Summary
Background
Human hair is highly responsive to stress, and human scalp hair follicles (HFs) contain a peripheral neuroendocrine equivalent of the systemic hypothalamic–pituitary–adrenal (HPA) stress axis. Androgenetic alopecia (AGA) is supposed to be aggravated by stress. We used corticotropin-releasing hormone (CRH), which triggers the HPA axis, to induce a stress response in human ex vivo male AGA HFs. Caffeine is known to reverse testosterone-mediated hair growth inhibition in the same hair organ culture model.
Objectives
To investigate whether caffeine would antagonize CRH-mediated stress in these HFs.
Methods
HFs from balding vertex area scalp biopsies of men affected by AGA were incubated with CRH (10−7 mol L−1) with or without caffeine (0·001% or 0·005%).
Results
Compared to controls, CRH significantly enhanced the expression of catagen-inducing transforming growth factor-β2 (TGF-β2) (P < 0·001), CRH receptors 1 and 2 (CRH-R1/2) (P < 0·01), adrenocorticotropic hormone (ACTH) (P < 0·001) and melanocortin receptor 2 (MC-R2) (P < 0·001), and additional stress-associated parameters, substance P and p75 neurotrophin receptor (p75NTR). CRH inhibited matrix keratinocyte proliferation and expression of anagen-promoting insulin-like growth factor-1 (IGF-1) and the pro-proliferative nerve growth factor receptor NGF-tyrosine kinase receptor A (TrkA). Caffeine significantly counteracted all described stress effects and additionally enhanced inositol trisphosphate receptor (IP3-R), for the first time detected in human HFs.
Conclusions
These findings provide the first evidence in ex vivo human AGA HFs that the stress mediator CRH induces not only a complex intrafollicular HPA response, but also a non-HPA-related stress response. Moreover, we show that these effects can be effectively antagonized by caffeine. Thus, these data strongly support the hypothesis that stress can impair human hair physiology and induce hair loss, and that caffeine may effectively counteract stress-induced hair damage and possibly prevent stress-induced hair loss.
Hair loss is deeply stressful for affected patients,1–5 while perceived stress might aggravate hair loss such as male and female pattern androgenetic alopecia (AGA) or diffuse alopecia.6–8 Animal models have shown that stress impairs hair growth by catagen induction mediated by substance P (SP), mast cell activation and nerve growth factor (NGF),9–13 as confirmed also in human hair follicles (HFs) ex vivo.14,15
Stress can be mediated by oxidative stress or stress hormones.16–18 The main actor of hormonal stress is corticotropin-releasing hormone (CRH).19 CRH inhibits growth and induces premature entry into the catagen phase in human scalp HFs ex vivo,20 which is consistent with the wide CRH expression and signalling in human skin,21,22 including direct proinflammatory activities.23,24 However, an established human hair stress model and related treatment principle are lacking so far.
HFs are skin appendages that survive and proliferate independently of the skin in vitro, for example in the human hair organ culture model.25,26 They undergo lifelong repeating growth cycles;27 synthesize, metabolize and process numerous (neuroendocrine) hormones;28–31 and consequently represent an essential part of the skin neuroendocrine system.19,32,33 Additionally, HFs (and skin) express a fully functional peripheral cutaneous equivalent of the central hypothalamic–pituitary–adrenal (HPA) stress axis [CRH → adrenocorticotropic hormone (ACTH) → cortisol],19–21,34,35 responsive even to ultraviolet B-induced stress.36 Finally, HFs (and the skin) manage locally occurring stress20,33,37 and therefore can serve excellently as a human ex vivo hair stress organ culture model. The HPA axis is initiated by CRH,38 which stimulates the pituitary CRH receptor type 1 (CRH-R1), leading to production and secretion of proopiomelanocortin (POMC)-derived peptides, including melanocyte-stimulating hormone and ACTH.39
The current study aimed now to establish a CRH-based hair stress model – for the first time in male ex vivo human HFs derived from AGA patients and to investigate the effects of a possible counteracting substance, the well-known phosphodiesterase inhibitor caffeine.40–42
Materials and methods
Reagents
All of the reagents used are listed in Appendix S1 (see Supporting Information).
Scalp biopsies
Whole human HFs (anagen VI) were microdissected from elective biopsies (0·5 × 1·5 cm) from the balding vertex region in the border area of the dense to the shedding area (androgen sensitive) of 18 men (age range 25–44 years, mean 35·4) with AGA in the moderate stage (Norwood–Hamilton stage III vertex and IV). The study was approved by the ethics committee of the University of Lübeck (reference 06-109) and written informed consent was obtained from the patients in accordance with the Declaration of Helsinki.
Hair follicle microdissection and organ culture
Extracted HFs were cultivated in a validated hair organ culture model as previously described (Appendix S2; see Supporting Information). 25,26,41,43,44
Experimental treatment was started by adding fresh medium containing CRH at 10−7 mol L−1 or 10−8 mol L−1, or CRH- and caffeine-free culture medium (control) to identify the most suppressive concentration of CRH on hair shaft elongation and matrix keratinocyte proliferation. CRH at 10−7 mol L−1 was most inhibitory, thus the following treatment conditions were established: 10−7 mol L−1 CRH, 10−7 mol L−1 CRH + 0·001% or 0·005% caffeine, or CRH- and caffeine-free culture medium (control). HFs were cultivated for 120 h at 37 °C and 5% CO2. Control and treatment media were changed every other day. Hair shaft elongation was measured every 24 h using a scaled microscopic eyepiece. At the end of the 120-h cultivation, follicles were frozen at −80° C and cryosections of 6 μm in thickness were performed for immunofluorescence. For reverse-transcriptase polymerase chain reaction (RT-PCR), 15 HFs per treatment condition were collected, placed in Eppendorf tubes and suspended in 600 μL TRIzol (Invitrogen, Carlsbad, CA, USA), then homogenized and frozen at −80° C until RT-PCR.
Real-time reverse-transcriptase polymerase chain reaction assay
Total RNA from HFs was extracted using TRIzol, the RNA concentration was quantified (NanoDrop 3300; Fisher Scientific, Wilmington, DE, USA) and 1 μg of total RNA was reverse transcribed with the SuperScript First-Strand Synthesis System (Applied Biosystems, Foster City, CA, USA). Primers for real-time PCR amplification were synthesized by Integrated DNA Technologies Inc. (Coralville, IA, USA) according to earlier published PCR detection of the CRH gene (Table S1; see Supporting Information).45 The reaction was performed in triplicate with the SYBR Green I Master Mix (Roche, Manheim, Germany) on a Light Cycler 480 (Roche). The amount of amplified product for each gene was compared with that of β-actin using a comparative ΔΔCT method ± SD, and the data are presented as the fold change.
Immunofluorescence labelling
For detection and visualization of protein expression in HFs, tyramide signal amplification (TSA) was applied for CRH-R1/2, insulin-like growth factor (IGF)-1, inositol triphosphate receptor (IP3-R), melanocortin receptor 2 (MC-R2), p75 neurotrophin receptor (p75NTR) and transforming growth factor (TGF)-β2, and the standard immunofluorescence procedure for ACTH, SP, NGF-tyrosine kinase receptor A (TrkA) and Ki-67. A detailed description of the procedures is provided in Appendix S3 and Table S2 (see Supporting Information).
Statistical analysis
A description of the statistical analyses is provided in Appendix S4 (see Supporting Information).
Results
Identification of growth and proliferation inhibitory concentrations of corticotropin-releasing hormone in male human androgenetic alopecia hair follicles in vitro
Incubation of male human AGA HFs in culture with CRH at 10−7 mol L−1 and 10−8 mol L−1 or with CRH-free medium (control) identified 10−7 mol L−1 as the most suppressive concentration regarding hair shaft elongation and matrix keratinocyte proliferation (Ki67 positivity; data not shown), corresponding to results in female human HFs.20
Corticotropin-releasing hormone suppressed, whereas caffeine significantly increased hair shaft elongation
CRH (10−7 mol L−1) slightly decreased hair shaft elongation by 5% after 120 h in culture compared with control (Figure 1). Caffeine 0·001% in co-culture with CRH significantly enhanced hair shaft elongation by 11% (P < 0·01) compared to control, and by 17% (P < 0·001) compared to CRH alone.
Figure 1.
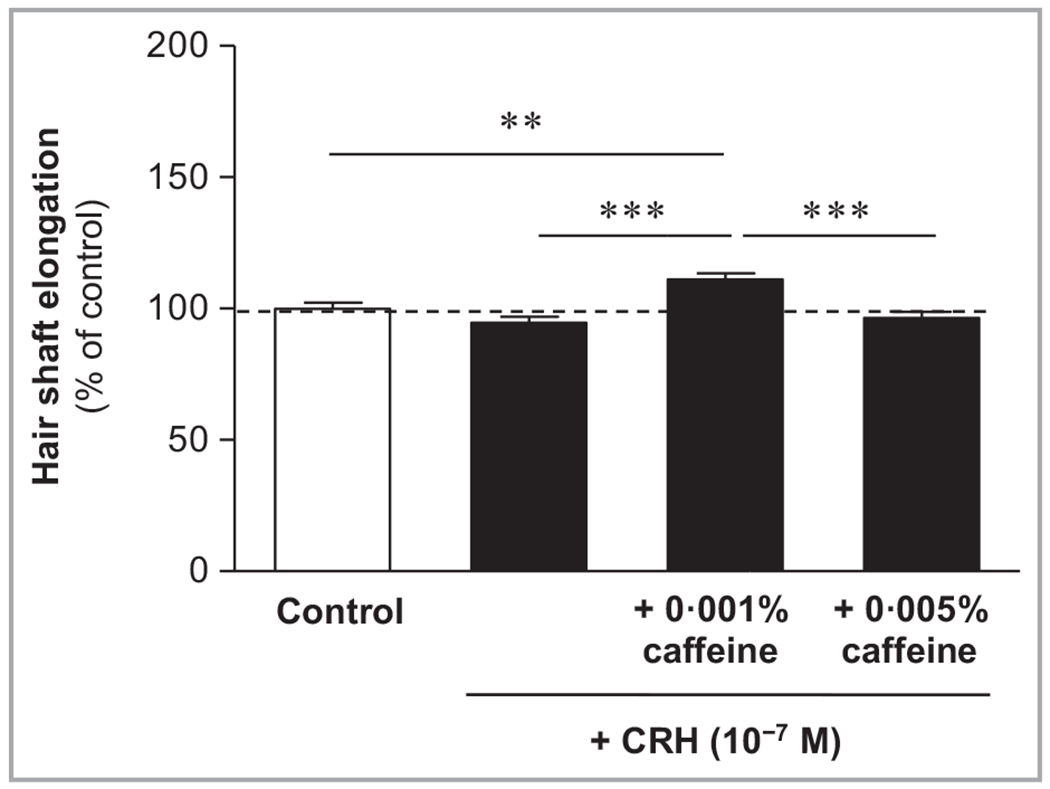
Effects of corticotropin-releasing hormone (CRH) and caffeine on hair shaft elongation. Hair follicles were incubated with the culture medium (control), CRH (10−7 mol L−1) alone or CRH in combination with caffeine (0·001% or 0·005%). CRH slightly reduced hair shaft elongation, while caffeine at the concentration of 0·001% significantly increased it compared with control at 120 h. Data are expressed as the pooled mean ± SEM of 18 independent experiments. Values are expressed as the percentage of the control value. Statistically significant variations are indicated as **P < 0·01, ***P < 0·001.
Corticotropin-releasing hormone reduced matrix keratinocyte proliferation (Ki-67) and insulin-like growth factor 1, while caffeine significantly counteracted these effects
CRH reduced matrix keratinocyte proliferation (Ki-67) (Figure 2) and protein expression of IGF-1 detected in the inner and outer root sheaths (Figure 3). Caffeine at 0·005% significantly enhanced the number of Ki-67-positive matrix keratinocytes by 65% (P < 0·05), and the IGF-1 positivity in the inner and outer root sheath by 47% (P < 0·001) compared to HFs incubated with CRH alone.
Figure 2.
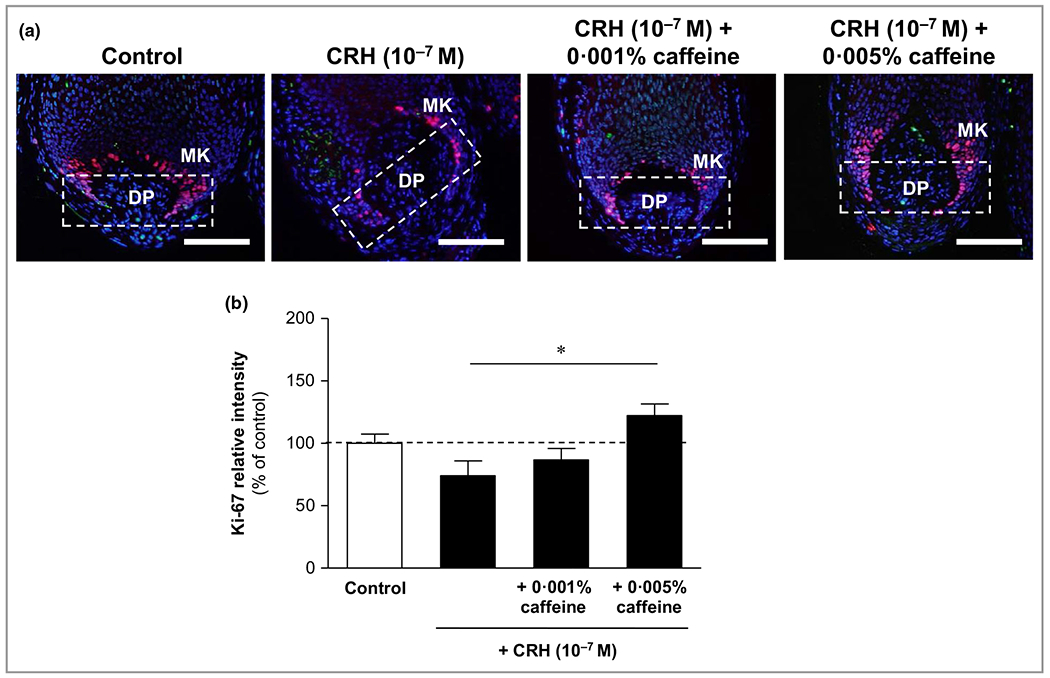
Effects of corticotropin-releasing hormone (CRH) and caffeine on matrix keratinocyte proliferation (Ki-67). (a) Hair follicles were stained with immunofluorescence-labelled antibodies for the proliferation marker Ki-67 (red fluorescence) in matrix keratinocytes (MKs) localized above and around the dermal papilla (DP). Bars = 50 μm. (b) Evaluation of the fluorescence intensity was conducted using Image J 1·38d software within the indicated white dashed rectangular areas. The data are expressed as the pooled mean ± SEM. *P < 0·05.
Figure 3.
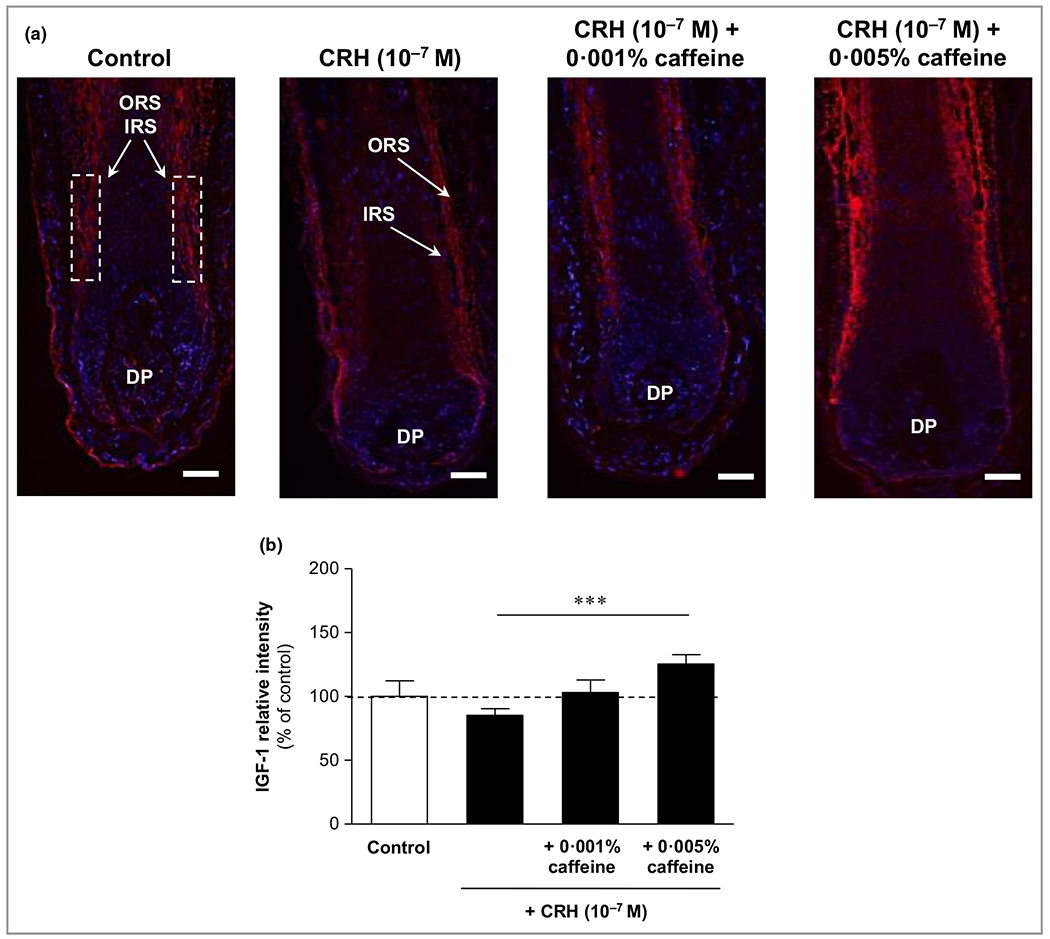
Effects of corticotropin-releasing hormone (CRH) and caffeine on insulin-like growth factor (IGF)-1 in organ-cultured hair follicles. (a) Hair follicles were stained with immunofluorescence-labelled antibodies for IGF-1 (red fluorescence) in the inner root sheath (IRS) and outer root sheath (ORS). DP, dermal papilla. Bars = 50 μm. (b) Evaluation of the fluorescence intensity was conducted using Image J 1·38d software within the indicated white dashed rectangular areas. The data are expressed as the pooled mean ± SEM. ***P < 0·001.
Corticotropin-releasing hormone significantly increased the catagen inducer transforming growth factor-β2, while caffeine significantly reduced it
The protein expression of the key catagen inducer TGF-β2 was significantly enhanced by CRH by 60% (P < 0·001) compared to control (Figure 4). Immunoreactivity was detected in the outer root sheath, and partly, but more weakly, in the inner root sheath. Caffeine 0·005% in co-incubation with CRH led to a significant reduction of CRH-induced TGF-β2 immunoreactivity, by 22% (P < 0·05).
Figure 4.
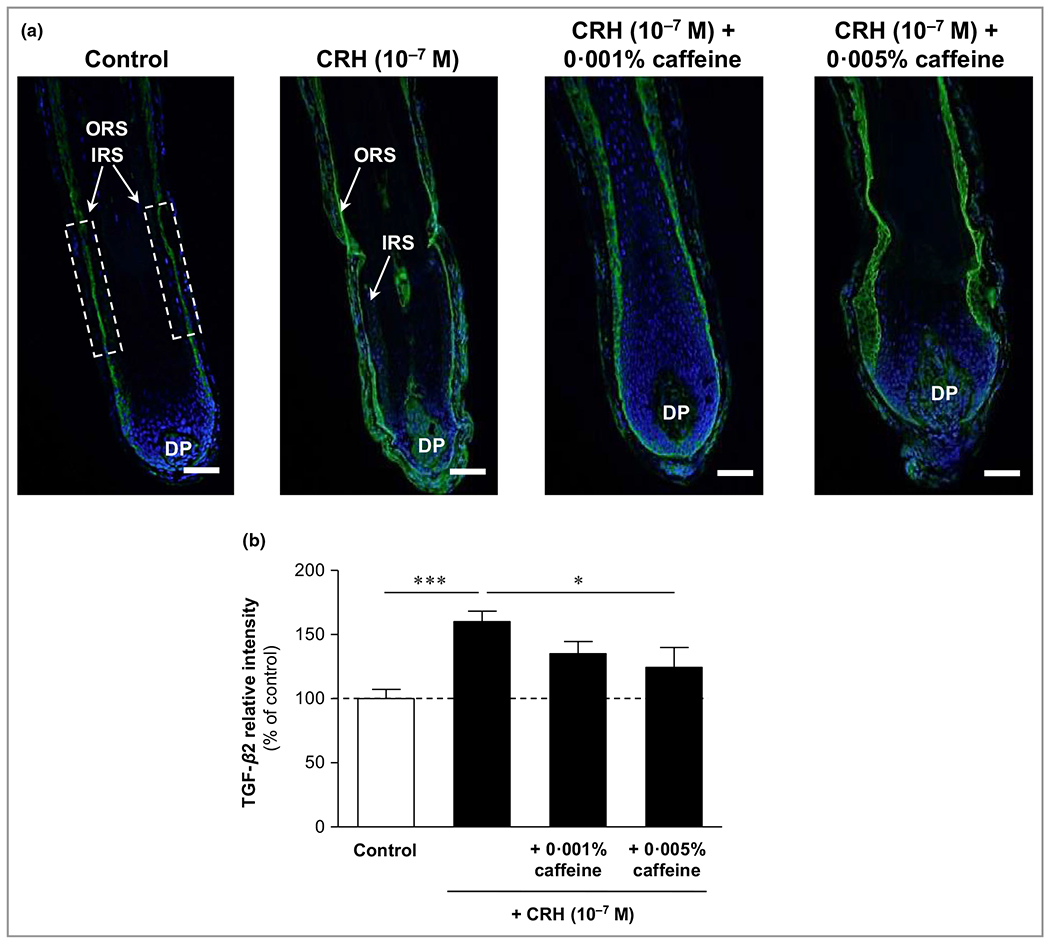
Effects of corticotropin-releasing hormone (CRH) and caffeine on the catagen-inducing factor transforming growth factor (TGF)-β2 in organ-cultured hair follicles. (a) Hair follicles were stained with immunofluorescence-labelled antibodies for TGF-β2 immunoreactivity (green fluorescence), which was detected in the outer root sheath (ORS) and partly in the inner root sheath (IRS). DP, dermal papilla. Bars = 50 μm. (b) Evaluation of the fluorescence intensity was conducted using Image J 1·38d software within the indicated white dashed rectangular areas. The data are expressed as the pooled mean ± SEM. *P < 0·05, ***P < 0·001.
Corticotropin-releasing hormone and caffeine modulated the hypothalamic–pituitary–adrenal axis-dependent stress parameters
Corticotropin-releasing hormone (CRH) receptor 1/2 was significantly upregulated by CRH but downregulated by caffeine
Cultivation of extracted HFs with CRH (10−7 mol L−1) and in combination with caffeine at 0·001% and 0·005% showed a strong impact on HPA axis-dependent stress parameters. Firstly, the expression of CRH-R1/2 was detected in the outer and inner root sheaths, with predominance in the outer root sheath, and was mainly membrane bound. Single fibroblasts in the dermal papilla were also CRH-R1/2 positive. CRH-R1/2 immunoreactivity was significantly enhanced by 23% (P < 0·01) compared with control after incubation with CRH 10−7 mol L−1. Co-incubation with caffeine 0·001% and 0·005% led to a distinct decrease of this effect by 33% (P < 0·001) (Figure 5).
Figure 5.
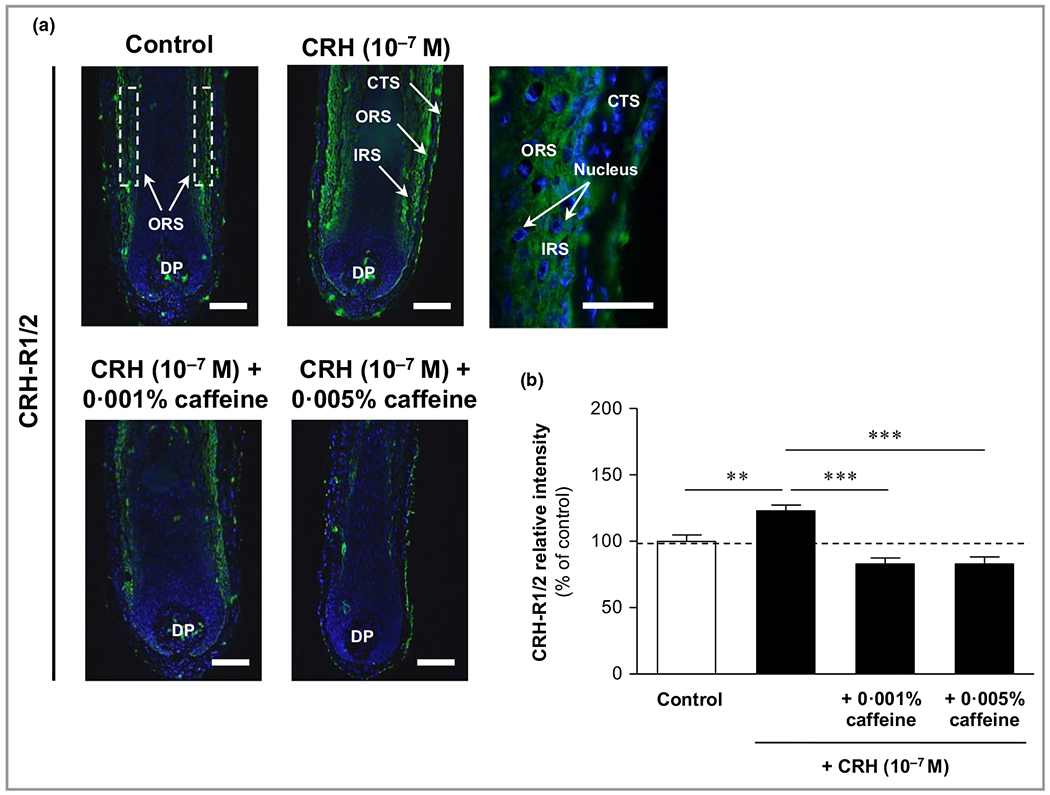
Effects of corticotropin-releasing hormone (CRH) and caffeine on the hypothalamic–pituitary–adrenal axis-dependent stress parameter CRH receptors (CRH1/2) in human hair follicles. (a) CRH-R1/2 immunoreactivity (green fluorescence) was detected in the outer root sheath (ORS) and inner root sheath (IRS) (white dashed rectangular areas), with membrane-bound and cytosolic detection (magnification in upper right image). Single fibroblasts in the dermal papilla (DP) were also CRH-R1/2 positive. CTS, connective tissue sheath. Bars = 50 μm. (b) Relative fluorescence intensity was measured with Image J 1·38d software within the dashed rectangular areas along the IRS and ORS. Data are presented as the mean ± SEM. **P < 0·01, ***P < 0·001.
Expression of inositol triphosphate receptor (IP3-R) was significantly enhanced by caffeine, but not by corticotropin-releasing hormone
The immunoreactivity of the intracellular second messenger receptor IP3-R was detected in the inner root sheath with cytosolic localization (Figure 6a), and was slightly reduced by CRH 10−7 mol L−1 compared with control (not significant) (Figure 6a, upper right image). Co-cultivation with caffeine at 0·001% and 0·005% led to a strong and significant enhancement of IP3-R immunoreactivity along the inner root sheath (Figure 6a, lower image panel). The enhancements compared to CRH-treated HFs were +24% (P < 0·01) and +29% (P < 0·001), respectively (Figure 6b).
Figure 6.
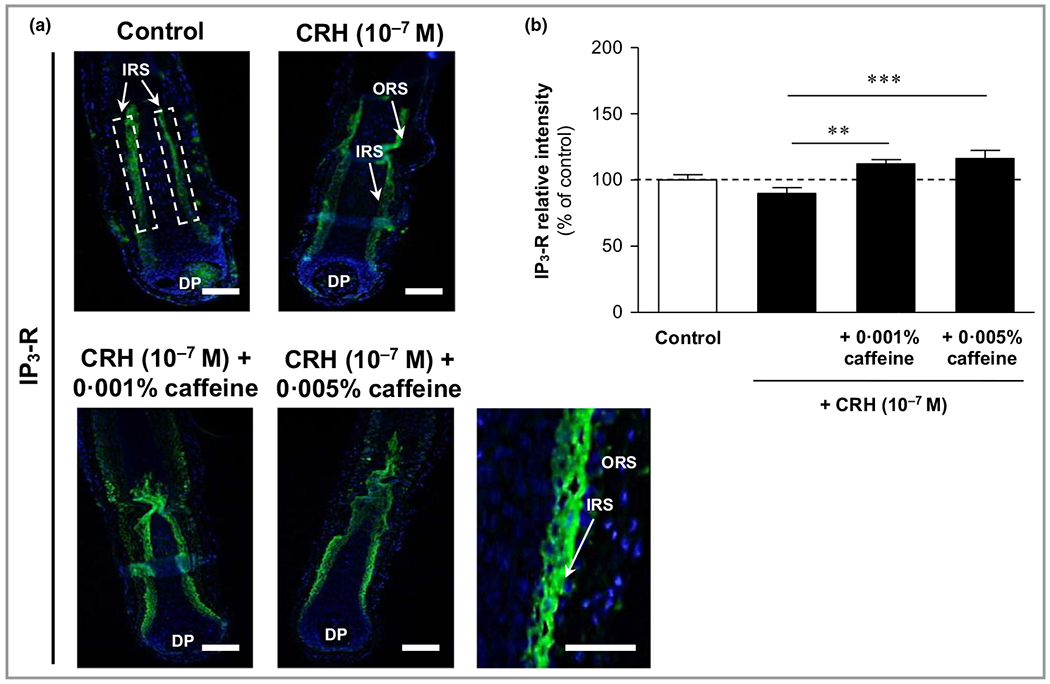
Effects of corticotropin-releasing hormone (CRH) and caffeine on the hypothalamic–pituitary–adrenal axis-dependent stress parameter inositol triphosphate receptor (IP3-R) in human hair follicles. (a) IP3-R immunoreactivity (green fluorescence) was detected in the inner root sheath (IRS) (white dashed rectangular areas) with cytosolic localization (magnification in lower right panel). DP, dermal papilla. Bars = 50 μm. (b) The relative fluorescence intensity was measured with Image J 1·38d software within the dashed rectangular areas along the inner root sheath (IRS). Data are presented as the mean ± SEM. **P < 0·01, ***P < 0·001.
Adrenocorticotropic hormone (ACTH) was significantly upregulated by corticotropin-releasing hormone and downregulated by caffeine co-administration
ACTH immunoreactivity was observed in the outer and inner root sheaths, as well as in matrix keratinocytes and in dermal papilla fibroblasts, with mainly intranuclear localization (Figure 7a, upper middle image). Weak ACTH immunoreactivity was also detected in the connective tissue sheath (Figure 7a, upper right image). ACTH in the outer and inner root sheaths was significantly increased by CRH 10−7 mol L−1, by 59% compared to control (P < 0·001) (Figure 7b), while co-cultivation with caffeine 0·005% led to a significant ACTH decrease by 23% compared to ACTH in HFs incubated with CRH 10−7 mol L−1 alone (P < 0·01) (Figure 7b).
Figure 7.
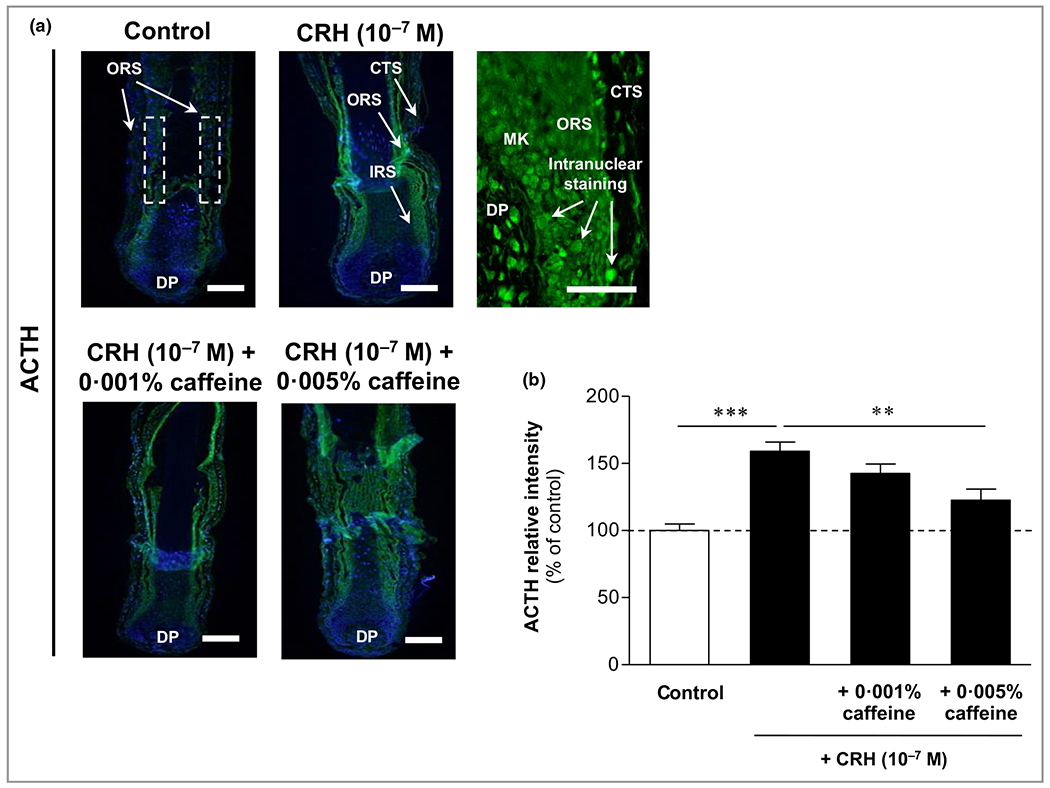
Effects of corticotropin-releasing hormone (CRH) and caffeine on the hypothalamic–pituitary–adrenal axis-dependent stress parameter adrenocorticotropic hormone (ACTH) in human hair follicles. (a) ACTH immunoreactivity (green fluorescence) was observed in the outer root sheath (ORS) and inner root sheath (IRS) (white dashed rectangular areas), as well as in matrix keratinocytes (MKs) and in fibroblasts in the dermal papilla (DP), with mainly intranuclear localization (magnification in upper right panel). Weak ACTH immunoreactivity was also detected in the connective tissue sheath (CTS) (magnification in upper right panel). Bars = 50 μm. (b) The relative fluorescence intensity was measured with Image J 1·38d software within the dashed rectangular areas along the ORS. Data are presented as the mean ± SEM. **P < 0·01, ***P < 0·001.
Melanocortin receptor 2 (MC-R2) was significantly upregulated by corticotropin-releasing hormone and downregulated by caffeine
MC-R2, the key receptor for ACTH, was detected membrane bound in the outer and inner root sheaths, with predominance in the outer parts of the inner root sheath (Figure 8a, upper panel). Weaker MC-R2 immunoreactivity was found in the connective tissue sheath (Figure 8a, upper right image). While MC-R2 was seen rather weakly in control HFs, it was significantly enhanced by CRH 10−7 mol L−1, by 23% compared with the control follicles (P < 0·001) (Figure 8b). Co-incubation with caffeine 0·001% significantly decreased MC-R2 by 11% compared to CRH alone (P < 0·01) (Figure 8b).
Figure 8.
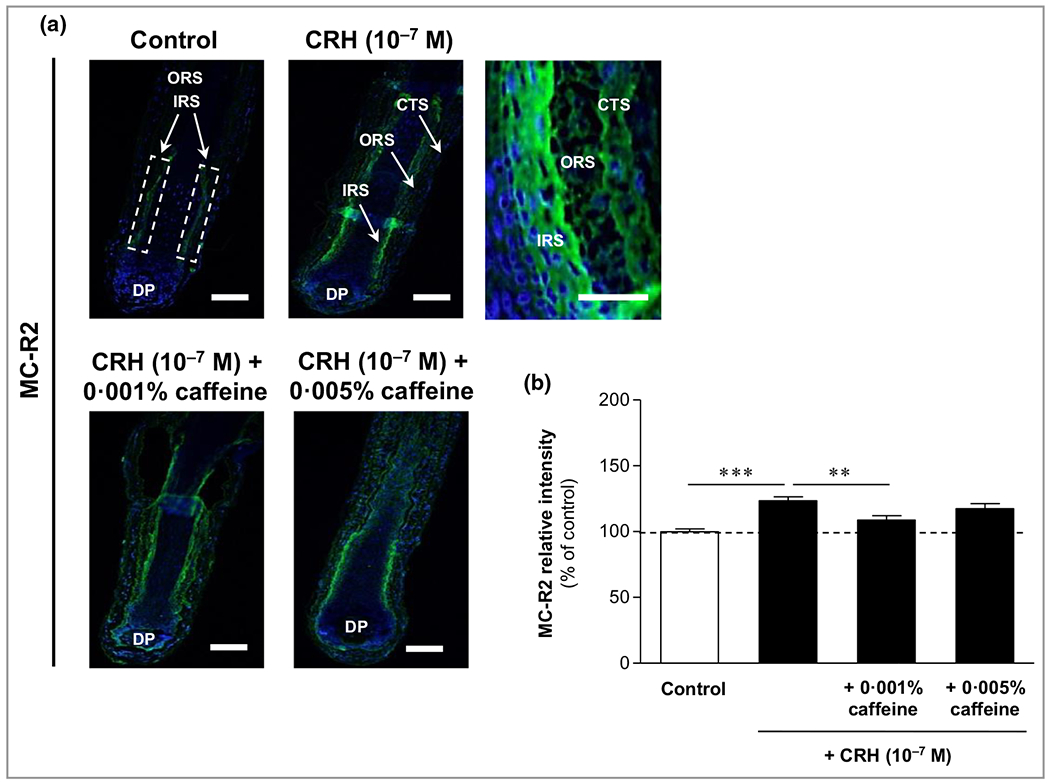
Effects of corticotropin-releasing hormone (CRH) and caffeine on the hypothalamic–pituitary–adrenal axis-dependent stress parameter melanocortin receptor 2 (MC-R2) in human hair follicles. (a) MC-R2 immunoreactivity (green fluorescence) was detected in the outer root sheath (ORS) and inner root sheath (IRS) (white dashed rectangular areas) with membrane-bound localization (magnification in upper right panel. Weaker MC-R2 immunoreactivity was found in the connective tissue sheath (CTS) (magnification in upper right panel). DP, dermal papilla. Bars = 50 μm. (b) The relative fluorescence intensity was measured with Image J 1·3 8d software within the dashed rectangular areas along the IRS. Data are presented as the mean ± SEM. **P < 0·01, ***P < 0·001.
The investigated stress parameters dependent on the HPA axis and their downstream positions along the HPA axis are presented in Figure 9.
Figure 9.
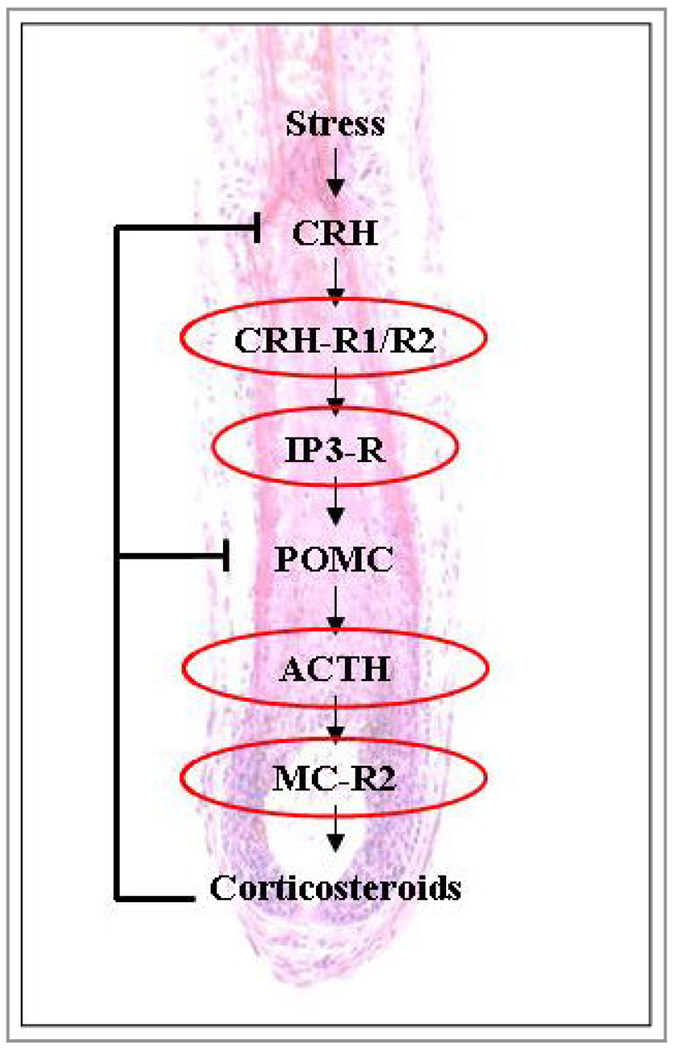
A representation of the downstream positions of the hypothalamic–pituitary–adrenal axis (HPA)-dependent stress parameters along the HPA axis. The red coloured ellipses indicate the stress parameters investigated in this study. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; CRH-R, CRH receptor; IP3-R, inositol triphosphate receptor; MC-R2, melanocortin receptor 2; POMC, proopiomelanocortin.
Corticotropin-releasing hormone and caffeine regulated the non-hypothalamic–pituitary–adrenal axis stress parameters substance P, p75 neurotrophin receptor and NGF-tyrosine kinase receptor A
Substance P was significantly enhanced by corticotropin-releasing hormone but reduced by caffeine
CRH and caffeine were identified to influence non-HPA-axis dependent stress parameters such as SP, p75NTR and TrkA. SP was detected in the Henle layer of the inner root sheath (Figure 10a, upper image panel). CRH (10−7 mol L−1) significantly increased SP in the Henle layer by 17% (P < 0·05) (Figure 10a, upper right panel, Figure 10b). Co-incubation with caffeine at 0·001% and 0·005% significantly decreased SP by 21% (P < 0·001) and 17% (P < 0·01), respectively (Figure 10).
Figure 10.
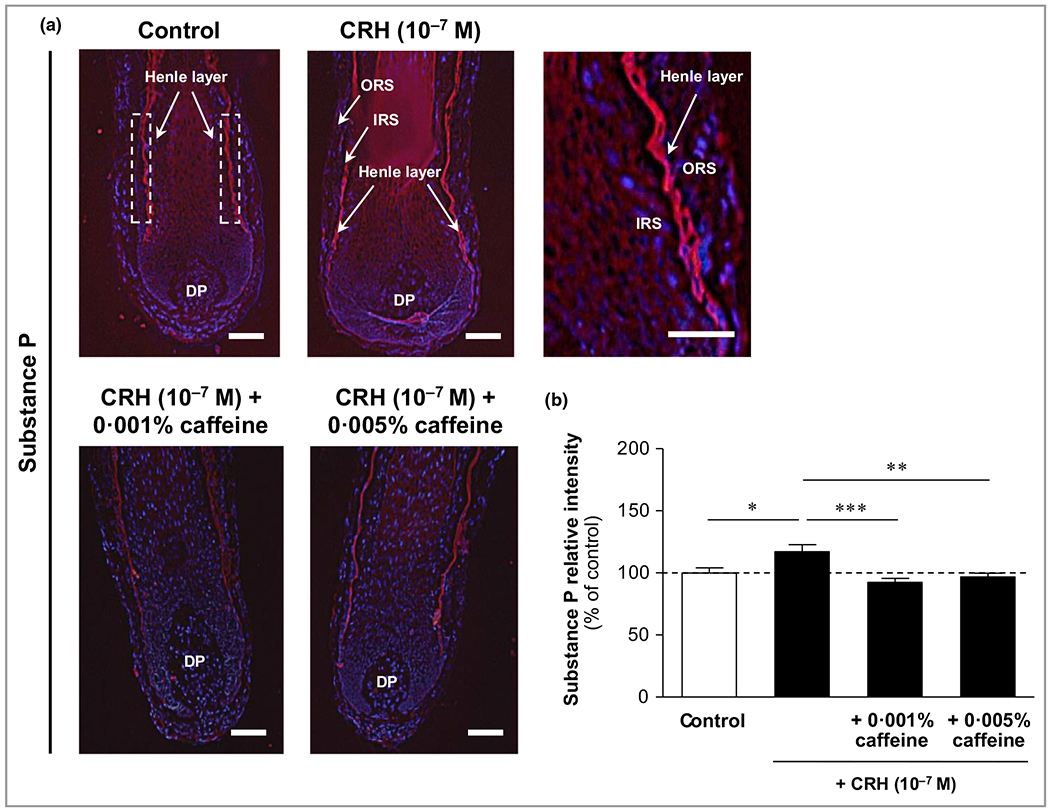
Effects of corticotropin-releasing hormone (CRH) and caffeine on the hypothalamic–pituitary–adrenal (HPA) axis-independent stress parameter substance P in human hair follicles. (a) Substance P immunoreactivity (red fluorescence) was detected precisely in the Henle layer of the inner root sheath (IRS) (white dashed rectangular areas in the upper left image, and magnification in the upper right panel). DP, dermal papilla. Bars = 50 μm. (b) The relative fluorescence intensity was measured with Image J 1·38d software within the dashed rectangular areas along the Henle layer of the inner root sheath (IRS). The data are presented as the mean ± SEM. *P < 0·05, **P < 0·01, ***P < 0·001.
Proapoptotic p75NTR was significantly induced by corticotropin-releasing hormone but reduced by caffeine
Immunoreactivity of the pro-apoptotic neurotrophin receptor p75NTR was detected in the outer root sheath with membrane-bound localization (Figure 11a, upper panel). It was significantly increased by CRH 10−7 mol L−1 by 27% (P < 0·01), while it was significantly reduced by co-incubation with caffeine 0·001%, by 24% (P < 0·01) (Figure 11b).
Figure 11.
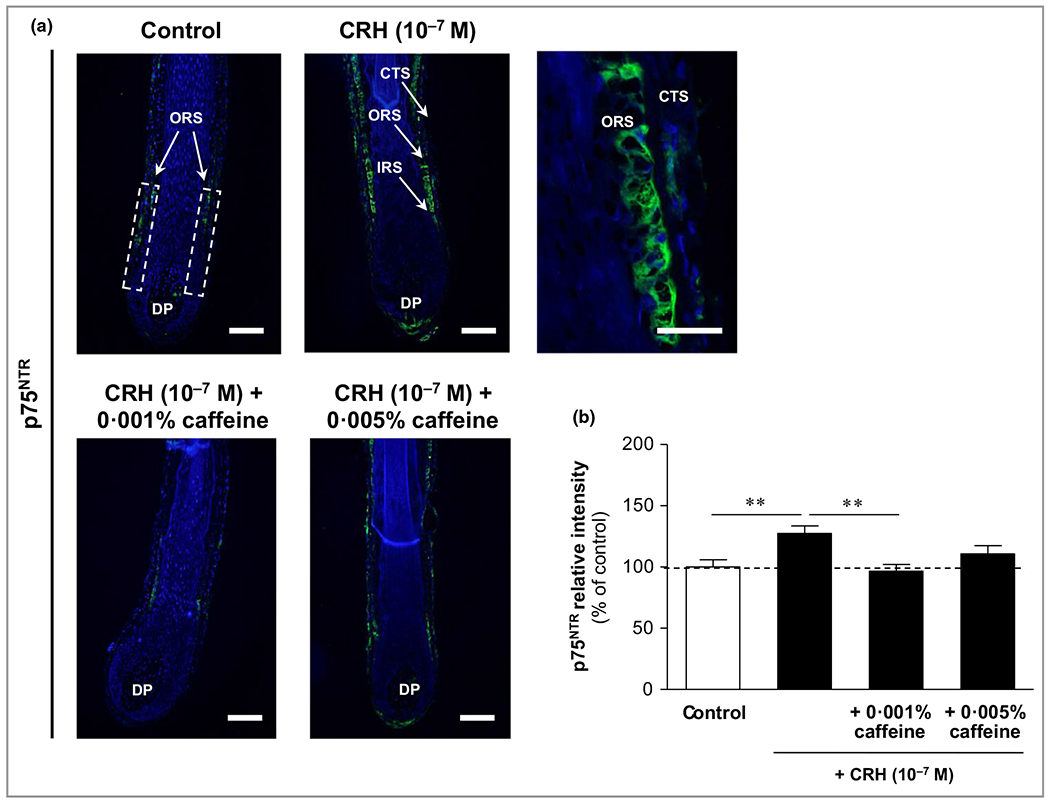
Effects of corticotropin-releasing hormone (CRH) and caffeine on the hypothalamic–pituitary–adrenal (HPA) axis-independent stress parameter p75 neurotrophin receptor (p75NTR) in human hair follicles. The immunoreactivity of p75NTR (green fluorescence) was detected in the outer root sheath (ORS) with membrane-bound localization (white dashed rectangular areas in the upper left image, and magnification in the upper right image). CTS, connective tissue sheath; DP, dermal papilla. Bars = 50 μm. (b) The relative fluorescence intensity was measured with Image J 1·38d software within the dashed rectangular areas along the outer root sheath (ORS). The data are presented as the mean ± SEM. **P < 0·01.
Antiapoptotic NGF-tyrosine kinase receptor A (TrkA) was enhanced by caffeine compared to corticotropin-releasing hormone (CRH), while CRH alone did not influence TrkA
Immunoreactivity of the antiapoptotic nerve growth factor receptor TrkA was detected in the outer root sheath with membrane-bound localization (Figure 12a). CRH 10−7 mol L−1 only slightly reduced TrkA compared to control (not significant), but caffeine 0·001% significantly enhanced TrkA immunoreactivity by 20% compared to CRH-treated HFs (P < 0·01) (Figure 12b). An overview of CRH- and caffeine-mediated effects in all immunofluorescence parameters is presented in Table 1.
Figure 12.
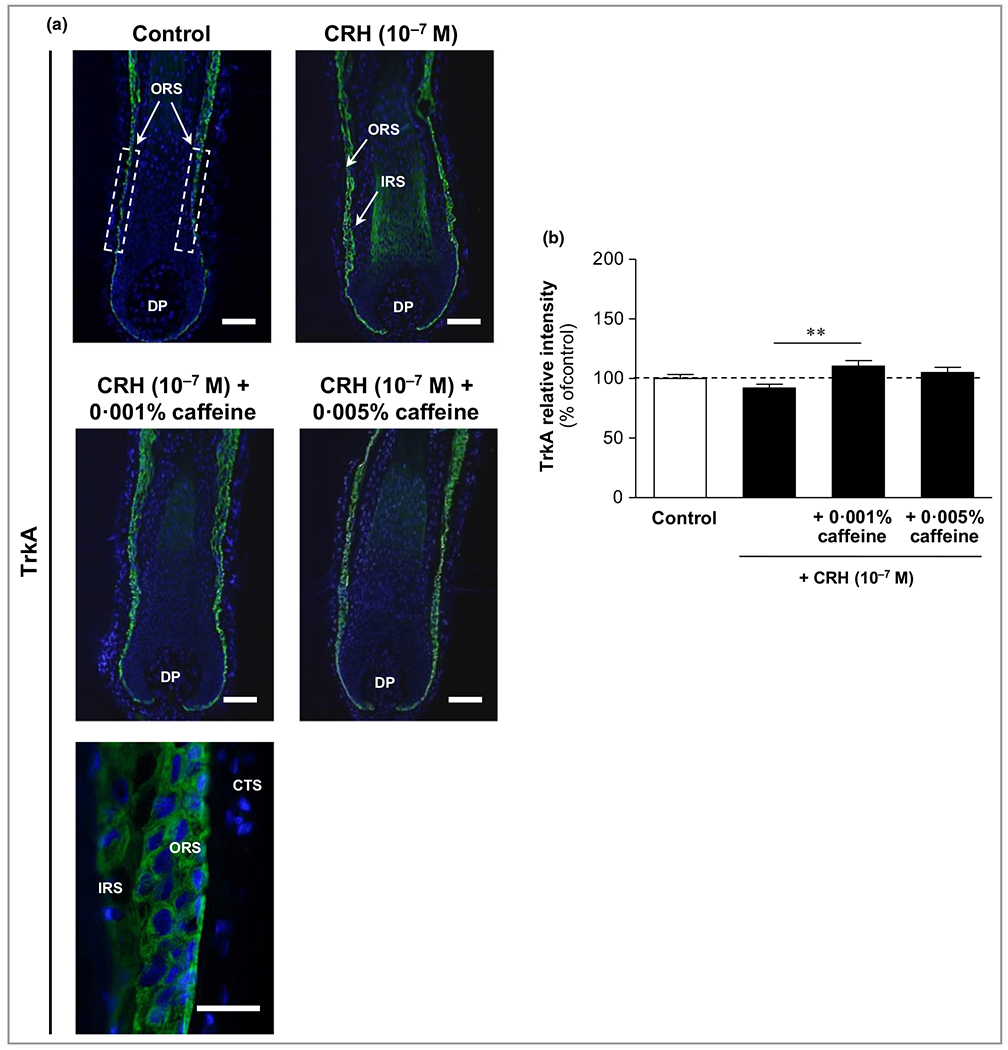
Effects of corticotropin-releasing hormone (CRH) and caffeine on the hypothalamic–pituitary–adrenal (HPA) axis-independent stress parameter NGF-tyrosine kinase receptor A (TrkA) in human hair follicles. (a) TrkA immunoreactivity (green fluorescence) was detected in the outer root sheath (ORS) with membrane-bound localization (white dashed rectangular areas in the upper left image, and magnification in the lower image). CTS, connective tissue sheath; DP, dermal papilla. Bars = 50 μm. (b) Relative fluorescence intensity was measured with Image J 1·38d software within the dashed rectangular areas along the outer root sheath (ORS). The data are presented as the mean ± SEM. **P < 0·01.
Table 1.
Overview of the effects of corticotropin-releasing hormone (CRH) 10−7 mol L−1 plus caffeine 0·001% or 0·005% on the investigated parameters assessed by immunofluorescence
| Parameter | CRH vs. control | CRH + caffeine 0·001% vs. CRH | CRH + caffeine 0·005% vs. CRH |
|---|---|---|---|
| HSE | ↓ | ↑ *** | – |
| Ki-67 | ↓ | ↓ | ↑ * |
| IGF-1 | ↓ | ↑ | ↑ *** |
| TGF-β2 | ↑ *** | ↓ | ↓ * |
| CRH-R1/2 | ↑ ** | ↓ *** | ↓ *** |
| IP3-R | ↓ | ↑ ** | ↑ *** |
| ACTH | ↑ *** | ↓ | ↓ ** |
| MC-R2 | ↑ *** | ↓ ** | ↓ |
| Substance P | ↑ * | ↓ *** | ↓ ** |
| p75NTR | ↑ ** | ↓ ** | ↓ |
| TrkA | ↓ | ↑ ** | ↑ |
CRH was used at 10−7 mol L−1 in all comparisons. Differences indicated with up or down arrows are ≥ 5% increases or decreases, respectively. Differences indicated with ‘–’ are no differences or differences < 5%. Significant differences are indicated as
P < 0·05,
P < 0·01,
P < 0·001.
ACTH, adrenocorticotropic hormone; CRH-R, CRH receptor; HSE, hair shaft elongation; IGF, insulin-like growth factor; IP3-R, inositol triphosphate receptor; MC-R2, melanocortin receptor 2; TGF, transforming growth factor; TrkA, NGF-tyrosine kinase receptor A.
Gene expression regulation of corticotropin-releasing hormone (CRH) receptors 1 and 2 in human hair follicles by CRH and caffeine
Co-cultivation of extracted HFs with caffeine 0·001% and CRH 10−7 mol L−1 led to a significant 200-fold upregulation of CHR-R1 and an approximately 1000-fold upregulation of CHR-R2 gene expression in human HFs compared with control HFs (Figure 13). CRH 10−7 mol L−1 alone had no effect on CHR-R1, but led to a significant 15-fold upregulation of CHR-R2 gene expression (Figure 13).
Figure 13.
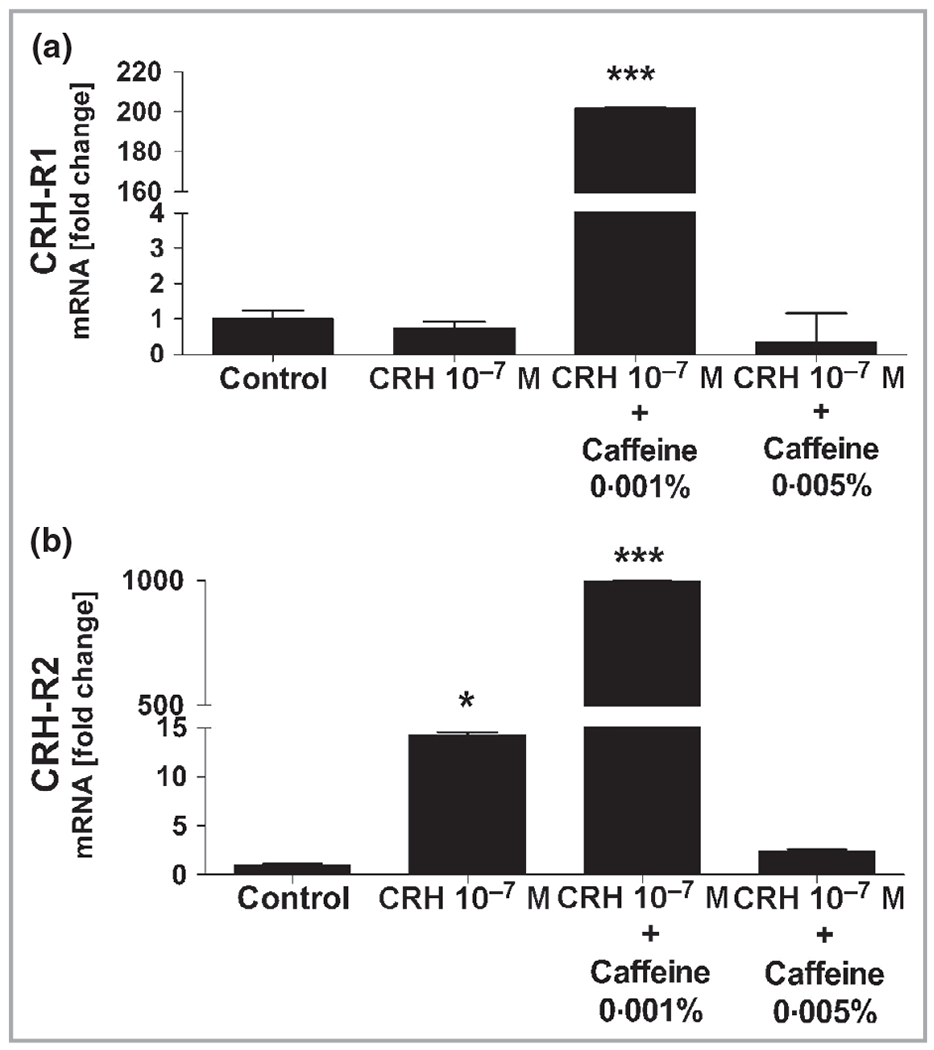
Gene expression of CRH-R1 and CRH-R2 in human hair follicles. Whole human hair follicles were lysed, then RNA was extracted and reverse transcribed for real-time polymerase chain reaction amplification for detection of the expression of (a) CRH-R1 and (b) CRH-R2. (a) CRH-R1 gene expression was 200-fold and significantly (***P < 0·001) upregulated in human hair follicles co-cultivated with caffeine 0·001% and corticotropin-releasing hormone (CRH) 10−7 mol L−1 compared to hair follicles incubated with control or only CRH 10−7 mol L−1. (b) CRH-R2 gene expression was 15-fold and significantly (*P < 0·05) upregulated in human hair follicles incubated with CRH 10−7 mol L−1 alone compared to controls, while it was approximately 1000-fold and significantly (***P < 0·001) upregulated in human hair follicles co-cultivated with caffeine 0·001% and CRH 10−7 mol L−1 compared to control or only CRH 10−7 mol L−1.
Discussion
While the original identification of the hair organ-specific local HPA-axis was shown earlier in female human hair follicles gained from routine face-lift surgery,20 the present study provides the first evidence for a functional HPA axis in HFs electively taken from the balding area of male human individuals with AGA, a condition found in every second man in European countries.46 Additionally, a new player along the HPA axis – IP3-R – was identified for the first time in human HFs.
While in the non-HPA-axis stress response of human organ cultured HFs a substance P mediated up-regulation of NGF and its apoptosis- and catagen-promoting receptor p75NTR and a down-regulation of the growth promoting, anti-apoptotic NGF receptor TrkA was shown earlier,15 the present study shows for the first time that CRH regulates SP, p75NTR and TrkA in human male AGA HFs.
Thus, the CRH-related human hair organ culture model has excellently proven to be a well-suited human hair stress model in a multifaceted way, and caffeine counteracted this stress response significantly. Caffeine was used at the same concentrations previously shown to be effective by our group in male or female human HFs.41,42
Consistent with our previous studies,41,42 we used anagen stage VI (terminal) HFs and not intermediate HFs as suggested by Miranda et al.47 The latter are suitable for investigation of hair growth stimulation, but are more sensible in stress induction (e.g. by CRH) or experimentally induced growth suppression (e.g. by testosterone41,42 or ultraviolet irradiation)48 as in the present and previous studies.
CRH induces ‘growth suppression’ in cell cultures of skin cells of different origin,49 with the most reported suppressive concentrations in cell culture and female HFs of 10−7 mol L−1 and 10−8 mol L−1.20,49,50 We confirmed the concentration of 10−7 mol L−1 as the most inhibitory. Concentrations higher than 10−7 mol L−1 were not tested by our group or others as they might be considered unphysiological.51
CRH plays an important role in skin and hair biology, as shown by hair-cycle-dependent expression of CRH-R1 and CRH-R2 in mice,52,53 and by a CRH-induced functional cascade along the HPA axis in human normal epidermal melanocytes and dermal fibroblasts.54,55
The CRH stress-mediated decrease of IGF-1 and increase of TGF-β2 and their counter-regulation by caffeine are in agreement with our own previous results in testosterone-treated AGA HFs.42
CRH 10−7 mol L−1 activated its own receptor CRH-R1/2 in the HF, corresponding to previous observations in human organ-cultured HFs as well as in in situ models.20,21,35 The normalization of CRH-R1/2 expression by caffeine might be explained by caffeine-mediated inhibitory gene regulation of CRH-R1/2 synthesis; however, we observed the opposite: caffeine lead to over-upregulation of the CRH-R1 and CRH-R2 genes. This might be explained by the assumption that caffeine would directly reduce CRH-R1/2 expression leading to reduced receptor density on the cell membrane, or, alternatively, caffeine might directly and competitively inhibit CRH binding at its receptor. The first hypothesis is more likely, as a reduced availability of free CRH receptors would reactively upregulate its gene expression, as shown by our results.
IP3-R is an intracellular cation channel that is located primarily at the membrane of the endoplasmic reticulum in a large range of cell types.56 Inositol-1,4,5-trisphosphate (IP3) is the most important ligand and leads after receptor binding to a fast release of Ca2+ ions, inducing a complex local and global Ca2+ signal response. IP3-R has additional activating ligands such as Ca2+ itself, ATP and cAMP.57 Via CRH-R1, CRH stimulates IP3 production, shown in human keratinocytes treated with CRH at 10−7 mol L−1.58 A similar effect with Ca2+ release might prove relevant also in HF keratinocytes. The slight CRH-induced reduction of its receptor, IP3-R, might be considered a negative feedback mechanism, observed also for example with the insulin receptor.59 Caffeine was reported to inhibit IP3-mediated cellular responses.60,61
However, as a phosphodiesterase inhibitor, caffeine increases cAMP,62 opens Ca2+ channels and increases intracellular Ca2+ levels.63 Thus, caffeine shows paradoxical effects on the intracellular Ca2+ balance, which might be dependent on the caffeine concentration. In our study, the increase of IP3-R by caffeine is most likely due to a decrease of intracellular Ca2+ and a consecutive upregulation of IP3-R as a positive feedback mechanism.
ACTH showed an immunoreactivity pattern in our HFs corresponding to previous reports,20,48 as well as its enhancement by CRH 10−7 mol L−1.20 The downregulating effect of caffeine might be mediated by phosphodiesterase inhibition because the activation of POMC gene expression and ACTH processing is mediated by the cAMP signal pathway.37,54,55
Also, the observed expression pattern of MC-R2 corresponded to the study of Ito et al.20 CRH might directly regulate MC-R2; however, an indirect stimulatory effect via ACTH was also described by Chakraborty and Pawelek.64 Caffeine showed a significant decrease of MC-R2 expression down to an almost normal level, thus confirming the potent stress inhibitory effects of caffeine at multiple points along the HPA axis.
SP immunoreactivity was significantly enhanced in the Henle layer by CRH. So far, it has been shown that SP can only be built in the dermal nerve fibres that surround the HF,65 seen also in alopecia areata scalp skin biopsies.66 Our extracted HFs were lacking the surrounding tissue and nerves, and therefore SP had to be derived from other sources. Such a source might be mast cells and human keratinocytes from the inner and outer root sheaths, which are known to liberate SP,67,68 or the connective tissue sheath, which is known to be an HF-associated reservoir of SP.15 Ito et al. reported mast cell activation and degranulation in human HFs by CRH 10−7 mol L−1,69 confirming our observation. To the best of our knowledge, this is the first report of a supposedly autocrine liberation of SP by the HF itself. The caffeine-induced reduction of SP might be mediated by its stimulatory effect on SP-degrading enzymes (e.g. endopeptidase and angiotensin-converting enzyme).70
The immunoreactivity localization of p75NTR in our study corresponds to that in previous studies.15,71 p75NTR mediates apoptotic signals72,73 and is strongly expressed in catagen and telogen.71
Expression of the transmembrane antiapoptotic high-affinity NGF tyrosine kinase receptor TrkA was found predominantly in the outer root sheath in the hair shaft area, as well as in the bulb region, which is also consistent with previous reports.15,71,74,75
The central role of the neurogenic non-HPA axis stress mediators SP, p75NTR and TrkA is described here for the first time in human male AGA HFs and confirms results from stressed mice developing hair loss.3,11,76 Also, the influence of caffeine on the neurogenic non-HPA axis stress response is a new observation.
In conclusion, our study shows for the first time a parallel CRH-induced stress response along the peripheral intrafollicular HPA axis and the neurogenic SP-mediated non-HPA axis in human HFs from men with AGA. Both stress responses are inhibited by caffeine, suggesting its potential for the clinical treatment of AGA or stress-induced hair loss.
Supplementary Material
What is already known about this topic?
Caffeine stimulates hair growth in male and female human hair follicles (HFs) in vitro.
What does this study add?
For the first time, corticotropin-releasing hormone induction of the hypothalamic–pituitary–adrenal (HPA) stress axis is documented in male human HFs from biopsies (balding vertex area) of men with androgenetic alopecia.
First time, the non-HPA neurogenic stress axis is shown in the same male human HFs.
Caffeine counteracts both stress axes.
Inositol trisphosphate receptor was newly identified in human HFs.
What is the translational message?
Stress can impair human hair physiology and induce hair loss.
Caffeine may effectively counteract stress-induced hair damage and possibly prevent stress-induced hair loss.
Acknowledgments
We greatly appreciate the valuable technical support of the technician Antje Winter-Keil.
Funding sources
This study was supported in part with funds from the Medical Faculty of the University of Lübeck, Germany, to T.W.F., and by a translational research grant from Dr. Kurt Wolff GmbH to T.W.F. and R.P. Partial support was obtained from the 1R01AR056666, 1R01AR073004-01A1 and R01 AR071189-01A1 grants from the National Institutes of Health to A.T.S.
Conflicts of interest
This study was supported in part by Dr. Kurt Wolff GmbH, and both T.W.F. and R.P. have served in a consultancy function for this company.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
Appendix S1 Reagents.
Appendix S2 Hair follicle microdissection and organ culture.
Appendix S3 Immunofluorescence labeling, tyramide signal amplification staining and standard immunofluorescence.
Appendix S4 Statistical analysis.
Table S1 List of quantitative real-time polymerase chain reaction forward and reverse primers used for gene-expression analysis of CRH-R1 and CRH-R2.
Table S2 Antibodies and staining techniques used for fluorescence labelling of the investigated proteins.
References
- 1.Fischer TW, Schmidt S, Strauss B, Elsner P. Hairdex: a tool for evaluation of disease-specific quality of life in patients with hair diseases. Hautarzt 2001; 52:219–27. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt S, Fischer TW, Chren MM et al. Strategies of coping and quality of life in women with alopecia. Br J Dermatol 2001; 144:1038–43. [DOI] [PubMed] [Google Scholar]
- 3.Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol 2004; 123:455–7. [DOI] [PubMed] [Google Scholar]
- 4.Paus R, Arck P. Neuroendocrine perspectives in alopecia areata: does stress play a role? J Invest Dermatol 2009; 129:1324–6. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos A, Chrousos GP. Stress-related skin disorders. Rev Endocr Metab Disord 2016; 17:295–304. [DOI] [PubMed] [Google Scholar]
- 6.York J, Nicholson T, Minors P, Duncan DF. Stressful life events and loss of hair among adult women, a case–control study. Psychol Rep 1998; 82:1044–6. [DOI] [PubMed] [Google Scholar]
- 7.Gatherwright J, Liu MT, Gliniak C et al. The contribution of endogenous and exogenous factors to female alopecia: a study of identical twins. Plast Reconstr Surg 2012; 130:1219–26. [DOI] [PubMed] [Google Scholar]
- 8.Gatherwright J, Liu MT, Amirlak B et al. The contribution of endogenous and exogenous factors to male alopecia: a study of identical twins. Plast Reconstr Surg 2013; 131:794e–801e. [DOI] [PubMed] [Google Scholar]
- 9.Shin H, Choi SJ, Cho AR et al. Acute stress-induced changes in follicular dermal papilla cells and mobilization of mast cells: implications for hair growth. Ann Dermatol 2016; 28:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters EM, Handjiski B, Kuhlmei A et al. Neurogenic inflammation in stress-induced termination of murine hair growth is promoted by nerve growth factor. Am J Pathol 2004; 165:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoe-motional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol 2006; 15:1–13. [DOI] [PubMed] [Google Scholar]
- 12.Joachim RA, Kuhlmei A, Dinh QT et al. Neuronal plasticity of the ‘brain–skin connection’: stress-triggered up-regulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathways. J Mol Med (Berl) 2007; 85:1369–78. [DOI] [PubMed] [Google Scholar]
- 13.Arck PC, Handjiski B, Kuhlmei A et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med (Berl) 2005; 83: 386–96. [DOI] [PubMed] [Google Scholar]
- 14.Peters EM, Stieglitz MG, Liezman C et al. p75 neurotrophin receptor-mediated signaling promotes human hair follicle regression (catagen). Am J Pathol 2006; 168:221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters EM, Liotiri EM, Bodó E et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol 2007; 171:1872–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang WY, Huang YC, Huang KS et al. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial–mesenchymal interaction. J Dermatol Sci 2017; 86:114–22. [DOI] [PubMed] [Google Scholar]
- 17.Trüeb RM. The impact of oxidative stress on hair. Int J Cosmet Sci 2015; 37 (Suppl. 2):25–30. [DOI] [PubMed] [Google Scholar]
- 18.Haslam IS, Jadkauskaite L, Szabó IL et al. Oxidative damage control in a human (mini-)organ: Nrf2 activation protects against oxidative stress-induced hair growth inhibition. J Invest Dermatol 2017; 137:295–304. [DOI] [PubMed] [Google Scholar]
- 19.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev 2000; 21:457–87. [DOI] [PubMed] [Google Scholar]
- 20.Ito N, Ito T, Kromminga A et al. Human hair follicles display a functional equivalent of the hypothalamic–pituitary–adrenal axis and synthesize cortisol. FASEB J 2005; 19:1332–4. [DOI] [PubMed] [Google Scholar]
- 21.Slominski A, Pisarchik A, Tobin DJ et al. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology 2004; 145:941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slominski AT, Zmijewski MA, Zbytek B et al. Key role of CRF in the skin stress response system. Endocr Rev 2013; 34:827–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-κB in human epidermal keratinocytes. J Endocrinol 2004; 181:R1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zbytek B, Slominski AT. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J Invest Dermatol 2007; 127:730–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philpott M, Green M, Kealey T. Human hair growth in vitro. J Cell Sci 1990; 97:463–71. [DOI] [PubMed] [Google Scholar]
- 26.Philpott M, Sanders D, Westgate G, Kealey T. Human hair growth in vitro: a model for the study of hair follicle biology. J Dermatol Sci 1994; 7 (Suppl.):S55–72. [DOI] [PubMed] [Google Scholar]
- 27.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev 2001; 81:449–94. [DOI] [PubMed] [Google Scholar]
- 28.Paus R, Langan EA, Vidali S et al. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med 2014; 20:559–70. [DOI] [PubMed] [Google Scholar]
- 29.Ohnemus U, Uenalan M, Inzunza J et al. The hair follicle as an estrogen target and source. Endocr Rev 2006; 27:677–706. [DOI] [PubMed] [Google Scholar]
- 30.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med 1999; 341:491–7. [DOI] [PubMed] [Google Scholar]
- 31.Foitzik K, Krause K, Nixon AJ et al. Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen. Am J Pathol 2003; 162:1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slominski A, Wortsman J, Luger T et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 2000; 80:979–1020. [DOI] [PubMed] [Google Scholar]
- 33.Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids 2015; 103:72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paus R, Botchkarev VA, Botchkareva NV et al. The skin POMC system (SPS). Leads and lessons from the hair follicle. Ann N Y Acad Sci 1999; 885:350–63. [DOI] [PubMed] [Google Scholar]
- 35.Ito N, Ito T, Betterman A et al. The human hair bulb is a source and target of CRH. J Invest Dermatol 2004; 122:235–7. [DOI] [PubMed] [Google Scholar]
- 36.Skobowiat C, Dowdy JC, Sayre RM et al. Cutaneous hypothalamic–pituitary–adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab 2011; 301:E484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol 2007; 265–6:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguilera G, Rabadan-Diehl C, Nikodemova M. Regulation of pituitary corticotropin releasing hormone receptors. Peptides 2001; 22:769–74. [DOI] [PubMed] [Google Scholar]
- 39.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci 1999; 885:312–28. [DOI] [PubMed] [Google Scholar]
- 40.Czok G. Coffee and its physiological effects. Arch Sci Med (Torino) 1972; 129:32–8. [PubMed] [Google Scholar]
- 41.Fischer TW, Hipler UC, Elsner P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int J Dermatol 2007; 46:27–35. [DOI] [PubMed] [Google Scholar]
- 42.Fischer TW, Herczeg-Lisztes E, Funk W et al. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and TGF-β2-/IGF-1-mediated regulation of hair cycle in male and female human hair follicles in vitro. Br J Dermatol 2014; 171:1031–43. [DOI] [PubMed] [Google Scholar]
- 43.Langan EA, Philpott M, Kloepper JE, Paus R. Human hair follicle organ culture: theory, application and perspectives. Exp Dermatol 2015; 24:903–11. [DOI] [PubMed] [Google Scholar]
- 44.Kloepper JE, Sugawara K, Al-Nuaimi Y et al. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol 2010; 19:305–12. [DOI] [PubMed] [Google Scholar]
- 45.Sehringer B, Zahradnik HP, Simon M et al. mRNA expression profiles for corticotrophin-releasing hormone, urocortin, CRH-binding protein and CRH receptors in human term gestational tissues determined by real-time quantitative RT-PCR. J Mol Endocrinol 2004; 32:339–48. [DOI] [PubMed] [Google Scholar]
- 46.Alfonso M, Richter-Appelt H, Tosti A et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin 2005; 21:1829–36. [DOI] [PubMed] [Google Scholar]
- 47.Miranda BH, Tobin DJ, Sharpe DT, Randall VA. Intermediate hair follicles: a new more clinically relevant model for hair growth investigations. Br J Dermatol 2010; 163:287–95. [DOI] [PubMed] [Google Scholar]
- 48.Lu Z, Fischer TW, Hasse S et al. Profiling the response of human hair follicles to ultraviolet radiation. J Invest Dermatol 2009; 129:1790–804. [DOI] [PubMed] [Google Scholar]
- 49.Slominski AT, Roloff B, Zbytek B et al. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol Anim 2000; 36:211–16. [DOI] [PubMed] [Google Scholar]
- 50.Quevedo ME, Slominski A, Pinto W et al. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim 2001; 37:50–4. [DOI] [PubMed] [Google Scholar]
- 51.Suda T, Tomori N, Yajima F et al. Immunoreactive corticotropin-releasing factor in human plasma. J Clin Invest 1985; 76:2026–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slominski A, Ermak G, Hwang J et al. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta 1996; 1289:247–51. [DOI] [PubMed] [Google Scholar]
- 53.Roloff B, Fechner K, Slominski A et al. Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. FASEB J 1998; 12:287–97. [DOI] [PubMed] [Google Scholar]
- 54.Slominski A, Zbytek B, Szczesniewski A et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab 2005; 288:E701–6. [DOI] [PubMed] [Google Scholar]
- 55.Slominski A, Zbytek B, Semak I et al. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol 2005; 162:97–102. [DOI] [PubMed] [Google Scholar]
- 56.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 1984; 312:315–21. [DOI] [PubMed] [Google Scholar]
- 57.Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium 1999; 26:237–51. [DOI] [PubMed] [Google Scholar]
- 58.Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol 2005; 203:118–26. [DOI] [PubMed] [Google Scholar]
- 59.Blackard WG, Guzelian PS, Small EE. Down regulation of insulin receptors. Trans Am Clin Climatol Assoc 1979; 90:94–101. [PMC free article] [PubMed] [Google Scholar]
- 60.Parker I, Ivorra I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol 1991; 433:229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang SS, Han KS, Ku BM et al. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res 2010; 70:1173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butcher RW, Sutherland EW. Adenosine 3′,5′-phosphate in biological materials. I. Purification and properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J Biol Chem 1962;237:1244–50. [PubMed] [Google Scholar]
- 63.Wojtal KA, Hoekstra D, van Ijzendoorn SC. cAMP-dependent protein kinase A and the dynamics of epithelial cell surface domains: moving membranes to keep in shape. Bioessays 2008; 30:146–55. [DOI] [PubMed] [Google Scholar]
- 64.Chakraborty A, Pawelek J. MSH receptors in immortalized human epidermal keratinocytes: a potential mechanism for coordinate regulation of the epidermal-melanin unit. J Cell Physiol 1993; 157:344–50. [DOI] [PubMed] [Google Scholar]
- 65.Schulze E, Witt M, Fink T et al. Immunohistochemical detection of human skin nerve fibers. Acta Histochem 1997; 99:301–9. [DOI] [PubMed] [Google Scholar]
- 66.Hordinsky MK, Kennedy W, Wendelschafer-Crabb G, Lewis S. Structure and function of cutaneous nerves in alopecia areata. J Invest Dermatol 1995; 104 (5 Suppl.):28–9. [DOI] [PubMed] [Google Scholar]
- 67.Bae S, Matsunaga Y, Tanaka Y, Katayama I. Autocrine induction of substance P mRNA and peptide in cultured normal human keratinocytes. Biochem Biophys Res Commun 1999; 263:327–33. [DOI] [PubMed] [Google Scholar]
- 68.Arck PC, Slominski A, Theoharides TC et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol 2006; 126:1697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito N, Sugawara K, Bodó E et al. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J Invest Dermatol 2010; 130:995–1004. [DOI] [PubMed] [Google Scholar]
- 70.Scholzen TE, Luger TA. Neutral endopeptidase and angiotensin-converting enzyme – key enzymes terminating the action of neuroendocrine mediators. Exp Dermatol 2004; 13 (Suppl. 4):22–6. [DOI] [PubMed] [Google Scholar]
- 71.Adly MA, Assaf HA, Hussein MR. Expression pattern of p75 neurotrophin receptor protein in human scalp skin and hair follicles: hair cycle-dependent expression. J Am Acad Dermatol 2009; 60:99–109. [DOI] [PubMed] [Google Scholar]
- 72.Botchkarev VA, Yaar M, Peters EM et al. Neurotrophins in skin biology and pathology. J Invest Dermatol 2006; 126:1719–27. [DOI] [PubMed] [Google Scholar]
- 73.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci 1995; 18:321–6. [PubMed] [Google Scholar]
- 74.Iacaruso MF, Galli S, Martí M et al. Structural model for p75NTR–TrkA intracellular domain interaction: a combined FRET and bioinformatics study. J Mol Biol 2011; 414:681–98. [DOI] [PubMed] [Google Scholar]
- 75.Adly MA, Assaf HA, Nada EA et al. Expression of nerve growth factor and its high-affinity receptor, tyrosine kinase A proteins, in the human scalp skin. J Cutan Pathol 2006; 33:559–68. [DOI] [PubMed] [Google Scholar]
- 76.Siebenhaar F, Sharov AA, Peters EM et al. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata). J Invest Dermatol 2007; 127:1489–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


