Abstract
Medical device coatings that resist protein adhesion and bacterial contamination are highly desirable in the healthcare industry. In this work, an antifouling zwitterionic terpolymer, 2-methacryloyloxyethyl phosphorylcholine-co-butyl methacrylate-co-benzophenone (BPMPC), is covalently grafted to a nitric oxide (NO) releasing antimicrobial biomedical grade copolymer of silicone-polycarbonate-urethane, CarboSil, to significantly enhance the biocompatibility, nonspecific protein repulsion and infection-resistant properties. The NO donor embedded into CarboSil is S-nitroso-N-acetylpenicillamine (SNAP) and covalent grafting of the BPMPC is achieved through rapid UV-cross-linking, providing a stable, hydrophilic coating that has excellent durability over a period of several weeks under physiological conditions. The protein adsorption test results indicate a significant reduction (~84–93%) of protein adhesion on the test samples compared to the control samples. Bacteria tests were also performed using the common nosocomial pathogen, Staphylococcus aureus. Test samples containing both NO donor and BPMPC show a 99.91 ± 0.06% reduction of viable bacteria when compared to control samples. This work demonstrates a synergistic combination of both antimicrobial and antifouling properties in medical devices using NO donors and zwitterionic copolymers that can be covalently grafted to any polymer surface.
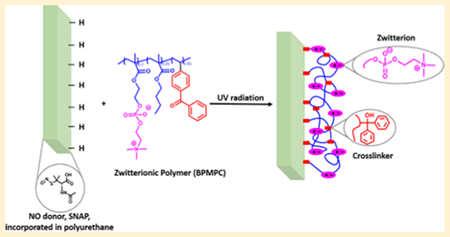
INTRODUCTION
The nonspecific adsorption of proteins has long been considered a grand challenge in many biomedical applications such as implants, contact lenses, catheters, and biosensors. In addition to medical device failure, the consequences of protein adsorption include thrombus formation, innate immune response, and bacterial infection.1,2 Preventing direct microbial contamination is also a highly desired characteristic of medical devices, implants, and hospital equipment.3–6 Although significant progress has been made in understanding and reducing adsorption and contamination, the Centers for Disease Control and Prevention (CDC) still reported that, in 2011, there were an estimated 722 000 healthcare-associated infections (HAIs) in U.S. acute care hospitals. Additionally, about 75 000 patients with HAIs died during their hospitalization.7 On any given day, approximately 1 out of every 25 patients in the U.S. contracts at least one infection during their hospital care. Therefore, materials demonstrating antifouling and antimicrobial effects are highly desirable.
In recent years, zwitterionic polymers have attracted attention due to their biomimetic nature, which provides excellent biocompatibility and antifouling properties compared to traditional materials like poly(ethylene glycol) (PEG). 8–10 Zwitterionic polymers, in which both cationic and anionic groups are on the same monomer residue, have a strong hydration ability which accounts for their ultralow fouling properties.11–16 The dipole arrangement of water molecules in the hydration shell formed via electrostatic interactions with the charged groups of the zwitterion are closer to free water than the directional arrangement of water molecules in the hydration shell formed via hydrogen bonds in case of PEG.17 The excellent hydrophilicity of zwitterionic polymers, however, provides a difficult challenge in coating hydrophobic materials, where coating delamination under physiological conditions has so far limited practical application.18
To explore the covalent grafting of zwitterionic polymers to various substrates ranging from hydrophilic to hydrophobic, we incorporated the benzophenone (BP) chromophore, a photoactive tethering reagent, into the polymeric backbone.19–24 The BP group can produce a diradical under low-intensity UV irradiation (350–365 nm) that abstracts an aliphatic hydrogen from a neighboring C—H bond to form a new C—C bond, without intensive UV oxidative damage to the polymer or substrates.20 Through this process, network polymer films can be grafted with excellent durability to a broad selection of C—H containing materials and surfaces, and has been used for many applications such as microfluidics,25,26 organic semiconductors,27 redox polymers,28,29 anti-icing polymers,30 and biosensors31,32
Nitric oxide (NO) is a well-known potent and nonspecific bactericidal agent due to its natural broad-spectrum antimicrobial properties with low risk for promoting bacterial resistance.33–35 NO utilizes several antimicrobial mechanisms including nitrosation of amines and thiols, lipid peroxidation, tyrosine nitration, and DNA cleavage.36 Major classes of current NO donors include organic nitrates, metal-NO complexes, N-nitrosamines, and S-nitrosothiols,37 S-nitroso-N-acetylpenicillamine (SNAP), a commonly studied S-nitrosothiol, exhibits significant antimicrobial and antithrombotic effects.38,39 In our previous studies, SNAP has been successfully doped into CarboSil polymer films, and these SNAP-doped polyurethane-based materials can release NO for extended periods (20 days) with very low levels of leaching. 38,40,41
In this work, we synthesized zwitterionic terpolymers (2-methacryloyloxyethyl phosphorylcholine-co-butyl methacrylate-co-benzophenone, BPMPC) that can be covalently grafted to antimicrobial, NO-releasing CarboSil (silicone-polycarbonate-urethane thermoplastic) upon UV-irradiation. The polymer-coated surfaces are characterized in detail, and the zwitterionic stability is assessed under physiological conditions. The protein repellency properties of these coatings are evaluated. At the same time, no SNAP degradation was observed during coating or UV irradiation, and the NO release profile remained above the physiological level for 2 weeks with the zwitterionic topcoat. Moreover, enhanced antimicrobial activity was demonstrated with bacteria testing.
EXPERIMENTAL SECTION
Materials.
4-Vinylbenzophenone (BP) was synthesized according to a previously reported method.30 2-Methacryloyloxyethyl phosphorylcholine (MPC), albumin from bovine serum (BSA), fluorescein isothiocyanate labeled bovine serum albumin (FITC-BSA), N-acetyl-d-penicillamine (NAP), sodium nitrite (NaNO2), concentrated sulfuric acid (conc. H2SO4), tetrahydrofuran (THF), sodium phosphate monobasic (NaH2PO4), sodium phosphate dibasic (Na2HPO4), potassium chloride, sodium chloride, and ethylenediamine tetraacetic acid (EDTA) were purchased from Sigma-Aldrich (St. Louis, MO). 2,2′-Azobis(2-methylpropionitrile) (AIBN) and n-butyl methacrylate (BMA) were bought from Alfa-Aesar (Haverhill, MA). Isobutyltrichlorosilane was purchased from Tokyo Chemical Industry (Portland, OR). Concentrated hydrochloric acid (conc. HCl), sodium hydroxide (NaOH), and methanol were bought from Fisher-Scientific (Hampton, NH). Potassium phosphate monobasic (KH2PO4) and lysozyme from egg white were purchased from BDH Chemicals—VWR International (West Chester, PA). CarboSil 2080A UR STPU (referred to as CarboSil hereon) was acquired from DSM Biomedical Inc. (Berkeley, CA). Milli-Q filter was used to obtain deionized(DI) water for all the aqueous solution preparations. Nitrogen and oxygen gas cylinders were purchased from Airgas (Kennesaw, GA). Staphylococcus aureus (ATCC 6538, S. aureus) was used for the bacterial experiments. LB Agar (LA), Miller and Luria broth (LB), Lennox were purchased from Fischer BioReagents (Fair Lawn, NJ). All the chemicals were used without further purification.
In brief, CarboSil polymers with 10 wt % SNAP (test samples) and no SNAP content (control samples) were prepared using solvent evaporation and/or spin coating method. These samples were then coated with a zwitterionic copolymer (referred to as BPMPC) which was covalently bonded to the CarboSil base polymers by UV-cross-linking. Surface analysis was performed on the films pre- and post- UV radiation to understand the cross-linking behavior of the polyzwitterionic system. Test and control samples with the BPMPC coating were analyzed for their NO release behavior. The samples were then tested for protein adhesion for 14 days in physiological conditions (37 °C in PBS) to evaluate antifouling properties of the topcoat. Finally, antimicrobial assay of the samples was done using a modified version of ASTM E2180 protocol.
Synthesis of NO Donor, SNAP.
S-nitroso-N-acetyl-d-penicillamine was synthesized using a revised approach for a method previously reported.38 1 M H2SO4 and 1 M HCl were mixed with an equimolar amount of NAP, methanol and NaNO2 aqueous solution. This reaction mixture was stirred for 20 min and then cooled for 7 h with a constant flow of air on the mixture. Precipitated green crystals of SNAP were filtered, collected, and dried in a covered vacuum desiccator. Dried crystals of SNAP were used for all experiments.
Synthesis of CarboSil Films Doped with SNAP.
CarboSil films containing 10 wt% SNAP were prepared using the solvent evaporation method. 700 mg of CarboSil was dissolved in 10 mL of THF to make the polymer solutions. 77 mg of SNAP was added to this solution for a final concentration of 10 wt % of SNAP. This polymer-SNAP blend was stirred in dark conditions until the SNAP crystals dissolved completely. The blend was then transferred into Teflon molds and allowed to let the solvent evaporate overnight in a fume hood. The overnight dried films were then cut into circular shapes of 0.8 cm diameter each. Each sample was immersed into a CarboSil solution without SNAP (40 mg mLfume hood. The overnight dried films were then cut into circular shapes of 0.8 cm diameter each. Each sample was immersed into a CarboSil solution without SNAP (40 mg mL−1 of polymer concentration in THF) to coat it (this was repeated thrice for each sample). The samples were dried overnight and then dried under vacuum for an additional 24 h. This added drying time was included to eliminate any remaining THF which can affect any following studies. The weight of each film was recorded before the topcoat application for all SNAP leaching behavior tests. The formulated samples were stored in the freezer (−18 °C) in the dark before experiments to prevent escape of SNAP or consequent loss of NO. These SNAP-incorporated films were used for NO release, SNAP leaching, and bacterial cell viability analyses. All samples used for the tests were less than a week old to ensure integrity of studies.
Synthesis of Zwitterionic Copolymer (BPMPC).
The polymer was synthesized by free radical polymerization. MPC (0.546 g, 1.85 mmol), n-BMA (0.105 mL, 0.66 mmol), and BP (0.027 g, 0.132 mmol) were dissolved in 5.3 mL ethanol (total monomer concentration 1.0 mmol mL−1) with the initiator AIBN (0.01 mmol mL−1) and the solution was poured into polymerization tube. After being degassed with argon for 30 min, the polymerization reaction was carried out under nitrogen flow at 60 °C for 16 h. The reaction was stopped by exposing the solution to air, cooled to room temperature, and poured into ethyl ether to precipitate the polymer. The white solid was collected by vacuum filtration and dried under vacuum for 12 h. Yield: 0.552 g, 83%. 1H NMR (D2O) was taken to confirm the polymer composition (Figure S1).
Cross-Linking of BPMPC with Substrates.
Silicon substrates were cut into 2.4 × 2.4 cm2 pieces and sonicated with deionized water, isopropanol, and acetone for 5 min each then dried under nitrogen, followed by plasma (Harrick Plasma PDC-32G) cleaning and treated with iBTS in toluene overnight before modification with the polymer. CarboSil substrates were coated with polymer without pretreatment.
Two coating methods were utilized when applying BPMPC on substrates: spin coating and spray coating. For spin coating, a polymer-modified film was developed on a functionalized silicon substrate using 0.5 mL BPMPC/ethanol solution (10 mg mL−1) at 1000 rpm for 30 s. Spray coating was applied for CarboSil films with and without SNAP. BPMPC/ethanol solution (2 mg mL−1) was sprayed using a spray gun from a distance of 10 cm onto vertically placed substrates to achieve uniform coating upon drying. We used spin coating in the protein adsorption experiments, and spray coating in SNAP/NO release and bacterial experiments, based on the method that afforded the smoothest, pinhole free coating on different forms of substrate. After that, the BPMPC substrates were irradiated with UV light (UVP, 254 nm, 6.5 mW cm−2) for 1 min to covalently bond the BPMPC to the surface. The substrates were rinsed with abundant ethanol to remove unattached BPMPC then dried under nitrogen.
Characterization of the Polymer Coatings.
The surface wettability was characterized by measuring the static water contact angle, which was obtained from a DSA 100 drop shape analysis system (KRÜSS) with a computer-controlled liquid dispensing system. One μL DI water droplets were deposited onto substrate surfaces, and the water contact angles were measured within 10 s through the analysis of photographic images. The cross-linking kinetics of BPMPC coating was investigated by a UV—vis spectroscopy (Varian) with 254 nm UV light. The thickness of the spin-coated polymer layer on the silicon substrates and CarboSil substrates were measured by a M-2000 V Spectroscopic Ellipsometer (J.A Woollam co., INC.) with a white light source at three incident angles (65°, 70°, and 75°). The thickness of the modified layer was measured and calculated using a Cauchy layer model. Infrared spectroscopy studies of polymer coated films were done using a Thermo-Nicolet model 6700 spectrometer equipped with a variable angle grazing angle attenuated total reflection (GATR-ATR) accessory (Harrick Scientific).
SNAP Leaching Study and NO-Release Profile.
The percentages of SNAP discharged from the samples were quantified by noting the absorbance of the PBS solutions (used to soak the samples) at 340 nm (characteristic absorbance maxima of S-NO group of SNAP). Each sample was weighed before coating with non-SNAP polymer solutions to determine the initial amount of SNAP in each film. The films were then immersed in vials containing PBS (pH 7.4 with 100 μM EDTA to prevent catalysis of NO release by metal ions) and stored at 37 °C. A UV—vis spectrophotometer (Thermoscientific Genesys 10S UV—vis) was utilized to quantify the absorbance of the buffer solutions in the required time intervals. The readings were converted to wt % of SNAP in die buffer utilizing the initial amount of SNAP present in each sample. One mL aliquots of the PBS solution in which the samples were soaked was used for each sample absorbance measurement to avoid any inconsistent readings, and three replicates were utilized for each quantification. The calibration graph with known amounts of SNAP in PBS (with EDTA) was used to interpolate the absorbance quantifications recorded from the study and convert them to concentrations of SNAP in die quantified sample.
SNAP incorporated in the polymers release NO in physiological conditions, and this release was measured and recorded in real time for the study using Sievers chemiluminescence NO analyzers (NOA 280i, GE Analytical, Boulder, CO, U.S.A.). The sample holder maintained dark conditions for the samples to prevent catalysis of the NO production by any light source. It was filled with 5 mL of PBS (pH 7.4 with 100 μM of EDTA) to soak the samples. EDTA acted as a chelating agent to prevent catalysis of NO production by metal ions in the PBS. This buffer solution was maintained at 37 °C by a temperature-regulated water jacket placed around the sample holder. Once a baseline of NO flux without the sample (prepared according to section 2.4) is established, the sample is then placed in die sample holder. Nitric oxide released by the sample in the sample holder was pushed and purged toward the analyzer by a continuous supply of nitrogen gas maintained at a constant flow rate of 200 mL min−1 through the sweep and bubble flows. The NO released by the sample is pushed toward the chemiluminescence detection chamber where the reactions shown in Figure S2 take place.
The voltage signal produced is converted to concentration of NO and displayed on the analyzer’s screen. Using the raw data in ppb form and NOA constant (mol ppb−1 s−1), die data in ppb are normalized for surface area of the sample and converted to NO flux units (× 10−10 mol cm−2 min−1). Data were collected in the time intervals mentioned and samples were stored in a PBS (with EDTA) solution at 37 °C in dark conditions between measurements. The PBS was replaced daily to avoid any accumulation of SNAP leached or NO released during the storage time. The instrument operating parameters were a cell pressure of 7.4 Torr, a supply pressure of 6.1 psig and a temperature of −12 °C. Three replicates were used for each measurement.
Protein Adhesion Assay.
Protein adsorption test is an important method for evaluating the blood adhesion. Therefore, the thickness change of substrates before and after incubation in protein solutions was monitored, as an indication of protein adsorption. Coated substrates were incubated in fibrinogen (1 mg mL−1) and lysozyme (1 mg mL−1) in PBS (pH 7.4, 0.01 M) solutions up to 14 days, followed by thickness measurement every day.
In the second approach, fluorescein isothiocyanate-bovine serum albumin (FITC-BSA, 2 mg mL−1) in PBS solution was used to evaluate the protein adsorption behavior on the surface of CarboSil substrate modified by BPMPC.42,43 Substrates were immersed in FITC-BSA solution for 1.5 h at 37 °C, then rinsed with DI water and dried with nitrogen. The substrates with protein were then analyzed by Nikon Eclipse NI—U fluorescence microscope (Nikon Instruments, Inc.), using a 5× objective lens, with the filter set (Ex/Em 470/525 nm). To confirm the long-term resistance to protein adsorption, the substrates were incubated in BSA (1 mg mL−1) PBS solution for up to 7 days at 37 °C before putting in FITC-BSA solution.
Viable Bacterial Adhension Assay.
Bacterial adhesion for each of the samples was calculated using serial dilution after an incubation period of 24 h. The method used to perform this assay was based on a modified version of the American Society for Testing and Materials E2180 protocol. S. aureus was used for antimicrobial evaluation of the samples. Bacteria were cultured in LB Broth (Lennox) at 37 °C and grown to ~106 colony-forming units (CFU) per mL as measured by optical density. The resulting overnight culture was collected by centrifugation (2500 g, 7 min) and resuspended in PBS. This resuspended bacterial suspension was used for incubation of polymer samples for 24 h.
After incubation with the bacterial solution, samples were washed gently with PBS to remove any unbound bacteria. The samples were then placed in 1 mL of PBS and homogenized for 1 min each to transfer any adhered bacteria to this new PBS solution. After homogenization, homogenate samples were serially diluted and plated onto LB Agar nutrient plates (37 °C). Viable bacterial count was determined by counting the colonies on each plate manually. Calculation of bacterial adhesion was done by counting number of colonies per cm2 of each sample.
Statistical Analysis.
All data are quantified as mean ± standard deviation with an n ≥ 3 for all trials. The results between the control and test films were analyzed by a comparison of means using student’s t-test. Values of p were obtained for the data analyzed and p < 0.05 was considered significant.
RESULTS AND DISCUSSION
The zwitterionic polymer (BPMPC) was synthesized by radical polymerization in ethanol (Scheme 1A). The copolymer composition was confirmed by 1H NMR spectroscopy, and consisted of 74:18:8 (MPC:nBMA:BP), which roughly matched the monomer feed ratio. This ratio provided the optimal antifouling result (discussed below) along with the most uniform coating on both hydrophobic and hydrophilic substrates. The polymer synthesis is simple and straightforward, no further purification is required besides precipitation, which makes large-scale production feasible. BPMPC is a hydrophilic polymer due to the high concentration of MPC, and has a high solubility in aqueous and alcohol solutions. The butyl methacrylate component in the terpolymer aids in uniformity and substrate wetting (both hydrophobic and hydrophilic), along with providing additional photochemical cross-linking sites. As described above, the benzophenone component of BPMPC acts as a cross-linker between the hydrophilic polymer and any organic substrate through C—H activation.
Scheme 1.
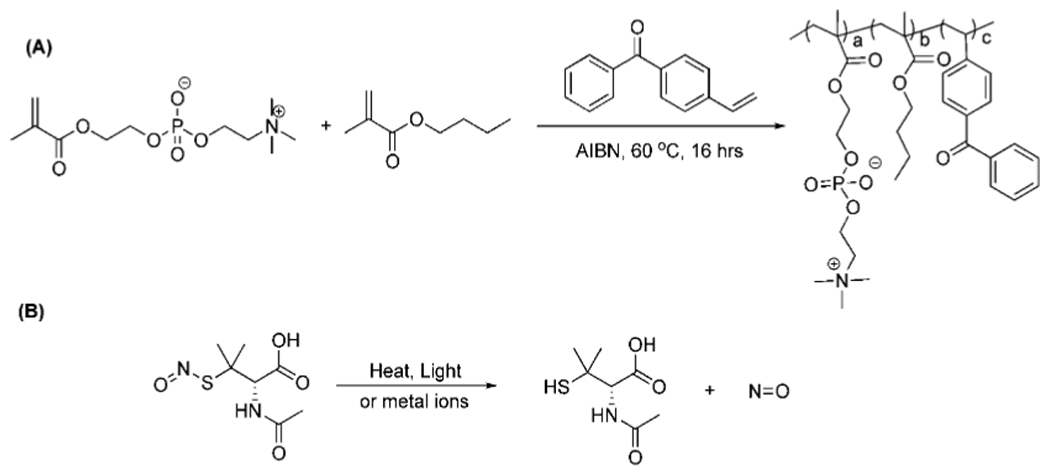
(A) Synthesis of the BPMPC Copolymer and (B) Chemical Structure of SNAP and NO Decomposition along with Innocuous N-Acetylpenicillamine Byproduct
The cross-linking kinetics of BPMPC was investigated by UV—vis spectroscopy on isobutyltrichlorosilane (iBTS) functionalized quartz substrates. The polymer solution (10 μL, 10 mg mL−1) was drop cast on alkylated quartz and the solvent allowed to evaporate. The UV cross-linking reaction was monitored by UV—vis, where the decreasing absorbance of the BP group at 255 nm occurs with increased irradiation time. Figure 1 shows the UV—vis spectra, where the absorbance maxima at 255 nm decreased dramatically from 0 to 120 s, and after 240 s, no further absorbance change was observed, even after prolonged irradiation. This result demonstrates that BPMPC cross-linking occurs with rapid kinetics, and only a few seconds are needed to covalently bond BPMPC to a variety of different substrates.
Figure 1.
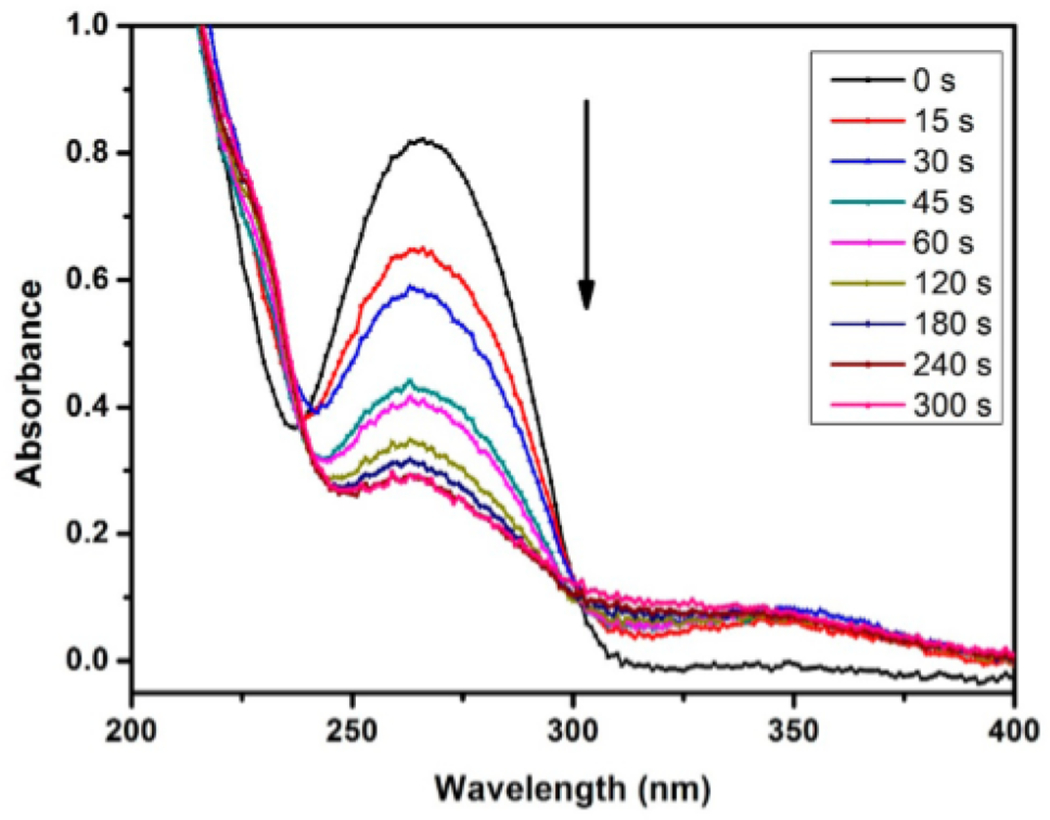
UV–vis absorption spectrum of BPMPC drop-cast onto a quartz substrate as a function of photochemical irradiation time at 254 nm (6.5 mW cm−2 intensity).
To further confirm the deposition and cross-linking of the BPMPC polymer, FTIR was conducted on coated substrates. In the IR spectra (Figure S3), absorption peaks of the carbonyl (1720 cm−1) and PC groups (1240, 1080, and 970 cm−1) were observed and assigned to the MPC units. The peak at (1650 cm−1) represents the C=O stretch of BP ketone. A significant reduction of this peak after irradiation further supports the formation of a network polymer of covalent linkage between BP and substrate.
To test the stability and durability of the coating, we monitored the water contact angle of the BPMPC coated silicon samples up to 14 days. The coated substrates were immersed in PBS solution and stirred in an incubator at 37 °C, subsequently rinsed with H2O and dried with nitrogen before measuring the water contact angle (Figure 2). The initial static contact angle for the bare CarboSil substrate is about 110°. A significant decrease in contact angle was observed after coating with BPMPC, from 110° to 50°, and this value of contact angle was maintained over a period of 14 days immersed in an agitated PBS solution, which suggests that the BPMPC coating was covalently bonded to the substrates and does not delaminate under physiological conditions.
Figure 2.
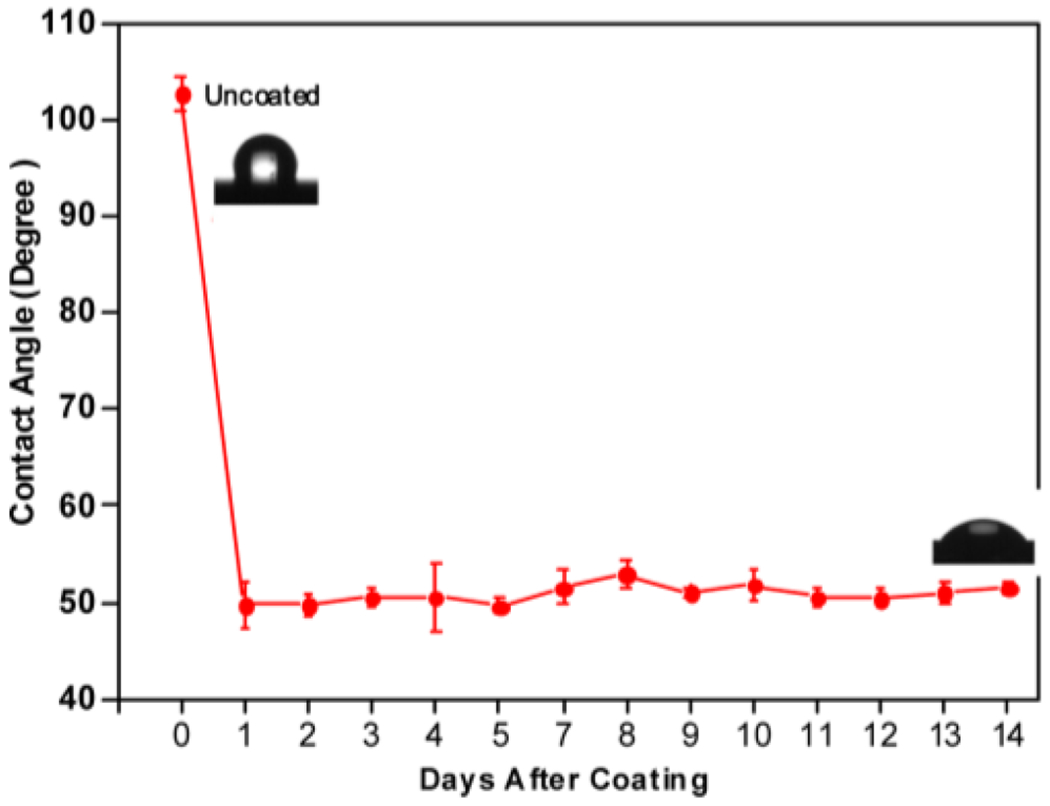
Contact angle measurement as a function of time for CarboSil coated with BPMPC and incubated at 37 °C in PBS under mild agitation.
The control samples used to test NO release behavior were coated only with CarboSil (the same polymer used to incorporate SNAP) while the test samples were coated with CarboSil and BPMPC. The samples were tested in lightly agitated conditions to simulate physiological conditions. The samples were tested for a period of 2 weeks to demonstrate sustainable release of NO from the combination of hydrophobic and hydrophilic polymers.
A SNAP leaching study was conducted first to measure the retention of SNAP in the control and test polymer films during the course of the study. Measurements were recorded every other day for 2 weeks of soaking in PBS (Figure 3A). A high amount of SNAP retention in the polymers ensures sustained release of NO from the polymer matrix and minimizes the risks (if any) associated with SNAP leaching.44 As seen in Figure 3A, for the initial measurement (day 0 on graph of Figure 3A) of leaching after 1 h of storage in 37 °C in PBS, a loss of 0.39 ± 0.06% and 0.47 ± 0.26% was recorded for the control and BPMPC-coated substrate, respectively. This initial higher leaching for the BPMPC-coated substrate is likely due to the hydrophilicity of the surface. However, SNAP leaching is almost identical between the control and test samples as supported by the data from 1 and 3 days of storage in 37 °C for BPMPC-coated test films (0.96 ± 0.26% and 1.44 ± 0.26% for day 1 and day 3, respectively) and control films (0.96 ± 0.05% and 1.55 ± 0.07% for day 1 and day 3, respectively).
Figure 3.
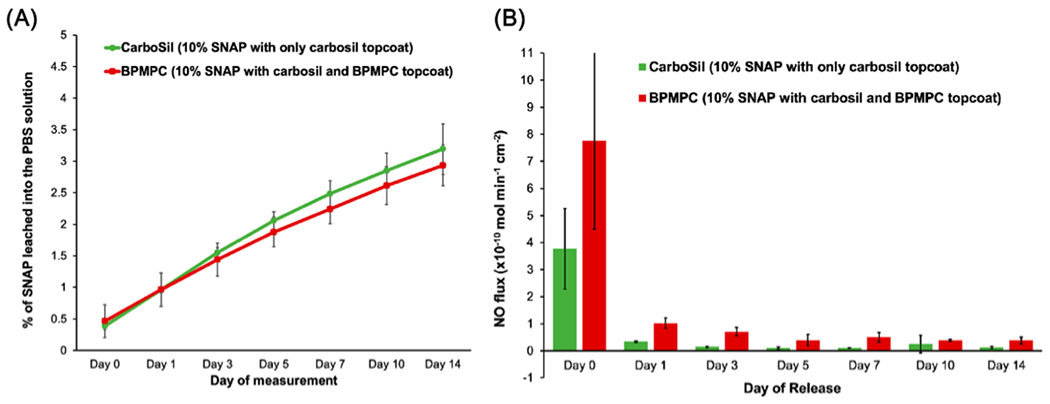
(A) SNAP leaching measured using UV–vis over 2 weeks and (B) Nitric oxide release measured over 2 weeks using chemiluminescence (n = 3 for both).
This trend of lower leaching of the SNAP molecules from the test films was observed over a 14-day period. It is also to be noted that at no point during the 14-day period were the samples kept at a temperature below 37 °C or in dry conditions. This was done to closely simulate physiological conditions for a continuous duration. The leaching for both the control and test samples remained very low (<3.5%) over the experiment duration but it is worth noting here that despite the expectation that the hydrophilic coating could cause a higher leaching of SNAP molecules from the NO donor containing polymer by attracting water molecules to the polymer surface, this was not the case. This is likely due to the ultrathin nature of the coating, which influences the aqueous interface, but not the bulk of the polymer film.
NO release measurements of the control and test samples were also carried out for a period of 14 days (Figure 3B). Measurements with a Sievers chemiluminescence NO analyzer is the standard characterization methodology accepted for polymers that release NO.45–47 It measures NO release in real time via the measurement of voltage produced by the photons on the reaction of NO with ozone. In this study, samples were stored at a constant temperature of 37 °C and in PBS to simulate physiological conditions.
The results indicated a general trend of higher NO release from the test samples (SNAP-containing material coated with CarboSil and BPMPC) compared to the control samples (SNAP-containing material coated with only CarboSil). Day 0 measurements indicate that the test samples had a flux of 7.75 ± 3.26 (× 10−10) mol cm−2 min−1 while control samples had 3.76 ± 1.50 (× 10−1) mol cm−2 min−1 (Table 1). This burst of NO release from test samples results from the hydrophilicity of the topcoat which attracts water molecules to the sample surface. Water molecules on the surface can accommodate release of NO as SNAP is more soluble (and prone to S—N=O bond cleavage) in aqueous conditions. After a day of storage, the control samples show a sharp decrease in NO flux (0.34 ± 0.03 × 10“−10 mol cm−2 min−1). This is seen because of the initial loss in SNAP molecules on day 0 and but inability to maintain a hydrated state for day 1. In contrast, BPMPC-coated substrates show three times the NO flux at 1.02 ± 0.02 × 10−10 mol cm−2 min−1. This difference in NO flux can result from the hydrophilic topcoat of test samples that maintains a hydrated surface layer, which facilitates the release of more NO. This trend of higher NO flux from test samples when compared to control samples can be seen through the 14-day study in Table 1 and the graph in Figure 3B.
Table 1.
Comparison of Nitric Oxide Release Kinetics between Control and Coated Samples
| 10% SNAP with only CarboSil topcoat (NO flux (× 10−10 mol min−1 cm−2) |
10% SNAP with CarboSil and BPMPC topcoat (NO flux (× 10−10 mol min−1 cm−2) |
|
|---|---|---|
| day 0 | 3.759 ± 1.491 | 7.746 ± 3.263 |
| day 1 | 0.335 ± 0.032 | 1.016 ± 0.198 |
| day 3 | 0.141 ± 0.023 | 0.706 ± 0.157 |
| day 5 | 0.110 ± 0.045 | 0.395 ± 0.208 |
| day 7 | 0.105 ± 0.008 | 0.498 ± 0.173 |
| day 10 | 0.247 ± 0.324 | 0.383 ± 0.040 |
| day 14 | 0.127 ± 0.035 | 0.380 ± 0.125 |
At the end of the 14-day study, test samples (0.38 ± 0.13 (× 10−10) mol cm−2 min−1) still release three times the NO flux compared to the control samples (0.13 ± 0.03 (× 10−10) mol cm−2 min−1). This propensity of higher release of NO from CarboSil top-coated with BPMPC along with the reduction in leaching of SNAP is very beneficial and combines the material properties of CarboSil (low SNAP leaching) with a higher, sustained release of NO due to the hydrophilic BPMPC topcoat.
As mentioned earlier, the BPMPC coating has excellent hydrophilicity, which helps inhibit the adsorption of proteins from solution. Fibrinogen and lysozyme were used as model proteins to evaluate the antifouling properties of the BPMPC coatings. Fibrinogen is a large (340 kDa, pI = 6.0) protein, and a key biomacromolecule in the coagulation cascade that rapidly adsorbs to foreign surfaces and binds to and activates platelets. Lysozyme is a small protein (14 kDa, pI = 12) that is positively charged under physiological pH. Figure 4A shows the adsorption thickness increase of Fibrinogen on CarboSil, CarboSil with 10% SNAP, BPMPC coated CarboSil, and BPMPC coated CarboSil with SNAP substrates, respectively. On the bare CarboSil films used as a control, the thickness increased about 3 nm after incubation for 24 h, and increased to over 30 nm after 2 weeks. The similar phenomenon was observed for CarboSil with SNAP films, which indicated a high amount of protein adsorption on surface, and protein accumulation over time. However, for the CarboSil films coated with BPMPC, the adsorption amount is significantly lower, only a 2 nm increase was observed after incubation for 2 weeks. The large difference in adsorption thickness confirmed that BPMPC coating has excellent protein resistance properties, even after UV activation. As expected, the BPMPC coated CarboSil with SNAP films also show low adsorption for fibrinogen. Moreover, a similar behavior was observed when films were subjected to lysozyme solution (Figure 4B). The thickness increase in control group was over 14 nm, while the coated group was less than 3 nm. The protein adsorption results indicate that the hydrophilic BPMPC surface layer provides excellent protein-resistant properties.
Figure 4.
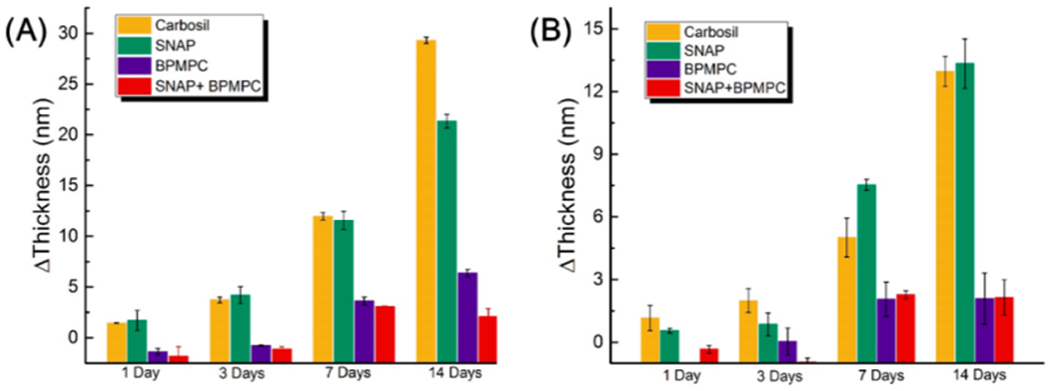
Thickness increase after incubation in (A) Fibrinogen solution and (B) in Lysozyme solution.
To further confirm the antifouling effectiveness of the durable BPMPC coating, fluorescence microscopy was utilized to evaluate the protein adsorption on the uncoated and coated CarboSil films using FITC labeled BSA protein. The fouling levels were compared between uncoated and BPMPC coated CarboSil films using the same excitation light intensity and exposure time. Figure 5A indicates protein adsorption on the control samples, and enhanced fluorescent signal (Figure 5B,C) was observed in the samples pretreated with BSA solution. These results demonstrate that after incubation in protein solution, a large amount of BSA was attached to the CarboSil samples, which facilitates the aggregation of FITC-BSA. On the contrary, protein adhesion to the surface of BPMPC modified samples was not observed (Figure 5D—F), even after incubation in BSA solution for 7 days. From all of these results collectively, the control films demonstrate large amounts of protein adsorption, while the BPMPC coated films display excellent antifouling properties.
Figure 5.
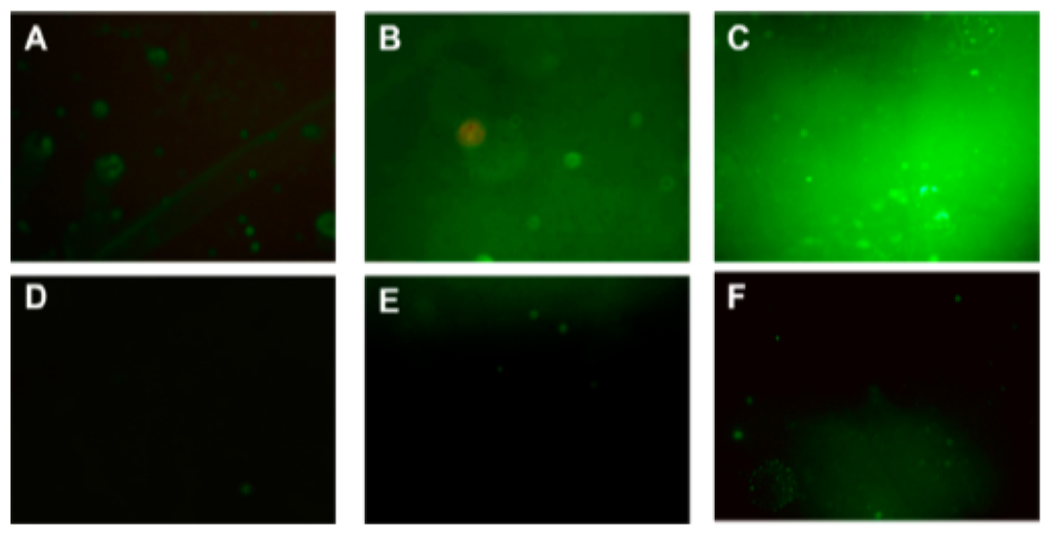
Fluorescence micrographs (magnification 10×) of uncoated films after (A) 90 min incubation, (B) 1 day in BSA solution before incubation, and (C) 7 days in BSA solution before incubation in 2 mg/mL FITC-BSA solution. Parts (D–F) are the coated film measured under the same experimental conditions.
Bacterial adhesion, which often results in biofilm formation, is a prevalent issue in moist and humid environments, including implanted devices. The basic nutrients important for bacterial growth may be resourced from the device material, bodily proteins that attach postimplantation, or other bodily macro-molecular contaminants that adhere to the surface of the device. Antimicrobial efficacy of the designed test samples was compared to the control samples to confirm their superior bactericidal and bacterial repulsion properties.
The samples were soaked in bacterial solutions containing ~106 CFU/mL of S. aureus. S. aureus is a commonly found nosocomial infection bacteria. It has been increasingly linked with healthcare-associated infections in the last two decades.48 They are most commonly associated with cardiac devices, intravascular catheters, and urinary catheters, among other prosthetic devices. This high prevalence of S. aureus along with its known affinity to proteins49,50 that foul medical devices has made it a very important pathogen used to evaluate the antimicrobial efficacy of medical device materials. For these reasons, bacterial adhesion study of the antifouling-biocide releasing polymer developed was done with S. aureus.
As mentioned in the Introduction, the NO molecules liberated by the decomposition of SNAP actively kill bacteria while the zwitterion topcoat repels protein adsorption, leading to enhanced antimicrobial efficacy. After 24 h of incubation, the antimicrobial effect of the test samples was clearly observed. NO releasing polymers with a top-coat of DPMPC showed a bactericidal efficiency of 99.91 ± 0.06% (~3 log reduction, Figure 6) compared to the control samples where a growth of ~106 CFU/cm2 was observed. This reduction is greater compared to films with only a BPMPC topcoat (70.15 ± 14.13%) and also films with only NO-releasing moieties (98.88 ± 0.54%). It can also be concluded from the results that BPMPC alone only reduces bacteria adhesion. However, because NO is not a contact active antimicrobial but a diffusing biocide, the SNAP-loaded samples also reduce bacterial adhesion significantly.
Figure 6.
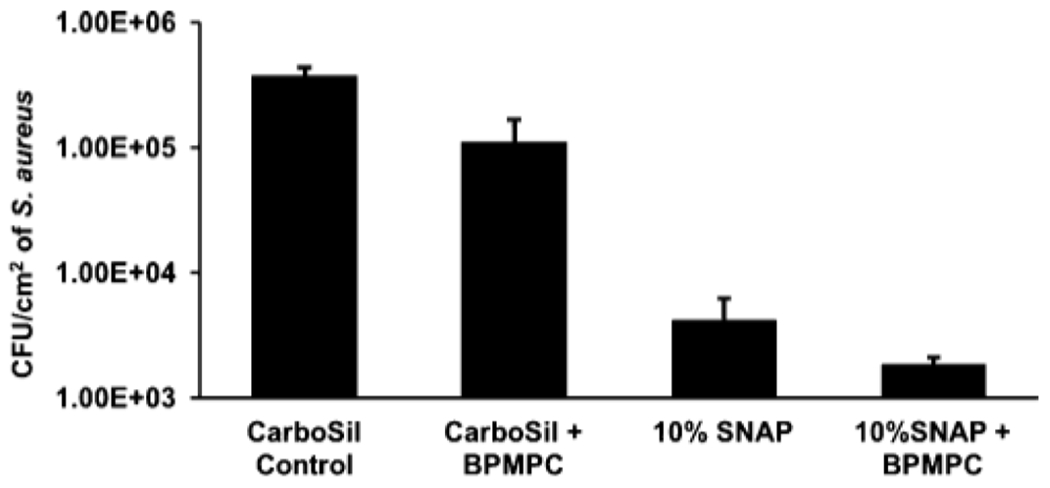
Antimicrobial efficacy of NO-releasing BPMPC coated samples relative to controls (n = 3).
These results are consistent with the theoretical expectations underlying the surface chemistry of BPMPC and bactericidal properties of NO. In summary, the synergistic effect of the modifiable NO-release kinetics from CarboSil’s surface and prevention of protein and/or bacterial adhesion due to BPMPC’s surface chemistry will significantly reduce undesired clinical consequences for implanted medical devices.
CONCLUSIONS
In conclusion, we have demonstrated that a combination of NO release and BPMPC can produce a material with antimicrobial ability and excellent antifouling properties. The formation of the covalent polymer network is rapid (less than 1 min) under mild UV conditions, and can be applied to various substrates, from hydrophilic to hydrophobic. More importantly, even though the BPMPC coating is around 50 nm, it resists moderate abrasion for over a week with retention of its antifouling property. Moreover, the NO release profile indicated a higher NO release form the BPMPC coated sample when compared to the control, with lower leaching of SNAP. The coatings were also challenged with protein adsorption tests for an extended time (up to 2 weeks), where antifouling properties remain. It is noteworthy that, the high killing efficiency of SNAP to S. aureus is enhanced by BPMPC coating. This one step photochemical attachment process of an antifouling coating to NO-releasing antimicrobial polyurethanes is a simple and scalable process that has application in both medical devices and other, industrial applications where antifouling and antimicrobial properties are desired.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the support from the National Institutes of Health, Grants K25HL111213 and R01HL134899. This project was also supported by the Centers for Disease Control and Prevention contract 200-2016-91933.
ABBREVIATIONS USED
- BPMPC
2-methacryloyloxyethyl phosphorylcholne-co-butyl methacrylate-co-benzophenone
- NO
nitric oxide
- SNAP
S-nitroso-N-acetylpenicillamine
- BP
4-vinylbenzophenone
- MPC
2-Methacryloyloxyethyl phosphorylcholine
- BSA
bovine serum albumin
- FITC-BSA
fluorescein isothiocyanate labeled bovine serum albumin
- NAP
N-acetyl-D-penicillamine
- NaNO2
sodium nitrite
- conc. H2SO4
concentrated sulfuric acid
- THF
tetrahydrofuran
- NaH2PO4
sodium phosphate monobasic
- Na2HPO4
sodium phosphate dibasic
- EDTA
ethylenediamine tetraacetic acid
- NaOH
sodium hydroxide
- KH2PO4
potassium phosphate monobasic
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.langmuir.7b02970.
1H NMR spectrum of BPMPC, nitric oxide chemiluminescence analyzer flowchart, and FTIR spectra of BPMPC coatings before and after UV exposure (PDF)
Notes
The authors declarse no competing financial interest.
REFERENCES
- (1).Cho WK; Kong B; Choi IS High Wfficient Non-biofouling Coating of Zwitterionic Polymer: Poly((3-(methacryloylamino)-propyl)-dimethyl(3-sulfopropyl)ammonium hydroxide). Langmuir 2007, 23, 5678. [DOI] [PubMed] [Google Scholar]
- (2).Nguyen AT; Baggerman J; Paulusse JM; van Rijn CJ; Zuilhof H Stable Protein-repellent Zwitterionic Polymer Brushes Grafted from Silicon Nitride. Langmuir 2011, 27 (6), 2587–94 [DOI] [PubMed] [Google Scholar]
- (3).Kenawy E-R; Worley SD; Broughton R The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules 2007, 8 (5), 1359–1384. [DOI] [PubMed] [Google Scholar]
- (4).Dastjerdi R; Montazer M A Review on the Application of Inorganic Nano-structured Materials in the Modification of Textiles: Focus on Anti-microbial Properties. Colloids Surf., B 2010, 79 (1), 5–18. [DOI] [PubMed] [Google Scholar]
- (5).Hetrick EM; Schoenfisch MH Reducing Implant-related Infections: Active Release Strategies. Chem. Soc. Rev 2006, 35 (9), 780–9. [DOI] [PubMed] [Google Scholar]
- (6).Yatvin J; Gao J; Locklin J Durable defense: robust and varied attachment of non-leaching poly″-onium″ bactericidal coatings to reactive and inert surfaces. Chem. Commun. (Cambridge, U. K.) 2014, 50 (67), 9433–42. [DOI] [PubMed] [Google Scholar]
- (7).Magill SS; Edwards JR; Bamberg W; Beldavs ZG; Dumyati G; Kainer MA; Lynfield R; Maloney M; McAllister-Hollod L; Nadle J; Ray SM; Thompson DL; Wilson LE; Fridkin SK Emerging Infections Program Healthcare-Associated, I.; Antimicrobial Use Prevalence Survey, T., Multistate Point-prevalence Survey of Health Care-associated Infections. N. Engl. J. Med 2014, 370 (13), 1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lowe S; O’Brien-Simpson NM; Connal L A Antibiofouling Polymer Interfaces: Poly(ethylene glycol) and Other Promising Candidates. Polym. Chem 2015, 6 (2), 198–212. [Google Scholar]
- (9).Jiang S; Cao Z Ultralow-fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and their Derivatives for Biological Applications. Adv. Mater 2010, 22 (9), 920–32. [DOI] [PubMed] [Google Scholar]
- (10).Gombotz WR; Guanghui G; Horbett TA; Hoffman AS Protein Adsorption to Poly(ethylene oxide) Surfaces. J. Biomed. Mater. Res 1991, 25, 1547–1562. [DOI] [PubMed] [Google Scholar]
- (11).Shao Q; Jiang S Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater 2015, 27 (1), 15–26. [DOI] [PubMed] [Google Scholar]
- (12).Zhang Z; Chao T; Chen SF; Jiang SY Superlow Fouling Sulfobetaine and Carboxybetaine Polymers on Glass Slides. Langmuir 2006, 22 (24), 10072–10077. [DOI] [PubMed] [Google Scholar]
- (13).Hucknall A; Rangarajan S; Chilkoti A In Pursuit of Zero: Polymer Brushes that Resist the Adsorption of Proteins. Adv. Mater 2009, 21 (23), 2441–2446. [Google Scholar]
- (14).Ladd J; Zhang Z; Chen S; Hower JC; Jiang S Zwitterionic Polymers Exhibiting High Resistance to Nonspecific Protein Adsorption from Human Serum and Plasma. Biomacromolecules 2008, 9 (5), 1357–1361. [DOI] [PubMed] [Google Scholar]
- (15).Holmlin RE; Chen XX; Chapman RG; Takayama S; Whitesides GM Zwitterionic SAMs that Resist Nonspecific Adsorption of Protein from Aqueous Buffer. Langmuir 2001, 17 (9), 2841–2850. [DOI] [PubMed] [Google Scholar]
- (16).He Y; Hower J; Chen SF; Bernards MT; Chang Y; Jiang SY Molecular Simulation Studies of Protein Interactions with Zwitterionic Phosphorylcholine Self-assembled Monolayers in the Presence of Water. Langmuir 2008, 24 (18), 10358–10364. [DOI] [PubMed] [Google Scholar]
- (17).Singha P; Locklin J; Handa H A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Ren PF; Yang HC; Liang HQ; Xu XL; Wan LS; Xu ZK Highly Stable, Protein-Resistant Surfaces via the Layer-by-Layer Assembly of Poly(sulfobetaine methacrylate) and Tannic Acid, Langmuir 2015, 31 (21), 5851–8. [DOI] [PubMed] [Google Scholar]
- (19).Turro NJ Modern Molecular Photochemistry; Benjamin/Cummings Publishing Co.: Menlo Park, CA, 1978. [Google Scholar]
- (20).Lin AA; Sastri VR; Tesoro G; Reiser A; Eachus R On the Crosslinking Mechanism of Benzophenone-containing Polyimides. Macromolecules 1988, 21 (4), 1165–1169. [Google Scholar]
- (21).Park M-K; Deng S; Advincula RC pH-Sensitive Bipolar Ion-Permselective Ultrathin Films. J. Am. Chem. Soc 2004, 126 (42), 13723–13731. [DOI] [PubMed] [Google Scholar]
- (22).Higuchi H; Yamashita T; Horie K; Mita I Photo-cross-linking Reaction of Benzophenone-containing Polyimide and Its Model Compounds. Chem. Mater 1991, 3 (1), 188–194. [Google Scholar]
- (23).Braeuchle C; Burland DM; Bjorklund GC Hydrogen Abstraction by Benzophenone Studied by Holographic Photochemistry. J. Phys. Chem 1981, 85 (2), 123–127. [Google Scholar]
- (24).Lin X; Fukazawa K; Ishihara K Photoreactive Polymers Bearing a Zwitterionic Phosphorylcholine Group for Surface Modification of Biomaterials. ACS Appl. Mater. Interfaces 2015, 7 (31), 17489–98. [DOI] [PubMed] [Google Scholar]
- (25).Samuel JDJS; Brenner T; Prucker O; Grumann M; Ducree J; Zengerle R; Rühe J Tailormade Microfluidic Devices Through Photochemical Surface Modification. Macromol. Chem. Phys 2010, 211 (2), 195–203. [Google Scholar]
- (26).Hu S; Ren X; Bachman M; Sims CE; Li GP; Allbritton NL Surface-Directed, Graft Polymerization within Microfluidic Channels. Anal. Chem 2004, 76 (7), 1865–1870. [DOI] [PubMed] [Google Scholar]
- (27).Virkar A; Ling M-M; Locklin J; Bao Z Oligothiophene Based Organic Semiconductors with Cross-linkable Benzophenone Moieties. Synth. Met 2008, 158 (21–24), 958–963. [Google Scholar]
- (28).Bunte C; Prucker O; Konig T; Ruhe J Enzyme Containing Redox Polymer Networks for Biosensors or Biofuel Cells: A Photochemical Approach. Langmuir 2010, 26 (8), 6019–6027. [DOI] [PubMed] [Google Scholar]
- (29).Bunte C; Ruhe J Photochemical Generation of Ferrocene-Based Redox-Polymer Networks. Macromol. Rapid Commun 2009, 30 (21), 1817–1822. [DOI] [PubMed] [Google Scholar]
- (30).Gao J; Martin A; Yatvin J; White E; Locklin J Permanently Grafted Icephobic Nanocomposites with High Abrasion Resistance. J. Mater. Chem. A 2016, 4 (30), 11719–11728. [Google Scholar]
- (31).Abu-Rabeah K; Atias D; Herrmann S; Frenkel J; Tavor D; Cosnier S; Marks RS Characterization of Electrogenerated Polypyrrole–Benzophenone Films Coated on Poly(pyrrole-methyl metacrylate) Optic-Conductive Fibers. Langmuir 2009, 25 (17), 10384–10389. [DOI] [PubMed] [Google Scholar]
- (32).Brandstetter T; Bohmer S; Prucker O; Bisse E; zur Hausen A; Alt-Morbe J; Ruhe J A Polymer-based DNA Biochip Platform for Human Papilloma Virus Genotyping.. J. Virol. Methods 2010, 163 (1), 40–48. [DOI] [PubMed] [Google Scholar]
- (33).Brisbois EJ; Bayliss J; Wu J; Major TC; Xi C; Wang SC; Bartlett RH; Handa H; Meyerhoff ME Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model. Acta Biomater. 2014, 10 (10), 4136–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pegalajar-Jurado A; Wold KA; Joslin JM; Neufeld BH; Arabea KA; Suazo LA; McDaniel SL; Bowen RA; Reynolds MM Nitric oxide-releasing polysaccharide derivative exhibits 8-log reduction against Escherichia coli, Acinetobacter baumannii and Staphylococcus aureus. J. Controlled Release 2015, 217, 228–234. [DOI] [PubMed] [Google Scholar]
- (35).Backlund CJ; Worley BV; Schoenfisch MH Anti-biofilm action of nitric oxide-releasing alkyl-modified poly (amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 2016, 29, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Fang FC Antimicrobial actions of nitric oxide. Nitric Oxide 2012, 27, S10. [Google Scholar]
- (37).Wang PG; Xian M; Tang X; Wu X; Wen Z; Cai T; Janczuk AJ Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev 2002, 102 (4), 1091–1134. [DOI] [PubMed] [Google Scholar]
- (38).Brisbois EJ; Handa H; Major TC; Bartlett RH; Meyerhoff ME Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As polymer. Biomaterials 2013, 34 (28), 6957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Broniowska KA; Hogg N The Chemical Biology of S-Nitrosothiols. Antioxid. Redox Signaling 2012, 17 (7), 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Singha P; Pant J; Goudie MJ; Workman CD; Handa H, Enhanced antibacterial efficacy of nitric oxide releasing thermoplastic polyurethanes with antifouling hydrophilic topcoats. Biomater. Sci 2017, 5, 1246 10.1039/C6BM00948D [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Brisbois EJ; Davis RP; Jones AM; Major TC; Bartlett RH; Meyerhoff ME; Handa H Reduction in Thrombosis and Bacterial Adhesion with 7 Day Implantation of -Nitroso-acetylpenicillamine (SNAP)-Doped Elast-eon E2As Catheters in Sheep. J. Mater. Chem. B 2015, 3 (8), 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sundaram HS; Han X; Nowinski AK; Ella-Menye JR; Wimbish C; Marek P; Senecal K; Jiang S One-step dip coating of zwitterionic sulfobetaine polymers on hydrophobic and hydrophilic surfaces. ACS Appl. Mater. Interfaces 2014, 6 (9), 6664–71. [DOI] [PubMed] [Google Scholar]
- (43).Diaz Blanco C; Ortner A; Dimitrov R; Navarro A; Mendoza E; Tzanov T Building an antifouling zwitterionic coating on urinary catheters using an enzymatically triggered bottom-up approach. ACS Appl. Mater. Interfaces 2014, 6 (14), 11385–93. [DOI] [PubMed] [Google Scholar]
- (44).Scatena R; Bottoni P; Pontoglio A; Giardina B Pharmacological modulation of nitric oxide release: new pharmacological perspectives, potential benefits and risks. Curr. Med. Chem 2010, 17 (1), 61–73 [DOI] [PubMed] [Google Scholar]
- (45).Wo Y; Li Z; Brisbois EJ; Colletta A; Wu J; Major TC; Xi C; Bartlett RH; Matzger AJ; Meyerhoff ME Origin of Long-Term Storage Stability and Nitric Oxide Release Behavior of CarboSil Polymer Doped with S-Nitroso-N-acetyl-d-penicillamine. AGS Appl. Mater. Interfaces 2015, 7 (40), 22218–22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Joslin JM; Lantvit SM; Reynolds MM Nitric Oxide Releasing Tygon Materials: Studies in Donor Leaching and Localized Nitric Oxide Release at a Polymer-Buffer Interface. ACS Appl. Mater. Interfaces 2013, 5 (19), 9285–9294. [DOI] [PubMed] [Google Scholar]
- (47).Privett BJ; Broadnax AD; Bauman SJ; Riccio DA; Schoenfisch MH Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26 (3), 169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Tong SY; Davis JS; Eichenberger E; Holland TL; Fowler VG Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev 2015, 28 (3), 603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ní Eidhin D; Perkins S; Francois P; Vaudaux P; Höök M; Foster TJ Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol 1998, 30 (2), 245–257.' [DOI] [PubMed] [Google Scholar]
- (50).Boland T; Latour RA; Stutzenberger FJ Molecular basis of bacterial adhesion. In Handbook of Bacterial Adhesion; Springer: New York, 2000; pp 29–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


