Abstract
Introduction
In cognitively normal (CN) adults, increased rates of amyloid beta (Aβ) accumulation can be detected in low Aβ (Aβ–) apolipoprotein E (APOE) ε4 carriers. We aimed to determine the effect of ε4 on the ability to benefit from experience (ie, learn) in Aβ– CNs.
Methods
Aβ– CNs (n = 333) underwent episodic memory assessments every 18 months for 108 months. A subset (n = 48) completed the Online Repeatable Cognitive Assessment‐Language Learning Test (ORCA‐LLT) over 6 days.
Results
Aβ– ε4 carriers showed significantly lower rates of improvement on episodic memory over 108 months compared to non‐carriers (d = 0.3). Rates of learning on the ORCA‐LLT were significantly slower in Aβ– ε4 carriers compared to non‐carriers (d = 1.2).
Discussion
In Aβ– CNs, ε4 is associated with a reduced ability to benefit from experience. This manifested as reduced practice effects (small to moderate in magnitude) over 108 months on the episodic memory composite, and a learning deficit (large in magnitude) over 6 days on the ORCA‐LLT. Alzheimer's disease (AD)–related cognitive abnormalities can manifest before preclinical AD thresholds.
Keywords: Alzheimer's disease, amyloid, apolipoprotein E, learning, memory
1. INTRODUCTION
In cognitively normal (CN) older adults, elevated amyloid beta (Aβ+) is associated with episodic memory dysfunction, hippocampal volume loss, accumulation of Aβ, and increased rate of progression to mild cognitive impairment (MCI) or dementia, relative to matched adults with low Aβ (Aβ–). 1 , 2 The severity of these clinical and biological manifestations of Aβ+ is increased further by the apolipoprotein E (APOE) ε4 allele 3 , 4 proposed to be a consequence of ε4 disrupting normal Aβ clearance. 5 Subtle but increased rates of Aβ accumulation over 3 to 4 years can also be detected in ε4 carriers who remain Aβ–, 6 raising the possibility that cognitive changes may be detectable in Aβ– ε4 carriers if the study design or cognitive assessments applied have sufficient sensitivity.
In the Australian Imaging, Biomarkers and Lifestyle (AIBL) study, CNs completed seven neuropsychological assessments over 108 months providing greater power than previous investigations of Aβ– groups to understand the effects of ε4 on cognition. 3 , 7 However, prospective investigations of cognitive change in AIBL, and in similar longitudinal cohorts, now show that in Aβ+ CNs, episodic memory remains stable over 5 to 6 years, whereas in matched Aβ– CNs, memory improves substantially over the same interval (ie, a practice effect). 8 , 9 , 10 , 11 Reduced practice effects are proposed to be a strong clinical marker of early Alzheimer's disease (AD) pathologic changes in preclinical AD, 9 , 11 and are therefore likely to occur in CN Aβ– ε4 carriers. However, we have argued that a more parsimonious conceptualization of observations of reduced practice effects is that in very early AD, deficits in the ability to benefit from experience (ie, to learn) are greater than deficits in memory retrieval; at least as when measured by standardized tests of episodic memory. 10 , 12 We challenged this hypothesis in preclinical AD, and found that deficits on a formal learning paradigm, evident over 6 days, were four times greater than the abnormal change in episodic memory detected across the prior 6 years. 12 Application of this learning model may therefore also inform understanding of any AD‐related cognitive dysfunction in CN Aβ– ε4 carriers.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using traditional (eg, PubMed) sources, meeting abstracts, and presentations. Studies reporting on the role of apolipoprotein E (APOE) in low amyloid beta (Aβ)– cognitively normal older adults were included. Studies on practice effects in the context of aging and Alzheimer's disease (AD) were also reviewed.
Interpretation: Our findings are novel in showing that reduced ability to benefit from experience (ie, learn) is evident in Aβ– ε4 carriers. This manifested as reduced practice effects over 108 months on the episodic memory composite, albeit of a small‐to‐moderate magnitude, and a learning deficit that was large in magnitude over 6 days on the Online Repeatable Cognitive Assessment‐Language Learning Test (ORCA‐LLT).
Future Directions: Future studies are required to determine the extent to which other neuroinflammatory, cerebrovascular, or neurodegenerative processes may be related to this learning deficit in Aβ– adults.
2. METHODS
2.1. Participants
Aβ– CN older adults (n = 333) enrolled in the AIBL study provided a blood sample for APOE genotyping, and underwent serial neuropsychological assessments every 18 months, for at least three timepoints. A subgroup of these participants (n = 48), naïve to Chinese, Japanese or Korean languages, also participated in a 6‐day learning challenge (Figure 1 summarizes the number of participants contacted, eligible, enrolled, and included in this analysis). No participant had progressed to MCI/AD. Recruitment and inclusion/exclusion criteria of AIBL have been described previously. 13 , 14 A clinical panel comprised of geriatricians, neurologists, and neuropsychologists determined the cognitive normality of participants by examining all available medical and neuropsychological information. This clinical panel was blind to genetic and neuroimaging information. Participants were classified as cognitively normal if they performed greater than –1 standard deviation on all neuropsychological tests when compared to Australian norms, had a Mini‐Mental State Examination (MMSE) score of 26 or greater, and a Clinical Dementia Rating (CDR) sum of boxes score of 0 or 0.5 (CDR sum of boxes score of 0.5 was acceptable if all neuropsychological tests were within normative ranges). Demographic characteristics are summarized in Table 1.
FIGURE 1.
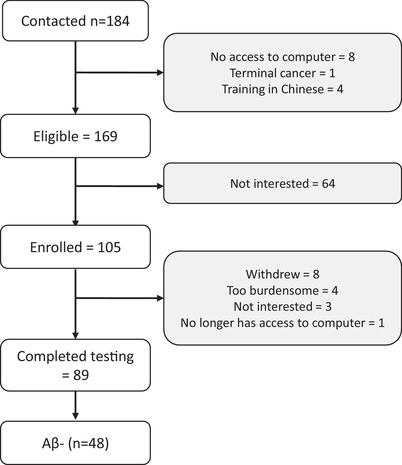
Flowchart of the number of participants contacted, enrolled, and completed Online Repeatable Cognitive Assessment‐Language Learning Test (ORCA‐LLT)
TABLE 1.
Demographic, clinical, cardiovascular, and neuroimaging characteristics
| AIBL CN sample | ORCA CN subsample | |||||
|---|---|---|---|---|---|---|
| Aβ‐ ε4‐ (n = 273) | Aβ‐ ε4+ (n = 60) | Aβ‐ ε4‐(n = 35) | Aβ‐ ε4+ (n = 13) | |||
| Mean (SD) | Mean (SD) | p | Mean (SD) | Mean (SD) | p | |
| N, Female (%) | 154 (46.2) | 33 (55.0) | .842 | 18 (60.0) | 5 (41.7) | .281 |
| Age at first assessment, y | 69.37 (5.87) | 67.69 (5.51) | .044 | 74.40 (4.93) | 73.85 (5.24) | .735 |
| Years of education | 12.36 (2.96) | 12.48 (3.05) | .790 | 13.85 (2.87) | 12.15 (3.11) | .083 |
| HADS‐anxiety† | 4.42 (2.85) | 4.79 (3.58) | .472 | 3.65 (2.97) | 4.00 (3.70) | .735 |
| HADS‐depression† | 2.60 (2.25) | 3.11 (2.44) | .211 | 2.32 (2.23) | 3.08 (2.66) | .331 |
| MMSE† | 28.86 (1.20) | 28.94 (1.10) | .616 | 29.12 (1.04) | 29.15 (1.21) | .919 |
| CDR Sum of Boxes† | 0.03 (0.16) | 0.04 (0.14) | .736 | 0.04 (0.19) | 0.19 (0.43) | .108 |
| Body mass index | 26.87 (4.15) | 26.25 (3.61) | .305 | 26.45 (3.67) | 25.39 (3.17) | .433 |
| Abdominal circumference, cm | 92.98 (13.28) | 92.44 (13.19) | .790 | 89.66 (11.27) | 90.70 (7.62) | .795 |
| Diastolic blood pressure, mmHg | 79.15 (10.33) | 77.24 (8.73) | .192 | 81.48 (9.18) | 78.60 (7.72) | .394 |
| Systolic blood pressure, mmHg | 136.52 (15.34) | 139.19 (15.22) | .230 | 136.74 (15.99) | 136.80 (7.76) | .991 |
| Centiloid† | 1.71 (9.87) | 2.70 (9.69) | .481 | 0.92 (4.63) | ‐0.60 (10.13) | .478 |
| Hippocampal volume, cm3† | 2.95 (0.29) | 2.97 (0.25) | .686 | 2.94 (0.25) | 3.01 (0.28) | .426 |
| N years between first PET scan and baseline AIBL cognitive assessment | 3.28 (2.54) | 2.61 (2.39) | .064 | – | – | – |
| N years between most recent PET scan and ORCA assessment | – | – | – | 1.31 (1.22) | 0.94 (0.84) | .319 |
| N AIBL cognitive assessments | 6.14 (1.20) | 6.27 (1.12) | .464 | 5.21 (2.31) | 4.69 (2.18) | .492 |
Abbreviations: AIBL, Australian Imaging, Biomarkers and Lifestyle Study; CDR, Clinical Dementia Rating; CN, cognitively normal; HADS, Hospital Anxiety and Depression Scale; MMSE, Mini‐Mental State Examination; ORCA, Online Repeatable Cognitive Assessment; PET, positron emission tomography; SD, standard deviation.
† obtained from the PET scan for the AIBL CN sample and closest PET scan to ORCA‐LLT assessment for the ORCA subsample; bolded values are significant at P < .05.
The AIBL study was approved by institutional research and ethics committees. 14 Human research ethics approval to conduct this study was obtained through Melbourne Health. 15 Informed consent was provided in writing prior to participation in this study.
2.2. Episodic memory composite
The rationale and validation of the AIBL episodic memory composite has been described. 3 Raw scores on the California Verbal Learning Test, Second Edition (CVLT‐II) delayed recall trial, the Logical Memory delayed recall trial, and the Rey Complex Figure Test (RCFT) 30‐minute delayed recall trial were standardized using the baseline mean and standard deviation of the Aβ– CN group, and averaged. As has been reported previously, identical forms of these memory tests were used at each assessment timepoint (administered in 18‐month intervals). 16 , 17
2.3. Learning test
The Online Repeatable Cognitive Assessment‐Language Learning Test (ORCA‐LLT) has also been described. 12 , 15 This test measured the ability to learn the English language equivalent of 50 Chinese characters over six sessions. Participants were required to determine whether the English word and Chinese character had the same meaning. Sessions consisted of two blocks of 200 trials of both correct and incorrect pairs with each block requiring approximately 10 minutes to complete. Within each block, each Chinese character was presented four times in random order. For two of these presentations, the Chinese character was paired with the correct spoken English word. For the remaining two presentations, incorrect spoken English words were selected at random from the other possible 49 words. Each day of the task provided unique sets of incorrect pairings, while the correct pairings stayed constant over time, to prevent off‐target learning of incorrect pairs. Thus, the ratio of correct to incorrect pairings for the first day was 4:2, for the second day was 8:2, for the third day was 12:2, for the fourth day was 16:2, for the fifth day was 20:2, and for the final day was 24:2. The order of trials was randomized for each session and participant. Participants were unaware of the underlying ratio of correct to incorrect pairings, and no feedback regarding accuracy of the decision was provided to participants. The primary outcome of the ORCA‐LLT was accuracy (percentage of correct responses).
2.4. Neuroimaging
Aβ imaging with positron emission tomography (PET) was conducted using one of four radioligands: Pittsburgh Compound B, florbetapir, flutemetamol, or navidea. The acquisition protocol for each radioligand has been detailed previously. 13 , 18 Threshold values for elevated Aβ deposition varied by radiotracer, so all standardized uptake value ratios (SUVR) were transformed onto the Centiloid scale using CapAIBL. 19 , 20 Aβ– was classified if Centiloid scores were <15 at the closest imaging visit relative to participants’ AIBL baseline cognitive assessment or ORCA assessment.
2.4.1. Procedure
Participants completed the AIBL neuropsychological battery every 18 months. A subgroup completed the ORCA‐LLT in their own homes through a web‐based application using either a laptop or desktop computer daily for 6 days. Assessors of the AIBL neuropsychological battery and the ORCA‐LLT were blind to Aβ neuroimaging and genetic results.
2.5. Data analysis
Analyses were conducted using R v.3.5.0. Although data distributions for raw proportion correct performance scores on the ORCA‐LLT were distributed normally (Shapiro‐Wilks test, Ps > .100 for all days), an arcsine square‐root transformation was applied prior to analyses. Arcsine square‐root transformations are used commonly for analyses of proportion correct scores as they increase the range of possible values when scales are bounded by chance (ie, 50%) and a perfect score (ie, 100%), which in turn can increase statistical power. 21 To ensure that this normalization did not distort outcomes, we repeated analyses using raw proportion correct data to determine the similarity of conclusions drawn from analyses using transformed data.
Differences between APOE ε4 carriers and non‐carriers in the rate of change on the episodic memory composite were determined using a linear mixed‐effects model (unstructured covariance matrix, maximum likelihood estimation, participant as random factor). Similarly, the difference between ε4 carriers and non‐carriers on the rate of learning on the ORCA‐LLT was determined using a linear mixed‐effects model. For the ORCA‐LLT, the Akaike information criterion (AIC) for the linear and quadratic models were 1534.18 and 1554.17, respectively, with the linear model demonstrating a significantly better fit (lower AIC values), χ2 = 52.99, P < .001. Similarly, for the episodic memory composite, the AIC for the linear and quadratic models were 4023.22 and 4042.91, with the quadratic model not significantly better than the linear model, χ2 = 1.53, P = .465. Age was included as a covariate in all models. The unit of time for both mixed‐effects models was test session (ie, months for the episodic memory composite and days for ORCA‐LLT). Cohen's d was used to express the magnitude of between‐group differences.
3. RESULTS
3.1. Sample characteristics
APOE ε4 carriers were slightly younger than ε4 non‐carriers, but were equivalent on other demographic, clinical, cardiovascular, and neuroimaging characteristics (Table 1). In the ORCA sub‐sample, ε4 carriers and non‐carriers were matched on all demographic, clinical and neuroimaging characteristics. The average completion rate for the ORCA‐LLT was 98% across all days, with the lowest completion rate observed on Day 6 (ie, 96%).
3.2. Effect of APOE ε4 on episodic memory and short‐term learning
For the episodic memory composite, a significant ε4 x time interaction was observed. Decomposition of the interaction indicated that Aβ– CN ε4 carriers demonstrated a significantly slower rate of improvement over 108 months compared to Aβ– CN ε4 non‐carriers (Figure 2A). This difference was small to moderate in magnitude (Table 2). Analyses of the ORCA‐LLT learning curves indicated that ε4 carriers showed significantly slower rates of learning compared to ε4 non‐carriers (modeled data in Figure 2B; raw data in Figure 2C), with the difference large in magnitude (Table 2). Re‐analyses of the ORCA‐LLT learning curves using untransformed data also yielded a significant interaction between APOE ε4 and time, albeit with a smaller effect size, d (95% confidence interval) = 1.18 (0.48, 1.84), P < .001.
FIGURE 2.
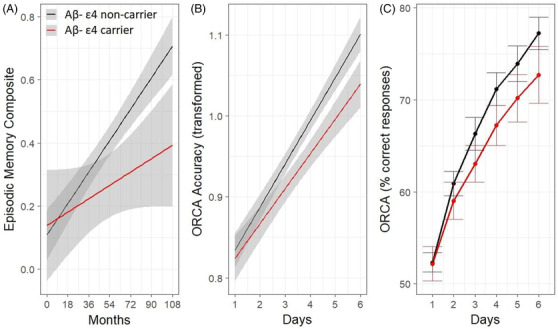
Effect of APOE ε4 on episodic memory performance over 108 months modeled using unadjusted estimates (A), and ORCA‐LLT performance over 6 days, modeled using linear mixed model (B), and raw group means (C). Shaded areas and error bars represent 95% confidence intervals. Aβ, amyloid beta; APOE, apolipoprotein E; ORCA‐LLT, Online Repeatable Cognitive Assessment‐Language Learning Test
TABLE 2.
Mean slopes (SD) and Cohen's d representing group mean slope differences from the mixed‐effects model on episodic memory performance in the broader AIBL sample, and accuracy of performance on the ORCA‐LLT
| AIBL CN full sample (outcome: EM composite) | ORCA CN subsample (outcome: ORCA‐LLT accuracy) | |||
|---|---|---|---|---|
| β (SE) | P | β (SE) | P | |
| APOE ε4 | –0.195 (0.117) | .096 | –0.311 (0.184) | .097 |
| Age | –0.291 (0.045) | <.001 | –0.028 (0.082) | .734 |
| Time | 0.148 (0.013) | <.001 | 0.752 (0.020) | <.001 |
| APOE ε4 × Time | –0.068 (0.031) | .027 | –0.147 (0.040) | <.001 |
| Mean (SD) | N | Mean (SD) | N | |
| Aβ‐ ε4 non‐carrier | 0.148 (0.215) | 273 | 0.752 (0.118) | 35 |
| Aβ‐ ε4 carrier | 0.079 (0.215) | 60 | 0.604 (0.126) | 13 |
| Cohen's d (95% CI) | 0.32 (0.04, 0.60) | 1.23 (0.53, 1.89) | ||
Abbreviations: AIBL, Australian Imaging, Biomarkers and Lifestyle (AIBL) study; APOE, apolipoprotein E; CI, confidence interval; CN, cognitively normal; EM, Episodic memory; ORCA‐LLT, Online Repeatable Cognitive Assessment‐Language Learning Test; SD, standard deviation; SE, standard error.
Notes: Bolded values are significant at P < .05; all β estimates reported have been standardized.
4. DISCUSSION
Our study shows that in Aβ– CN older adults, the APOE ε4 allele is associated with a reduced ability to learn, or put more broadly, as a reduced ability to benefit from experience. One manifestation of this is a reduction in the practice effect expected from 9 years of retesting on neuropsychological tests that yield the AIBL episodic memory composite (Figure 2A). However, despite the considerable length of follow‐up, number of reassessments using the same versions of the memory tests, and sample size, the magnitude of the reduced practice effect in the Aβ– CN ε4 group was only small to moderate (d = 0.3). More proactively, in ε4 carriers, the reduced ability to benefit from experience was evident from only 6 days of testing on the ORCA‐LLT. In the ORCA paradigm, the failure to benefit from experience manifested in the substantially lower ability of the Aβ– CN ε4 carriers to learn a set of 50 Chinese character‐English word pairs, with the magnitude of this reduction much larger (d = 1.2) than that observed for the episodic memory composite (Figure 2B).
Reduced practice effects on episodic memory tests have been observed previously in preclinical AD groups from AIBL and other prospective studies, 8 , 9 , 10 although the magnitude of these reductions (d = 0.4) have been only slightly larger than those observed between the current sample of Aβ– ε4 carriers and non‐carriers. Recent investigations into Aβ– individuals have identified subsets with faster Aβ accumulation, 7 particularly in ε4 carriers, 6 although no study has observed memory decline in either AD risk groups when individuals who progressed to MCI/AD were excluded. 7 The large deficit in learning observed in Aβ– ε4 carriers on the ORCA‐LLT is qualitatively similar to that reported previously, albeit with a slightly reduced magnitude, in the comparison of older adults with preclinical AD to Aβ– controls (ie, d > 2). 12 While this learning deficit likely reflects the deleterious effects of accumulating Aβ on the neurons or synapses necessary for the acquisition of new information, the precise biological basis of this interaction requires further exploration.
The learning paradigm used in the ORCA‐LLT was based on experimental psychological models; 22 , 23 however, the modification to require aspects of language learning makes the outcomes of this study directly generalizable to the functional aspects of daily living of older adults at risk of developing AD. 15 The large learning deficit observed in older adults who carried a strong genetic risk factor for AD, but for whom Aβ levels had not reached current thresholds of abnormality, suggest that the earliest AD‐related cognitive dysfunction in otherwise CN older adults will be evident when they are required to acquire new and complex information, such as learning aspects of a new language. Future studies will be required to determine the extent to which acquisition of other novel and complex information would be similarly impaired (eg, learning a new technical procedure).
There are several limitations to consider when interpreting the results of our study. First, only a small subset of Aβ– ε4 carriers completed the ORCA‐LLT as the number of ε4 carriers who remained Aβ– after 12 to 13 years of follow‐up in AIBL was substantially reduced. However, despite our small sample size, we observed a high rate of completion (98%) on the ORCA‐LLT. Additionally, our previous observation that short‐term learning deficits in preclinical AD were very large (d > 2), 12 which provided reassurance that even with this relatively small sample size, we would have sufficient power to observe a qualitatively similar deficit in Aβ– ε4 carriers. When considered together with the reduced practice effect observed over years, the learning deficits observed over days provides an important foundation for challenges of this approach in larger samples of older, or even middle‐aged, Aβ– ε4 carriers, which may help to further clarify the nature and magnitude of this effect and elucidate its bases in models of AD pathogenesis. Second, individuals with substantial cardiovascular disease were excluded from enrollment into the AIBL study. As such, while it is unlikely that the learning deficit observed here in Aβ– ε4 carriers could be attributed to cardiovascular disease, it will be important for future studies to determine the extent to which other neuroinflammatory, cerebrovascular, or neurodegenerative processes may be related to this learning deficit. Finally, while the ORCA‐LLT was designed to be a prospective measure of learning, we did not examine the extent to which performance on the ORCA‐LLT changes over longer periods of time (eg, 1–2 years). It will be important for future studies to determine whether the nature and magnitude of learning rates change over time.
These limitations notwithstanding, the consistent observation that AD risk factors such as Aβ accumulation and APOE ε4 are associated with substantial learning deficits, that can be detected over days, supports the hypothesis that cognitive dysfunction in early AD manifests as a failure to benefit from experience. Furthermore, the presence of this large learning deficit in Aβ– CN ε4 carriers shows, perhaps for the first time, that AD‐related clinical abnormalities can manifest strongly in CN individuals even before they reach thresholds that currently define preclinical AD.
CONFLICTS OF INTEREST
YY Lim, J Baker, A Mills, L Bruns Jr, C Fowler, J Fripp, SR Rainey‐Smith, D Ames, CL Masters, and P Maruff report no conflicts of interest related to the article.
FUNDING
Funding for the AIBL study was provided in part by the study partners (Australian Commonwealth Scientific Industrial and Research Organization [CSIRO], Edith Cowan University [ECU], Mental Health Research Institute [MHRI], Alzheimer's Australia [AA], National Ageing Research Institute [NARI], Austin Health, CogState Ltd., Hollywood Private Hospital, Sir Charles Gardner Hospital). The study also received support from the National Health and Medical Research Council (NHMRC) and the Dementia Collaborative Research Centres program (DCRC2), as well as ongoing funding from the Science and Industry Endowment Fund (SIEF).
YYL reports grants from the National Health and Medical Research Council (GNT1111603, GNT1147465). Funding for the ORCA study was provided by the Dementia Australia Research Foundation and the Victorian Medical Research Acceleration Fund.
ACKNOWLEDGMENTS
Alzheimer's Australia (Victoria and Western Australia) assisted with promotion of the AIBL study and the screening of telephone calls from volunteers. We acknowledge the financial support of the Cooperative Research Centre (CRC) for Mental Health. The CRC program is an Australian Government Initiative. We thank all those who participated in the study for their commitment and dedication to helping advance research into the early detection and causation of AD.
Lim YY, Baker JE, Mills A, et al. Learning deficit in cognitively normal APOE ε4 carriers with LOW β‐amyloid. Alzheimer's Dement. 2021;13:e12136. 10.1002/dad2.12136
REFERENCES
- 1. Jack CRJ, Wiste HJ, Therneau TM, Weigand SD, Knopman DS, Mielke MM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. J Am Med Assoc. 2019;321:2316‐2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker JE, Lim YY, Pietrzak RH, Hassenstab J, Snyder PJ, Masters CL, et al. Cognitive impairment and decline in cognitively normal older adults with high amyloid‐β: a meta‐analysis. Alzheimer's & Dementia: diagnosis. Alzheimers Dement (Amst). 2016;6:108‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim YY, Kalinowski P, Pietrzak RH, Laws SM, Burnham S, Ames D, et al. Association of β‐amyloid and apolipoprotein E ε4 with memory decline in preclinical Alzheimer disease. JAMA Neurol. 2018;75:488‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, et al. Amyloid and APOE E4 interact to influence short‐term decline in preclinical Alzheimer's disease. Neurology. 2014;82:1760‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim YY, Mormino EC. the Alzheimer's Disease Neuroimaging Initiative. APOE genotype and early β‐amyloid accumulation in older adults without dementia. Neurology. 2017;89:1028‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landau S, Horng A, Jagust WJ. Memory decline accompanies subthreshold amyloid accumulation. Neurology. 2018;90:e1452‐e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. Absence of practice effects in preclinical Alzheimer's disease. Neuropsychology. 2015;29:940‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duff K, Hammers DB, Dalley BCA, Suhrie KR, Atkinson TJ, Rasmussen KM, et al. Short‐term practice effects and amyloid deposition: providing information above and beyond baseline cognition. J Prev Alzheimers Dis. 2017;4:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker JE, Pietrzak RH, Laws SM, Ames D, Villemagne VL, Rowe CC, et al. Visual paired associate learning deficits associated with elevated beta‐amyloid in cognitively normal older adults. Neuropsychology. 2019. epub. [DOI] [PubMed] [Google Scholar]
- 11. Jutten RJ, Grandoit E, Foldi NS, Sikkes SAM, Jones RN, Choi SE, et al. Lower practice effects as a marker of cognitive performance and dementia risk: a literature review. Alzheimer's & Dementia: diagnosis. Alzheimers Dement (Amst). 2020;12:e12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim YY, Baker JE, Bruns L Jr, Mills A, Fowler C, Fripp J, et al. Association of deficits in short‐term learning and Aβ and hippocampal volume in cognitively normal adults. Neurology. 2020. epub. [DOI] [PubMed] [Google Scholar]
- 13. Rowe CC, Bourgeat P, Ellis KA, Brown B, Lim YY, Mulligan R, et al. Predicting Alzheimer disease with β‐amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol. 2013;74:905‐913. [DOI] [PubMed] [Google Scholar]
- 14. Ellis KA, Bush AI, Darby D, De Fazio D, Foster J, Hudson P, et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr. 2009;21:672‐687. [DOI] [PubMed] [Google Scholar]
- 15. Baker JE, Bruns Jr L, Hassenstab J, Masters CL, Maruff P, Lim YY. Use of an experimental language acquisition paradigm for standardized neuropsychological assessment of learning: a pilot study in young and older adults. J Clin Exp Neuropsychol. 2020;42:55‐65. [DOI] [PubMed] [Google Scholar]
- 16. Ellis KA, Rowe CC, Villemagne VL, Martins RN, Masters CL, Salvado O, et al. Addressing population aging and Alzheimer's disease through the Australian Imaging Biomarkers and Lifestyle study: collaboration with the Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement. 2010;6:291‐296. [DOI] [PubMed] [Google Scholar]
- 17. Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. Effect of amyloid on memory and non‐memory decline from preclinical to clinical Alzheimer's disease. Brain. 2014;137:221‐231. [DOI] [PubMed] [Google Scholar]
- 18. Villemagne VL, Pike KE, Chételat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bourgeat P, Dore V, Fripp J, Ames D, Masters CL, Salvado O, et al. Implementing the centiloid transformation for 11 C‐PiB and β‐amyloid 18 F‐PET tracers using CapAIBL. Neuroimage. 2018;183:387‐393. [DOI] [PubMed] [Google Scholar]
- 20. Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous MDS, Jagust WJ, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tabachnick BG, Fidell LS. Using multivariate statistics (5th ed.). Needham Heights: Allyn & Bacon; 2006. [Google Scholar]
- 22. Breitenstein C, Knecht S. Development and validation of a language learning model for behavioral and functional‐imaging studies. J Neurosci Methods. 2002;114:173‐179. [DOI] [PubMed] [Google Scholar]
- 23. Breitenstein C, Jansen A, Deppe M, Foerster AF, Sommer J, Wolbers T, et al. Hippocampus activity differentiates good from poor learners of a novel lexicon. Neuroimage. 2005;25:958‐968. [DOI] [PubMed] [Google Scholar]


