Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, in December 2020 and coronavirus disease 19 (COVID-19) was later announced as pandemic by the World Health Organization (WHO). Since then, several studies have been conducted on the prevention and treatment of COVID-19 by potential vaccines and drugs. Although, the governments and global population have been attracted by some vaccine production projects, the presence of SARS-CoV-2-specific antiviral drugs would be an urge necessity in parallel with the efficient preventive vaccines. Various nonspecific drugs produced previously against other bacterial, viral, and parasite infections were recently evaluated for treating patients with COVID-19. In addition to therapeutic properties of these anti-COVID-19 compounds, some adverse effects were observed in different human organs as well. Not only several attentions were paid to antiviral therapy and treatment of COVID-19, but also nanomedicine, immunotherapy, and cell therapy were conducted against this viral infection. In this review study, we planned to introduce the present and potential future treatment strategies against COVID-19 and define the advantages and disadvantages of each treatment strategy.
Keywords: SARS-CoV-2; COVID-19; Antiviral therapy, Immunotherapy; Cell therapy; Nanomedicine; Antivirals
Graphical Abstract
1. Introduction
Human coronavirus 229E (HCoV-229E) (classified in the genus Alphacoronavirus) and HCoV-OC43 (Betacoronavirus lineage 2a member) identified in the 1960s. Then in March 2003, severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) (Betacoronavirus lineage 2b member) appeared, while human coronavirus NL63 (HCoV-NL63) (Alphacoronavirus lineage 1b member) characterized in 2004, HCoV-HKU1 (Betacoronavirus lineage 2a member) discovered in 2005, and at last Middle East respiratory syndrome coronavirus (MERS-CoV) emerged in 2012 (Betacoronavirus lineage 2c member). Further experimental and clinical studies revealed that CoVs including SARS-CoVs and MERS-CoV are the threat agents for great public health [1]. Since the sudden outbreak of coronavirus disease 2019 (COVID-19) by severe acute respiratory syndrome coronavirus (SARS-CoV-2) as the causative agent in Wuhan, China, in December 2020, the disease was spread rapidly around the world [2]. Immediately and on March 11, 2020, the World Health Organization (WHO) declared the COVID-19 outbreak as a pandemic viral infection [3]. SARS-CoV-2, is the seventh human coronavirus described to date as the etiological agent of COVID-19 characterized by a pulmonary infection in humans [4]. Within this regard, evidence was rapidly reported that the patients are being affected by an infection with a novel betacoronavirus, which was nominated as SARS-CoV-2 [5], [6], [7], which represents a pandemic threat to global public health [7]. The sequence of novel SARS-CoV-2 has been shown to be 75–80% identical to SARS-CoV-1% and 40% identical to MERS-CoV [8]. SARS-CoV-2 and SARS-CoV-1 share the same host receptor, human angiotensin-converting enzyme 2 (ACE2), which is the primary target of the virus. The virus binding to the lung epithelial cells with ACE2 receptors and fusion with the cell membrane are the first steps of viral infection [9].
The coronavirus disease 2019 (COVID-19) pandemic passed all borders and has spread to almost all countries of the world [10]. At the time of publication (March 2021), there have been more than 118,000,000 people have been infected with the virus and more than 2,620,000 deaths have been reported [11].
Generally, coronavirus-infecting human can be classified into low and highly pathogenic CoVs. Low pathogenic CoVs infect upper airway and cause seasonal respiratory illness, while highly pathogenic CoVs such as severe acute respiratory syndrome CoV (SARS-CoV) and Middle East respiratory syndrome CoV (MERS-CoV), infect the lower respiratory tract and may lead to acute respiratory distress syndrome (ARDS) and acute lung injury (ALI), and even death [12]. SARS-CoV-2 infection disrupts normal immune responses, leading to cytokine storm with various local and systematic damage of tissues and organs [13]. Local damages primarily affect the lower respiratory tract and present as pneumonia with the associated symptoms including fever, cough, expectoration, and hemoptysis. In addition to pulmonary damages of COVID-19, the incidence of other symptoms are occurred, includes heartbeat arrhythmia, hepatocellular injury, acute kidney injury, myocardial dysfunction, neurological illnesses, and gastrointestinal symptoms [14].
There are numerous potential approaches to prevent or treat COVID-19 such as vaccines, monoclonal antibodies, small-molecule drugs, interferon therapies, peptides, and oligonucleotides. Medications are being developed to target the spike, membrane, nucleocapsid, or envelope proteins. For individuals exhibiting signs of the disease, including cough, fever, fatigue, rhinitis, and other symptoms typically begin to manifest after few days. About 75% of COVID-19 patients exhibit symptoms as diagnosed by computed tomography scan (CT scan). In COVID-19 infection, the pneumonia frequently presents in patients during the second or third week of symptomatic so the main symptoms of viral pneumonia include ground glass abnormalities, interlobular involvement, alveolar exudates, decreased oxygen saturation, blood gas deviations, and patchy consolidation. Lymphopenia is frequently observed together with an elevation in proinflammatory cytokines and inflammatory proteins such as C-reactive protein or CRP. Although, mechanical ventilators are used for patients with pulmonary failure, oxygen therapy and treatment of symptoms constitute the mainstay of therapy. Severe acute respiratory distress, pneumonia, renal failure and death are associated with severe forms of the infection. It is challenging to compute the number of asymptomatic individuals infected with COVID-19 [10].
2. Biology of coronaviruses
Coronaviruses are a large family of viruses that vary greatly in genotypic and phenotypic characteristics [1], [15], [16]. This family of viruses target the human respiratory tract [1]. Coronaviruses are classified into four classes: alphacoronaviruses, betacoronaviruses, gammacoronviruses, and deltacoronaviruses. SARS-CoV, SARS-CoV-2, and MERS-CoV are classified in the betacoronavirus [17]. These viruses belong to the Coronaviridae family and Orthocoronavirinae subfamily, which are enveloped viruses containing single-stranded positive-sense RNA ( Table 1) [18].
Table 1.
Classification of coronaviruses.
| Order | Nidovirales | |||||
| Family | Arteriviridae | Roniviridae | Mesoniviridae | Coronaviridae | ||
| Subfamily | _ | _ | _ | Coronavirinae | Torovirinae | |
| Genus | Arterivirus | Okarivirus | α-Mesonivirus | Alphacoronavirus | Bafinivirus | |
| Betacoronavirus (The specific subtypes) | SARS-COV | Torovirus | ||||
| MERS-COV | ||||||
| SARS-COV-2 | ||||||
| Deltacoronavirus | ||||||
| Gammacoronavirus | ||||||
The viral genome is about 27–32 kb that encode structural proteins, including membrane (M), envelope (E), nucleocapsid (N), and spike (S) proteins, and nonstructural proteins (NSPs), which play a major role in viral entry and replication in the host cell of birds, mammals, and humans [15], [16]. CoVs are the largest RNA viruses have been identified in various hosts such as avian, bats, mice, dogs, cats, camels, and masked palm civets. CoVs transmission from animals to humans has made them a zoonotic virus [19].
Similar to SARS-CoV and MERS-CoV, the novel virus attacks to lower respiratory tract and causes viral pneumonia. However, gastrointestinal tract, heart, kidneys, liver, and central nervous system may also be affected, and organ failure may occur [5], [20]. The new coronavirus primarily targets the respiratory system and systemically spreads to the heart, liver, and kidneys through the lungs [21]. This is due to the high expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lungs and bronchial branches cells in the respiratory tract. Other systems in the body, including the cardiovascular system (CVS), central nervous system (CNS), gastrointestinal tract (GIT), female and male reproductive systems, can be infected due to the presence of virus-targeted receptors in their cells [22], [23], [24]. Endothelial cells and CVS have a high expression of ACE2, which is effective in regulating blood pressure and myocardial contractility. By binding SARS-CoV-2 to the ACE2 as the surface receptor of these cells, a series of downstream ACE2 signals are activated. For example, the RAS-ERK and AP-1 pathways are activated, which ultimately activate the CC motif chemokine ligand 2 (CCL2) that is a pro-fibrosis factor and may cause heart inflammation and heart fibrosis [23], [25]. The CNS may be infected by four different ways [26], including:
-
1.
Direct infection can occur through the blood circulatory and neural pathways. SARS-CoV-2 causes infection by increasing the permeability of the blood-brain barrier (BBB) through the cytokine storm mechanism. In the latter case, the sensory nerve ending is the primary target for viral infection, which may lead to anterograde or delayed axonal transmission by motor kinesin and dyneins [23], [26], [27].
-
2.
Hypoxia damage: As a result of viral infection in lung tissue, disorders of alveolar gas exchange originate a lack of oxygen in the CNS and elevate anaerobic metabolism in the mitochondria of brain cells. The lack of oxygen eventually leads to high blood pressure (headache), sleepiness (drowsiness), and swelling of the olfactory bulbs (loss of taste), which can cause severe CNS damage [23], [26], [27].
-
3.
During the COVID-19 infection, the brain's immune cells are activated, resulting in a severe cytokine storm, leading to severe brain damage [23], [26].
-
4.
Binding of SARS-CoV-2 to ACE2 of capillary endothelium may damage the BBB and facilitate viral entry by invading the vascular system [23], [27]. Consequently, SARS-CoV-2 reaches CNS through destroying the BBB and attacking to the endothelial layer [27].
SARS-CoV-2 can use an alternative route through the olfactory bulb instead of the common blood circulation system. In this pathway, the virus may enter the CNS via the cribriform plate of the olfactory bulb and pass the neurons along with blood vessels and epithelial cells [27]. Tissues with high expression of ACE2 and TMPRSS2 genes may be more vulnerable to COVID-19 infection, especially those tissues and organs with higher association between ACE2 and TMPRSS2 genes expression [28]. ACE2 is highly expressed in the reproductive organs, especially in the uterus, placenta, and fetal interface of pregnant women. So apart from the transmission through droplets and contact, the possibility of mother-to-child and sexual transmission also exists. Angiotensin II (Ang II), Ang-(1−7), and ACE2 regulate follicle development and ovulation, modulate luteal angiogenesis and degeneration, and also influence the regular changes in endometrial tissue and embryo development. Taking these functions into account, SARS-CoV-2 may disturb the female reproductive functions through regulating ACE2 [29]. It has been reported that COVID-19 is usually accompanied by high levels of interleukin (IL)−6, IL-8, tumor necrosis factor-α (TNF-α), and other cytokines, which trigger a procoagulant state that is unfavorable to the development of blastocyst or fetus in a normal human uterus. An epidemiological study demonstrated that coronaviruses could have adverse effects on fetuses and infants, including intrauterine growth restriction, preterm delivery, spontaneous abortion, and even death [14].
During the COVID-19 pandemic, the binding of SARS-CoV-2 to ACE2 receptor counteracts preeclampsia in the reproductive system of pregnant women and increases mortality rate [23]. With this consideration, the regulatory effects of COVID-19 on ACE2 may disturb the female reproductive functions and induce infertility, menstrual disorder and fetal distress [29]. In the human reproductive system, especially in the germ and somatic cells of testicles, the expression of ACE2 is high. In addition, transmembrane protease serine 2 (TMPRSS2), that assists in the virus-cell fusion process needs to be present. However, the expression of TMPRSS2 is rare in testicular tissue (15). Therefore, there are doubts about whether the testicle is a vulnerable organ in COVID-19 [22], [30].
Bats host the largest number of coronaviruses and seem to be immune to coronavirus diseases [31]. Bats are resistant to RNA viruses based on the evolution of their multifaceted antiviral immune system. The evolution of the immune system has created unique antiviral responses in this mammal. This evolution occurs at the gene and protein levels in bats. The evolution of flight in bats and concomitant with infectious viruses has shaped its distinct immune response [32], [33].
After the emergence of COVID-19, the complete SARS-CoV-2 genome was registered on the National Center for Biotechnology Information (NCBI) database (GenBank: MN908947.3). In addition, information about the structure and glycosylation pattern of the proteins and host cell communication was provided by Wu et al. in 2020 [34].
3. Structure of SARS-CoV-2
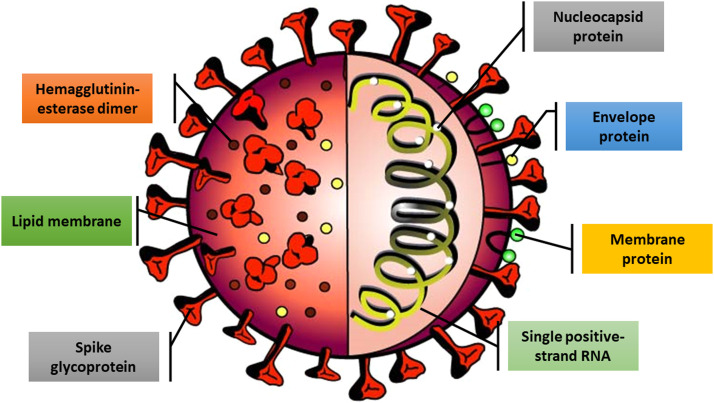
Similar to most other betacoronaviruses, their genome is made up of approximately 30,000 nucleotides. Four structural proteins, including N, M, S, and E proteins, and several NSPs are encoded by the genome ( Fig. 1) [35], [36].
Fig. 1.
Schematic structure of SARS-CoV-2. The SARS-CoV-2 virus contains different structural and non-structural proteins.
The genetic material of the virus is enclosed in a lipid envelope. The capsid is a protein coat, containing nuclear capsid or N proteins. N proteins bind to viral single positive-strand RNA and play an important role in virus replication and transcription. These proteins help the virus take over host cells. M protein with the highest amount at the cell surface of the virus affects the viral assembly. Protein E is also a small membrane protein that is involved in the viral assembly and the interaction of virus with the host cell through the permeability of the host cell membrane. Hemagglutinin-esterase (HE) dimer, which is located on the surface of the virus, in spite of its negligible importance in virus replication, is considered essential for infecting host cell [36]. The viral membrane is studied with S glycoprotein that gives coronaviruses a crown-like appearance. The coronavirus name is a derivative from the Latin corona, describing its characteristic structure of surface projections on the viral envelope giving it an appearance similar to a crown [9]. This glycosylated protein is the prime viral interacting protein with host cell targets such as angiotensin-converting enzyme 2 (ACE2), CD26, Ezrin, cyclophilins, and other cell adhesion factors. In addition, S protein is important for cell adhesion and viral virulence [37], [38], [39].
4. Mechanism of COVID-19 infection
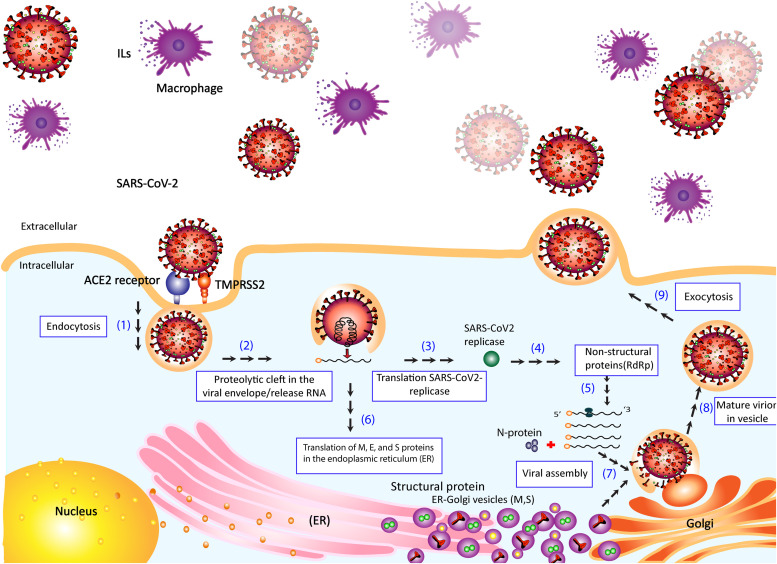
In both SARS-CoV and SARS-CoV-2, the S protein binds to ACE2 as its receptor on host cells [40]. Cryo-EM structure analysis has revealed that the binding affinity of S protein to ACE2 in SARS-CoV-2 is about 10–20 times higher than that of the S protein from SARS-CoV [41]. Since the process of viral infection begins with the interaction of S protein and ACE2 on the host cell surface, this step is of particular importance. In studies with the presence of serine protease, the invasive process of the virus is facilitated by the S protein [42], [43]. During infection, NSPs including RNA-dependent RNA polymerase (RdRp), 3C-like protease (3CLpro), and papain-like protease (PLpro) are encoded by the viral genome [42], [43]. Once the virus enters the host cell, the viral genome (single-stranded positive RNA) is released and consequently the viral proteins are produced by employing the host cell's protein translational system. The viral proteinases, including 3CLpro and PLpro cleave proteins to their effector forms [42]. PLpro acts as a de-ubiquitinating enzyme or deubiquitinase and may ubiquitinate certain host cell proteins, including interferon factor 3 and nuclear factor kappa B (NF-κB), resulting in immune suppression [42], [44]. To make more viral genomic RNA, RdRp enzyme synthesizes a full-length negative-strand RNA template to be used in the next steps ( Fig. 2) [41].
Fig. 2.
Schematic representation of the entrance and propagation of SARS-CoV-2 in host cells.
The viral S protein binds to the ACE2 receptor of the host cell. Transmembrane serine protease2 (TMPRSS2) cleaves viral S glycoprotein to facilitate viral activation. As the virus enters the cell by endocytosis, a proteolytic cleft is produced in the viral envelope, causing the RNA genome to be released in the cytoplasm. The RNA is translated by host machinery to produce the replicase and structural proteins. Host and SARS-CoV-2 proteases cleave the replicase into NSPs such as RNA-dependent RNA polymerase (RdRp). RdRp mediates RNA replication and amplification for SARS-CoV-2. Viral polypeptides and NSPs are translated from the viral genome using RNA-dependent RNA polymerase (RdRp). The viral genome is also translated into structural proteins (S, M, and N) to assemble a new virus. The translation of M, E, and S proteins occurs in the endoplasmic reticulum (ER) resulting in their accumulation in the ER-Golgi compartment (ERGIC) as well as cis-Golgi apparatus. N protein is produced in the cytoplasm, which is combined with the newly generated genome to form ribonucleoproteins (RNPs). As the new virus is assembled (mature virion), the virus is fused into the plasma membrane using the vesicles in the intermediate ERGIC, and the virus is released eventually. The severe outcome in COVID-19 and immunopathology of SARS-CoV-2 can be associated to monocyte-macrophage cells.
5. Antiviral therapy for COVID-19
COVID-19 has become a global pandemic and has spread at an exponential rate and on the other hand, there are still no effective drugs to treat this viral infection even though some non-specific therapeutic options are exist [10].
All potential drugs with antiviral efficacy on SARS-CoV-2 have been listed in Table 2.
Table 2.
Drugs with antiviral efficacy on SARS-CoV-2.
| No. | Drug | The main objective | The main use of drug | References |
|---|---|---|---|---|
| 1 | Chloroquine and Hydroxychloroquine | Inhibit glycosylation of host receptors, proteolytic processing, and endosomal acidification | Malaria and chronic inflammatory diseases including systemic lupus erythematosus (sle) and rheumatoid arthritis (ra) | [45] |
| 2 | Azithromycin | Broad-spectrum antibiotic with anti-inflammatory properties a | Variety of community-acquired respiratory tract, skin and soft tissue, and sexually transmitted disease infections. | [46] |
| 3 | Lopinavir/Ritonavir | Acts against the viral 3CL protease. | HIV, SARS, MERS | [47] |
| 4 | Ribavirin | Guanine analog | SARS, MERS | [48] |
| Inhibition RNA-dependent RNA polymerase for replicating viral genome | ||||
| 5 | Tocilizumab | Specifically binds to soluble and membrane-bound IL-6 receptors (IL-6R), thus blocking IL-6 signalling and its mediated inflammatory response | Rheumatic diseases, such as rheumatoid arthritis | [49] |
| 6 | Baricitinib | Jak kinas inhibitor | SARS | [50] |
| 7 | Remdesivir | Inhibition RNA-dependent RNA polymerase | Hepatitis B and C, Ebola, and Marburg virus, human immunodeficiency virus (HIV) | [51] |
| 8 | Favipiravir | A purine nucleic acid analogue that inhibits RdRp | Arenavirus, Bunyavirus, Flavivirus, and Filoviruses causing hemorrhagic fever and Ebola | [52] |
| 9 | Abidol | Inhibiting the fusion of viral cells into the target cell membrane(inhibition S-protein- ACE2), and preventing the virus from entering the target cell | Influenza, SARS | [53] |
| 10 | Ruxolitinib | Janus kinase inhibitor | Intermediate or high-risk myelofibrosis | [54] |
| 11 | Teicoplanin | A lipoglycopeptide used for treatment of gram-positive bacterial infections, especially in staphylococcal infections | MERS-CoV and SARS-CoV, ebola, influenza, and hepatitis C viruses as well as flavivirus and HIV | [55], [56] |
| 12 | Ivermectin | Boosts the immune system by increasing the production of IL-1 and other cytokines, as well as activation of superoxide anion production and augmentation of lymphocyte response to mitogens. | An antifungal drug, RNA viruses such as respiratory syncytial virus (RSV), dengue, influenza, rabies, and Zika viruses | [57], [58], [59] |
| 13 | Corticosteroid | Anti-inflammatory and immunosuppressive properties | Primary and secondary hemophagocytic lymphohistiocytosis (HLH) syndrome and inflammation associated with acute respiratory distress syndrome (ARDS) | [60], [61] |
| 14 | Doxycycline | Inhibitory action on metalloproteases and modulating effects of pro-inflammatory cytokines IL-6, IL-8, and TNF-α | Antibiotic effects (bacteriostatic action by inhibition of bacterial protein synthesis, anti-inflammatory effects, anti-RNA viruses and ARDS | [62], [63] |
5.1. Chloroquine and hydroxychloroquine
Chloroquine (CQ) and Hydroxychloroquine (HCQ) have a long-standing history in the prevention and treatment of malaria and the treatment of chronic inflammatory diseases, including systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [64]. HCQ is derived from CQ and both compounds have been used for treating COVID-19 disease [65].
Compared with QC, the derivative HQC has fewer side effects, drug-drug interactions, and toxicity [66]. Studies on Vero E6 cells have shown that the antimalarial drug CQ, between its half-maximal effective concentration (EC50) of 1.1 μM and its 90% maximal effective concentrations (EC90) of 6.9 μM, is effective against COVID-19 [67]. HCQ is more effective (EC50 5.47 μM) [65]. The mechanism of action of the two drugs on viral cells is to inhibit glycosylation of host receptors, proteolytic processing, and endosomal acidification. CQ and HCQ, also have immunomodulatory effects through attenuation of cytokine production and inhibition of autophagy and lysosomal activity in host cells [68], [69]. In addition, nucleic acid replication is inhibited by these drugs.
The use of CQ in high doses or along with macrolides may cause side effects, including cardiac toxicity [70]. Meanwhile, bundle-branch block, incomplete or complete atrioventricular block, Quiet Torpedo (QT) prolongation and subsequent “torsade de pointes” (a specific electrocardiographic form of polymorphic ventricular tachycardia) and cardiomyopathy (hypertrophy and congestive heart failure) can be other side effects, which are caused by CQ/HCQ in patients [71]. Patients with COVID-19 who have previous underlying diseases, including diabetes, obesity, and cardiovascular disease, are more at risk of side effects by prescription of CQ/HCQ [71]. Based on the provided information on the CQ and HCQ, they were no longer supported further for treating COVID-19. However, given the increasing demand for these two drugs and the number of people who use CQ and HCQ, they must necessarily consider the clinical and social effects of long-term hearing effects [72]. To prove the efficacy and safety of CQ and HCQ for treating COVID-19, near twenty-three clinical trials have been conducted in China [73], [74], [75]. Despite the side effects of CQ, the drug indicated to be effective in treating and curing 100 patients with COVID-19 [74]. These observations showed that this drug should be prescribed at a dose of 500 mg twice per day for 10 days to patients with mild, moderate and severe COVID-19, who were not contraindicated in CQ.
Several inconsistent treatment studies have shown that cardiac side effects may be increased with HCQ and azithromycin [76], [77]. On April 24, 2020, the US Food and Drug Administration (FDA) issued a statement on the treatment of CQ and HCQ for treating COVID-19 out of hospital or clinical trials: “HCQ and CQ can cause abnormal heart rhythms such as prolonged QT and ventricular tachycardia." The FDA noted that QT prolongation is more common in people receiving azithromycin and those with underlying heart or kidney diseases [78].
A recent controversial study by Prodromos et al., on the possible side effects of HCQ in patients with COVID-19 revealed that not only HCQ azithromycin is not cardiotoxic and does not cause cardiac arrhythmia and mortality, but also protect the heart with reducing cholesterol, thrombosis, and arrhythmia in the treated patients [79].
Clinical studies of CQ and HCQ in COVID-19 infection reported conflicting results. Moderate certainty evidence suggests that HCQ, with or without azithromycin, lacks efficacy in reducing short-term mortality in patients hospitalized with COVID-19 or risk of hospitalization in outpatients with COVID-19 [80].
Despite of promising in vitro results, the last update of international randomized controlled trials (RCTs) for COVID-19 treatments launched by WHO concluded that HCQ had little or no effect on overall mortality, initiation of ventilation, and duration of hospital stay in hospitalized patients, whereas potential effectiveness at the early stage of the diseases should be confirmed [66]. In summary, although CQ or HCQ have been promoted as potential anti-COVID-19 drugs, the evidence for their clinical effectiveness is insufficient in COVID-19. The FDA has revoked emergency use authorization (EUA) for CQ and HCQ as the known risks outweighs potential benefits with their use. Since CQ and its derivatives affect a multitude of mechanisms in the lung, further studies are necessary to identify structurally similar drugs that are safe in viral associated diseases including COVID-19 [81].
5.2. Azithromycin
Although the preponderance of evidence indicates that there is no benefit of HCQ for treating COVID-19, fewer studies have evaluated azithromycin along with HCQ, a broad-spectrum antibiotic with anti-inflammatory properties [82]. Azithromycin is an antibacterial compound, which exhibits significant anti-inflammatory properties against bacterial lipopolysaccharide (LPS)-induced inflammation in pneumonia [83], [84]. According to a study by Stellari et al., pretreatment of patients with COVID-19 with azithromycin can significantly decrease LPS-induced lung bioluminescence and airway cell infiltration, reduce the concentration of proinflammatory cytokines in bronchoalveolar lavage and inhibit NF-κB nuclear translocation [84]. Studies on the antiviral activity of azithromycin have also been conducted on RNA viruses such as Zika virus, rhinovirus and SARS-CoV-2. In the case of COVID-19, this antibiotic had no direct effect on patients' recovery process, while some scientists have suggested that the antibacterial properties of azithromycin have been clinically effective in the experimental treatment of severe acute respiratory syndrome, which occurs in patients with COVID-19 [82]. However, not all current treatment guidelines agree on the use of azithromycin in improving community-acquired pneumonia [82], [85]. Nevertheless, there are some reports that hospitals have begun including azithromycin along with CQ or HCQ to treat patients with COVID-19 [86].
Based on duality, it is required that the clinical pharmacology and characteristics of azithromycin could be considered in clinical trials alone or combined with other agents to ensure its efficacy for treating patients with COVID-19 and to increase the probability of achievement to a definitive treatment protocol [86].
In studies conducted on patients with COVID-19, preclinical data showed that consumption of 600-mg HCQ per day was effective in 70% of patients (n = 20). The use of azithromycin also increased the therapeutic effect of HCQ [87]. Azithromycin, as a weak base has antimicrobial properties and is commonly used for treating patients with chronic pulmonary disorders [60].
5.3. Lopinavir/ritonavir
Lopinavir and ritonavir (LPV/r or Kaletra) were approved by FDA in 2000 for treating human immunodeficiency virus-1 (HIV-1) infection [88]. LPV acts against the viral 3CL protease [89], [90]. Two serum concentrations of LPV including 9.6 µg/mL (peak level) and 5.5 µg/mL (trough level) were effective in the inhibition of SARS-CoV [91]. Lopinavir-1 is an HIV protease inhibitor and is usually administered with another protease inhibitor (ritonavir). After the entrance of the viral particles into the cells, LPV blocks the post-entry step in the MERS-CoV and inhibits its replication cycle [92].
Ritonavir increases the serum concentration of LPV by inhibiting cytochrome P450 [93]. In addition, by increasing the serum concentration of LPV, ritonavir inhibits the CYP3A-mediated metabolism of LPV. Combination in LPV/r showed that the antiviral activity is still similar to that of LPV alone, suggesting that the effect is largely driven by LPV [90], [94].
In vitro studies have shown that lopinavir has antiviral activity in SARS-CoV and MERS-CoV [92], [93], [95], so, the patients with SARS-CoV were treated with LPV/r. The outputs showed favorable clinical results compared with the control group who has not received LPV/r. Although XT Ye et al. reported that Kaletra or LPV/r may be more effective for treating COVID-19 infections [96], more studies are needed on a larger scale to clarify its effectiveness.
For further evaluation of LPV within the epidemiological and clinical aspects, the LPV was prescribed to ten patients with COVID-19. Of course, the drug was discontinued in three patients due to side effects. After administration of Kaletra, the levels of potassium, albumin and lymphocytes were increased. In addition, the eosinophil count returned to its normal range. Meanwhile, following the administration of lopinavir, an increase in the eosinophil count may be a sign of improvement in patients with COVID-19 [97].
5.4. Ribavirin
Due to the availability and low cost, ribavirin is used as a drug to treat coronavirus infections [98]. Ribavirin is an analog of guanine and can inhibit viral RNA-dependent RNA polymerase. Its approved activity against other coronaviruses makes it a potential candidate for COVID-19 treatment.
The challenges related to the administration of ribavirin for treating SARS-CoV and MERS-CoV infections were studied in 2003 and 2013, respectively. Those findings led to its assessment as controversial for treating COVID-19 infections [98]. To treat SARS-CoV using ribavirin, an in vitro study revealed that a combination therapy with high dose concentrations of ribavirin (eg, 1.2–2.4 g orally every 8 h) is required to inhibit viral replication [99]. Among 30 clinical trials on ribavirin, 26 studies showed ineffectiveness of the treatment on SARS-CoV infection and in 4 studies some side effects including hematologic and liver toxicity was observed [99].
For treating MERS with ribavirin and in the combination with interferons, no discernible effect on clinical outcomes or viral clearance was demonstrated [100], [101]. Among the possible studies related to COVID-19 treatment procedures, drug combination approach using ribavirin is used extensively. In addition and according to the available evidence, the in vitro antiviral activity of ribavirin against SARS-CoV-2 strain WIV04 has been demonstrated [67], [98].
The drug potential in low doses is well managed based on treatment synergies [67], [98]. Also, the efficacy and safety of combined interferon beta-1b (IFN-β1B), Kaletra, and ribavirin for treating patients with COVID-19 was assessed [102]. The study was conducted in phase II of clinical trials on adult patients with COVID-19 in six Hong Kong hospitals. In addition, the project was a multicenter, prospective, open-label, and randomized study. In this study, the combination of lopinavir, ritonavir, ribavirin, and interferon-β1B (IFN-β1B) was prescribed. The primary endpoint was the time to provide a nasopharyngeal swab negative for RT-PCR.
Compared to other COVID-19 treatment strategies, triple-drug administration has been effective in shortening the hospitalization period of acute and mild to moderate patients with COVID-19, reduction of symptoms, and duration of viral shedding. Meanwhile, the presence of IFN-β1B as a backbone for double antiviral treatment was warranted [102].
5.5. Tocilizumab
Tocilizumab (TCZ) or Actemra is a monoclonal antibody which is widely used in treatment of rheumatic diseases such as rheumatoid arthritis. TCZ is one of the drugs approved in the United States on August 30, 2017, for severe life-threatening cytokine release syndrome caused by chimeric antigen receptor (CAR) T-cell Immunotherapy [103]. Interleukin-6 (IL-6) is highly expressed in patients with SARS and MERS as well as COVID-19 [104], [105], [106]. TCZ can be prescribed to patients with COVID-19 who are in the risk of cytokine storm. TCZ as a recombinant human monoclonal antibody binds to soluble and membrane-bound IL-6 receptors (IL-6R), and stops IL-6 signals and production of intermediate inflammatory molecules [107]. TCZ is considered a pharmaceutical option for treating patients with COVID-19, however, during the treatment procedures, clinicians should evaluate the safety and efficacy of TCZ.
During the administration of TCZ in patients with COVID-19, screening and monitoring parameters, especially latent tuberculosis test (TB) should be performed by IFN-g release assay (IGRA) before and during the treatment. Studies on phase III trials associated with TCZ revealed that it will be crucial in the reduction of severe respiratory symptoms in patients with COVID-19 [108].
During the treatment of patients with COVID-19 through TCZ therapy, some laboratory parameters including C-reactive protein (CRP) and IL-6 concentrations should be assessed before and after TCZ therapy. In addition, TCZ was used along with methylprednisolone in some patients with COVID-19. The studies have shown that the level of IL-6 decreased in patients after taking TCZ, while the level of IL-6 increased significantly in patients who were not treated with TCZ. TCZ appears to be an effective treatment option in patients with COVID-19 at high risk of cytokine storm. Meanwhile, a repeated dose determination of TCZ is recommended for patients with high IL-6 [107].
5.6. Baricitinib
Baricitinib is one of the leading pharmaceuticals recommended for treating pneumonia associated with COVID-19 [68], [109]. This compound is known as a safe drug with high affinity to infected cells [68]. Regarding the SARS virus, the most important receptor for glycoprotein S binding was the angiotensin-converting enzyme 2 (ACE2) in the human cells. In the SARS-CoV-2 and due to the structural similarity of glycoprotein S with that found in SARS virus, ACE2 plays the major receptor for viral entrance. This viral receptor is widely present in kidney cells, heart, blood vessels and especially in lung AT2 alveolar epithelial cells [110]. These cells are prone to viral infections such as SARS, so, they are effective in the reproduction and transmission of viral particles through endocytosis [68], [111].
SARS-CoV-2 invades and enters the cell through endocytosis. Two important promoter factors in endocytosis are two kinases, including AP-2 associated protein kinase 1 (AAK1) and cyclin G-associated kinase (GAK) [112], [113]. Kinase inhibitors such as baricitinib prevent the entrance of viral particles to host cells and assembly of the viral particles, thus reduce viral infection [114]. To achieve an inhibitory dose in plasma, it is sufficient to prescribe 2–4 mg/day of baricitinib [68]. Meanwhile, due to the risks posed by bacitracin in patients with COVID-19, it will not be an effective drug for treating COVID-19. Some studies have demonstrated that baricitinib should not be used in patients with neutrophil and lymphocyte counts less than 1 × 109 cells/L and 0.5 × 109 cells/L, respectively [115], [116]. The progression of infection in patients with COVID-19 can be affected by lymphocytopenia. Baricitinib causes anemia in several patients. In addition, the incidence of anemia can occur in non-survivor patients with COVID-19 with 26% probability. Hence, the usage of baricitinib in COVID-19 may further reduce the number of patients [117].
According to the statistics, the level of creatine kinase (normal: < 175 U/L) in patients who took bacitracin exceeded the permissible level. Alternatively, elevated CK levels pose a risk for initiating baricitinib therapy [117]. There are limited data on the potential effects of bacitracin in the elderly aged over 75 years. In studies conducted by Zhou et al., mortality was higher in elderly patients [115] .
Co-infection, as well as the risk of reactivation of latent infections, is an important risk in the management of COVID-19 [118]. Studies have shown that baricitinib reactivates viruses, including the varicella zoster, herpes simplex, and Epstein-Barr virus strains. There is also a risk of reactivation of latent infections. Zhou et al. proved that approximately 50% of the patients with COVID-19, also experienced a secondary infection [115].
5.7. Remdesivir
Remdesivir, formally known as GS-5734 with similar structure to tenofovir alafenamide, is a nucleotide analog of adenosine 5-monophosphate [119]. This molecule has a broad-spectrum antiviral activity against hepatitis B and C, Ebola, and Marburg viruses [120], as well as human immunodeficiency virus (HIV) [121]. This agent was initially approved during the screening process for its antiviral activity against coronaviridae and flaviviridae. With the advent of the Ebola virus and further research on the effective drug, researchers have found that remdesivir can be an effective compound for treatingthe Ebola virus infection due to its low EC50 and host polymerase selectivity against the Ebola virus [122]. Meanwhile, in vitro and in vivo studies on remdesivir has shown an anti-viral inhibition activity against SARS-CoV-2 [123]. The mechanism of action of remdesivir is interference with RdRp activity through the inhibition of the virus replication [123]. In the presence of this pharmaceutical molecule, prolongation of the viral RNA is prevented [120]. The first case of COVID-19 in Washington, USA, was compassionately treated with intravenous (IV) administration of remdesivir to stop the progression of pneumonia on day 7 of hospitalization [124]. Studies have shown that remdesivir is a potential therapy for COVID-19 due to its in vitro potent and broad-spectrum, activity against several SARS-CoV-2 strains with EC50 and EC90 values of 0.77 μM and 1.76 μM, respectively [125], [126].
Despite the side effects, remdesivir has yielded acceptable results for treating patients with COVID-19. However, further studies are needed to prescribe remdesivir with minimal side effects on patients with COVID-19 [123]. An in vivo study in mice with MERS-CoV infection showed that remdesivir is an effective drug in the prevention of pulmonary hemorrhage and is capable of reducing the viral titer [90]. Albeit, it should not be neglected that remdesivir was not originally designed to target COVID-19 [120].
To evaluate the safety and pharmacokinetics of remdesivir, the drug was evaluated in single- and multiple-doses associated to phase I clinical trial. The results revealed that IV infusion of 3–225 mg remedsivir posed no adverse risk to the kidneys or liver [127]. Remdesivir was approved by FDA on May 01, by Japan on May 07, 2020, and subsequently by various European countries and Canada [128]. Further studies are needed on its safe and efficient prescription in children and pregnant women.
Since April 19, 2020, several studies have been conducted in the United States to investigate the effect of remdesivir on the treatment of patients with COVID-19. In a recent study by Grein et al., the efficacy of remdesivir was evaluated in 61 patients with COVID-19. Several moderate side effects such as renal impairment, rash, diarrhea, increment of hepatic enzymes, and hypotension have been reported in 32 patients. Also, 12 patients experienced severe side effects, including septic shock, multiple-organ-dysfunction syndrome, hypotension, and acute kidney injury. In addition, the treatment was stopped in four patients due to deteriorating preexisting renal failure and multiple organ failure as well as increased transaminases in two patients, including one patient with a maculopapular rash [129].
5.8. Favipiravir
Favipiravir is a purine nucleic acid analog and a pyrazine carboxamide derivative (6-fluoro-3-hydroxy-2-pyrazine carboxamide). Previously, favipiravir was known as T-705-RTP that is related to ribosylated and phosphorylated prodrug, which intracellularly forms the active metabolite favipiravir-ribofuranosyl-5'-triphosphate (Favipiravir-RTP). In Japan, favipiravir is prescribed as a broad-spectrum antiviral drug to treat influenza. A study showed that the half-maximal inhibitory concentration (IC50) of favipiravir can inhibit RdRp from the influenza virus, whereas human DNA polymerases α, β, and γ subunits are not inhibited by favipiravir at up to 100 µg/mL [130].
Favipiravir is effective in the inhibition of RNA viruses, including arenavirus, bunyavirus, flavivirus, and filoviruses causing hemorrhagic fever [131]. With the emergence of Ebola virus, favipiravir was used to treat patients, which showed significant effects on the treatment of the disease [132]. Some studies have been done on the treatment of COVID-19 with favipiravir. In one study, Wang et al. demonstrated that favipiravir requires a high therapeutic concentration compared with remdesivir (EC50 = 61.88 μM) [67]. However, in previous in vivo studies in mice infected with the Ebola virus, favipiravir showed a considerable efficacy in the reduction of viral response and mortality [133], [134].
In a non-randomized trial, 80 patients with COVID-19 were treated with favipiravir. In this group of patients, the duration of treatment was shorter than in the control group treated with LPV/r [135].
5.9. Abidol-umifenovir
One of the approved antiviral agents in China and Russia is Abidol. This compound works against a large number of enveloped and non-enveloped viruses such as influenza, SARS, and Lassa viruses [136], [137]. Abidol exerts its antiviral properties by inhibiting the fusion of viral particles into the target cell membrane and preventing the virus from entering the target cell [137]. There have been few reports of this drug's effect on patients with COVID-19.
A limited number of COVID-19 studies report data about patients receiving LPV/r and arbidol. It is challenging to identify whether patients have recovered naturally or the recovery process is associated with medications. Studies by Zhen Zhu et al. have shown that arbidol monotherapy is more effective than LPV/r for treating patients with COVID-19 [136]. Contrary to that, Deng’s reported that the efficacy of LPV/r alone is higher than the combination of arbidol and LPV/r for treating patients with COVID-19 [138].
5.10. Ruxolitinib
Ruxolitinib is commonly used for treating patients with intermediate or high-risk myelofibrosis [21]. Ruxolitinib as a Janus kinase (JAK) inhibitor was prescribed in a phase III clinical trial of patients with COVID-19 associated with cytokine storm. However, due to the broad immunosuppressive effects of JAK kinase inhibitors, the US National Institute of Health (NIH) did not recommend the application of ruxolitinib for control of cytokine storm in patients with COVID-19 [58].
5.11. Teicoplanin
Teicoplanin is a lipoglycopeptide used for treating gram-positive bacterial infections, especially in staphylococcal infections. Some studies have shown that this protein can show anti-viral activity against coronaviruses such as MERS-CoV and SARS-CoV. In addition, several studies have been conducted on the effect of teicoplanin on Ebola, influenza, and hepatitis C viruses as well as flavivirus and HIV [55], [56].
According to a study by Zhou et al. on the coronaviruses, teicoplanin inhibits the low pH cleavage of the viral spike protein in the early step of viral life cycle. The mechanism of action is the inhibition of cathepsin L in the late endosomes and consequently prevents the release of genomic viral RNA and replication cycle. An in vitro study showed that 1.66 µM of teicoplanin is required to inhibit the replication of 50% of viruses (IC50). Comparing the in vitro and in vivo conditions revealed that a higher blood concentration (8.78 µM) of teicoplanin is needed to reduce blood pH in human [58], [139]. Based on the similarity between the infectious process of the SARS-Cov-2 and other coronavirus strains, teicoplanin has been used as a potential therapeutic agent [139].
5.12. Ivermectin
Ivermectin is an antifungal drug, which is prescribed as a treatment for cutaneous larva migrans [57]. Ivermectin boosts the immune system by increasing the production of IL-1 and other cytokines, as well as activation of superoxide anion production and augmentation of lymphocyte response to mitogens [58]. Ivermectin has been reported to be effective in treating infections caused by RNA viruses such as respiratory syncytial virus, dengue, influenza, rabies, and Zika viruses [59], [140].
Ivermectin inhibits the replication of human immunodeficiency virus (HIV) by inhibiting the interaction of the HIV-1 integrase and α/β1 heterodimer of importin. Notably, importin is responsible for the nuclear import of integrase [58].
In an in vitro study by Caly et al., ivermectin was used as a potential replication inhibitor of SARS-CoV-2. They showed that the addition of 5 μM ivermectin to virus-infected Vero/hSLAM cells was capable of reducing SARS-CoV-2 RNA level about 5000-fold, compared to controls within 48 h [140].
According to another study by Chaccour et al., the concomitant prescription of HCQ and ivermectin could be effective for treating COVID-19. This could be related to the mechanism of action of both drugs. HCQ prevents the entrance of viral particles to host cells in the first phase of viral attack, while ivermectin prevents the genome replication of the virus [57]. In addition and within this context, ivermectin appears to be an immunosuppressive drug with no adverse drug interactions. However, no in vitro or in vivo studies have been conducted in this regard.
In a study by Alam et al., ivermectin along with doxycycline was applied for treating patients with COVID-19. In this research, 100 patients (64 males and 36 females) were enrolled with a predefined inclusion and exclusion criteria. During the medications, all patients were tested twice (4 and 18 days of starting the medication). Based on the results obtained from RT-PCR and other screening methods, the combination of ivermectin and doxycycline provided better viral clearance in patients with COVID-19 with mild and moderate symptoms [141].
In another study by Rahman et al., a comparison was done on the viral clearance between ivermectin with doxycycline and HCQ with azithromycin in patients with COVID-19. One group of the patients received ivermectin with doxycycline and another group received HCQ with azithromycin. Based on the results obtained from PCR tests, viral clearance of ivermectin with doxycycline was more effective than HCQ with azithromycin [142].
5.13. Doxycycline
The anti-inflammatory properties of doxycycline and other components of tetracycline has been demonstrated for several inflammatory airway diseases such as ARDS. Doxycycline (a semisynthetic derivative of tetracycline) would seem to be a valid alternative to azithromycin. In fact, in addition to its well-defined antibiotic effects (bacteriostatic action by inhibition of bacterial protein synthesis), in vitro studies have shown that doxycycline has anti-inflammatory effects at low (20–40 mg/day) and high (100 or 200 mg/day) doses with inhibitory action on metalloproteases, in particular MMP-9, which is likely required for initial viral entry into the cell. Doxycycline also modulate pro-inflammatory cytokines IL-6, IL-8, and TNF-α [62], [63]. In addition, low-dose doxycycline has been found to inhibit expression of CD147/EMMPRIN, which may be necessary for SARS-CoV-2 entry into T lymphocytes. Structural analysis demonstrates that doxycycline has the potentials to inhibit PLpro and 3CLpro, which both are essential for viral replication and lifecycle [143].
Given the risks of hydroxychloroquine and azithromycin in combination, doxycycline could be a better alternative to azithromycin [63]. Paul A et al., reported a series of four patients with COVID-19 infection and known high-risk pulmonary disease who were placed on standard doses of doxycycline for a course between 5 and 14 days. Patients were initiated on therapy with doxycycline. A standard antimicrobial dose of doxycycline (100 mg twice daily or daily) was administered to four patients in this case series. It is difficult to establish a definitive causal link between use of doxycycline and accelerated recovery or decreased morbidity from SARS-CoV-2 infection in a small case series. The patients had a number of high-risk features, predictive of severe disease and increased risk of mortality. There was no concomitant use of any other antibiotics, antiviral agents, antimalarial drugs, zinc, or any supplements postulated to be beneficial in therapy for SARS-CoV-2 in these four patients. These reported cases were the first correlative relationship between doxycycline and potential reduction of severity of symptoms in COVID-19 patients. Despite the safety of doxycycline, the general use of this drug is not recommended for the treatment of COVID-19 patients, unless under the direct guidance and supervision of a physician [143].
5.14. Corticosteroids and non-steroidal anti-inflammatory drugs
The role of corticosteroids for treating COVID-19 is controversial. The prescription of corticosteroids in primary and secondary hemophagocytic lymphohistiocytosis (HLH) syndrome and treatment of inflammation associated with acute respiratory distress syndrome (ARDS) has led to the application of these drugs for treating COVID-19 [60]. There is currently no conclusive evidence for the use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) for treatingthe patients with COVID-19, whereas corticosteroids are regarded useful in the early acute stages of the infection [144]. Corticosteroids can reduce inflammatory responses in ARDS; however, the side effects of NSAIDs, including the possibility of secondary infections and impairment of viral clearance can exclude them from treatment options [144], [145].
6. Immunotherapy of COVID-19
In immunotherapy, immune system components are used to fight the pathogenic agent. Immunotherapy has provided an appropriate response for treating cancer and viral infections. Meanwhile, a couple of challenges for treating patients with COVID-19, including dysfunctional viral immune response and severe inflammation should be considered. Strengthening the immune system using immune-boosting substances has benefits in COVID-19 treatment. The development of new immunosuppressive therapies for targeting viral infections and modifying dysfunctional immune responses could improve the clinical outcome of patients with COVID-19. This new approach has been dubbed as "COVID-19 immunotherapy", which is one of the new treatment approaches with a variety of potentials. Several methods such as plasma therapy and cytokine therapy can be used as COVID-19 immunotherapeutic strategies.
6.1. Plasma therapy
As newer antibiotics, antivirals, and vaccines emerged, the use of convalescent serum or plasma as a frontline therapy decreased (149).
The sera of the infection recovered patients contain plenty of antibodies against the pathogens. In this treatment method, the patient’s serum is infused to the recently infected patient with the same pathogen, so that the specific antibodies neutralize the pathogen in the recipient [146].
During the outbreak of Ebola virus in 2014 and SARS in 2002, plasma therapy was proposed as an experimental treatment against these viral infections [147], [148], [149]. Some evaluations were performed to analyze the clinical effectiveness of convalescent plasma, serum, or hyperimmune immunoglobulin for the treatment of severe viral acute respiratory infections including those due to SARS coronavirus, Spanish influenza A (H1N1), avian influenza A (H5N1), and pandemic influenza A (H1N1) in 2009 [118]. In all cases, hyperimmune immunoglobulin was able to demonstrate a statistically significant reduction in the odds of mortality among those who were treated with convalescent plasma or serum [151]. During SARS-CoV infection, it was thought that convalescent plasma improve the outcome of infected patients. Previous studies on patients with SARS-CoV infection suggested that convalescent plasma may be useful for patients with SARS so showed improvements in survival and resulted in a shorter hospital stay. A protocol for the application of convalescent plasma as a therapeutic option for MERS was suggested [152]. The recent outbreak of Ebola virus disease (EVD) in West Africa has been the worst ever witnessed. By September 9, 2015, a total of 28,183 cases and 11,306 deaths had been reported. The high case fatality rate (40–60%) highlights the need for effective EVD-specific treatments. Such interventions would facilitate the rapid tracing of contacts of patients and the implementation of measurements to control the spread of an outbreak. The WHO has prioritized the evaluation of treatment with convalescent whole blood or plasma derived from patients who have recovered from EVD. Such treatment has been used successfully for other serious infectious diseases with appropriate safeguards. In 1995, the largest case series involved eight patients who were treated with convalescent whole blood during the Kikwit outbreak of EVD that seven patients were survived. However, it was not possible to assess whether the low case fatality rate was due to treatment with convalescent whole blood or other factors, such as characteristics of the patients or the period during the illness at which treatment was given [153].
In the Pandemic of Covid-19, plasma therapy has been used for treating COVID-9 patients [154]. In an initial study, five patients with COVID-19 with ARDS underwent plasma therapy and clinical outcomes were compared before and after convalescent plasma (CP) transfusion The results showed improvement in the patients' clinical condition [154]. Due to the limitations in the sample and the experimental design, it is not possible to give a definite opinion about the potential effectiveness of this type of treatment and more clinical observations will be needed.
In a study by Duan et al. in ten severe adult cases, the results showed that a dose of 200 mL CP was well tolerated and could significantly increase or maintain neutralizing antibodies at a desirable level. This treatment was capable of reducing viremia within 7 days. After the application of this treatment method, clinical and paraclinical symptoms improved rapidly within 3 days. Radiological studies also showed varying degrees of absorption of lung lesions within 7 days. According to these observations, CP can be expected as a life-saving option in patients with severe COVID-19 [155].
6.2. Cytokine-based immunotherapy
Interferon (IFN)-based immunotherapy could be a safe choice for multiple sclerosis (MS) patients with mild-to-moderate disease activity during the COVID-19 pandemic. At the first stage of viral disease, the expression of IFNs could be an appropriate innate immune response to the virus. Afterward, the main immune response to the virus is mediated by lymphocytes, monocytes, and macrophages. IFNs induce interference with viral replication and previous evidence suggests that IFN-β is mildly effective in animal models of MERS-CoV whenever used along with lopinavir and ritonavir, so the capability of IFN-β for treating COVID-19 could be a useful investigation [156]. The prescription of IFNs in the early stages of COVID-19 can elicit a stronger antiviral response and prevent infection spreading to other cells. However, the use of IFNs in the severe phase of the immune response can trigger hyperactivation of immune response and intensify the cytokine storm. Therefore, this treatment is not recommended in the acute phase of infection [157]. SARS-CoV-2 inhibits the production of type 1 IFNs by disrupting IFN signaling pathways through the inhibition of signal transducers and transcription activators such as signal transducer and activator of transcription 1 (STAT1) and Interferon regulatory factor 3 (IRF3).
IFN production level varies according to age. Due to the low excitability threshold of IFN in children, the mortality rate of COVID-19 would be lower in children. In contrast, the high excitability threshold of IFN results in a higher rate in adults. Inhibition of IFN activity by the virus and inadequate production of IFN during the infection is an important challenge of the immune response to COVID-19. An in vitro study showed that SARS-CoV-2 was more sensitive to treatment with IFN-α and -β than many other pathogenic viruses. From a clinical perspective, the initial triple treatment of COVID-19 with IFN-β1b, lopinavir, and ritonavir has been reported to be more effective than lopinavir-ritonavir. The mentioned combination effectively prevents infection and reduces disease progression to severe stages. Based on the results obtained from in vitro and in vivo experiments, the effectiveness of treatment with type I IFNs was approved so IFN-based immunotherapy has been considered in clinical trials of patients with COVID-19. Numerous clinical trials are evaluating the effects of recombinant forms of human type 1 IFNs (IFN-α and -β) for treating early-stage patients with COVID-19 [119].
Type III IFN, also known as IFN-λ, contributes in immune response to viral infections. The importance of IFN-λ function in health and disease has been difficult to analyze, meanwhile, it seems that the presence of IFN-λ is critical to prepare a balance in the antiviral response to SARS-CoV-2 in the respiratory tract [158]. IFN-λ activates inflammatory transcription factors leading to the stimulation of the Janus kinase (JAK) through the STAT signaling pathway. Based on previous clinical trials for application of IFN-λ in chronic viral hepatitis, IFN-λ has been identified as a candidate for immunotherapy of some viral infections such as COVID-19. Accordingly, Peginterferon-λ (PEG-IFN-λ), has been used in COVID-19 clinical trials to suppress the viral replication and development of cytokine storm [157], [158].
Cytokine storm as the hallmark of ARDS, is an uncontrolled systemic inflammatory response triggered by some immune system cells due to the release of proinflammatory cytokines and chemokines in some patients with COVID-19. In this regard, high expression levels of cytokines and chemokines, including IL1-β, IL1RA, IL7, IL8, IL9, IL10, FGF2, GC-SF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGFB, TNF-α, and VEGFA are observed in the serum of patients with COVID-19 [159]. The inability of the immune system to control this condition has led to the death of many patients with COVID-19. The immune response features in COVID-19 are similar to the immune response in SARS-CoV and MERS-CoV infections [8].
An urgent attention has been paid to provide suitable control protocols for SARS-CoV-2; in this regard, the focus has shifted to using IFNs to control viral infection [159]. Anti-inflammatory agents have been effective in the control of various cytokine storm syndrome cytokines. The target of these drugs is usually IL-1, IL-6, IL-18, and IFN-γ. Although, there is no definitive drug for treating a cytokine storm in patients with COVID-19, the administration of anti-inflammatory agents requires further investigation. Meanwhile, the application of anti-IL-6 antibody has been suggested for effective blockade of IL-6 and control of ARDS in patients with COVID-19 [159].
According to the IFN-I therapy studies conducted on the SARS-CoV and MERS-CoV infections, type 1 IFNs show high-potential for treating patients with COVID-19 [160]. In this regard, IFN-α2b was used as a therapeutic agent for treating patients with COVID-19 according to a study conducted in Wuhan, China [161]. In this study, 77 hospitalized adults with COVID-19 were treated with IFN-α2b (5 mU b.i.d.), or arbidol (200 mg t.i.d), or a combination of both pharmaceuticals. Hematological, biochemical and immunological parameters were evaluated. IFN-α2b therapy, either alone or concomitantly with arbidol, significantly reduced the duration of the detection of SARS-CoV-2 virus in the upper respiratory tract and also reduced the blood levels of the inflammatory markers such as IL-6 and CRP. Referring to the conducted studies, IFN could be considered as a pharmaceutical option for treating patients with COVID-19 [161].
7. Cell therapy
Cell-based therapies are classified into two major categories, including allogeneic and autologous sources. Both categories can be applied with a minimum manipulation in clinical purposes. The local production for small-scale autologous facilities can be located close to patients with dedicated set. One of the major disadvantages of autologous cell therapy is the cost-effectiveness and inadequacy of target scale in the treatment process. The preparation of autologous cell therapies is done manually and takes time. Other disadvantages of autologous cell-based therapy methods are long cultivation periods, large numbers of technicians, and necessity for elevation of contamination risk. The initial source of the provided cells in both cell-based technics can be expanded using the existing equipment and available technologies for future applications. Therefore, cell therapy is a cost-effective treatment method and performing in the larger cell volumes is more laborious; moreover, the cell stability can be a limitation, too [162].
7.1. Stem cell therapy
Nowadays, cell-based therapy methods such as stem cell therapy are considered potential therapeutic strategies for treating some severe diseases. Due to the superior properties of mesenchymal stem cells (MSCs) and their conditioned medium compared to other cellular therapies, the application of MSC-based therapy has further expanded in the field of cell therapy [163].
MSCs are readily available and can be isolated and stored from various tissues such as bone marrow and adipose tissue. MSCs are multipotent stem cells and can be easily expanded to clinical applications in a desired period. Therapeutic application of MSCs has been documented in several clinical trials. Clinical trials using MSCs have not shown any adverse effects in the patients [163].
In patients with COVID-19, the cytokine storm occurs after over-production of inflammatory agents by the immune system. MSCs control the release and activity of cytokine in this condition which is provided through endogenous repair with compensatory properties of the stem cell products.
By intravenous injection of MSCs, the pulmonary environment is restored, alveolar epithelial cells are protected, pulmonary fibrosis is inhibited and COVID-19 pneumonia is treated. Therefore, MSC-based therapy can be a potential novel treatment ideal for clinical trials or at least along with other treatment methods for patients with COVID-19 [163].
In a study by Liang et al. on a 65-year-old woman with COVID-19, human umbilical cord MSCs (hUCMSCs) were used. The allogenic hUCMSCs were given three times (5 × 107 cells each time) with a 3-day interval, with daily injection of thymosin α1 and antibiotics. After treatment with MSCs, most experimental indicators and computerized tomography (CT) images showed an improvement in the inflammation and symptoms. The patient was then transferred out of intensive care unit (ICU) and the throat swab test for RT-PCR test was reported negative 4 days later. The results suggested that hUCMSCs could be a potential treatment option alone or in combination with other immune modulators for acute patients with COVID-19 [164].
In a study by Leng et al., they investigated the effect of MSC transplantation on pneumonia-associated patients with COVID-19. The period of treatment lasted for 14 days. Within 2 days post-transplantation, the patients' pulmonary function had improved dramatically. After treatment, the CRP level decreased, peripheral lymphocyte count was increased, cytokine-secreting immune cells such as CXCR3+CD4+ T and CXCR3+CD8+ T cells were over-activated, and CXCR3+ natural killer (NK) cells disappeared within 3–6 days. Meanwhile, the group of immunological cells including CD14+, CD11c+, CD11bmid, and regulatory dendritic cells (DCs) dramatically increased. During the treatment, the level of IL-10 as an immunomodulatory cytokine was increased, while, the level of TNF-α was decreased. Therefore, intravenous transplantation of MSCs can be a potential and safe treatment method against acute pneumonia-associated patients with COVID-19 [165].
Exosomes are secretory components studied for their similarity to the paracrine effect of MCSs for treating various diseases. These 30–150 nm nanoparticles are involved in cellular communication and are responsible for transporting many biological materials, including mRNA and protein molecules [166].
The use of MSC-derived exosomes as cell-free therapeutics offers several advantages over their cellular counterparts, including high stability, low immunogenicity, ease of storage, and ability to cross the blood-brain barrier. MSCs-derived exosomes could be a new intervention idea to treat severe conditions of COVID-19 through modulation of the immune system and antimicrobials [126]. Finally, controlled experiments are needed to evaluate the possible treatment of COVID-19 with MSCs, their conditioned medium and MSC-derived exosomes [167], [168].
7.2. NK cell therapy
NK cells are a subset of innate immunity lymphocytes that comprise 10−15% of total peripheral blood leukocytes. They are categorized as first-line defense components against viruses. They are naturally activated during the initial immune response to virus-infected cells and promote the infected cells toward apoptosis [169]. The most distinct features of the NK immune response are MHC independence and ready availability to combat virally infected cells. Their functionality could be enhanced by macrophage-derived cytokines and type I IFNs. Since the outbreak of COVID-19, scientists have considered the use of NK cells as an effective cell therapy method for treating patients with COVID-19 [157].
The chimeric antigen receptor (CAR) is a genetically engineered receptor that is widely used for treating various cancers. Engineered NK cells expressing CAR molecules can specifically target virus antigen-expressing cells. Since NK cells play an important role in antiviral immune response to SARS-CoV-2, CAR-NK engineered cells have been proposed as a new approach to the treatment of COVID-19. ACE-2 can be a target antigen that can be used to design CAR-NK cells against SARS-CoV-2. NCT04324996 is a phase I/II clinical trial study initiated to evaluate the efficacy of Universal Off-the-shelf NKG2D-ACE2 CAR-NK cells for treating COVID-19 pneumonia [157], [170].
7.3. DC therapy
As members of the innate immune system, type 1 DCs (DC-1) exert their antiviral immune response by producing IL-6 and IFN, and acting as antigen-presenting cells. The application of engineered DCs is mainly a hot topic in cancer therapy, however, its application in infectious disease is possible, too. DCs may activate NK cells by expressing NKG2D [171]. However, the hypersecretion of IL-6 is considered the major mechanism that contributes to the progression of respiratory inflammation and lung tissue damage in ARDS. Application of DC-blocking agents and using engineered DCs could be considered for inhibition of the proinflammatory effects of patients with severe COVID-19 [157].
7.4. Macrophage therapy
Macrophages are categorized into type 1 macrophages (M1) with proinflammatory functions, and type 2 macrophages (M2) with anti-inflammatory properties. Commonly, these cells could be developed from monocytes in the in vitro conditions. Similarly, in the in vivo condition the circulating monocytes enter tissues and differentiate into macrophages. During the COVID-19 pandemic, M1 macrophages contribute to severe inflammation by secretion of proinflammatory cytokines such as IL-6 and IL-1β. To suppress the hyperinflammatory condition, macrophages can be modified in two ways. The first method could be the modulation of the M1 macrophages to secrete lower levels of proinflammatory cytokines and the second approach could be the application of M2 macrophages to suppress the inflammation of the lungs. Considering the application of macrophages for treating COVID-19, macrophage therapy could be considered in further studies [157].
8. Nanomedicine for treatment of COVID-19
Recently, nanomedicine has entered all fields of medicine and commonly applied as an interdisciplinary method for differential diagnosis and imaging. Nano-dimensional biomolecules such as polymeric nanoparticles, liposomes, nanosurfactants, nanocrystals and protein nanoparticles have been used as carriers to aid drug delivery. The diversity and variety of nanoparticles, and the interaction of these materials with biological tissues and molecules has extended their applications in medicine. Theoretically, these particles can effectively inactivate the virus by preventing it’s attachment to the host cell. Blockade of virus surface proteins by targeted nanoparticles, especially those expressed by the virus, can reduce the burden of the virus [172]. Metal nanoparticles are capable of preventing viral attachment to the cell surface and inhibit their entry into the host cell, thus impeding virus replication following their entrance. These findings are based on the reaction of nanoparticles consisting of titanium (Ti), silver (Ag), gold (Au) and zinc (Zn) against HIV, smallpox, influenza, herpes simplex, respiratory syncytial virus, transmissible gastrointestinal virus, and zika virus. The mechanism of action is based on the binding of nanoparticles to the viral envelope or its protein, thereby disrupting the interaction of the virus with the host cell. The effectiveness of the treatment depends on the size, shape and surface charge of the nanoparticles; however, safety issues regarding the virus concentration must be taken to prevent the cytotoxicity of the host cells. The main limitations of metal nanoparticles against viruses are their lack of specificity and, as a result, can cause host cytotoxicity, which can be addressed by organic nanoparticles.
According to above discussions, various antimicrobials such as CQ, lopinavir, ritonavir, ribavirin, and remedsivir have been tested in clinical trials for COVID-19 and have shown promising results. Antimicrobial nanocapsules may also help in the development of safe treatments for COVID-19 and other viral diseases [173]. Y Han et al. declared that ACE2-Based Peptide Inhibitors could be conjugated to nanoparticles to provide better efficacy and consequently applied as antiviral drugs or vaccine carriers in COVID-19 [174]. In a study by Lammers et al., nanoscale dexamethasone has been described as an effective drug for treating COVID-19. The researchers defined the positive effect of the nanoformulated dexamethasone for treating diseases such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, liver fibrosis, wound healing, and cancer. In addition, the nanoformulated dexamethasone can be effective for treating patients with COVID-19 [175], [176].
For treating patients with COVID-19, a combination of drugs has been used as a therapeutic method. This treatment strategy has some benefits, including lower dosages of individual drugs with limited side effects, achieving multiple and complimenting therapeutic targets, and reducing the likelihood of resistance to antibiotics. The nanocarriers are believed to be useful in delivering multiple drugs with different physical and chemical properties, and can complement the benefits of simultaneous drug delivery [177], [178]. The flexibility provided by various nanomaterials and manufacturing techniques allows the design of drug compounds loaded on nanocarriers with excellent control over the maintenance of synergistic drug ratios, pharmacokinetic overlap, and reduction of combined side effects [23].
9. Conclusion
Since the emergence of COVID-19 in December 2020, many academic groups and pharmaceutical companies focused their efforts and budgets on designing of the pharmaceutical compounds for treatment and production of various forms of anti-COVID-19 vaccines. Although some pharmaceutical companies such as Pfizer-BioNTech, Moderna, and AstraZeneca reported the production of anti-COVID-19 vaccines with different definitive immunogenicity percentages [179], the immunogenicity of vaccines does not cover all the global population and the elimination of COVID-19 by vaccination may be far from access of WHO and governments for next years. Therefore, accessibility to the potential, safe, and efficient COVID-19-specific drugs accessible to all geographic regions and lower-income economies would be urgent planes to combat against COVID-19 in parallel with prevention strategies such as vaccination. The best recommendation for treatment of COVID-19 patients is to utilize an efficient present antiviral drug accompany with anti-inflammatory and anti-coagulant agents till discovery of a SARS-CoV-2-specific drug. To reach the ultimate target, regarding the elimination of COVID-19, researchers from all nationalities and pharmaceutical companies accompany with WHO and charity foundations must be joined together and share their experiences on drug designing for treatment of COVID-19.
Author’s contribution
RZE designed and supervised the study. MSM drafted the first version of the manuscript. AHM and HN designed the figures and performed artworks on the manuscript. JH checked the clinical-associated statements of the manuscript and RF revised the immunological-associated statements of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of conflicting interests
The authors declare that they have no competing interests. The funding organizations had no role in the design of study.
Acknowledgments
The study was supported by the national COVID-19 committee of the National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran.
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zolfaghari Emameh R., Eftekhari M., Nosrati H., Heshmatnia J., Falak R. Identification and characterization of a silent mutation in RNA binding domain of N protein coding gene from SARS-CoV-2. BMC Res. Notes. 2021;14(1):10. doi: 10.1186/s13104-020-05439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karim A., et al. Knowledge and attitude towards COVID-19 in Bangladesh: population-level estimation and a comparison. Nature. 2020;5(4):536–544. [Google Scholar]
- 8.Zolfaghari Emameh R., Nosrati H., Eftekhari M., Falak R., Khoshmirsafa M. Expansion of single cell transcriptomics data of SARS-CoV infection in human bronchial epithelial cells to COVID-19. Biol. Proced. Online. 2020;22:16. doi: 10.1186/s12575-020-00127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danesh-Meyer H.V., McGhee C.N. Implications of coronavirus disease 2019 for ophthalmologists. Am. J. Ophthalmol. 2021;223:108–118. doi: 10.1016/j.ajo.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ita K. Coronavirus Disease (COVID-19): current status and prospects for drug and vaccine development. Arch. Med. Res. 2021;52(1):15–24. doi: 10.1016/j.arcmed.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y.M., Xu G., Wang B., Liu B.C. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. J. Intern. Med. 2021;289(2):147–161. doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zolfaghari Emameh R., Falak R., Bahreini E. Application of system biology to explore the association of neprilysin, angiotensin-converting enzyme 2 (ACE2), and carbonic anhydrase (CA) in pathogenesis of SARS-CoV-2. Biol. Proced. Online. 2020;22:11. doi: 10.1186/s12575-020-00124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F., Lu H., Zhang Q., Li X., wang T., Liu Q., Yang Q., Qiang L. Impact of COVID-19 on female fertility: a systematic review and meta-analysis protocol. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-045524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan. F1000Research. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zolfaghari Emameh R., Nosrati H., Taheri R.A. Combination of biodata mining and computational modelling in identification and characterization of ORF1ab polyprotein of SARS-CoV-2 isolated from Oronasopharynx of an Iranian Patient. Biol. Proced. Online. 2020;22:8. doi: 10.1186/s12575-020-00121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang S., Zhang X., Du L. Therapeutic antibodies and fusion inhibitors targeting the spike protein of SARS-CoV-2. Expert Opin. Ther. Targets. 2020:1–7. doi: 10.1080/14728222.2020.1820482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarandi P.K., Zinatizadeh M.R., Zinatizadeh M., Yousefi M.H., Rezaei N. SARS-CoV-2: from the pathogenesis to potential anti-viral treatments. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y.-R., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil. Med. Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barraco F., Greil R., Herbrecht R., Schmidt B., Reiter A., Willenbacher W., Raymakers R., Liersch R., Wroclawska M., Pack R., Burock K., Karumanchi D., Gisslinger H. Real‐world non‐interventional long‐term post‐authorisation safety study of ruxolitinib in myelofibrosis. Br. J. Haematol. 2020;191(5):764–774. doi: 10.1111/bjh.16729. [DOI] [PubMed] [Google Scholar]
- 22.de Carvalho R.C., Groner M.F., Camillo J., Ferreira P.R.A., Fraietta R. The interference of COVID-19 in the male reproductive system: important questions and the future of assisted reproduction techniques. Clinics. 2020;75 doi: 10.6061/clinics/2020/e2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;14(7):7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 24.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oudit G.Y., Crackower M.A., Backx P.H., Penninger J.M. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc. Med. 2003;13(3):93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 26.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das G., Mukherjee N., Ghosh S. Neurological insights of COVID-19 pandemic. ACS Chem. Neurosci. 2020;11(9):1206–1209. doi: 10.1021/acschemneuro.0c00201. [DOI] [PubMed] [Google Scholar]
- 28.Hu R., et al. Differential expression and immune correlation analysis of COVID-19 receptor ACE2 and TMPRSS2 genes in all normal and tumor tissues. Eur. Rev. Med. Pharmacol. 2021;25(3):1724–1731. doi: 10.26355/eurrev_202102_24882. [DOI] [PubMed] [Google Scholar]
- 29.Jing Y., Run-Qian L., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G., Fei C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020;26(6):367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illiano E., Trama F., Costantini E. Could COVID‐19 have an impact on male fertility? Andrologia. 2020;52 doi: 10.1111/and.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong A., Li X., Lau S., Woo P. Global epidemiology of bat coronaviruses. Viruses. 2019;11(2):174. doi: 10.3390/v11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerjee A., Baker M.L., Kulcsar K., Misra V., Plowright R., Mossman K. Novel insights into immune systems of bats. Front. Immunol. 2020;11:26. doi: 10.3389/fimmu.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subudhi S., Rapin N., Misra V. Immune system modulation and viral persistence in bats: understanding viral spillover. Viruses. 2019;11(2):192. doi: 10.3390/v11020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robson B. Computers and viral diseases. Preliminary bioinformatics studies on the design of a synthetic vaccine and a preventative peptidomimetic antagonist against the SARS-CoV-2 (2019-nCoV, COVID-19) coronavirus. Comput. Biol. Med. 2020;119 doi: 10.1016/j.compbiomed.2020.103670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anthony S.J., Johnson C.K., Greig D.J., Kramer S., Che X., Wells H., Hicks A.L., Joly D.O., Wolfe N.D., Daszak P., Karesh W., Lipkin W.I., Morse S.S., Mazet J.A.K., Goldstein T. Global patterns in coronavirus diversity. Virus Evol. 2017;3(1):vex012. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song W., Gui M., Wang X., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14(8) doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millet J.K., Kien F., Cheung C.Y., Siu Y.L., Chan W.L., Li H., Leung H.L., Jaume M., Bruzzone R., Malik Peiris J.S., Altmeyer R.M., Nal B. Ezrin interacts with the SARS coronavirus spike protein and restrains infection at the entry stage. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong X., et al. Glycan shield and fusion activation of a deltacoronavirus spike glycoprotein fine-tuned for enteric infections. J. Virol. 2018;92(4) doi: 10.1128/JVI.01628-17. e01628-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., Banovich N., Barbry P., Brazma A., Desai T., Duong T.E., Eickelberg O., Falk C., Farzan M., Glass I., Haniffa M., Horvath P., Hung D., Kaminski N., Krasnow M., Kropski J.A., Kuhnemund M., Lafyatis R., Lee H., Leroy S., Linnarson S., Lundeberg J., Meyer K., Misharin A., Nawijn M., Nikolic M.Z., Ordovas-Montanes J., Pe’er D., Powell J., Quake S., Rajagopal J., Tata P.R., Rawlins E.L., Regev A., Reyfman P.A., Rojas M., Rosen O., Saeb-Parsy K., Samakovlis C., Schiller H., Schultze J.L., Seibold M.A., Shalek A.K., Shepherd D., Spence J., Spira A., Sun X., Teichmann S., Theis F., Tsankov A., van den Berge M., von Papen M., Whitsett J., Xavier R., Xu Y., Zaragosi L.E., Zhang K. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Báez-Santos Y.M., John S.E.S., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee T.-W., Cherney M.M., Huitema C., Liu J., James K.E., Powers J.C., Eltis L.D., James M.N.G. Crystal structures of the main peptidase from the SARS coronavirus inhibited by a substrate-like aza-peptide epoxide. J. Mol. Biol. 2005;353(5):1137–1151. doi: 10.1016/j.jmb.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plantone D., Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin. Drug Investig. 2018;38(8):653–671. doi: 10.1007/s40261-018-0656-y. [DOI] [PubMed] [Google Scholar]
- 46.Duran J.M., Amsden G.W. Azithromycin: indications for the future? Expert Opin. Drug Deliv. 2000;1(3):489–505. doi: 10.1517/14656566.1.3.489. [DOI] [PubMed] [Google Scholar]
- 47.Nutho B., Mahalapbutr P., Hengphasatporn K., Pattaranggoon N.C., Simanon N., Shigeta Y., Hannongbua S., Rungrotmongkol T. Why are lopinavir and ritonavir effective against the newly emerged Coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry. 2020;59(18):1769–1779. doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- 48.De Wilde A.H., Falzarano D., Zevenhoven-Dobbe J.C., Beugeling C., Fett C., Martellaro C., Posthuma C.C., Feldmann H., Perlman S., Snijder E.J. Alisporivir inhibits MERS-and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017;228:7–13. doi: 10.1016/j.virusres.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott L.J. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77(17):1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jorgensen S.C.J., Tse C.L.Y., Burry L., Dresser L.D. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID‐19. Pharmacotherapy. 2020;40(8):843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giovane R.A., Rezai S., Cleland E., Henderson C.E. Current pharmacological modalities for management of novel coronavirus disease 2019 (COVID‐19) and the rationale for their utilization: a review. Rev. Med. Virol. 2020;30(5) doi: 10.1002/rmv.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westover J.B., Sefing E.J., Bailey K.W., Van Wettere A.J., Jung K.H., Dagley A., Wandersee L., Downs B., Smee D.F., Furuta Y., Bray M., Gowen B.B. Low-dose ribavirin potentiates the antiviral activity of favipiravir against hemorrhagic fever viruses. Antivir. Res. 2016;126:62–68. doi: 10.1016/j.antiviral.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu S., Guo X., Geary K., Zhang D. Emerging therapeutic strategies for COVID-19 patients. Discoveries. 2020;8(1) doi: 10.15190/d.2020.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison C.N., Schaap N., Mesa R.A. Management of myelofibrosis after ruxolitinib failure. Ann. Hematol. 2020;99:1177–1191. doi: 10.1007/s00277-020-04002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C., Tao L., Zhang H. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291(17):9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colson P., Raoult D. Fighting viruses with antibiotics: an overlooked path. Int. J. Antimicrob. Agents. 2016;48(4):349–352. doi: 10.1016/j.ijantimicag.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patrì A., Fabbrocini G. Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment? J. Am. Acad. Dermatol. 2020;82(6) doi: 10.1016/j.jaad.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jean S.-S., Hsueh P.-R. Old and re-purposed drugs for the treatment of COVID-19. Expert Rev. Anti Infect. 2020;18(9):843–847. doi: 10.1080/14787210.2020.1771181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: immunology and treatment options. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kapugi M., Cunningham K. Corticosteroids. Orthop. Nurs. 2019;38(5):336–339. doi: 10.1097/NOR.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 62.Conforti C., Giuffrida R., Zalaudek I., Di Meo N. Doxycycline, a widely used antibiotic in dermatology with a possible anti‐inflammatory action against IL‐6 in COVID‐19 outbreak. Dermatol. Ther. 2020;33(4) doi: 10.1111/dth.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malek A.E., Granwehr B., Kontoyiannis D.P. Doxycycline as a potential partner of COVID-19 therapies. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gasmi A., et al. Chloroquine and hydroxychloroquine in the treatment of COVID-19: the never-ending story. Appl. Microbiol. Biotechnol. 2021:1–11. doi: 10.1007/s00253-021-11094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Rawling M., Savory E., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rolain J.-M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents. 2007;30(4):297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gendrot M., Javelle E., Clerc A., Savini H., Pradines B. Chloroquine as a prophylactic agent against COVID-19? Int. J. Antimicrob. Agents. 2020;55(6) doi: 10.1016/j.ijantimicag.2020.105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gevers S., Kwa M.S.G., Wijnans E., van Nieuwkoop C. Safety considerations for chloroquine and hydroxychloroquine in the treatment of COVID-19. Clin. Microbiol. Infect. 2020;26(9):1276–1277. doi: 10.1016/j.cmi.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Luca P., Scarpa A., De Bonis E., Cavaliere M., Viola P., Gioacchini F.M., Ralli M., Ettore C., Claudia C. Chloroquine and hydroxychloroquine ototoxicity; potential implications for SARS-CoV-2 treatment. A brief review of the literature. Am. J. Otolaryngol. 2020 doi: 10.1016/j.amjoto.2020.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao J., Tian Z., yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of covId-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 75.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antivir. Res. 2020;177 doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F., Hajjar L.A., Pinto R.C., Balieiro A.A.S., Pacheco A.G.F., Santos J.D.O., Naveca F.G., Xavier M.S., Siqueira A.M., Schwarzbold A., Croda J., Nogueira M.L., Romero G.A.S., Bassat Q., Fontes C.J., Albuquerque B.C., Daniel-Ribeiro C.T., Monteiro W.M., Lacerda M.V.G. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.8857. e208857-e208857. [DOI] [PubMed] [Google Scholar]
- 78.Lofgren S.M., Nicol M.R., Bangdiwala A.S., Pastick K.A., Okafor E.C., Skipper C.P., Pullen M.F., Engen N.W., Abassi M., Williams D.A., Nascene A.A., Axelrod M.L., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Cheng M.P., Zarychanski R., Schwartz I.S., Silverman M., Chagla Z., Kelly L.E., McDonald E.G., Lee T.C., Hullsiek K.H., Boulware D.R., Rajasingham R. Safety of hydroxychloroquine among outpatient clinical trial participants for COVID-19. OFID. 2020;7(11):ofaa500. doi: 10.1093/ofid/ofaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prodromos C.C. Hydroxychloroquine is protective to the heart, not harmful: a systematic review. New Microbes New Infect. 2020;37 doi: 10.1016/j.nmni.2020.100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kashour Z., Riaz M., Garbati M.A., AlDosary O., Tlayjeh H., Gerberi D., Murad M.H., Sohail M.R., Kashour T., Tleyjeh I.M. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. J. Antimicrob. Chemother. 2021;76(1):30–42. doi: 10.1093/jac/dkaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma P., McAlinden K.D., Ghavami S., Deshpande D.A. Chloroquine: autophagy inhibitor, antimalarial, bitter taste receptor agonist in fight against COVID-19, a reality check? Eur. J. Pharmacol. 2021;897 doi: 10.1016/j.ejphar.2021.173928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oldenburg C.E., Doan T. Azithromycin for severe COVID-19. Lancet. 2020;396(10256):936–937. doi: 10.1016/S0140-6736(20)31863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ivetić Tkalčević V., Bošnjak B., Hrvačić B., Bosnar M., Marjanović N., Ferenčić Ž., Šitum K., Čulić O., Parnham M.J., Eraković V. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur. J. Pharmacol. 2006;539(1–2):131–138. doi: 10.1016/j.ejphar.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 84.Stellari F.F., Sala A., Donofrio G., Ruscitti F., Caruso P., Topini T.M., Francis K.P., Li X., Carnini C., Civelli M., Villetti G. Azithromycin inhibits nuclear factor‐κB activation during lung inflammation: an in vivo imaging study. Pharmacol. Res. Perspect. 2014;2(5) doi: 10.1002/prp2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sultana J., Cutroneo P.M., Crisafulli S., Puglisi G., Caramori G., Trifirò G. Azithromycin in COVID-19 patients: pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf. 2020;43(8):691–698. doi: 10.1007/s40264-020-00976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Damle B., Vourvahis M., Wang E., Leaney J., Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clin. Pharmacol. Ther. 2020;108(2):201–211. doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morris J.L., Kraus D.M. New antiretroviral therapies for pediatric HIV infection. J. Pediatr. Pharmacol. Ther. 2005;10(4):215–247. doi: 10.5863/1551-6776-10.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baden L.R., Rubin E.J. Covid-19 — the search for effective therapy. N. Engl. J. Med. 2020;382(19):1851–1852. doi: 10.1056/NEJMe2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hurst M., Faulds D. Lopinavir. Drugs. 2000;60(6):1371–1379. doi: 10.2165/00003495-200060060-00009. [DOI] [PubMed] [Google Scholar]
- 92.De Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boffito M., Arnaudo I., Raiteri R., Bonora S., Sinicco A., Di Garbo A., Reynolds H.E., Hoggard P.G., Back D.J., Perri G.D. Clinical use of lopinavir/ritonavir in a salvage therapy setting: pharmacokinetics and pharmacodynamics. Aids. 2002;16(15):2081–2083. doi: 10.1097/00002030-200210180-00015. [DOI] [PubMed] [Google Scholar]
- 95.Chen F., Chan K.H., Jiang Y., Kao R.Y.T., Lu H.T., Fan K.W., Cheng V.C.C., Tsui W.H.W., Hung I.F.N., Lee T.S.W. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ye X., et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur. Rev. Med. Pharmacol. Sci. 2020;24(6):3390–3396. doi: 10.26355/eurrev_202003_20706. [DOI] [PubMed] [Google Scholar]
- 97.Hong R., et al. Clinical characterization and risk factors associated with cytokine release syndrome induced by COVID-19 and chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2020:1–11. doi: 10.1038/s41409-020-01060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khalili J.S., Zhu H., Mak N.S.A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID‐19. J. Med. Virol. 2020;92(7):740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9) doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morra M.E., Van Thanh L., Kamel M.G., Ghazy A.A., Altibi A.M.A., Dat L.M., Thy T.N.X., Vuong N.L., Mostafa M.R., Ahmed S.I., Elabd S.S., Fathima S., Le Huy Vu T., Omrani A.S., Memish Z.A., Hirayama K., Huy N.T. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta‐analysis. Rev. Med. Virol. 2018;28(3) doi: 10.1002/rmv.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., Jose J., Alraddadi B., Almotairi A., Al Khatib K., Abdulmomen A., Qushmaq I., Sindi A.A., Mady A., Solaiman O., Al-Raddadi R., Maghrabi K., Ragab A., Al Mekhlafi G.A., Balkhy H.H., Al Harthy A., Kharaba A., Gramish J.A., Al-Aithan A.M., Al-Dawood A., Merson L., Hayden F.G., Fowler R. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin. Infect. Dis. 2020;70(9):1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hung I.F.-N., Lung K.C., Tso E.Y.K., Liu R., Chung T.W.H., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., Shum H.P., Chan V., Wu A.K.L., Sin K.M., Leung W.S., Law W.L., Lung D.C., Sin S., Yeung P., Yip C.C.Y., Zhang R.R., Fung A.Y.F., Yan E.Y.W., Leung K.H., Ip J.D., Chu A.W.H., Chan W.M., Ng A.C.K., Lee R., Fung K., Yeung A., Wu T.C., Chan J.W.M., Yan W.W., Chan W.M., Chan J.F.W., Lie A.K.W., Tsang O.T.Y., Cheng V.C.C., Que T.L., Lau C.S., Chan K.H., To K.K.W., Yuen K.Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J., Zhang C., Li H., Xia X., Kong S., Liao J., Jia H., Pang X., Song Y., Tian Y., Wang B., Wu C., Yuan H., Zhang Y., Li Y., Sun W., Zhang Y., Zhu S., Wang S., Xie Y., Ge S., Zhang L., Hu Y., Xie M. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 104.Castilletti C., Bordi L., Lalle E., Rozera G., Poccia F., Agrati C., Abbate I., Capobianchi M.R. Coordinate induction of IFN-α and-γ by SARS-CoV also in the absence of virus replication. Virology. 2005;341(1):163–169. doi: 10.1016/j.virol.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shi S., PENG J., LI Y., QIN C., LIANG G., XU L., YANG Y., WANG J., SUN Q. The expression of membrane protein augments the specific responses induced by SARS-CoV nucleocapsid DNA immunization. Mol. Immunol. 2006;43(11):1791–1798. doi: 10.1016/j.molimm.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tseng C.-T.K., Perrone L.A., Zhu H., Makino S., Peters C.J. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J. Immunol. 2005;174(12):7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- 107.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID‐19: a single center experience. J. Med. Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alzghari S.K., Acuña V.S. Supportive treatment with tocilizumab for COVID-19: a systematic review. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Danilczyk U., Penninger J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ. Res. 2006;98(4):463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 111.Pu S.-Y., Xiao F., Schor S., Bekerman E., Zanini F., Barouch-Bentov R., Nagamine C.M., Einav S. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antivir. Res. 2018;155:67–75. doi: 10.1016/j.antiviral.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sorrell F.J., Szklarz M., Abdul Azeez K.R., Elkins J.M., Knapp S. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24(3):401–411. doi: 10.1016/j.str.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mesa R.A. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs. 2010;13(6):394. [PubMed] [Google Scholar]
- 114.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chaplin S. Baricitinib: a new oral treatment for rheumatoid arthritis. Prescriber. 2017;28(6):44–46. [Google Scholar]
- 117.Praveen D., Chowdary P.R., Aanandhi M.V. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khorramdelazad H., Kazemi M.H., Najafi A., Keykhaee M., Zolfaghari Emameh R., Falak R. Immunopathological similarities between COVID-19 and influenza: investigating the consequences of Co-infection. Microb. Pathog. 2021;152 doi: 10.1016/j.micpath.2020.104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., Zhang L., Neville S., Carra E., Lew W., Ross B., Wang Q., Wolfe L., Jordan R., Soloveva V., Knox J., Perry J., Perron M., Stray K.M., Barauskas O., Feng J.Y., Xu Y., Lee G., Rheingold A.L., Ray A.S., Bannister R., Strickley R., Swaminathan S., Lee W.A., Bavari S., Cihlar T., Lo M.K., Warren T.K., Mackman R.L. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo [2, 1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. J. Med. Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 120.Yoo J.-H. Uncertainty about the efficacy of Remdesivir on COVID-19. J. Korean Med. Sci. 2020;35(23) doi: 10.3346/jkms.2020.35.e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ko W.-C., Rolain J.M., Lee N.Y., Chen P.L., Huang C.T., Lee P.I., Hsueh P.R. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tchesnokov E., Feng J., Porter D., Götte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11(4):326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singh A.K., Singh A., Singh R., Misra A. Remdesivir in COVID-19: a critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(4):641–648. doi: 10.1016/j.dsx.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J., Liang Z., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019 nCoV) in vitro. Cell Res. 2019;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jacobs M., Rodger A., Bell D.J., Bhagani S., Cropley I., Filipe A., Gifford R.J., Hopkins S., Hughes J., Jabeen F., Johannessen I., Karageorgopoulos D., Lackenby A., Lester R., Liu R.S.N., MacConnachie A., Mahungu T., Martin D., Marshall N., Mepham S., Orton R., Palmarini M., Patel M., Perry C., Peters S.E., Porter D., Ritchie D., Ritchie N.D., Seaton R.A., Sreenu V.B., Templeton K., Warren S., Wilkie G.S., Zambon M., Gopal R., Thomson E.C. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388(10043):498–503. doi: 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lamb Y.N. Remdesivir: first approval. Drugs. 2020;80:1355–1363. doi: 10.1007/s40265-020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49(3):981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sissoko D., Laouenan C., Folkesson E., M’Lebing A.B., Beavogui A.H., Baize S., Camara A.M., Maes P., Shepherd S., Danel C., Carazo S., Conde M.N., Gala J.L., Colin G., Savini H., Bore J.A., Le Marcis F., Koundouno F.R., Petitjean F., Lamah M.C., Diederich S., Tounkara A., Poelart G., Berbain E., Dindart J.M., Duraffour S., Lefevre A., Leno T., Peyrouset O., Irenge L., Bangoura N., Palich R., Hinzmann J., Kraus A., Barry T.S., Berette S., Bongono A., Camara M.S., Chanfreau Munoz V., Doumbouya L., Souley Harouna, Kighoma P.M., Koundouno F.R., Réné Lolamou, Loua C.M., Massala V., Moumouni K., Provost C., Samake N., Sekou C., Soumah A., Arnould I., Komano M.S., Gustin L., Berutto C., Camara D., Camara F.S., Colpaert J., Delamou L., Jansson L., Kourouma E., Loua M., Malme K., Manfrin E., Maomou A., Milinouno A., Ombelet S., Sidiboun A.Y., Verreckt I., Yombouno P., Bocquin A., Carbonnelle C., Carmoi T., Frange P., Mely S., Nguyen V.K., Pannetier D., Taburet A.M., Treluyer J.M., Kolie J., Moh R., Gonzalez M.C., Kuisma E., Liedigk B., Ngabo D., Rudolf M., Thom R., Kerber R., Gabriel M., Di Caro A., Wölfel R., Badir J., Bentahir M., Deccache Y., Dumont C., Durant J.F., El Bakkouri K., Gasasira Uwamahoro M., Smits B., Toufik N., Van Cauwenberghe S., Ezzedine K., Dortenzio E., Pizarro L., Etienne A., Guedj J., Fizet A., Barte de Sainte Fare E., Murgue B., Tran-Minh T., Rapp C., Piguet P., Poncin M., Draguez B., Allaford Duverger T., Barbe S., Baret G., Defourny I., Carroll M., Raoul H., Augier A., Eholie S.P., Yazdanpanah Y., Levy-Marchal C., Antierrens A., Van Herp M., Günther S., de Lamballerie X., Keïta S., Mentre F., Anglaret X., Malvy D. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13(3) doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oestereich L., Lüdtke A., Wurr S., Rieger T., Muñoz-Fontela C., Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir. Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 134.Smither S.J., Eastaugh L.S., Steward J.A., Nelson M., Lenk R.P., Lever M.S. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antivir. Res. 2014;104:153–155. doi: 10.1016/j.antiviral.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 135.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Lu J., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boriskin Y., Leneva I., Pecheur E.I., Polyak S. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008;15(10):997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 138.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J. Infect. 2020;81(1):e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baron S.A., Devaux C., Colson P., Raoult D., Rolain J.M. Teicoplanin: an alternative drug for the treatment of COVID-19? Int. J. Antimicrob. Agents. 2020;55(10.1016) doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaccour C., Hammann F., Ramón-García S., Rabinovich N.R. Ivermectin and COVID-19: keeping rigor in times of urgency. Am. J. Trop. Med. Hyg. 2020;102(6):1156–1157. doi: 10.4269/ajtmh.20-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alam M.T., Murshed R., Bhiuyan E., Saber S., Alam R.F., Robin R.C. A case series of 100 COVID-19 positive patients treated with combination of ivermectin and doxycycline. J. Bangladesh Coll. Phys. Surg. 2020;38:10–15. [Google Scholar]
- 142.Rahman M.A., Iqbal S.A., Islam M.A., Niaz M.K., Hussain T., Siddiquee T.H. Comparison of viral clearance between ivermectin with doxycycline and hydroxychloroquine with azithromycin in COVID-19 patients. J. Bangladesh Coll. Physicians Surg. 2020:5–9. [Google Scholar]
- 143.Yates P.A., Newman S.A., Oshry L.J., Glassman R.H., Leone A.M., Reichel E. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620951053. 1753466620951053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Q., et al. Glucocorticoid application in the treatment of coronavirus disease 2019 (COVID-19): the pros and cons. Med. J. Wuhan Univ. 2020;41(4) [Google Scholar]
- 146.Rabaan A.A., Al-Ahmed S.H., Sah R., Al-Tawfiq J.A., Al-Qaaneh A.M., Al-Jamea L.H., Woodman A., Al-Qahtani M., Haque S., Harapan H., Bonilla-Aldana D.K., Kumar P., Dhama K., Rodriguez-Morales A.J. Recent advances in vaccine and immunotherapy for COVID-19. Hum. Vaccines Immunother. 2020;16:3011–3022. doi: 10.1080/21645515.2020.1825896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;323(16):1561–1562. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 149.Cherry J.D., Krogstad P. Sars: the first pandemic of the 21 st century. Pediatr. Res. 2004;56(1):1–5. doi: 10.1203/01.PDR.0000129184.87042.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Winkler A.M., Koepsell S.A. The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease. Curr. Opin. Hematol. 2015;22(6):521–526. doi: 10.1097/MOH.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 152.Al-Tawfiq J.A., Arabi Y. Convalescent plasma therapy for coronavirus infection: experience from MERS and application in COVID-19. Hum. Vaccines Immunother. 2020;16:2973–2979. doi: 10.1080/21645515.2020.1793712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Van Griensven J., Edwards T., de Lamballerie X., Semple M.G., Gallian P., Baize S., Horby P.W., Raoul H., Magassouba N., Antierens A., Lomas C., Faye O., Sall A.A., Fransen K., Buyze J., Ravinetto R., Tiberghien P., Claeys Y., De Crop M., Lynen L., Bah E.I., Smith P.G., Delamou A., De Weggheleire A., Haba N. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N. Engl. J. Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Korsukewitz C., Reddel S.W., Bar-Or A., Wiendl H. Neurological immunotherapy in the era of COVID-19—looking for consensus in the literature. Nat. Rev. Neurol. 2020;16(9):493–505. doi: 10.1038/s41582-020-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Esmaeilzadeh A., Elahi R. Immunobiology and immunotherapy of COVID‐19: a clinically updated overview. J. Cell. Physiol. 2020;236:2519–2543. doi: 10.1002/jcp.30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Andreakos E., Tsiodras S. COVID‐19: lambda interferon against viral load and hyperinflammation. EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202012465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou Q., Chen V., Shannon C.P., Wei X.S., Xiang X., Wang X., Wang Z.H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-α2b Treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Golchin A. Cell-based therapy for severe COVID-19 patients: clinical trials and cost-utility. Stem Cell Rev. Rep. 2020:1–7. doi: 10.1007/s12015-020-10046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev. Rep. 2020:1–7. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., Li J., Yu C., Nie F., Ma Z., Yang M., Xiao M., Nie P., Gao Y., Qian C., Hu M. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine. 2020;99(31) doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu B., Zhang X., Li X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014;15(3):4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gupta S., et al. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev. Rep. 2020:1–11. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gupta A., et al. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Hum. Cell. 2020:1–12. doi: 10.1007/s13577-020-00407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cerwenka A., Lanier L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 2001;1(1):41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 170.Feins S., Kong W., Williams E.F., Milone M.C., Fraietta J.A. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol. 2019;94(s1):3–9. doi: 10.1002/ajh.25418. [DOI] [PubMed] [Google Scholar]
- 171.Draghi M., Pashine A., Sanjanwala B., Gendzekhadze K., Cantoni C., Cosman D., Moretta A., Valiante N.M., Parham P. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J. Immunol. 2007;178(5):2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 172.Al-Halifa S., Gauthier L., Arpin D., Bourgault S., Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019;10:22. doi: 10.3389/fimmu.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Witika B.A., Makoni P.A., Mweetwa L.L., Ntemi P.V., Chikukwa M.T.R., Matafwali S.K., Mwila C., Mudenda S., Katandula J., Walker R.B. Nano-biomimetic drug delivery vehicles: potential approaches for COVID-19 treatment. Molecules. 2020;25(24):5952. doi: 10.3390/molecules25245952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Han Y., Král P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14(4):5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lammers T., Sofias A.M., van der Meel R., Schiffelers R., Storm G., Tacke F., Koschmieder S., Brümmendorf T.H., Kiessling F., Metselaar J.M. Dexamethasone nanomedicines for COVID-19. Nat. Nanotechnol. 2020;15(8):622–624. doi: 10.1038/s41565-020-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yetisgin A.A., Cetinel S., Zuvin M., Kosar A., Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25:2193. doi: 10.3390/molecules25092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abd Elkodous M., El-Sayyad G.S., Abdel-Daim M.M. Engineered nanomaterials as fighters against SARS-CoV-2: the way to control and treat pandemics. Environ. Sci. Pollut. Res. 2020:1–7. doi: 10.1007/s11356-020-11032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang M.-Y., Zhao R.R., Fang Y.F., Jiang J.L., Yuan X.T., Shao J.W. Carrier-free nanodrug: a novel strategy of cancer diagnosis and synergistic therapy. Int. J. Pharm. 2019;570 doi: 10.1016/j.ijpharm.2019.118663. [DOI] [PubMed] [Google Scholar]
- 179.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14(10):12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]