Abstract
Imperata cylindrica is a medicinal plant native to southwestern Asia and the tropical and subtropical zones. To date, 72 chemical constituents have been isolated and identified from I. cylindrica Among these compounds, saponins, flavonoids, phenols, and glycosides are the major constituents. Investigations of pharmacological activities of I. cylindrica revealed that this edible medicinal herb exhibits a wide range of therapeutic potential including immunomodulatory, antibacterial, antitumor, anti-inflammatory, and liver protection activities both in vivo and in vitro. The purpose of this review is to provide an overview of I. cylindrica studies until 2019. This article also intends to review advances in the botanical, phytochemical, and pharmacological studies and industrial applications of I. cylindrica, which will provide a useful bibliography for further investigations and applications of I. cylindrica in medicines and foods.
Keywords: Imperata cylindrica, edible medicinal herb, glycosides, immunomodulatory, antitumor
1. Introduction
The genus Imperata cylindrica. a member of Gramineae family, is a perennial rhizomatous plant that can grow on soils with a vast range of nutrients and moisture [1]. It is widely distributed in southwestern Asia and is specifically native to the tropical and subtropical zones. The dried rhizomes of Imperata cylindrica have been commonly used in China named “Bai mao gen”, in Korean named Mo-Geun-Chu-Chul-Mul, and in Japan as a crude drug named “Boukon (Imperata rhizome)”. The existing literature indicates that these herbal plants could be taken both internally and externally and it is regarded as a safe and effective treatment strategy. The rhizome of Imperata has been recorded in multiple versions of Chinese Pharmacopoeia, at the same time, it is also traditionally and ethnically used as a medicinal herb in Japan and Korea. In traditional Chinese medicine (TCM), the rhizome of Imperata could be used either alone or in combination with other herbal medicines to cure hematuresis, jaundice with damp-heat pathogen, emesis, hemorrhage and reducing fever and causing diuresis, anti-fever, and anti-inflammatory. A variety of classical formulas containing I. cylindrica is widely used in TCM clinic. For instance, a combination of I. cylindrica, Panax quinquefolius, Rehmannia glutinosa, and Wolfiporia cocos is commonly used for the treatment of hematuresis. The combination of I. cylindrica, Artemisia capillaris Thunb, and Gardenia jasminoides Ellis could significantly dispels dampness and heat. Thus, this is used widely in jaundice with damp-heat pathogen.
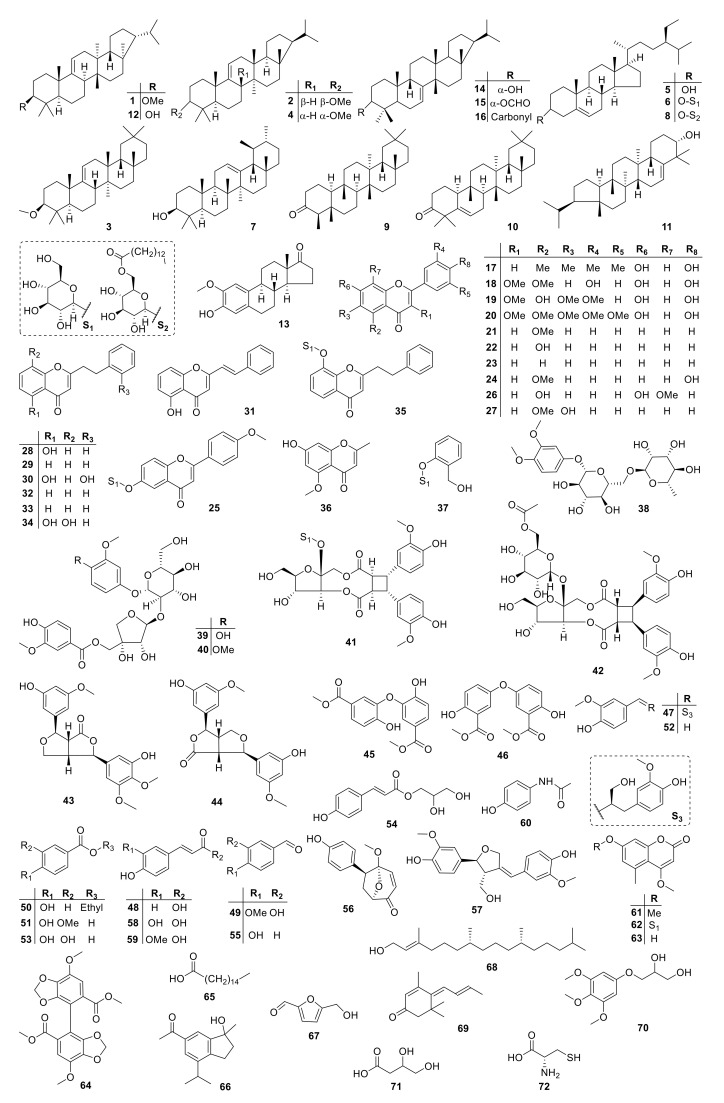
Until now, a total of 72 compounds have been isolated and identified from the I. cylindrica plants, and the major phytochemical constituents identified in I. cylindrica are saponins, glycosides, coumarins, flavonoids, and phenols. (Figure 1). Modern pharmacology investigations on I. cylindrica have indicated that several substances from I. cylindrica. exhibit a wide range of biological activities such as hemostasis, improvement of urination, anti-inflammatory, antibacterial, anticancer, enhancement of the immune system, etc.
Figure 1.
Constituents isolated from I. cylindrica.
In this review, we intended to systematically present the advances achieved in phytochemical studies in recent decades, along with all the compounds listed. Additionally, we now provide a systematic overview of the available information of I. cylindrica including traditional uses, botany, nutritional composition, physiochemical, pharmacology, and industrial use.
2. Botany
2.1. Botanical Traits
I. cylindrica is a member of Gramineae family. According to “The Plant List” (www.theplantlist.org, Dec. 2019) I. cylindrica is the only accepted name for the plant with relative to other synonyms including I. cylindrica var. Africana (Andersson) C.E.Hubb., I. cylindrica var. condensata (Steud.) Hack., I. cylindrica var. cylindrica, I. cylindrica var. europaea (Andersson) Asch. & Graebn., etc.
I. cylindrica is a perennial herb about 25–120 cm tall. Its culms often have 1–4-noded and its tough and scaly rhizomes spread widely. The leaf blades of I. cylindrica are often stiffly erect with a flat or rolled shape. The margins of culm blades are scabrid with pubescence on its adaxial surface. The flowers of I. cylindrical are panicle cylindrical with copious numbers of hairs. The spikelets are about 2.5–6 mm long. Glumes with long silky hairs on the back have 5–9-veined. The length of the lower ciliate ovate-lanceolate lemma is two-thirds of a glum and the length of the denticulate ciliate upper lemma is one half of a glum. The anthers are about 2.2–4 mm with purplish black color.
2.2. Traditional Uses
Generally, I. cylindrica plants have been used in traditional systems of medicine in oriental area. It was first recorded in the Chinese medical classic “Shen Nong’s herbal Classic”(Han Dynasty), which is listed as a middle grade herbal. It is described sweet in taste, cold in nature, and attributive to the lung, stomach, and bladder meridians, with relieving fever, and a diuretic effect. For the treatment of fever, polydipsia, vomiting blood, blood stasis, lung heat, difficulty in micturition, edema, jaundice, etc. Based on “Shen Nong’s herbal Classic (Han Dynasty)” I. cylindrica was used to invigorating spleen and replenishing qi. Complying with “Ming Yi Bie Lu” (Han Dynasty), I. cylindrica was used to irregular menses. According to “Ben Cao Gang Mu” (Ming Dynasty) the main function of I. cylindrica was to diuresis to eliminate edema, hemostasis. In “Ben Jing Feng Yuan” (Qing Dynasty), the I. cylindrical was described as a treatment for frequent vomiting. The usual processing methods of I. cylindrical are decocting with water, and I. cylindrical is also considered for external use. A therapy dosage of 9–30 g of I. cylindrica has been recommended in the 2015 edition of Chinese Pharmacopoeia.
For the purpose of compatibility of medicines, I. cylindrica was typically matched with the Morus alba L. for treatment of asthma. Moreover, I. cylindrica can be used in a combination with Puerariae lobatae Radix to relieve high fever with sweet. Besides, I. cylindrica and Rhizoma phragmitis can be used to lower the negative Qi to stop vomiting.
3. Nutritional and Physiochemical Composition
3.1. Nutritional Composition
Apart from above constituents, nutrient substances including crude fiber, carbohydrates, sugars, fatty acid, and trace elements have been proved to be contained in the I. cylindrica, indicating the low-calorie and health-promoting properties of I. cylindrica. The carbohydrates and sugars are energetic sources and add flavor to plants, whose presence reveals the potential of I. cylindrica as functional foods [2]. The inorganic nutrient analysis of the aboveground living parts of I. cylindrica showed that Ca2+, Na+, Mg2+, Fe3+, and K+ are the main trace elements in the plant [3]. Moreover, I. cylindrica can survive in seawater salt concentrations and simultaneously have high nutraceutical potential, for instance, some of these species have already been considered edible for cattle [2].
3.2. Physiochemical and Structural Features
The detailed phytochemical and nutritional analyses of I. cylindrica had been carried out. Among the constituents isolated from I. cylindrica, saponins, glycosides, flavonoids, coumarins, and phenols are the primary types. All compounds are summarized and compiled in Table 1, and the structure of them has been detailed in Figure 1.
Table 1.
Constituents isolated from I. cylindrica
| NO. | Name | CAS | Formula | References |
|---|---|---|---|---|
| Saponins | ||||
| 1 | Arundoin | 4555-56-0 | C31H52O | [4] |
| 2 | Cylindrin | 17904-55-1 | C31H52O | [4] |
| 3 | 26-Norolean-9(11)-ene, 3-methoxy-13-methyl-, (3β)- | 2392-92-9 | C31H52O | [4] |
| 4 | (8α)-Arborinol methyl ether | 27570-19-0 | C31H52O | [5] |
| 5 | β-Sitosterol | 83-46-5 | C29H50O | [6] |
| 6 | Daucosterol | 474-58-8 | C35H60O6 | [6] |
| 7 | α-Amyrin | 638-95-9 | C30H50O | [6] |
| 8 | 3-O-β-D-glucopyranosyl-6′-tetradecanoate | 120000-27-3 | C49H86O7 | [6] |
| 9 | Friedelin | 559-74-0 | C30H50O | [7] |
| 10 | Alnusenone | 508-09-8 | C30H48O | [8] |
| 11 | Simiarenol | 1615-94-7 | C30H50O | [8] |
| 12 | Fernenol | 4966-00-1 | C30H50O | [8] |
| 13 | 2-Methoxyestrone | 362-08-3 | C19H24O3 | [9] |
| 14 | 14-epiarbor-7-en-3β-ol | - | C30H50O | [10] |
| 15 | 14-epiarbor-7-en-3β-yl formate | - | C31H50O2 | [10] |
| 16 | 14-epiarbor-7-en- 3-one | - | C30H48O | [10] |
| Flavonoids | ||||
| 17 | Tricin | 520-32-1 | C17H14O7 | [6] |
| 18 | Caryatin | 1486-66-4 | C17H14O7 | [6] |
| 19 | Jaceidin | 10173-01-0 | C18H16O8 | [6] |
| 20 | 4H-1-Benzopyran-4-one, 7-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,5,6-trimethoxy- | 58130-92-0 | C19H18O8 | [6] |
| 21 | 5-Methoxyflavone | 42079-78-7 | C16H12O3 | [7] |
| 22 | 5-Hydroxyflavone | 491-78-1 | C15H10O3 | [11] |
| 23 | Flavone | 525-82-6 | C15H10O2 | [11] |
| 24 | 2-(4-Hydroxyphenyl)-5-methoxy-4H-1-benzopyran-4-one | 106848-87-7 | C16H12O4 | [11] |
| 25 | 4H-1-Benzopyran-4-one, 6-(β-D-glucopyranosyloxy)-2-(4-methoxyphenyl)- | 1588495-01-5 | C22H22O9 | [11] |
| 26 | 5,7-Dihydroxy-8-methoxyflavone | 632-85-9 | C16H12O5 | [11] |
| 27 | 6-Hydroxy-5-methoxy-2-phenyl-4H-1-benzopyran-4-one | 118021-60-6 | C16H12O4 | [11] |
| 28 | 5-hydroxy-2-(2-phenylethyl) chromone | 877673-99-9 | C17H14O3 | [12] |
| 29 | Flidersiachromone | 61828-53-3 | C17H14O2 | [12] |
| 30 | 5-Hydroxy-2-[2-(2-hydroxyphenyl)ethyl]-4H-1-benzopyran-4-one | 357637-15-1 | C17H14O4 | [12] |
| 31 | 5-Hydroxy-2-(2-phenylethenyl)-4H-1-benzopyran-4-one | 389136-26-9 | C17H12O3 | [12] |
| 32 | Flindersiachromone | 61828-53-3 | C17H14O2 | [11] |
| 33 | 4H-1-Benzopyran-4-one, 5-hydroxy-2-(2-phenylethyl)- | 877673-99-9 | C17H14O3 | [11] |
| 34 | 8-Hydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one | 1588494-99-8 | C17H14O3 | [11] |
| 35 | 4H-1-Benzopyran-4-one, 8-(β-D-glucopyranosyloxy)-2-(2-phenylethyl) | 1588495-00-4 | C23H24O8 | [11] |
| 36 | Maritimin | 3449-40-9 | C11H10O4 | [13] |
| Glycosides | ||||
| 37 | Salicin | 138-52-3 | C13H18O7 | [8] |
| 38 | 3,4-Dimethoxyphenyl 1-O-α-L-rhamnopyranosyl-(1→6)-O-β-D-glucopyranoside | 872885-48-8 | C20H30O12 | [11] |
| 39 | Seguinoside K | 257888-26-9 | C26H32O15 | [14] |
| 40 | Gymnetinoside D | 1588477-87-5 | C27H34O15 | [14] |
| 41 | Deacetylimpecyloside | 1622990-04-8 | C32H38O17 | [14] |
| 42 | Impecyloside | 1070967-17-7 | C34H40O18 | [15] |
| Phenols | ||||
| 43 | Graminone B | 161407-73-4 | C21H22O8 | [16] |
| 44 | Graminone A | 161407-72-3 | C20H20O7 | [16] |
| 45 | Cylindol A | 159225-89-5 | C16H14O7 | [17] |
| 46 | Cylindol B | 159225-90-8 | C16H14O7 | [17] |
| 47 | Imperanene | 163634-08-0 | C19H22O5 | [18] |
| 48 | 4-Hydroxy-cinnamic acid | 57-10-3 | C16H32O2 | [19] |
| 49 | Isovanillin | 621-59-0 | C8H8O3 | [6] |
| 50 | Ethyl p-hydroxybenzoate | 120-47-8 | C9H10O3 | [7] |
| 51 | Vanillic acid | 121-34-6 | C8H8O4 | [7] |
| 52 | p-Vinylguaiacol | 7786-61-0 | C9H10O2 | [20] |
| 53 | Protocatechuic acid | 99-50-3 | C7H6O4 | [8] |
| 54 | 1-O-p-Coumaroylglycerol | 63529-09-9 | C12H14O5 | [8] |
| 55 | 4-Hydroxybenzaldehyde | 123-08-0 | C7H6O2 | [11] |
| 56 | Impecylone | 1622990-03-7 | C14H14O4 | [14] |
| 57 | Impecylenolide | 1622990-05-9 | C20H20O7 | [14] |
| 58 | Caffeic acid | 331-39-5 | C9H8O4 | [13] |
| 59 | Ferulic acid | 1135-24-6 | C10H10O4 | [13] |
| 60 | N-Acetyl-p-aminophenol | 103-90-2 | C8H9 NO2 | [21] |
| Coumarins | ||||
| 61 | Siderin | 53377-54-1 | C12 H12 O4 | [22] |
| 62 | 7-O-Glucosyloxy-4-methoxy-5-methylcoumarin | 41680-13-1 | C17 H20 O9 | [8] |
| 63 | 7-Hydroxy-4-methoxy-5-methylcoumarin | 41680-12-0 | C11 H10 O4 | [11] |
| Other compounds | ||||
| 64 | Dimethyl 4,4′-dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-dicarboxylate | 73536-69-3 | C20 H18 O10 | [19] |
| 65 | Palmitic acid | 57-10-3 | C16 H32 O2 | [19] |
| 66 | Cylindrene | 158204-49-0 | C15 H20 O2 | [17] |
| 67 | 5-Hydroxymethylfurfural | 67-47-0 | C6 H6 O3 | [7] |
| 68 | Phytol | 150-86-7 | C20 H40 O | [20] |
| 69 | Tabanone | 13215-88-8 | C13 H18 O | [20] |
| 70 | 3-(3,4,5-Trimethoxyphenoxy)-1,2-propanediol | 68576-87-4 | C12 H18 O6 | [8] |
| 71 | 3, 4-Dihydroxybutyric acid | 1518-61-2 | C4 H8 O4 | [8] |
| 72 | L-Cysteine | 52-90-4 | C3 H7 NO2 S | [21] |
3.2.1. Saponins
I. cylindrical contain multiple triterpenoids. To date, 16 triterpenoid compounds have been isolated from I. cylindrica (1–16). Among them, most of compounds are pentacyclic triterpenoid sapogenins except for compounds (5), (6) and (8). The basic skeleton of the pentacyclic triterpenoid sapogenins could be divided into fernane and arborane. Arundoin (1) and cylindrin (2) are the first set of compounds isolated from I. cylindrica, which were methyl ethers of fernane type pentacyclic triterpenoid sapogenins, and friedelin (9) is an arborane type pentacyclic triterpenoid saponin. Additionally, some other triterpenoids such as 14-epiarbor-7-en-3β-ol (14), 14-epiarbor-7-en-3β-yl formate (15), and 14-epiarbor-7-en- 3-one (16) could also be found in this category. The contents of the triterpenoids are shown in Table 1.
3.2.2. Flavonoids
Flavonoids are a typical category of compounds present in the I. cylindrica plants. Among them, mainly chromone and other characteristic structure of flavonoids (17–36).
The chromone 28, 29, 32, and 36 have been isolated from I. cylindrica. Compound 28 showed significant neuroprotective activity against glutamate-induced neurotoxicity at 10.0 µm concentration in primary cultures of rat cortical cells (Jeong Seon et al., 2006). Some characteristic flavonoids have been separated and identified from I. cylindrica as well, such as tricin (17), caryatin (18), jaceidin (19), 5-methoxyflavone (21), 5-hydroxyflavone (22), flavone (23), and so on.
3.2.3. Glycosides
Biologically important glycosides have been less widespread in the I. cylindrica. To date, 6 types of glycosides compounds (37–42) have been isolated from the plants including impecyloside (42) as a lignin glycoside, seguinoside K (39), gymnetinoside D (40), and deacetylimpecyloside (41).
3.2.4. Phenols
Phenols are widely distributed in I. cylindrica. Approximately 18 kinds of phenols (43–60) have been isolated from I. cylindrical. Among them, simple phenols and phenolic acids can be found such as vanillic acid (51), caffeic acid (58), ferulic acid (59), protocatechuic acid (53), and so on. Moreover, the phenylpropanoids 1-O-p-Coumaroylglycerol (54) and biphenyl ether cylindol A (45) and cylindol B (46) also have been isolated from I. cylindrica. Along with the lignans graminone A (44) and graminone B (43), where graminone B at 10−4 M gave a 50% inhibition of the contractile response of the aorta isolated from rabbit to KCI (30 mM). Imperanene (47) exhibited the platelet aggregation activity.
3.2.5. Coumarins
Three coumarins, siderin (61), 7-O-glucosyloxy-4-methoxy-5-methylcoumarin (62), 7-hydroxy-4-methoxy-5-methylcoumarin (63) have been isolated from I. cylindrica, and all the coumarins belong to simple coumarins, 4, 7-dimethoxy-5-methylcoumarin as a common scaffold. Among them, compound 61 has been proved to have antibacterial activity [23].
3.2.6. Others
In addition to above-mentioned compounds, a sesquiterpenoid cylindrene (66) with vasoconstriction activity, was isolated again from rhizomes of this plant [17]. A phytotoxic tabanone (69) was isolated [20] to inhibit growth of frond area of duckweed, root growth of garden onion, and fresh weight gain of garden lettuce with 50% inhibition values of 0.094, 3.6, and 6.5 mM, respectively. The target site of tabanone remains unknown, but its mode of action results in rapid loss of membrane integrity and subsequent decrease in the rate of photosynthetic electron flow. The antimicrobial palmitic acid (65) and phytol (68) also have been isolated from I. cylindrica.
4. Progress of Pharmacological Studies on I. cylindrica
The traditional medicinal applications of I. cylindrica have inspired many pharmacological investigations. Extracts and isolated compounds from I. cylindrica plants exhibited multiple bioactivities—including diuretic activity, hemostasis, anti-inflammatory, antitumor, antibacterial, immunomodulatory. Here, we summarized the progress and list as follow.
4.1. Diuretic Activity
The diuretic activity of I. cylindrica plants was found to be related to their traditional uses for treating edema and difficulty in micturition. I. cylindrica water extract possessed diuretic activity in normal rabbit, the diuretic effect was the most obvious after administration for 5 to 10 days. This effect may be related to the nervous system, cutting off the peripheral nerves of the kidney, and its diuretic effect is lost. Additionally, the water extract can alleviate glomerular vasospasm, increase renal blood flow and renal filtration rate [24].
4.2. Hemostasis
The extract of I. cylindrica displayed hemostasis effect on rat blood heat hemorrhage model induced by dry yeast suspension combined with absolute ethanol. In addition, the aqueous extract of I. cylindrica can significantly shorten its prothrombin time, thrombin time and activated partial thromboplastin time, and its hemostasis is related to endogenous, exogenous thrombin, and internal and external sources. Other studies have shown that aqueous extract of I. cylindrica can enhance the activity of endogenous and exogenous coagulation system, up-regulated the expression of TXB2, reduced the level of 6-keto-PGF1α, promoted platelet aggregation, and enhanced hemostasis [25].
4.3. Anti-Inflammatory
Inflammation has been reported to be relative to various diseases. The aqueous extracts of I. cylindrica was reported to have anti-inflammatory activity by relieving the auricular edema in mice induced by dimethylbenzene, ameliorate the paw-swelling in rats by carrageenan, significantly blocking the increased permeability of celiac blood capillary by glacial acetic acid and was able to remarkably resist paw swelling in rats induced by zymosan A [26]. Some researchers studied the protective effect of I. cylindrica extract on renal tissues in rats with Adriamycin nephrosis. It was found that the ethyl acetate extracts of Imperatae Rhizoma had protective effect in rats with adriamycin nephrosis. It may be related to reducing the expression of NF-κB p65 and TGF-β1 and the content of TNF-α in renal tissues of rats reduce inflammation of kidney tissue [27].
In 2020, Putri et al. reported that the ethanol extract of cogongrass (I. cylindrica L.) roots could decrease the sepsis score and didnot cause any harm in body weight [28].
4.4. Antioxidant Activity
The good antioxidant capacity of I. cylindrica was found by Fenton method and potassium ferricyanide reduction method. Its IC50 for scavenging of hydroxyl radicals was 0.0948 mg/mL, while IC50 of ascorbic acid was 0.1096 mg/mL; in the ferricyanide considerable reduction method, the extract exhibited reducing power comparable to that of the ascorbic acid [29].
Other studies have shown that aqueous extract of I. cylindrica can enhance the activity of superoxide dismutase (SOD) in liver and brain tissue of mice with alcoholism, inhibiting the activity of hydroxyl radicals, reducing the level of malondialdehyde, improving the body’s antioxidant capacity, and alleviating the pathological damage of free radicals to liver and brain tissue. It suggested that I. cylindrica has a protective effect on liver and brain damage caused by alcoholism.
The hydroalcoholic root extract of I. cylindrica showed antioxidant activity of 14.33 ± 0.045 (10 µg/mL) to 36.56 ± 0.053 (50 µg/mL) by DPPH assay. Antioxidant activity was also confirmed with reduction by FRAP assays [30].
4.5. Antitumor
4.5.1. Crude Extract
The aqueous extracts of I. cylindrica and polysaccharides can inhibit the growth of proliferation of human hepatoma cell line SMMC-7721, HepG2 cells and solid tumors in H22 mice. They also can increase the Interleukin (IL) 2 secretion level of leukocyte-mediated peripheral blood of tumor-bearing mice, suggesting that I. cylindrica extracts have anti-hepatic tumor effects. The different concentrations of I. cylindrica water extract were reported to dose-dependently suppress hepatoma SMMC-7721 cells. The mechanism of action is to increase S period ratio at the same time reduce the G2/M phase cell proportion, and inducing apoptosis of the cell [31]. In addition, the extract of I. cylindrica was proved to against cancer cells, when tested at 20 g/mL [32]. It was able to inhibit more that 50% the proliferation of the three tested cancer cells (MiaPaCa-2, CEM/ADR5000, CCRF-CEM). The lowest IC50 values of 6.86 g/mL on MiaPaCa-2 and 3.91 g/mL on CCRF-CEM cells were obtained with X. aethiopica, while the corresponding value of 6.56 g/mL was obtained with P. capense on CEM/ADR5000 cells. In addition, the extracts of I. cylindrica inhibited the proliferation of the tested cancer cell lines including sensitive and drug-resistant phenotypes [33]. The recorded IC50 ranges were 3.28 mg/mL (against HCT116 (p53−/−) cells) to 33.43 mg/mL (against HepG2 cells), and the extracts of I. cylindrica induced apoptosis in CCRF-CEM cells via the alteration loss of MMP whilst that of Piper capense also enhanced the production of ROS.
The I. cylindrica extracts also have anti-cancer effects on human oral squamous carcinoma cell line SCC-9 in vitro [34]. It treatment caused cytotoxicity and induced cell death in vitro in SCC-9 cells in a dose-dependent manner. Additionally, this treatment also significantly reduced the clonogenic potential and inhibited cell proliferation by arresting the cell cycle in the G2/M phase.
4.5.2. Isolated Compounds
Arundoin (1) played a role in upregulating the expression levels of cleaved caspase-3, cleaved caspase-9 and Bax, downregulating Bcl-2, in a dose-dependent manner, indicated that arundoin can inhibit the proliferation of LNCap cells and induce apoptosis (Chen D.-K., 2017).Another research pointed that arundoin (1) can increase the cracking of polyadenosine diphosphate ribose polymerase(PARP) and block the proliferation of PC3 cells, promoting apoptosis of prostate cancer cell linePC3 [35].
2-Methoxyestrone (13) and tricin (17) exhibited considerable growth inhibitory activities against BT-549 and HT-29 cancer cell lines in vitro [9]. The half-maximal inhibitory concentration (IC50) of compounds 13 and 17 on BT-549 breast cancer cell line (72 h) was 102 µM and 68 µM, respectively. IC50 of compounds 13 and 17 on a HT-29 colon cancer cell line (72 h) was 147µM and 114 µM, respectively. Daucosterol (6) was reported to inhibit the proliferative ability of HL-7702, SMMC-7721, and HepG2 cells in a concentration-dependent manner, with IC50 values of 214.99, 143.40, and 138.73 µg/mL, respectively [36]. Therefore, these findings suggested that compound 6 significantly suppressed the proliferation ability of hepatocellular carcinoma (HCC) cells in a concentration-dependent manner. Besides, it was found that compound 6 inhibited the proliferation of human breast cancer cell line MCF-7 and gastric cancer cell lines MGC803, BGC823 and AGS in a dose-dependent manner. Furthermore, compound 6 inhibits murine hepatoma H22 cell growth in ICR mice. Treatment of compound 6 induced intracellular ROS generation and autophagy, but not apoptotic cell death [37].
4.6. Antibacterial
The extracts of I. cylindrica possessed antibacterial activity in inhibiting Quorum-sensing metabolites of Chromobacterium violaceum CV026 with antibacterial zone of 12 mm and Quorum-sensing inhibition zone of 20 mm; however, it showed no inhibition on Pseudomonas aeruginosa PA01 [38]. The different solvent extracts of Imperatae rhizoma possessed antibacterial activity in five tested strains. Among them, ethyl acetate extract had the best bacteriostatic effect on Klebsiella aerogenes, boiling extract against Escherichia coli, acetone extract for Staphylococcus aureus, boiled extract for Bacillus subtilis, and 50% ethanol extract for Enterobacter aerogenes, inhibition zone of 12.0, 13.0, 13.5, 13.5, and 15.0 mm, respectively.
4.7. Immunomodulatory
In the innate immunity, complement system is of significant importance. Components of complement can be activated sequentially by the classical pathway, the lectin pathway and the alternative pathway, the activated components of complement mainly mediate complement-dependent cytotoxicity to achieve clearance of pathogenic microorganisms and abnormal cells. It was found that the mineral ether extract of I. rhizoma and the ethyl acetate extract of I. rhizoma can reduce the activity of complement C1, C2, C4, C3, and C5 and inhibit the activation of the classical pathway of complement [7].
Macrophages are a subset of innate immune cells that the body mediates non-specific cellular immune responses, which exert direct phagocytosis and indirect secretion of cytokine-mediated immune regulation. NIH mice were orally administrated with I. rhizoma decoction up to 20th day, then 5% starch and 9% NaCl were intraperitoneally injected, after one hour, 1% chicken red blood cells were injected. It is found that the number of peritoneal macrophages, percentage of phagocytosis and phagocytic index were significantly increased. It suggests that I. rhizoma extract can enhance the non-specific immunity of the body. In addition, another research reported that water extract of I. rhizoma can inhibit the formation of nitric oxide in lipopolysaccharide-induced mouse macrophages RAW264.7 [39].
In the adaptive immune response, the immunomodulatory effects of I. rhizoma extract are mainly reflected in the subpopulation structure, proliferative capacity and secretion of related cytokines of T lymphocytes. Animal experiments showed that I. rhizoma decoction can increase the proportion of CD4+ T cells in immunodeficient mice, decrease the proportion of CD8+ T cells, adjust the ratio of CD +4 /CD8 + T cells to balance and restore their immune function. Meanwhile, I. rhizoma decoction can also increase the proportion of helper T cells (Th cells) in the spleen of NIH mice and increase the secretion of IL-2 [40].
4.8. Liver Protection Activity
The methanol extract of I. rhizoma can inhibit the damage of carbon tetrachloride to the liver without toxic side effects [6]. A report has shown that Rhizoma Imperata has a renal protective effect on the rat model of IgA nephropathy established by bovine serum albumin plus staphylococcal enterotoxin B method (Yin et al., 2011). Compared with the model group, URBC, 24hUP, Scr, BUN, PAF, and TGF-β1 expression were significantly decreased, while serum IL-2 was significantly increased (p < 0.01) in the treatment groups. It was suggested that I. cylindrica decoction can significantly reduce hematuria, proteinuria of the IgA nephropathy rats, reduce the pathological changes and improve renal function. The mechanism may be related to the elevation of IL -2 secretion of RI and inhibition of renal TGF-β1 secretion expression, etc.
4.9. Other Activity
The extract of the leaves of I. cylindrica possessed an antihypertensive action with a significant dose-dependent reduction in amplitude of contraction of smooth muscle cells of rabbit jejunum in comparison with standard antihypertensive drug, adrenaline [41]. The heart pressure of the cats was significantly reduced with 160 and 320 mg/mL of the extract from 266 to 180 mmHg (p = 0.012) but there was no effect on the heart rate. The minimum effective dose EC50 was 0.013. Ethanolic leaf extract of I. cylindrica exhibited vasodilative antihypertensive properties similar to mechanism of action of adrenaline. The extract could be used to manage hypertension.
Moreover, the extract of I. cylindrica played a role in regulating lipid metabolism and tolerance to hypoxia [42,43]. The polysaccharide of Rhizoma Imperatae can reduce the levels of glycated hemoglobin, triglyceride, total cholesterol, and low-density lipoprotein cholesterol in the serum of diabetic mice, increase the levels of hepatic glycogen and high-density lipoprotein cholesterol, and regulate the disorder of glucose and lipid metabolism. When mice were orally administered with I. cylindrica extract at the dose of 50, 100, 200, 400 mg/kg and propranolol at the dose of 20 mg/kg, the oxygen consumption was reduced respectively by 2.48 %, 23.14%, 10.74%, 5.79%, and 28.93% for 0 to 5 min, and 2 .11 %, 21.13%, 16.90%, 38.73%, and 9.86% for 5 to 10 min. The survival rates of mice with hypoxia for 30 min were 0, 0 ,20%, 10%, 50%, 20% when receiving saline, propranolol of 20 mg/ kg and RIP of 50, 100, 200, 400 mg·kg−1. The survival time was prolonged by 33.16%, 37.31%, and 35.23% in mice with hypoxia of propranolol and RIP of 200 and 400 mg/kg groups
5. Industrial Applications
Based on the record in “Compendium of material medica” and “ben jing”, I. cylindrica is considered to be effective in encouraging a variety of physical functions. These include stopping vomiting, improving renal function, increasing urination, clearing heat and toxins, relieving fever, and promoting hemostasis. Thus, it could be widely used for the treatment of fever and thirst, vomiting blood, epistaxis, pulmonary heat and asthma. Nowadays, a modern prescription “Qingre Tonglin Pian” has been approved by China Food and Drug Administration (Z20050124) for the hot-water drench treatment. This formula is mainly composed of an extract from I. cylindrica roots. I. cylindrica is utilized in other countries besides China. In India, it is one of the key ingredients in India’s various traditional medicinal formula and has multiple pharmacological applications. Generally, it is used as diuretic and an anti-inflammatory agent. Foodservice industry applications of I. cylindrica provide a novel choice for people to select health-care foods such as jellies, vinegar, beverages, sauces, flavoring agents, tea, or wines that contain some derivatives of I. cylindrica (Table 1). I. cylindrica is also used in the cosmetic industry. For instance, I. cylindrica root antibacterial powder is used to make natural non-toxic toothpaste. Furthermore, fermented liquor used for Chinese herbal compounds that contained I. cylindrica is used to make oil-control and acne-treatment cosmetic products. Selected patents regarding pharmacological applications of I. cylindrica is presented in Table 2.
Table 2.
Patents list of products containing constituents from I. cylindrical and their claimed pharmacological properties
| Application | Main Composition | Pharmacological Properties | Publish Number |
|---|---|---|---|
| Jelly | I. cylindrica roots, Mume fructus, Mori fructus, Sophorae Flavescentis radix, edible gelatin, Glyceryl Monostearate | Dispelling the effects of alcoholism | CN 107484997 A |
| Vinegar | I. cylindrica, Lycium barbarum, Ziziphus jujube, brewed rice vinegar with acidity of 3.5–4% | Relieving hangover and protecting the stomach | CN 109423430 A |
| Soft beverage | I. cylindrica roots, Momordica grosvenori, Siraitia grosvenorii fruits, Chrysanthemum morifolium flowers, crystal sugar | Clearing heat and detoxicating, cooling blood and hemostasis | CN 106418063 A |
| Solid beverage | sugar cane powder and I. cylindrica powder | Clearing heat and detoxicating, promoting urination, and salivation, relieving restlessness | CN 105495260 A |
| Sauce | I. cylindrica | Clearing heat and detoxicating, tonifying middle-jiao and qi | CN 104543941 A |
| Flavoring agents | I. cylindrica flowers, Phyllanthus Emblica fruits, antelope horn, Panax Ginseng leaves, edible vinegar essence, sesame oil, prebiotics | Tranquillizing heart and nourishing stomach | CN 106107858 A |
| Tea | I. cylindrica roots, Paeonia suffruticosa root barks, Saposhnikovia divaricata roots | Clearing heat and cooling blood, reinforcing disease resistance and preventing cold | CN 106689560 A |
| Tea | I. cylindrica root, Lonicera japonica flowers, crystal sugar | Clearing heat and toxic materials and relieving sore throat | CN 106689557 A |
| Wine | Imperata cylindrical, Hedyotis diffusa, Semen Plantaginis, 5 kg of pure grain spirit | Eliminating heat and toxin, removing dampness and treating stranguria | CN 103725482 A |
| Edible toothpaste | Imperata cylindrica root antibacterial powder, vitamin powder, fish gelatin | Preventing periodontal disease, antisepsis and containing no toxic chemicals | CN 106214600 A |
6. Conclusions
In recent years, people have increasingly paid attention to the problems of multidrug resistance and side effects of drugs in existing drugs, forcing researchers and stakeholders to constantly try to develop safe and effective drugs. A wide variety of plants are traditionally and ethnopharmacologically used for treating various disorders, and some proved to be effective. Therefore, natural medicine is undoubtedly the best excavation point. The plant of I. cylindrica is an excellent medicinal plant with multiple bioactive constituents. The plant has been used for treating various diseases and extensive research has been initiated. Although various aspects of this plant are constantly making progress, the development and discovery of a new drug, even an allopathic phytomedicine from I. cylindrica, will require more detailed preliminary studies in both the preclinical and clinical venues.
The present review summarizes a comprehensive overview of the reported pharmacognosy, phytochemistry, and pharmacological activities of the plant I. cylindrical. Up to now, 72 compounds from I. cylindrica were identified, among them flavonoids and saponins are the main compositions. The reports on the bioactivities of extracts and compounds from I. cylindrica are chronicled, the main bioactive components are lignans and flavonoids, which may contribute either directly or indirectly to the biological effects of the I. cylindrica genus. Studies implemented through in vivo and in vitro experiments have demonstrated the bioactivities of I. cylindrica plants, most of which support their traditional medicine uses. Pharmacological studies are mainly focused on the anti-inflammatory, antitumor, antiviral and antioxidant activity, and liver protection activity. In addition, some studies indicated that it has immunomodulatory functions.
Nevertheless, some issues still remain unclarified. Firstly, the traditional diuretic and hemostasis actions of I. cylindrica require more modern pharmacology studies to clarify its underlying mechanism. As current activity studies mostly focus on in vitro, more in vivo studies and more clinical applications are needed to further optimize modern clinical drug standards. In addition, it should be noted that during our review, we observed that some studies have conflicting results, which suggest that these studies may not provide reproducible data. Moreover, study protocols were improperly described and there was heterogeneity in study protocols. These factors could hinder the replication of experiments. Strong scientific methodologies are required before confirmatory decisions can be made on the potential of I. cylindrica. Secondly, there are a limited number of active ingredients that have been pharmacological studies. Therefore, we need to answer if these identified substances can achieve the equivalent effect of I. cylindrica, or, if not, to what extent. Thirdly, the traditional using of TCM are commonly combined with other TCM in conventional therapies. The interactions and underlying mechanisms between I. cylindrica and other TCM should be further investigated by future studies. Furthermore, the current studies of this plant have primarily focused on its small molecule compounds, but have rarely investigated its macromolecular compounds. Thus, future studies of I. cylindrica could devote more effort to its macromolecular compounds, especially its polysaccharides. Fourthly, due to the complex composition of agents used in herbal medicine, a unified international identification of I. cylindrica is needed to control the quality of this herbal medicine and establishing a standardized fingerprint of this plant might be indispensable. Finally, although I. cylindrica is currently widely distributed and cultivated in many areas of the world, it is also important to manage the use of this plant and avoid overexploitation.
We believe that if we can solve the above questions in depth and reveal the medical potential of I. cylindrica, as well as other TCM, we can explore and utilize the precious medical reserve that TCM left to the human beings. Simultaneously, we hope this review highlights the importance of I. cylindrica and provides some directions for the future development of this plant.
Abbreviations
| MIC | minimum inhibitory concentration |
| MMP | matrix metalloproteinase |
| MRSA | methicillin-resistant Staphylococcus aureus |
| NF-κB | nuclear factor-κB |
| ROS | reactive oxygen species |
| RSV | respiratory syncytial virus |
| SOD | superoxide dismutase |
| SsaA | staphylococcal secretory antigen |
| Syk | spleen tyrosine kinase |
| TBARS | thio-barbituric acid reactive substances |
| TCM | traditional Chinese medicine |
| TLR2 | toll-like receptor |
| TNF-α | tumor necrosis factor alpha |
| TGF-β | transforming growth factor-β |
| UV | ultraviolet |
Author Contributions
Conceptualization and writing the draft: D.S.; Data collection and analysis: D.S. and Y.-K.J.; Writing-review and editing: D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03043708).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Santoso D., Adiningsih S., Mutert E., Fairhurst T., Noordwijk M.V. Soil fertility management for reclamation of Imperata grasslands by smallholder agroforestry. Agrofor. Syst. 1996;36:181–202. doi: 10.1007/BF00142873. [DOI] [Google Scholar]
- 2.Faustino M.V., Faustino M.A.F., Pinto D. Halophytic Grasses, a New Source of Nutraceuticals? A Review on Their Secondary Metabolites and Biological Activities. Int. J. Mol. Sci. 2019;20:1067. doi: 10.3390/ijms20051067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaltout K.H., Galal T.M., El-Komi T.M. Phenology, biomass and nutrients of Imperata cylindrica and Desmostachya bipinnata along the water courses in Nile Delta, Egypt. Rend. Lincei. Sci. Fis. Nat. 2016;27:215–228. doi: 10.1007/s12210-015-0459-5. [DOI] [Google Scholar]
- 4.Ohmoto T., Nishimoto K., Ito M., Natori S. Triterpene methyl ethers from rhizome of Imperata cylindrica var media. Chem. Pharm. Bull. 1965;13:224–226. [PubMed] [Google Scholar]
- 5.Ohmoto T. Triterpenoids and the related compounds from Gramineous plants. VI. Yakugaku Zasshi. 1969;89:1682–1687. doi: 10.1248/yakushi1947.89.12_1682. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed G.A., Adbel-Lateff A., Fouad M.A., Ibrahim S.R.M., Elkhayat E.S., Okino T. Chemical composition and hepato-protective activity of Imperata cylindrica Beauv. Pharmacogn. Mag. 2009;5:28–36. [Google Scholar]
- 7.Fu L., Chen L., Liu R., Chen D. Chemical constituents of Rhizoma Imperatae and their anti-complementary activity. J. Chin. Med. Mater. 2010;33:1871–1874. [PubMed] [Google Scholar]
- 8.Liu X., Zhang B., Chou G., Yang L., Wang Z. Chemical constituents from Imperata cylindrica. China J. Chin. Mater. Med. 2012;37:2296–2300. [PubMed] [Google Scholar]
- 9.Wang Y., Shen J.Z., Chan Y.W., Ho W.S. Identification and Growth Inhibitory Activity of the Chemical Constituents from Imperata Cylindrica Aerial Part Ethyl Acetate Extract. Molecules. 2018;23:1807. doi: 10.3390/molecules23071807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai Y., Shinozaki J., Takano A., Masuda K., Nakane T. Three novel 14-epiarborane triterpenoids from Imperata cylindrica Beauv. var. major. Phytochem. Lett. 2018;26:74–77. doi: 10.1016/j.phytol.2018.05.002. [DOI] [Google Scholar]
- 11.Liu R.-H., Chen S.-S., Ren G., Shao F., Huang H.-L. Phenolic compounds from roots of Imperata cylindrica var. major. Chin. Herb. Med. 2013;5:240–243. [Google Scholar]
- 12.Yoon J.S., Lee M.K., Sung S.H., Kim Y.C. Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrica. J. Nat. Prod. 2006;69:290–291. doi: 10.1021/np0503808. [DOI] [PubMed] [Google Scholar]
- 13.An H.-J., Nugroho A., Song B.-M., Park H.-J. Isoeugenin, a novel nitric oxide synthase inhibitor isolated from the rhizomes of Imperata cylindrica. Molecules. 2015;20:21336–21345. doi: 10.3390/molecules201219767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X., Zhang B.-F., Yang L., Chou G.-X., Wang Z.-T. Four new compounds from Imperata cylindrica. J. Nat. Med. 2014;68:295–301. doi: 10.1007/s11418-013-0793-9. [DOI] [PubMed] [Google Scholar]
- 15.Lee D.-Y., Han K.-M., Song M.-C., Lee D.-G., Rho Y.-D., Baek N.-I. A new lignan glycoside from the rhizomes of Imperata cylindrica. J. Asian Nat. Prod. Res. 2008;10:299–302. doi: 10.1080/10286020701783419. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga K., Shibuya M., Ohizumi Y. Graminone B, a novel lignan with vasodilative activity from Imperata cylindrica. J. Nat. Prod. 1994;57:1734–1736. doi: 10.1021/np50114a020. [DOI] [PubMed] [Google Scholar]
- 17.Matsunaga K., Ikeda M., Shibuya M., Ohizumi Y. Cylindol A, a novel biphenyl ether with 5-lipoxygenase inhibitory activity, and a related compound from Imperata cylindrica. J. Nat. Prod. 1994;57:1290–1293. doi: 10.1021/np50111a019. [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga K., Shibuya M., Ohizumi Y. Imperanene, a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica. J. Nat. Prod. 1995;58:138–139. doi: 10.1021/np50115a022. [DOI] [PubMed] [Google Scholar]
- 19.Wang M., Wang S., Sun Q., Wu L. Studies on the chemical constituents of the rhizomes of Imperata cylindrica. Zhongguo Yaowu Huaxue Zazhi. 1996;6:192–194. [Google Scholar]
- 20.Cerdeira A.L., Cantrell C.L., Dayan F.E., Byrd J.D., Duke S.O. Tabanone, a new phytotoxic constituent of cogongrass (Imperata cylindrica) Weed Sci. 2012;60:212–218. doi: 10.1614/WS-D-11-00160.1. [DOI] [Google Scholar]
- 21.Ma J., Sun H., Liu H., Shi G.-N., Zang Y.-D., Li C.-J., Yang J.-Z., Chen F.-Y., Huang J.-W., Zhang D., et al. Hepatoprotective glycosides from the rhizomes of Imperata cylindrica. J. Asian Nat. Prod. Res. 2018;20:451–459. doi: 10.1080/10286020.2018.1471065. [DOI] [PubMed] [Google Scholar]
- 22.Wang M., Wang S., Sun Q., Wu L. Chemical constituents of Imperata cylindrica rhizomes. J. Chin. Pharm. Sci. 1996;5:53–55. [Google Scholar]
- 23.Chowdhury R., Hasan C.M., Rashid M.A. Antimicrobial activity of Toona ciliata and Amoora rohituka. Fitoterapia. 2003;74:155–158. doi: 10.1016/S0367-326X(02)00322-2. [DOI] [PubMed] [Google Scholar]
- 24.Shah N.T., Pandya T.N., Sharma P.P., Patel B.P., Acharya R. Mootrala Karma of Kusha [Imperata cylindrical Beauv.] and Darbha [Desmostachya bipinnata Stapf.]—A comparative study. AYU. 2012;33:387–390. doi: 10.4103/0974-8520.108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojha S.N., Nagore D.H., Ganu G.P. In vitro and in vivo Anticoagulant activity of Imperata cylindrica a novel anticoagulant lead from natural origin. PHCOG J. 2010;2:38–43. [Google Scholar]
- 26.Yue X.R., Hou Z.X., Liu P. Anti-inflammatory effect of Imperata cylindrica. Chin. J. Clin. Rehabil. 2006;10:85–87. [Google Scholar]
- 27.Chen L.Y., Chen Z., Wang C.Q., Luo Y., Luo Y.H., Duan M., Liu R.H. Protective Effects of Different Extracts of Imperatae Rhizoma in Rats with Adriamycin Nephrosis and Influence on Expression of TGF-β1, and NF-κB p65. J. Chin. Med. Mater. 2015;38:2342. [PubMed] [Google Scholar]
- 28.Putri M., Anggraeni N., Tresna M.D., Ramli G.A., Kusmiati M., Andriane Y., Hendryanny E., Hassan A.H., Damayanti M.M., Sutadipura N., et al. Cogongrass (Imperata cylindrica L.) Ethanol Extract on Sepsis Mice Model Body Weight and Sepsis Score. Glob. Med. Health Commun. 2020;8:206–210. doi: 10.29313/gmhc.v8i3.6604. [DOI] [Google Scholar]
- 29.Zhou X.R., Wang J.H., Jiang B., Shang J., Zhao C.Q. A study of extraction process and in vitro antioxidant activity of total phenols from Rhizoma Imperatae. Afr. J. Tradit. Complement. Altern. Med. 2013;10:175–178. doi: 10.4314/ajtcam.v10i4.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayalakshmi S., Mishra A.K., Mishra A., Singla R.K., Ghosh A.K. In-Vitro Evaluation of Antioxidant Activity of Five Drugs of Trinpanchmool. Pharmacologyonline. 2011;2:1153–1159. [Google Scholar]
- 31.Bao Y.R., Wang S., Meng X.S., Yang X.X., Wang Y. Effect of flavonoids of Lalang Grass Rhizome Water Extract on cell cycle and apoptosis induction of human hepatoma cell line SMMC-7721. Lishizhen Med. Mater. Med. Res. 2013;7:1584–1586. [Google Scholar]
- 32.Kuete V., Krusche B., Youns M., Voukeng I., Fankam A.G., Tankeo S., Lacmata S., Efferth T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 2011;134:803–812. doi: 10.1016/j.jep.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Victor K., Sandjo L.P., Benjamin W., Thomas E. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J. Ethnopharmacol. 2013;149:245–253. doi: 10.1016/j.jep.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Keshava R., Muniyappa N., Gope R., Ramaswamaiah A.S. Anti-Cancer Effects of Imperata cylindrica Leaf Extract on Human Oral Squamous Carcinoma Cell Line SCC-9 in Vitro. Asian Pac. J. Cancer Prev. 2016;17:1891–1898. doi: 10.7314/APJCP.2016.17.4.1891. [DOI] [PubMed] [Google Scholar]
- 35.Chen D.K. Apoptotic effect of arundoin on human prostatic cancer cells by activating Poly ADP ribose polymerase. Chin. Tradit. Herb. Drugs. 2017;48:1183–1187. [Google Scholar]
- 36.Zeng J., Liu X., Li X., Zheng Y., Liu B., Xiao Y. Daucosterol Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Wnt/beta-Catenin Signaling. Molecules. 2017;22:862. doi: 10.3390/molecules22060862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao C., She T., Wang L., Su Y., Qu L., Gao Y., Xu S., Cai S., Shou C. Daucosterol inhibits cancer cell proliferation by inducing autophagy through reactive oxygen species-dependent manner. Life Sci. 2015;137:37–43. doi: 10.1016/j.lfs.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Koh K.H., Tham F.Y. Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J. Microbiol. Immunol. Infect. 2011;44:144–148. doi: 10.1016/j.jmii.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Hui L., Banbury L.K., Leach D.N. Inhibitory effects of 12 hemostatic Chinese herbs on nitric oxide production in LPS-activated RAW264.7 macrophages. J. China Pharm. 2007;18:117–130. [Google Scholar]
- 40.Yin Y.S., Ou J., Wei J.Z., Li Y.Q., Li X.L., Li Q.C., Li K.H. Effects of Rhizoma Imperatae and its Compound Decoction on the Model of IgA Nephropathy in Rats. Lishizhen Med. Mater. Med. Res. 2011;11:2659–2662. [Google Scholar]
- 41.Mak-Mensah E.E., Komlaga G., Terlabi E.O. Antiypertensive action of ethanolic extract of Imperata cylindrica leaves in animal models. J. Med. Plants Res. 2010;4:1499–1504. [Google Scholar]
- 42.Cui J., Chao L.I., You J., Xiao-Dong X.U. Effects of Imperata cylindrica Polysaccharides on Glucose and Lipid Metabolism in Diabetic Mice. Food Sci. 2012;33:302–305. [Google Scholar]
- 43.Sun L.Y., Liu Z.L., Sun J.X., Gao Y.S., Zhao Z.T. Effect of polysaccharides of Rhizoma imperatae on hypoxia tolerance in mice. Chin. J. Hosp. Pharm. 2008;2:96–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.