Abstract
Background
The combined effects of bilateral corticospinal tract (CST) reorganization and interhemispheric functional connectivity (FC) reorganization on motor recovery of upper and lower limbs after stroke remain unknown.
Material/Methods
A total of 34 patients underwent magnetic resonance imaging (MRI) examination at weeks 1, 4, and 12 after stroke, with a control group of 34 healthy subjects receiving 1 MRI examination. Interhemispheric FC in the somatomotor network (SMN) was calculated using the resting-state functional MRI (rs-fMRI). Fractional anisotropy (FA) of bilateral CST was recorded as a measure of reorganization obtained from diffusion tensor imaging (DTI). After intergroup comparisons, multiple linear regression analysis was used to explore the effects of altered FA and interhemispheric FC on motor recovery.
Results
Interhemispheric FC restoration mostly occurred within 4 weeks after stroke, and FA in ipsilesional remained CST consistently elevated within 12 weeks. Multivariate linear regression analysis showed that the increase in both interhemispheric FC and ipsilesional CST-FA were significantly correlated with greater motor recovery from week 1 to week 4 following stroke. Moreover, only increased FA of ipsilesional CST was significantly correlated with greater motor recovery during weeks 4 to 12 after stroke compared to interhemispheric FC.
Conclusions
Our results show dynamic structural and functional reorganizations following motor stroke, and structure reorganization may be more related to motor recovery at the late subacute phase. These results may play a role in guiding neurological rehabilitation.
Keywords: Diffusion Tensor Imaging, Pyramidal Tracts, Recovery of Function, Stroke
Background
Although the mortality rate has declined in the past 30 years, stroke remains the most important cause of adult disability [1]. Most stroke patients generally experience a certain degree of motor recovery within the first several months following stroke, even without treatment. Several studies have shown that reorganization of structure and functional connection in the motor system significantly contributes to the restoration of motor functions after stroke [2–4].
The corticospinal tract (CST) is the major descending pathway and plays a key role in motor control. In some research, changes in microstructural integrity were found using fractional anisotropy (FA) extracted from diffusion tensor imaging (DTI) [5–7]. The elevated FA values were assumed to reflect extensive white matter reorganization and improvement in the structural integrity of nerve fibers during stroke recovery [8–10]. Some previous studies have found that FA in perilesional or contralesional CST is positively correlated with motor performance in murine models or in patients at the chronic stage after stroke [8,9,11,12].
Numerous studies have investigated the altered interhemispheric functional connectivity (FC) in the somatomotor network (SMN) using resting-state functional MRI (rs-fMRI) after stroke, which has been shown to be related to motor performance [13–17]. In fact, almost all patients exhibit a decrease in interhemispheric FC at the initial stage after stroke. Subsequently, increased interhemispheric FC shows a positive association with motor recovery in the subacute or chronic phase of stroke [18–20].
Although reorganization in CST or interhemispheric FC is highly relevant to motor recovery after stroke, their combined effects on motor recovery over time remain unknown. In the current study, we combined rs-fMRI and DTI techniques to explore longitudinal changes in interhemispheric FC and bilateral CST across a continuum from acute to subacute stages of stroke and to examine the combined effect of these 2 processes on motor recovery over time.
Material and Methods
Participants
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, and all participants signed written informed consent before their enrolment. Stroke patients were prospectively recruited at the Department of Neurology of Guangxi Medical University between January 2019 and December 2019. Patients were included in this study if they: (1) were between 18 and 80 years old; (2) had their first-ever unilateral ischemic stroke confirmed by MRI scan, and the lesion locations were restricted to the internal capsule and neighboring regions; (3) exhibited clinically detectable motor deficits based on neurological examination; (4) had sufficient cognition to participate in the study and take the Mini-Mental State Examination (MMSE) ≥21. Patients were excluded if they had: (1) previous motor impairments; (2) a history of neurological or psychiatric disease; (3) MRI contraindications, such as claustrophobia, among others; and (4) heart and renal failure, malignancies, as well as other intercurrent severe illnesses that preclude active participation in research.
Age- and sex-matched healthy controls were also recruited if they: (1) exhibited a normal neurological examination; (2) denied having suffered from a neurological or mental illness; and (3) did not have MRI contraindications.
Clinical Evaluation
We recorded participants’ baseline characteristics, including age and sex, lesions, and vascular risk factors, as well as parameters from neurological examination using the National Institutes of Health Stroke Scale (NIHSS) and Mini-Mental State Examination (MMSE) at admission. Patients were discharged from the neurology ward after a period of stabilization. Subsequently, they underwent conventional stroke rehabilitation treatment at the outpatient department or rehabilitation wards. Each patient’s synergistic motor control of the paretic extremities was evaluated using the upper- (scale 0–66) and lower- (scale 0–34) extremity section of the Fugl-Meyer Assessment (FMA), 2 h before each MRI scan. The total possible FMA score was 0 to 100. In addition, the degree of disability in performing daily living activities was assessed based on the Barthel Index (BI), which uses a scale ranging from 0 to 100.
MRI Data Acquisition
We obtained rs-fMRI and DTI data from all enrolled patients on weeks 1, 4, and 12 after stroke onset, hereafter denoted W1, W4, and W12, respectively, with a control group of 34 healthy subjects receiving 1 MRI examination. Images were obtained using Siemens Prisma 3.0 Tesla MR scanners (Siemens, Erlangen, Germany) in the Radiology Department at the First Affiliated Hospital of Guangxi Medical University.
A gradient echo-planar imaging (EPI) sequence was used for rs-fMRI scanning (TR/TE=2000/30 ms, flip angle = 90° field of view (FOV) = 240×240 mm, number of slices=38, slice thickness=3 mm, gap=1 mm, and number of time points=240, total scan time=6 min 20 s). These 38 slices covered the entire cerebral cortex. High-resolution structural scans were obtained with T1-weighted brain volume sequence (TR/TE =8/3.5 ms, flip angle=11°, FOV=256×256 mm, slice thickness=1 mm, no gap, voxel resolution=1.0×1.0×1.0 mm). A single-shot echo-planar imaging (EPI) sequence which consisted of 30 directions (TR/TE=9600/92 ms, FOV =256×256 mm, flip angle=90°, slice thickness=3 mm, no gap, and b value: 0 and 1000 s/mm2) was used for DTI scanning. During the scanning, all subjects were instructed to close their eyes but not sleep. In addition, they were asked not to think about anything.
Lesion Analysis
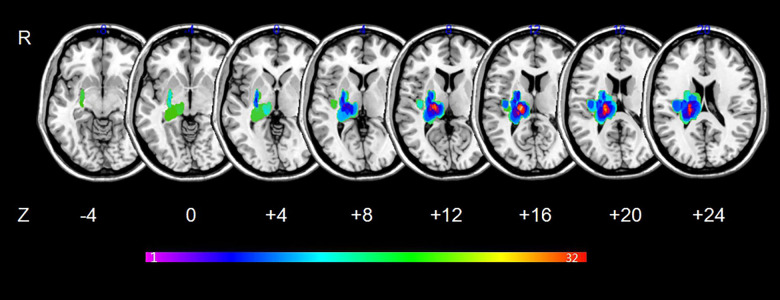
MRIcron software (www.mricro.com) was used to outline the lesion overlap images for all stroke patients. More details about the processing of lesion overlap maps have been reported in a previous publication [21]. Maps of the lesion overlap are shown in Figure 1.
Figure 1.
Lesion overlapped across the 34 stroke patients. Color bar indicates the number of subjects having lesions in each voxel.
DTI Data Pre-processing
DTI data were preprocessed using the FMRIB’s Software Library (FSL) version 5.0.9 (www.fmrib.ox.ac.uk/fsl/). Briefly, pre-processing included eddy current correction, head motion correction, and brain extraction. After pre-processing, diffusion indices were calculated for each voxel, and diffusion tensors were reconstructed using the linear least-square fitting algorithm [22]. Then whole-brain fiber tractography was performed using Diffusion Toolkit (http://www.trackvis.org/dtk/). The CST tracts were tracked using the regions of interest (ROIs) approach implemented in the Diffusion Toolkit (http://www.trackvis.org/dtk/). Specifically, 3 ROIs, selected based on previous studies [19,23], were placed in the precentral gyrus, the posterior limb of the internal capsule (PLIC), and the cerebral peduncle on the individual axial FA map. Exact anatomic positions were defined according to a neuroanatomical atlas [24]. Fibers passing through all ROIs were defined as the CST. We only extracted FA values of CST from the PLIC using the Trackvis software, as the CST fibers in PLIC have a relatively coherent arrangement. Further details on image reconstruction can be found in a previous publication [19].
rs-fMRI data pre-processing
Pre-processing of rs-fMRI data was performed using MATLAB version 9.1 (http://www.mathworks.com) and Statistical Parametric Mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm) [25]. Briefly, we removed the first 5 functional volumes and initial pre-processing for the remaining volumes included compensation for slice-dependent time shifts and correction of head motion. Participants with a maximum displacement of less than 2 mm and a maximum rotation of less than 2.0° were selected for this study. The pre-processing steps included: resampling to the standard space of the Montreal Neurological Institute (MNI) (voxel size=3×3×3 mm); selection of a band pass frequency filter (0.01–0.08 Hz) to eliminate unwanted frequencies; and application of the 8mm gaussian kernel for spatial smoothing. Then, linear regression analysis was performed to remove several nuisance variables from the smoothened and filtered data including head motion parameters, as well as signals of the whole brain, white matter, and cerebrospinal fluids. The residual BOLD time course was also recorded for FC analysis. The ROIs were selected in the somatomotor network (SMN) along the left and right primary motor cortex (M1) as previously defined [26]. (left: MNI coordinates, −11, −26 and 78; radius=6 mm; right: MNI coordinates, 11, −26, and 78; radius=6mm). The average time series for the left and right seed areas were extracted and their linear correlation coefficients (r) were calculated. Fisher r-z transformation was then performed to convert the r to a normally distributed z score. The z scores were subsequently used as the FC of each time point for statistical analysis.
Statistical Analysis
All statistical analyses were performed using SPSS version 23.0. Differences in FC, FA, BI, and FMA indicators at week 1 between the stroke group and healthy controls were analyzed by two-sample independent t tests. Data of each parameter at 3 timepoints were compared using a repeated-measures ANOVA in the stroke group. Linear correlation analysis was also implemented to assess the relationship between percentage changes in FA and those in FC in weeks 1–4 and 4–12. A multivariate linear regression method was then applied to explore the effects of percentage changes in FA and interhemispheric FC on percentage changes in FMA in weeks 1–4 and 4–12. With reference to our previous study [27], the percentage change was calculated as: {[(W4-W1)/W1]×100%} in weeks 1–4, and {[(W12-W4)/W1]×100%} in weeks 4–12. Values of P<0.05 were considered statistically significant. Because the initial motor impairment (initial FMA score) is a comprehensive parameter reflecting characteristics of the lesions, the initial FMA score was included in multivariable models based on the literature [12]. The multicollinearity was tested using SPSS when conducting multiple linear regression analyses. If multicollinearity was found among covariates (Variance Inflation Factor, VIF >5), variables with VIF >5 were excluded.
Results
Clinical Data
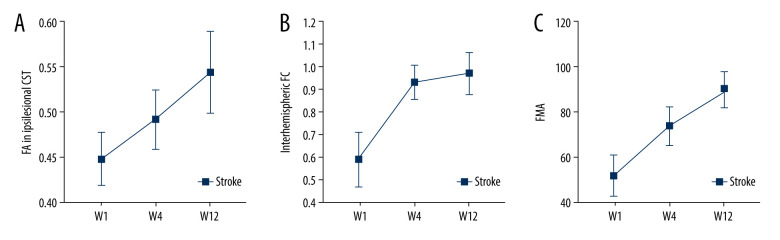
The process for patient selection and the resulting data are summarized in Figure 2, whereas the general clinical baseline characteristics across patient groups and healthy controls are outlined in Table 1. The lesion was on the left side in 18 cases, and the right side in 16. The sizes of lesions ranged from 0.8 to 54.3 cm3, median 9.4 cm3. Overall, most patients exhibited mild to moderate stroke, with a median NIHSS score of 7 (Inter Quartile Range 4–13). All stroke patients had received conventional rehabilitation with or without acupuncture for at least 4 weeks, with 15 patients in rehabilitation wards and 19 in the outpatient department. Twenty patients completed conventional rehabilitation for 8 weeks. FMA and BI scores in stroke patients further showed a progressive increase across the 3 time points (Figure 3C, Table 2).
Figure 2.
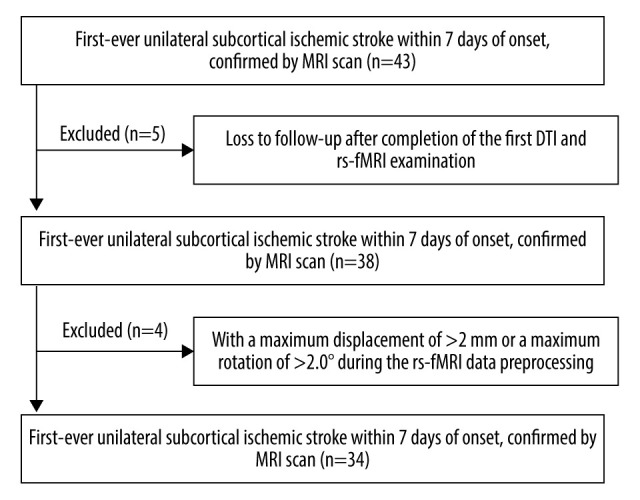
Patient selection.
Table 1.
Baseline characteristics of patients and controls.
| Characteristics | Patients, n=34 | Controls, n=34 | P value |
|---|---|---|---|
| Age (years) | 63.4 [52.1–73.8] | 61.2 [49.3–70.5] | 0.532* |
| Female N (%) | 14 (41.2%) | 16 (47.1%) | 0.808** |
| Vascular risk factors N (%) | |||
| Hypertension | 23 (67.6%) | 18 (52.9%) | 0.215** |
| Diabetes | 19 (55.9%) | 13 (38.2%) | 0.145** |
| Hypercholesterolemia | 20 (58.8%) | 15 (44.1%) | 0.225** |
| NIHSS admission | 7 [4–13] | – | – |
| MMSE | ≥21 | – | – |
| Lesion size (cm3) | 9.4 [3.2–35.1] | – | – |
| Lesion side (left) N (%) | 18 (52.9%) | – | – |
NIHSS – National Institutes of Health Stroke Scale; MMSE – Mini-Mental State Examination. Continuous variables presented as median [P25–P75].
Assessed by Mann-Whitney U test;
assessed by chi-square test.
Figure 3.
Changes over time. FA – fractional anisotropy, FMA – Fugl-Meyer Assessment, FC – functional connectivity. FA values increased longitudinally in ipsilesional CST (A). Interhemispheric FC restoration happened mostly within 4 weeks after stroke (B). The Fugl-Meyer Assessment (FMA) scores of stroke patients improved gradually (C).
Table 2.
Clinical and imaging indicators of subjects at different time points.
| Controls (n=34) | Patients W1 (n=34) | Patients W4 (n=34) | Patients W12 (n=34) | |
|---|---|---|---|---|
| FMA (0–100) | – | 51.315±9.209 | 73.355±8.854** | 89.703±7.870**,*** |
| Barthel index (0–100) | – | 54.732±12.937 | 77.708±8.634** | 86.368±14.409**,*** |
| Interhemispheric FC | 1.01±0.043 | 0.587±0.119* | 0.929±0.077*,** | 0.969±0.093*,**,*** |
| FA in ipsilesional CST | 0.547±0.058 | 0.448±0.029* | 0.492±0.033*,** | 0.544±0.045**,*** |
| FA in contralesional CST | 0.547±0.058 | 0.538±0.055 | 0.544±0.048 | 0.548±0.040 |
FA – fractional anisotropy; FMA – Fugl-Meyer Assessment; FC – functional connectivity. The FA of healthy controls were derived from averages of bilateral CST measurements.
Compared with the control group, P<0.05 obtained by two-sample independent t tests;
compared with week 1, P<0.05 obtained by RM-ANOVA;
compared with week 4, P<0.05 obtained by RM-ANOVA.
CST Reorganization
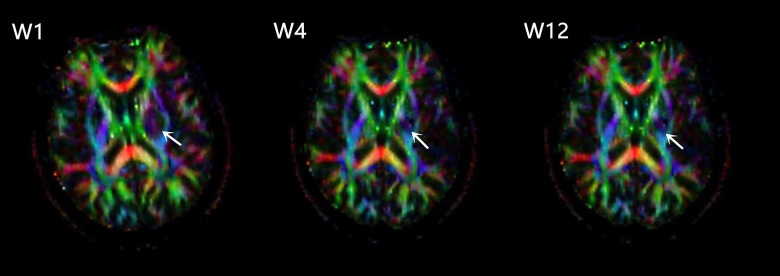
FA values in the ipsilesional remaining CST showed a decline during the first week and then increased longitudinally over the next 12 weeks after stroke (Figures 3A and 4, Table 2). Results further revealed that mean percentage changes of FA values in the ipsilesional remaining CST across 1–4 weeks (22.68±2.50%) and 4–12 weeks (23.01±1.90%) were not significantly different (P=0.524). In addition, contralesional CST did not show a significant change from week 1 to week 12 after stroke (Table 2).
Figure 4.
FA pseudocolor pictures of one stroke patient at W1, W4, W12, levels of the internal capsule. Arrows indicate the increased FA in the perilesional CST.
Reorganization of the Interhemispheric FC
A significantly lower interhemispheric FC was found in stroke patients relative to healthy controls in the first week, after which a longitudinal increase through week 12 was evident. Interhemispheric FC restoration occurred mostly within 4 weeks after stroke, with only a minor recovery in the following 8 weeks (Figure 3B, Table 2). The difference in mean percentage changes of interhemispheric FC between weeks 1–4 (58.26±10.56%) and weeks 4–12 (6.81±3.98%) was statistically significant (P<0.001).
Correlation Analysis
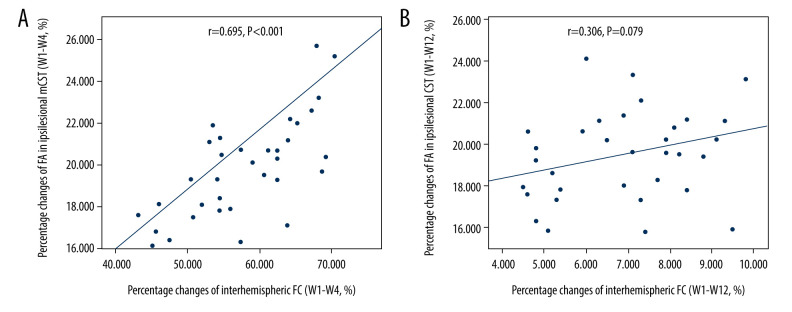
We found a positive correlation between percentage changes of FA in ipsilesional remaining CST and those of interhemispheric FC in patients during weeks 1 to 4 after stroke (r=0.695, P<0.001, Figure 5A). However, no correlation was observed between them from weeks 4 to 12 (r=0.306, P=0.079, Figure 5B).
Figure 5.
Significantly positive correlation between percentage changes of FA in ipsilesional remaining CST and those of interhemispheric FC during weeks 1 to 4 after stroke (A). No correlation between them from weeks 4 to 12 (B). FA – fractional anisotropy, FC – functional connectivity, CST – corticospinal tract.
Multivariate Linear Regression
The initial FMA score was removed from the multivariable model due to multicollinearity. Multivariate linear regression analysis showed that the increase in both interhemispheric FC and ipsilesional CST-FA were significantly correlated with the greater change of FMA between weeks 1 and 4 following stroke. Moreover, only increased FA of ipsilesional CST was significantly correlated with the greater change of FMA at weeks 4–12 after stroke compared to interhemispheric FC (Table 3).
Table 3.
Multiple linear regression of factors influencing motor recovery.
| Model 1 | Model 2 | ||
|---|---|---|---|
| R2 | 0.701 | R2 | 0.309 |
| Total (P value) | <0.001 | Total (P value) | <0.001 |
| Percentage changes of FA(W1–W4) Beta (P value) |
0.304 (0.011) | Percentage changes of FA(W4–W12) Beta (P value) |
0.557 (0.001) |
| Percentage changes of interhemispheric FC(W1–W4) Beta (P value) |
0.647 (<0.001) | Percentage changes of interhemispheric FC(W4–W12) Beta (P value) |
0.086 (0.571) |
Beta – standardized regression coefficients. Model 1 with percentage changes of FMA(W1–W4) as dependent variable. Model 2 with percentage changes of FMA(W4–W12) as dependent variable.
Discussion
The key findings of the current study included: (1) Ipsilesional remaining CST and interhemispheric FC underwent reorganization within 12 weeks after stroke and showed different time courses; and (2) The combined effects of these 2 processes on motor recovery varied with time. These findings can provide a better understanding of motor recovery mechanisms. Taken together, the results of this study may play a role in guiding neurological rehabilitation.
Previous studies have shown that stroke is accompanied by microstructural changes in the white matter, even in undamaged areas [8,28,29]. In ipsilesional white matter areas, DTI studies using stroke animal models have reported significantly increased FA values in the perilesional white matter, with postmortem histological sections indicating the presence of high-density axons and myelin in this area [8,30,31]. The present study showed an increase in longitudinal FA in ipsilesional remaining CST regions, consistent with the findings of Lu et al [32], who placed the ROIs within the CST but outside of the lesion to extract the FA values. These findings indicate ipsilesional remaining CST undergoes remodeling after stroke. However, it is not clear whether contralesional corticospinal pathways undergo reorganization after stroke. A previous study using severely impaired long-term stroke patients showed higher FA values in contralesional CST compared to normal controls, and this was associated with favorable motor outcomes [9]. However, the results of the current study indicated that FA values in the contralesional CST were relatively stable from weeks 1 to 12 after stroke. In the present study, our patient population mainly exhibited mild to moderate stroke, unlike in previous studies that showed contralesional CST reorganization based on subjects that mostly had a severe stroke. This variation in stroke severity possibly explains the variation in the range of CST reorganization.
Several longitudinal studies have demonstrated that interhemispheric FC in the SMN first decreases in the early stages after stroke, then increases in the following weeks or months [15,18,33]. Some studies that selected subjects similar to those in the current research have explored the dynamic evolution of interhemispheric FC after stroke [15,33,34]. For example, Golestani et al [15] used resting-state fMRI scans on patients across 3 time points (<24 h, 7 days, and 90 days) after stroke, and found that interhemispheric FC reestablished normality after 1 week in some patients who had a mild stroke. In another study, mild to moderate stroke patients who underwent rs-fMRI at week 5 and week 26 after stroke exhibited no change in interhemispheric FC, although their motor performance scores improved during this period [34]. In the current study, we selected participants with mild to moderate stroke and found that their interhemispheric FC improved longitudinally. Notably, the interhemispheric FC restoration occurred mostly within 4 weeks after stroke. These findings suggest that the first few weeks after stroke are the best time window for interhemispheric FC reorganization for patients with mild to moderate stroke.
A significant correlation between increased interhemispheric FC and motor recovery has been demonstrated by numerous studies [17,20,32,33,35,36]. Among those, only a few studies have reported a strong correlation between elevated FA values in CST and improved motor function within 10 weeks [35] or 3 months [32]. As post-stroke motor recovery may reflect a complex interaction of structural and functional reorganization in brain motor networks, we used multiple linear regression to explore the combined effects of structural and functional reorganization on motor recovery. Our results showed that the reorganization in both interhemispheric FC and ipsilesional CST were significantly correlated with greater motor recovery during weeks 1 to 4 following the stroke. Moreover, ipsilesional CST reorganization was more significantly correlated with greater motor recovery at weeks 4–12 after stroke compared to interhemispheric FC. These results may be related to the different time courses of structural and functional reorganization in the brain motor network. Recently, Lin et al [12] reported this difference in the time course of reorganization beyond 3 months after stroke. Their results showed that CST reorganization may occur from 3 to 12 months after stroke and is related to improved motor function, while interhemispheric FC does not change during this period. The present study focused on the longitudinal changes in bilateral CST and interhemispheric FC within 12 weeks after stroke, revealing that interhemispheric FC restoration mainly occurred within 4 weeks after stroke, with only a minor recovery observed during the following 8 weeks. However, increased FA values in ipsilesional remaining CST were comparable across these 2 stages. Our study complements those of Lin et al
Several studies have revealed a close relationship between changes in CST and upstream interhemispheric FC in SMN [19,32,35,37–39]. For example, CST impairment was found to be positively correlated to decreased somatomotor interhemispheric FC within 4 weeks after subcortical stroke [37]. A study by Van et al [35] evaluated the links between brain reorganization and neurological function recovery from days 3 to 70 after stroke in mice. They found a significant positive correlation between increased interhemispheric FC in SMN and elevated FA in the ipsilesional CST. In the current study, our results revealed a positive correlation between CST reorganization and increased interhemispheric FC during weeks 1 to 4 following stroke. However, there was no correlation between these 2 processes at weeks 4–12. Therefore, although CST reorganization is closely related to that of interhemispheric FC, it is likely that changes unrelated to the reorganization of FC also occur.
Our study had some limitations. Firstly, for the study sample was a narrow range of mild to moderate stroke patients, so the generalizability of results is probably limited. Secondly, participants’ lesions were located in subcortical regions, implying that our results cannot be generalized to individuals with a stroke occurring in the cortex. Finally, many independent variables might affect the long-term motor outcomes, such as lesion volume, lesion location, different rehabilitation programs, and the duration of rehabilitation, which could significantly affect the outcome. Future studies in a more general stroke population are needed to validate our findings.
Conclusions
Our results show the dynamic structural and functional reorganizations following motor stroke, and suggest that structure reorganization may be more related to motor recovery at the late subacute phase. These results may play a role in guiding neurological rehabilitation.
Acknowledgments
The authors thank the patients and healthy volunteers who participated in the study.
Footnotes
Conflict of Interests
None.
Source of support: This study was supported by the National Key Research and Development Program of China (No. 2018YFC1311305)
References
- 1.Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res. 2017;120(3):439–48. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 2.Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 3.Jang SH. A review of diffusion tensor imaging studies on motor recovery mechanisms in stroke patients. Neurorehabilitation. 2011;28(4):345–52. doi: 10.3233/NRE-2011-0662. [DOI] [PubMed] [Google Scholar]
- 4.Thiel A, Vahdat S. Structural and resting-state brain connectivity of motor networks after stroke. Stroke. 2015;46(1):296–301. doi: 10.1161/STROKEAHA.114.006307. [DOI] [PubMed] [Google Scholar]
- 5.Dijkhuizen RM, van der Marel K, Otte WM, et al. Functional MRI and diffusion tensor imaging of brain reorganization after experimental stroke. Transl Stroke Res. 2012;3(1):36–43. doi: 10.1007/s12975-011-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buch ER, Rizk S, Nicolo P, et al. Predicting motor improvement after stroke with clinical assessment and diffusion tensor imaging. Neurology. 2016;86(20):1924–25. doi: 10.1212/WNL.0000000000002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guggisberg AG, Nicolo P, Cohen LG, et al. Longitudinal structural and functional differences between proportional and poor motor recovery after stroke. Neurorehabil Neural Repair. 2017;31(12):1029–41. doi: 10.1177/1545968317740634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Zijden JP, van der Toorn A, van der Marel K, et al. Longitudinal in vivo MRI of alterations in perilesional tissue after transient ischemic stroke in rats. Exp Neurol. 2008;212(1):207–12. doi: 10.1016/j.expneurol.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Human Brain Mapp. 2009;30(11):3461–74. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung WB, Han YH, Chung JJ, et al. Spatiotemporal microstructural white matter changes in diffusion tensor imaging after transient focal ischemic stroke in rats. NMR Biomed. 2017;30:6. doi: 10.1002/nbm.3704. [DOI] [PubMed] [Google Scholar]
- 11.Borich MR, Mang C, Boyd LA. Both projection and commissural pathways are disrupted in individuals with chronic stroke: Investigating microstructural white matter correlates of motor recovery. BMC Neuroscience. 2012;13:107. doi: 10.1186/1471-2202-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin LY, Ramsey L, Metcalf NV, et al. Stronger prediction of motor recovery and outcome post-stroke by cortico-spinal tract integrity than functional connectivity. PLoS One. 2018;13(8):e0202504. doi: 10.1371/journal.pone.0202504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter AR, Astafiev SV, Lang CE, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365–75. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park CH, Chang WH, Ohn SH, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42(5):1357–62. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golestani AM, Tymchuk S, Demchuk A, et al. Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair. 2013;27(2):153–63. doi: 10.1177/1545968312457827. [DOI] [PubMed] [Google Scholar]
- 16.Tang C, Zhao Z, Chen C, et al. Decreased functional connectivity of homotopic brain regions in chronic stroke patients: A resting state fMRI study. PLoS One. 2016;11(4):e0152875. doi: 10.1371/journal.pone.0152875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desowska A, Turner DL. Dynamics of brain connectivity after stroke. Rev Neurosci. 2019;30(6):605–23. doi: 10.1515/revneuro-2018-0082. [DOI] [PubMed] [Google Scholar]
- 18.van Meer MP, van der Marel K, Wang K, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30(11):3964–72. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Qin W, Zhang J, et al. Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke. 2015;46(4):1045–51. doi: 10.1161/STROKEAHA.114.007044. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Park E, Lee A, et al. Alteration and role of interhemispheric and intrahemispheric connectivity in motor network after stroke. Brain Topogr. 2018;31(4):708–19. doi: 10.1007/s10548-018-0644-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z, Tang C, Yin D, et al. Frequency-specific alterations of regional homogeneity in subcortical stroke patients with different outcomes in hand function. Hum Brain Mapp. 2018;39(11):4373–84. doi: 10.1002/hbm.24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 23.Xi SD, Zhu YL, Chen C, et al. The plasticity of the corticospinal tract in children with obstetric brachial plexus palsy after Botulinum Toxin A treatment. J Neurol Sci. 2018;394:19–25. doi: 10.1016/j.jns.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Mai JK, Majtanik M, Paxinos G. Atlas of the human brain. Academic Press; 2015. [Google Scholar]
- 25.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hacker CD, Laumann TO, Szrama NP, et al. Resting state network estimation in individual subjects. Neuroimage. 2013;82:616–33. doi: 10.1016/j.neuroimage.2013.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang Z, Zeng J, Zhang C, et al. Progression of pathological changes in the middle cerebellar peduncle by diffusion tensor imaging correlates with lesser motor gains after pontine infarction. Neurorehabil Neural Repair. 2009;23(7):692–98. doi: 10.1177/1545968308331142. [DOI] [PubMed] [Google Scholar]
- 28.Guleria S, Gupta RK, Saksena S, et al. Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. J Neurosci Res. 2008;86(10):2271–80. doi: 10.1002/jnr.21664. [DOI] [PubMed] [Google Scholar]
- 29.Radlinska BA, Blunk Y, Leppert IR, et al. Changes in callosal motor fiber integrity after subcortical stroke of the pyramidal tract. J Cereb Blood Flow Metab. 2012;32(8):1515–24. doi: 10.1038/jcbfm.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Q, Zhang ZG, Ding GL, et al. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32(3):1080–89. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Ding G, Jiang Q, Li L, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28(8):1440–48. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Q, Huang G, Chen L, et al. Structural and functional reorganization following unilateral internal capsule infarction contribute to neurological function recovery. Neuroradiology. 2019;61(10):1181–90. doi: 10.1007/s00234-019-02278-x. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Qin W, Chen H, et al. Contribution of the resting-state functional connectivity of the contralesional primary sensorimotor cortex to motor recovery after subcortical stroke. PLoS One. 2014;9(1):e84729. doi: 10.1371/journal.pone.0084729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nijboer TCW, Buma FE, Winters C, et al. No changes in functional connectivity during motor recovery beyond 5 weeks after stroke: A longitudinal resting-state fMRI study. PLoS One. 2017;12(6):e0178017. doi: 10.1371/journal.pone.0178017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Meer MP, Otte WM, van der Marel K, et al. Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. J Neurosci. 2012;32(13):4495–507. doi: 10.1523/JNEUROSCI.3662-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Wang Y, Liao C, et al. Longitudinal brain functional connectivity changes of the cortical motor-related network in subcortical stroke patients with acupuncture treatment. Neural Plast. 2017;2017 doi: 10.1155/2017/5816263. 5816263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter AR, Patel KR, Astafiev SV, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair. 2012;26(1):7–19. doi: 10.1177/1545968311411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JL, Schlaug G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front Neurol. 2013;4:178. doi: 10.3389/fneur.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ovadia-Caro S, Villringer K, Fiebach J, et al. Longitudinal effects of lesions on functional networks after stroke. J Cereb Blood Flow Metab. 2013;33(8):1279–85. doi: 10.1038/jcbfm.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]