Abstract
Study design:
Cross-sectional design.
Objectives:
To examine personal factors, secondary health conditions, and environmental factors as potential correlates of adherence to a 12-week home-based exercise trial in people with spinal cord injury.
Setting:
Home
Methods:
Participants (n=28) were prescribed a set of exercise videos that they were asked to complete three times each week for 12 weeks (36 total sessions). The videos were accessible through a custom-designed mobile application and included movements targeting strength, cardiorespiratory fitness, and balance that were accompanied with music. Watched video minutes were automatically recorded on the web-based platform. At baseline, participants completed self-report questionnaires that measured personal (e.g., age, self-efficacy) and environmental (e.g., barriers) factors and secondary health conditions (e.g., depression). Data were analyzed using quantile (median) regression analysis.
Results:
Race (African American; β = −65.62, p = 0.004), community barriers (β = −9.12, p = 0.026), anxiety (β = −3.84, p = <0.001), depression (β = −1.42, p = 0.038), physical function (β = −1.35, p = 0.048), and self-efficacy (β = −0.61, p = 0.007) were associated with a lower number of exercise video minutes. Pain intensity (β = 2.03, p = 0.032), pain interference (β = 1.84, p = 0.012), and age (β = 1.13, p = 0.013) were associated with a higher number of exercise video minutes. Total variance explained by the model was 77% (pseudo R2 = 0.77).
Conclusions:
Factors associated with lower and higher adherence to home-based exercise should guide future research efforts in creating more precision-based approaches for self-managed home exercise.
Introduction
In recent years, internet-based interventions targeting health issues such as nutrition, smoking, physical activity, or multiple health behaviors have become popular [1]. These interventions have several advantages for people with spinal cord injury (SCI) because of their interactive designs, ability to tailor information to individual users, the potential to reach large groups of people with SCI at relatively low cost, and the ease with which users can get involved, that is, people with SCI can use the intervention at home, which is the most convenient place to obtain regular lifestyle health guidance [2]. Other benefits of home-based programs include their low cost (compared to onsite programs), the elimination of travel time, and the ability for individuals to work at a self-selected pace in a comfortable environment [2, 3].
It is well documented that people with SCI engage in lower levels of physical activity compared to groups without disabilities [4, 5]. Systematic reviews have documented that people who use wheelchairs, in particular, experience a plethora of physical activity barriers that span across each level of the socioecological model: intrapersonal (e.g., lack of self-efficacy, fear, pain, or fatigue); interpersonal (e.g., lack of social support, negative attitudes); institutional (e.g., lack of knowledge of individuals within institutions/organizations); community (e.g., lack of accessible features in fitness and recreation venues); and policy (e.g., poor/no transportation systems, lack of funding for exercise programs) [4].
Given the low rate of physical activity participation among people with SCI, new strategies for promoting this important health behavior are critically needed. Technology is a strategy that can play a substantial role in addressing exercise barriers in the delivery of home-based exercise to people with SCI. Advances in information and communication technology (ICT) such as ubiquitous videoconferencing software, mobile phones and their applications, and wearable monitoring devices enhance the ability of health professionals to better disseminate and monitor the implementation of exercise programs in the community [6]. For people with SCI, ICT can enhance the convenience and accessibility of exercise programs [7]. Such technology is relevant since most people with SCI have a computer with access to the Internet [8]. However, to our knowledge, no study has implemented a remotely delivered, self-managed exercise program using an innovative form of adapted exercise, such as movement-to-music (M2M).
M2M consists of routines choregraphed to music that follow a systematic framework to ensure every muscle group is used to progressively improve physical functioning by targeting three fitness components: balance, cardiorespiratory endurance, and strength. The program has demonstrated significant improvements in physical outcomes among people with multiple sclerosis when compared to adapted yoga [9], but the program has not been used in people with SCI. The SUPER-HEALTH project [10], which stands for Scale-Up Project Evaluating Responsiveness to Home Exercise And Lifestyle Tele-Health, is a 48-week ongoing exercise trial assessing the utilization of M2M remotely delivered to various disability groups, including wheelchair users with either a traumatic SCI or non-traumatic SCI (spina bifida).
SUPER-HEALTH was designed to circumvent the majority of barriers confronted by people with SCI. The study did this by promoting home exercise that can be done conveniently at any time of the day; eliminating the need for transportation to engage in exercise; customizing routines to the unique functional needs of the participant; and using music to facilitate enjoyment. While the assumption is that eliminating common barriers to participating in exercise will provide individuals with SCI a stable and consistent pathway to being regularly active, there are no studies that have examined participant adherence to home exercise (i.e., completion of the prescribed exercise volume) in people with SCI. Therefore, the purpose of this study was to examine the potential mediators/moderators of home-based exercise adherence in a group of adults with SCI. We hypothesized that a model of regression for predicting exercise video minutes in this population would include significant predictors for personal factors, secondary health conditions, and environmental factors.
Methods
The intervention protocol was approved by the University’s Institutional Review Board (IRB-160923002) and was registered with ClinicialTrials.gov (#NCT03024320) as a phase III clinical trial. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. The study design is cross sectional, including data from participants throughout the first 12 weeks. The first 12-week period was chosen because it is the critical phase that determines whether participants will adopt physical activity behavior into their lifestyle.
Study Procedures
Data were obtained from a cohort of 28 people with SCI (traumatic SCI, n=20; spina bifida, n=8) from an ongoing scale-up, hybrid type 1 effectiveness trial. These 28 participants were randomized to receive 12-weeks of intervention (as opposed to a 12-week non-intervention control group). Intervention participants were given a computer tablet that came preinstalled with a custom-designed web application [11]. The application released a new M2M exercise video each week for the participant to use to meet their weekly exercise goal. When a new routine was introduced, the routine was guided by an M2M instructor that provided verbal instruction and explained each movement pattern. The following week, the same routine was guided by an M2M instructor with SCI but with no verbal instruction, and a new guided routine was also delivered. The routines delivered to participants were designed specifically for wheelchair users and choregraphed to music. The M2M videos incorporated ‘public domain’ music to avoid copyright issues. The first week of the intervention included a prescription of 48 minutes of exercise video content, which was progressed up to 112 minutes at week 12. Additionally, the application included health promotion articles. Participants were provided with a tablet stand to position their tablet for an easy view of the videos, as well as a Fitbit charge HR 2/3 to monitor their daily step/push counts (see figure 1). Further intervention details can be found elsewhere [10].
Figure 1.
Picture of person with SCI participating in the home-based exercise intervention using the provided tablet on an adjustable stand.
Participants
Participants were primarily recruited from the Integrating Biology and Bedside (i2B2) database, which included over 400 patients with SCI from the Physical Medicine and Rehabilitation outpatient clinics affiliated with a university-based medical rehabilitation center. In addition to the mail-outs, there were multiple methods of recruitment at the rehabilitation clinics. First, passive recruitment involved informational brochures and a study advertisement in the waiting area of the clinics. The second method for clinic-based recruitment involved informing the physicians and physicians’ staff of the study for them to encourage patients to join.
Participants in this study met the following inclusion criteria: 1) traumatic or non-traumatic spinal cord injury, 2) aged 18–70 yrs., and 3) use of wheelchair as primary means of mobility. The study included the following exclusion criteria: 1) accumulating more than 60 minutes of moderate/vigorous physical activity per week; 2) not within working age (18 to 70 yrs. of age); 3) enrolled in a structured exercise program over the past 6 months; 4) unable to use at least one set of extremities to exercise; 5) unable to converse and read English; 6) medically unstable to perform home exercise as determined by their physician; or 7) cognitive impairment that may preclude self-directed daily activities.
Measures
Questionnaires were automatically emailed to participants at baseline by the REDCap system. Data for the present study were categorized into personal factors, secondary health conditions, and environmental factors. Personal factors included disability type (spinal cord injury or spina bifida), age in years, sex, race, and physical function measured via the National Institutes of Health (NIH) Patient-Reported Outcome Measurement Information System (PROMIS) short form. Other questionnaires for personal factors supported constructs of the Social Cognitive Theory and included exercise goal-setting (Exercise Goal-setting Scale) [12], exercise planning (Exercise Planning and Scheduling Scale) [13], outcome expectations for exercise (Multidimensional Outcomes Expectations for Exercise Scale) [12], and exercise self-efficacy (Exercise Self-Efficacy Scale) [14]. Questionnaires for secondary conditions included anxiety, depression, fatigue, sleep disturbance, and pain, which were measured via NIH PROMIS short forms [15]. Lastly, measures on environmental factors included Ability to Participate in Social Roles (NIH PROMIS), barriers (Barriers to Physical Activity Questionnaire for People with Mobility Impairment) [16], and social support (Social Provisions Scale) [17].
Analysis
For the dependent variable, the median number of minutes of exercise video viewed were first calculated for each week then the total median across the 12-week period (adherence). Independent variables included personal factors (disability type, age, sex, race, self-efficacy, self-regulation, outcome expectations, physical functioning), secondary health conditions (anxiety, depression, fatigue, sleep disturbance, pain interference, and pain intensity), and environmental factors (barriers, social support). Descriptive statistics include medians and interquartile ranges of the continuous variables and frequencies and percentages of categorical variables. The dependent variable was positively skewed due to outliers, a few people who spent more than an hour a week watching videos; therefore quantile (median) regression was used to analyze the data. Quantile (median) regression is more robust to outliers, and it does not rely on the assumption about the distribution of the residuals [18]. The model is specified below:
Data were analyzed using Stata 16. Significance level of 0.05 was used in evaluating the statistical tests.
For descriptive purposes, participants were categorized as high adherence, sub-optimal adherence, low adherence, and non-adherence to the 12-week home-based exercise intervention. High adherence was operationally defined as video minutes viewed at or above 80% of what was prescribed (≥ 80%); sub-optimal adherence between 80% and 40%; low adherence between 40% and 20%; and non-adherence at less than 20%.
Results
Recruitment for the study included 460 flyers sent out to people with SCI with 47 responding to the flyers and an additional 18 from other sources (see figure 2). For enrollment, 65 people were screened, 43 people completed baseline, and 28 people were randomized to the exercise intervention (M2M).
Figure 2.
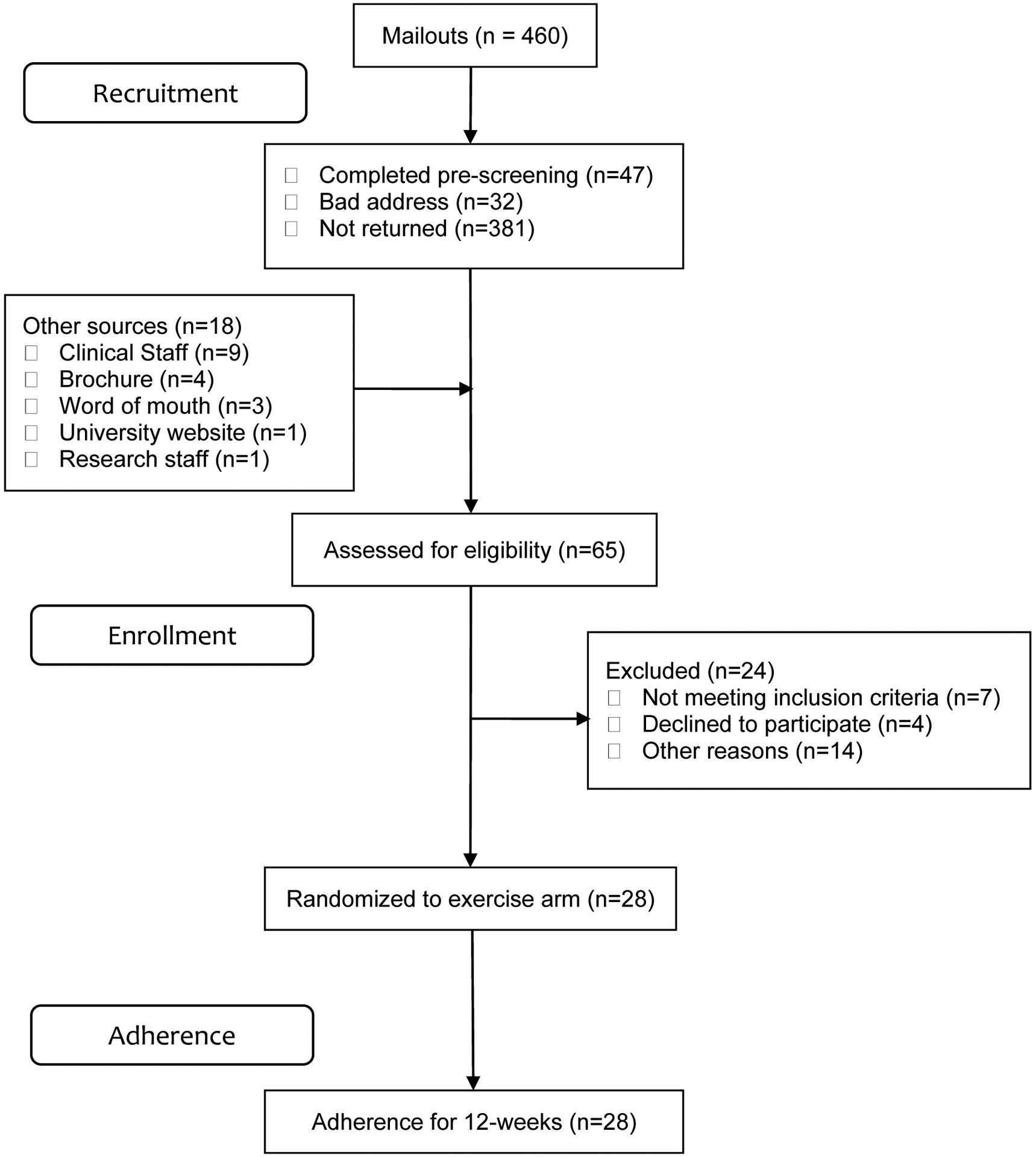
CONSORT diagram of study
Participant Data
There were 19 females and 9 males in the study (table 1). The median age of participants was 44 years with the majority being white while four were African Americans. Participants scored one standard deviation below the mean on PROMIS physical functioning (median=37) and ability to participate in social roles measures (median=42). Their pain (median=54) and fatigue (median=55) scores were a half standard deviation above the mean.
Table 1.
Descriptive statistics of the sample (N=28)
| Variables | Frequency (%) / Median (IQR)a |
|---|---|
| Personal Factors | |
| Disability type | |
| Traumatic SCI | 19 (68%) |
| Spina Bifida | 9 (32%) |
| Sex | |
| Male | 8 (29%) |
| Female | 20 (71%) |
| Race | |
| White | 24 (85%) |
| African American | 4 (15%) |
| Age | 44.00 (30.50 – 54.00) |
| Exercise goal setting | 22.00 (15.50 – 28.00) |
| Exercise planning and scheduling | 26.50 (24.00 – 29.00) |
| Exercise self-efficacy | 87.50 (62.50 – 100.00) |
| Outcomes expectations | 4.27 (3.83 – 4.57) |
| Physical function | 37.20 (29.80 – 44.85) |
| Secondary Health Conditions | |
| Anxiety | 53.20 (43.20 – 57.40) |
| Depression | 50.90 (38.20 – 55.50) |
| Fatigue | 54.60 (46.90 – 59.40) |
| Sleep disturbance | 50.10 (47.90 – 54.30) |
| Pain interference | 54.05 (40.70 – 64.15) |
| Pain intensity | 46.30 (40.20 – 52.10) |
| Environmental Factors | |
| Social provision scale | 82.50 (75.00 – 89.50) |
| Able to participate in social roles | 42.00 (36.10 – 44.50) |
| Barriers | |
| Intrapersonal | 1.94 (1.04 – 3.15) |
| Interpersonal | 0.76 (0 – 1.92) |
| Organizational | 0 (0 – 1.92) |
| Community | 0.35 (0 – 1.09) |
IQR = Interquartile Range
Correlates of Exercise Video Minutes
Median regression analysis demonstrated that self-efficacy, depression, anxiety, physical functioning, and community barriers had statistically significant negative correlations with average exercise video minutes, whereas age and pain were identified as positive correlates (see table 2). African Americans, relative to Whites, spent less time viewing exercise videos (β=−65.62, p-value=0.004). A one unit increase in the exercise self-efficacy scale (β=−0.6, p-value=0.007) was associated with shorter minutes of video viewed. Similarly, depression (β=−1.4, p-value=0.038), anxiety (β=−3.84, p-value=0.000), physical functioning (β=−1.35, p-value=0.048), and community barriers (β=−9.12, p-value=0.026) were associated with shorter viewed video minutes. In contrast, each additional year in age was associated with longer periods of viewing exercise videos (β=1.1, p-value=0.013). Additionally, higher pain intensity (β=1.89, p-value=0.022) and pain interference (β=1.84, p-value=0.012) scores were associated with longer minutes of video watched. A notable trend included lower video minutes for participants able to participate in social activity (β=−1.84, p-value=0.052).
Table 2.
Median regression of exercise video minutes with personal and environmental factors and secondary health conditions (n=28)
| Variables | Coefficients | p-value |
|---|---|---|
| Personal Factors | ||
| Disability type | ||
| Traumatic SCI | ref | |
| Spina Bifida | 9.76 | 0.443 |
| Sex | ||
| Male | ref | |
| Female | 4.00 | 0.674 |
| Race | ||
| White | Ref | |
| African American | −65.62 | 0.004c |
| Age | 1.13 | 0.013b |
| Physical functioning | −1.35 | 0.048b |
| Exercise goal-setting | −1.02 | 0.188 |
| Exercise planning and scheduling | 1.75 | 0.134 |
| Exercise self-efficacy | −0.61 | 0.007c |
| Outcome expectations for exercise | 6.01 | 0.220 |
| Environmental Factors | ||
| Social Support | −0.70 | 0.221 |
| Able to participate in social activities | −1.84 | 0.052a |
| Barriers | ||
| Intrapersonal | 0.49 | 0.861 |
| Interpersonal | −3.47 | 0.354 |
| Organizational | 0.95 | 0.609 |
| Community | −9.12 | 0.026b |
| Secondary Health Conditions | ||
| Anxiety | −3.84 | 0.000c |
| Depression | −1.42 | 0.038b |
| Fatigue | −0.35 | 0.513 |
| Sleep disturbance | −1.80 | 0.111 |
| Pain interference | 1.84 | 0.012b |
| Pain intensity | 1.89 | 0.022b |
p <0.1;
p < 0.05;
p < 0.01;
Psuedo-R2 = 0.77;
Descriptive Adherence
Regarding adherence (number of minutes of exercise performed weekly), the median minutes of exercise videos watched per week for the 12-week period was 21 minutes (figure 3). Median minutes of exercise video prescribed across the 12-week intervention was 93 minutes. For the adherence groups, 2 people (7%) were classified as high adherence (>74 minutes), 7 as sub-optimal adherence (25%; 37–74 minutes), 5 as low adherence (18%; 18–37 minutes), and 14 as non-adherence (50%; <18 minutes).
Figure 3.
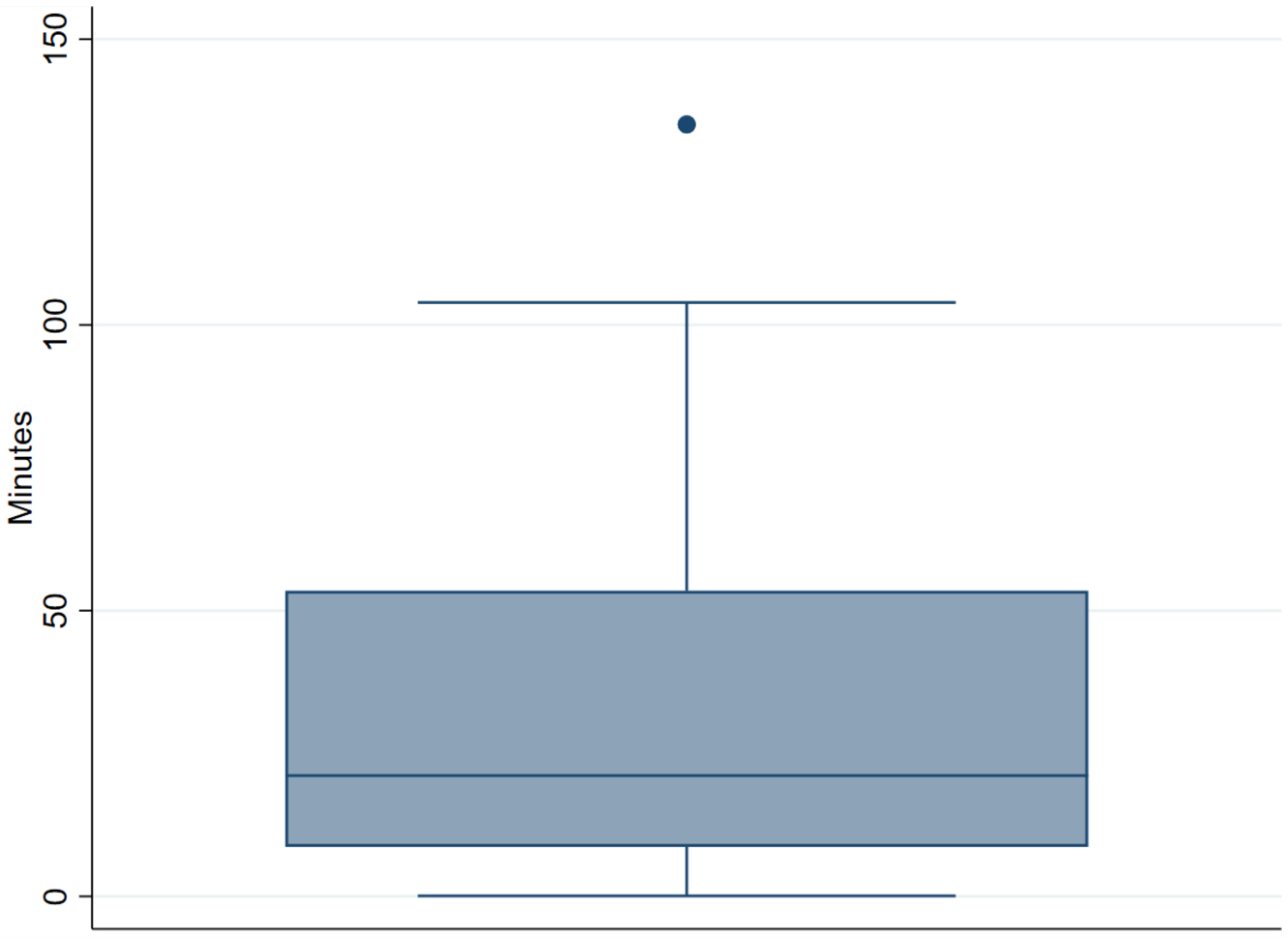
Average minutes of exercise video viewed for the 12-week period
Discussion
This is the first study to examine predictors of adherence to a self-managed and home-based exercise program using rhythmic movement patterns to music. The M2M program incorporated telehealth technology to bypass common barriers to exercise, such as lack of transportation and access to knowledgeable staff. Despite removing these barriers, study findings demonstrated that adherence was low and could be predicted by certain demographic, health, functional, and environmental factors.
The current study included potential predictors of physical activity among people with SCI based on those that have been analyzed in previous studies [20, 21]. In agreement with those studies, personal factors accounted for the strongest predictors [21]. In contrast, self-regulatory behaviors (goal setting and planning) were not identified as significant predictors of participation as shown in previous studies [20]. This may have been due to the nature of the intervention, which was home-based, included built-in goals and schedule (e.g., videos delivered weekly 3 times a week), and provided participants with all of the resources needed to perform exercise so their planning was minimized (i.e., exercise videos, tablet, wrist weights).
Another contrasting finding from previous studies was that higher self-efficacy values were correlated with lower exercise minutes. Previous research demonstrated that higher self-efficacy was associated with higher rates of physical activity in people with SCI [20]. However, this may have been due to participants with higher levels of self-efficacy having more opportunities or access to physical activity outside the home, something that could be explored in future research.
Another finding was that community barriers (e.g., accessible facilities, equipment, and cost) were associated with lower exercise minutes. Given that the exercise intervention was focused on eliminating these self-reported barriers, the perceived notion of community barriers among our participants may have had an indirect effect on their level of interest in participating in the home-based exercise program. Lastly, higher scores of pain interference and pain intensity were correlated with more exercise video minutes, which could possibly be interpreted that people who were experiencing pain were more motivated to improve their pain levels by participating in more exercise [22].
Adherence to video minutes was lower than expected across the sample with half of the participants adhering to less than 20 percent of the prescribed video minutes. However, using the physical activity guidelines for people with SCI of 20 minutes of aerobic exercise twice per week [23], one third of our sample met these international guidelines. This is a positive finding given that our target group was not getting any regular exercise and increasing that to 33% of participants obtaining enough minutes of exercise that provides cardiorespiratory benefits is a good starting point upon which to build future research [23].
Not surprisingly, higher scores for anxiety, depression, and physical functioning at baseline were associated with lower exercise video minutes among study participants. An opportunity is being missed when baseline factors such as these are not being used to individualize the intervention (i.e., behavior change techniques, dosing, delivery) in order to improve participant adherence. These findings provide researchers and health professionals with an opportunity to design intervention strategies that are tailored based upon baseline data [24].
Researchers interested in exercise adherence in SCI populations should consider using adaptive intervention designs in home and community-based interventions (e.g., stepped-care designs). These designs can serve as a mechanism for tailoring and ‘titrating’ the intervention based on participant data that are obtained during early intervention stages. Adaptive designs have become popular for targeting low-response behaviors such as smoking cessation and weight loss [25, 26]. Almirall et al. [27] introduced the Sequential Multiple Assignment Randomized Trial (SMART), a type of research design for creating adaptive interventions. These SMART designs help investigators determine when and how to alter treatment variables such as dose, time, and delivery method in order to develop an adaptive intervention [27]. Adaptive intervention designs are also purposed to increase adherence to the intervention by augmenting or switching programs for low- or non-responders.
The SMART design entails a first-stage treatment decision that either compares the length of the first stage or compares the effect of two evidence-based interventions, which can be individualized based on baseline measures associated with adherence (e.g., personal factors, secondary health conditions). A primary tailoring variable (e.g., weeks of first treatment stage) is tested that leads to a second stage treatment decision (i.e., adherence level), which compares an augmentation of the current treatment versus switching to an alternative evidence-based intervention. There is considerable evidence that under the right circumstances and with the appropriate dosing and monitoring, exercise behavior can be modified in cohorts with low and non-participation rates [28]. To our knowledge, these designs have not been utilized in exercise interventions for people with SCI.
There is a clear and pressing need for designing future home-based exercise interventions that have a greater level of customization/tailoring for participants who have low to non-participation rates. With the growing popularity of SMART interventions, use of baseline variables and decision support tools that use algorithmic technology to provide greater customization of exercise interventions to people with SCI may increase their rates of exercise adherence and participation.
Strengths and Limitations
This study analyzed preliminary data from a remotely delivered exercise program aimed at improving access to exercise for people with SCI including spina bifida. There are several limitations that should be considered as part of this study. Given the sample size, the generalizability of the study findings was limited. The sample consisted of people living in the Deep South of the United States, where the study was conducted, and thus, the findings may only apply to people living in similar settings (e.g., limited access to healthcare, high disability prevalence, and high prevalence of chronic disease). Another limitation was the higher percentage of females to males, which differs from the higher incidence of SCI among men [29]. A third limitation was the lack of some potential predictors of participation, such as enjoyment, which is a primary component of M2M but was not part of our regression model.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the UAB/Lakeshore Research Collaborative for supporting this project.
Funding
This research was sponsored by a grant from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R01HD085186) and a Mentored Career Development Award from the National Center Advancing Translational Research (KL2 TR 003097).
Footnotes
Data Archiving
This study presents preliminary findings on a sub-population of a larger trial. A supplementary file with de-identified data from which the primary results were derived is attached.
Statement of Ethics
The intervention protocol was approved by the University’s Institutional Review Board (IRB-160923002) and was registered with ClinicialTrials.gov (#NCT03024320) as a phase III clinical trial. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Webb T, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: A systemic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Internet Med Res. 2010;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miriam W, Martin-Diener E, Bauer G, Braun-Fahrländer C, Martin BW. Comparison of trial participants and open access users of a web-based physical activity intervention regarding adherence, attrition, and repeated participation. J Internet Med Res. 2010;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive-behavior therapy for health problems: A systematic review. J Behav Med. 2008;39:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin Ginis KA, Ma JK, Latimer-Cheung AE, Rimmer JH. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health Psych Rev. 2016;10(4):478–94. [DOI] [PubMed] [Google Scholar]
- 5.Spinal Cord Injury Research Evidence. Physical Activity Participation Levels in SCI 2020 [Available from: https://scireproject.com/evidence/rehabilitation-evidence/physical-activity/increasing-physical-activity-participation-in-sci/physical-sci/.
- 6.Rimmer JH, Lai B, Young HJ. Bending the arc of exercise and recreation technology toward people with disabilities. Arch Phys Med Rehabil. 2016;97(9 Suppl):S247–51. [DOI] [PubMed] [Google Scholar]
- 7.Lai B, Rimmer J, Barstow B, Jovanov E, Bickel CS. Teleexercise for persons with spinal cord injury: a mixed-methods feasibility case series. JMIR Rehabil Assist Technol. 2016;3(2):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman N, Jette AM, Houlihan B, Williams S. Computer and internet use by persons after traumatic spinal cord injury. Arch Phys Med Rehabil. 2008. Aug 1;89(8):1492–8 [DOI] [PubMed] [Google Scholar]
- 9.Young H-J, Mehta TS, Herman C, Wang F, Rimmer JH. The effects of M2M and adapted yoga on physical and psychosocial outcomes in people with multiple sclerosis. Arch Phys Med Rehabil. 2019;100(3):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rimmer JH, Mehta T, Wilroy J, Lai B, Young H-J, Kim Y, et al. Rationale and design of a Scale-Up Project Evaluating Responsiveness to Home Exercise And Lifestyle Tele-Health (SUPER-HEALTH) in people with physical/mobility disabilities: a type 1 hybrid design effectiveness trial. BMJ Open. 2019;9(3):e023538–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai B, Wilroy J, Young H-J, Howell J, Rimmer JH, Mehta T, et al. A mobile app to promote adapted exercise and social networking for people with physical disabilities: usability study. JMIR Form Res. 2019;3(1):e11689–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh Y, Weikert M, Dlugonski D, Sandroff B, Motl RW. Social cognitive correlates of physical activity: findings from a cross-sectional study of adults with relapsing-remitting multiple sclerosis. J Phys Act Health. 2011;8(5):626–35. [DOI] [PubMed] [Google Scholar]
- 13.Rovniak LS, Anderson ES, Winett RA, Stephens RS. Social cognitive determinants of physical activity in young adults: a prospective structural equation analysis. Ann Behav Med. 2002;24(2):149–56. [DOI] [PubMed] [Google Scholar]
- 14.Motl RW, Snook EM, McAuley E, Scott JA, Douglass ML. Correlates of physical activity among individuals with multiple sclerosis. Ann Behav Med. 2006;32(2):154–61. [DOI] [PubMed] [Google Scholar]
- 15.Health Measures. PROMIS (Patient-Reported Outcome Measurement Information System). http://www.healthmeasures.net/index.php?option=com_content&view=category&layout=blog&id=147&Itemid=806 [Accessed 10 April 2020]
- 16.Vasudevan V, Rimmer JH, Kviz F. Development of the barriers to physical activity questionnaire for people with mobility impairments. Disabil Health J. 2015;8(4):547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu C-Y, Motl RW, Ditchman N. Validation of the social provisions scale in people with multiple sclerosis. Rehabil Psychol. 2016;61(3):297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalali N, Babanezhad M. Quantile regression due to skewness and outliers. Appl Math Sci. 2011;5(39):1974–51. [Google Scholar]
- 19.Lai B, Cederberg K, Vanderbom KA, Bickel CS, Rimmer JH, Motl RW. Characteristics of adults with neurologic disability recruited for exercise trials: a secondary analysis. Adapt Phys Act Q. 2018;35(4):476–97. [DOI] [PubMed] [Google Scholar]
- 20.Martin Ginis KA, Latimer AE, Arbour-Nicitopoulos KP, Bassett RL, Wolfe DL, Hanna SE. Determinants of physical activity among people with spinal cord injury: a test of social cognitive theory. Ann Behav Med. 2011;42(1):127–33. [DOI] [PubMed] [Google Scholar]
- 21.Martin Ginis KA, Arbour-Nicitopoulos KP, Latimer-Cheung AE, Buchholz AC, Bray SR, Craven BC, et al. Predictors of leisure time physical activity among people with spinal cord injury. Ann Behav Med. 2012;44(1):104–18. [DOI] [PubMed] [Google Scholar]
- 22.Tawashy AE, Eng JJ, Lin KH, Tang PF, Hung C. Physical activity is related to lower levels of pain, fatigue and depression in individuals with spinal-cord injury: a correlational study. Spinal Cord. 2009;47(4):301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56(4):308–21. [DOI] [PubMed] [Google Scholar]
- 24.Tomasone JR, Flood SM, Ma JK, Scime NV, Burke SM, Sleeth L, et al. Physical activity self-management interventions for adults with spinal cord injury: Part 1 – A systematic review of the use and effectiveness of behavior change techniques. Psychol Sport Exerc. 2018;37:274–285. [Google Scholar]
- 25.Fu SS, Rothman AJ, Vock DM, Lindgren B, Almirall D, Begnaud A, et al. Program for lung cancer screening and tobacco cessation: study protocol of a sequential, multiple assignment, randomized trial. Contemporary Clinical Trials. 2017;60:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherwood NE, Butryn ML, Forman EM, Almirall D, Seburg EM, Lauren Crain A, et al. The BestFIT trial: A SMART approach to developing individualized weight loss treatments. Contemp Clin Trials. 2016;47:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med. 2014;4(3):260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobbs N, Godfrey A, Lara J, Errington L, Meyer TD, Rochester L, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med. 2013;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance: 2019 SCI data sheet. https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202019%20-%20Final.pdf [Accessed 10 April 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


