Abstract
Objective
Survivors of extremely preterm birth are at risk of re-hospitalization but risk factors in the Canadian population are unknown. Our objective is to identify neonatal, sociodemographic, and geographic characteristics that predict re-hospitalization in Canadian extremely preterm neonates.
Methods
This is a retrospective analysis of a prospective observational cohort study that included preterm infants born 22 to 28 weeks’ gestational age from April 1, 2009 to September 30, 2011 and seen at 18 to 24 months corrected gestational age in a Canadian Neonatal Follow-Up Network clinic. Characteristics of infants re-hospitalized versus not re-hospitalized are compared. The potential neonatal, sociodemographic, and geographic factors with significant association in the univariate analysis are included in a multivariate model.
Results
From a total of 2,275 preterm infants born at 22 to 28 weeks gestation included, 838 (36.8%) were re-hospitalized at least once. There were significant disparities between Canadian provincial regions, ranging from 25.9% to 49.4%. In the multivariate logistic regression analysis, factors associated with an increased risk for re-hospitalization were region of residence, male sex, bronchopulmonary dysplasia, necrotizing enterocolitis, prolonged neonatal intensive care unit (NICU) stay, ethnicity, Indigenous ethnicity, and sibling(s) in the home.
Conclusion
Various neonatal, sociodemographic, and geographic factors predict re-hospitalization of extremely preterm infants born in Canada. The risk factors of re-hospitalization provide insights to help health care leaders explore potential preventative approaches to improve child health and reduce health care system costs.
Keywords: Extremely preterm, Infant, Neonatal follow-up, NICU, Re-hospitalization, Risk factors
Improvements in obstetric, perinatal, and neonatal care have led to significant increases in survival among extremely preterm infants born less than 29 weeks’ gestational age (GA) (1). However, more than half of these surviving infants develop at least one major morbidity during their initial hospitalization including bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and posthemorrhagic hydrocephalus, making them vulnerable to long-term health problems (2,3). Following discharge from the neonatal intensive care unit (NICU), extremely preterm infants are more likely to use hospital and community health services during their first years of life compared to their term-born counterparts (4–6). Indeed, 50 to 75% of extremely preterm infants get re-hospitalized (7–10) most often for respiratory infections, surgery, and gastro-intestinal tract problems (7,9). Not surprisingly, infants with BPD, especially on home oxygen, are at increased risk for re-hospitalization (8,10–12).
These studies have been conducted in countries with different health care systems; the Canadian health care system is characterized by universal health care coverage. However, the Canadian system has significant geographical disparities due to health care being under provincial jurisdiction. A study of high-risk children demonstrated that planning of discharge and postdischarge resources could diminish re-hospitalization rate and costs (13). Describing patterns of re-hospitalization is essential to identify high-risk infants, design preventative strategies that could reduce health care costs and improve care across Canada’s vast area. We aimed to describe re-hospitalization rates in a Canadian cohort of extremely preterm infants born 22 to 28 weeks’ GA and to explore the risk factors for hospital re-admission at discharge from NICU.
METHODS
Study design and population
This study is a retrospective analysis of a prospective observational cohort study by the Canadian Institute of Health Research team in Maternal Infant Care (MiCare) which linked national neonatal data collected by the Canadian Neonatal Network (CNN) with outcomes at 18 to 24 months corrected GA collected by the Canadian Neonatal Follow-Up Network (CNFUN) for all 26 NICUs with a neonatal follow-up program. Institutional review board approval for the MiCare study data collection was obtained at all sites. Written informed consent was obtained from participating families at the CNFUN visit as per local institutional review board requirements. The Research Ethics Boards at the Children’s Hospital of Eastern Ontario (CHEO) Research Institute and Ottawa Health Science Network approved this study.
Study participants assessments
CNFUN procedures and neurodevelopmental outcomes have been previously reported (14). The 18 to 24 months corrected age assessment included a caregiver completed questionnaire on sociodemographic characteristics and health care resource use including re-hospitalization, duration of hospitalization and reason for admission from neonatal hospitalization discharge (i.e., first day at home) up to the day of the visit. In addition to a history, the visit included a neurological examination and a developmental assessment.
Outcome and risk factors definitions
Operation manuals for both the CNN and CNFUN database included detailed data collection definitions to guide data abstractors (14). The primary outcome was re-hospitalization after NICU final discharge to home from the last hospital. Re-hospitalization was defined as a child admission on a short- or long-term unit for at least one night after the initial NICU hospitalization up to the date of the 18- to 24-month assessment. Re-hospitalization included an admission from emergency to a short stay care unit (which is not an observation room) or to a paediatric day centre where treatment or investigation of certain diseases are ambulatory and on a daily basis for a specified period (i.e., for treatment of pyelonephritis, moderate cellulitis). An observation extended to the emergency room was not included. Primary reasons for re-hospitalization recorded included mostly acute medical conditions that required unplanned re-hospitalization and surgical conditions that excluded minor day procedures (see Supplementary Table S1). From the information included in the CNFUN database, we could not differentiate between elective and unplanned admission, nor could we determine whether admission in an intensive care unit was required. The information on re-hospitalization was self-reported by parents at the 18 months corrected GA visit and cross-checked with medical chart review when available. The definitions used for the risk factors were: GA calculated by using a hierarchy of in vitro fertilization date, early antenatal ultrasound dating, last menstrual date, obstetric estimate, and neonatal estimate in that sequence; antenatal steroid use defined as any corticosteroid administration before birth; small for GA (SGA) defined as birth weight < 10th percentile (15); severe neurological injury as grade 3 or 4 intraventricular hemorrhage (16) and/or periventricular leukomalacia (17) on head ultrasound; severe retinopathy of prematurity (ROP) as stage 3 or greater or treatment with laser or injections of anti-vascular endothelial growth factor (18); BPD as oxygen needs at 36 weeks GA or at hospital discharge if discharged prior to 36 weeks GA; NEC as stage 2 or 3 according to Bell’s criteria (19); and, living over 100 km from the NICU.
Data analysis
Infant characteristics and outcomes as well as family sociodemographic and geographic characteristics were compared between infants with and without re-hospitalization. Frequency or mean (standard deviation) were reported. Difference between the two groups was assessed by Pearson Chi-square for categorical variables and Student t-test or ANOVA F-test for continuous variables. Rows with count number less than 5 were not included in the final results. Infants were categorized into six geographic Canadian provincial regions (20) to avoid inadvertent identification of specific hospitals. Infant characteristics, neonatal outcomes, sociodemographic status and the reason for readmission were compared across the geographic regions using Pearson Chi-square for categorical variables or ANOVA F-test for continuous variables.
Univariate logistic analyses were done to identify risk factors for re-hospitalization. Clinically relevant factors were entered into the multivariate analysis by stepwise selection using the lack-of-fit (Hosner) method to get the final model. In case of collinearity between variables (e.g., BPD and home oxygen), the medical condition was kept in the model. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. For comparisons between regions, the one with the lowest rate of readmission (British Columbia) was used for reference. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) with two-sided significance level 0.05.
RESULTS
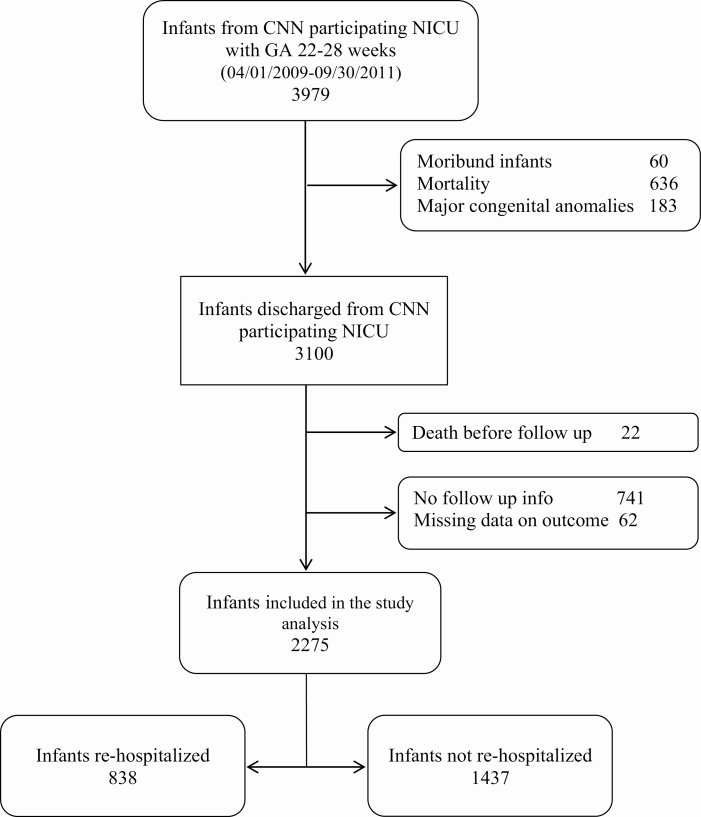
As shown in Figure 1, after the exclusion of infants with major congenital malformations, 3,100/3,979 (77.9%) infants were discharged alive from CNN participating NICUs during the study period. At the 18- to 24-month visit, 22 had died, and 741 were lost to follow-up leaving 2,337 infants seen. Among them, 62 had incomplete data on hospitalization. Infants with missing data were less premature, less likely to have received antenatal corticosteroids, less likely to have BPD or ROP, had younger mothers and more likely to live over 100 km from NICU than those included in the analyses (Supplementary Table S2).
Figure 1.
Flow diagram of study population.
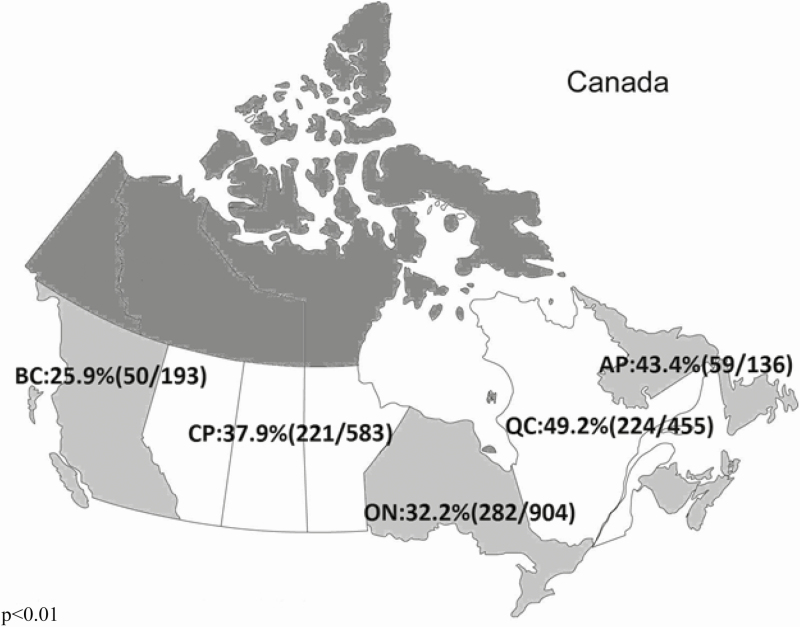
Among the 2275 remaining infants, 838 (36.8%) were re-hospitalized at least once for a total of 1162 re-hospitalizations. Of infants re-hospitalized, 492 (58.7%) had only one admission, 185 (22%) two admissions, and 161 (20%) had three or more (range: 1 to 14). Figure 2 illustrates rates of re-hospitalization by Canadian provincial region, which ranged from 25.9% in British Columbia (BC) to 49.4% in Quebec.
Figure 2.
Re-hospitalization rates across Canada by provincial regions. BC British Columbia; CP (Canadian Prairies) Manitoba, Saskatoon, and Alberta; ON Ontario; QC Quebec; AP (Atlantic Provinces) Newfoundland and Labrador, Prince Edward Island, and Nova Scotia.
Table 1 shows the characteristics that are associated with a risk for re-hospitalization after NICU discharge compared to those never re-hospitalized. In addition to the geographic and family sociodemographic characteristics, infant medical conditions including BPD, severe neurological injury on head ultrasound and use of medical assistive technologies (e.g., continuous positive airway pressure [CPAP], tracheostomy, gavage feeding) were higher in the group re-hospitalized. Table 2 describes the characteristics of the study population by geographic regions including neonatal medical complications that could influence the risk for re-hospitalization. The small number of infants (n=4) living in northern Canada for whom follow-up data were available prevented meaningful statistical analysis and results so were not included in the tables.
Table 1.
Characteristics of infants with at least one re-hospitalization after NICU discharge versus those never readmitted
| Infants with at least one re-hospitalization (N=838) | Infants never re-hospitalized (N=1,437) | P-value | |
|---|---|---|---|
| Neonatal characteristics | |||
| Gestational age (weeks), mean (SD) | 26.1 (1.4) | 26.5 (1.3) | <0.01 |
| Small for gestational age; n, (%) | 71 (8.5) | 95 (6.6) | 0.10 |
| Male; n, (%) | 468 (55.9) | 725 (50.5) | 0.01 |
| Antenatal steroids; n, (%) | 730 (89.7) | 1,276 (90.8) | 0.41 |
| Broncho-pulmonary dysplasia; n, (%) | 456 (54.6) | 570 (39.7) | <0.01 |
| Home oxygen; n, (%) | 227 (27.2) | 139 (9.7) | <0.01 |
| Respirator CPAP/Tracheostomy; n, (%) | 21 (2.5) | 11 (0.8) | <0.01 |
| Severe neurological injury; n, (%) | 114 (13.9) | 125 (9.0) | <0.01 |
| Necrotizing enterocolitis; n, (%) | 86 (10.3) | 76 (5.3) | <0.01 |
| Gavage feeding at NICU discharge; n, (%) | 71 (8.5) | 28 (2.0) | <0.01 |
| LOS in the NICU (days), mean (SD) | 90.0 (45.5) | 73.5 (36.7) | <0.01 |
| Sociodemographic characteristics; n, (%) | |||
| At least one caregiver born in Canada | 615 (73.4) | 887 (61.7) | <0.01 |
| Caucasian | 529 (69.8) | 787 (66.0) | <0.01 |
| Indigenous | 45 (5.9) | 42 (3.5) | |
| Others | 184 (24.3) | 364 (30.5) | |
| Paid employment | 615 (81.6) | 1,014 (85.6) | 0.01 |
| Social welfare | 99 (13.1) | 106 (9.0) | |
| Other | 40 (5.3) | 65 (5.5) | |
| Education some college or higher | 546 (70.4) | 974 (73.3) | 0.14 |
| High school and lower | 230 (29.6) | 354 (26.7) | |
| Sibling living in home | 518 (62.6) | 803 (56.9) | 0.01 |
| Geographic characteristics; n | |||
| Atlantic provinces1 | 59 | 77 | <0.01 |
| Quebec | 224 | 231 | |
| Ontario | 282 | 622 | |
| Canadian prairies2 | 221 | 362 | |
| British Columbia | 50 | 143 | |
| Living over 100 km from NICU | 166 (21.6) | 230 (17.5) | 0.02 |
CPAP Continuous positive airway pressure; LOS Length of stay; NICU Neonatal Intensive Care Unit; SD Standard deviation.
1Atlantic Provinces: Newfoundland & Labrador, Prince Edward Island, and Nova Scotia.
2Canadian prairies: Manitoba, Saskatchewan, and Alberta.
Table 2.
Characteristics of study populations in relation to regions
| Atlantic provinces1 (N = 136 | Quebec (N=455) | Ontario (N=904) | Canadian prairies2 (N=583) | British Columbia (N=193) | p-value | |
|---|---|---|---|---|---|---|
| Neonatal characteristics | ||||||
| Gestational age (weeks), mean (SD) | 26.2 (1.5) | 26.3 (1.4) | 26.4 (1.3) | 26.5 (1.3) | 26.3 (1.5) | 0.13 |
| Birth weight (grams), mean (SD) | 930 (247) | 913 (214) | 934 (221) | 963 (229) | 941 (229) | 0.02 |
| Antenatal steroids, n (%) | 120 (88.9) | 412 (92.8) | 802 (90.3) | 503 (88.3) | 165 (92.2) | 0.19 |
| Small for gestational age, n (%) | 11 (8.1) | 38 (8.4) | 66 (7.3) | 34 (5.8) | 17 (8.9) | 0.59 |
| Male sex, n (%) | 61 (44.8) | 239 (52.5) | 475 (52.5) | 311 (53.3) | 107 (55.7) | 0.13 |
| Multiple, n (%) | 41 (30.2) | 128 (28.1) | 266 (29.4) | 154 (26.4) | 55 (28.5) | 0.64 |
| Broncho-pulmonary dysplasia, n (%) | 66 (48.5) | 210 (46.3) | 357 (39.5) | 325 (55.8) | 65 (33.9) | <0.01 |
| Home oxygen, n (%) | 13 (9.7) | 122 (26.9) | 95 (10.5) | 127 (21.8) | 9 (4.7) | <0.01 |
| Severe brain injury, n (%) | 23 (17.0) | 31 (6.9) | 104 (11.7) | 64 (11.6) | 16 (8.5) | 0.01 |
| Necrotizing enterocolitis, n (%) | 13 (9.6) | 47 (10.4) | 57 (6.3) | 37 (6.4) | 8 (4.2) | 0.03 |
| Severe retinopathy of prematurity, n (%) | 27 (21.8) | 65 (15.2) | 88 (11.6) | 71 (19.0) | 16 (15.8) | 0.01 |
| Late-onset sepsis, n (%) | 58 (42.7) | 147 (32.3) | 231 (25.6) | 156 (26.8) | 40 (20.7) | <0.01 |
| LOS in the NICU (day), mean (SD) | 97.3 (46.9) | 97.6 (43.2) | 70.8 (37.4) | 73.7 (36.8) | 83.6 (41.7) | <0.01 |
| Sociodemographic characteristics | ||||||
| Employment status: employed, n (%) | 89 (66.9) | 278 (67.2) | 496 (66.7) | 286 (50.2) | 118 (62.1) | <0.01 |
| Education; some college and higher, n (%) | 95 (70.9) | 295 (66.0) | 602 (79.3) | 372 (65.3) | 155 (81.6) | <0.01 |
| Ethnicity: Caucasian, n (%) | 116 (88.6) | 320 (71.4) | 405 (67.4) | 365 (63.3) | 109 (57.4) | <0.01 |
| Living over 100 km from NICU, n (%) | 49 (37.4) | 53 (12.3) | 80 (10.1) | 163 (29.9) | 48 (26.1) | <0.01 |
LOS Length of stay; NICU Neonatal Intensive Care Unit; SD Standard deviation.
1Atlantic Provinces: Newfoundland & Labrador, Prince Edward Island, and Nova Scotia.
2Canadian prairies: Manitoba, Saskatchewan, and Alberta.
Table 3 shows variations in the reasons for re-hospitalization by geographic regions. Re-hospitalization for respiratory disorders (62%) including infectious (bronchiolitis, pneumonia, laryngitis/croup, and other) and noninfectious (apnea, brief resolved unexplained event, unstable BPD, and other) causes was the most frequent indication across all Canadian regions, followed by the need for surgical interventions (40%). Inguinal hernia repair was the most common surgery performed.
Table 3.
Common reasons for re-hospitalization per infants in relation to regions in Canada
| Reasons; n (%) | All regions | Atlantic provinces1 | Quebec | Ontario | Canadian prairies2 | British Columbia | P-value |
|---|---|---|---|---|---|---|---|
| Respiratory disorders | 518 (61.8) | 34 (57.6) | 157 (70.1) | 155 (55.0) | 136 (61.5) | 34 (68.0) | 0.01 |
| Infections other than respiratory system | 85 (10.1) | 8 (13.6) | 23 (10.3) | 33 (11.7) | 18 (8.1) | 3 (6.0) | 0.60 |
| Gastro-intestinal | 65 (7.8) | 4 (6.8) | 25 (11.2) | 17 (6.0) | 19 (8.6) | 0 (0) | 0.09 |
| Neurological | 20 (2.4) | 3 (5.1) | 1 (0.5) | 6 (2.1) | 10 (4.5) | 0 (0) | 0.02 |
| Surgery | 333 (39.7) | 24 (40.7) | 79 (35.3) | 116 (41.1) | 100 (45.3) | 14 (28.0) | 0.10 |
| Inguinal hernia repair | 102 (12.2) | 8 (13.6) | 26 (11.6) | 38 (13.5) | 25 (11.3) | 5 (10.0) | 0.94 |
| Ventriculoperitoneal shunts | 27 (3.2) | 4 (6.8) | 2 (0.9) | 14 (5.0) | 7 (3.2) | 0 (0) | 0.06 |
| Other type of surgery | 234 (27.9) | 15 (25.4) | 53 (23.7) | 76 (27.0) | 79 (35.8) | 11 (22.0) | 0.06 |
| Other reasons3 | 141 (16.8) | 12 (20.3) | 33 (14.7) | 47 (16.7) | 39 (17.7) | 10 (20.0) | 0.84 |
1Atlantic Provinces: Newfoundland & Labrador, Prince Edward Island, and Nova Scotia.
2Canadian prairies: Manitoba, Saskatchewan, and Alberta.
3Other reasons for re-hospitalization included: accident, trauma, choking, reflux, roseola, retinal problem, sleep study.
Univariate and multivariate logistic regressions are shown in Table 4. Lower GA, male gender, BPD, severe brain lesions, NEC, longer length of stay (LOS), family sociodemographic characteristics including at least one caregiver born in Canada, Indigenous ethnicity, depending on social assistance and having a sibling living at home, as well as the Canadian region of follow-up and the living distance from the NICU were associated with increased odds of re-hospitalization on the univariate analysis. Sensitivity analyses using the postal code and Statistics Canada definition of rural versus urban did not find an association of location with re-hospitalization. From the multivariate analysis, in addition to the Canadian regions, only NEC, having a sibling living in the home, BPD, at least one caregiver born in Canada, Indigenous ethnicity and LOS were still associated with re-hospitalization. When entering these factors in the model, the multivariate analysis shows that the lower GA was not significantly associated with increased risks of re-hospitalization.
Table 4.
Univariate and multivariate logistic regression analysis model of risk factors for re-hospitalization up to 18 to 24 months corrected gestational age
| Neonatal factors | Univariate analysis OR (95% CI) | Multivariate analysis OR (95% CI) |
|---|---|---|
| Gestational Age (per additional week) | 0.82 (0.77, 0.87) | 0.93 (0.85, 1.01) |
| Small for gestational age | 1.31 (0.95, 1.80) | - |
| Male | 1.24 (1.05, 1.47) | 1.29 (1.06, 1.56) |
| Antenatal steroids | 0.89 (0.66, 1.18) | - |
| Bronchopulmonary disease | 1.83 (1.54, 2.17) | 1.38 (1.12, 1.69) |
| Home oxygen | 3.46 (2.74, 4.36) | - |
| Respirator CPAP/Tracheostomy | 3.34 (1.60, 6.97) | - |
| Severe brain lesion | 1.64 (1.25, 2.15) | - |
| Necrotizing enterocolitis | 2.05 (1.49, 2.83) | 1.50 (1.04, 2.16) |
| Gavage feeding at NICU discharge | 4.66 (2.98, 7.27) | - |
| Ileostomy | 6.07 (1.99, 18.5) | - |
| LOS in the NICU (every one more week) | 1.07 (1.06, 1.09) | 1.04 (1.02, 1.07) |
| Sociodemographic factors | ||
| At least one caregiver born in Canada | 1.71 (1.42, 2.06) | 1.37 (1.01, 1.87) |
| Caucasian vs. Indigenous | 0.63 (0.41, 0.97) | 0.56 (0.35, 0.89) |
| Others vs. Indigenous | 0.47 (0.30, 0.75) | 0.52 (0.31, 0.89) |
| Social welfare vs. paid employment | 1.54 (1.15, 2.06) | - |
| Other vs. paid employment | 1.02 (0.68, 1.52) | |
| Education some college or higher vs High school and lower | 0.86 (0.71, 1.05) | - |
| Sibling living in home vs. no sibling | 1.27 (1.07, 1.52) | 1.40 (1.15, 1.71) |
| Geographic factors | ||
| By region of follow-up | ||
| Atlantic provinces1 vs. British Columbia | 2.19 (1.37, 3.50) | 2.00 (1.21, 3.31) |
| Quebec vs. British Columbia | 2.77 (1.91, 4.02) | 2.57 (1.73, 3.83) |
| Ontario vs. British Columbia | 1.30 (0.91, 1.84) | 1.72 (1.17, 2.53) |
| Canadian prairies2 vs. British Columbia | 1.75 (1.21, 2.51) | 1.71 (1.16, 2.53) |
| Living area over 100 km from NICU | 1.30 (1.04, 1.63) | - |
1Atlantic Provinces: Newfoundland & Labrador, Prince Edward Island, and Nova Scotia
2Canadian prairies: Manitoba, Saskatchewan, and Alberta
CI Confidence interval; CPAP Continuous positive airway pressure; LOS Length of stay; NICU Neonatal intensive care unit; OR Odds ratio.
DISCUSSION
Our study shows that slightly more than one-third of premature infants born <29 weeks born in Canada were re-hospitalized during their first 18 months after discharge from NICU with rates comparable to other studies. The EPIPAGE study showed a readmission rate of 47.3% in the first nine months post NICU discharge (7). A study of premature infants born < 29 weeks’ gestation in the province of Quebec between 2003 and 2004 showed a similar re-hospitalization rate of 50.5% compared to 49.2% almost a decade later in the current study (21).
The rate of postdischarge re-hospitalization in the first 18 to 24 months corrected age varied significantly by Canadian geographic regions. Geographic variation in infant hospital readmission rates has also been reported in term born babies in Canada (22) and for low birth weight infants in other countries (23). The disparity in health care system delivery and resource availability may explain some of the variations. One explanation could be the nature of follow-up care; close ambulatory follow-up can decrease the need for emergency room visits and potentially hospital admission where detection and treatment of early signs for respiratory deterioration may prevent the need for admission. However, more studies are needed to explore theses hypotheses with our population of preterm infants. Though our univariate analyses showed increased re-hospitalization rates for infants living in remote areas similar to other studies (24), when adjusted for other confounding factors including morbidities and sociodemographic and geographical factors, living over 100 km from the NICU was no longer significant which would be consistent with the finding of an increased risk of prematurity and severe neonatal morbidity amongst infants with a remote area address (25).
Similarly to the results of Rasler et al. (25), we found that complications related to prematurity rather than GA predicted re-hospitalization. In the multivariate analysis, gestational age was not a risk factor for re-hospitalization likely because postnatal factors including developing NEC, BPD, prolonged NICU stay and other sociodemographic and geographical factors contribute more to the risk of readmission of extremely preterm infants. Our study showed the expected correlation between neonatal morbidities such as BPD and NEC and re-hospitalization rates. The association between BPD and re-hospitalization has been demonstrated in multiple studies (7,8,21,25,26). Preterm infants, especially those with BPD have varying degrees of lung injury with increased susceptibility to infection and bronchospasm (12). Infants with NEC are at higher risk of needing an ostomy, malabsorption, short bowel syndrome, and failure to thrive known to increase health resource utilization (27) but the rate of post NICU discharge re-hospitalization has not been well described. Our findings certainly support the need to study preventive approaches for BPD, NEC, and other neonatal complications to reduce re-hospitalization rates. Lastly, in accordance with previous studies, we found that male gender is a risk factor for re-hospitalization, yet it is unclear if this is attributable to their higher rate of inguinal hernia or underlying co-morbidities (2,24,28).
Taking into consideration the multivariate analysis showing that geographic variations existed despite different rates of neonatal characteristics, Table 2 may help to better understand how the neonatal morbidities and the geographical risk factors interplay. Although premature infants in Quebec had a higher re-hospitalization rate, they had a significantly lower mean birth weight and a higher incidence of NEC than British Columbia with a lower re-hospitalization rate and significantly lower NEC and BPD rates.
In addition to neonatal risk factors, we confirmed the finding by Vohr et al. (28) that family composition, such as the presence of sibling(s) in the home, increased the odds of re-hospitalization probably due to the increased risk of household infection transmission. Our study did not confirm the findings of Brooks-Gunn et al.’s (29) study that showed that infants from more impoverished families were more likely to be re-hospitalized. We did not find any relationship between the educational status or the employment status of the care provider and re-admission rates. It is possible that universal health care coverage mitigates the effect of social disparities on hospital access.
As found in other studies (2,7,21,28,30,31), we found that respiratory disorders were the principal reasons for re-hospitalization in early life and surgery, mainly inguinal hernia repair, was the second most common reason for admission accounting for almost 40% of the re-hospitalizations. Our finding on hernia repair corroborates other publications as an important reason for re-hospitalization in children born preterm (26,31).
The inclusion of a large national cohort of infants in a demographically and geographically diverse area with different health care delivery systems is a strength of this study. It provides insight into the geographical variations within Canada. This study has some limitations. Of the 3,078 infants who survived to 18 months, data were available for 2,275 (74%) with significant differences between those with and without follow-up (Supplementary Table S2). Children lost to follow-up had fewer risk factors for re-hospitalization and hence, the re-hospitalization rate could have been overestimated. However, the risk factors of re-hospitalization are less likely to have been affected by the loss of follow-up. Re-hospitalization was self-reported by parents, which may have led to under-reporting. CNFUN sites were encouraged to confirm hospital readmissions but this was not always possible. Our definition of re-hospitalization does not allow distinguishing between unplanned and elective admissions for procedures or investigations. However, the vast majority of re-hospitalization in our cohort were related to unplanned admissions. Lastly, our results may not be generalizable to different health care systems in other countries without universal health coverage.
Conclusions
Over one-third of children born in Canada at less than 29 weeks’ gestation are re-hospitalized in the first 18 to 24 months after their expected date of delivery. The risk of re-hospitalization is associated with various neonatal, sociodemographic and geographic factors. Knowing about these risk factors can help health care providers identify infants at highest risk, to plan for resource allocation and ensure that preventative measures such as vaccination of the child and family or hand hygiene are rigorously respected and reinforced or more complex approaches such as enhanced medical home care is readily accessible to families and their high risk infants. Reducing hospitalization will certainly contribute to improving the quality of life for the infants and their families.
Supplementary Material
Acknowledgements
We would like to thank Junmin Yang for his help with the statistical analysis.
Contributor Information
Canadian Neonatal Follow-Up Network:
Thevanisha Pillay, Anne Synnes, Leonora Hendson, Amber Reichert, Jaya Bodani, Sibasis Daspal, Diane Moddemann, Chukwuma Nwaesei, Thierry Daboval, Sarah McKnight, Kevin Coughlin, Linh Ly, Edmond Kelly, Saroj Saigal, Karen Thomas, Paige Church, Ermelinda Pelausa, M Khairy, Thuy Mai Luu, Charlotte Demers, Alyssa Morin, Dr., Sylvie Bélanger, Roderick Canning, Luis Monterrosa, Hala Makary, Jehier Afifi, Phil Murphy, and Charles Janeway
Funding:
There are no funders to report for this submission.
Potential Conflicts of Interest:
All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Canadian Neonatal Follow-Up Network
Thevanisha Pillay, MD, Victoria General Hospital, Victoria, B.C.
Anne Synnes, MDCM, MHSC, British Columbia Women’s Hospital, Vancouver, B.C.
Leonora Hendson MB.BCH, MSc, Alberta’s Children’s Hospital, Foothills Medical Centre, Calgary, AB
Amber Reichert, MD, Glenrose Rehabilitation Hospital, Edmonton, AB
Jaya Bodani, MD, Regina General Hospital, Regina, SK
Sibasis Daspal, MD, Royal University Hospital, Saskatoon, SK
Diane Moddemann, MD, Cecilia de Cabo, Winnipeg Health Sciences Centre, St. Boniface General Hospital, Winnipeg, MB
Chukwuma Nwaesei, MD, Windsor Regional Hospital, Windsor, ON
Thierry Daboval, MD, Children’s Hospital of Eastern Ontario, Ottawa, ON
Sarah McKnight, Kingston General Hospital, Kingston, ON
Kevin Coughlin, MD, Children’s Hospital London Health Sciences Centre, London, ON
Linh Ly, MD, Hospital for Sick Children, Toronto, ON
Edmond Kelly, MD, Mount Sinai Hospital, Toronto, ON
Saroj Saigal, Karen Thomas, MD, Hamilton Health Sciences Centre, Hamilton, ON
Paige Church, MD, Sunnybrook Health Sciences Centre, Toronto, ON
Ermelinda Pelausa, MD, Jewish General Hospital, Montréal, QC
M Khairy MD, Montréal Children’s Hospital, Royal Victoria Hospital, Montréal, QC
Thuy Mai Luu MD, MSc, Centre Hospitalier Universitaire Sainte-Justine, Montréal, QC
Charlotte Demers and Dr. Alyssa Morin, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, QC
Sylvie Bélanger, MD, Centre Hospitalier Universitaire de Québec, Québec City, QC
Roderick Canning, MD, Moncton Hospital, Moncton, N.B.
Luis Monterrosa, MD, Saint John Regional Hospital, Saint John, N.B.
Hala Makary, MD, Dr. Everett Chalmers Hospital, Fredericton, N.B.
Jehier Afifi, MB BCh, MSc, IWK Health Centre, Halifax, N.S.
Phil Murphy, Charles Janeway Children’s Health and Rehabilitation Centre, St. John’s, NL.
References
- 1. The Canadian Neonatal Network Le Réseau Néonatal Canadien. Annual Report 2017 Rapport Annuel. <http://www.canadianneonatalnetwork.org/Portal/LinkClick.aspx?fileticket=XhPMIxFgc2M%3D&tabid=39> (Accessed April 12, 2019).
- 2. Kuint J, Lerner-Geva L, Chodick G, Boyko V, Shalev V, Reichman B; Israel Neonatal Network . Rehospitalization through childhood and adolescence: Association with neonatal morbidities in infants of very low birth weight. J Pediatr 2017;188:135–141.e2. [DOI] [PubMed] [Google Scholar]
- 3. Stensvold HJ, Klingenberg C, Stoen R, et al. Neonatal morbidity and 1-year survival of extremely preterm infants. Pediatrics 2017;139(3):e20161821. [DOI] [PubMed] [Google Scholar]
- 4. Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med 2003;349(10):959–67. [DOI] [PubMed] [Google Scholar]
- 5. Spencer N, Coe C. Parent reported longstanding health problems in early childhood: A cohort study. Arch Dis Child 2003;88(7):570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCormick MC, Bernbaum JC, Eisenberg JM, Kustra SL, Finnegan E. Costs incurred by parents of very low birth weight infants after the initial neonatal hospitalization. Pediatrics 1991;88(3):533–41. [PubMed] [Google Scholar]
- 7. Lamarche-Vadel A, Blondel B, Truffer Pet al. ; EPIPAGE Study Group. Re-hospitalization in infants younger than 29 weeks’ gestation in the EPIPAGE cohort. Acta Paediatr 2004;93(10):1340–5. [DOI] [PubMed] [Google Scholar]
- 8. Chien YH, Tsao PN, Chou HC, Tang JR, Tsou KI. Rehospitalization of extremely-low-birth-weight infants in first 2 years of life. Early Hum Dev 2002;66(1):33–40. [DOI] [PubMed] [Google Scholar]
- 9. Luu TM, Lefebvre F, Riley P, Infante-Rivard C. Continuing utilisation of specialised health services in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2010;95(5):F320–5. [DOI] [PubMed] [Google Scholar]
- 10. Elder DE, Hagan R, Evans SF, Benninger HR, French NP. Hospital admissions in the first year of life in very preterm infants. J Paediatr Child Health 1999;35(2):145–50. [DOI] [PubMed] [Google Scholar]
- 11. Carbonell-Estrany X, Quero J, Bustos G, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: A prospective study. IRIS Study Group. Pediatr Infect Dis J 2000;19(7):592–7. [DOI] [PubMed] [Google Scholar]
- 12. Grégoire MC, Lefebvre F, Glorieux J. Health and developmental outcomes at 18 months in very preterm infants with bronchopulmonary dysplasia. Pediatrics 1998;101(5):856–60. [DOI] [PubMed] [Google Scholar]
- 13. Mosquera RA, Avritscher EB, Samuels CL, et al. Effect of an enhanced medical home on serious illness and cost of care among high-risk children with chronic illness a randomized clinical trial. JAMA - J Am Med Assoc 2015;77030(24):2640–8. [DOI] [PubMed] [Google Scholar]
- 14. Synnes A, Luu TM, Moddemann D, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child - Fetal Neonatal Ed 2016;0:F1–6. 10.1136/archdischild-2016-311228 [DOI] [PubMed] [Google Scholar]
- 15. Kramer MS, Platt RW, Wen SW, et al. ; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001;108(2):E35. [DOI] [PubMed] [Google Scholar]
- 16. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92(4):529–34. [DOI] [PubMed] [Google Scholar]
- 17. Dubowitz LM, Bydder GM, Mushin J. Developmental sequence of periventricular leukomalacia. Correlation of ultrasound, clinical, and nuclear magnetic resonance functions. Arch Dis Child 1985;60(4):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jefferies A. Retinopathy of prematurity: Recommendations for screening. Paediatr Child Health 2010;15(10):667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Canada, Government of Canada S. Standard Geographical Classification (SGC 2016 - Introduction. 2018. <https://www.statcan.gc.ca/eng/subjects/standard/sgc/2016/introduction> (Accessed February 18, 2019).
- 21. Liu S, Wen SW, McMillan D, Trouton K, Fowler D, McCourt C. Increased neonatal readmission rate associated with decreased length of hospital stay at birth in Canada. Can J Public Health 2000;91(1):46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tseng YH, Chen CW, Huang HL, et al. Incidence of and predictors for short-term readmission among preterm low-birthweight infants. Pediatr Int 2010;52(5):711–7. [DOI] [PubMed] [Google Scholar]
- 23. Lundberg B, Lindgren C, Palme-Kilander C, Örtenstrand A, Bonamy AK, Sarman I. Hospital-assisted home care after early discharge from a Swedish neonatal intensive care unit was safe and readmissions were rare. Acta Paediatr 2016;105(8):895–901. [DOI] [PubMed] [Google Scholar]
- 24. Lisonkova S, Haslam MD, Dahlgren L, Chen I, Synnes AR, Lim KI. Maternal morbidity and perinatal outcomes among women in rural versus urban areas. CMAJ 2016;188(17–18):E456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ralser E, Mueller W, Haberland C, et al. Rehospitalization in the first 2 years of life in children born preterm. Acta Paediatr 2012;101(1):e1–5. [DOI] [PubMed] [Google Scholar]
- 26. Mowitz ME, Dukhovny D, Zupancic JAF. The cost of necrotizing enterocolitis in premature infants. Semin Fetal Neonatal Med 2018;23(6):416–9. [DOI] [PubMed] [Google Scholar]
- 27. Laugier O, Garcia P, Boucékine M, et al. Influence of socioeconomic context on the rehospitalization rates of infants born preterm. J Pediatr 2017;190:174–179.e1. [DOI] [PubMed] [Google Scholar]
- 28. Vohr BR, Yatchmink YE, Burke RT, et al. Factors associated with rehospitalizations of very low birthweight infants: Impact of a transition home support and education program. Early Hum Dev 2012;88(7):455–60. [DOI] [PubMed] [Google Scholar]
- 29. Davis AS, Hintz SR, Goldstein RF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Outcomes of extremely preterm infants following severe intracranial hemorrhage. J Perinatol 2014;34(3):203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ambalavanan N, Carlo WA, McDonald SA, Yao Q, Das A, Higgins RD; Generic Database and Follow-up Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Identification of extremely premature infants at high risk of rehospitalization. Pediatrics 2011;128(5):e1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hack M. Rehospitalization of the very-low-birth-weight infant. Am J Dis Child 1981;135(3):263–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.