activates a multifaceted intracellular signal transduction network in T cells. Initiation of signaling propagation is critically fine-tuned by the activity of the non-receptor Src family protein tyrosine kinase Lck. Activated TCR recruits Lck to phosphorylate tyrosine residues within the immunoreceptor tyrosine-based activation motif (ITAM) of CD3 and ζ chains, which initiates the formation of various signaling complexes that are driven by PPI 1. These complexes subsequently trigger a signaling cascade that regulates T cell proliferation, development, and homeostasis. Due to its pivotal role in establishing the threshold for initiation of T cell signaling, dysregulation of Lck is often implicated in various immune diseases 2, 3. Despite extensive studies, full characterization of TCR-inducible interacting partners of Lck remains incomplete 4.
Mass spectrometry-based proximity labeling techniques, such as APEX, BioID, and TurboID, are increasingly utilized for comprehensive identification of PPI networks 5. Contrary to conventional co-immunoprecipitation approaches, proximity labeling allows for the study of PPI in living cells with detection of transient interactions occurring within an estimated short-range labeling radius of 10–20 nm 6, 7. In short timescale proximity labeling experiments, APEX and TurboID are highly-efficient as sufficient labeling can be achieved within minutes rather than the several hours that is required by BioID to achieve a similar degree of labeling 8. Following APEX proximity labeling, the usage of anti-biotin antibodies to enrich for biotinylated peptides allows for specific determination of interactors through the detection of biotinylation sites 9, 10. Despite its high efficiency and specificity, APEX presents technical challenges when used in the study of T cells due to its use of hydrogen peroxide, which activates lymphocyte functions promiscuously and may introduce potential toxicity 5. The recently developed TurboID method utilizes a mutant biotin ligase, which exhibits enhanced labeling efficiency via 14 mutations in its reactive biotin-5’-AMP binding motif, in combination with the subsequent affinity purification of biotinylated proteins by streptavidin 11, avoiding the technical challenge experienced using APEX. When compared alongside the previously mentioned methodologies TurboID is better-suited for the temporal resolution required for TCR activation interactome studies as TCR activation results in rapid response of TCR-proximal proteins. Furthermore, as Lck activation is a receptor-proximal signaling event, its regulation is a key determinant in establishing an appropriate threshold for the immune response 1, 4. Given that TCR stimulation drives nucleation of various PPI initiated by Lck, functional insights can be elucidated by the examination of TCR-induced Lck-interacting partners in T cells.
Here, we utilized a Lck-TurboID proximity labeling fusion protein to quantitatively characterize the short-range interactome of Lck upon TCR stimulation for 30 minutes in living Jurkat T cells. Previous proteomic interactome studies in T cells have encountered several challenges, such as non-physiological stimulation using pervanadate 12 and long labeling times 13. Moreover, many Lck interactors were previously characterized in cell lysates, potentially resulting in artifactual interactions. While spectral counting is often used in many proximity labeling proteomic studies, we exploited the increased accuracy and quantitative sensitivity of intensity-based label free quantitation 14–19 to discern Lck interactome dynamics, which is critical in the investigation of low-abundance or transient interactors. Using protein intensity-based quantitation of Lck-TurboID, we identified 27 TCR stimulation-induced Lck-proximal interactors. While some of the proteins identified in this study are canonical interactors of Lck, a majority of these proteins were not previously characterized as proximal interactors. Further inspection of these putative interactors revealed potentially novel regulatory mechanisms in the modulation of TCR stimulation-induced signaling pathways, including cytoskeletal rearrangement, ubiquitination of TCR signaling proteins, activation of the MAPK cascade, coalescence of the LAT signalosome, and the formation of the immunological synapse. Collectively, these results validate the utility and discovery power of Lck-TurboID.
Experimental Section
Plasmid construction
Guide RNAs targeting an exon region of Lck were designed and cloned into pSpCas9(BB)-2A-GFP vector or PX458 (Addgene, 48138) for targeted deletion of Lck using CRISPR-Cas9 20. Lck and TurboID cDNA were amplified by PCR from pCMV-SPORT6-Lck (antibodies-online Inc, ABIN3804434) and V5-TurboID-NES_pCDNA3 (Addgene, 107169), respectively, using Phusion polymerase and 10 μM forward and reverse primers. PCR products were verified by gel electrophoresis and purified using Monarch PCR & DNA Cleanup Kit (New England BioLabs, T1030). Purified PCR products were digested accordingly and ligated into pEF6 mammalian expression vector (Thermo Fisher Scientific, V96120) to form pEF6-Lck-TurboID. Plasmid sequences were verified by DNA sequencing. All oligonucleotides used in this work were purchased from Sigma-Aldrich and are listed in Table S1. Plasmids PX458-Lck (#159430) and pEF6-Lck-TurboID (#159433) are deposited to Addgene.
Cell culture and generation of stable cell line
Jurkat T cells (clone E6–1) were obtained from the American Tissue Culture Collection (ATCC) and cultured in RPMI 1640 containing 2.05 mM L-glutamine supplemented with 100 U/mL penicillin G, 100 μg/mL streptomycin, 2 mM L-Glutamine (HyClone) and 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich), in a humidified incubator with 5% CO2 at 37°C. Lck-deficient Jurkat cells were generated using PX458-Lck as indicated above via CRISPR/Cas9 targeted gene knockout 20. Jurkat cells were transfected with the construct via electroporation (260 V, 1250 μF) and allowed to recover in antibiotic-free RPMI media for 48 hours before single cell fluorescence-activated cell sorting (FACS). Expanded single clones were screened for the loss of Lck protein expression by Western blot and subsequently sequenced to confirm that the genomic DNA targeted by CRISPR/Cas9 resulted in the disruption of the coding frame to generate Lck-deficient stable clone (J.Lck-) (Figure S1). J.Lck- was reconstituted with pEF-Lck-TurboID or pEF-Lck-WT via electroporation followed by limited dilution with selection in Blasticidin (10 mg/mL). Stable clones were verified by accessing Lck expression via Western blotting. J.Lck.WT was generated similarly using pEF6-Lck-WT and used as a control. To reduce background biotinylation, J.Lck.TurboID and J.Lck.WT cells were grown in biotin-free RPMI (MyBioSource, MBS653376) supplemented with 100 U/mL penicillin G, 100 μg/mL streptomycin, 2 mM L-Glutamine (HyClone), 2.5% (v/v) heat-inactivated fetal bovine serum (Sigma-Aldrich) and 7.5% (v/v) heat-inactivated dialyzed fetal bovine serum (Hyclone) for 2 weeks prior to proximity labeling experiments. Cells used in this work were confirmed mycoplasma-free using the Universal Mycoplasma Detection Kit from ATCC according to manufacturer’s instruction and were authenticated by ATCC using Short Tandem Repeat (STR) profiling.
Western blotting
Cells were lysed with sample loading buffer (2% w/v sodium dodecyl sulfate (SDS), 62.5 mM Tris-HCl, 10% v/v glycerol, 2.5% v/v 2-mercaptoethanol, 0.005% w/v bromophenol blue) to obtain total cell lysate samples, which were separated by SDS-PAGE using ClearPAGE 4–20% polyacrylamide gradient gels (Expedeon) and transferred onto an Immobilon-FL PVDF membrane (EMD Millipore). Membranes were then blocked with Odyssey Blocking Buffer (Li-Cor) before incubation with primary antibodies for 1 hour at room temperature and subsequent incubation with IRDyes-conjugated secondary antibodies (Li-Cor) for 1 hour at room temperature. Mouse anti-GAPDH (clone GAPDH-71.1) and rabbit anti-GAPDH (G9545) were from Sigma-Aldrich. IRDye 800CW-conjugated streptavidin was from Li-Cor, while the anti-Lck (clone L22B1), anti-p44/42 MAPK (Erk1/2, clone 3A7), and anti-phospho-p44/42 MAPK (Erk1/2, Thr202/Tyr204, clone D13.14.4E) antibodies were from Cell Signaling Technologies. Blots were visualized using the Odyssey CLx Imaging System and quantified using ImageStudio software (Li-Cor).
TurboID proximity labeling and T cell receptor stimulation
Five replicates of 3×107 J.Lck.TurboID cells were washed with DPBS, resuspended in 6×107 cells/mL in DPBS, and allowed to rest for 20 minutes in 37°C. To initiate proximity labeling and TCR stimulation simultaneously, equal volumes of 2 mM D-biotin (Thermo Fisher Scientific, AAA1420703) dissolved in DPBS containing anti-TCR antibody (C305 IgM) were added to the replicates respectively to a final concentration of 1 mM biotin and 1 μg/mL of C305 over a course of 30 minutes at 37°C. Proximity labeling without the addition C305 antibody was included as a control samples without TCR stimulation. For pervanadate (PV) treatment was used as a positive control in immunoblots, wherein cells were incubated with 500 μM PV (prepared by mixing equal volume of 1 mM sodium orthovanadate and 1 mM hydrogen peroxide) for 10 minutes at 37°C.
Sample processing for proteomic analysis
Immediately after proximity labeling, cells were washed three times with 10 mL of DPBS, lysed in 1 mL of 1.2% SDS in DPBS, and boiled at 95°C for 5 minutes. Lysates were then sonicated at 30-W for 30 seconds and clarified via centrifugation at 20000×g for 10 minutes at 20°C. Protein concentration of the supernatant was determined by Pierce BCA Protein Assay (Thermo Fisher Scientific, 23225). Lysate was diluted 6-fold in DPBS and transferred to 100 μL of streptavidin agarose beads (Thermo Fisher Scientific, 20353) for a 3-hour incubation period on a rocking platform at room temperature. Beads were then centrifuged at 1800×g for 5 minutes at 20°C, washed once with 4 mL of 0.2% SDS in DPBS, twice with 10 mL of DPBS, and once with 10 mL of milliQ water. Beads were subsequently resuspended in 8 M urea in 20 mM HEPES (pH 8), reduced in 10 mM DTT for 15 minutes at 65°C, and alkylated in 20 mM iodoacetamide for 30 minutes at room temperature. Urea concentration in lysate was reduced to 2 M by dilution in 20 mM HEPES (pH 8) before addition of 2 μg of trypsin (Promega, V5113) at 37°C overnight for on-bead tryptic digestion. Digested peptides were collected and acidified to 0.15% TFA prior to desalting using Sep-Pak C18 Cartridge (Waters, WAT023590) and subsequent solvent evaporation using speedvac vacuum concentrator.
Liquid chromatography-mass spectrometry
Peptides were separated on a 15 cm (75 um ID) reversed phase analytical column packed in-house with XSelect CSH C18 2.5 μm resin (Waters) using an UltiMate 3000 RSLCnano system (ThermoFisher Scientific), at a flow rate of 300 nL/min. Peptides were eluted with a 65-minute gradient from 5% to 30% Buffer B, followed by a 5-minute gradient from 30% to 95% Buffer B, in a total method duration of 90 minutes (Buffer A = 0.1% [v/v] formic acid, 0.5% [v/v] acetonitrile, 99.4% [v/v] water; Buffer B = 0.1% [v/v] formic acid, 99.9% [v/v] acetonitrile). Data was acquired on an Orbitrap Eclipse Tribrid mass spectrometer (Thermo Fisher Scientific) by applying 2.2 kV of electrospray voltage in positive ion mode with advanced peak determination. MS scans with a 375–1400 m/z scan range for precursor ions of charge states 2–5 were acquired on the Orbitrap detector in profile mode at a resolution of 120,000, maximum injection time of 50 milliseconds, automatic gain control (AGC) of 800,000, and a dynamic exclusion time of 20 seconds in a data-dependent mode with a 2-second cycle time. Up to top 9 precursor ions were fragmented by higher-energy collision dissociation (30% energy) and isolated within a mass isolation window of 0.7 m/z. MS/MS scans were collected in centroid mode on the ion trap detector with a scan range of 200–1300 m/z, a dynamic maximum injection time of 12 or 35 milliseconds, a dynamic AGC target of 10,000 or 20,000, depending on the precursor ions intensity.
Database Search Parameters and Acceptance Criteria for Identifications
Raw files were processed in MaxQuant 21 version 1.6.10.43 using the integrated peptide search engine Andromeda 22. MS/MS spectra were searched against a human UniProt database (Homo sapiens, last modified 12/01/2019) comprised of 74,811 forward protein sequences. A false discovery rate (FDR) of 1% was set on peptide spectrum match (PSM) using a reverse decoy protein sequence database approach. Protein groups identification was also set at 1% FDR. The following parameters were used: precursor ion tolerance of 3 ppm, ITMS MS/MS fragment ion mass tolerance of 0.5 Da, trypsin enzyme cleavage specificity, a maximum of two possible missed cleavages, a minimum peptide length of 7 amino acids, maximum peptide mass of 4600 Da, fixed modification of carbamidomethylation on cysteine, variable modifications of oxidation on methionine and phosphorylation on serine, threonine and tyrosine, and a maximum of 5 modifications per peptide. “Match-between-runs” was enabled with a retention time window of 0.7 minutes. Protein intensity was quantified using LFQ with default parameters (minimum ratio count of 2, fast LFQ enabled, minimum of 3 neighbors, average of 6 neighbors, normalized) 19.
Data Analysis
ProteinGroups.txt (Table S2) from MaxQuant was imported into Perseus 23 version 1.6.10.43. Protein groups originating from reverse hits, only identified by site, and common or potential contaminants (such as keratin, ribosome-associated proteins) were removed prior to logarithmic transformation of the LFQ protein intensities. Only protein groups with at least 3 out of 5 replicates in which the protein intensities were quantified for each condition were analyzed. Missing protein intensity values were imputed from the normal distribution of each replicate (width 0.3; downshift 1.8) before p values were obtained using a two-sample unpaired student’s t-test. Subsequently, p values were corrected to q-values using permutation-based FDR multiple hypothesis testing correction. Protein groups with a q-value less than 0.15 were considered significant, and further binned into three categories: high stringency (q < 0.05), medium stringency (0.05 ≤ q < 0.10) or low stringency (0.10 ≤ q < 0.15). To further improve the confidence of significant hits, only protein groups that exceed a minimum threshold of 0.2 in |log2(fold change)| were further analyzed. A fold-change cutoff of 0.2 represents one standard deviation of the distribution of log2(fold change) across all proteins (Figure S2A), as previously described 24. Significantly changing proteins were used for Fisher’s exact test to determine the enriched Gene Ontology Biological Process categories confined within 1% FDR using the Benjamini-Hochberg correction. Protein motif analysis were generated using InterProScan 25. Heatmaps were generated using the webtool Morpheus (https://software.broadinstitute.org/morpheus). All other analyses and graphical plotting were performed in R or Microsoft Excel.
Results and Discussion
Validation of Lck-TurboID
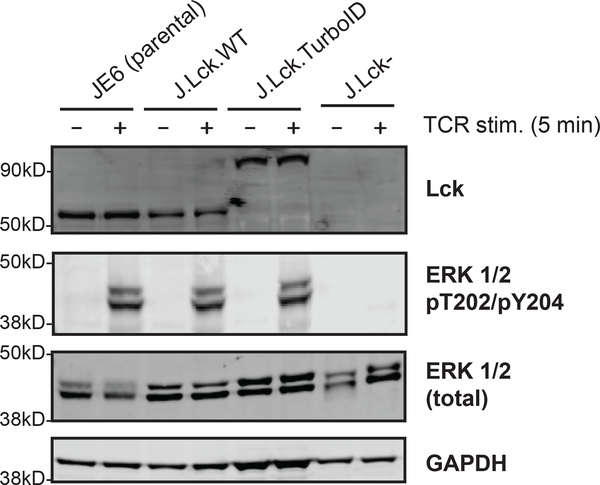
To systematically investigate the interactome of Lck in a TCR stimulation inducible manner, Lck-TurboID was transfected into Lck-deficient Jurkat T cells (J.Lck-) for proximity labeling (Figure 1). J.Lck- cells were generated using CRISPR-Cas9 targeted knockout to remove any competitive effects that may arise from the endogenous Lck (Figure S1). To minimize artifacts introduced by the fusion of a 35 kD TurboID biotin ligase to Lck, we first investigated the expression of Lck-TurboID compared to wild type Lck. Based on immunoblot data, Lck-TurboID (~93 kD) was expressed at similar levels as wild type Lck in the corresponding wild type control and parental JE6 (Figure 2). Since Lck is the critical initiating tyrosine kinase in T cells, dysfunction of Lck could lead to aberrant TCR signaling. To ensure that Lck remained functional in Lck-TurboID cells, we probed for the phosphorylation of tyrosine (pTyr) and threonine (pThr) on the TEY tripeptide motif of extracellular signal-regulated kinase 1 (ERK1) and extracellular signal-regulated kinase 2 (ERK2). Phosphorylation of these residues on ERK1 and ERK2 are signature readouts of TCR activation modulated by Lck activity 26. Using phospho-specific antibodies, we observed that phosphorylation of ERK-1/2 appeared to be equally robust in Lck-TurboID cells and the wild type controls (Figure 2), demonstrating that the function of Lck in TCR signaling remains intact in Lck-TurboID. These observations are consistent with published reports of Lck fusion with a C-terminal fluorescent biosensor construct demonstrating full functionality of Lck in a Förster resonance energy transfer experiment 27.
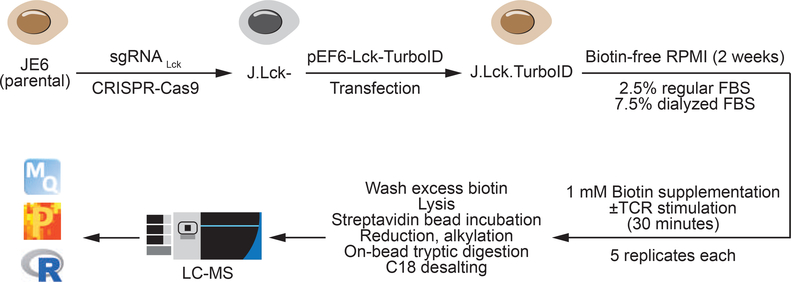
Figure 1: Design and workflow of Lck-TurboID.
J.Lck.TurboID cells were generated by the transfection of pEF6-Lck-TurboID into Lck-deficient Jurkat E6–1 T cells made using CRISPR-Cas9. J.Lck.TurboID cells were grown in biotin-free RPMI with 2.5% regular:7.5% dialyzed serum for 2 weeks before 1 mM biotin supplementation ± TCR stimulation for 30 minutes. Cells were washed, lysed, enriched via streptavidin and processed for mass spectrometry as described.
Figure 2: Validation of J.Lck.TurboID.
Lck, ERK 1/2, and ERK 1/2 pThr202/pTyr204 abundance were compared on an immunoblot for J.Lck.TurboID alongside JE6 parental, J.Lck.WT, and Lck-deficient controls. Cells were stimulated using anti-TCR C305 antibody for 5 minutes. GAPDH was used as a loading control.
Optimization of proximity labeling condition using dialyzed serum
We first set out to determine the optimal biotin incubation time for proximity labeling. To minimize background biotinylation and to maximize the signal-to-noise ratio, cells were grown in biotin-free RPMI, 10% FBS media for 2 weeks, after which, cells were supplemented with 1 mM D-biotin for 0, 10, 30, 60 and 120 minutes. Consistent with a previous report 11, immunoblot revealed that 10–30 minutes of biotin incubation time provided visibly sufficient biotinylated protein for enrichment and subsequent proteomic analysis (Figure 3A). However, the level of background biotinylation prior to the addition of exogenous biotin (0 minutes) was significantly higher in Lck-TurboID (Figure 3A) than was previously observed with TurboID expressed in the cytosol of HEK293T cells 11. While this previous study did reveal higher background biotinylation with TurboID targeted to specific organelles such as the mitochondria and the endoplasmic reticulum, cytosolic TurboID in Jurkat cells was not examined. This observed difference may be attributable cell-type specific differences in either biotin uptake or expression of biotin transporters 28, 29. We also observed increased background biotinylation in J.Lck cells reconstituted with Lck-TurboID when compared to Lck-BioID (Figure 3B). These observations are consistent with the previously established ~3–23-fold increased specific activity of TurboID compared to BioID 11, 30.
Figure 3: Optimization of J.Lck.TurboID.
(A) J.Lck.TurboID cells were incubated in 1 mM biotin for 0, 10, 30, 60, or 120 minutes in regular RPMI media alongside a JE6 control. Biotinylation was assessed by streptavidin via immunoblot, with GAPDH as loading control. (B) J.Lck.TurboID and J.Lck.BioID were compared directly examining the effects of biotin incubation time (10 minutes, 6 hours, 24 hours) and TCR stimulation (5 minutes). Biotinylation and Lck expression was assessed by immunoblot, with GAPDH acting as loading control.
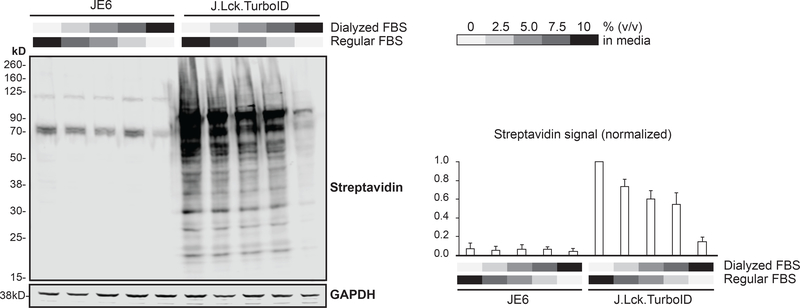
Since cells were already grown in biotin-free RPMI, 10% FBS to preemptively reduce background biotinylation, we hypothesized that the basal biotinylation primarily originated from free biotin present in FBS and that this issue could be mitigated by using dialyzed serum. The dialysis of serum removes small molecules, including biotin 31, and is commonly used in Stable Isotopic Labeling by Amino acids in Cell culture (SILAC) 32 experiments. To maintain the proportion of serum in the media at 10% (v/v), we titrated the ratio of dialyzed:regular serum and examined the levels of background biotinylation after 2 weeks. Increasing the ratio of dialyzed:regular serum in the media reduced the background biotinylation proportionally (Figure 4) while cells grown in the dialyzed serum alone exhibited reduced growth rates, similar to previous observations 30. Due to the need to obtain a large number of cells in the proteomic experiment, we chose to use a ratio of 7.5:2.5% in dialyzed:regular serum to minimize background biotinylation while maintaining regular cell growth.
Figure 4: Reduction of background biotinylation.
J.Lck.TurboID and JE6 cells were grown in biotin-free RPMI containing different ratios of dialyzed:regular fetal serum for 2 weeks. Background biotinylation without the addition of exogenous biotin was assessed by immunoblot. Representative image of three replicates is shown. Streptavidin signal normalized to GAPDH abundance was quantified and represented in a bar chart. (n=3)
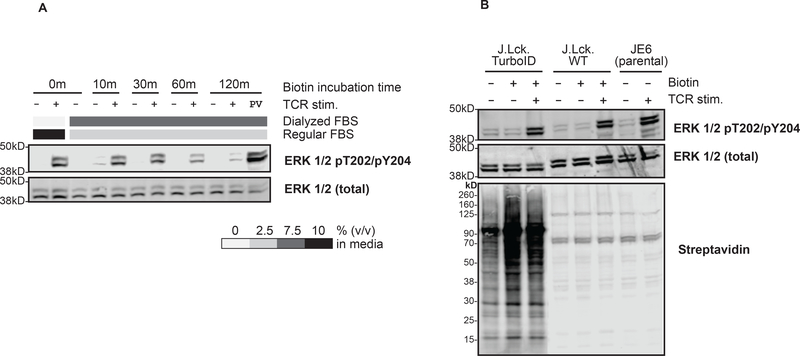
We next optimized biotin incubation time of TCR stimulated Lck-TurboID cells grown in the presence of dialyzed serum. The data revealed that 30 minutes of biotin incubation was optimal, as ERK-1/2 phosphorylation was unaffected by 30 minutes of biotin incubation in the presence of dialyzed serum and was similar to the parental control and its corresponding wild type (Figure 5A, B). In contrast, ERK-1/2 phosphorylation was noticeably inhibited when cells were pulsed with biotin for 60 or 120 minutes, suggesting attenuated TCR signaling at these incubation times (Figure 5A). Collectively, these results confirm that Lck-TurboID is a valid construct for proximity labeling of Lck with 30 minutes of biotin incubation for the investigation of Lck interactors in TCR-induced signaling.
Figure 5: Validation of J.Lck.TurboID signaling in dialyzed serum.
(A) J.Lck.TurboID cells were incubated in 0, 10, 30, 60, 120 minutes in 1 mM biotin in biotin-free RPMI containing different ratios of dialyzed:regular fetal serum with or without TCR stimulation (5 minutes) or pervanadate (PV) treatment as a positive control. ERK 1/2 pThr202/pTyr204 abundance was assessed via immunoblot, using the unphosphorylated ERK 1/2 as loading control. (B) J.Lck.TurboID, J.Lck.WT and JE6 cells were incubated in 30 minutes in 1 mM biotin in biotin-free RPMI media containing 2.5% regular serum and 7.5% dialyzed serum with or without TCR stimulation (5 minutes). ERK 1/2 pThr202/pTyr204 abundance was assessed via immunoblot, using the unphosphorylated ERK 1/2 as loading control. Biotinylation was assessed by streptavidin via immunoblot.
Assessment of proteomic data
To interrogate the interactome of Lck upon TCR stimulation, we performed 30 minutes of proximity labeling of Lck-TurboID, with 5 replicates each of stimulated (1 mM biotin + 1 ug/mLC305 anti-TCR antibody) and unstimulated control (1 mM biotin). Histograms of protein intensities derived from MaxQuant revealed a normal distribution of protein intensities in each of the replicates, allowing for parametric testing such as Pearson correlation and student’s t-test (Figure S3). Reproducibility between replicates, including all TCR signaling proteins as annotated by the Kyoto Encyclopedia of Genes and Genomes (KEGG), within the same condition was high. This was evidenced by Pearson correlations of at least 0.95 across all pairwise comparisons (Figure S4) and a median coefficient of variation of approximately 11% in both conditions (Figure S5). Protein intensities of each replicate for all KEGG TCR proteins identified in this work are represented in a heatmap (Figure S6).
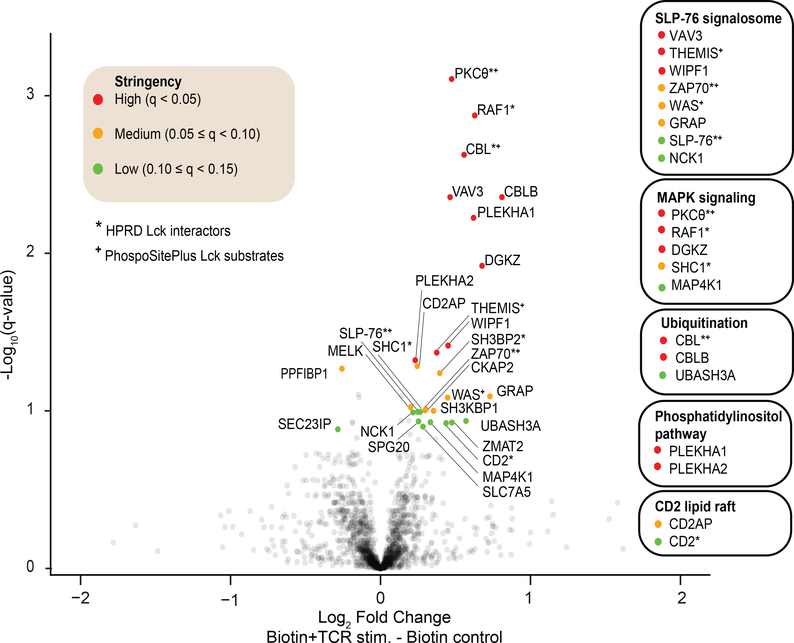
Overall, we identified 1,770 proteins in both TCR-stimulated and unstimulated Lck-TurboID cells (Table S3). Of these, we identified 29 proteins that displayed a significant change in abundance, with a minimum fold change of 0.2 and a maximum q-value of 0.15. We defined fold-change cutoff at 0.2 to represent one standard deviation of the distribution of log2(fold change) across all 1,770 proteins (Figure S2), as described previously 24. The overall narrow distribution of fold change was likely due to the short labeling time. These 29 proteins were further binned based on q-value into high stringency (q < 0.05; n=10), medium stringency (0.05 ≤ q < 0.10; n=9) and low stringency (0.10 ≤ q < 0.15; n=10) groups (Figure 6). While the majority of the significantly changing proteins (27 out of 29) had an increased fold change in the TCR-stimulated condition, this result was not attributable to artifactual or systematic increase in the TCR-stimulated samples as there was an approximately equal number of proteins that exhibited an increased or decreased fold change (882 up vs 888 down) (Figure 6). This result is consistent with the consensus that TCR stimulation induces the engagement of Lck interaction with various downstream effector proteins to propagate signaling pathways 1. Of the 29 significantly changing proteins, 10 proteins were either verified HPRD Lck interactors 33 or PhosphoSitePlus Lck substrates 34, further adding confidence to this dataset as the majority of these proteins also belonged to the high stringency group. Most importantly, the 29 identified proteins were not characterized as short-range Lck interactors in living cells, likely due to their previous characterization in cell lysates.
Figure 6: Volcano plot of Lck interactome proteomic data.
Protein intensities of TCR-stimulated J.Lck.TurboID cells relative to unstimulated control was represented in a volcano plot (negative log10 q-value plotted as function of log2 fold change). Proteins that have a q-value less than 0.15 and a minimum of |log2 fold change| of 0.2 are considered significantly changing, and are further binned into high, medium or low stringency based on their q-values as indicated. Lck interactors and substrates according to the Human Protein Reference Database (HPRD) and PhosphoSitePlus, respectively, are also annotated. A select group of proteins are also clustered based on their known biological functions in T cells by referencing existing literature.
Proteomic analysis revealed bona fide and novel TCR inducible Lck interactors
The identified TCR inducible Lck interactors were subdivided into several clusters based on biological function (Figure 6), constructing potential regulatory nodes for Lck within T cells. From these clusters, we noted an apparent increase of SLP-76 signalosome proteins associating with Lck in the stimulated condition, such as Zap-70 and SLP-76, many of which are either known Lck substrates or interactors 1, 4. It was recently reported that upon TCR stimulation, Lck, via its SH2 domain, binds to and phosphorylates Zap70, followed by subsequent association with the SLP-76/LAT adaptor complex, acting as a molecular bridge between Zap-70 and LAT 35. Identification of various known Lck interactors that had an increased association with Lck upon TCR stimulation, such as Zap-70, SLP-76, Nck1, WAS, WIPF1, GRAP 36, supported the validity of using Lck-TurboID to study TCR-inducible Lck interactors.
Vav3
Interestingly, guanine nucleotide exchange factor Vav3 had significantly increased association with Lck upon TCR stimulation (Figure 6). This observation corroborated a previous study reporting that Vav3 translocated to the membrane to associate with SLP-76 via its SH2 domain following T cell activation in Jurkat cells, and that Vav3 recruitment required Lck expression and CD3 stimulation but not CD28 stimulation 37. The monoclonal antibody C305 used in our study also specifically reacts to the CD3 chains of TCR 38, making results from both studies highly comparable. The same report also described increased membrane translocation of Vav3 upon anti-CD3 stimulation, while Vav1 (another member of the Vav family of proteins) translocation remained unchanged 37. This observation is in full agreement with our data, wherein we observed a ~40% increase (fold-change = 1.38; q = 0.0044) in Vav3, whereas change in Vav1 abundance was insignificant (q = 0.23). While Vav1 is well characterized, much less is known about Vav3. Vav3 has been implicated in the formation of cytoskeletal structures via actin re-localization. Vav3 binds and activates Rho GTP-binding proteins, including RhoA and RhoG, though promoting nucleotide exchange. Activation of Rho family proteins has been shown to be important in modulating cytoskeletal rearrangement, amplifying signaling cascades, and regulating gene expression 39, 40. Although it was demonstrated that activation of Vav3 requires tyrosine phosphorylation by Lck, these data were generated from in vitro kinase assays using a constitutively active form of Lck 39, 41. These studies also did not demonstrate close proximity between Lck and Vav3 and were susceptible to artifacts arising from non-native cellular environments. Here, we provide direct evidence demonstrating proximity between Vav3 and Lck upon TCR stimulation in living cells, revealing uncanonical but potentially functional interaction of Lck-Vav3 in the process of signal transduction upon receptor engagement via the rearrangement of cytoskeletal structures.
THEMIS
THEMIS is another protein that was observed to have a statistically increased association with Lck upon TCR stimulation (Figure 6) (fold-change = 1.30; q = 0.0427). THEMIS can act as a positive or a negative regulator of TCR signaling through its ability to either activate thymocytes for positive selection or dampen TCR signaling 42. Mechanistically, THEMIS is reported to associate with LAT and to be phosphorylated by Lck and Zap-70 in vitro 43, confirming the hypothesis of THEMIS as a bona fide Lck substrate. A mass spectrometric analysis of THEMIS-immunoprecipitated complex in thymocytes C57BL/6 mice similarly revealed SLP-76 and CBL as enriched interacting partners specifically in the stimulated state 44, consistent with the data presented here. In other studies, THEMIS was reported to be a key component of the TCR complex and crucial for T cell development, likely through its interaction with Grb2 and LAT and regulation of SHP-1 and Vav1 activity 45–47. Moreover, the canonical mechanism posits that the colocalization of Lck and LAT within a coalesced lipid raft domain requires TCR activation 48. Although we did not observe an elevated short range interaction of these THEMIS-interacting proteins upon TCR stimulation, previous studies have shown that constitutively active Lck 49 engages in a pre-formed complex with LAT and its interacting partners prior to TCR stimulation 50, 51. Consistent with these data, LAT, SHP-1, Vav1, and Grb2-related adaptor protein 2 (GRAP2) were confidently identified and quantified in all of our samples as part of Lck interactome (Table S3). From our results, we hypothesize that a subpopulation of non-active Lck becomes activated upon stimulation and further engages with the LAT complex to recruit more downstream interactors such as SLP-76, THEMIS and Protein Kinase C Theta (PKCθ). Overall, we provide additional insights into the functionally elusive regulator of TCR signaling THEMIS by showing enhanced proximal interaction between Lck and THEMIS upon TCR engagement.
MAPK signaling activators
We also discovered a cluster of proteins that associates with Lck more upon TCR stimulation which is also involved in the upstream activation of MAP kinase signaling, including PKCθ, RAF1, DGKZ, SHC1, and MAP4K1. Notably, PKCθ, RAF1, and SHC1 are classified as Lck interactors in HPRD (Figure 6). We detected a statistically significant increase in protein intensity for PKCθ (fold-change = 1.39; q = 0.0008), RAF1 (fold-change = 1.55; q = 0.0013), and DGKZ (fold-change = 1.60; q = 0.0120). Lck activation leads to the nucleation of the LAT complex, followed by the recruitment of effectors such as SLP-76, Vav1 GTP exchange factor, and PLCg1, leading to Ca2+ mobilization, diacylglycerol (DAG) production, PKCθ activation, mitogen-activated protein kinase (MAPK) cascade (Ras/Raf/MAPKs) activation. This ultimately leads to the activation of transcription factors such as nuclear factor of activated T cells (NFAT) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) to modulate gene expression 52. In Jurkat T cells, PKCθ translocates to membrane lipid rafts upon TCR:CD3 stimulation to be tyrosine-phosphorylated by Lck specifically 53, 54. In vitro binding assays additionally indicate direct association between Lck and PKCθ via Lck SH2/SH3 domains and is enhanced by TCR activation 53, which is consistent with our data. PKCθ plays an essential role in Ras activation, which in turn activates Raf 55. SHC1, on the other hand, is an adaptor protein that functions by coupling tyrosine kinases to Ras 56. Since PKCθ can also be activated by DAG, DGKZ has been reported to be associate with PKCθ to regulate DAG availability 57. Altogether, these data support the hypothesis that upon TCR activation, a protein complex consisting of Lck, PKCθ, DGKZ, Raf and the adaptor SHC1 allows for efficient integration and amplification of MAPK signaling.
Ubiquitination-related proteins
Notably, we identified three ubiquitination-related proteins with significantly enhanced association with Lck upon TCR activation, namely CBL (fold-change = 1.47; q = 0.0024), CBLB (fold-change = 1.75; q = 0.0044) and UBASH3A (fold-change = 1.48; q = 0.1159). CBL and CBLB are members of the RING-type E3 ubiquitin protein ligase family and function by simultaneously binding to an E2 ubiquitin-conjugating enzyme and a substrate protein, leading to ubiquitin-directed proteasomal degradation of the target substrate 58. Ubiquitination of TCR proximal proteins is recognized as an important regulatory mechanism to terminate TCR-driven signaling network, allowing the immune system to return to the basal state 59. Previous studies identified Lck as the tyrosine kinase that phosphorylates CBL in Jurkat cells 60. Furthermore, enhanced association between Lck and CBL upon TCR activation was observed in cell lysates 61, congruent with our proximity labeling results. UBASH3A interacts with the proline-rich region of CBL via its SH3 domain to regulate its activity 62. This interaction was similarly observed in an affinity purification experiment from cell lysates wherein UBASH3A appeared as one of the few shared interactors between CBL and CBLB 63. Interestingly, UBASH3A has been proposed as a putative adaptor linking CBL and Src 62, but this association between these proteins has yet to be directly observed or characterized. Here, we provide evidence of a physiological interaction between a Src family kinase Lck and CBL/CBLB, forming a complex possibly stabilized by UBASH3A, upon TCR engagement to facilitate the negative regulation of activated Lck for ubiquitination and subsequent targeting to lysosomes, elucidating a potentially critical role of a previously unappreciated adaptor protein UBASH3A.
CD2 lipid raft
Our results revealed that Lck had an increased association with CD2 (fold-change = 1.35; q = 0.1192) and CD2AP (fold-change = 1.19; q = 0.0520) upon TCR engagement (Figure 6). TCR engagement is followed by the formation of an immunological synapse, where TCR-associated molecules and selected cytosolic signaling effectors are clustered to allow for more efficient signal transduction and amplification 64. The formation of immunological synapse requires repositioning of membrane lipid rafts, during which CD2 exhibits inducible-translocation to lipid rafts and becomes integral to the structure of the immunological synapse 65, 66. Notably, CD2 translocation to the immunological synapse of TCR leads to physical association with Lck 67, supporting our findings. An affinity purification proteomic study of Lck similarly identified CD2 as a Lck interactor in primary mouse CD4+ T cells 36. CD2 participates in T cell adhesion and facilitates signal transduction to allow cell proliferation. It has been shown that the proline-rich domain of CD2 associates with the SH3 domain of Lck 68 and adaptor protein CD2AP 69. CD2 interaction with CD2AP, which is induced by TCR activation, was proposed to connect the cytoplasmic tail of CD2 to the cytoskeleton 69. Incidentally, Lck has both a N-terminus membrane anchor and a cytoplasmic SH3-SH2-kinase region, where a Lck:CD2:CD2AP association would be consistent with published data. Altogether, we show here for the first time a short range TCR-inducible interaction of CD2 and CD2AP with Lck in living T cells that might be crucial in the formation of immunological synapse.
Motif Analysis
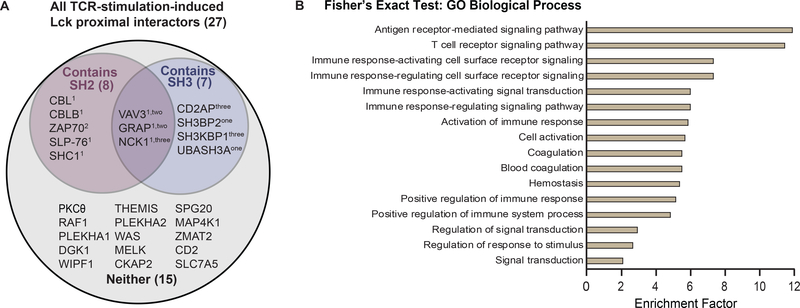
Many adaptor proteins exhibit increased association with Lck upon TCR stimulation. Lck-interacting adaptor proteins typically contain either SH2 or SH3 domains, allowing for binding to pTyr residues or proline-rich regions, respectively. To further investigate the domain composition of the 27 TCR-induced Lck proximal interactors, we performed a motif scan analysis on these proteins using InterProScan 25. We found that 12 of the 27 proteins have either SH2 or SH3 domain, possibly to allow binding to Lck pTyr sites or its proline-rich region (Figure 7A, Table S4). This result is notable as Lck Tyr192, Tyr394, Tyr505 phosphosites have been shown to play a regulatory role in modifying the conformation of Lck and hence its ability to associate with its interacting partners 4, 70. The number of SH2- and SH3-domain-containing proteins identified here are represented in a higher proportion (29.6% for SH2, 25.9% for SH3) relative to the distribution of all SH2- and SH3-domain-containing proteins among the predicted 19,823 proteins encoded by the human genome (0.6% for SH2, 1.1% for SH3) 71, 72, suggesting that Lck preferentially associates with adaptors containing SH2 or SH3 domains. For instance, Vav3 can function as an effective adaptor for Lck because of its SH2 domain, SH3 domain, tyrosine-kinase-binding region, proline-rich region, and multiple tyrosine phosphorylation sites 40, 73. Conversely, we observed two proteins that have decreased association with Lck upon TCR stimulation, namely Liprin-beta-1 (PPFIBP1) and SEC23-interacting protein (SEC23IP); However, no particular motif or biological insights can be confidently drawn from these proteins. Altogether, our data shows that Lck preferentially associates with interactors containing SH2 or SH3 domains, many of which are characterized as adaptor proteins.
Figure 7: Additional validation of Lck-TurboID proteomic data.
(A) InterProScan motif analysis of proteins with significantly increased interaction with Lck. Venn diagram of the proteins with enhanced association with Lck upon TCR stimulation containing SH2 domain, SH3 domain, both, or neither. The number of the corresponding domain present in each protein is indicated as a superscript. (B) GO Biological Process of significantly changing proteins. Fisher’s exact test was performed on the proteins that are significantly changing to determine if there is a non-random association between these protein groups and all terms in the Gene Ontology biological process (GOBP). The top 16 enriched pathways alongside their enrichment factors were plotted accordingly. Proteins which are categorized under each pathway are listed in Table S5.
GO Biological Process
To comprehensively examine the relevant physiological pathways to all 29 significantly changing proteins, we performed a Fisher’s exact test to determine if there is a non-random association between these proteins and all terms in the Gene Ontology biological process (GOBP) 74. This enrichment analysis provides insight towards the common functional roles of this cluster of proteins. To mitigate enrichment biases for the immune system, the reference space for enrichment is appropriately chosen to be the proteome identified and quantified in this experiment instead of the whole human proteome 23. To maintain high statistical stringency, FDR was set at 1% using the Benjamini-Hochberg methodology. Many biological processes that are directly related to T cell signaling were highly enriched, such as antigen receptor-mediated signaling pathway, T cell receptor signaling pathway, and immune response-regulating cell surface receptor signaling pathway (Figure 7B, Table S5). Interestingly, Vav3 is listed to be involved in all top 16 enriched GOBPs assessed here, except in the T cell signaling pathway. Our data provides evidence of statistically enhanced proximal interaction between Lck and Vav3 in living T cells, potentially elucidating previously uncharacterized functional roles of Vav3 in canonical T cell signaling pathways.
Conclusion
In summary, we developed a Lck-TurboID proximity labeling fusion protein in living Jurkat T cells to quantitatively characterize the short-range interactome of Lck upon TCR stimulation of 30 minutes. This work represents the first application of TurboID in the context of Lck, one of the most important kinases in T cells. Here, we employed protein intensity-based label-free quantitation to confidently identify 27 TCR stimulation-induced Lck-proximal interactors, with varying degrees of statistical stringency. While some of these proteins were known Lck interactors, a bulk of the identified proteins had not been previously characterized as canonical Lck interactors, such as Vav3, UBASH3A, CD2AP, validating the utility and discovery power of Lck-TurboID. Further examination of these interactors revealed potentially novel regulatory mechanisms in Lck activity modulation, such as cytoskeletal rearrangement, ubiquitination of TCR signaling proteins, activation of the MAPK cascade, coalescence of the LAT signalosome, and formation of the immunological synapse. TurboID also revealed that Lck preferentially associates with adaptor proteins containing SH2 or SH3 domains upon TCR stimulation, and that Vav3 may be an important but previously unappreciated participant in the canonical TCR signaling pathway. Overall, a similar strategy of proximity labeling as presented by this study may be broadly applied to other systems to infer putative functional insights from protein interactome.
Supplementary Material
Figure S1: CRISPR knockout of Lck disrupts of coding frames in both Lck alleles.
Figure S2: (A) Density plot and (B) S-curve distribution of log2 fold change in protein intensities.
Figure S3: Histogram of log2 protein intensities for all proteins in each sample replicate.
Figure S4: Multi-scatter plot of protein intensities of each sample replicate.
Figure S5: Coefficients of variation (%) of protein intensities of replicates samples.
Figure S6: Heatmap representing protein intensities of each replicate of all KEGG annotated TCR signaling proteins.
File S1: Uncropped Western blot images.
Table S1: List of primers used in the generation of plasmids.
Table S2: LFQ protein intensities.
Table S3: Protein intensities with q-values.
Table S4: InterProScan protein motif analysis.
Table S5: Fisher’s exact test of enriched GO biological pathways.
Acknowledgements
The authors wish to thank Dr. Ricky Edmondson and Dr. Samuel G. Mackintosh from University of Arkansas for Medical Sciences (UAMS) for running the proteomic samples. We thank Dr. Arthur Weiss for kindly providing J.Lck.WT cell line and C305 antibody used in this study. We acknowledge financial support from NIH grants R01AI083636, P01AI091580, and P20GM121293.
Footnotes
SUPPORTING INFORMATION:
The following supporting information is available free of charge at ACS website http://pubs.acs.org
Data Availability
The mass spectrometry proteomic data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository 75 with the dataset identifier PXD020759.
References
- 1.Chakraborty AK; Weiss A, Insights into the initiation of TCR signaling. Nat Immunol 2014, 15 (9), 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palacios EH; Weiss A, Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 2004, 23 (48), 7990–8000. [DOI] [PubMed] [Google Scholar]
- 3.Bommhardt U; Schraven B; Simeoni L, Beyond TCR Signaling: Emerging Functions of Lck in Cancer and Immunotherapy. Int J Mol Sci 2019, 20 (14), 3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtney AH; Lo WL; Weiss A, TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem Sci 2018, 43 (2), 108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinkle-Mulcahy L, Recent advances in proximity-based labeling methods for interactome mapping. F1000Res 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DI; Birendra KC; Zhu W; Motamedchaboki K; Doye V; Roux KJ, Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc Natl Acad Sci U S A 2014, 111 (24), E2453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martell JD; Deerinck TJ; Sancak Y; Poulos TL; Mootha VK; Sosinsky GE; Ellisman MH; Ting AY, Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol 2012, 30 (11), 1143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears RM; May DG; Roux KJ, BioID as a Tool for Protein-Proximity Labeling in Living Cells. Methods in molecular biology (Clifton, N.J.) 2019, 2012, 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DI; Cutler JA; Na CH; Reckel S; Renuse S; Madugundu AK; Tahir R; Goldschmidt HL; Reddy KL; Huganir RL; Wu X; Zachara NE; Hantschel O; Pandey A, BioSITe: A Method for Direct Detection and Quantitation of Site-Specific Biotinylation. Journal of proteome research 2018, 17 (2), 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Udeshi ND; Pedram K; Svinkina T; Fereshetian S; Myers SA; Aygun O; Krug K; Clauser K; Ryan D; Ast T; Mootha VK; Ting AY; Carr SA, Antibodies to biotin enable large-scale detection of biotinylation sites on proteins. Nat Methods 2017, 14 (12), 1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branon TC; Bosch JA; Sanchez AD; Udeshi ND; Svinkina T; Carr SA; Feldman JL; Perrimon N; Ting AY, Efficient proximity labeling in living cells and organisms with TurboID. Nat Biotechnol 2018, 36 (9), 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roncagalli R; Hauri S; Fiore F; Liang Y; Chen Z; Sansoni A; Kanduri K; Joly R; Malzac A; Lahdesmaki H; Lahesmaa R; Yamasaki S; Saito T; Malissen M; Aebersold R; Gstaiger M; Malissen B, Quantitative proteomics analysis of signalosome dynamics in primary T cells identifies the surface receptor CD6 as a Lat adaptor-independent TCR signaling hub. Nat Immunol 2014, 15 (4), 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Sage V; Cinti A; Valiente-Echeverria F; Mouland AJ, Proteomic analysis of HIV-1 Gag interacting partners using proximity-dependent biotinylation. Virol J 2015, 12, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CH; Chien MJ; Chang YC; Cheng YH; Li FA; Mou KY, Combining Proximity Labeling and Cross-Linking Mass Spectrometry for Proteomic Dissection of Nuclear Envelope Interactome. Journal of proteome research 2020, 19 (3), 1109–1118. [DOI] [PubMed] [Google Scholar]
- 15.Prikas E; Poljak A; Ittner A, Mapping p38alpha mitogen-activated protein kinase signaling by proximity-dependent labeling. Protein Sci 2020, 29 (5), 1196–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillingham AK; Bertram J; Begum F; Munro S, In vivo identification of GTPase interactors by mitochondrial relocalization and proximity biotinylation. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannix KM; Starble RM; Kaufman RS; Cooley L, Proximity labeling reveals novel interactomes in live Drosophila tissue. Development 2019, 146 (14), dev176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H; Glatter T; Gstaiger M; Nesvizhskii AI, SAINT-MS1: protein-protein interaction scoring using label-free intensity data in affinity purification-mass spectrometry experiments. Journal of proteome research 2012, 11 (4), 2619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox J; Hein MY; Luber CA; Paron I; Nagaraj N; Mann M, Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Molecular & cellular proteomics : MCP 2014, 13 (9), 2513–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ran FA; Hsu PD; Wright J; Agarwala V; Scott DA; Zhang F, Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013, 8 (11), 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox J; Mann M, MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 2008, 26 (12), 1367–72. [DOI] [PubMed] [Google Scholar]
- 22.Cox J; Neuhauser N; Michalski A; Scheltema RA; Olsen JV; Mann M, Andromeda: a peptide search engine integrated into the MaxQuant environment. Journal of proteome research 2011, 10 (4), 1794–805. [DOI] [PubMed] [Google Scholar]
- 23.Tyanova S; Temu T; Sinitcyn P; Carlson A; Hein MY; Geiger T; Mann M; Cox J, The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods 2016, 13 (9), 731–40. [DOI] [PubMed] [Google Scholar]
- 24.Keilhauer EC; Hein MY; Mann M, Accurate protein complex retrieval by affinity enrichment mass spectrometry (AE-MS) rather than affinity purification mass spectrometry (AP-MS). Molecular & cellular proteomics : MCP 2015, 14 (1), 120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones P; Binns D; Chang HY; Fraser M; Li W; McAnulla C; McWilliam H; Maslen J; Mitchell A; Nuka G; Pesseat S; Quinn AF; Sangrador-Vegas A; Scheremetjew M; Yong SY; Lopez R; Hunter S, InterProScan 5: genome-scale protein function classification. Bioinformatics (Oxford, England) 2014, 30 (9), 1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kortum RL; Rouquette-Jazdanian AK; Samelson LE, Ras and extracellular signal-regulated kinase signaling in thymocytes and T cells. Trends Immunol 2013, 34 (6), 259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paster W; Paar C; Eckerstorfer P; Jakober A; Drbal K; Schutz GJ; Sonnleitner A; Stockinger H, Genetically encoded Forster resonance energy transfer sensors for the conformation of the Src family kinase Lck. Journal of immunology (Baltimore, Md. : 1950) 2009, 182 (4), 2160–7. [DOI] [PubMed] [Google Scholar]
- 28.Mall GK; Chew YC; Zempleni J, Biotin requirements are lower in human Jurkat lymphoid cells but homeostatic mechanisms are similar to those of HepG2 liver cells. J Nutr 2010, 140 (6), 1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manthey KC; Griffin JB; Zempleni J, Biotin supply affects expression of biotin transporters, biotinylation of carboxylases and metabolism of interleukin-2 in Jurkat cells. J Nutr 2002, 132 (5), 887–92. [DOI] [PubMed] [Google Scholar]
- 30.May DG; Scott KL; Campos AR; Roux KJ, Comparative Application of BioID and TurboID for Protein-Proximity Biotinylation. Cells 2020, 9 (5), 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biotin and fatty acid requirements for lymphocyte response. Nutr Rev 1980, 38 (3), 126–7. [DOI] [PubMed] [Google Scholar]
- 32.Ong SE; Blagoev B; Kratchmarova I; Kristensen DB; Steen H; Pandey A; Mann M, Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Molecular & cellular proteomics : MCP 2002, 1 (5), 376–86. [DOI] [PubMed] [Google Scholar]
- 33.Peri S; Navarro JD; Amanchy R; Kristiansen TZ; Jonnalagadda CK; Surendranath V; Niranjan V; Muthusamy B; Gandhi TK; Gronborg M; Ibarrola N; Deshpande N; Shanker K; Shivashankar HN; Rashmi BP; Ramya MA; Zhao Z; Chandrika KN; Padma N; Harsha HC; Yatish AJ; Kavitha MP; Menezes M; Choudhury DR; Suresh S; Ghosh N; Saravana R; Chandran S; Krishna S; Joy M; Anand SK; Madavan V; Joseph A; Wong GW; Schiemann WP; Constantinescu SN; Huang L; Khosravi-Far R; Steen H; Tewari M; Ghaffari S; Blobe GC; Dang CV; Garcia JG; Pevsner J; Jensen ON; Roepstorff P; Deshpande KS; Chinnaiyan AM; Hamosh A; Chakravarti A; Pandey A, Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res 2003, 13 (10), 2363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornbeck PV; Zhang B; Murray B; Kornhauser JM; Latham V; Skrzypek E, PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 2015, 43 (Database issue), D512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo W-L; Shah NH; Ahsan N; Horkova V; Stepanek O; Salomon AR; Kuriyan J; Weiss A, Lck promotes Zap70-dependent LAT phosphorylation by bridging Zap70 to LAT. Nature Immunology 2018, 19 (7), 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voisinne G; Kersse K; Chaoui K; Lu L; Chaix J; Zhang L; Goncalves Menoita M; Girard L; Ounoughene Y; Wang H; Burlet-Schiltz O; Luche H; Fiore F; Malissen M; Gonzalez de Peredo A; Liang Y; Roncagalli R; Malissen B, Quantitative interactomics in primary T cells unveils TCR signal diversification extent and dynamics. Nat Immunol 2019, 20 (11), 1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charvet C; Canonigo AJ; Billadeau DD; Altman A, Membrane localization and function of Vav3 in T cells depend on its association with the adapter SLP-76. The Journal of biological chemistry 2005, 280 (15), 15289–99. [DOI] [PubMed] [Google Scholar]
- 38.Weiss A; Stobo JD, Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med 1984, 160 (5), 1284–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Movilla N; Bustelo XR, Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol 1999, 19 (11), 7870–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng L; Sachdev P; Yan L; Chan JL; Trenkle T; McClelland M; Welsh J; Wang LH, Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol Cell Biol 2000, 20 (24), 9212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yabana N; Shibuya M, Adaptor protein APS binds the NH2-terminal autoinhibitory domain of guanine nucleotide exchange factor Vav3 and augments its activity. Oncogene 2002, 21 (50), 7720–9. [DOI] [PubMed] [Google Scholar]
- 42.Choi S; Cornall R; Lesourne R; Love PE, THEMIS: Two Models, Different Thresholds. Trends Immunol 2017, 38 (9), 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paster W; Brockmeyer C; Fu G; Simister PC; de Wet B; Martinez-Riano A; Hoerter JA; Feller SM; Wulfing C; Gascoigne NR; Acuto O, GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. Journal of immunology (Baltimore, Md. : 1950) 2013, 190 (7), 3749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zvezdova E; Mikolajczak J; Garreau A; Marcellin M; Rigal L; Lee J; Choi S; Blaize G; Argenty J; Familiades J; Li L; Gonzalez de Peredo A; Burlet-Schiltz O; Love PE; Lesourne R, Themis1 enhances T cell receptor signaling during thymocyte development by promoting Vav1 activity and Grb2 stability. Science signaling 2016, 9 (428), ra51. [DOI] [PubMed] [Google Scholar]
- 45.Choi S; Warzecha C; Zvezdova E; Lee J; Argenty J; Lesourne R; Aravind L; Love PE, THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat Immunol 2017, 18 (4), 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paster W; Bruger AM; Katsch K; Gregoire C; Roncagalli R; Fu G; Gascoigne NR; Nika K; Cohnen A; Feller SM; Simister PC; Molder KC; Cordoba SP; Dushek O; Malissen B; Acuto O, A THEMIS:SHP1 complex promotes T-cell survival. EMBO J 2015, 34 (3), 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brockmeyer C; Paster W; Pepper D; Tan CP; Trudgian DC; McGowan S; Fu G; Gascoigne NR; Acuto O; Salek M, T cell receptor (TCR)-induced tyrosine phosphorylation dynamics identifies THEMIS as a new TCR signalosome component. The Journal of biological chemistry 2011, 286 (9), 7535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schade AE; Levine AD, Lipid raft heterogeneity in human peripheral blood T lymphoblasts: a mechanism for regulating the initiation of TCR signal transduction. Journal of immunology (Baltimore, Md. : 1950) 2002, 168 (5), 2233–9. [DOI] [PubMed] [Google Scholar]
- 49.Nika K; Soldani C; Salek M; Paster W; Gray A; Etzensperger R; Fugger L; Polzella P; Cerundolo V; Dushek O; Hofer T; Viola A; Acuto O, Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 2010, 32 (6), 766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kabouridis PS, Selective interaction of LAT (linker of activated T cells) with the open-active form of Lck in lipid rafts reveals a new mechanism for the regulation of Lck in T cells. Biochem J 2003, 371 (Pt 3), 907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munoz P; Navarro MD; Pavon EJ; Salmeron J; Malavasi F; Sancho J; Zubiaur M, CD38 signaling in T cells is initiated within a subset of membrane rafts containing Lck and the CD3-zeta subunit of the T cell antigen receptor. The Journal of biological chemistry 2003, 278 (50), 50791–802. [DOI] [PubMed] [Google Scholar]
- 52.Sommers CL; Samelson LE; Love PE, LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. BioEssays : news and reviews in molecular, cellular and developmental biology 2004, 26 (1), 61–7. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y; Witte S; Liu YC; Doyle M; Elly C; Altman A, Regulation of protein kinase Ctheta function during T cell activation by Lck-mediated tyrosine phosphorylation. The Journal of biological chemistry 2000, 275 (5), 3603–9. [DOI] [PubMed] [Google Scholar]
- 54.Bi K; Tanaka Y; Coudronniere N; Sugie K; Hong S; van Stipdonk MJ; Altman A, Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol 2001, 2 (6), 556–63. [DOI] [PubMed] [Google Scholar]
- 55.Isakov N; Altman A, Protein kinase C(theta) in T cell activation. Annu Rev Immunol 2002, 20, 761–94. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed SBM; Prigent SA, Insights into the Shc Family of Adaptor Proteins. J Mol Signal 2017, 12, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo B; Prescott SM; Topham MK, Association of diacylglycerol kinase zeta with protein kinase C alpha: spatial regulation of diacylglycerol signaling. J Cell Biol 2003, 160 (6), 929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thien CB; Langdon WY, c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J 2005, 391 (Pt 2), 153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohapatra B; Ahmad G; Nadeau S; Zutshi N; An W; Scheffe S; Dong L; Feng D; Goetz B; Arya P; Bailey TA; Palermo N; Borgstahl GE; Natarajan A; Raja SM; Naramura M; Band V; Band H, Protein tyrosine kinase regulation by ubiquitination: critical roles of Cbl-family ubiquitin ligases. Biochim Biophys Acta 2013, 1833 (1), 122–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao Y; Qiao G; Tang J; Tang R; Guo H; Warwar S; Langdon WY; Tao L; Zhang J, Protein Tyrosine Phosphatase SHP-1 Modulates T Cell Responses by Controlling Cbl-b Degradation. Journal of immunology (Baltimore, Md. : 1950) 2015, 195 (9), 4218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao N; Miyake S; Reddi AL; Douillard P; Ghosh AK; Dodge IL; Zhou P; Fernandes ND; Band H, Negative regulation of Lck by Cbl ubiquitin ligase. Proc Natl Acad Sci U S A 2002, 99 (6), 3794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feshchenko EA; Smirnova EV; Swaminathan G; Teckchandani AM; Agrawal R; Band H; Zhang X; Annan RS; Carr SA; Tsygankov AY, TULA: an SH3- and UBA-containing protein that binds to c-Cbl and ubiquitin. Oncogene 2004, 23 (27), 4690–706. [DOI] [PubMed] [Google Scholar]
- 63.Voisinne G; Garcia-Blesa A; Chaoui K; Fiore F; Bergot E; Girard L; Malissen M; Burlet-Schiltz O; Gonzalez de Peredo A; Malissen B; Roncagalli R, Co-recruitment analysis of the CBL and CBLB signalosomes in primary T cells identifies CD5 as a key regulator of TCR-induced ubiquitylation. Mol Syst Biol 2016, 12 (7), 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grakoui A; Bromley SK; Sumen C; Davis MM; Shaw AS; Allen PM; Dustin ML, The immunological synapse: a molecular machine controlling T cell activation. Science (New York, N.Y.) 1999, 285 (5425), 221–7. [DOI] [PubMed] [Google Scholar]
- 65.Yang H; Reinherz EL, Dynamic recruitment of human CD2 into lipid rafts. Linkage to T cell signal transduction. The Journal of biological chemistry 2001, 276 (22), 18775–85. [DOI] [PubMed] [Google Scholar]
- 66.Janes PW; Ley SC; Magee AI; Kabouridis PS, The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol 2000, 12 (1), 23–34. [DOI] [PubMed] [Google Scholar]
- 67.Nunes RJ; Castro MA; Goncalves CM; Bamberger M; Pereira CF; Bismuth G; Carmo AM, Protein interactions between CD2 and Lck are required for the lipid raft distribution of CD2. Journal of immunology (Baltimore, Md. : 1950) 2008, 180 (2), 988–97. [DOI] [PubMed] [Google Scholar]
- 68.Bell GM; Fargnoli J; Bolen JB; Kish L; Imboden JB, The SH3 domain of p56lck binds to proline-rich sequences in the cytoplasmic domain of CD2. J Exp Med 1996, 183 (1), 169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dustin ML; Olszowy MW; Holdorf AD; Li J; Bromley S; Desai N; Widder P; Rosenberger F; van der Merwe PA; Allen PM; Shaw AS, A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 1998, 94 (5), 667–77. [DOI] [PubMed] [Google Scholar]
- 70.Courtney AH; Amacher JF; Kadlecek TA; Mollenauer MN; Au-Yeung BB; Kuriyan J; Weiss A, A Phosphosite within the SH2 Domain of Lck Regulates Its Activation by CD45. Molecular cell 2017, 67 (3), 498–511 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jadwin JA; Ogiue-Ikeda M; Machida K, The application of modular protein domains in proteomics. FEBS Lett 2012, 586 (17), 2586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Omenn GS; Lane L; Overall CM; Corrales FJ; Schwenk JM; Paik YK; Van Eyk JE; Liu S; Pennington S; Snyder MP; Baker MS; Deutsch EW, Progress on Identifying and Characterizing the Human Proteome: 2019 Metrics from the HUPO Human Proteome Project. Journal of proteome research 2019, 18 (12), 4098–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt MHH; Dikic I, The Cbl interactome and its functions. Nat Rev Mol Cell Biol 2005, 6 (12), 907–18. [DOI] [PubMed] [Google Scholar]
- 74.Ashburner M; Ball CA; Blake JA; Botstein D; Butler H; Cherry JM; Davis AP; Dolinski K; Dwight SS; Eppig JT; Harris MA; Hill DP; Issel-Tarver L; Kasarskis A; Lewis S; Matese JC; Richardson JE; Ringwald M; Rubin GM; Sherlock G, Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000, 25 (1), 25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vizcaino JA; Cote RG; Csordas A; Dianes JA; Fabregat A; Foster JM; Griss J; Alpi E; Birim M; Contell J; O’Kelly G; Schoenegger A; Ovelleiro D; Perez-Riverol Y; Reisinger F; Rios D; Wang R; Hermjakob H, The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res 2013, 41 (Database issue), D1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: CRISPR knockout of Lck disrupts of coding frames in both Lck alleles.
Figure S2: (A) Density plot and (B) S-curve distribution of log2 fold change in protein intensities.
Figure S3: Histogram of log2 protein intensities for all proteins in each sample replicate.
Figure S4: Multi-scatter plot of protein intensities of each sample replicate.
Figure S5: Coefficients of variation (%) of protein intensities of replicates samples.
Figure S6: Heatmap representing protein intensities of each replicate of all KEGG annotated TCR signaling proteins.
File S1: Uncropped Western blot images.
Table S1: List of primers used in the generation of plasmids.
Table S2: LFQ protein intensities.
Table S3: Protein intensities with q-values.
Table S4: InterProScan protein motif analysis.
Table S5: Fisher’s exact test of enriched GO biological pathways.
Data Availability Statement
The mass spectrometry proteomic data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository 75 with the dataset identifier PXD020759.