Abstract
Understanding the mechanisms that drive transition from acute to chronic pain is essential to identify new therapeutic targets. The importance of endogenous resolution pathways acting as a “brake” to prevent development of chronic pain has been largely ignored. We examined the role of IL-10 in resolution of neuropathic pain induced by cisplatin. In search of an underlying mechanism, we studied the effect of cisplatin and IL-10 on spontaneous activity (SA) in DRG neurons. Cisplatin (2 mg/kg daily for 3 days) induced mechanical hypersensitivity that resolved within 3 weeks. In both sexes, resolution of mechanical hypersensitivity was delayed in Il10−/− mice, in WT mice treated intrathecally with neutralizing anti-IL-10 antibody, and in mice with cell-targeted deletion of IL-10R1 on advillin-positive sensory neurons. Electrophysiologically, small to medium-sized DRG neurons from cisplatin-treated mice displayed an increase in the incidence of spontaneous activity. Cisplatin treatment also depolarized the resting membrane potential, and decreased action potential voltage threshold and rheobase, while increasing ongoing activity at −45 mV and the amplitude of depolarizing spontaneous fluctuations (DSFs). In vitro addition of IL-10 (10 ng/ml) reversed the effect of cisplatin on SA and on the DSFs amplitudes, but unexpectedly had little effect on the other electrophysiological parameters affected by cisplatin. Collectively, our findings challenge the prevailing concept that IL-10 resolves pain solely by dampening neuroinflammation and demonstrate in a model of chemotherapy-induced neuropathic pain that endogenous IL-10 prevents transition to chronic pain by binding to IL-10 receptors on sensory neurons to regulate their activity.
Introduction.
Chronic pain affects between 30% and 50% of the population worldwide [50]. The immune system has been shown to play a pivotal role in the onset and maintenance of chronic pain [14,20,46]. Immune cells release pro-inflammatory mediators that sensitize peripheral sensory neurons and spinal cord neurons via binding to receptors for cytokines, chemokines and alarmins on these neurons [7,13,42,43,67]. Mice lacking receptors for these mediators are protected from neuropathic pain [1,53,63].
The immune system is, however, not always detrimental in chronic pain. An emerging body of literature indicates that the resolution of pain is an active process that depends on the participation of immune cells [3,9,23,25,26,28,62]. For example, we and others have shown that the lack of IL-10-producing macrophages and/or CD8+ T cells prevents the resolution of mechanical hypersensitivity induced by inflammation or chemotherapeutic drugs [3,9,23,26,28,62].
The anti-nociceptive effect of exogenous administration of IL-10 after nerve injury, inflammation, and paclitaxel treatment is well-established [16,23,30,37,58,65,74]. In addition, endogenous IL-10 is essential for exercise-induced analgesia [2,31,49], prevents neuropathic pain in young rodents [35] and promotes axon regeneration after injury [36].
IL-10 signals via binding to the IL-10 receptor (IL-10R), a hetero-tetramer of two IL-10R1 and two IL-10R2 subunits. IL-10R1 is the ligand binding subunit and is necessary for cells to be responsive to IL-10 [38]. IL-10R2 is shared with receptors for other cytokines. IL-10R is expressed by many different cell types [38,48]. The mechanisms via which IL-10 alleviates pain, including the identity of the target cells, remain elusive. Most of the previous studies have attributed beneficial effects of IL-10 on pain to its capacity to suppress the production of pro-inflammatory cytokines. Indeed, IL-10 administration suppresses neuroinflammation and microglia activation in models of chronic pain [16,37,58]. There is also evidence that IL-10 acts directly on sensory neurons [23,48]. However, it is not known whether direct effects of IL-10 on sensory neurons are required for its beneficial effects on chronic pain and whether IL-10 signaling to sensory neurons affects their activity.
Chronic pain is associated with neuronal hyperactivity, including spontaneous activity (SA) recorded in vivo from somatosensory neurons of humans [22] and rodents [8,15,45,60,64]. The SA associated with pain is often generated in the somata of DRG neurons in vivo [5,59]. This SA persists in vitro, both in rodent [4,5,33,51,71] and in human DRG neurons [39,57] and is strongly implicated in driving persistent pain [39,69]. Nociceptor SA generated at resting membrane potential (RMP) in the absence of extrinsic stimulation or ongoing activity (OA) resulting from extrinsic stimulation both depend upon enhancement of depolarizing spontaneous fluctuations (DSFs). DSFs were recently recognized as important contributors to nociceptor hyperactivity in painful conditions [40]. It is unknown whether cisplatin-induced neuropathic pain is associated with SA and enhancements of DSFs in DRG neurons.
Here we hypothesized that cisplatin-induced neuropathic pain is associated with hyperactivity of DRG neurons, and that a direct action of IL-10 on sensory neurons is required for resolution of cisplatin-induced neuronal hyperactivity and mechanical hypersensitivity.
Methods
All experimental protocols were approved by the Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center and conformed to the US National Institutes of Health guidelines on the ethical care and use of animals and ARRIVE guidelines.
Animal model.
Male and female 9-week old WT (JAX#000664) and Il10−/− (JAX#002251) mice were purchased from Jackson laboratory (Maine). To generate the AvilCre+/−;Il10rafloxflox (Il10raDRG-KO) mice, AvilCre+/− mice [27,75] (generously provided by Dr. Hui-Lin Pan, MD Anderson Cancer Center) were bred with Il10rafloxflox [41] mice. Both strains were on C57BL/6 background. Experimental animals were obtained from AvilCre+/−;Il10rafloxflox sir crossed with Il10rafloxflox dam mice. The breeding generated 50% of AvilCre+/−;Il10rafloxflox (Il10raDRG-KO) pups and 50% of control Il10rafloxflox pups. No sex difference was observed in the proportion of each genotype. CIPN was induced by intraperitoneal administration of 2 mg/kg cisplatin (Teva pharmaceuticals) daily for 3 days (identified as days 0, 1 and 2 in figures) as previously described [26].
Experimental design.
Mice were randomly assigned to experimental groups. The sample size was estimated based on previous publications [4,23,26,40]. Il10−/− mice were compared to WT mice obtained from Jackson labs at the same time. Il10raDRG-KO were compared to their Cre-negative control littermates (Il10rafloxflox).
Mechanical pain sensitivity was assessed using von Frey filaments as previously described [11,19,26]. Briefly, mice were placed in opaque boxes (10 × 10 × 10 cm). After a 30 min habituation period, von Frey filaments were applied, and the paw withdrawal threshold was calculated using the “up & down” method. Female and male mice were tested separately, and the data were pooled as no differences were detected between sexes. Behavioral testing was performed by experimenters blinded to treatment and genotype.
Intrathecal injection.
Neutralizing anti-IL-10 (10 μg Sigma, #15145) antibody or control IgG (Sigma) were injected intrathecally under anesthesia (isoflurane 2.5%) as previously described [23,24,29] on days 8, 9 and 10 after the first dose of cisplatin.
Double immunofluorescence staining.
On day 25 after the first cisplatin injection, animals were euthanized with CO2 and perfused with ice-cold PBS and4% paraformaldehyde in PBS. Lumbar DRG were fixed in paraformaldehyde for 48h and cryoprotected with sucrose. DRG were sliced with a Leica CM3050 S Cryostat to 8 μm sections. Slices were stained with mouse monoclonal anti-IL-10 (Santa Cruz, sc-28371, 1:100) and polyclonal rabbit anti-NeuN (Abcam #ab177487, 1:500) overnight at 4°C followed by Alexa Fluor 488 Goat-anti-mouse and Fluor 594 Goat-anti-rabbit (Alexa, Invitrogen, A-11029, 1:500 and A-11037, 1:500) [16]. Two controls were used: omission of the primary antibody and DRG slices from Il10raDRG-KO mice. Images were captured with a CTR4000 Leica confocal microscope.
Sensory neuron isolation.
On day 10 after the first injection, PBS- and cisplatin-treated WT mice were euthanized with C02 and perfused with ice-cold PBS. DRGs were quickly and carefully removed and trimmed in ice-cold DMEM, digested with Trypsin (0.3 mg/ml, Worthington, #LS003702) and collagenase D (1.5 mg/ml, Sigma, #11088858001) for 40 min at 34°C. Debris was removed by two successive centrifugations (6 minutes at 600 rpm) and cells were plated onto coverslips coated with 0.01% poly-L-ornithine (Sigma, P4957) and incubated overnight at 37°C with 5% CO2 in serum free DMEM as previously described [4].
Electrophysiology.
Patch-clamp recordings were performed as previously reported [4,40]. Small to medium size neurons (diameter < 30 μm) were recorded with whole-cell patch-clamp configuration at room temperature (~21 °C) with an EPC10 amplifier (HEKA Elektronik GmbH). Patch pipettes were pulled from borosilicate glass capillaries with a P-97 puller (Sutter Instruments) and fire polished to achieve a final electrode resistance of 3–8 MΩ when filled with a solution containing the following (in mM): 134 KCl, 1.6 MgCl2, 13.2 NaCl, 3 EGTA, 9 HEPES, 4 Mg-ATP, and 0.3 Na-GTP (pH 7.2 adjusted with KOH, 300 mOsM, adjusted with sucrose). The bath solution contained (in mM): 140 NaCl, 3 KCl, 1.8 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose (pH 7.4 adjusted with NaOH, 320 mOsM). The whole-cell patch-clamp configuration was achieved under voltage-clamp by rupture of the membrane after a tight seal (>1 GΩ) was obtained. Membrane capacitance was then measured, and capacitance and series resistance were electronically compensated afterward. The calculated liquid junction potential was 4.3 mV and was not corrected. Spontaneous activity (SA) was measured in current-clamp mode and was defined as at least 1 spike occurring within 1 min with no current injection (I = 0). Ongoing activity (OA) during modest depolarization was then tested by injecting sufficient current to hold neurons at −45 mV (± 2 mV) for 30 seconds. Discharge of one or more action potentials was considered OA. Neurons were identified as rapidly accommodating (RA) or nonaccomodating (NA) by injecting a series of 2-second depolarizing pulses of depolarizing current (5 pA increments) from a holding potential of −60 mV up to twice rheobase as described previously (Odem et al., 2018). We excluded from our analysis RA neurons because they never exhibit SA [40]. RA neurons represented 4% of the 26 neurons patched from PBS-treated wild-type mice). The remaining NA neurons had electrophysiological properties identical to those shown previously in rats to be highly enriched in capsaicin-sensitive cells that are likely to be primary nociceptors [40].
For IL-10 treatments, cells isolated from cisplatin-injected mice were incubated for 15–30 min with 10 ng/ml of recombinant IL-10 (BioVision #4156–10) before recording. Recombinant IL-10 was reconstituted in 3 mM Tris PH8 and diluted into PBS following manufacturer instructions. We acknowledge that the lack of a carrier protein may have reduced the working concentration of IL-10 in vitro. This dose was chosen based on its ability to inhibit microglia and astrocyte activation in vitro (without the use of protein carrier) [16].
Quantitative-real-time polymerase chain reaction (qPCR).
Sciatic nerve (SN), lumbar DRG and spinal cord tissues were quickly dissected and snap-frozen in liquid nitrogen. RNA was isolated using the Trizol-Chloroform (#15596–026, Invitrogen) method. One μg of RNA was reverse transcribed into cDNA using High Capacity cDNA Reverse Transcription kit (#4368813, Applied Bioscience). PCR was performed using PrimeTime Gene Expression Master Mix (#1055770, Integrated DNA Technologies, Coralville, IA) and validated PrimeTime qPCR primer assays for Gapdh, Il10 and Il10ra (Integrated DNA Technologies).
Statistical analysis.
All data are presented as mean ± S.E.M. For behavioral experiments differences between groups were assessed using repeated measures two-way ANOVA followed by Bonferroni correction. For biochemical experiments unpaired t tests were used. For electrophysiological experiments, differences in incidence of SA or OA between populations were assessed by Fisher’s exact test with Bonferroni correction. Differences between groups for the other electrophysiological modalities were tested with one-way ANOVA with Sidak’s correction for multiple comparisons for normally distributed data, otherwise a Kruskal-Wallis test with Dunn’s correction for multiple comparisons was used. Differences were considered statistically significant at p<0.05. In graphs, statistical differences are indicated as *=p<0.05, **=p<0.01 and ***=p<0.001. Prism (GraphPad software, La Jolla, CA) was used for plotting graphs and statistical analysis.
Results
Endogenous IL-10 is necessary for the resolution of cisplatin-induced mechanical pain hypersensitivity
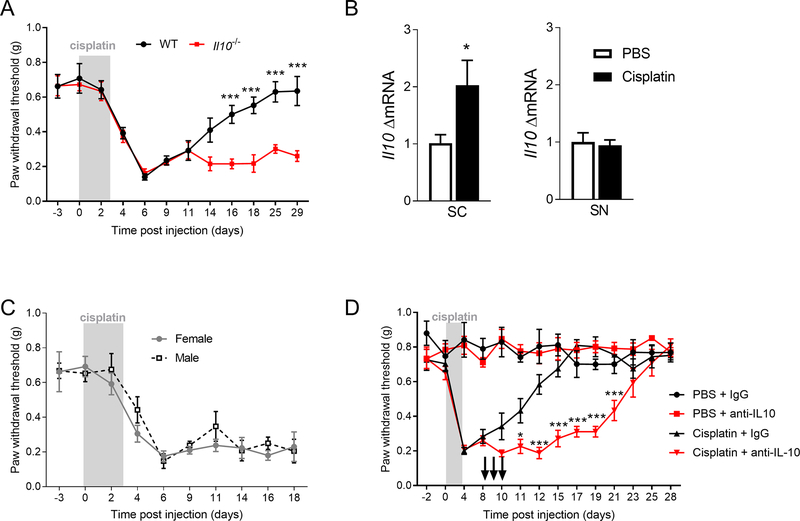
Administration of cisplatin (2 mg/kg for 3 days) induced mechanical hypersensitivity that resolved after 3 weeks (Figure 1A). Resolution of pain hypersensitivity in cisplatin-treated mice was associated with an increase in Il10 mRNA in the spinal cord but not in the sciatic nerve (Figure 1B, unpaired t test, F(3, 4) = 6.98, p<0.05). Il10 mRNA was not detectable in the DRG. To determine the contribution of endogenous IL-10 to the resolution of mechanical hypersensitivity, we used female and male WT and Il10−/− mice. The maximal intensity of mechanical hypersensitivity was similar in WT and Il10−/− mice but the resolution of mechanical pain hypersensitivity was delayed in Il10−/− mice (Figure 1A, repeated measure two-way ANOVA, interaction genotype x time F(11, 110) = 5.55, p<0.001). No differences were found between female and male mice (Figure 1C). To test whether IL-10 was acting at the level of the spinal cord/DRG to promote resolution of hypersensitivity, we intrathecally injected a neutralizing anti-IL-10 antibody on day 8, 9 and 10 after the first injection of cisplatin. Inhibition of IL-10 signaling in the spinal cord/DRG markedly delayed the resolution of mechanical pain hypersensitivity in female and male mice (Figure 1D, repeated measures two-way ANOVA, interaction genotype x antibody F(39, 208) = 7.14, p<0.001).
Figure 1.
IL-10 is critical for resolution of cisplatin-induced mechanical pain hypersensitivity. A. Mechanical sensitivity measured by von Frey hairs in WT and Il10−/− mice in response to cisplatin (2 mg/kg daily for 3 days) (n=4M+4F/group), repeated measure two-way ANOVA, interaction genotype x time F(11, 110) = 5.55, p<0.0001. B. mRNA levels of Il10 in spinal cord (SC) and sciatic nerve (SN) on day 8 after start of cisplatin treatment (n=5/groups), for SC unpaired t test, F (3, 4) = 6.98, p=0.044. C. Mechanical sensitivity measured by von Frey hairs in female and male Il10−/− mice in response to cisplatin (n=4/group). D. Effect of intrathecal administration of neutralizing anti-IL-10 antibody or control IgG (10 μg/mouse/day, on day 8, 9 and 10 after start of cisplatin treatment) on mechanical sensitivity after cisplatin or PBS administration in female and male mice (n= 4F+4M/group), repeated measure two-way ANOVA, interaction genotype x antibody F (39, 208) = 7.14, p<0.0001. The arrows represent the intrathecal injection. Data are shown as mean ± SEM.
Isolated DRG neurons from cisplatin-treated mice exhibit spontaneous and evoked hyperactivity
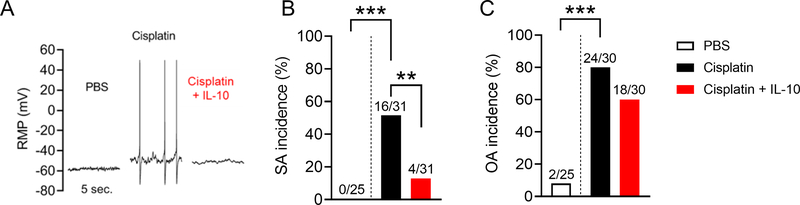
To test whether IL-10 may regulate neuronal hyperactivity associated with neuropathic pain, we first tested the hypothesis that cisplatin treatment induces hyperactivity of DRG neurons. We isolated neurons from lumbar DRG of PBS- and cisplatin-treated mice on day 10 and cultured the cells overnight. DRG neurons from cisplatin-treated mice showed a dramatic increase in the incidence of SA (Figure 2A–B, Fisher’s exact test, p<0.001). Cisplatin treatment also increased the incidence of OA as observed when neurons were artificially depolarized from their normal resting membrane potential (RMP) to −45 mV for 30 sec (Figure 2C Fisher’s exact test, p<0.001).
Figure 2.
IL-10 reverses cisplatin treatment-induced nociceptor hyperactivity by reducing the incidence of spontaneous activity (SA) in dissociated sensory neurons. A. Examples of SA in isolated DRG neurons 10 days after the first injection of PBS or cisplatin. Recombinant IL-10 (10 ng/ml) was added 15–30 min before recording. B, C. Percentage of neurons exhibiting SA at RMP (B) and ongoing activity (OA) when held at −45 mV (C) are increased in cisplatin-treated mice. IL-10 bath application significantly attenuated the effects of cisplatin on the incidence of SA but not OA (B). The ratio above each bar indicates the number of neurons with SA or OA/total sampled (PBS-treated mice n=3 and cisplatin n=6). For SA incidence comparisons, Fisher’s exact test, p<0.001 PBS vs cisplatin and p<0.01 cisplatin vs cisplatin + IL-10. For OA, Fisher’s exact test, p<0.0001 PBS vs cisplatin. Data are shown as mean ± SEM.
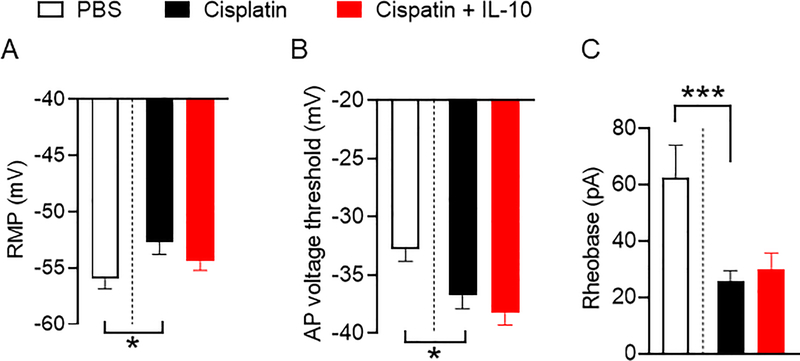
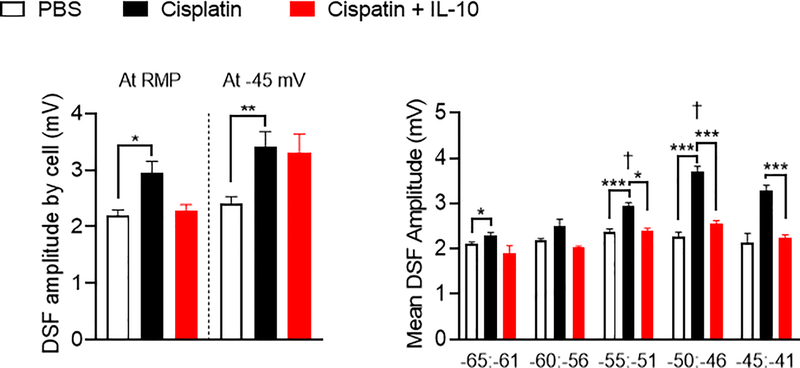
In principle, SA or OA in the absence of transient depolarizing inputs can be promoted by one or more of only three general electrophysiological alterations [40]: (i) depolarization of RMP, (ii) hyperpolarization of the voltage threshold for generating action potentials (APs), and (iii) enhanced amplitude of depolarizing spontaneous fluctuations (DSFs) of membrane potential that bridges the gap between RMP and AP threshold. The results in Figures 3 show that cisplatin induced all three alterations. We observed sustained depolarization of the RMP (Figure 3A, PBS vs. cisplatin: one-way ANOVA followed by Sidak’s multiple correction test p<0.05), hyperpolarization of the AP voltage threshold (Figure 3B, one-way ANOVA followed by Sidak’s multiple correction test p<0.01, PBS vs. cisplatin: p<0.05 ), which together help explain the decrease in AP current threshold (rheobase) (Figure 3C, Kruskal-Wallis test with Dunn’s correction for multiple comparisons p=0.001; PBS vs. cisplatin: p=0.001). In addition, neurons isolated from cisplatin-treated mice exhibited greater mean DSF amplitudes as measured either at RMP or when artificially depolarized to −45 mV compared to DRG neurons from PBS-treated mice (Figure 4A, Kruskal-Wallis test with Dunn’s correction for multiple comparisons, p<0.05 at rest and p=0.01 at −45 mV). The increase in DSF amplitudes in DRG neurons of cisplatin-treated mice was voltage dependent, being most prominent at RMP. (Figure 4B, between −55 and −40 mV: Kruskal-Wallis test with Dunn’s correction for multiple comparisons p<0.001, respectively for each bin compared with the next depolarized bin, p<0.10, p<0.001, p<0.001, p>0.10). This is in line with our previous observations in DRG neurons from rats [40] and mice [6] after spinal cord injury.
Figure 3.
Cisplatin treatment promotes neuronal hyperactivity by multiple electrophysiological alterations. Data were collected from DRG neurons isolated 10 days after the first injection of PBS or cisplatin. Recombinant IL-10 (10 ng/ml or 0.5 nM) was added 15–30 min before recording. A. Resting membrane potential (RMP, mV). PBS vs. cisplatin: one-way ANOVA followed by Sidak’s multiple correction test p=0.038. B. Action potential (AP) voltage threshold (mV): one-way ANOVA followed by Sidak’s multiple correction test p=0.0022, PBS vs. cisplatin: p=0.025. C. Rheobase (pA) Kruskal-Wallis test with Dunn’s correction for multiple comparisons p=0.0003; PBS vs. cisplatin: p=0.0005. Data are shown as mean ± SEM.
Figure 4.
Amplitudes of depolarizing spontaneous fluctuations (DSFs) are enhanced by in vivo cisplatin treatment and normalized by in vitro IL-10 incubation at depolarized membrane potentials. A. Average DSF amplitudes per neuron increased in cisplatin-treated mice at RMP and when neurons were artificially depolarized to −45 mV, (mice: PBS n=3 and cisplatin n=6) without being reversed by IL-10 incubation. Kruskal-Wallis test with Dunn’s correction for multiple comparisons p=0.0195, PBS vs. cisplatin: p=0.0155. B. Mean DSF amplitudes binned by RMP ranging from −65 and −41 mV from DRG neurons isolated from PBS and cisplatin treated mice exposed to vehicle or IL-10 (10 ng/ml) in vitro. f denotes statistical significance for cisplatin-treated neurons from the marked bin compared with the previous one. Data are shown as mean ± SEM.
IL-10 reverses cisplatin-induced neuronal hyperactivity primarily by reducing the amplitude of the DSFs
Given that IL-10 is required for resolution of pain, we tested whether bath application of IL-10 could reverse the hyperactivity observed in DRG neurons from cisplatin-treated mice. Bath application of recombinant IL-10 (10 ng/ml) to DRG neurons from cisplatin-treated mice for 15–30 min significantly reduced the incidence of SA at RMP from 52% to 13% of neurons (Figure 2, SA Fisher’s exact test, p<0.01). Bath application of IL-10 had no significant effect on OA at −45 mV (Figure 2). IL-10 also did not reverse the effects of cisplatin treatment on RMP, AP voltage threshold, and rheobase (Figure 3).
IL-10 bath application reduced DSF amplitudes in neurons from cisplatin-treated mice having RMPs between −55 and −41 mV, compatible with the average RMP recorded (Figure 4B, Kruskal-Wallis test with Dunn’s correction for multiple comparisons for −55 to −51 mV and −50 to −46 mV bins with respectively p=0.05 and p<0.001, and one-way ANOVA followed by Sidak’s multiple correction test for −45 to −41 mV bin with p<0.001). IL-10 did not significantly affect the overall DSF amplitudes recorded at RMP or when neurons were depolarized artificially to −45 mV (Figure 4A).
Cisplatin increased IL-10R1 expression in sensory neurons
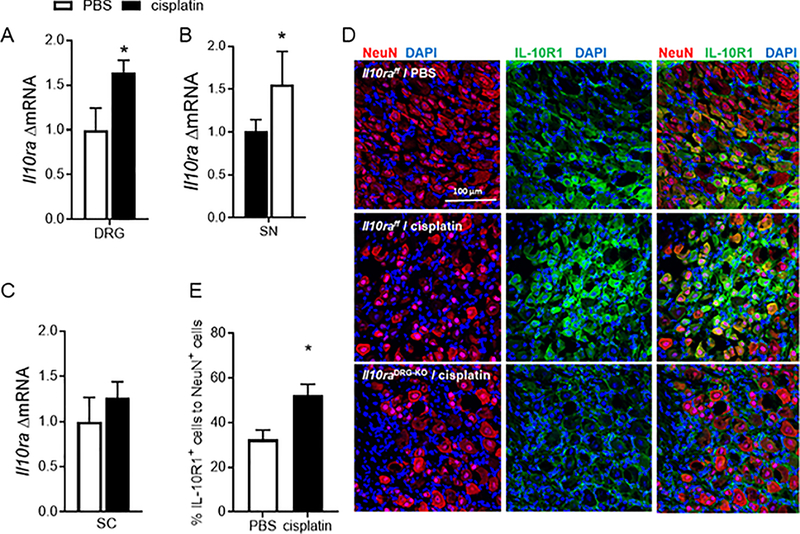
Because IL-10 has a direct effect on neuronal activity, we next investigated whether resolution of cisplatin-induced mechanical hypersensitivity was associated with changes in the expression of IL-10R1 in DRG and spinal cord. Il10ra mRNA levels increased in the DRG and sciatic nerve but not in spinal cord in response to cisplatin administration (Figure 5A–C, for DRG unpaired t test, F(6,7) = 2.91 p<0.05; for SN unpaired t test, F(7,5) = 10.8 p<0.05). Next, we performed double immunofluorescence analysis to assess IL-10R1 expression on sensory neurons. IL-10R1 is expressed by sensory neurons and satellite glial cells in naïve mice (Figure 5D). The percentage of IL-10R1-positive neurons increased after cisplatin treatment (Figure 5E, Unpaired t test, F(3,3) = 1.56 p<0.05). To validate the specificity of the staining, we used mice that do not express IL-10R1 on Advillin-positive sensory neurons (Il10RaDRG-KO mice) (Figure 5D). IL-10R1 staining was not detectable on sensory neurons in the DRG of these mice, while staining on cells around the neurons, most likely satellite glia, was not affected. The expression of IL-10R1 is upregulated at mRNA and protein level from day 8 and persisted for a least 2 weeks.
Figure 5.
Cisplatin increases IL-10R1 expression on sensory neurons A. mRNA levels of Il10ra in dorsal root ganglia (DRG) (n=8/group) unpaired t test, F(6,7) = 2.91 p=0.03, B sciatic nerve (SN) (n=8/group) unpaired t test, F(7,5) = 10.8 andC, spinal cord (SC) (n=9/group) on day 8 after start of cisplatin treatment.D, Immunofluorescence analysis of IL-10R1 on DRG neurons. Representative images of DRG sections labeled with anti-IL-10R1 (green) and anti-NeuN antibodies (red) to identify neuronal cells in control (Il10rafloxflox) and Il10DRG-KO mice on day 25 after cisplatin or PBS administration. E. Quantification of the percentage of IL-10R1- and NeuN-positive cells (n= 3F+3M/group) Unpaired t test, F (3,3) = 1.56 p=0.021. Data are shown as mean ± SEM.
Sensory neuron IL-10R1 is critical for resolution of cisplatin-induced mechanical pain hypersensitivity
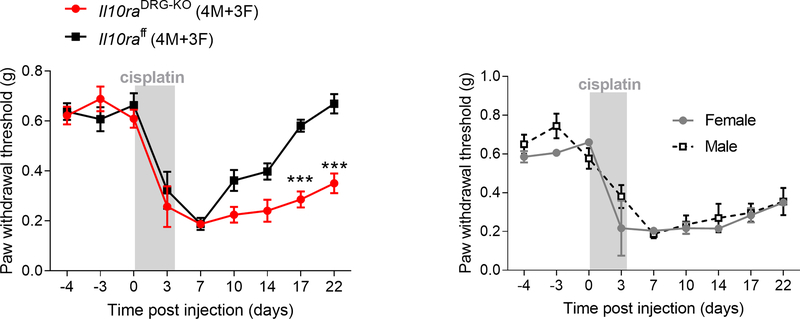
Because IL-10R1 is expressed on sensory neurons (Figure 5D) and in vitro addition of IL-10 reduced spontaneous activity in sensory neurons isolated from cisplatin-treated mice, we determined the contribution of IL-10R1 on sensory neurons to the resolution of pain hypersensitivity. We monitored the resolution of mechanical hypersensitivity in control (Il10raflox) and Il10DRG-KO mice treated with cisplatin. The lack of IL-10R1 in sensory neurons delayed the resolution in female and male mice (Figure 6A, repeated measure two-way ANOVA interaction time x genotype F(8,72) = 5.09, p<0.001). No differences were observed between sexes (Figure 6B). Baseline pain thresholds and severity of mechanical pain were similar in both genotypes (Figure 6A).
Figure 6.
Genetic deletion of Il10ra from Advillin-positive sensory neurons delays the resolution of cisplatin-induced mechanical hypersensitivity. A. Mechanical pain threshold in control (Il10rafloxflox) and Il10raDRG-KO mice after cisplatin treatment (2 mg/kg daily for 3 days) (n= 3F+4M/group), repeated measure two-way ANOVA interaction time x genotype F (8,72) = 5.09, p<0.0001. Resolution of mechanical pain threshold is delayed in mice that lack IL-10R1 on sensory neurons while baseline pain thresholds and severity of mechanical pain were similar in both genotypes. B. Mechanical sensitivity measured by von Frey hairs in female and male Il10raDRG-KO mice in response to cisplatin. Data are shown as mean ± SEM.
Discussion
It is well established that sensory neurons respond to proinflammatory cytokines such as IL-1β or TNFα produced by glia in the spinal cord or by infiltrating immune cells in spinal cord, DRG or nerve with increased excitability and pain hypersensitivity [7,17,21]. There is also ample evidence that exogenous administration of IL-10 reduces pain-related signaling and this is thought to be mediated through suppression of the production of proinflammatory cytokines by glia. Here we demonstrate for the first time that signaling by the anti-inflammatory cytokine IL-10 to specific IL-10 receptors expressed on sensory neurons is required for resolution of mechanical hypersensitivity in cisplatin-treated male and female mice. We also show for the first time that cisplatin induces hyperactivity, including SA, in DRG neurons, a phenomenon closely linked to the maintenance of neuropathic pain [15,39,57,69]. Notably, IL-10 reverses the increase in SA induced by treatment of mice with cisplatin. Our present findings reinforce the concept that resolution of pain is an active mechanism that involves neuroimmune interactions [3,9,18,23,24,26]. These findings also challenge the concept that glia and/or immune cells are the main target cells mediating resolution of pain in response to IL-10.
We did not observe sex differences in the pro-resolution effect of endogenous IL-10 signaling. The lack of sex effects is consistent with previous findings on the role of T cells in the resolution of chemotherapy-induced peripheral neuropathy (CIPN), nerve injury-induced pain sensitivity, and the analgesic effect of IL-10 observed in both sexes following spared nerve injury [18,26,65]. The data in the figure 1D suggest that IL-10 actively inhibits pain hypersensitivity, because the hypersensitivity resolved after we stopped dosing the neutralizing antibody. The kinetics of the resolution after the last dose of anti-IL-10 are consistent with the described half-life of ~10 days for IgG [34].
Resolution of cisplatin-induced pain hypersensitivity is associated with upregulation of Il10 in the spinal cord and Il10ra in the DRG and sciatic nerve indicating that resolution is an active mechanism and not the result of a simple disappearance of the triggers of pain. Our data clearly show that peripheral sensory neurons express IL-10R1 and that expression of these receptors in Advillin-positive neurons is required for the resolution of mechanical pain hypersensitivity. While Advillin is expressed by both sensory and sympathetic neurons, single cell RNA-seq data show that Il10ra is expressed in sensory but not sympathetic neurons [70] ruling out a potential effect of sympathetic neurons in our model. It should be noted that there is some delayed reduction in allodynia in the IL-10R1DRG-KO mice. This may be due to some residual expression of IL-10R1 on sensory neurons. However, we cannot exclude the possibility that IL-10R1 on other cells contributes to recovery as well.
Our findings expand previous findings by us and others that indicated that IL-10 reduces pain via a direct action on sensory neurons [23,48,68]. This neuronal effect of IL-10 adds to the proposed ability of this cytokine to suppress pain by suppressing production of proinflammatory cytokines by microglia thereby reducing neuroinflammation [55,58,74]. Moreover, our findings open up the possibility that reduction of microglial activity in response to IL-10 is mediated in part by suppression of SA in sensory neurons [61,66]. Consistent with our present findings, endogenous IL-10 is also necessary for the resolution of inflammatory pain [3,62]. In contrast to our findings in CIPN and inflammatory pain models, resolution of pain hypersensitivity after chronic constriction injury (CCI) is not altered in Il10−/− mice [56]. These data indicate that the role of endogenous IL-10 in resolution of pain may be disease/pain model specific. This is perhaps not surprising; the mechanisms underlying pain development differ among pain models, and therefore it is likely that mechanisms underlying resolution also differ.
We and others showed previously that chemotherapy-induced peripheral neuropathy is not associated with microglial activation, making it unlikely that suppression of microglial activity contributes to the beneficial effects of IL-10 reported here [10,12,44,72,73]. Our current findings reveal a novel and direct mechanism of action of IL-10 on sensory neuron activity to resolve pain. We performed the first comprehensive electrophysiological characterization of isolated sensory neurons from cisplatin-treated mice. Our study reveals that cisplatin treatment induced strong hyperactivity in sensory neurons as shown by the enhanced incidence of SA, a phenomenon previously observed in models of peripheral and central neuropathic pain induced by surgical damage to the nerve [8,15,45,60,64]. Cisplatin also induced sustained OA measured at −45 mV, a novel measure of neuronal hyperactivity [40]. These findings indicate that cisplatin-induced neuronal hyperactivity is associated with the same fundamental alterations of neuronal excitability as previously described in nociceptors isolated from rats and mice after spinal cord injury, i.e. depolarization of RMP, lowered AP voltage threshold, and increased DSF amplitudes [6,40]. The mechanisms underlying large, irregular, low-frequency DSFs are complex and currently under investigation. DSF mechanisms in small, capsaicin-sensitive and/or isolectin B4-binding DRG neurons (probable nociceptors) (Odem, 2018) clearly differ from the mechanisms of high-frequency, sinusoidal, subthreshold membrane potential oscillations (SMPOs) described by Devor and colleagues in large DRG neurons (primarily non-nociceptive) [34].
Bath application of IL-10 for 15–30 min was sufficient to reverse SA in sensory neurons from cisplatin-treated mice. We showed previously that perfusion of DRG neurons from paclitaxel-treated rats with IL-10 blocked SA already after a few seconds, but the dose of IL-10 used in that study was an order of magnitude higher than in the present study [23]. IL-10 can alter the mRNA expression of ion channels implicated in the regulation of neuronal excitability, such as Nav1.8 and Cav2.2 channels [48,68]. However, the rapid effect of IL-10 on SA observed in our current and previous study suggests a non-genomic effect of IL-10. To our knowledge, direct effects of IL-10 on the function of Nav1.8 and Cav2.2, or other channels regulating neuronal excitability have not been reported. It is noteworthy that IL-10 has been reported to regulate neuronal activity in the hippocampus as well. Similarly to our observations, IL-10 reduced hippocampal neuronal activity within 10 min [32,52]. In these studies, IL-10 stimulates the activation of potassium channel BKα1 and the GABA release.
Intriguingly, IL-10 reduced SA by reversing the cisplatin-induced increase in DSF amplitudes without substantially altering RMP, rheobase, and AP voltage threshold. This result reinforces growing evidence that enhancement of DSFs is particularly important for nociceptor hyperactivity [6,40]. Our findings support the hypothesis that IL-10 may regulate the activity and/or trafficking of ion channels involved in generating or modulating DSFs.
In 20%−30% of patients treated with chemotherapy, neuropathy persists after cessation of treatment and this reduces the quality of life of cancer survivors [47]. It is important to note that a study comparing patients with painful neuropathy, painless neuropathy and controls showed that the circulating levels of IL-10 were higher in patients with painless neuropathy than in the other groups (patients with neuropathies induced by chemotherapy and other etiologies were included in this study) [54].
In conclusion, we report for the first time that IL-10 acts directly on sensory neurons to resolve neuronal hyperactivity and pain hypersensitivity induced by cisplatin. On the basis of these findings, we propose that stimulation of IL-10 signaling, or administration of IL-10 may be developed into a safe therapeutic strategy to prevent or treat chronic chemotherapy-induced neuropathic pain.
Supplementary Material
Supplementary Figure 1. Avil and Il10ra expression in the mouse central nervous system from mousebrain.org [70].
Acknowledgement
We wish to thank Dr. Hui-Lin Pan (MD Anderson Cancer Center) for providing the AdvillinCre mice. This study was supported by grants from the NIH R01 NS073939 (A.K., C.J.H and R.D.), R01 CA227064 (A.K., C.J.H), R01 NS111521 (ETW) and R01 NS091759 (Carmen Dessauer and E.T.W.), Mission Connect, a program of TIRR Foundation (A.B.), Cyrus Scholar Award (G.L.) and American Pain Society (G.L.)
Footnotes
Conflict of Interest statement.
The authors declare no conflict of interest
Resolution of pain depends on endogenous IL-10 signaling, IL-10 binds to its receptor on sensory neurons to reverse cisplatin induced-neuronal hyperactivity and -pain hypersensitivity.
References.
- [1].Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA 2003;100:7947–7952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alvarez P, Bogen O, Green PG, Levine JD. Nociceptor interleukin 10 receptor 1 is critical for muscle analgesia induced by repeated bouts of eccentric exercise in the rat. Pain 2017;158:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bang S, Xie Y-K, Zhang Z-J, Wang Z, Xu Z-Z, Ji R-R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest 2018;128:3568–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bavencoffe A, Li Y, Wu Z, Yang Q, Herrera J, Kennedy EJ, Walters ET, Dessauer CW. Persistent Electrical Activity in Primary Nociceptors after Spinal Cord Injury Is Maintained by Scaffolded Adenylyl Cyclase and Protein Kinase A and Is Associated with Altered Adenylyl Cyclase Regulation. J Neurosci 2016;36:1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 2010;30:14870–14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Berkey SC, Herrera JJ, Odem MA, Rahman S, Cheruvu SS, Cheng X, Walters ET, Dessauer CW, Bavencoffe AG. EPAC1 and EPAC2 promote nociceptor hyperactivity associated with chronic pain after spinal cord injury. Neurobiology of Pain 2020;7:100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji R-R, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci 2008;28:14062–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Blumberg H, Janig W. Discharge pattern of afferent fibers from a neuroma. Pain 1984;20:335–353. [DOI] [PubMed] [Google Scholar]
- [9].Boué J, Blanpied C, Brousset P, Vergnolle N, Dietrich G. Endogenous opioid-mediated analgesia is dependent on adaptive T cell response in mice. J Immunol 2011;186:5078–5084. [DOI] [PubMed] [Google Scholar]
- [10].Boyette-Davis JA, Walters ET, Dougherty PM. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag 2015;5:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [12].Chiu GS, Maj MA, Rizvi S, Dantzer R, Vichaya EG, Laumet G, Kavelaars A, Heijnen CJ. Pifithrin-μ Prevents Cisplatin-Induced Chemobrain by Preserving Neuronal Mitochondrial Function. Cancer Res 2017;77:742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013;501:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune Cytokines and Their Receptors in Inflammatory Pain. Trends in Immunology 2018;39:240–255. [DOI] [PubMed] [Google Scholar]
- [15].Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 2006;26:1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eijkelkamp N, Steen-Louws C, Hartgring SAY, Willemen HLDM, Prado J, Lafeber FPJG, Heijnen CJ, Hack CE,van Roon JAG, Kavelaars A IL4–10 Fusion Protein Is a Novel Drug to Treat Persistent Inflammatory Pain. J Neurosci 2016;36:7353–7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 1988;334:698–700. [DOI] [PubMed] [Google Scholar]
- [18].Fischer R, Sendetski M, Del Rivero T, Martinez GF, Bracchi-Ricard V, Swanson KA, Pruzinsky EK, Delguercio N, Rosalino MJ, Padutsch T, Kontermann RE, Pfizenmaier K, Bethea JR. TNFR2 promotes Treg-mediated recovery from neuropathic pain across sexes. Proc Natl Acad Sci USA 2019;116:17045–17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Garriga J, Laumet G, Chen S-R, Zhang Y, Madzo J, Issa J-PJ, Pan H-L, Jelinek J. Nerve Injury-Induced Chronic Pain Is Associated with Persistent DNA Methylation Reprogramming in Dorsal Root Ganglion. J Neurosci 2018;38:6090–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ji R-R, Chamessian A, Zhang Y-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016;354:572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 2006;26:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kleggetveit IP, Namer B, Schmidt R, Helas T, Rückel M, Ørstavik K, Schmelz M, Jørum E. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain 2012;153:2040–2047. [DOI] [PubMed] [Google Scholar]
- [23].Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 2016;36:11074–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Laumet G, Edralin JD, Chiang AC-A, Dantzer R, Heijnen CJ, Kavelaars A. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology 2018;43:2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A. CD3+ T cells are critical for the resolution of comorbid inflammatory pain and depression-like behavior. Neurobiology of Pain 2020;7:100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A. Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain 2019;160:1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Laumet G, Garriga J, Chen S-R, Zhang Y, Li D-P, Smith TM, Dong Y, Jelinek J, Cesaroni M, Issa J-P, Pan H-L. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015;18:1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laumet G, Ma J, Robison AJ, Kumari S, Heijnen CJ, Kavelaars A. T Cells as an Emerging Target for Chronic Pain Therapy. Front Mol Neurosci 2019;12:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Laumet G, Zhou W, Dantzer R, Edralin JD, Huo X, Budac DP, O’Connor JC, Lee AW, Heijnen CJ, Kavelaars A. Upregulation of neuronal kynurenine 3-monooxygenase mediates depression-like behavior in a mouse model of neuropathic pain. Brain Behav Immun 2017;66:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 2007;21:686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Leung A, Gregory NS, Allen L-AH, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain 2016;157:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Levin SG, Konakov MV, Godukhin OV. Role of BK(Ca) Potassium Channels in the Mechanisms of Modulatory Effects of IL-10 on Hypoxia-Induced Changes in Activity of Hippocampal Neurons. Bull Exp Biol Med 2016;160:643–645. [DOI] [PubMed] [Google Scholar]
- [33].Li Y, Tatsui CE, Rhines LD, North RY, Harrison DS, Cassidy RM, Johansson CA, Kosturakis AK, Edwards DD, Zhang H, Dougherty PM. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain 2017;158:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, Wedgwood RJ. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med 1988;112:634–640. [PubMed] [Google Scholar]
- [35].McKelvey R, Berta T, Old E, Ji R-R, Fitzgerald M. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J Neurosci 2015;35:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mietto BS, Kroner A, Girolami EI, Santos-Nogueira E, Zhang J, David S. Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury. J Neurosci 2015;35:16431–16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 2005;21:2136–2148. [DOI] [PubMed] [Google Scholar]
- [38].Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- [39].North RY, Li Y, Ray P, Rhines LD, Tatsui CE, Rao G, Johansson CA, Zhang H, Kim YH, Zhang B, Dussor G, Kim TH, Price TJ, Dougherty PM. Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 2019;142:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Odem MA, Bavencoffe AG, Cassidy RM, Lopez ER, Tian J, Dessauer CW, Walters ET. Isolated nociceptors reveal multiple specializations for generating irregular ongoing activity associated with ongoing pain. Pain 2018;159:2347–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pils MC, Pisano F, Fasnacht N, Heinrich J-M, Groebe L, Schippers A, Rozell B, Jack RS, Müller W. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur J Immunol 2010;40:443–448. [DOI] [PubMed] [Google Scholar]
- [42].Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol 2017;38:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ramer MS, Murphy PG, Richardson PM, Bisby MA. Spinal nerve lesion-induced mechanoallodynia and adrenergic sprouting in sensory ganglia are attenuated in interleukin-6 knockout mice. Pain 1998;78:115–121. [DOI] [PubMed] [Google Scholar]
- [44].Robinson CR, Zhang H, Dougherty PM. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 2014;274:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Roza C, Laird JMA, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol (Lond) 2003;550:921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10:1361–1368 [DOI] [PubMed] [Google Scholar]
- [47].Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014;155:2461–2470. [DOI] [PubMed] [Google Scholar]
- [48].Shen K-F, Zhu H-Q, Wei X-H, Wang J, Li Y-Y, Pang R-P, Liu X-G. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol 2013;247:466–475. [DOI] [PubMed] [Google Scholar]
- [49].da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos ARS. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol 2015;51:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].de Souza JB, Grossmann E, Perissinotti DMN, de Oliveira Junior JO, da Fonseca PRB, de P Posso I Prevalence of Chronic Pain, Treatments, Perception, and Interference on Life Activities: Brazilian Population-Based Survey. Pain Research and Management 2017. doi: 10.1155/2017/4643830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain 1996;65:235–242. [DOI] [PubMed] [Google Scholar]
- [52].Suryanarayanan A, Carter JM, Landin JD, Morrow AL, Werner DF, Spigelman I. Role of interleukin-10 (IL-10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology 2016;107:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA 2005;102:5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Uçeyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology 2007;69:42–49. [DOI] [PubMed] [Google Scholar]
- [55].Vale ML, Marques JB, Moreira CA, Rocha FAC, Ferreira SH, Poole S, Cunha FQ, Ribeiro RA. Antinociceptive effects of interleukin-4, −10, and −13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther 2003;304:102–108. [DOI] [PubMed] [Google Scholar]
- [56].Vanderwall AG, Noor S, Sun MS, Sanchez JE, Yang XO, Jantzie LL, Mellios N, Milligan ED. Effects of spinal non-viral interleukin-10 gene therapy formulated with D-mannose in neuropathic interleukin-10 deficient mice: behavioral characterization, mRNA and protein analysis in pain relevant tissues. Brain Behav Immun 2018;69:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vaso A, Adahan H-M, Gjika A, Zahaj S, Zhurda T, Vyshka G, Devor M. Peripheral nervous system origin of phantom limb pain. Pain 2014;155:1384–1391. [DOI] [PubMed] [Google Scholar]
- [58].Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain 1998;74:35–42. [DOI] [PubMed] [Google Scholar]
- [59].Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain 1983;17:321–339. [DOI] [PubMed] [Google Scholar]
- [60].Wall PD, Gutnick M. Properties of afferent nerve impulses originating from a neuroma. Nature 1974;248:740–743. [DOI] [PubMed] [Google Scholar]
- [61].Wen Y-R, Suter MR, Kawasaki Y, Huang J, Pertin M, Kohno T, Berde CB, Decosterd I, Ji R-R. Nerve Conduction Blockade in the Sciatic Nerve Prevents but Does Not Reverse the Activation of p38 Mitogen-activated Protein Kinase in Spinal Microglia in the Rat Spared Nerve Injury Model: Anesthesiology 2007;107:312–321 [DOI] [PubMed] [Google Scholar]
- [62].Willemen HLDM, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain 2014;15:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wolf G, Gabay E, Tal M, Yirmiya R, Shavit Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain 2006;120:315–324. [DOI] [PubMed] [Google Scholar]
- [64].Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci 2001;21:RC140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wu H-Y, Mao X-F, Tang X-Q, Ali U, Apryani E, Liu H, Li X-Y, Wang Y-X. Spinal interleukin-10 produces antinociception in neuropathy through microglial β-endorphin expression, separated from antineuroinflammation. Brain Behav Immun 2018;73:504–519. [DOI] [PubMed] [Google Scholar]
- [66].Xie W, Strong JA, Zhang J-M. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience 2009;160:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Xu Z-Z, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji R-R. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 2015;21:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yang J, Xie M-X, Hu L, Wang X-F, Mai J-Z, Li Y-Y, Wu N, Zhang C, Li J, Pang R-P, Liu X-G. Upregulation of N-type calcium channels in the soma of uninjured dorsal root ganglion neurons contributes to neuropathic pain by increasing neuronal excitability following peripheral nerve injury. Brain Behav Immun 2018;71:52–65. [DOI] [PubMed] [Google Scholar]
- [69].Yang Q, Wu Z, Hadden JK, Odem MA, Zuo Y, Crook RJ, Frost JA, Walters ET. Persistent pain after spinal cord injury is maintained by primary afferent activity. J Neurosci 2014;34:10765–10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular Architecture of the Mouse Nervous System. Cell 2018;174:999–1014.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang JM, Song XJ, LaMotte RH. An in vitro study of ectopic discharge generation and adrenergic sensitivity in the intact, nerve-injured rat dorsal root ganglion. Pain 1997;72:51–57. [DOI] [PubMed] [Google Scholar]
- [72].Zheng FY, Xiao W-H, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 2011;176:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhou W, Kavelaars A, Heijnen CJ. Metformin Prevents Cisplatin-Induced Cognitive Impairment and Brain Damage in Mice. PLoS ONE 2016;11:e0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther 2008;15:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zurborg S, Piszczek A, Martínez C, Hublitz P, Al Banchaabouchi M, Moreira P, Perlas E, Heppenstall PA. Generation and characterization of an Advillin-Cre driver mouse line. Mol Pain 2011;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Avil and Il10ra expression in the mouse central nervous system from mousebrain.org [70].