Abstract
Background.
Ghana was among the first African nations to introduce monovalent rotavirus vaccine (RV1) into its childhood immunization schedule in April 2012. We aimed to assess the impact of vaccine introduction on rotavirus and acute gastroenteritis (AGE) hospitalizations and to estimate vaccine effectiveness (VE).
Methods.
Using data from 2 teaching hospitals, monthly AGE and rotavirus admissions by age were examined 40 months before and 31 months after RV1 introduction using interrupted time-series analyses. From January 2013, we enrolled children <2 years of age who were eligible for RV1 from a total of 7 sentinel sites across the country. To estimate VE, we fit unconditional logistic regression models to calculate odds ratios of vaccination by rotavirus case-patient status, controlling for potential confounders.
Results.
Vaccine coverage ranged from 95% to 100% for dose 1 and 93% to 100% for dose 2. In the first 3 years after vaccine introduction, the percentage of hospital admissions positive for rotavirus fell from 48% in the prevaccine period to 28% (49% adjusted rate reduction; 95% confidence interval [CI], 32%–63%) postvaccination among <5-year-olds. With high vaccine coverage, it was not possible to arrive at robust VE estimates; any-dose VE against rotavirus hospitalization was estimated at 60% (95% CI, −2% to 84%; P = .056).
Conclusions.
Results from the first 3 years following RV1 introduction suggest substantial reductions of pediatric diarrheal disease as a result of vaccination. Our VE estimate is consistent with the observed rotavirus decrease and with efficacy estimates from elsewhere in sub-Saharan Africa.
Keywords: Ghana, rotavirus, surveillance, case-control, vaccine effectiveness
Rotavirus is the leading cause of severe childhood acute gastroenteritis (AGE), and remains the fourth most common cause of childhood mortality worldwide [1]. Since 2009, the World Health Organization has recommended the use of rotavirus vaccine globally, especially in countries with high child mortality due to AGE. Currently, 2 live attenuated oral rotavirus vaccines are licensed globally: RV1, the human monovalent strain vaccine (Rotarix; GlaxoSmithKline Biologics, Rixenstart, Belgium) and RV5, a pentavalent bovine-human reassortant vaccine (RotaTeq; Merck Vaccines, Whitehouse Station, New Jersey) [2].
In sub-Saharan Africa, double-blind randomized controlled trials of both RV5 and RV1 vaccines in children demonstrated protection against rotavirus AGE. RV1 demonstrated a vaccine effectiveness (VE) of 61% (95% confidence interval [CI], 44%–73%) in preventing hospital admissions against severe AGE in studies conducted in South Africa and Malawi, whereas RV5 demonstrated a VE of 39% (95% CI, 19%–54%) [3, 4] in trials in Ghana, Kenya, and Mali. In the Ghana arm of the trial, VE was 56% (95% CI, 28%–73%). These efficacy values were, however, lower than the 70%–92% attained in high-income countries, such as the United States and Europe [5, 6]. In South Africa, the first sub-Saharan African country to introduce RV1 as part of the national immunization program, VE was estimated at 57% (95% CI, 40%–68%) [7]. The lower vaccine performance observed in these low- and middle-income countries on the African continent is consistent with VE estimates in lower-income settings in Latin America and Asia [8, 9].
Despite the lower VE of rotavirus vaccines observed in Africa, Latin America, and Asia, rotavirus vaccines have potential to save lives in Africa and reduce morbidity given the high rotavirus disease burden in sub-Saharan countries [10, 11]. An impact study of RV1 in South Africa estimated that 13 000–20 000 hospitalizations were prevented in the 2 years after RV1 introduction [10]. The aim of our study was to assess the impact of the vaccine introduction on rotavirus and AGE hospitalizations and to estimate VE of RV1 in Ghana since its introduction at the end of April 2012.
METHODS
Surveillance
Ghana is a low- to middle-income tropical West African nation with a total population of approximately 27 million and an annual birth cohort of approximately 800 000 [12]. Sentinel surveillance for rotavirus gastroenteritis was conducted in the 2 largest tertiary care hospitals in Ghana, the Korle Bu Teaching Hospital and the Komfo Anokye Teaching Hospital. The 2000-bed Korle Bu Teaching Hospital is the largest in Ghana, located in the capital city of Accra. Also included as part of the Korle Bu surveillance was Princess Marie Louise Children’s Hospital in Accra. The 1000-bed Komfo Anokye Teaching Hospital is located within the Ashanti region of central Ghana in the city of Kumasi and is the second-largest hospital in Ghana.
Since 2009, both hospitals have served as sentinel surveillance sites implementing the World Health Organization–recommended protocol [13]. Children <5 years old admitted with diarrhea (≥3 loose stools in a 24-hour period) and/or vomiting with a duration ≤7 days were enrolled in the surveillance program. Surveillance was conducted 24 hours a day in the emergency department and inpatient units, aiming to capture all children <5 years old admitted for severe AGE who required hospital admission or intravenous fluid rehydration. Physicians, nurses, and surveillance officers also reviewed log books to further identify and enroll children presenting with a chief complaint of vomiting or diarrhea and admitted overnight. Children identified >48 hours after admission were not enrolled because of the risk of including nosocomial infections.
Stool specimens were collected within 48 hours of admission, stored at 2°C–8°C, and tested for rotavirus using a commercially available enzyme immunoassay (EIA) (ProSpecT; Oxoid, Cambridge, United Kingdom) at the medical virology laboratories of Korle Bu Teaching Hospital and Komfo Anokye Teaching Hospital. Rotavirus-positive stool specimens and 10% EIA-negative specimens were transferred to the Noguchi Memorial Institute for Medical Research in Accra for genotyping and retesting for quality control, respectively. Genotyping was performed by reverse transcription polymerase chain reaction as described elsewhere [3].
Vaccine Effectiveness Evaluation
From January 2013, to achieve study power and improve geographic representation, we also enrolled children from other hospitals in Kumasi (Agogo Presbyterian Hospital); Ho in the Volta region (Ho Municipal Hospital, Ho Regional Hospital and Hohoe District Hospital); and the only hospital in the Kassena Nankana district of the Upper East Region (Navrongo War Memorial Hospital). Including the 2 surveillance sites described above, there were a total of 7 sentinel sites across the country that recruited participants.
The study was conducted through a case-control design at the 7 hospitals described above. All vaccine-eligible children (ie, born on or after 1 April 2012, and ≥6 weeks old) who were hospitalized after 1 January 2013 for AGE were enrolled and had their stool tested for rotavirus. Children who received a dose of the vaccine within 14 days of hospital admission were excluded. Case patients were defined as children born on or after 1 April 2012 who were hospitalized for rotavirus diarrhea or received intravenous hydration in the emergency department, and controls were defined as children with rotavirus test-negative diarrhea.
Data Collection
After written informed consent was obtained from caregivers, a questionnaire was administered to collect demographic and socioeconomic factors, medical history and history of present illness, and vaccination history. The vaccination history was considered confirmed if a copy of the vaccination card or a vaccination clinic record was produced. Any missing information was obtained through review of medical records and at least 3 attempts were made to follow up by phone or in person.
Statistical Methods
Trends Analysis
Monthly rotavirus admissions by age were examined 40 months before (January 2009–March 2012) and 31 months after (year 1: April 2012–March 2013; year 2: April 2013–March 2014; year 3: April 2014–December 2014) introduction of rotavirus vaccination using an interrupted time-series analyses, with the third period being only 9 months in duration. Because of laboratory diagnostic sensitivity concerns, 2009 data from the Komfo Anokye Teaching Hospital were excluded from the rotavirus trends analysis, but were retained for the AGE analysis. A generalized linear model was fit to the time-series data, assuming that monthly counts of admissions were Poisson distributed. We adjusted for seasonality by including calendar month and accounted for total diarrhea admissions by considering the log of nonrotavirus admissions as the exposure. We controlled for possible secular trends in surveillance sensitivity by including a sequential monthly term in the model. This assumed a linear and monotonic secular trend and, therefore, may result in a conservative estimate of vaccine-associated impact.
The rate ratio of rotavirus admissions in the vaccine era was calculated using an indicator variable for the year after rotavirus vaccine introduction, with prevaccine time as the referent. We investigated changes in rates by age group (<1 year, 1 to <2 years, 2 to <5 years) because vaccine coverage during the early years of an immunization program and disease rates vary substantially by age.
Case-Control Analysis
We performed bivariate analyses to assess for differences in demographic and socioeconomic factors comparing cases and test-negative controls using Wilcoxon rank-sum or χ2 tests for significance. Our primary objective was to estimate VE of 1 or 2 doses of RV1 against rotavirus hospitalization. Many children were still being vaccinated up to age 6 months and with substantial numbers of cases, to avoid residual confounding by age, we restricted analysis to children aged 6 months to <2 years at time of presentation. To estimate VE, we fit unconditional logistic regression models to calculate odds ratios (ORs) of vaccination by rotavirus case-patient status, with associated 95% CIs [14]. All models controlled for age and hospital. We also considered socioeconomic factors significant in bivariate analysis as potential confounders. We used backward stepwise elimination to retain factors that resulted in a ≥10% change in the primary VE outcome. However, none aside from region met this criteria. Age was retained as a confounder a priori; we also considered birth month/year as a confounder. Neither age nor birth month/year affected the OR estimate. Vaccine effectiveness was calculated as (1 − OR) × 100%. Statistical significance was designated as a P value <.05. Analyses were done with Stata software version 13.0 (StataCorp, College Station, Texas).
For secondary objectives, we also estimated VE stratified by age (6–11 months and 1 to <2 years) and doses of vaccine received (exactly 2). To investigate a potential gradient in protection by severity, we repeated all analyses for VE against for moderate to severe and severe rotavirus hospitalization, defined as hospital admission with rotavirus detected in stool by EIA and with a clinical severity score of ≥10 on a modified 20-point Vesikari scoring scale [15].
Ethical Analysis
This study was approved by the institutional review boards of the Centers for Disease Control and Prevention (Atlanta, Georgia), and the Noguchi Memorial Institute for Medical Research, University of Ghana (Accra). Study personnel obtained informed consent from the parents or legal guardian of the child.
RESULTS
Rotavirus vaccine was introduced nationwide in Ghana in late April 2012, and a high vaccine coverage was achieved at the onset among age-eligible children. Vaccine coverage among age-eligible controls aged ≥6 months born during the second quarter of 2012 (April–June) was 97% and 90% for 1-dose and 2-dose vaccine, respectively. For subsequent quarterly birth cohorts, vaccine coverage among controls aged ≥6 months ranged from 95% to 100% for dose 1 and 93% to 100% for dose 2 (Figure 1). Vaccination was generally timely, with the first dose administered between the sixth and 15th week of life for 92% (567/614) of vaccinated children and the second dose administered between the 10th and 35th week of life for 96% (584/607) of vaccinated children (Figure 1).
Figure 1.
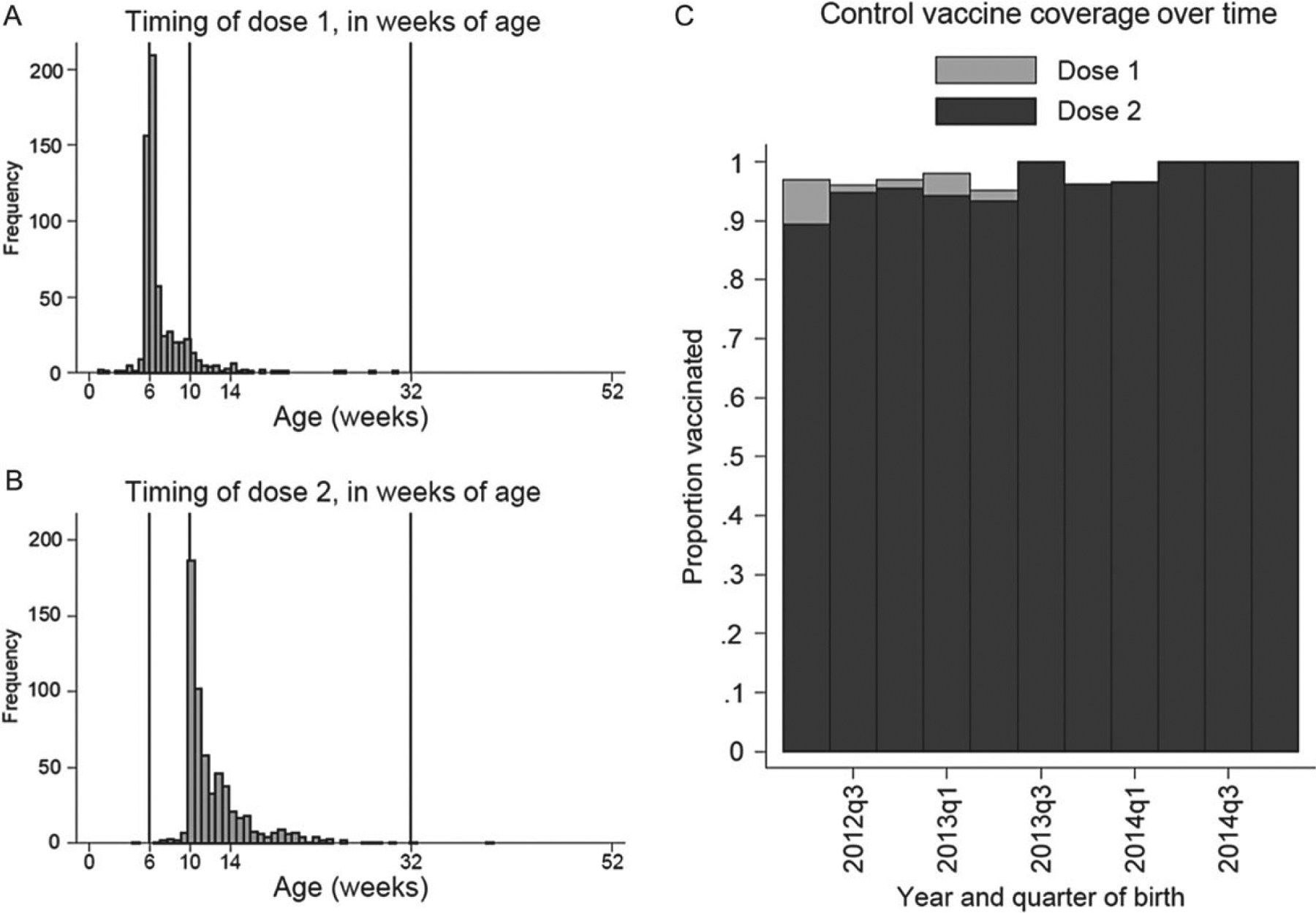
Age in weeks of receipt of dose 1 (A) and dose 2 (B) of monovalent rotavirus vaccine, and 1-dose and 2-dose vaccine coverage by quarter of birth (C).
Rotavirus Trends
Over the 2009–2014 surveillance period, a total of 3929 children aged <5 years were included whose stool was tested for rotavirus, of whom 1646 were positive (42%). Overall, of the 1646 rotavirus cases detected in surveillance, 1011 (61%) occurred in the first year of life and 516 (31%) occurred in the second year of life. AGE and rotavirus-associated hospitalizations decreased following vaccine introduction (Table 1; Figure 2A and 2B).
Table 1.
Trends in Rotavirus Vaccine Coverage and Rotavirus Positivity, and Relative Risk of Hospitalization for Rotavirus Gastroenteritis by Age Among Children Aged <5 Years, Ghana, April 2010–December 2014
| Age Group | Prevaccinea (Jan 2009-Mar 2012) | Year 1 Postvaccine (Apr 2012-Mar 2013) | Year 2 Postvaccine (Apr 2013-Mar 2014) | Year 3 Postvaccine (Apr 2014-Dec 2014) | Total Postvaccine (Apr 2012-Dec 2014) |
|---|---|---|---|---|---|
| <1 y | |||||
| RV+/No. (%) | 852/1688 (50) | 65/251 (26) | 60/211 (28) | 34/140 (24) | 159/602 (26) |
| RV rate reductiona,b (95% CI) | 1 | 58% (43%−70%) | 63% (45%−74%) | 73% (55%−84%) | 56% (38%−69%) |
| AGE rate reductionb (95% CI) | 1 | 51% (30%−65%) | 57% (20%−76%) | 52% (−18% to 80%) | 52% (36%−64%) |
| 1 to <2 y | |||||
| RV+/No. (%) | 386/820 (47) | 50/186 (27) | 51/151 (34 | 29/84 (35%) | 130/421 (32) |
| RV rate reductiona,b (95% CI) | 1 | 43% (15%−62%) | 29% (−26% to 60%) | 31% (−57% to 70%) | 44% (21%−60%) |
| AGE rate reductionb (95% CI) | 1 | 30% (0%−51%) | 42% (−3% to 68%) | 51% (−16% to 80%) | 23% (−4% to 44%) |
| 2 to <5 y | |||||
| RV+/No. (%) | 81/252 (32) | 11/46 (24) | 17/49 (35) | 10/51 (20) | 38/146 (26) |
| RV rate reductiona,b (95% CI) | 1 | 38% (−43% to 73%) | 29% (−173% to 76%) | 56% (−141% to 92%) | 19% (−70% to 61%) |
| AGE rate reductionb (95% CI) | 1 | 30% (−18% to 60%) | 9% (−123% to 63%) | 80% (−552% to 50%) | 50% (22%−68%) |
| Total (<5 y) | |||||
| RV+/No. (%) | 1319/2760 (48) | 126/483 (26) | 128/411 (31) | 73/275 (27) | 327/1169 (28) |
| RV rate reductiona,b (95% CI) | 1 | 54% (38%−65%) | 52% (30%−66%) | 64% (42%−87%) | 49% (32%−63%) |
| AGE rate reductionb (95% CI) | 1 | 43% (20%−43%) | 48% (9%−71%) | 43% (−30% to 75%) | 44% (28%−64%) |
Vaccine was introduced nationally in April 2012.
Abbreviations: AGE, acute gastroenteritis; CI, confidence interval; RV, rotavirus.
Komfo Anokye Teaching Hospital rotavirus testing data were excluded from 2009 analysis.
Compared to prevaccine period, adjusting for hospital and calendar month and secular time trends.
Figure 2.
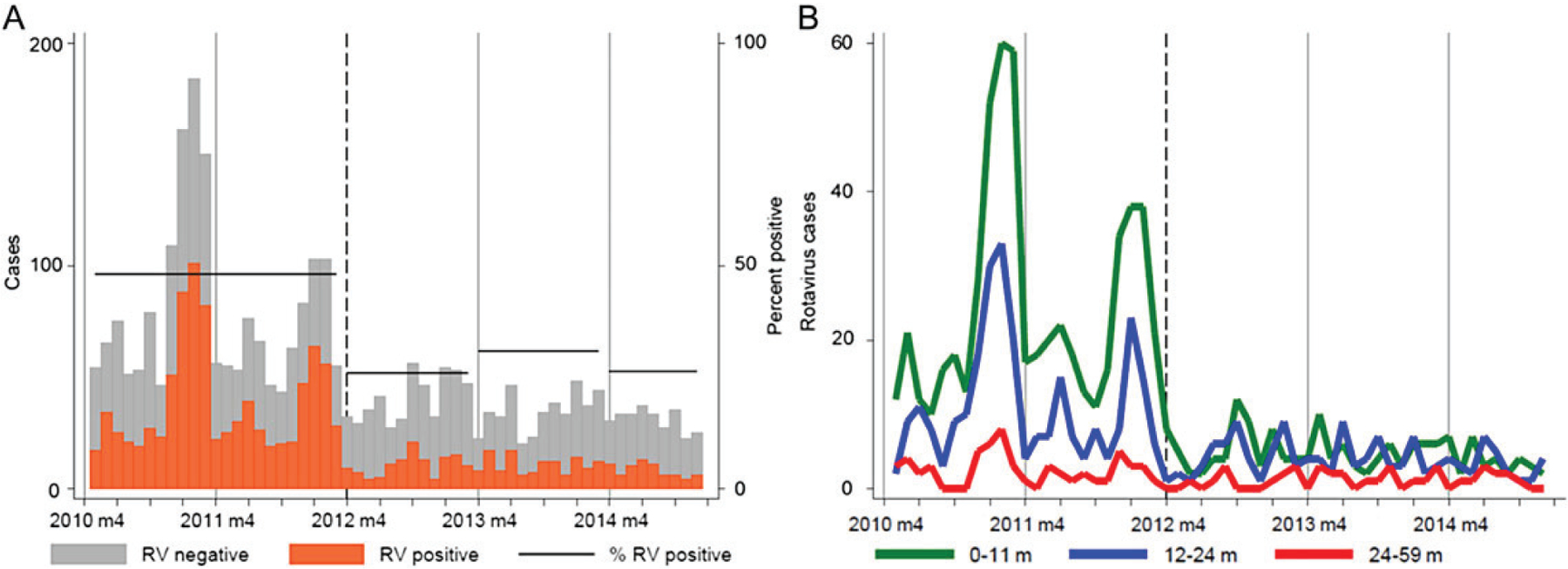
A, Rotavirus (RV)–positive and RV-negative hospital admissions among children <5 years of age. B, RV-positive hospital admissions by age. Ghana, January 2010–December 2014. Vaccine was introduced nationally in April 2012 (dashed lines).
In the first 3 years after vaccine introduction, the percentage of hospital admissions positive for rotavirus fell from 48% in the prevaccine period to 28% (adjusted rate reduction [aRR], 49%; 95% CI, 32%–63%) postvaccination among <5-year-olds. These patterns were most pronounced for <1-year-olds, among whom rotavirus prevalence fell from 50% in the prevaccine period to 26% (aRR, 56%; 95% CI, 38%–69%) postvaccination. Rotavirus prevalence also decreased among children aged 1 to <2 years, from 47% prevaccine to 32% (aRR, 44%; 95% CI, 21%–60%) postvaccination. Overall, rotavirus-associated hospitalizations were not significantly reduced in children aged 2 to <5 years. We found no statistical evidence of reductions in rotavirus hospitalizations in cohorts too old to have been vaccinated (eg, 1- to <5-year-olds in year 1 postvaccination or 2- to <5-year-olds in year 2 postvaccination), although all point estimates were in the direction of reductions.
AGE rates were significantly reduced among children <5 years of age (44%; 95% CI, 28%–64%) and <1 year of age (52%; 95% CI, 36%–64%), although less than the reductions against rotavirus cases.
From 2009 to 2014, a total of 572 specimens were genotyped. In all seasons, there was considerable diversity of G and P types (Supplementary Figure 1). G1P[8] was the most common genotype in all prevaccine seasons (2009: n = 31 [30%]; 2010: n = 25 [31%]; 2011: n = 14 [30%]). In postvaccination seasons, G12P [8] was the most common strain (2013: n = 28 [26%]; 2014: 29 [37%]). G12P[6], G2P[4], and G3P[6] were also common in postvaccination years.
Vaccine Effectiveness
A total of 1021 children were enrolled in the case-control study; 947 (93%) submitted a stool sample (96%), of whom 870 (92%) had card-documented vaccine status. Of these 870, 825 (95%) were admitted >14 days after their most recent dose of rotavirus vaccine (Figure 3). As explained above, we restricted all VE analyses to children aged 6 months to <2 years. Of these remaining 657 children, 207 (32%) tested positive for rotavirus (ie, were case patients); 161 had moderate-to-severe disease (severity score ≥10), and 13 had severe disease (severity score ≥15).
Figure 3.
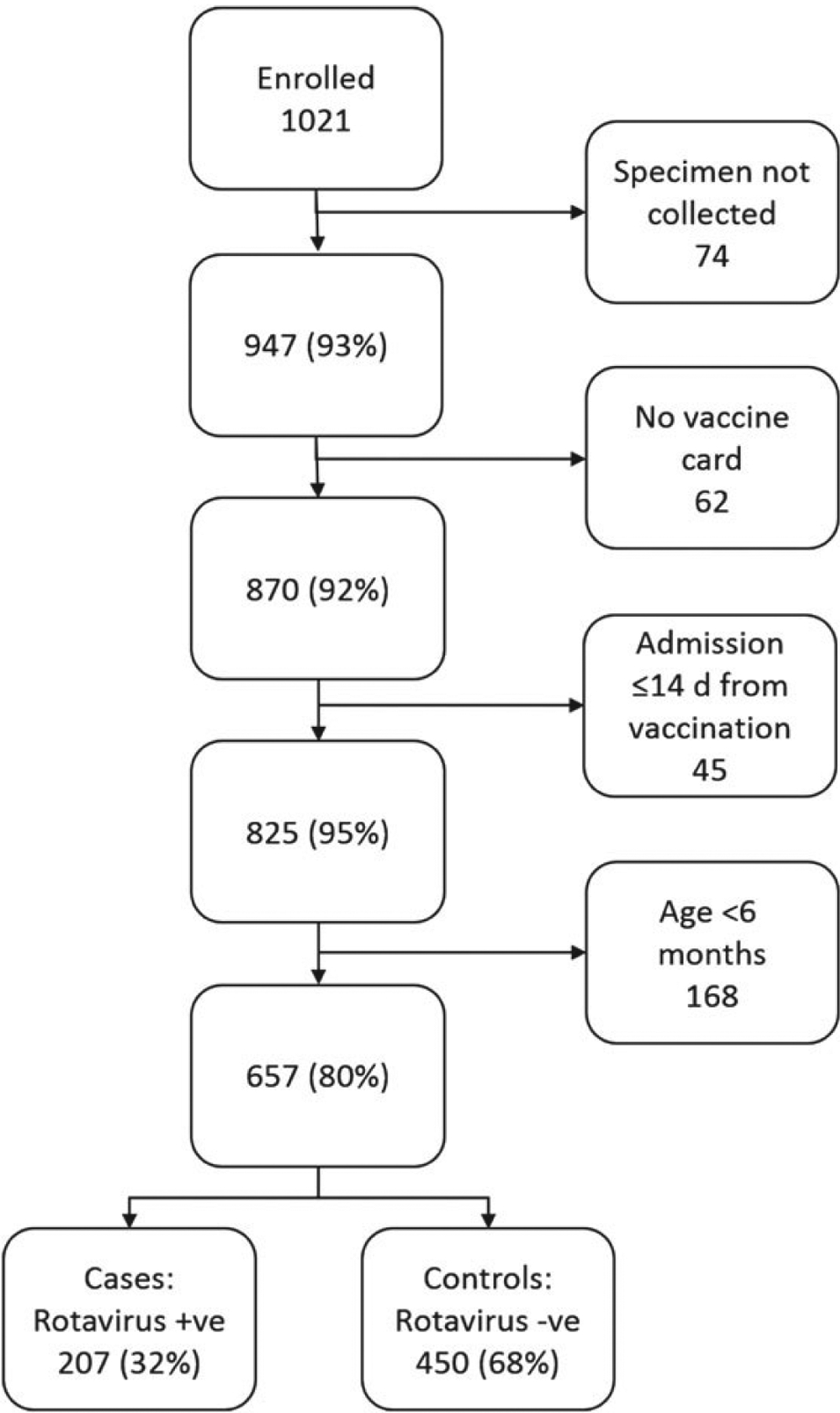
Enrollment and exclusion flowchart for case-control study. Abbreviations: +ve, positive; −ve, negative.
Controls and cases were similar in terms of age (Supplementary Figure 1), percentage female sex, and most other sociodemographic characteristics. There was evidence that cases came from smaller households, were of slightly lower birthweight, and had fathers who were less likely to have completed primary education. Cases had a higher maximum number of diarrhea and vomiting episodes in a 24-hour period, were more likely to have vomiting, were less likely to receive oral rehydration solution, and had higher severity scores. There were no differences between cases and controls in terms of vaccination receipt (Table 2).
Table 2.
Sociodemographic and Clinical Characteristics, and Coverage of Other Vaccines Among Cases and Controls
| Characteristic | Cases (n = 207) | Controls (n = 450) | P Value |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age, moa | 11.3 (0.3) | 11.5 (0.2) | .63 |
| Female sex | 48% | 40% | .064 |
| No. of rooms in householda | 5 (4.5) | 5 (3) | .58 |
| No. of children in householda | 1 (2) | 1 (3) | <.001 |
| Mother’s age, ya | 27 (10) | 28 (9) | .12 |
| Birthweight, kga | 2.9 (0.7) | 3.0 (0.7) | <.001 |
| Maternal education, secondary or greater | 63% | 69% | .29 |
| Mother married | 86% | 82% | .14 |
| Paternal education, secondary or greater | 69% | 76% | .05 |
| Ever breastfed | 99% | 98% | .15 |
| Currently breastfed | 92% | 88% | .24 |
| Clinical characteristics | |||
| Diarrhea, da | 2 (2) | 3 (2) | <.001 |
| Maximum diarrhea episodes in 24 ha | 5 (2) | 4 (3) | .014 |
| Vomiting | 91% | 63% | <.001 |
| Vomiting, da | 2 (2) | 2 (2) | .54 |
| Maximum vomiting episodes in 24 ha | 4 (3) | 3 (2) | <.001 |
| Received ORS | 47% | 60% | .009 |
| Modified Vesikari severity scorea | 12 (3) | 10 (5) | <.001 |
| HIV infected | 4% | <1% | .01 |
| Vaccination | |||
| Rotavirus dose 1 | 95% | 97% | .21 |
| Rotavirus dose 2 | 95% | 95% | .83 |
| Pentavalentb dose 1 | 100% | 99% | .17 |
| Pentavalentb dose 2 | >99% | 99% | .58 |
| Pentavalentb dose 3 | >99% | 98% | .13 |
| PCV dose 1 | 97% | 98% | .37 |
| PCV dose 2 | 94% | 95% | .24 |
Abbreviations: HIV, human immunodeficiency virus; ORS, oral rehydration solution; PCV, pneumococcal conjugate vaccine.
Mean (standard deviation).
Haemophilus influenzae type B, pertussis, tetanus, hepatitis B, and diphtheria vaccine.
The prevalence of rotavirus (and therefore the case-control ratio) varied markedly by site. Prevalence ranged from 10% (21/193) among recruits from hospitals in the Ho/Volta region to 49% (103/211) in Navrongo (Table 3; Figure 4). Overall, ≥1 dose of vaccine coverage was 95% among cases and 97% among controls. Coverage varied by region, but notably was higher among controls within each region. Region, therefore, was a likely confounder of case-control status and important to control for in the VE analysis. Because many cases occurred in the Navrongo region despite high vaccine coverage, we investigated for evidence of different vaccine efficacy by region (ie, effect modification), but found none.
Table 3.
Cases and Controls and Vaccine Coverage by Region
| Region | Cases | Controls | Total No. | ||
|---|---|---|---|---|---|
| No. (%a) | Vaccinatedb, No. (%) | No. (%a) | Vaccinatedb, No. (%) | ||
| Kumasi | 40 (39) | 37 (93) | 61 (59) | 60 (98) | 101 |
| Ho/Volta | 21 (10) | 19 (90) | 172 (90) | 165 (96) | 193 |
| Accra | 43 (28) | 40 (93) | 109 (72) | 105 (96) | 152 |
| Navrongo | 103 (49) | 101 (98) | 108 (51) | 107 (99) | 211 |
| Total | 207 (32) | 197 (95) | 450 (68) | 437 (97) | 657 |
As a percentage of all eligible and enrolled.
With at least 1 dose of RV1.
Figure 4.
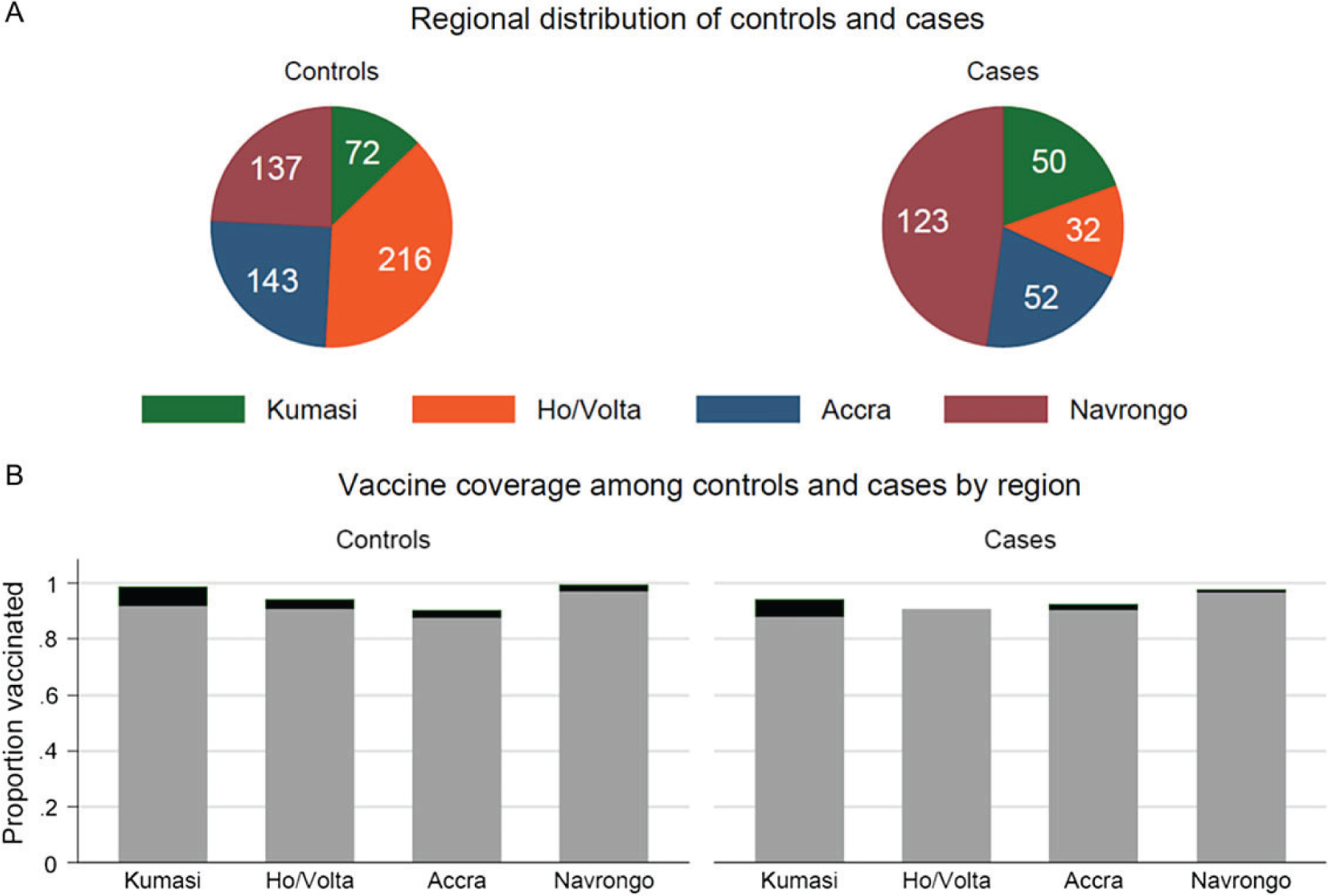
Regional distribution (A) and vaccine coverage (B) among cases and controls.
With high vaccine coverage and between-site heterogeneity, it was not possible to arrive at robust estimates of VE. We calculated any-dose VE against rotavirus hospitalization of 60% (95% CI, −2% to 84%; P = .056; Table 4). Two-dose VE estimates were less precise and tended toward the null. Although the statistical evidence was weak, there was a pattern of higher VE against more severe disease and in the first year of life relative to VE in the second year of life.
Table 4.
Effectiveness of Monovalent Rotavirus Vaccine Overall, by Number of Doses and Age Against All Rotavirus Hospitalization, Moderate to Severe Rotavirus Hospitalization, and Severe Rotavirus Hospitalization
| Dose | Controls Vaccinated/No. (%) | All Rotavirus Hospitalizations | Moderate to Severe Rotavirus Hospitalizations | Severe Rotavirus Hospitalizations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated/No. (%) | VE, % (95% CI) | p Value | Vaccinated/No. (%) | VE, % (95% CI) | p Value | Vaccinated/No. (%) | VE, % (95% CI) | p Value | ||
| Any dose | 437/450 (97) | 197/207 (95) | 60 (−2 to 84) | .056 | 151/159 (95) | 62 (−2 to 86) | .056 | 12/13 (92) | 80 (−156 to 98) | .22 |
| 2 doses | 426/448 (95) | 196/207 (94) | 18 (−81 to 63) | .62 | 150/159 (94) | 26 (−72 to 68) | .48 | 12/13 (92) | 52 (−318 to 95) | .52 |
| Any dose: age 6–11 mo | 243/249 (98) | 101/107 (94) | 78 (2–95) | .048 | 74/79 (94) | 83 (15–96) | .031 | 5/6 (83) | 95 (25–99) | .03 |
| Any dose: age 1-<2y | 224/240 (93) | 106/112 (95) | 50 (−57 to 84) | .95 | 86/90 (96) | 40 (−126 to 84) | .59 | 7/7 (100) | … | … |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
DISCUSSION
Ghana was among the first African nations—and the first in West Africa—to introduce rotavirus vaccination into its routine childhood immunization schedule. These results from the first 3 years following introduction are suggestive of impacts of vaccination on reducing pediatric diarrheal disease in general and rotavirus gastroenteritis specifically. Immediately following nationwide vaccine introduction, coverage among age-eligible children was impressively high among the populations served by the study facilities. Rotavirus hospitalizations among children aged <5 years decreased soon after the implementation of vaccination in a pattern consistent with vaccine impact. Decreases were most pronounced among children <1 year of age in the first year. Impacts were more modest in the second and third–fifth years of life, but may be acceptable in terms of public health importance, as 61% and 93% of rotavirus cases occurred by the first and second years of life, respectively. Although not reaching statistical significance, our primary estimate of VE (60%; 95% CI, −2% to 84%) is consistent with the observed 49% decrease in rotavirus hospitalizations and 44% decrease in AGE hospitalizations, given approximately 95% vaccine coverage.
A strength of this study is the combination of surveillance with case-control data, allowing us to examine both trends and vaccine performance. We had 2–3 years of surveillance data prior to vaccine introduction. Expanding this surveillance infrastructure to a total of 7 facilities, we transitioned to perform a case-control study, by using a common protocol for surveillance in all sites and making considerable effort to collect vaccination history data from all eligible enrolled subjects. We used rotavirus test-negative children with AGE as our comparison group. Compared to community or hospital controls, test-negative controls have the advantages of being straightforward to recruit, having similar care-seeking behavior as cases, and having less likelihood of bias in ascertainment of vaccination status, as the study team remains blinded to the subjects’ case/control status.
The study was subject to limitations. The most critical of these was the very high vaccine coverage immediately after the RV1 vaccine was introduced in Ghana. With coverage among rotavirus age-eligible negative controls at 95% overall and as high as 99% in certain regions, we were unable to enroll a sufficiently large comparison group of unvaccinated individuals needed to calculate VE. Although this high coverage is clearly a mark of success of Ghana’s immunization program, it presents a major challenge for an observational case-control study. Second, analysis of trends was limited geographically in that only 2 urban sites from the 2 largest cities in Ghana (Accra and Kumasi) conducted consistent surveillance over a long enough time period. High vaccine coverage in these 2 sites may not be representative of more rural parts of Ghana. Rotavirus epidemiology could also differ, perhaps with a lower force of infection and older age distribution in less densely populated areas. Third, we had only 2 years of prevaccination data and <3 years of postvaccination data from these 2 surveillance sites. A longer prevaccination series would give further confidence that these observations are indeed a result of mass immunization. We recommend continued prospective surveillance to evaluate the longer term impact of vaccination.
Although there are certain concerns about the accuracy and precision of these VE estimates due to the high vaccination coverage rates in Ghana, it is interesting to note that the VE is consistent with estimates from the limited clinical trials and observational studies in Ghana and elsewhere in Africa [16]. RV1 was trialed in Malawi and South Africa, where VE against severe rotavirus gastroenteritis was estimated at 49.4% and 76.9%, respectively [17]. The efficacy of 3-dose pentavalent (RV5) vaccine against severe rotavirus gastroenteritis was 39% in sub-Saharan Africa and 56% in the Ghana arm of the trial. In postintroduction evaluations of routine RV1 use in Africa, VE has been estimated at 64% (95% CI, 24%–83%) in Malawi [18] and 54% (95% CI, 32%–68%) among children <1 year of age in South Africa [7]. Our observed 49% reduction in rotavirus hospitalizations in Ghana is also very similar to that of the other early-introducing African countries. In South Africa, reductions of 54% and 58% in the first and second years (2010 and 2011), respectively, after vaccine introduction were observed [19], and in Malawi, reductions of 43% were observed in the second season (2014) following vaccine introduction. Indeed, 50% reductions were observed in preliminary analysis in one of our surveillance sites (Korle Bu Teaching Hospital) in Ghana [20].
Taken together, these data suggest a reduction in rotavirus-associated hospitalizations commensurate with high coverage (approximately 95%), with a vaccine of moderate efficacy (approximately 50%–60%). This analysis has implications for Ghana, as it is set to graduate out of Gavi support eligibility and will increasingly be responsible for self-financing its national vaccination program. These early-impact data support sustained use of rotavirus vaccine. In combination with findings from other early-introducing countries in sub-Saharan Africa, these data should be encouraging for other countries in the region considering introducing rotavirus vaccination into their national programs. This study also has implications for evaluating impact in those countries. Surveillance should be performed consistently for a number of years prior to vaccine introduction, and case-control evaluations of VE should be started as early as possible after vaccine introduction and should be conducted in selected subpopulations that do not historically have very high vaccine coverage. We recommend continued surveillance for rotavirus gastroenteritis in Ghanaian hospitals to monitor vaccine uptake and to assess the medium- and long-term impacts of rotavirus vaccination.
Supplementary Material
Acknowledgments.
The authors are thankful to the surveillance and laboratory teams at the participating hospitals: Komfo Anokye Teaching Hospital and Agogo Presbyterian Hospital (Bernard Arhin, Kwabena Adjei Asante); Korle Bu Teaching Hospital (Anna Aba Hayford, Makafui Seshie, Juanita Adams, Anita Achaempong); Ho and Hohoe hospitals (Eric Agboli); Navrongo War Memorial Hospital (Edward Sobe, George Atia); and the Princess Marie Louise Children’s Hospital (Gifty Okine). We are also grateful to the rotavirus study group at the Noguchi Institute for Medical Research, University of Ghana, for data entry, management, and strain characterization, and to all parents and children who participated in the study.
Financial support. This work was supported by Gavi, the Vaccine Alliance, through a grant to the CDC Foundation.
Supplement sponsorship. This article appears as part of the supplement “Health Benefits of Rotavirus Vaccination in Developing Countries,” sponsored by PATH and the CDC Foundation through grants from the Bill and Melinda Gates Foundation and GAVI, the Vaccine Alliance.
Footnotes
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The views expressed by the authors do not necessarily reflect the views of PATH, the CDC Foundation, the Bill and Melinda Gates Foundation, or GAVI, the Vaccine Alliance.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Immunization, vaccines and biologicals. Geneva, Switzerland: WHO, 2010. [Google Scholar]
- 3.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 4.Steele AD, Neuzil KM, Cunliffe NA, et al. Human rotavirus vaccine Rotarix provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infect Dis 2012; 12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortese MM, Immergluck LC, Held M, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics 2013; 132:e25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin Infect Dis 2013; 57:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groome MJ, Page N, Cortese MM, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis 2014; 14:1096–104. [DOI] [PubMed] [Google Scholar]
- 8.de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010; 340:c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301:2243–51. [DOI] [PubMed] [Google Scholar]
- 10.Mukaratirwa A, Berejena C, Nziramasanga P, et al. Epidemiologic and genotypic characteristics of rotavirus strains detected in children less than 5 years of age with gastroenteritis treated at 3 pediatric hospitals in Zimbabwe during 2008–2011. Pediatr Infect Dis J 2014; 33(suppl 1):S45–8. [DOI] [PubMed] [Google Scholar]
- 11.Tsolenyanu E, Seheri M, Dagnra A, et al. Surveillance for rotavirus gastroenteritis in children less than 5 years of age in Togo. Pediatr Infect Dis J 2014; 33(suppl 1):S14–8. [DOI] [PubMed] [Google Scholar]
- 12.Gavi, the Vaccine Alliance. Ghana Gavi country hub 2015. Available at: http://www.gavi.org/country/ghana/. Accessed 6 October 2015.
- 13.World Health Organization. Generic protocols for (i) hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children and (ii) a community-based survey on utilization of health care services for gastroenteritis in children: field test version. Available at: http://www.who.int/immunization/diseases/rotavirus/generic_protocols/en/. Accessed 6 October 2015.
- 14.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: Wiley; 2000. xii, 373. [Google Scholar]
- 15.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259–67. [DOI] [PubMed] [Google Scholar]
- 16.Soares-Weiser K, Maclehose H, Bergman H, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev 2012; 11:CD008521. [DOI] [PubMed] [Google Scholar]
- 17.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 18.Bar-Zeev N, Kapanda L, Tate JE, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis 2015; 15:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Msimang VM, Page N, Groome MJ, et al. Impact of rotavirus vaccine on childhood diarrheal hospitalization after introduction into the South African public immunization program. Pediatr Infect Dis J 2013; 32:1359–64. [DOI] [PubMed] [Google Scholar]
- 20.Enweronu-Laryea CC, Boamah I, Sifah E, Diamenu SK, Armah G. Decline in severe diarrhea hospitalizations after the introduction of rotavirus vaccination in Ghana: a prevalence study. BMC Infect Dis 2014; 14:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


