Abstract
Autism spectrum disorder (ASD) is a highly prevalent neurodevelopmental disorder characterized by core symptoms of impaired social behavior and communication. Recent studies have suggested that the oxytocin system, which regulates social behavior in mammals, is potentially involved in ASD. Mouse models of ASD provide a useful system for understanding the associations between an impaired oxytocin system and social behavior deficits. However, limited studies have shown the involvement of the oxytocin system in the behavioral phenotypes in mouse models of ASD. We have previously demonstrated that a mouse model that carries the ASD patient-derived de novo mutation in the pogo transposable element derived with zinc finger domain (POGZWT/Q1038R mice), showed ASD-like social behavioral deficits. Here, we have explored whether oxytocin (OXT) administration improves impaired social behavior in POGZWT/Q1038R mice and found that intranasal oxytocin administration effectively restored the impaired social behavior in POGZWT/Q1038R mice. We also found that the expression level of the oxytocin receptor gene (OXTR) was low in POGZWT/Q1038R mice. However, we did not detect significant changes in the number of OXT-expressing neurons between the paraventricular nucleus of POGZWT/Q1038R mice and that of WT mice. A chromatin immunoprecipitation assay revealed that POGZ binds to the promoter region of OXTR and is involved in the transcriptional regulation of OXTR. In summary, our study demonstrate that the pathogenic mutation in the POGZ, a high-confidence ASD gene, impairs the oxytocin system and social behavior in mice, providing insights into the development of oxytocin-based therapeutics for ASD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13041-021-00769-8.
Keywords: Social behavior, Autism spectrum disorder, POGZ, De novo mutation, Paraventricular nucleus, Oxytocin, Oxytocin receptor, ChIP
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by core symptoms of impaired social behavior and communication. Its molecular pathology is largely unclear [1]. Although the prevalence rate of ASD is considerable, there are no pharmacological therapeutics for the core symptoms of ASD.
The neuropeptide oxytocin plays a central role in social behavior [2, 3]. Genetic variation in the oxytocin system is associated with social behavior in humans [4]. Recent clinical studies have suggested a potential therapeutic effect of oxytocin in ASD [5]. In mice, targeted disruption of genes encoding oxytocin and its receptor impairs social behavior [6]. Thus, mouse models of ASD provide a useful system for understanding the associations between an impaired oxytocin system and social behavior deficits. However, limited studies have shown the effectiveness of oxytocin in the behavioral phenotypes in mouse models of ASD [7–9].
The pogo transposable element derived with zinc finger domain (POGZ) is one of the most frequently de novo mutated genes in patients with ASD [10], making POGZ a high-confidence and strong candidate ASD gene (SFARI database). We previously generated a mouse model that carried a pathogenic de novo mutation of POGZ identified in a patient with ASD (POGZWT/Q1038R mouse) and found ASD-like behavioral abnormalities in these mice [11], emphasizing the relevance of POGZWT/Q1038R mouse as an ASD model with high construct and face validity. This study explored whether oxytocin administration improves impaired social behavior in POGZWT/Q1038R mice.
Results
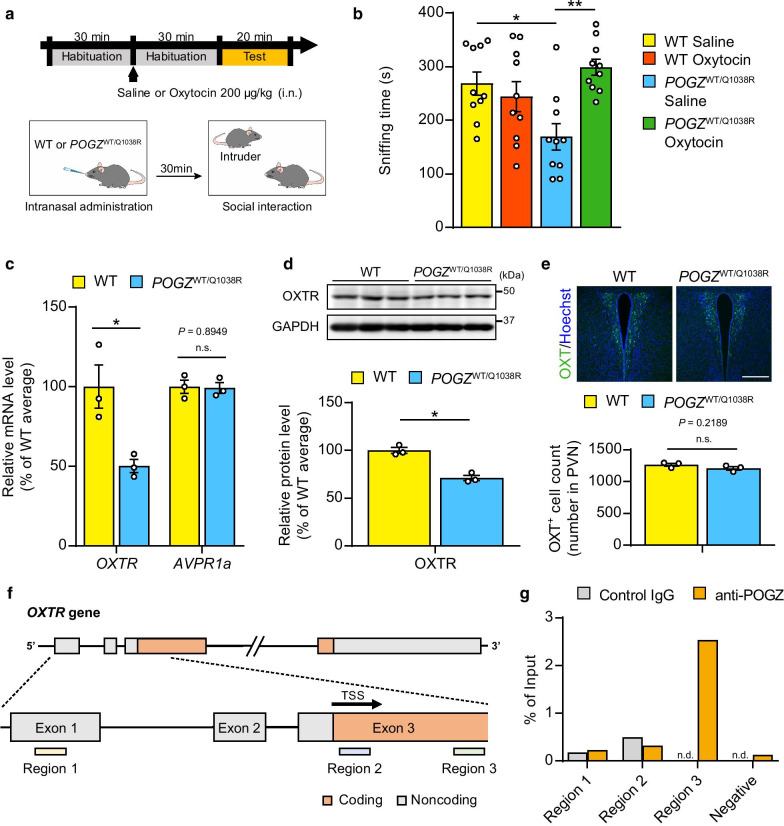
To examine the effect of oxytocin on social behavior deficits in POGZWT/Q1038R mice, we performed a reciprocal social interaction test 30 min after intranasal administration of saline or 200 μg/kg of oxytocin (Fig. 1a). The dose of oxytocin administration was determined based on the previous report [7, 8]. Consistent with our previous study [11], we confirmed that POGZWT/Q1038R mice treated with saline spent significantly less time sniffing the intruder mice than their WT littermates. We found that intranasal administration of oxytocin successfully ameliorated the decreased sniffing time in POGZWT/Q1038R mice, but it did not significantly affect the sniffing time in WT mice (Fig. 1b).
Fig. 1.
Oxytocin ameliorates impaired social interaction in POGZWT/Q1038R mice. a Time course of the reciprocal social interaction test. The test was performed 30 min after intranasal administration of oxytocin (200 μg/kg) or saline. The duration of sniffing was measured during the test. i.n. intranasally, WT wild-type. b Time spent sniffing in the reciprocal social interaction test after oxytocin treatment (each n = 10). c qPCR analysis of the expression levels of OXTR and AVPR1a mRNA in the brain (each n = 3). OXTR, oxytocin receptor; AVPR1a, vasopressin receptor 1A. d Representative western blotting and quantification of decreased OXTR in POGZWT/Q1038R mice. GAPDH was used as loading control (each n = 3). Values were normalized to the expression levels of GAPDH. Uncropped western blot images are shown in Additional file 2: Fig. S1. e Representative image of OXT (green) and Hoechst 33258 (blue) immunofluorescence in the PVN and stereological counts of OXT-expressing cells in the PVN region of WT and POGZWT/Q1038R mice (each n = 3; scale bar, 300 μm). f Schematic diagram of the position of the qPCR amplicons by each primer set in the mouse OXTR promoter region. TSS translation start site. g ChIP assay coupled with qPCR for the quantification of DNA fragments precipitated by an anti-POGZ antibody (n = 1 mice). The results are presented as percentage of the input DNA. n.d. not detected. Data are presented as the mean ± SEM. Statistical significance was analyzed by two-way ANOVA, followed by Bonferroni Dunn post hoc tests (b, F1, 36 = 11.67) and Student’s t-test (c–e). *P < 0.05, **P < 0.01
To characterize the oxytocin system in POGZWT/Q1038R mice, we first measured the expression levels of oxytocin receptor (OXTR) and vasopressin receptor 1A (AVPR1a), which are receptors for oxytocin (OXT) [12]. We found that the OXTR mRNA and protein expression was significantly decreased in POGZWT/Q1038R mice compared to that in WT littermates (Fig. 1c, d). There was no significant difference in the expression levels of AVPR1a mRNA between the genotypes (Fig. 1c). We then measured the number of OXT-expressing neurons in the paraventricular nucleus (PVN), a major brain region in OXT synthesis [12]. In contrast to previously reported mouse models of ASD, in which social behavior was curable by OXT [8, 9], we did not detect significant changes in the number of OXT-expressing neurons between the PVN of POGZWT/Q1038R mice and that of WT mice (Fig. 1e).
According to its amino acid sequences and putative domain structures, POGZ is suggested to be involved in transcriptional regulation. Given that the human POGZ-Q1042R (Q1038R in mice) mutation reduces the DNA-binding activity of POGZ [13], we hypothesized that POGZ-Q1038R mutation disrupts the binding of POGZ to DNA, resulting in decreased OXTR gene expression. To assess whether POGZ binds to the OXTR promoter, we performed chromatin immunoprecipitation (ChIP) assays coupled with qPCR (Fig. 1f). Based on a previous study on the OXTR promoter [14], we performed qPCR at three sites, upstream (Region 1), directly below (Region 2), and downstream (Region 3) of the translation start site (TSS) in the promoter region of OXTR. We found that the DNA sequence containing Region 3 was enriched by anti-POGZ antibody immunoprecipitation, whereas the DNA sequences containing Regions 1 and 2 were not enriched (Fig. 1g). These data suggest that POGZ binds to the OXTR promoter downstream of the TSS and regulates OXTR gene expression.
Discussion
A growing body of evidence suggests that oxytocin/vasopressin family peptides are promising therapeutic agents for ASD [4, 5]. However, the possible associations between ASD-associated genetic mutations and the oxytocin system are largely unclear. In this study, we showed that impaired social behavior caused by pathogenic mutation in POGZ, a high-confidence ASD gene, can be treated with oxytocin even in the adults. Our study provides insights into the development of oxytocin-based therapeutics for ASD.
Although epigenetic regulation affects the transcription of OXTR [12], limited information is available on intracellular and extracellular signals as well as the molecular mechanisms that regulate the transcription of OXTR. The downstream pathways of OXTR underlying the regulation of social behavior are also unclear. We found that the pathogenic POGZ mutation identified in a patient with ASD caused decreased OXTR expression (Fig. 1c, d). Unlike OXTR-null mice [6], only a slight but significant decrease in OXTR expression is suggested to impair social interaction. Unraveling the precise molecular phenotype of POGZ-mediated regulation of OXTR expression will help understand the association between impaired social behavior and OXTR signaling.
Given that histone H3 lysine 9 acetylation and trimethylation, markers for increased and repressed gene expression, respectively, are enriched at the Region 3 in the OXTR promoter [14], POGZ binds to the Region 3 and may be involved in the regulation of OXTR expression through epigenetic mechanisms. Although our results suggest that POGZ positively regulates OXTR expression and that POGZ-Q1038R mutation disrupts the transcriptional function of POGZ, recent functional analysis using a luciferase assay showed that POGZ represses transcription [15]. This discrepancy can be reconciled by further investigation of the molecular phenotype underlying the Q1038R mutation.
Our study suggest that pathogenic mutation in one of the high-confidence ASD genes impairs the oxytocin system. Further studies on the possible effects of pathogenic mutations in high-confidence ASD genes on oxytocin signaling are needed to confirm the generalizability of the current findings. Considering the etiological heterogeneity of ASD, focusing on the role of the oxytocin system in ASD can be beneficial for the neurobiological and molecular mechanism-based stratification of patients with ASD, which is important for accelerating therapeutic development.
Supplementary Information
Additional file 1. Detailed materials and methods.
Additional file 2: Fig. S1. Raw images of entire membrane of immunoblotting
Acknowledgements
We thank Drs. Shinya Ayabe, Masaru Tamura, Shigeharu Wakana and Atsushi Yoshiki (RIKEN BioResource Research Center) for generating the POGZWT/Q1038R mice.
Abbreviations
- ASD
Autism spectrum disorder
- ChIP
Chromatin immunoprecipitation
- i.n.
Intranasally
- n.d.
Not detected
- OXT
Oxytocin
- OXTR
Oxytocin receptor
- POGZ
Pogo transposable element derived with zinc finger domain
- PVN
Paraventricular nucleus
- qPCR
Quantitative PCR
- TSS
Translation start site
- WT
Wild-type
Authors’ contributions
KK, KM, HH, and TN designed the experiments and wrote the manuscript. KK, KM, MB, MK, TT, KN, YA, KS, AH-T, AK, RH, and KT designed and performed the biochemical and animal behavior experiments and analyzed the data. All authors read and approved the final manuscript.
Funding
This work was supported in part by JSPS KAKENHI, Grant numbers JP17H03989 (H.H.), JP18H02574 (T.N.), JP20H03556 (K.S.), JP20H03391 (A.K.), JP20K21479 (A.K.), JP20H00492 (H.H.), and JP20K21464 (T.N.); MEXT KAKENHI, Grant numbers JP18H05416 (H.H. and T.N.), JP19H05217 (A.K.), and JP19H05218 (T.N.); AMED, Grant numbers JP20dm0107122 (H.H.), JP20dm0207061 (H.H.), and JP20gm1310003 (T.N.); Platform Project for Supporting Drug Discovery and Life Science Research (BINDS), Grant number JP20am0101084 (H.H.); and Grants from the Takeda Science Foundation (H.H. and T.N.), the Asahi Glass Foundation (A.K. and T.N.), and the Naito Foundation (T.N.). This study was also supported in part by the Center for Medical Research and Education, Graduate School of Medicine, Osaka University.
Availability of data and materials
Detailed materials and methods are included in Additional file 1. All data supporting the finding of this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Animal experiments were performed in accordance with the guidelines for animal use issued by the Committee of Animal Experiments at Osaka University (#28-1-15).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hitoshi Hashimoto, Email: hasimoto@phs.osaka-u.ac.jp.
Takanobu Nakazawa, Email: tn207427@nodai.ac.jp.
References
- 1.Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis Primers. 2020;6:5. doi: 10.1038/s41572-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walum H, Young LJ. The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci. 2018;19:643–654. doi: 10.1038/s41583-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cataldo I, Azhari A, Esposito G. A review of oxytocin and arginine-vasopressin receptors and their modulation of autism spectrum disorder. Front Mol Neurosci. 2018;11:27. doi: 10.3389/fnmol.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamasue H, Okada T, Munesue T, Kuroda M, Fujioka T, Uno Y, et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Mol Psychiatry. 2020;25:1849–1858. doi: 10.1038/s41380-018-0097-2. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell HK, Aulino EA, Freeman AR, Miller TV, Witchey SK. Oxytocin and behavior: lessons from knockout mice. Dev Neurobiol. 2017;77(2):190–201. doi: 10.1002/dneu.22431. [DOI] [PubMed] [Google Scholar]
- 7.Hara Y, Ago Y, Higuchi M, Hasebe S, Nakazawa T, Hashimoto H, et al. Oxytocin attenuates deficits in social interaction but not recognition memory in a prenatal valproic acid-induced mouse model of autism. Horm Behav. 2017;96:130–136. doi: 10.1016/j.yhbeh.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Peñagarikano O, Lázaro MT, Lu X-H, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hörnberg H, Pérez-Garci E, Schreiner D, Hatstatt-Burklé L, Magara F, Baudouin S, et al. Rescue of oxytocin response and social behavior in a mouse model of autism. Nature. 2020;584:252–256. doi: 10.1038/s41586-020-2563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satterstrom FK, Kosmicki JA, Wang J, Breen MS, Rubeis SD, An J-Y, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180(3):568–84.e23. doi: 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumura K, Seiriki K, Okada S, Nagase M, Ayabe S, Yamada I, et al. Pathogenic POGZ mutation causes impaired cortical development and reversible autism-like phenotypes. Nat Commun. 2020;11:859. doi: 10.1038/s41467-020-14697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 2018;98(3):1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura K, Nakazawa T, Nagayasu K, Gotoda-Nishimura N, Kasai A, Hayata-Takano A, et al. De novo POGZ mutations in sporadic autism disrupt the DNA-binding activity of POGZ. J Mol Psychiatry. 2016;4:1. doi: 10.1186/s40303-016-0016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glendining KA, Jasoni CL. Maternal high fat diet-induced obesity modifies histone binding and expression of Oxtr in offspring hippocampus in a sex-specific manner. Int J Mol Sci. 2019;20(2):329. doi: 10.3390/ijms20020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suliman-Lavie R, Title B, Cohen Y, Hamada N, Tal M, Tal N, et al. Pogz deficiency leads to transcription dysregulation and impaired cerebellar activity underlying autism-like behavior in mice. Nat Commun. 2020;11:5836. doi: 10.1038/s41467-020-19577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Detailed materials and methods.
Additional file 2: Fig. S1. Raw images of entire membrane of immunoblotting
Data Availability Statement
Detailed materials and methods are included in Additional file 1. All data supporting the finding of this study are available from the corresponding author on reasonable request.