Abstract
Objective
To describe the clinical features and assess the determinants of severity and in-hospital mortality of patients with coronavirus disease 2019 (COVID-19) from a unique setting in Ethiopia.
Methods
Consecutive patients admitted to a COVID-19 isolation and treatment centre were included in this study. The overall clinical spectrum of COVID-19, and factors associated with risk of severe COVID-19 and in-hospital mortality were analysed.
Results
Of 2617 quarantined patients, three-quarters (n = 1935, 74%) were asymptomatic and only 114 (4.4%) presented with severe COVID-19. Common characteristics among the 682 symptomatic patients were cough (n = 354, 50.6%), myalgia (n = 212, 31.1%), headache (n = 196, 28.7%), fever (n = 161, 23.6%), dyspnoea (n = 111, 16.3%), anosmia and/or dysgeusia (n = 90, 13.2%), sore throat (n = 87, 12.8%) and chest pain (n = 77, 11.3%). Factors associated with severe COVID-19 were older age [adjusted relative risk (aRR) 1.78, 95% confidence interval (CI) 1.61–1.97; P < 0.0001], diabetes (aRR 2.00, 95% CI 1.20–3.32; P = 0.007), cardiovascular disease (aRR 2.53, 95% CI 1.53–4.17; P < 0.0001), malignancy (aRR 4.57, 95% CI 1.62–12.87; P = 0.004), surgery/trauma (aRR 23.98, 95% CI 10.35–55.57; P < 0.0001) and human immunodeficiency virus infection (aRR 4.24, 95% CI 1.55–11.61; P = 005). Factors associated with risk of in-hospital mortality included older age (aRR 2.37, 95% CI 1.90–2.95; P < 0.001), malignancy (aRR 6.73, 95% CI 1.50–30.16; P = 0.013) and surgery/trauma (aRR 59.52, 95% CI 12.90–274.68; P < 0.0001).
Conclusions
A significant proportion of cases of COVID-19 were asymptomatic, and key comorbid conditions increased the risk of severe COVID-19 and in-hospital mortality. These findings could help in the design of appropriate management strategies for patients.
Keywords: COVID-19, Africa, Comorbidities, Clinical features, Ethiopia, SARS-CoV-2, Mortality
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus that emerged in Wuhan, China in 2019, has resulted in an unprecedented global pandemic of respiratory illness, termed ‘coronavirus disease 2019’ (COVID-19) (Huang et al., 2020, Zhu et al., 2020, Wang et al., 2020). As of 3 February 2021, more than 104 million cases of COVID-19 and 2.26 million COVID-19-related deaths have been reported worldwide (World Health Organization, 2021). The first case of COVID-19 in Ethiopia was reported on 13 March 2020, and there had been 138,861 cases of COVID-19 and 2116 (1.5%) COVID-19-related deaths as of 3 February 2021.
Previous reports from China demonstrated that most patients with SARS-CoV-2 infection are asymptomatic or develop mild COVID-19, approximately 14% develop severe COVID-19 that requires hospitalization and oxygen support, and 5% require admission to an intensive care unit (Huang et al., 2020, Zhu et al., 2020, Wang et al., 2020). In severe cases, COVID-19 can be complicated by acute respiratory distress syndrome, sepsis and septic shock, and multi-organ failure, including acute kidney injury and cardiac injury (Cummings et al., 2020, Grasselli et al., 2020, Huang et al., 2020, Richardson et al., 2020, Wu et al., 2020, Zhu et al., 2020, Wang et al., 2020). The exact proportion of asymptomatic cases of COVID-19 remains unknown but it is estimated to be between 18% and 80% (Gandhi et al., 2020, Nikolai et al., 2020).
Most clinical reports of COVID-19 from high-income countries (HICs) are derived from symptomatic patients admitted to hospitals. In HICs, older age and underlying comorbidities due to non-communicable diseases (NCDs), such as hypertension, cardiovascular disease and diabetes, have been reported as risk factors for severe disease and death (Cummings et al., 2020, Grasselli et al., 2020, Richardson et al., 2020, Wu et al., 2020). However, in Sub-Saharan Africa (SSA), much less information is available on the symptomatology of COVID-19 (Abayomi et al., 2020, Elimian et al., 2020, Kirenga et al., 2020, Nachega et al., 2020). Literature is emerging to suggest significantly lower COVID-19-related morbidity and mortality in SSA compared with HICs (Diop et al., 2020, Fonte et al., 2020, Mbow et al., 2020, Njenga et al., 2020). Various hypotheses for this have been suggested, including 20-year lower average age of population in SSA, general under-reporting of (causes of) mortality in SSA, significantly reduced coverage of COVID-19 testing in SSA, different genetic backgrounds, different immune o-activation status and potential protection from helminth infections, and cross-protection due to non-pathogenic coronaviruses (Gutman et al., 2020, Hays et al., 2020, Margolin et al., 2020, Mbow et al., 2020). In low-to-middle-income countries (LMICs), communicable diseases, such as human immunodeficiency virus (HIV), tuberculosis, malaria and other neglected infectious diseases, are highly prevalent (Gutman et al., 2020, Hays et al., 2020). Moreover, NCDs are being identified with increasing frequency in SSA (Gutman et al., 2020).
The objective of this study was to describe the clinical features, associated risk factors, and morbidity and mortality among patients with COVID-19 in Ethiopia.
Methods
Study design and participants
This retrospective cohort study analysed a consecutive series of patients with COVID-19 admitted to Kuyha COVID-19 Isolation and Treatment Centre, Mekelle University College of Health Sciences, Mekelle City, Tigray State, in northern Ethiopia. Following the declaration of a pandemic situation by the World Health Organization (WHO), the Ethiopian Ministry of Health implemented mass screening of all travellers, people who had been in contact with confirmed cases of COVID-19, and people in high-risk settings (e.g. healthcare workers). Cases confirmed by polymerase chain reaction (PCR) between 10 May and 16 October 2020 were included. In this unique setting, all cases were either quarantined for 10–14 days or admitted to hospital in Kuyha, depending on their clinical presentation. After this time, management guidelines were changed and only people with symptoms were admitted.
Sociodemographic, clinical and laboratory data were collected using standardized case record forms adapted from the International Severe Acute Respiratory and Emerging Infection Consortium’s case record form (International Severe Acute Respiratory and Emerging Infection Consortium, 2020). A patient’s clinical status was stratified following WHO criteria as asymptomatic, mild/moderate, severe (dyspnoea, respiratory rate ≥30 breaths/min, O2 saturation ≤93%, lung infiltrates ≥50% of lung fields within 24–48 h) or critical (respiratory failure, septic shock, multiple organ failure) (World Health Organization, 2020). For this study, asymptomatic and mild/moderate cases were considered as non-severe cases, and severe and critical cases were considered as severe cases. All data were entered into electronic medical records.
Statistical analysis
Baseline characteristics for continuous variables were expressed as median and interquartile range (IQR), and those for categorical variables were expressed as count and percentage. For categorical variables, comparisons were performed using Chi-squared test or Fisher’s exact test whenever any expected cell count was <5. Continuous variables were compared using Mann–Whitney U-test or Kruskal–Wallis test, as appropriate.
Factors associated with severe COVID-19 and in-hospital mortality were analysed using Poisson regression analysis. Using univariate analysis, the relative risk (RR) of severe disease or in-hospital mortality was analysed with respect to baseline demographic data, clinical characteristics and comorbid conditions. Next, a multi-variate regression analysis [adjusted relative risk (aRR)] was undertaken (with backward stepwise elimination), including all variables that were significant (P < 0.10) on univariate analysis. Two-sided P < 0.05 was considered to indicate significance. Data were analysed using STATA Version 15.0 (StataCorp, College Station, TX, USA).
Results
Characteristics of study participants
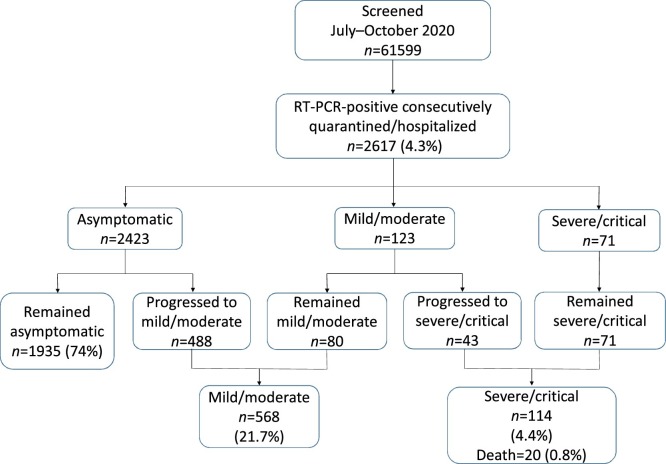
During the study period, 61,599 individuals were tested for SARS-CoV-2. Of these, 2617 (4.3%) patients who tested positive on reverse transcriptase PCR (RT-PCR) for SARS-CoV-2 were included in the study (Figure 1 , Table 1 ). The majority of the study population were male (63.3%), and among the 960 females enrolled, 27 (2.8%) were pregnant. The median age of the cohort was 29 (IQR 24–38) years, and the majority (66.9%) of participants were in the age range 20–39 years. The proportion of children aged <20 years was 9.2% (n = 242), with a median age of 17 (IQR 14–18) years, and those aged ≤10 years constituted only 1.1%, with a median age of 4 (IQR 1–7) years. The proportion of patients aged ≥60 years was only 5.7%, with a median age of 67 (IQR 62–75) years. Many patients reported a history of recent travel (57.9%), and very few (4.6) reported contact history with a known confirmed case of COVID-19.
Figure 1.
Flow diagram of the clinical spectrum of coronavirus disease 2019. RT-PCR, reverse transcription polymerase chain reaction.
Table 1.
Baseline sociodemographic and clinical characteristics of patients with coronavirus disease 2019 in northern Ethiopia.
| Characteristic | All cases | Non-severe cases (asymptomatic and mild/moderate) | Severe cases (severe and critical) | P-value |
|---|---|---|---|---|
| n = 2617 | n = 2503 | n = 114 | ||
| Sociodemographic features | ||||
| Gender (male), n (%) | 1657 (63.3) | 1569 (62.7) | 88 (77.2) | 0.002 |
| Age in years [median (IQR)] | 29 (24–38) | 29 (24–38) | 55 (38–74) | <0.00001 |
| Age group [years, n (%)] | ||||
| <20 | 242 (9.2) | 234 (9.4) | 8 (7.0) | <0.0001 |
| 20–39 | 1752 (66.9) | 1730 (69.1) | 22 (19.3) | |
| 40–59 | 475 (18.2) | 443 (17.7) | 32 (28.1) | |
| ≥60 | 148 (5.7) | 96 (3.8) | 52 (45.6) | |
| Healthcare workers, n (%) | 229 (8.8) | 224 (9.0) | 5 (4.4) | 0.092 |
| Urban residents, n (%) | 698 (26.8) | 669 (26.9) | 29 (25.4) | 0.392 |
| History of travel, n (%) | 1514 (57.9) | 1409 (56.3) | 105 (92.1) | <0.0001 |
| History of contact with COVID-19 case, n (%) | 119 (4.6) | 117 (4.8) | 2 (1.8) | 0.143 |
| Clinical symptoms and signs | ||||
| Fever, n (%) | 161 (6.2) | 127 (5.1) | 34 (29.8) | <0.0001 |
| Dyspnoea, n (%) | 111 (4.2) | 35 (1.4) | 76 (66.7) | <0.0001 |
| Cough [any type, n (%)] | 345 (13.2) | 274 (11.0) | 71 (62.3) | <0.0001 |
| Non-productive cough | 191 (7.3) | 157 (6.3) | 34 (29.8) | <0.0001 |
| Productive cough | 154 (5.9) | 117 (4.7) | 37 (32.5) | <0.0001 |
| Haemoptysis, n (%) | 13 (0.5) | 10 (0.4) | 3 (2.6) | 0.001 |
| Chest pain, n (%) | 77 (2.9) | 56 (2.2) | 21 (18.4) | <0.0001 |
| Sore throat, n (%) | 87 (3.3) | 74 (3.0) | 13 (11.4) | <0.0001 |
| Headache, n (%) | 196 (7.5) | 159 (6.4) | 37 (32.5) | <0.0001 |
| Nasal congestion, n (%) | 59 (2.3) | 55 (2.2) | 4 (3.5) | 0.356 |
| Anosmia and/or dysgeusia, n (%) | 90 (3.4) | 74 (3.0) | 16 (14.0) | <0.0001 |
| Diarrhoea, n (%) | 24 (0.9) | 20 (0.8) | 4 (3.5) | 0.003 |
| Myalgia, n (%) | 212 (8.1) | 149 (6.0) | 63 (55.3) | <0.0001 |
| Temperature (median °C, IQR) | 36.0 (36.0–36.7) | 36.0 (36.0–36.7) | 36.3 (36.0–36.7) | 0.6756 |
| Systolic blood pressure (median mmHg, IQR) | 115 (108–125) | 115 (109–125) | 118 (110–125) | 0.9616 |
| Diastolic blood pressure (median mmHg, IQR) | 75 (67–80) | 75 (67–80) | 76 (69–80) | 0.2523 |
| Respiratory rate (median breaths/min, IQR) | 21 (19–22) | 21 (19–22) | 22 (20–23) | 0.1654 |
| Heart rate (median beats/min, IQR) | 80 (74–88) | 80 (73–88) | 81 (74–90) | 0.3292 |
IQR, interquartile range.
Most notably, 92.6% of the cohort population were asymptomatic at the time of diagnosis (Figure 1). The remaining subjects had either mild/moderate symptoms (4.7%) or presented with severe disease (2.7%). During quarantine, 488 patients progressed from asymptomatic to mild/moderate clinical presentation, while 43 cases with an initial mild/moderate clinical presentation developed severe/critical disease. Overall, almost one-quarter of the cohort (74%) remained asymptomatic throughout the quarantine period (median 12 days, IQR 10–14 days), while 21.7% had mild/moderate disease and only 4.4% had severe/critical disease. Of the 114 severe cases, only 15 (13.2%) required mechanical ventilation. Patients with severe clinical presentation were predominantly male and older than patients with non-severe clinical presentation (Table 1). Healthcare workers represented 8.8% of the infected population, and the majority presented with no symptoms or mild/moderate symptoms. Among patients who presented with symptoms, the most common were cough (50.6%), myalgia (31.1%), headache (28.7%), fever (23.6%), dyspnoea (16.3%), anosmia and/or dysgeusia (13.2%), sore throat (12.8%) and chest pain (11.3%) (Table 2 ). Diarrhoea (3.5%) and haemoptysis (1.9%) were reported with lower frequencies. However, among patients with severe COVID-19, the most common symptoms were cough (96.5%), dyspnoea (66.7%), myalgia (55.3%), headache (32.5%), fever (29.8%), chest pain (18.4%), and anosmia and/or dysgeusia (14.0%).
Table 2.
Comparison of sociodemographic and clinical characteristics of mild/moderate versus severe cases of coronavirus disease 2019 in northern Ethiopia.
| Characteristic | All symptomatic cases | Mild/moderate cases | Severe cases (severe and critical) | P-value |
|---|---|---|---|---|
| n = 682 | n = 568 | n = 114 | ||
| Sociodemographic features | ||||
| Gender (male), n (%) | 471 (69.1) | 383 (67.4) | 88 (77.2) | 0.040 |
| Age in years [median (IQR)] | 32 (26–46) | 30 (25–40) | 55 (38–74) | <0.00001 |
| Age group [years, n (%)] | ||||
| <20 | 36 (5.3) | 28 (4.9) | 8 (7.0) | <0.0001 |
| 20–39 | 418 (61.3) | 396 (69.7) | 22 (19.3) | |
| 40–59 | 139 (20.4) | 107 (18.9) | 32 (28.1) | |
| ≥60 | 89 (13.0) | 37 (6.5) | 52 (45.6) | |
| Healthcare workers, n (%) | 110 (16.1) | 105 (18.5) | 5 (4.4) | <0.0001 |
| Urban residents, n (%) | 29 (25.4) | 0.107 | ||
| History of travel, n (%) | 493 (74.3) | 396 (71.0) | 97 (91.5) | <0.0001 |
| History of contact with COVID-19 case, n (%) | 26 (4.1) | 24 (4.5) | 2 (2.0) | 0.231 |
| Clinical symptoms and signs | ||||
| Fever, n (%) | 161 (23.6) | 127 (22.4) | 34 (29.8) | 0.087 |
| Dyspnoea, n (%) | 111 (16.3) | 35 (6.2) | 76 (66.7) | <0.0001 |
| Cough (any type, n (%)) | 354 (50.6) | 274 (48.2) | 110 (96.5) | <0.0001 |
| Non-productive cough | 191 (28.0) | 157 (27.6) | 34 (29.8) | 0.636 |
| Productive cough | 154 (22.6) | 117 (20.6) | 37 (32.5) | 0.006 |
| Haemoptysis, n (%) | 13 (1.9) | 10 (1.8) | 3 (2.6) | 0.535 |
| Chest pain, n (%) | 77 (11.3) | 56 (9.9) | 21 (18.4) | 0.008 |
| Sore throat, n (%) | 87 (12.8) | 74 (13.0) | 13 (11.4) | 0.635 |
| Headache, n (%) | 196 (28.7) | 159 (28.0) | 37 (32.5) | 0.337 |
| Nasal congestion, n (%) | 59 (8.7) | 55 (9.7) | 4 (3.5) | 0.032 |
| Anosmia and/or dysgeusia, n (%) | 90 (13.2) | 74 (13.0) | 16 (14.0) | 0.772 |
| Diarrhoea, n (%) | 24 (3.5) | 20 (3.5) | 4 (3.5) | 0.995 |
| Myalgia, n (%) | 212 (31.1) | 149 (26.2) | 63 (55.3) | <0.0001 |
| Temperature (median oC, IQR) | 36.0 (36.0–36.7) | 36.0 (36.0–36.7) | 36.3 (36.0–36.7) | 0.9616 |
| Systolic blood pressure (median mmHg, IQR) | 115 (108–125) | 116 (110–125) | 115 (105–1125) | 0.7106 |
| Diastolic blood pressure (median mmHg, IQR) | 76 (69–76) | 78 (70–81) | 76 (69–80) | 0.3882 |
| Respiratory rate (median breaths/min, IQR) | 22 (20–22) | 21 (18–22) | 22 (20–23) | 0.0047 |
| Heart rate (median beats/min, IQR) | 81 (75–90) | 82 (75–90) | 81 (74–90) | 0.6978 |
IQR, interquartile range.
Comorbidities and other conditions
In total, 285 (10.9%) patients with COVID-19 had at least one comorbidity, and 30 (10.5%) of these patients had at least two comorbidities (Table 3 ). NCDs comprised the majority of comorbidities (87.7%), with diabetes (3.1%), cardiovascular disease (including hypertension) (3.1%) and chronic obstructive lung disease (including asthma) (2.8%) being the most common Of the female patients with COVID-19, 2.8% were pregnant. Overall, comorbid conditions were significantly higher among those who presented with severe disease (53.5%) compared with those with non-severe clinical presentation (9.0%) (P < 0.0001). In addition, despite low frequencies, communicable diseases, particularly HIV, were reported more frequently among severe (3.5%) cases of COVID-19 compared with non-severe (0.8%) cases (P = 0.003). Of the 24 patients co-infected with HIV-1 and SARS-CoV-2, 21 (88%) were already on antiretroviral therapy and three were newly diagnosed.
Table 3.
Comorbidities and underlying conditions among patients with coronavirus disease 2019.
| Characteristic | All cases | Non-severe cases (asymptomatic and mild/moderate) | Severe cases (severe and critical) | P-value |
|---|---|---|---|---|
| i = 2617 | n = 2503 | n = 114 | ||
| At least one comorbidity, n (%) | 285 (10.9) | 224 (9.0) | 61 (53.5) | <0.0001 |
| At least two comorbidities, n (%) | 30 (1.2) | 18 (0.7) | 12 (10.5) | <0.0001 |
| Non-communicable disease comorbidities, n (%) | 250 (9.6) | 191 (7.6) | 59 (51.8) | <0.0001 |
| Communicable disease comorbidities, n (%) | 39 (1.5) | 35 (1.4) | 4 (3.5) | 0.069 |
| Diabetes, n (%) | 82 (3.1) | 61 (2.4) | 21 (18.4) | <0.0001 |
| Cardiovascular disease, including hypertension, n (%) | 82 (3.1) | 54 (2.2) | 28 (24.6) | <0.0001 |
| Chronic obstructive lung disease, including asthma, n (%) | 72 (2.8) | 66 (2.6) | 6 (5.3) | 0.094 |
| Chronic liver disease, n (%) | 5 (0.2) | 5 (0.2) | 0 (0.0) | 0.633 |
| Chronic kidney disease, n (%) | 16 (0.6) | 12 (0.5) | 4 (3.5) | <0.0001 |
| Malignancy, n (%) | 9 (0.3) | 5 (0.2) | 4 (3.5) | <0.0001 |
| Surgery/trauma, n (%) | 13 (0.5) | 7 (0.3) | 6 (5.3) | <0.0001 |
| Pregnancy, n (%) | 27 (2.8) | 26 (2.8) | 1 (3.9) | 0.747 |
| Human immunodeficiency virus, n (%) | 24 (0.9) | 20 (0.8) | 4 (3.5) | 0.003 |
| Tuberculosis, n (%) | 8 (0.3) | 8 (0.3) | 0 (0.0) | 0.545 |
| Malaria, n (%) | 9 (0.3) | 8 (0.3) | 1 (0.9) | 0.320 |
Factors associated with severe COVID-19 and in-hospital mortality
Of the 2617 patients with COVID-19, only 114 (4.4%) presented with severe disease. Risk factors associated with severe COVID-19 are summarized in Table 4 . On multi-variate analysis, the risk factors significantly associated with severe COVID-19 were older age (aRR 1.78, 95% CI 1.61–1.97; P < 0.0001) and having any comorbid conditions (aRR 3.56, 95% CI 2.36–5.38; P < 0.0001), particularly NCDs (aRR 3.64, 95% CI 2.39–5.54; P < 0.0001). Of the NCDs, diabetes (aRR 2.00, 95% CI 1.20–3.32; P = 0.007), cardiovascular disease (aRR 2.53, 95% CI 1.53–4.17; P < 0.0001), malignancy (aRR 4.57, 95% CI 1.62–12.87; P = 0.004) and surgery/trauma (aRR 23.98, 95% CI 10.35–55.57; P < 0.0001) were associated with increased risk of severe COVID-19. HIV-1 was the only communicable disease associated with severe COVID-19 (aRR 4.24, 95% CI 1.55–11.61; P = 0.005).
Table 4.
Factors associated with severity of coronavirus disease 2019 among patients in northern Ethiopia.
| Characteristic | Unadjusted relative risk (95% CI) | P-value | Adjusted relative risk (95% CI) | P-value |
|---|---|---|---|---|
| Gender (male versus female) | 1.96 (1.27–3.04) | 0.003 | 1.33 (0.85–2.07) | 0.212 |
| Age (per 10 years older) | 1.92 (1.76–2.09) | <0.0001 | 1.78 (1.61–1.97) | <0.0001 |
| Healthcare workers (versus others) | 1.09 (0.72–1.63) | 0.696 | ||
| Pregnancy | 1.38 (0.19–10.20) | 0.751 | ||
| At least one comorbiditya | 9.42 (6.52–13.61) | <0.0001 | 3.56 (2.36–5.38) | <0.0001 |
| At least two comorbiditiesa | 10.15 (5.58–18.45) | <0.0001 | 2.66 (1.44–4.89) | 0.002 |
| Non-communicable disease comorbiditiesa | 10.16 (7.03–14.67) | <0.0001 | 3.64 (2.39–5.54) | <0.0001 |
| Communicable disease comorbiditiesa | 2.40 (0.89–6.52) | 0.085 | ||
| Diabetesb | 6.98 (4.35–11.21) | <0.0001 | 2.00 (1.20–3.32) | 0.007 |
| Cardiovascular disease, including hypertensionb | 10.07 (6.57–15.42) | <0.0001 | 2.53 (1.53–4.17) | <0.0001 |
| Chronic obstructive lung disease, including asthma | 1.96 (0.86–4.47) | 0.108 | ||
| Chronic liver disease | 2.06e-09 (0.00–) | 1.000 | ||
| Chronic kidney diseaseb | 5.91 (2.18–16.03) | <0.0001 | 2.25 (0.81–6.24) | 0.118 |
| Malignancyb | 10.54 (3.89–28.58) | <0.0001 | 4.57 (1.62–12.87) | 0.004 |
| Surgery/traumab | 11.13 (4.89–25.32) | <0.0001 | 23.98 (10.35–55.57) | <0.0001 |
| Human immunodeficiency virusb | 3.93 (1.45–10.65) | 0.007 | 4.24 (1.55–11.61) | 0.005 |
| Tuberculosis | 2.05e-09 (0.00–) | 1.000 | ||
| Malaria | 2.56 (0.36–18.36) | 0.348 |
CI, confidence interval.
Adjusted for gender and age.
Adjusted for gender, age, diabetes, cardiovascular disease, malignancy and surgery/trauma.
Of the 2617 patients in the cohort, only 20 (0.8%) died during in-hospital admission Table 5 . Five (0.2%) patients were either transferred out to other facilities or lost to follow-up. Overall, 2592 (99.9%) of the admitted patients recovered and were discharged. None of the children aged <20 years died, two deaths (10%) were among patients aged 20–39 years, four (20%) deaths were among patients aged 40–59 years, and the remaining 14 (70%) deaths were among patients aged ≥60 years. On multi-variate analysis, the factors significantly associated with increased risk of in-hospital mortality were older age (aRR 2.37, 95% CI 1.90–2.95; P < 0.001), malignancy (aRR 6.73, 95% CI 1.50–30.16; P = 0.013) and surgery/trauma (aRR 59.52, 95% CI 12.90–274.68; P < 0.0001) Table 5.
Table 5.
Factors associated with in-hospital mortality among patients with coronavirus disease 2019 in Ethiopia.
| Characteristic | Unadjusted relative risk | P-value | Adjusted relative risk | P-value |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Gender (male versus female) | 2.32 (0.78–6.93) | 0.133 | 1.37 (0.45–4.18) | 0.583 |
| Age (per 10 years older) | 2.33 (1.90–2.86) | <0.0001 | 2.37 (1.90–2.95) | <0.001 |
| Healthcare workers (versus others) | 0.55 (0.07–4.10) | 0.559 | ||
| Pregnancy | 2.25e-09 | 1.000 | ||
| At least one comorbiditya | 10.00 (4.14–24.13) | <0.0001 | 2.50 (0.98–6.37) | 0.055 |
| At least two comorbiditiesa | 21.56 (7.20–64.48) | <0.0001 | 4.86 (1.61–14.64) | 0.005 |
| Non-communicable disease comorbiditiesa | 11.57 (4.80–27.93) | <0.0001 | 2.73 (1.06–6.99) | 0.037 |
| Communicable disease comorbiditiesa | 2.29e-09 | 1.000 | ||
| Diabetes | 7.73 (2.58–23.12) | <0.0001 | 2.50 (0.77–8.14) | 0.129 |
| Cardiovascular disease, including hypertension | 7.73 (2.58–23.12) | <0.0001 | 1.36 (0.40–4.63) | 0.623 |
| Chronic obstructive lung disease, including asthma | 3.93 (0.91–16.93) | 0.066 | ||
| Chronic liver disease | 2.06e-09 (0.00–) | 1.000 | ||
| Chronic kidney disease | 2.32e-09 | 1.000 | ||
| Malignancyb | 32.20 (7.47–138.76) | <0.0001 | 6.73 (1.50–30.16) | 0.013 |
| Surgery/traumab | 22.26 (5.16–95.92) | <0.0001 | 59.52 (12.90–274.68) | <0.0001 |
| Human immunodeficiency virus | 231e-09 | 1.000 | ||
| Tuberculosis | 2.05e-09 (0.00–) | 1.000 | ||
| Malaria | 2.32e09 | 1.000 |
Adjusted for gender and age.
Adjusted for gender, age, diabetes, cardiovascular disease, malignancy and surgery/trauma.
Discussion
To the best of the authors’ knowledge, this is one of the first large cohort studies on COVID-19 undertaken in SSA on a relatively representative population. This was possible due to the unique setting of compulsory quarantine measures for all who tested positive for SARS-CoV-2. The true proportion of asymptomatic patients is not known (Gandhi et al., 2020, Nikolai et al., 2020). The present study noted a high proportion (74%) of asymptomatic cases. This finding is similar to a recent report from Addis Ababa, the epicentre of SARS-CoV-2 infection in Ethiopia, where the investigators reported that 78% of cases were asymptomatic (Abrahim et al., 2020). Moreover, the findings are consistent with previous reports showing high proportions of asymptomatic carriers of SARS-CoV-2, ranging from 66% to 88% (Barrett et al., 2020, Ing et al., 2020, Lytras et al., 2020, Bagget et al., 2020), but higher than those reported from Nigeria, Uganda, the Middle East, Europe and the USA, ranging from 45% to 58% (Abayomi et al., 2020, Almazeedi et al., 2020, Kirenga et al., 2020, Mizumoto et al., 2020, Moriarty et al., 2020). The implications of high proportions of asymptomatic cases for the spread of COVID-19 in SSA remain to be determined. Nonetheless, it has been suggested that a high proportion of asymptomatic (or presymptomatic) cases fuels the pandemic through super-spreading events (Gandhi et al., 2020, Nikolai et al., 2020). Tracking and tracing super-spreaders and their contacts and subsequent isolation/quarantine are a cornerstone of interrupting the transmission cycle of the pandemic. This is particularly challenging when super-spreaders are aymptomatic. It has been reported that asymptomatic cases transmit with 42% lower relative risk than symptomatic cases, with an unknown proportion of super-spreaders amongst the asymptomatic cases (Buitrago-Garcia et al., 2020).
One of the challenges with respect to describing the overall clinical spectrum of COVID-19 in a particular geographical setting is that a significant proportion of patients progress from one status of clinical presentation to another. Indeed, the actual proportion of patients initially diagnosed as asymptomatic but who subsequently become symptomatic (also known as ‘presymptomatic cases’) is not known. This study provides more information on this topic. A previous study from China with a small sample size showed that a significant proportion (14/24, 60%) of infected patients were presymptomatic and developed COVID-19 symptoms within 1–3 weeks (Hu et al., 2020). Likewise, a report from Japan showed that 11/96 (11.5%) asymptomatic cases who transferred from the cruise ship Diamond Princess to a hospital developed clinical signs and symptoms a median of 4 days after the first positive PCR test (Sakurai et al., 2020). In addition, a study from Kuwait reported 35/508 (6.9%) presymptomatic patients (Almazeedi et al., 2020). Interestingly, the present study identified 488 (18.7%) asymptomatic patients who subsequently developed symptoms, indicating that these patients had been presymptomatic at the time of PCR testing. The wide variation in the proportion of presymptomatic cases seen in different countries may be related to the diverse clinical status of COVID-19 included in the reports.
The present cohort mainly included male patients, similar to previous studies (Abayomi et al., 2020, Almazeedi et al., 2020, Cummings et al., 2020, Gandhi et al., 2020, Grasselli et al., 2020, Nachega et al., 2020, Nikolai et al., 2020, Richardson et al., 2020, Wu et al., 2020). The median age was 29 years, which was lower compared with other studies from Africa (33–46 years) (Abayomi et al., 2020, Elimian et al., 2020, Kirenga et al., 2020, Nachega et al., 2020), the Middle East (41 years) (Almazeedi et al., 2020) and China (49 years) (Huang et al., 2020), and much lower compared with studies from HICs (approximately 62 years) (Cummings et al., 2020, Grasselli et al., 2020, Richardson et al., 2020). Younger median age is an important factor explaining the high proportion of asymptomatic cases observed. In the present study, the median age of patients with severe COVID-19 (60 years) was similar to other settings (Abayomi et al., 2020, Almazeedi et al., 2020, Cummings et al., 2020, Elimian et al., 2020, Gandhi et al., 2020, Grasselli et al., 2020, Kirenga et al., 2020, Nachega et al., 2020, Nikolai et al., 2020, Richardson et al., 2020, Wu et al., 2020). The proportion of children aged <20 years in the present study was 9.1%, which was higher compared with studies from Democratic Republic of Congo (DRC) and Nigeria (Abayomi et al., 2020, Nachega et al., 2020), but lower compared with studies from Nigeria and Uganda (Elimian et al., 2020, Kirenga et al., 2020). Typically, SARS-CoV-2 infection manifests as asymptomatic or mild disease in the majority of children (Wald et al., 2020). As such, the higher proportion of children included in the present study and studies from other African countries (Elimian et al., 2020, Kirenga et al., 2020) may be related to the inclusion of all patients with COVID-19, including a significant proportion of asymptomatic cases. The proportion of patients aged ≥60 years was low (5.7%) in the present study compared with studies from DRC and Nigeria (Abayomi et al., 2020, Nachega et al., 2020).
Among the cohort participants who were symptomatic, the most common symptoms noted at presentation were cough (50.6%), myalgia (31.1%), headache (28.7%), fever (23.6%), dyspnoea (16.3%), anosmia and/or dysgeusia (13.2%), sore throat (12.8%) and chest pain (11.3%). Cough (96.5%), dyspnoea (66.7%), myalgia (55.3%), headache (32.5%), fever (29.8%), chest pain (18.4%), and anosmia and/or dysgeusia (14.0%) were the most common symptoms reported among those who developed severe COVID-19. Anosmia and/or dysgeusia was less common in patients with non-severe COVID-19 compared with patients with severe COVID-19 in the study cohort. This was similar to the findings of studies from Nigeria (Abayomi et al., 2020) and South Africa (Parker et al., 2020), but differed from the study from DRC which reported that none of the study participants displayed anosmia and/or dysgeusia (Nachega et al., 2020). Diarrhoea was reported in 3.5% of patients in the present study, which was similar to the findings reported from Nigeria and Uganda (Abayomi et al., 2020, Kirenga et al., 2020).
The proportion of cases with severe COVID-19 in the study cohort was low (4.4%) in comparison with the study from DRC (Nachega et al., 2020), but similar compared with studies from Uganda, Nigeria and Kuwait (Abayomi et al., 2020, Almazeedi et al., 2020, Elimian et al., 2020, Kirenga et al., 2020). However, severe COVID-19 requiring hospitalization has been reported to occur in 10–20% of settings in HICs (Garg et al., 2020, Huang et al., 2020, Kimball et al., 2020, Mizumoto et al., 2020, Zhu et al., 2020). Consistent with previous reports (Abayomi et al., 2020, Almazeedi et al., 2020, Cummings et al., 2020, Gandhi et al., 2020, Grasselli et al., 2020, Nachega et al., 2020, Nikolai et al., 2020, Richardson et al., 2020, Wu et al., 2020), the present findings showed that a significant proportion of patients in the cohort with pre-existing comorbid conditions developed severe COVID-19 or died. Overall, NCDs were more prevalent than communicable diseases. The most common NCDs among patients with severe COVID-19 were cardiovascular disease (including hypertension) (24.6%), diabetes (18.4%) and chronic obstructive lung disease (including asthma) (5.3%). The overall proportion of these comorbid conditions in both non-severe and severe cases of COVID-19 in the study cohort was lower than reported previously from both HICs (Cummings et al., 2020, Richardson et al., 2020, Wu et al., 2020) and LMICs (Abayomi et al., 2020, Nachega et al., 2020, Parker et al., 2020). In addition, although only a small number of cases were observed in this study, co-infection with HIV-1 was found to be an independent and significant risk factor for the severity of COVID-19. This study did not find any association between tuberculosis and the severity of COVID-19, and HIV and tuberculosis did not contribute to in-hospital mortality. The finding that HIV did not affect in-hospital mortality was similar to the results from DRC and South Africa (Nachega et al., 2020, Parker et al., 2020), but differed compared with a study from Western Cape Province in South Africa (Boulle et al., 2020), where both HIV and tuberculosis independently increased the risk of COVID-19-related death. Such differences may be attributed to the overall low proportion of deaths observed in the study cohort or studies from other African countries (Nachega et al., 2020, Parker et al., 2020) in comparison with the cohort from Cape Town, South Africa (Boulle et al., 2020). In addition, this study did not find any association between malaria and the severity of COVID-19; however, the number of cases of malaria was too small to draw firm conclusions. The proportion of healthcare workers infected with SARS-CoV-2 in the study cohort was 8.8%, which was lower compared with a study from Nigeria (14.3%) (Elimian et al., 2020). Although it has been reported that healthcare workers are at increased risk of developing severe COVID-19 (Rothan and Byrareddy, 2020), no increased risk for severe COVID-19 or mortality was noted in the study cohort.
As of 3 February 2021, there had been 3,614,270 cases of COVID-19 and 92,472 COVID-19-related deaths in SSA, representing a case fatality rate of 2.6% (World Health Organization, 2021). The overall mortality rate among patients with COVID-19 in the study cohort was significantly lower (0.8%), and also lower than the national Ethiopian case fatality rate (1.5%), in comparison with studies from DRC (13.2%) (Nachega et al., 2020), Nigeria (9.2%) (Elimian et al., 2020) and South Africa (26.7%) (Parker et al., 2020). The main reason for the lower mortality rate observed in the study cohort compared with other SSA countries may be related to the inclusion of a significant proportion of asymptomatic cases in the study cohort. The in-hospital mortality rate among severe cases of COVID-19 in the study cohort was lower (28.6%) compared with the mortality rate among severe cases of COVID-19 from DRC (45%) (Nachega et al., 2020) and South Africa (47.6%) (Parker et al., 2020). In addition, no deaths were reported in children aged <20 years in the study cohort, compared with 11.8% and 2.4% in studies from DRC and Nigeria, respectively (Nachega et al., 2020, Elimian et al., 2020). Such differences may be attributed to the younger age, lower prevalence of comorbid conditions, and predominantly asymptomatic or mild SARS-CoV-2 infections in the study cohort. In addition, co-infection with parasites that are known to modulate the immune response and reduce hyperinflammation associated with increased risk of severity of COVID-19 may play a significant role (Hays et al., 2020). Prospective studies are underway to see whether co-infection with parasites reduces the severity of COVID-19.
The main strength of this study was the enrolment of all cases of COVID-19 confirmed by RT-PCR who were admitted to a single centre for treatment and/or quarantine, irrespective of the severity of disease. As such, it was possible to determine the true proportions of asymptomatic cases and severe cases. Another strength of the current study was the large sample size compared with previous studies from SSA. However, this study also had a few limitations. First, data were missing due to the retrospective nature of the study, thus precluding detailed analyses of factors that may impact the risk of severe COVID-19 and in-hospital mortality. Second, incomplete data of key laboratory markers limited the power of risk factor association analyses. Third, the sample size of patients with communicable diseases was too small to conclude that such diseases, particularly malaria and tuberculosis, do not influence the severity of COVID-19. Fourth, due to the retrospective nature of this study, it was not possible to measure the antibody seroconversion rate of patients who remained asymptomatic. Finally, the study may not be generalizable to the rest of SSA, as admission criteria differ between countries.
Conclusion
To the best of the authors’ knowledge, this study is one of the first comprehensive assessments of the clinical spectrum of COVID-19 admitted to a single centre in SSA for treatment and/or quarantine. The study also provided information on the most relevant comorbid risks related to NCDs for severe COVID-19; the findings were similar to those reported previously from HICs and LMICs (Abayomi et al., 2020, Elimian et al., 2020, Grasselli et al., 2020, Nachega et al., 2020, Richardson et al., 2020, Wu et al., 2020). More importantly, this study found that co-infection with HIV-1, which is highly prevalent in SSA (70% of global prevalence) (Margolin et al., 2020), is strongly associated with increased risk of severe COVID-19. Future cohort studies are needed to address the effect of other highly prevalent infections in SSA, including tuberculosis and malaria, on patients with COVID-19.
Conflict of interest
None declared.
Funding
This project was funded, in part, by the European and Developing Countries Clinical Trials Partnership (EDCTP)–European Union (Profile-Cov project, Grant #: RIA-2020EF-2095).
Ethical approval
The study protocol was reviewed and approved by the Health Research Ethics Review Committee of Mekelle University College of Health Sciences (#ERC 1822/2020). As the nature of the study was retrospective, consent was not obtained from individual study participants and approved by the Ethics Review Committee. Nonetheless, all personal identifiers were removed from the original sources.
Acknowledgments
The authors wish to thank the staff of the Kuyha COVID-19 Isolation and Treatment Centre, Mekelle University College of Health Sciences for their cooperation.
References
- Abayomi A., Odukoya O., Osibogun A., Wright O., Adebayo B., Abdus-Salam I. Presenting symptoms and predictors of poor outcome among 2184 patients with COVID-19 in Lagos State, Nigeria. Int J Infect Dis. 2020;102:226–232. doi: 10.1016/j.ijid.2020.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahim S.A., Tessema Demoz G., Defar A., Hussen A., Ejeta E., Demoz G. Time to recovery and its predictors among adults hospitalized with COVID-19: a prospective cohort study in Ethiopia. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazeedi S., Al-Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagget T.P., Keys H., Sporn N., Gaeta J.M. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;23:2191–2192. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E.S., Horton D.B., Roy J., Gennaro M.L., Brooks A., Tischfield J. Prevalence of SARS-CoV-2 in previously undiagnosed health care workers at the onset of the U.S. COVID-19 epidemic. medRxiv. 2020 doi: 10.1101/2020.04.20.20072470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle A., Davies M.-A., Hussey H., Ismail M., Morden E., Vundle Z. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago-Garcia D., Egli-Gany D., Counotte M.J., Hossmann S., Imeri H., Ipekci A.M. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop B.Z., Ngom M., Pougué Biyong C., Pougué Biyong J.N. The relatively young and rural population may limit the spread and severity of COVID-19 in Africa: a modelling study. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elimian K.O., Ochu C.L., Ebhodaghe B., Myles P., Crawford E.E., Igumbor E. Patient characteristics associated with COVID-19 positivity and fatality in Nigeria: retrospective cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-044079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonte L., Acosta A., Sarmiento M.E., Ginori M., Garcia G., Norazmi M.N. COVID-19 lethality in Sub-Saharan Africa and helminth immune modulation. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.574910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19. N Engl J Med. 2020;382:22–23. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019–COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman J.R., Lucchi N.W., Cantey P.T., Steinhardt L.C., Samuels A.M., Kamb M.L. Malaria and parasitic neglected tropical diseases: potential syndemics with COVID-19? Am J Trop Med Hyg. 2020;103:572–577. doi: 10.4269/ajtmh.20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R., Pierce D., Giacomin P., Loukas A., Bourke P., McDermott R. Helminth coinfection and COVID-19: an alternate hypothesis. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infection with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing A.J., Cocks C., Green J.P. COVID-19: in the footsteps of Ernest Shackleton. Thorax. 2020;75:693–694. doi: 10.1136/thoraxjnl-2020-215091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Severe Acute Respiratory and Emerging Infection Consortium . ISARIC; 2020. COVID-19 CRF. Available at: https://isaric.tghn.org/COVID-19-CRF/. [Accessed 1 March 2020] [Google Scholar]
- Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K. Asymptomatic and pre-symptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility–King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirenga B., Muttamba W., Kayongo A., Nsereko C., Siddharthan T., Lusiba J. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytras T., Dellis G., Flountzi A., Hatzianastasiou S., Nikolopoulou G., Tsekou K. High prevalence of SARS-CoV-2 infection in repatriation flights to Greece from three European countries. J Travel Med. 2020;27:taaa054. doi: 10.1093/jtm/taaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin E., Burgers W.A., Sturrock E.D., Mendelson M., Chapman R., Douglass N. Prospects for SARS-CoV-2 diagnostics, therapeutics and vaccines in Africa. Nat Rev Microbiol. 2020;18:690–704. doi: 10.1038/s41579-020-00441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbow M., Lell B., Jochems S.P., Cisse B., Mboup S., Dewals B.G. COVID-19 in Africa: dampening the storm? Science. 2020;369:624–626. doi: 10.1126/science.abd3902. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty L.F., Plucinski M.M., Marston B.J., Kurbatova E.V., Knust B., Murray E.L. Public health responses to COVID-19 outbreaks on cruise ships — worldwide, February–March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:347–352. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega J.B., Ishoso D.K., Otokoye J.O., Hermans M.P., Machekano R.N., Sam-Agudu N.A. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the Democratic Republic of the Congo. Am J Trop Med Hyg. 2020;103:2419–2428. doi: 10.4269/ajtmh.20-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolai L.A., Meyer C.G., Kremsner P.G., Velavan T. Asymptomatic SARS coronavirus 2 infection: invisible yet invincible. Int J Infect Dis. 2020;100:112–116. doi: 10.1016/j.ijid.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njenga M.K., Dawa J., Nanyingi M., Gachohi J., Ngere I., Letko M. Why is there low morbidity and mortality of COVID-19 in Africa? Am J Trop Med Hyg. 2020;103:564–569. doi: 10.4269/ajtmh.20-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A., Koegelenberg C.F.N., Moolla M.S., Louw E.H., Mowlana A., Nortje A. High HIV prevalence in an early cohort of hospital admissions with COVID-19 in Cape Town, South Africa. S Afr Med J. 2020;110:982–987. doi: 10.7196/SAMJ.2020.v110i10.15067. [DOI] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A., Sasaki T., Kato S., Hayashi M., Tsuzuki S.-I., Ishihara T. Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med. 2020;383:885–886. doi: 10.1056/NEJMc2013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald E.R., Schmit K.M., Gusland D.Y. A pediatric infectious disease perspective on COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019‐nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID-19) Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-jointmission-on-covid-19-final-report.pdf. [Accessed 28 March 2020] [Google Scholar]
- World Health Organization . WHO; Geneva: 2021. Coronavirus disease (COVID-2019) situation reports. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Accessed 3 February 2021] [Google Scholar]
- Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Xia J., Zhou X., Xu S. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]