Abstract
Aim:
With the advent of highly active and combination antiretroviral therapy have substantially increased the life expectancy of patients infected with human immunodeficiency virus (HIV). However, this has brought into sharp contrast the incidence of several ‘Non-acquired immunodeficiency syndrome (AIDS) diseases such as NeuroAIDS which identifies a group of neurological disorders caused primarily by HIV-mediated damage to the central and peripheral nervous systems. Given the patients depleted immune condition, the use and abuse of drug and addictive substances such as tobacco smoking can further deteriorates their overall health and accelerate the progression and severity of the disease. In this review we detail the pathogenesis, progression and characteristics of HIV and the impact of tobacco smoking as a risk factor for the progression of the disease to NeuroAIDS. This is a poorly understood aspect of HIV-related complications that needs to be addressed.
Subjects and methods:
Review of theoretical approaches and knowledge synthesis.
Results:
Tobacco smoking is highly prevalent in HIV patients when compared to the general population. The oxidative damage and inflammatory stress caused by chronic smoking on the cerebrovascular system have been well established. Considering that HIV patients have an impaired immune system and smokers per se are more susceptible to viral and bacterial inflammatory neuropathologies than non-smokers, it is conceivable that tobacco smoking as a risk factor for the progression of HIV into NeuroAIDS and related neurological impairments.
Conclusion:
Tobacco smoke (TS) may bring about a synergistic effect in the context of persistent inflammatory state and cerebrovascular damage which facilitate HIV infection and progression to NeuroAIDS when compared to non-smokers.
Keywords: Blood-Brain Barrier, Oxidative Stress, Inflammation, Smoking, Brain Disorders, Abuse, Cognitive
INTRODUCTION
HIV/AIDS
AIDS is caused by HIV in humans. It belongs to the Lentivirus subgroup of the Retrovirus family and is classified into two major types HIV-1 and HIV-2 (Sharp and Hahn 2011). As on date, AIDS remains a major health concern. Around 20.9 million people received antiretroviral therapy in June 2017 and 36.7 million people were living with HIV in 2016 worldwide(UNAIDS 2017). An infection occurs when the virus makes its way into the body either through exposure to certain body fluids or through ‘percutaneous/intravenous inoculations’ (Cohen et al. 2011). Once the virus is inside the body, its primary target is the immune system. It specifically infects and kills CD4+ or T cells, thereby gradually weakening the immune system until the body loses its ability to resist opportunistic infections or malignancies (Prevention 2018). An AIDS diagnosis is made when a patient exhibits one or more of these afflictions in addition to a severely reduced CD4+ count of 200 cells/mm3 or less. Though, there is no cure for AIDS yet, currently available treatment for AIDS is known as highly active antiretroviral therapy, or HAART (Abuse 2012) also called combined antiretroviral therapy (cART).
Etiology and Pathogenesis:
A brief and acute infection followed by a protracted asymptomatic phase and finally a rapid decline in CD4+ cell count are three stages that constitute the pattern of a typical HIV infection (Hernandez-Vargas and Middleton 2013). The pathogenesis of HIV infection involves a three-pronged attack on the immune system viz. immunodeficiency (through decline in CD4+ count), immunosuppression (i.e., inadequate action of remaining CD4+ cells) and persistent immune activation (prompted by viral replication). Moreover, an environment of continual inflammation and coagulation persists, which can lead to risk of morbidity and mortality arising from AIDS as well as non-AIDS illnesses (Lane 2010). This environment of chronic inflammation could lead to immunosenescence and aging, thereby increasing the chances of opportunistic infections (Barré-Sinoussi et al. 2013). Inadequate immune response to HIV infection allows ongoing viral replication, driving continued immune activation in a self-sustaining cycle. Thus, patients with HIV infection are at increased risk of morbidity and mortality from a variety of pathological conditions / opportunistic infections (Affairs 2018) such as Tuberculosis, Hepatitis B and C, toxoplasmosis, Kaposi’s sarcoma, pneumonia, pulmonary candidiasis etc. and it has become increasingly clear that inflammation (endogenous and or derived from external stimuli such as cigarette smoking) further contributes to this increased risk.
Morbidity and Mortality:
Severe impairment of immune function is the defining feature of a HIV infection. It manifests itself in the form of accelerated cardiovascular, cerebrovascular, renal and bone disease. Atherogenesis facilitated by the virus also plays a part in organ dysfunction leading to premature death (Data Collection on Adverse Events of Anti et al. 2010; F. Onen and Turner Overton 2011) have reported that, although the decrease in mortality observed in HIV-positive populations in the post-cART era is much lower than that observed in the pre-cART era, AIDS-related illness were the most common cause of death. The tendency to engage in highly risky behavior such as smoking, alcohol and drug use was also observed to be associated with higher death outcomes especially when coupled with cerebrovascular diseases and non-AIDS illnesses. On the contrary, another study (Maartens et al. 2014) reported that AIDS was not the cause of death in half the people receiving antiretroviral therapy in high-income countries. Instead, non-AIDS-defining cancers (23·5%), cardiovascular disease (15·7%), and liver disease (14·1%) were major reasons for mortality. Though ART is able to arrest rapid immune decline, many impairments continue to persist, most prominently; inflammation. People accessing ART appear to be at an increased risk for developing certain non-AIDS illnesses such as Hypertension, Diabetes mellitus and insulin resistance, Cardiovascular disease, Pulmonary hypertension, Cancer, Osteopenia, osteoporosis, Liver failure, Kidney failure, Peripheral neuropathy, Frailty, Cognitive decline and dementia compared with non-HIV infected population. The risk is majorly attributed to patients with lower CD4 cell counts (Deeks and Phillips 2009).
Risk factors:
On a global level, the abuse of drugs and the risk of acquiring HIV/AIDS are essentially interrelated (Winstanley et al. 2006). Moreover, alcohol misuse, smoking and drug addiction in HIV infected people are also highly implicated in non-adherence and reduced response to the ART regimen, thereby leading to disease progression (Kumar et al. 2015) and eventually leading to non-AIDS events causing morbidity and mortality (Collaboration and Article 2010). The most commonly abused substances amongst the HIV infected population, viz. opioids, cocaine, morphine, methamphetamine, tobacco and alcohol increase the threat of acquiring a HIV infection through indulgence in high risk behavior. Furthermore, neurocognitive disorders are increasingly observed in HIV positive individuals with drug addictions. Opioid use promotes entry and replication of virus into immune cells thereby causing neuroinflammation. Morphine along with viral protein gp120 is reported to cause oxidative stress and damage to DNA. Abuse of cocaine leads to rapid AIDS progression which further exacerbates to neurodegenerative dysfunction. Major consequences of alcohol consumption for HIV infected individuals are reduced adherence to ART and viral proliferation. The main component of TS, nicotine impairs synaptic plasticity in neurons exposed to the virus (Atluri 2016). A significant statistic with regard to smoking is that, the rate of cigarette smoking is exceedingly high in HIV infected individuals (40–75%) when compared with the general population and is known to aggravate HIV pathogenesis (Ande et al. 2015; Pacek 2016).
RESULTS AND DISCUSSION
NeuroAIDS:
NeuroAIDS is a group of neurological disorders resulting from chronic HIV infection in the central nervous system (CNS). Its manifestation ranges from mild to moderate impairments involving cognition and motor function to full blown disability, retardation and paralysis of lower limbs. These disorders are broadly classified into several categories such as AIDS dementia complex (ADC), HIV-associated cognitive-motor complex (HIVCMC) and HIV associated neurocognitive disorders (HAND) (Shapshak et al. 2011). Studies have also shown that HIV patients have diminished working and prospective memory in addition to difficulties in processing emotional-conflicts (Thomas et al. 2013).
Etiology and Pathogenesis:
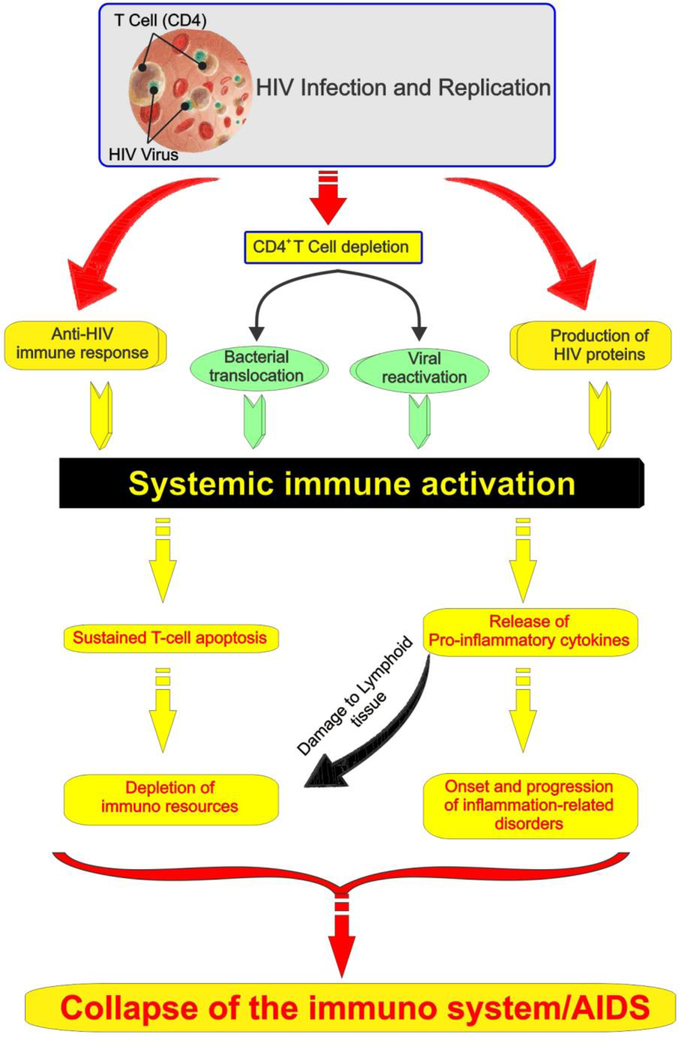
The Blood Brain Barrier (BBB) is a protective feature of the brain that prevents pathogens or unwanted substances from entering it. It is hypothesized that the invasion of BBB by the virus in an HIV infection causes dysregulation which is temporary at first, but its extent progresses with viral replication. Additionally, viral proteins and inflammatory cytokines augment the dysfunction (see also Fig. 1), thereby increasing viral permeability which has a permanent impact on BBB integrity highlighted by immune activation and immunosenescence (Strazza et al. 2011). One popular theory about the mechanism of HIV entry across the BBB is the ‘‘Trojan horse hypothesis’’ wherein it is hypothesized that the virus traffics across the BBB by infecting and commuting within CD4+ cells. As such, it is observed that around 40–60% of HIV infected individuals’ progress to NeuroAIDS despite receiving ART (Atluri et al. 2015).
Figure 1: Schematic model of HIV pathogenesis.
The model encompasses both prodromal factors (yellow) triggering immune activation and downstream consequences including immune depletion which concur in promoting HIV progression and activity.
It has been well established that NeuroAIDS is not caused by viral infection of neurons. Rather, the neurotoxic nature of the virus, viral proteins gp120, Tat and oxidative stress exerted by cytokines, chemicals and toxins secreted by the brain endothelial cells are processes which give rise to breakdown of BBB and consequently neurocognitive dysfunction (Banks et al. 2006; McRae 2016). Banks et all. have reported that viral protein Tat present in the blood, entered the BBB and effected brain dysfunction on buildup (Banks et al. 2005) while others (Zlokovic 2008) suggested that the loss of occludin and ZO-1 triggered by Tat could be the primary cause of BBB breakdown. Another study reported that injecting HIV-1 matrix protein p17 into the brains of mice impaired their cognitive and behavioral functionalities (Zeinolabediny et al. 2017).
Macrophages/microglia and a small proportion of astrocytes are the main cell types that HIV infects. Apart from the effects of viral proteins, disruption observed in the BBB was also due to “endothelial apoptosis, misguided astrocyte end feet, and dysregulation of lipoxygenase/cyclooxygenase, Large Conductance, Ca2+-Activated K+ (BKCa) channels, and ATP receptor activation within astrocytes.” Astrocyte participation in CNS dysfunction through gap junction mediated mechanism was also observed (Eugenin et al. 2011).
Treatment:
It is pertinent to note that, inhibition of viral replication and hence replication mediated BBB damage by HAART in patients leads to reduced neurocognitive dysfunction (Avison et al. 2004). However, the rate of incidence of HAND in HIV patients has not reduced significantly when compared with other AIDS related illnesses. The current ART has not achieved adequate potency to eradicate the virus within the CNS and specifically across the BBB since HIV replication is known to occur in astrocytes and microglia (Watkins and Treisman 2015). The diagnosis of NeuroAIDS is done by psychological function tests and recognizing clinical symptoms. In addition to lacking in specificity, these tests also fail to incorporate phenotypic variations. Currently, biomarkers are in the process of development to be able to better ascertain the scale of neurodegeneration due to HIV infection (Rahimian and He 2017). However, due to the dearth of stable biomarkers for NeuroAIDS, diagnosis and treatment is severely impeded (Pendyala et al. 2007).
Risk factors:
Blood Brain Barrier impairment in HIV infection of the CNS is further aggravated by drug abuse. Current ART cannot target viral reservoirs in the CNS due to physical barriers and efflux function. As mentioned before, drug abuse and HIV infection are inherently interconnected and studies suggest that progression and exacerbation of HIV infectivity occurs in light of drug abuse leading to increased mortality and morbidity (Zhang et al. 2015) (see Table 1)
Table 1.
Drugs of abuse associated with exacerbation of HIV infection
| No. | Drug type | Effect | Through | Article |
|---|---|---|---|---|
| 1 | Intravenous Drugs | BBB dysfunction ↑ | TNF-a | (35) |
| 2 | Cocaine, Methamphetamine, Morphine | Oxidative stress & BBB permeability ↑ | gp120 neurotoxicity | (36–39) |
| 3 | Morphine | Expression of Dendric cell markers ↑ | CD11c, macDC-SIGN, CD83 | (40) |
| 4 | Methamphetamine, N-Methyl-3,4-methylene-dioxyamphetamine (MDMA), cocaine, nicotine | BBB dysfunction ↑ | change in expression & conformation of TJ proteins, glial & astrocyte activation, inducing neuroinflammatory pathways | (41–43) |
| 5 | Methamphetamine | BBB dysfunction ↑ | Increasing viral load | (44) |
| 6 | Methamphetamine | BBB dysfunction ↑ | MMP level ↑ microglial activation, neuronal & synaptic injury | (45) |
| 7 | Cocaine | BBB leakage ↑ | Transmigration of leukocytes/monocytes into CNS | (46) |
| 8 | Cocaine | Monocyte adhesion and trafficking ↑ | activated leukocyte cell adhesion molecule (ALCAM) | (47) |
| 9 | Cocaine | Increased permeability of endothelial barrier ↑ | PDGF (platelet derived growth factor) implication in activating mitogen activated protein kinases & Egr-1 pathways | (48, 49) |
| 10 | Cocaine | PDGF-B expression | Notch-1 activation | (49) |
| 11 | Cannabinoid agonists | Decrease the permeability of BMVECs, prevent downregulation of TJ proteins such as ZO-1, claudin-5 & JAM-1. Inhibit transmigration of human monocytes across BBB & block BBB permeability in vivo | Inhibition of calcium influx by substance P & CB(2) receptor (CB2R) | (50–52) |
| 12 | Nicotine | Dysfunction in BMVECs & EPCs (Endothelial Progenitor Cells) | nicotine+gp120 = increased serum levels of ubiquitin C-terminal hydrolase 1 (UCHL1) | (53) |
| 13 | Nicotine | BBB integrity disruption | nicotine + protease inhibitors = oxidative stress in endothelial cells & multiple efflux transporters | (54) |
TS: Components and their adverse effects
Mathers et al. reported that, TS is probably the single most significant source of toxic chemical exposure to humans (Mathers and Loncar 2006). The World Health Organization forecasts that cigarettes will kill nearly 9 million people per year globally by the year 2030.”
The fundamental addictive substance in TS is nicotine. However, TS also contains thousands of constituents that have been reported to cause toxicity to cerebrovascular, cardiovascular and pulmonary systems within the body. These toxic effects are orchestrated by inflammation, oxidative stress and atherosclerosis. The harmful traits of nicotine have been emphasized in arrested neurodevelopment in children and neuropathology in Alzheimer’s disease. The addictiveness of nicotine stems from its action on nicotinic acetylcholine receptors (Swan and Lessov-Schlaggar 2007).
TS is a mix of gases and particles that can be divided into two phases – gaseous and particulate. The gaseous phase consists > 1015 free radicals/gram and particulate phase consists of > 1017 free radicals/puff. Particulate radicals within the body more than their gaseous counterparts (Ambrose and Barua 2004).
Cigarette smoke is known to contain close to 7000 compounds with varying effects and levels of toxicity that have not been completely understood. While vinyl chloride, hydrogen cyanide and arsenic are a hazard for the brain; acrolein, acetaldehyde, formaldehyde and cadmium endanger the pulmonary system with additional effects on the CNS. Ammonia, cresol, catechol, carbon monoxide, hydroquinone, lead, methyl ethyl ketone, nitric oxide, phenol, styrene, toluene and butane are other chemicals that affect the cardiovascular system or CNS (Bernhard et al. 2005; Fowles and Dybing 2003).
Others have also observed that the exposure to heavy metals present in TS poses a hazard for acquisition of Alzheimer’s disease through oxidative stress (Liu et al. 2006). Moreover, the incidence of ADC was found to be elevated in case of smokers (Burns et al. 1996).
Mechanism of TS toxicity:
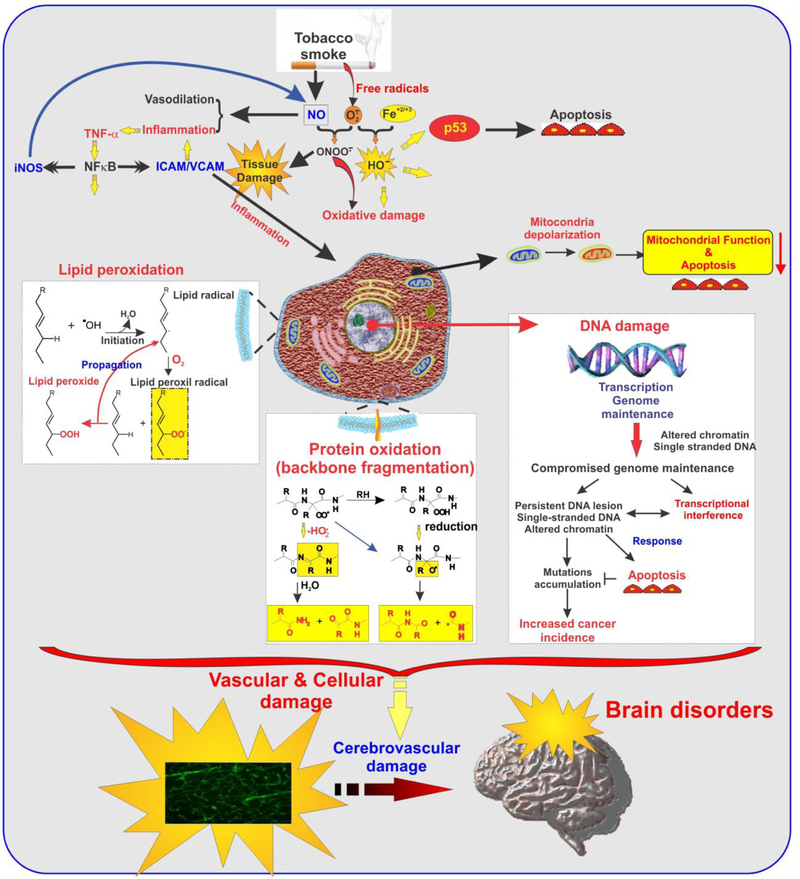
TS is known to cause endothelial dysfunction and damage to the vascular system (see Fig. 2). It is an inflammatory agent that causes oxidative stress thereby causing BBB impairment and can lead to a state of coagulation in blood which can be especially dangerous to the cerebrovascular system in the presence of other factors/infectious agents that affect BBB (Hossain et al. 2009; Kaisar et al. 2015; Kaisar et al. 2018; Kaisar et al. 2017; Mazzone et al. 2010). Furthermore, nicotine can cause absence of immune response in T cells through activation of protein tyrosine kinases (PTKs) and IP3-sensitive Ca21 stores in T cells (Kalra et al. 2000).
Figure 2: TS-induced cellular inflammatory responses and oxidative damage.
Schematic representation of multiple pathways by which chronic exposure to TS can induce cellular damage and inflammation, thus affecting the cerebrovascular system including BBB integrity and immune responses.
TS contains Reactive Oxygen Species (ROS) in the form of free oxygen radicals or reactive anions. Their interaction with molecules containing oxygen atoms gives rise to free radical formation. Accumulation of ROS leads to a) Increase in oxidants b) decrease in antioxidant function and c) Inability to repair damage from oxidation. Superoxide dismutase (SOD), catalase, glutathione (GSH) peroxidase, ascorbic acid (vitamin C), and α-tocopherol (vitamin E) repair oxidative damage in normal conditions (Hossain et al. 2009; Kaisar et al. 2015; Naik et al. 2015).
High levels of superoxide, hydroxyl radical, hydrogen peroxide, and peroxynitrite are generated by the burning of cigarettes leading to exposure of endothelial surfaces to ROS (Banzet et al. 1999; Colles et al. 2001; Peluffo et al. 2009; Raij et al. 2001). The damage caused by ROS-mediated interactions with macromolecules (Circu and Aw 2010) includes lipoperoxidation of polyunsaturated fatty acids in membrane lipids, protein oxidation, DNA strand breakage (Chen et al. 2004; DeMarini 2004; Pryor et al. 1998), RNA oxidation, mitochondrial depolarization and apoptosis. In addition mutations to nuclear protein p53 and DNA damage due to carcinogens also occurs (Pfeifer et al. 2002). Additional studies have reported that supplementing antioxidants protects from the oxidative harm caused by TS to some extent, thereby giving credence to the theory of ROS induced toxicity of TS. TS constituents trigger a complex pro-inflammatory response by recruiting leukocytes to the site of inflammation via cytokine signaling (such as IL-1β and TNF-α), matrix metalloproteinase upregulation (e.g., MMP-1 and MMP-9), and by promoting the adhesion and binding of monocytes to the endothelial wall of blood vessels thereby promoting atherosclerosis (Nordskog et al. 2003). Increased expression of selectins, VCAM-1, and ICAM-1 is seen with the activation of endothelial cells which causes monocytes to adhere to the inside walls of blood vessels (McMullen et al. 2000). Increased levels of WBCs primary neutrophils and monocytes are seen in smokers (Masubuchi et al. 1998) especially neutrophils which secrete free radicals, elastase and collagenase trigger immune response and cause EC injury. Smokers are more vulnerable to inflammatory neuropathologies caused by viruses and bacteria in comparison to non-smokers (Stampfli and Anderson 2009) despite having an increased white blood cell count. This may be due to weakening of the host immune system in addition to BBB impairment thereby assisting the pathogenesis of bacterial / viral mediated neurological disorders (Chen et al. 2002; Iles et al. 2001; Robbins et al. 2006). Thrombosis is another risk that is posed to smokers. The same is mediated by platelet activation which is characterized by increased levels of platelet activating factor, Von Willebrand factor, catecholamines, and thromboxane which seriously endangers brain microvasculature in the absence of vascular tone regulatory mechanisms (Girdhar et al. 2008; Togna et al. 2008). Cigarette smoking can also cause increased levels of C-reactive protein (CRP) that promotes endothelial impairment by lowering production of Nitric Oxide (NO) and reducing its activity by upregulation of nuclear factor ҡB (NF-ҡβ) signaling in ECs while attenuating endothelial progenitor cell survival and differentiation. Apart from the harmful effects of Nicotine on BBB impairment which are already well established (Abbruscato et al. 2002; Hawkins et al. 2004; Kaisar et al. 2017), ROS promotes oxidation of low density lipoprotein that can cause cell injury and atherosclerotic lesions (Colles et al. 2001; Howard et al. 1998). ROS causes breakdown of BBB by modifying tight junctions, activation of matrix metalloproteinases and disintegration of basal membrane (Pun et al. 2009). ROS and Nicotine display a synergistic effect in causing BBB impairment and dysfunction (Yin et al. 2008). Exposure to TS causes an increased expression of inflammatory response genes such as chemokines, pro-inflammatory cytokines and STAT3 (Hossain et al. 2009).
Impact of TS on HIV infectivity/AIDS progression:
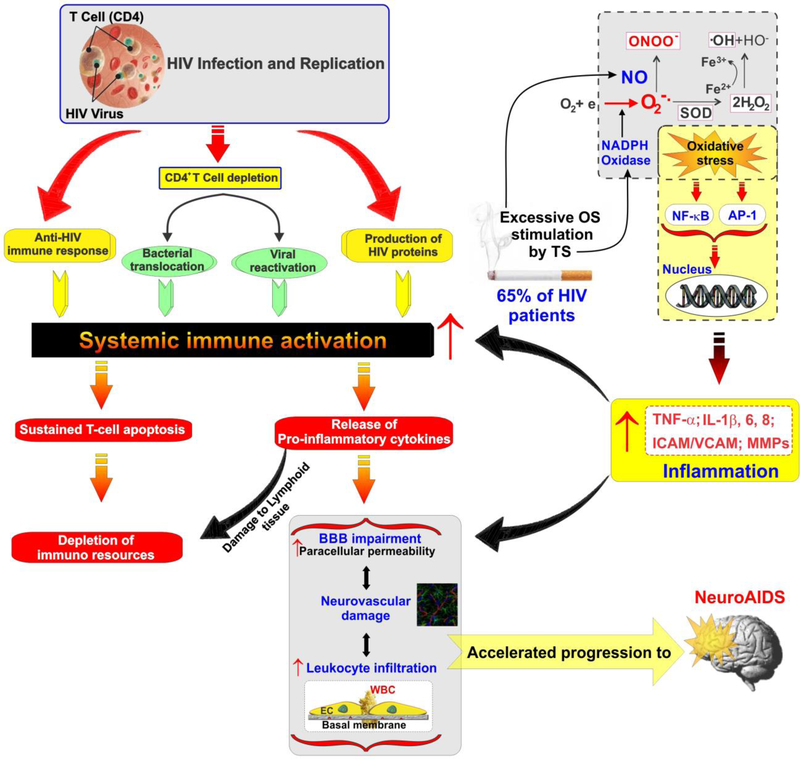
TS and its harmful effects in the context of HIV/AIDS is an area that has witnessed conflicting reports regarding disease progression. Though it has shown that a nicotine concentration of 300 μM increased HIV-1 expression (Abbud et al. 1995; Taylor et al. 1986) using in vitro alveolar macrophages and microglial cells rock et al. have demonstrated that concentrations of nicotine closer to blood plasma levels of smokers (0.23 μM) do not have any effect on HIV expression (Rock et al. 2008). Similarly, others have reported that oxidative stress induced by TS can result in disease progression (Boelaert et al. 1996; Miller et al. 1997). However, blood samples obtained from HIV smokers and non-smokers were found to show not much difference in oxidative stress or antioxidant capacity (Cole et al. 2005; Earla et al. 2014) although the metabolism of nicotine was found to be increased in HIV infected individuals who smoked. Further, others have also reported that due to metabolizing activity of cytochrome P450 enzymes on nicotine and antiretroviral drugs, these may not achieve therapeutic levels in the plasma of HIV patients due to elevated levels of ROS thereby augmenting viral replication (Ande et al. 2013; Pal et al. 2011). Mitochondrial toxicity can result from both antiretroviral drugs and HIV infection (Lopez et al. 2004) leading to oxidative stress, telomere shortening and thereby immunosenescence (Shammas 2011; Zanet et al. 2014). Replication of the virus is augmented by the nicotine and other TS components by activation of cellular gene expression (Rock et al. 2008; Zhao et al. 2010). Nicotine induces oxidative stress in macrophages by CYP2A6 metabolism and is involved in viral replication (Jin et al. 2012). In addition to reduced HAART function (Feldman et al. 2006) HIV infected smokers were found to be more prone to pneumonia, oral candidiasis, hairy leukoplakia, vascular diseases, AIDS-related malignancies and neuropathy (Conley et al. 1996; Elzi et al. 2006). Peroxynitrite resulting from the reaction between endogenous superoxide and NO (released by TS) can trigger the activation of the NF-kB pathway in lymphocytes (Hasnis et al. 2007). In addition, when mRNA microarray analysis of TS exposed human T cells was carried out, various genes linked with HIV infection were found to be upregulated while those associated with immune functions were down regulated (see also Table 2 and Fig. 3). These studies revealed that IL-8 mRNA, the activator protein 1 (AP-1) components c-Jun, High FOS-like antigen 1 (FOSL1) and 2 (FOSL2) levels, chemotaxis mechanisms through interleukin-8 (IL-8), transforming growth factor type 1 (TGF1) in microglial cells preincubated with nicotine were up-regulated whereas other cytokines and chemokines, the expression of the NF-B component p50 and IB, innate immunity, and transforming growth factor (TGF) pathway were downregulated (Zhao et al. 2010).
Table 2.
TS-associated biochemical alteration which can positively impact HIV progression
| Target | Expression | Effect | Ref. |
|---|---|---|---|
| TXNIP | ↓ | Tobacco smoke inhibits TXNIP and increases reducing function of Trx. Higher levels of Trx is found in the plasma of HIV infected individuals. | (110, 111) |
| Thioredoxin-related transmembrane protein 1 | ↑ | Responsible for downregulation of CD4 facilitated by HIV | (112) |
| TAX 1 binding protein 1 (TAX1BP1) |
↑ | Binds to HIV Long Terminal Repeats indirectly | (113) |
| Calmodulin1 | ↑ | Enhances apoptosis by binding to HIV gp160 | (114) |
| TGF-β1 | ↓ | Enhancement of viral expression mediated by nicotine | (94) |
| HSP90 beta 1 | ↑ | HIV Tat transactivation mediated by positive transcription elongation factor b (P-TEFb) | (115) |
| CD59 (protectin) | ↑ | Protects virus from complement-mediated destruction | (116) |
| NF-kB signaling pathway | ↑ | Oxidative stress and lipid peroxidation caused by tobacco smoke and its effect on virus | (109) |
| Phospholipid hydroperoxide glutathione peroxidase (PHGPX) | ↓ | protects membrane lipids from peroxidation | (117) |
| Antioxidant potential of TS | ↑ | Strengthening of viral infectivity by TS mediated protection against degradation & oxidative action | (104) |
Figure 3: Model of HIV pathogenesis and progression incorporating the enhancing activity of chronic smoking.
Note how the oxidative stress and pro-inflammatory activity promoted by tobacco smoking can positively affect multiple aspects of HIV infection and progression to NeuroAIDS.
Tobacco smoking in HIV infected population: Reports and studies
Though smoking is highly prevalent amongst the HIV infected population, it remains to be seen whether it has an additive effect in the progression of HIV infection to AIDS and other subsequent ailments such as NeuroAIDS. Though, there isn’t a direct relationship between smoking and HIV acquisition, there seems to be enhanced risk for susceptibility to several comorbidities. One major finding was that, though the CD4 cell counts in HIV infected smokers was higher initially, the rates of decline in CD4 counts were much faster than non-smokers. Due to the success of ART for treatment of HIV/AIDS, factors such as smoking are expected to have greater influence on morbidity and mortality (Reynolds 2009). The susceptibility of HIV infected individuals to pulmonary infections and malignancies is also abnormally heightened. Moreover, response to ART is reduced to a great extent giving rise to cardio and cerebrovascular diseases and other non-AIDS illnesses (Rahmanian et al. 2011). As such, TS and HIV infection may bring about a synergistic effect in the context of persistent inflammatory state when compared to smokers who are not infected by HIV (Shirley et al. 2013) (see also Fig. 3).
CONCLUSION
The rates of AIDS related illnesses and death in people infected with HIV have reduced substantially with the advent of highly active and combination antiretroviral therapy. However, this has brought into sharp contrast the incidence of several characteristically ‘Non-AIDS’ diseases due to increased life expectancy amongst the HIV patients. One such condition is NeuroAIDS which is categorized as a group of neurological disorders caused primarily by HIV-mediated damage to the central and peripheral nervous systems. Its clinical manifestation ranges from mild to moderate impairments involving cognition and motor function to full blown disability, retardation and paralysis of lower limbs. Given the patients depleted immune condition, the use and abuse of drug and addictive substances further deteriorates their overall health.
In this contest, tobacco smoking is highly prevalent in HIV patients when compared to the general population. The oxidative damage and inflammatory stress caused by chronic smoking on the cerebrovascular system have been well established. There is however, a paucity of studies so far looking at the effects of TS-mediated enhancement of viral infectivity leading to progression of neurological dysfunction. We believe that, in light of the association between large numbers of HIV infected smokers progressing to NeuroAIDS, the discovery of common biomarkers for both NeuroAIDS and smoking can reveal novel aspects of synergy between the two. This has the potential for the development of better treatments and outcomes while putting additional emphasis on developing and promoting smoking cessation programs for HIV infected individuals.
Highlights:
NeuroAIDS identifies a group of neurological disorders caused primarily by HIV-mediated damage to the central and peripheral nervous systems.
Tobacco smoking as a risk factor for the progression of HIV into NeuroAIDS and related neurological impairments.
Smokers are more susceptible to viral and bacterial inflammatory neuropathologies than non-smokers.
TS may bring about a synergistic effect in the context of persistent inflammatory state and cerebrovascular damage which facilitate HIV infection and progression to NeuroAIDS when compared to smokers.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health/ National Institute on Drug Abuse 2R01-DA029121-01A1 to Dr. Luca Cucullo.
Abbreviations:
- AIDS
Acquired immunodeficiency syndrome
- AP-1
Activator protein 1
- ART
Antiretroviral Therapy
- BBB
Blood-Brain Barrier
- BKCa
Large Conductance, Ca2+-Activated K+ Channels
- IL-8
Interleukin 8
- FOSL1
High FOS-like antigen 1
- FOSL2
High FOS-like antigen 2
- CNS
Central Nervous System
- OS
Oxidative stress
- TS
TS
- AIDS
Acquired immunodeficiency syndrome
- HIV
Human immunodeficiency virus
- HAART
Highly active antiretroviral therapy
- cART
Combined antiretroviral therapy
- ADC
AIDS dementia complex
- HIVCMC
HIV-associated cognitive-motor complex
- HAND
HIV associated neurocognitive disorders
- CRP
C-reactive protein
- ROS
Reactive oxygen species
- PTKs
Protein tyrosine kinases
- TGF1
Transforming growth factor type 1
Footnotes
Competing Interests:
The authors declare no competing interests.
Ethical approval:
This article does not contain any studies with human participants or animals performed by the author.
References
- Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP (2002) Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells J Pharm Sci 91:2525–2538 doi: 10.1002/jps.10256 [DOI] [PubMed] [Google Scholar]
- Abbud RA, Finegan CK, Guay LA, Rich EA (1995) Enhanced production of human immunodeficiency virus type 1 by in vitro-infected alveolar macrophages from otherwise healthy cigarette smokers J Infect Dis 172:859–863 [DOI] [PubMed] [Google Scholar]
- Abuse NIoD (2012) How Does Drug Abuse Affect the HIV Epidemic?
- Affairs USDoV (2018) AIDS-defining illnesses
- Ambrose JA, Barua RS (2004) The pathophysiology of cigarette smoking and cardiovascular disease: An update vol 43. doi: 10.1016/j.jacc.2003.12.047 [DOI] [PubMed] [Google Scholar]
- Ande A et al. (2015) Effect of mild-to-moderate smoking on viral load, cytokines, oxidative stress, and cytochrome P450 enzymes in HIV-infected individuals PLoS ONE 10 doi: 10.1371/journal.pone.0122402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande A, McArthur C, Kumar A, Kumar S (2013) Tobacco smoking effect on HIV-1 pathogenesis: role of cytochrome P450 isozymes Expert Opin Drug Metab Toxicol 9:1453–1464 doi: 10.1517/17425255.2013.816285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri VSR (2016) Editorial : HIV and Illicit Drugs of Abuse Frontiers in Microbiology doi: 10.1089/aid.2009.0211 [DOI] [PMC free article] [PubMed]
- Atluri VSR, Hidalgo M, Samikkannu T, Kurapati KRV, Jayant RD, Sagar V, Nair MPN (2015) Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update Frontiers in Cellular Neuroscience 9:1–10 doi: 10.3389/fncel.2015.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Greenberg RN, Berger JR (2004) Neuroimaging correlates of HIV-associated BBB compromise vol 157. doi: 10.1016/j.jneuroim.2004.08.025 [DOI] [PubMed] [Google Scholar]
- Banks WA, Ercal N, Otamis Price T (2006) The Blood-Brain Barrier in NeuroAIDS Current HIV Research 4:0–0 [DOI] [PubMed] [Google Scholar]
- Banks WA, Robinson SM, Nath A (2005) Permeability of the blood-brain barrier to HIV-1 Tat Experimental Neurology 193:218–227 doi: 10.1016/j.expneurol.2004.11.019 [DOI] [PubMed] [Google Scholar]
- Banzet N, Francois D, Polla BS (1999) TS induces mitochondrial depolarization along with cell death: effects of antioxidants Redox Rep 4:229–236 doi: 10.1179/135100099101534945 [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F, Ross AL, Delfraissy JF (2013) Past, present and future: 30 years of HIV research vol 11. doi: 10.1038/nrmicro3132 [DOI] [PubMed] [Google Scholar]
- Bernhard D, Rossmann A, Wick G (2005) Metals in cigarette smoke IUBMB Life 57:805–809 doi: 10.1080/15216540500459667 [DOI] [PubMed] [Google Scholar]
- Boelaert JR, Piette J, Weinberg GA, Sappey C, Weinberg ED (1996) Iron and oxidative stress as a mechanism for the enhanced production of human immunodeficiency virus by alveolar macrophages from otherwise healthy cigarette smokers J Infect Dis 173:1045–1047 [DOI] [PubMed] [Google Scholar]
- Burns DN et al. (1996) Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS J Acquir Immune Defic Syndr Hum Retrovirol 13:374–383 [DOI] [PubMed] [Google Scholar]
- Chen HW, Chien ML, Chaung YH, Lii CK, Wang TS (2004) Extracts from cigarette smoke induce DNA damage and cell adhesion molecule expression through different pathways Chem Biol Interact 150:233–241 doi: 10.1016/j.cbi.2004.09.014 [DOI] [PubMed] [Google Scholar]
- Chen YH, Chen SH, Jong A, Zhou ZY, Li W, Suzuki K, Huang SH (2002) Enhanced Escherichia coli invasion of human brain microvascular endothelial cells is associated with alternations in cytoskeleton induced by nicotine Cell Microbiol 4:503–514 [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis Free Radic Biol Med 48:749–762 doi: 10.1016/j.freeradbiomed.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Shaw GM, McMichael AJ, Haynes BF (2011) Acute HIV-1 Infection New England Journal of Medicine 364:1943–1954 doi: 10.1056/NEJMra1011874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SB, Langkamp-Henken B, Bender BS, Findley K, Herrlinger-Garcia KA, Uphold CR (2005) Oxidative stress and antioxidant capacity in smoking and nonsmoking men with HIV/acquired immunodeficiency syndrome Nutr Clin Pract 20:662–667 doi: 10.1177/0115426505020006662 [DOI] [PubMed] [Google Scholar]
- Collaboration ATC, Article M (2010) Causes of Death in HIV‐1–Infected Patients Treated with Antiretroviral Therapy, 1996–2006: Collaborative Analysis of 13 HIV Cohort Studies Clinical Infectious Diseases 50:1387–1396 doi: 10.1086/652283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles SM, Maxson JM, Carlson SG, Chisolm GM (2001) Oxidized LDL-induced injury and apoptosis in atherosclerosis. Potential roles for oxysterols Trends Cardiovasc Med 11:131–138 [DOI] [PubMed] [Google Scholar]
- Conley LJ, Bush TJ, Buchbinder SP, Penley KA, Judson FN, Holmberg SD (1996) The association between cigarette smoking and selected HIV-related medical conditions AIDS 10:1121–1126 [PubMed] [Google Scholar]
- Data Collection on Adverse Events of Anti HIVdSG et al. (2010) Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study AIDS 24:1537–1548 doi: 10.1097/QAD.0b013e32833a0918 [DOI] [PubMed] [Google Scholar]
- Deeks SG, Phillips AN (2009) HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity BMJ (Online) 338:288–292 doi: 10.1136/bmj.a3172 [DOI] [PubMed] [Google Scholar]
- DeMarini DM (2004) Genotoxicity of TS and TS condensate: a review Mutat Res 567:447–474 doi: 10.1016/j.mrrev.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Earla R, Ande A, McArthur C, Kumar A, Kumar S (2014) Enhanced nicotine metabolism in HIV-1-positive smokers compared with HIV-negative smokers: simultaneous determination of nicotine and its four metabolites in their plasma using a simple and sensitive electrospray ionization liquid chromatography-tandem mass spectrometry technique Drug Metab Dispos 42:282–293 doi: 10.1124/dmd.113.055186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzi L et al. (2006) A smoking cessation programme in HIV-infected individuals: a pilot study Antivir Ther 11:787–795 [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC, Berman JW (2011) Human Immunodeficiency Virus Infection of Human Astrocytes Disrupts Blood-Brain Barrier Integrity by a Gap Junction-Dependent Mechanism Journal of Neuroscience 31:9456–9465 doi: 10.1523/JNEUROSCI.1460-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen NF, Turner Overton E (2011) A Review of Premature Frailty in HIV-Infected Persons; Another Manifestation of HIV-Related Accelerated Aging Current Aging Science 4:33–41 doi: 10.2174/1874609811104010033 [DOI] [PubMed] [Google Scholar]
- Feldman JG et al. (2006) Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study Am J Public Health 96:1060–1065 doi: 10.2105/AJPH.2005.062745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles J, Dybing E (2003) Chemical Constituents of Cigarette Smoke Tobacco Control:424–431 doi: 10.1136/tc.12.4.424 [DOI] [PMC free article] [PubMed]
- Girdhar G, Xu S, Jesty J, Bluestein D (2008) In vitro model of platelet-endothelial activation due to cigarette smoke under cardiovascular circulation conditions Ann Biomed Eng 36:1142–1151 doi: 10.1007/s10439-008-9503-2 [DOI] [PubMed] [Google Scholar]
- Hasnis E, Bar-Shai M, Burbea Z, Reznicka Z (2007) Mechanisms underlying cigarette smoke-induced NF-kappaB activation in human lymphocytes: the role of reactive nitrogen species. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 58 Suppl 5:275–287 [PubMed] [Google Scholar]
- Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP (2004) Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution Brain Res 1027:48–58 doi: 10.1016/j.brainres.2004.08.043 [DOI] [PubMed] [Google Scholar]
- Hernandez-Vargas EA, Middleton RH (2013) Modeling the three stages in HIV infection Journal of Theoretical Biology doi: 10.1016/j.jtbi.2012.11.028 [DOI] [PubMed]
- Hossain M et al. (2009) TS: A critical etiological factor for vascular impairment at the blood-brain barrier Brain Research 1287:192–205 doi: 10.1016/j.brainres.2009.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G et al. (1998) Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study JAMA 279:119–124 [DOI] [PubMed] [Google Scholar]
- Iles K, Poplawski NK, Couper RT (2001) Passive exposure to TS and bacterial meningitis in children J Paediatr Child Health 37:388–391 [DOI] [PubMed] [Google Scholar]
- Jin M et al. (2012) A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV + smokers J Neuroimmune Pharmacol 7:289–299 doi: 10.1007/s11481-011-9283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MA, Prasad S, Cucullo L (2015) Protecting the BBB endothelium against cigarette smoke-induced oxidative stress using popular antioxidants: Are they really beneficial? Brain Res 1627:90–100 doi: 10.1016/j.brainres.2015.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MA, Sivandzade F, Bhalerao A, Cucullo L (2018) Conventional and electronic cigarettes dysregulate the expression of iron transporters and detoxifying enzymes at the brain vascular endothelium: In vivo evidence of a gender-specific cellular response to chronic cigarette smoke exposure Neurosci Lett 682:1–9 doi: 10.1016/j.neulet.2018.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MA et al. (2017) Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox Biol 13:353–362 doi: 10.1016/j.redox.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra R, Singh SP, Savage SM, Finch GL, Sopori ML (2000) Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca(2+) stores. The Journal of pharmacology and experimental therapeutics 293:166–171 [PubMed] [Google Scholar]
- Kumar S, Rao P, Earla R, Kumar A, City K, City K (2015) Drug–drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems Expert Opinion on Drug Metabolism and Toxicology 11:343–355 doi: 10.1517/17425255.2015.996546.Drug [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HC (2010) Pathogenesis of HIV infection: total CD4+ T-cell pool, immune activation, and inflammation. Topics in HIV medicine : a publication of the International AIDS Society, USA 18:2–6 [PubMed] [Google Scholar]
- Liu G et al. (2006) Metal exposure and Alzheimer’s pathogenesis doi: 10.1016/j.jsb.2005.12.011 [DOI] [PubMed]
- Lopez S et al. (2004) Mitochondrial effects of antiretroviral therapies in asymptomatic patients Antivir Ther 9:47–55 [PubMed] [Google Scholar]
- Maartens G, Celum C, Lewin SR (2014) HIV infection: Epidemiology, pathogenesis, treatment, and prevention The Lancet 384:258–271 doi: 10.1016/S0140-6736(14)60164-1 [DOI] [PubMed] [Google Scholar]
- Masubuchi T et al. (1998) Smoke extract stimulates lung epithelial cells to release neutrophil and monocyte chemotactic activity Am J Pathol 153:1903–1912 doi: 10.1016/S0002-9440(10)65704-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030 PLoS Medicine 3:2011–2030 doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P, Tierney W, Hossain M, Puvenna V, Janigro D, Cucullo L (2010) Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: Expanding the awareness of smoking toxicity in an underappreciated area vol 7. doi: 10.3390/ijerph7124111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen CB, Fleming E, Clarke G, Armstrong MA (2000) The role of reactive oxygen intermediates in the regulation of cytokine-induced ICAM-1 surface expression on endothelial cells Mol Cell Biol Res Commun 3:231–237 doi: 10.1006/mcbr.2000.0216 [DOI] [PubMed] [Google Scholar]
- McRae M (2016) HIV and viral protein effects on the blood brain barrier Tissue Barriers 4:e1143543 doi: 10.1080/21688370.2016.1143543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ER 3rd, Appel LJ, Jiang L, Risby TH (1997) Association between cigarette smoking and lipid peroxidation in a controlled feeding study Circulation 96:1097–1101 [DOI] [PubMed] [Google Scholar]
- Naik P, Sajja RK, Prasad S, Cucullo L (2015) Effect of full flavor and denicotinized cigarettes exposure on the brain microvascular endothelium: a microarray-based gene expression study using a human immortalized BBB endothelial cell line BMC Neurosci 16:38 doi: 10.1186/s12868-015-0173-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordskog BK, Blixt AD, Morgan WT, Fields WR, Hellmann GM (2003) Matrix-degrading and pro-inflammatory changes in human vascular endothelial cells exposed to cigarette smoke condensate Cardiovasc Toxicol 3:101–117 [DOI] [PubMed] [Google Scholar]
- Pacek LRC, Patricia A. (2016) Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living with HIV 12:87–92 doi: 10.1016/j.coviro.2015.09.001.Human [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Kwatra D, Minocha M, Paturi DK, Budda B, Mitra AK (2011) Efflux transporters-and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals Life Sci 88:959–971 doi: 10.1016/j.lfs.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo G, Calcerrada P, Piacenza L, Pizzano N, Radi R (2009) Superoxide-mediated inactivation of nitric oxide and peroxynitrite formation by TS in vascular endothelium: studies in cultured cells and smokers Am J Physiol Heart Circ Physiol 296:H1781–1792 doi: 10.1152/ajpheart.00930.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala G, Want EJ, Webb W, Siuzdak G, Fox HS (2007) Biomarkers for neuroAIDS: The widening scope of metabolomics vol 2. doi: 10.1007/s11481-006-9041-3 [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P (2002) TS carcinogens, DNA damage and p53 mutations in smoking-associated cancers Oncogene 21:7435–7451 doi: 10.1038/sj.onc.1205803 [DOI] [PubMed] [Google Scholar]
- Prevention CfDCa (2018) About HIV / AIDS Hiv/Aids:2–3
- Pryor WA, Stone K, Zang LY, Bermudez E (1998) Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage Chem Res Toxicol 11:441–448 doi: 10.1021/tx970159y [DOI] [PubMed] [Google Scholar]
- Pun PB, Lu J, Moochhala S (2009) Involvement of ROS in BBB dysfunction Free Radic Res 43:348–364 doi: 10.1080/10715760902751902 [DOI] [PubMed] [Google Scholar]
- Rahimian P, He JJ (2017) HIV/neuroAIDS biomarkers Progress in Neurobiology 157:117–132 doi: 10.1016/j.pneurobio.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P (2011) Cigarette Smoking in the HIV-Infected Population Proceedings of the American Thoracic Society 8:313–319 doi: 10.1513/pats.201009-058WR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij L, DeMaster EG, Jaimes EA (2001) Cigarette smoke-induced endothelium dysfunction: role of superoxide anion J Hypertens 19:891–897 [DOI] [PubMed] [Google Scholar]
- Reynolds NR (2009) Cigarette smoking and HIV: more evidence for action. [Review] [91 refs] AIDS Education and Prevention 21:Suppl-21 doi: 10.1521/aeap.2009.21.3_supp.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CS, Bauer CM, Vujicic N, Gaschler GJ, Lichty BD, Brown EG, Stampfli MR (2006) Cigarette smoke impacts immune inflammatory responses to influenza in mice Am J Respir Crit Care Med 174:1342–1351 doi: 10.1164/rccm.200604-561OC [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Aravalli RN, Hu S, Sheng WS, Peterson PK (2008) Potentiation of HIV-1 expression in microglial cells by nicotine: Involvement of transforming growth factor-β1 Journal of Neuroimmune Pharmacology 3:143–149 doi: 10.1007/s11481-007-9098-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammas MA (2011) Telomeres, lifestyle, cancer, and aging Curr Opin Clin Nutr Metab Care 14:28–34 doi: 10.1097/MCO.0b013e32834121b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapshak P et al. (2011) Editorial NeuroAIDS review vol 25. doi: 10.1097/QAD.0b013e328340fd42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Hahn BH (2011) Origins of HIV and the AIDS pandemic Cold Spring Harbor Perspective in Medicine doi: 10.1101/cshperspect.a006841 a006841 [pii] [DOI] [PMC free article] [PubMed]
- Shirley DK, Kaner RJ, Glesby MJ (2013) Effects of smoking on non-AIDS-related morbidity in HIV-infected patients Clinical Infectious Diseases 57:275–282 doi: 10.1093/cid/cit207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfli MR, Anderson GP (2009) How cigarette smoke skews immune responses to promote infection, lung disease and cancer Nat Rev Immunol 9:377–384 doi: 10.1038/nri2530 [DOI] [PubMed] [Google Scholar]
- Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR (2011) Breaking down the barrier: The effects of HIV-1 on the blood-brain barrier vol 1399. doi: 10.1016/j.brainres.2011.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN (2007) The effects of TS and nicotine on cognition and the brain vol 17. doi: 10.1007/s11065-007-9035-9 [DOI] [PubMed] [Google Scholar]
- Taylor RG, Woodman G, Clarke SW (1986) Plasma nicotine concentration and the white blood cell count in smokers Thorax 41:407–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM, Snyder AZ, Ances BM (2013) Pathways to neurodegeneration: Effects of HIV and aging on resting-state functional connectivity Neurology 80:1186–1193 doi: 10.1212/WNL.0b013e318288792b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togna AR, Latina V, Orlando R, Togna GI (2008) Cigarette smoke inhibits adenine nucleotide hydrolysis by human platelets Platelets 19:537–542 doi: 10.1080/09537100802272626 [DOI] [PubMed] [Google Scholar]
- UNAIDS (2017) Fact sheet -Latest global and regional statistics on the status of the AIDS epidemic. Unaids:8 doi:2017 [Google Scholar]
- Watkins CC, Treisman GJ (2015) Cognitive impairment in patients with Aids – Prevalence and severity HIV/AIDS -Research and Palliative Care 7:35–47 doi: 10.2147/HIV.S39665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Gust SW, Strathdee SA (2006) Drug abuse and HIV/AIDS: international research lessons and imperatives Drug and Alcohol Dependence 82:1–5 doi: 10.1016/S0376-8716(06)80001-3 [DOI] [PubMed] [Google Scholar]
- Yin W, Ghebrehiwet B, Weksler B, Peerschke EI (2008) Regulated complement deposition on the surface of human endothelial cells: effect of TS and shear stress Thromb Res 122:221–228 doi: 10.1016/j.thromres.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanet DL et al. (2014) Association between short leukocyte telomere length and HIV infection in a cohort study: No evidence of a relationship with antiretroviral therapy Clin Infect Dis 58:1322–1332 doi: 10.1093/cid/ciu051 [DOI] [PubMed] [Google Scholar]
- Zeinolabediny Y et al. (2017) HIV-1 matrix protein p17 misfolding forms toxic amyloidogenic assemblies that induce neurocognitive disorders Scientific Reports 7 doi: 10.1038/s41598-017-10875-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YL, Ouyang YB, Liu LG, Chen DX (2015) Blood-brain barrier and neuro-AIDS European Review for Medical and Pharmacological Sciences 19:4927–4939 [PubMed] [Google Scholar]
- Zhao L, Li F, Zhang Y, Elbourkadi N, Wang Z, Yu C, Taylor EW (2010) Mechanisms and genes involved in enhancement of HIV infectivity by TS Toxicology 278:242–248 doi: 10.1016/j.tox.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2008) The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders Neuron 57:178–201 doi: 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]