Abstract
Purpose:
Wide field swept source OCT angiography (WF SS-OCTA) imaging was compared with ultrawide-field (UWF) fluorescein angiography (FA) imaging to better understand changes in retinal non-perfusion before and after panretinal photocoagulation (PRP) in treatment-naïve eyes with proliferative diabetic retinopathy (PDR).
Design:
Prospective, observational, consecutive case series.
Participants:
Patients with treatment-naïve PDR.
Methods:
Patients were imaged using the SS-OCTA 12x12mm scan pattern at baseline and 1 week, 1 month, and 3 months after PRP. UWF FA was obtained at baseline and 3 months after PRP. Selected eyes were imaged using five SS-OCTA 12x12mm scans to create a posterior pole montage, and 5 eyes also underwent SS-OCTA imaging at 6 months and 1 year. Areas of retinal non-perfusion (RNP) were drawn independently by two masked graders, and analysis of variance (ANOVA) tests were used to compare areas of RNP over time.
Main Outcome Measures:
Area and boundaries of RNP visualized using WF SS-OCTA and UWF FA
Results:
From January 2018 through January 2019, WF SS-OCTA was performed on 20 eyes with treatment-naïve PDR from 15 patients. Areas of RNP identified on UWF FA images co-localized with RNP areas visualized on WF SS-OCTA images. There were no statistically significant changes in RNP area on WF SS-OCTA images through 3 months after PRP. Even eyes that were severely ischemic at baseline had no significant changes in RNP area one year after PRP.
Conclusions:
RNP in PDR can be identified at baseline and imaged serially after PRP using WF SS-OCTA. Retinal perfusion in PDR does not change significantly after PRP. The ability of WF SS-OCTA to longitudinally evaluate RNP areas provides additional justification for adopting WF SS-OCTA as the sole imaging modality for clinical management of PDR.
INTRODUCTION
Diabetic retinopathy (DR) is the most common cause of blindness among working-age adults in most developed countries.1 Vision loss in DR occurs because retinal ischemia can lead to retinal atrophy, diabetic macular edema (DME), and neovascularization (NV) with subsequent vitreous hemorrhage and tractional detachment. In proliferative diabetic retinopathy (PDR), panretinal photocoagulation (PRP) to areas of peripheral retinal ischemia causes regression of NV and reduces rates of vision loss.2 An alternative or supplemental therapy for PDR is intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) drugs, which can also induce regression of NV and reduce vision loss.3
Since the complications of DR result from retinal ischemia, there is great interest in determining whether current therapies have the ability to halt ischemic progression or even reverse it. Studies in the 1980s using laser Doppler velocimetry suggested that PRP leads to a decrease in retinal blood flow,4,5 and this finding was replicated recently using laser speckle flowgraphy6 and Doppler OCT.7 In contrast, different investigators using Doppler OCT8 and OCT angiography (OCTA) have reported either no effect9 or an increase in macular perfusion10–11 after PRP. There is also disagreement regarding the effect of anti-VEGF injections on retinal non-perfusion (RNP). Authors using ultrawide-field (UWF) fluorescein angiography (FA) in large prospective studies have detected a possible reversal of RNP with monthly anti-VEGF injections,12,13 whereas smaller, retrospective studies using OCTA have found no effect.14–16 Importantly, multiple studies comparing OCTA and FA have indicated that OCTA can more accurately delineate RNP areas than FA.14,17
Published studies using OCTA to investigate the effect of PRP or anti-VEGF injections on RNP have been limited by small and heterogeneous patient cohorts,9–11,15–16 the 3x3mm or 6x6mm field of view when using OCTA,9–11,15,16 and a single or a few follow-up time-points after treatment.9–11,14–16 To our knowledge, only one published study has used wide-field (WF) OCTA to study the effect of a therapeutic intervention on areas of diabetic RNP, but in that study, the extent of RNP was assessed at only one follow-up time-point after 3 anti-VEGF injections.14
We previously reported a prospective, observational, consecutive case series of treatment-naive PDR eyes treated with PRP and evaluated using UWF FA (at baseline and 3 months) and WF SS-OCTA at baseline, 1 week, 1 month, and 3 months.18 Our first report compared the two imaging modalities and described the critical benefits of WF SS-OCTA over UWF FA for identifying NV and monitoring eyes longitudinally. The current study compared the ability of WF SS-OCTA versus UWF FA in the longitudinal evaluation of RNP in treatment-naïve PDR eyes treated with PRP, including 1 year of follow-up of select eyes.
METHODS
This study was performed in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act of 1996, and was approved by the Institutional Review Board of the University of Miami Miller School of Medicine. Informed consent was obtained from all patients. Patients with treatment-naive PDR seen in the resident physician clinics of 4 of the authors (J.F.R., N.L.S., J.W.H., K.C.F.) were recruited and treated at the baseline visit with a single session of 360-degree PRP. Further details of patient recruitment, demographics, BCVA measurements, imaging protocol, and PRP protocol were previously reported.18 In brief, ultra wide-field (UWF) FA (Optos, Inc., Marlborough, MA), UWF fundus photography (Optos, Inc.), and WF SS-OCTA (PLEX® Elite 9000, Carl Zeiss Meditec, Inc., Dublin, CA) were performed at baseline. SS-OCTA was repeated 1 week, 1 month, and 3 months after PRP. UWF FA and UWF fundus photography were repeated at 3 months. In select eyes, SS-OCTA was repeated 6 months and 1 year after PRP.
The 12x12mm WF SS-OCTA en face images centered on the fovea and segmented to include the total retinal vasculature were used for analysis of RNP. The 12x12mm SS-OCTA images from the baseline, 1 week, 1 month, and 3 month visits were cropped using large vessel landmarks to correct for small differences in the areas of the imaged retina at different visits. The images were then assembled in a random order, two graders (J.F.R. and H.A.K.) were masked to patient identity and time-point of the SS-OCTA images, and the images were independently graded for the areas of RNP using ImageJ v1.52 (NIH, Bethesda, MD). The definition used for RNP was complete absence of capillaries in an area larger than the width of a retinal vein at its exit from the disc. The foveal avascular zone was not included as an area of RNP. Graders adjusted the contrast and brightness of the images at their discretion. Small areas of OCTA images with poor quality because of overlying NV, vitreous hemorrhage, cataract, or poor fixation were excluded from the analysis of that image. Any image with obscuration of >10% of retinal area was omitted entirely from the analysis. After all images were independently graded, the two graders conferred and came to a consensus on areas of RNP, using an adjudicator (P.J.R.) when necessary. In a secondary analysis, the grading protocol was repeated using the consensus RNP areas from the baseline image for each eye as a reference to determine RNP areas at the 1 week, 1 month, and 3 month time-points.
Eyes lacking data from all 4 time-points were excluded from statistical analysis. ImageJ was used to calculate pixels of RNP. This value was divided by the total number of pixels in the image to arrive at the percentage of the area occupied by RNP (RNP%). Analysis of variance (ANOVA) was performed using Excel (Microsoft, Redmond, WA). Two-factor ANOVA without replication (i.e., repeated measures) was used to compare RNP% of the cohort between time-points. Single-factor ANOVA was used to compare mean RNP% between subjects.
RESULTS
Twenty treatment-naive PDR eyes of 15 patients were imaged from January 2018 through May 2018, and those eyes not lost to follow-up were imaged through January 2019. PRP was performed at the baseline visit.18 There were no additional treatments through the 3-month visit except for one or two anti-VEGF injections in 6 eyes for subfoveal DME.18 A total of 69 12x12mm WF SS-OCTA en face images depicting the total retinal vasculature were graded. This was less than the expected 80 images (4 time-points from 20 eyes) because 2 eyes of the same patient were lost to follow-up after 1 week and 7 images from 4 eyes were excluded because of vitreous hemorrhage that developed after the baseline visit. Baseline and 3 month UWF FA were available for 17 of the 20 eyes.
We first qualitatively compared the distribution of RNP on WF SS-OCTA and UWF FA images. UWF FA at baseline (Figure 1A) highlighted areas of RNP that were evident only as “featureless retina” on UWF fundus photography (Figure 1C). In every case, areas of RNP seen on UWF FA were captured on the corresponding WF SS-OCTA (e.g., Figure 1A and 2A which were taken at the baseline visit, and Figure 1B and 2B which were taken at the 3 month visit). In all 20 eyes there were areas of RNP within the field of view (FOV) of the 12x12mm WF SS-OCTA images. The extent of RNP within the 12x12mm FOV varied greatly between eyes because some eyes had severe macular ischemia at baseline (Figure 1A) while others did not. The WF SS-OCTA montages, which incorporated 5 overlapping 12x12mm SS-OCTA scans, were able to visualize all areas of RNP seen on corresponding UWF FA images up to 6-8 disc diameters from the disc superiorly, temporally, and inferiorly (compare Figure 3A to 3D/3E, and Figure 3B to 3F/3G). The WF SS-OCTA distribution of RNP corresponded to the distribution of RNP seen on UWF FA at both the baseline and 3 month visits. Areas of RNP at the 3 month visit were easier to interpret on WF SS-OCTA images than on the corresponding UWF FA images because of staining of PRP scars on FA (Figure 1B, 3F/3G). Thus, regions on the UWF FA images showing areas of RNP within the posterior pole could be imaged at baseline and after PRP in PDR eyes using WF SS-OCTA as well.
Figure 1. Ultrawide-field (UWF) fluorescein angiogram (FA) and fundus photographs of a representative treatment-naïve eye with proliferative diabetic retinopathy (PDR) before and after panretinal photocoagulation (PRP).
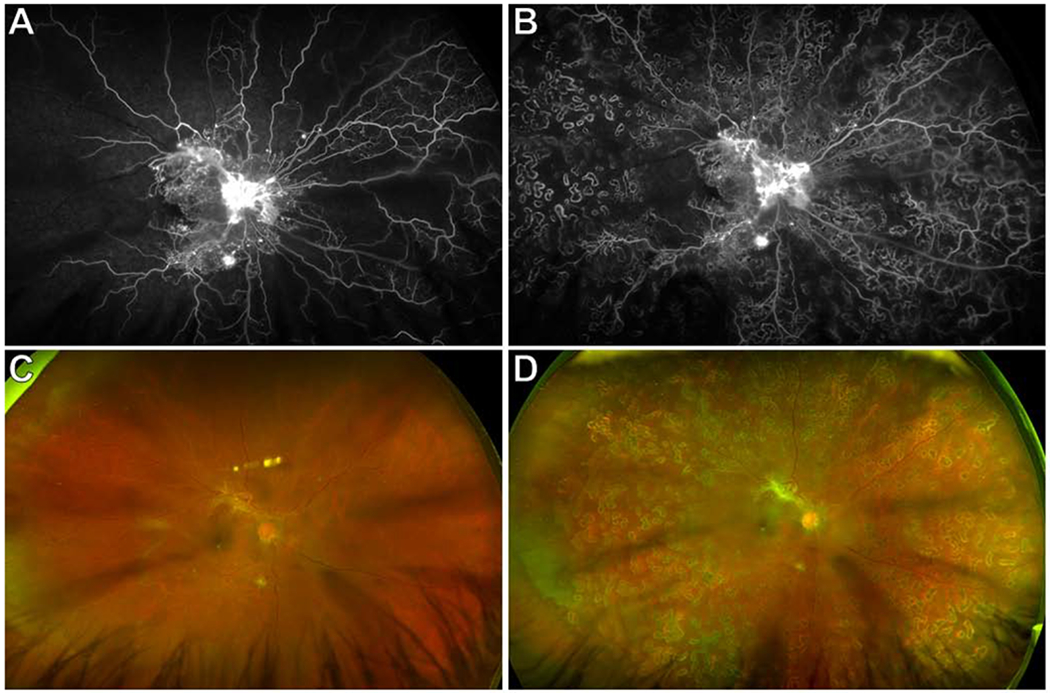
(A) Baseline UWF FA showed neovascularization (NV) of the disc, multifocal NV elsewhere (NVE), and extensive retinal non-perfusion (RNP) involving the macula. Areas of RNP were only evident as “featureless fundus” on the corresponding UWF fundus photograph (C). There was a cortical cataract.
(B) UWF FA 3 months after PRP showed 360 degree dense PRP scars, persistent NVD and NVE, and stable RNP. The corresponding UWF fundus photograph is shown in (D).
Figure 2. Wide-field (WF) swept-source (SS) OCT angiography (OCTA) 12x12mm total retinal vasculature images of proliferative diabetic retinopathy (PDR) eye through 1 year of follow-up after panretinal photocoagulation (PRP).
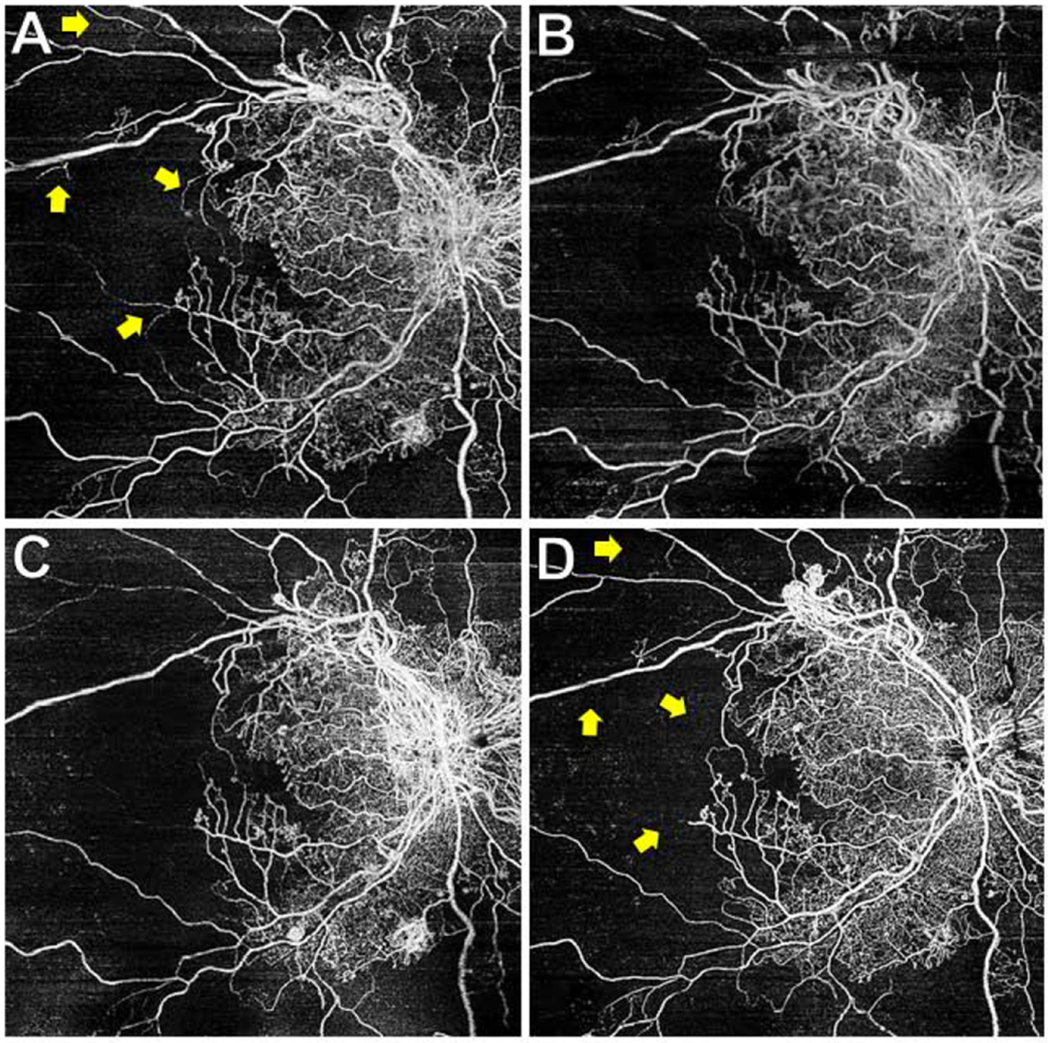
The eye shown in Figure 1 underwent WF SS-OCTA scans at the baseline (A), 3 month (B), 6 month (C), and 1 year (D) follow-up visits. This eye received additional PRP after the 3 month visit, and between the 6 month and 1 year visits received 2 anti-VEGF injections (for vitreous hemorrhage) and underwent cataract extraction. Retinal non-perfusion remained stable at all visits and through 1 year. BCVA at 1 year was 20/30+2. Some small areas of large-caliber vessel dropout were noted (yellow arrows). The area of signal dropout near the top of (B) were omitted from the RNP analysis.
Figure 3. Wide-field (WF) swept-source (SS) OCT angiography (OCTA) posterior pole montages and ultrawide-field (UWF) fluorescein angiograms (FA) of a representative eye with proliferative diabetic retinopathy (PDR) after panretinal photocoagulation (PRP).
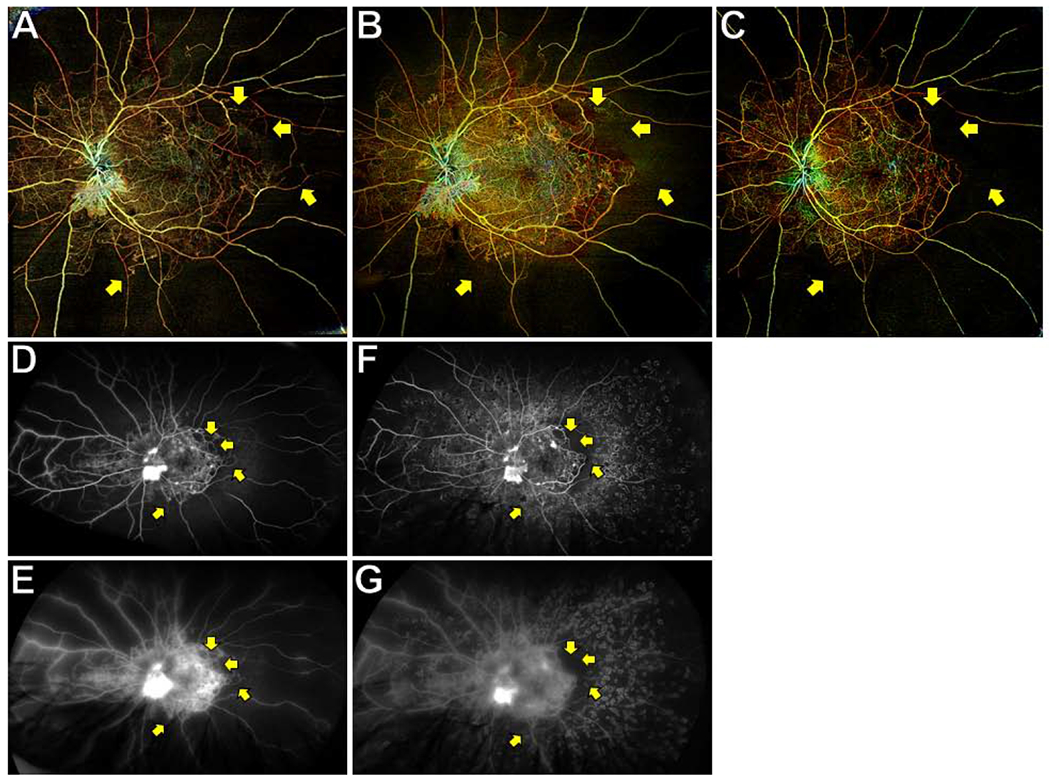
WF SS-OCTA posterior pole montages of total retinal vasculature at the baseline (A) and 3 month (B) visits showed retinal non-perfusion (RNP) that corresponded with UWF FAs taken on the same visits (D and E are early and late frames of baseline FA; F and G are early and late frames of 3 month FA). RNP was easier visualized on the 3 month OCTA image (B) than the 3 month FA because of staining of PRP scars (F). RNP was stable at the 3 month and 6 month (C) visits, though some small areas of large-caliber vessel dropout were noted (yellow arrows).
Repeat WF SS-OCTA imaging was performed at 6 months and 1 year in 5 eyes (the other 15 eyes were lost to follow-up before 1 year). After the 3 month visit, these 5 eyes were treated as needed with additional PRP and/or anti-VEGF injections at the discretion of the treating physician (J.F.R.). One eye also underwent cataract extraction. No further UWF FA imaging was performed because it was judged unnecessary. In eyes with 1 year of follow-up, there was minimal change in area of RNP on SS-OCTA images at any of the 6 time-points (baseline, 1 week, 1 month, 3 month, 6 months, 1 year) (Figure 2, 3A–C). Small changes in the dropout of capillaries and large-caliber vessels were seen in a few eyes with severe macular ischemia (Figures 2, 3A–C). These changes were also observed on the corresponding UWF FA images (Figure 1B, 3D–G). The correspondence between UWF FA and WF SS-OCTA images suggests that WF SS-OCTA can reliably monitor RNP and large-caliber vessel dropout for at least 1 year after PRP.
We then quantitatively analyzed RNP over time after PRP. We restricted our analysis to the 11 eyes that had SS-OCTA scans without media opacity or poor signal strength at the baseline, 1 week, 1 month, and 3 month visits. Data from a representative eye is shown in Figure 4. The consensus RNP% (i.e., RNP area divided by total image area) for each of the 11 eyes averaged for all 4 visits ranged from 1.24 to 56.4% (mean 16.0; std dev 18.3) (Figure 5). There were highly statistically significant differences in mean RNP% between eyes (P < 0.001), in keeping with the variable severity of baseline macular ischemia. However, there were no statistically significant differences in RNP% over time (mean RNP% was 15.7, 15.9, 15.5, and 16.8% at baseline, 1 week, 1 month, and 3 months, respectively; P = 0.244). Similarly, when a secondary analysis was performed using the consensus baseline areas of RNP to grade RNP areas at the 3 follow-up visits, there were no statistically significant differences in RNP% over time (mean RNP% was 15.8, 16.0, 15.9, and 16.6% at baseline, 1 week, 1 month, and 3 months, respectively; P = 0.383). As seen in Figure 4, small areas of variability were seen between visits, but these were not statistically different and likely reflected inherent imprecision of the imaging and/or RNP grading rather than physiologically valid changes in RNP.
Figure 4. Retinal non-perfusion (RNP) areas on wide-field (WF) swept-source (SS) OCT angiography (OCTA) of a representative eye with proliferative diabetic retinopathy (PDR) after panretinal photocoagulation (PRP).
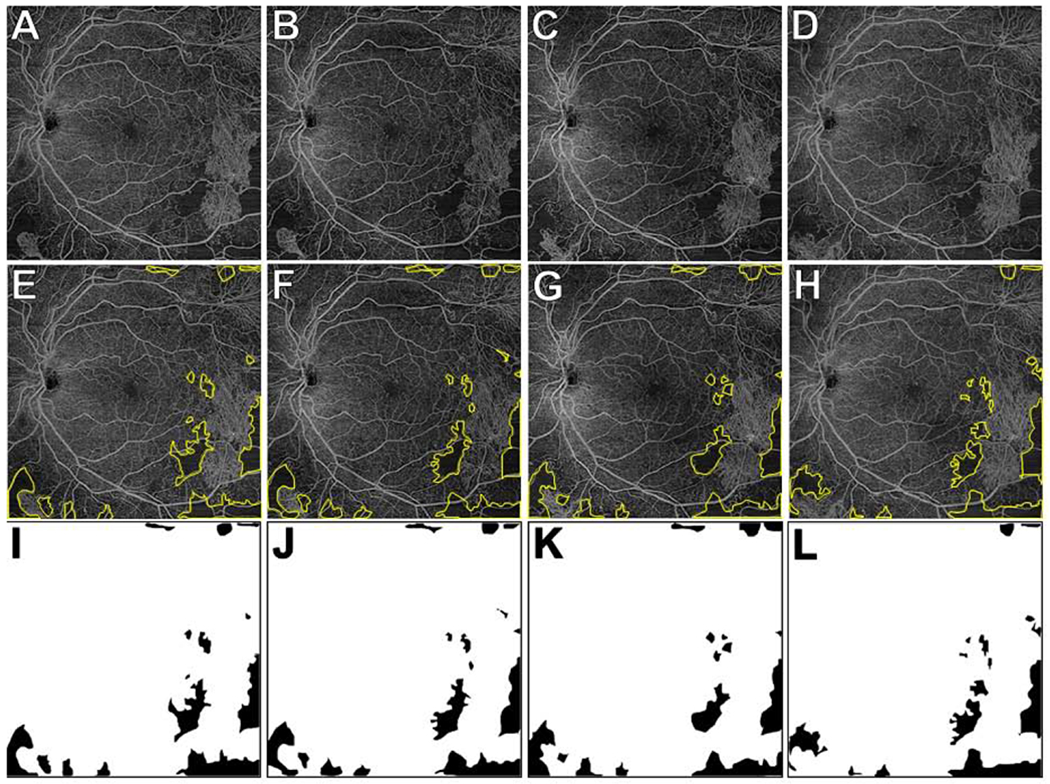
(A-D) WF SS-OCTA 12x12mm total retinal vasculature images at baseline (A), 1 week (B), 1 month (C), and 3 months (D). The consensus graded RNP areas are outlined in yellow in (E-H) and as black areas in (I-L). In this eye, the RNP% was 7.78%, 7.69%, 7.56%, and 6.70% at the baseline, 1 week, 1 month, and 3 month visits, respectively. There were small differences between visits in RNP areas but these likely reflected the inherent imprecision of the imaging and/or RNP analysis rather than physiological changes in RNP.
Figure 5. Percent retinal non-perfusion (RNP%) measured with wide-field (WF) swept-source (SS) OCT angiography (OCTA) in 11 eyes with proliferative diabetic retinopathy (PDR) through 3 months after panretinal photocoagulation (PRP).
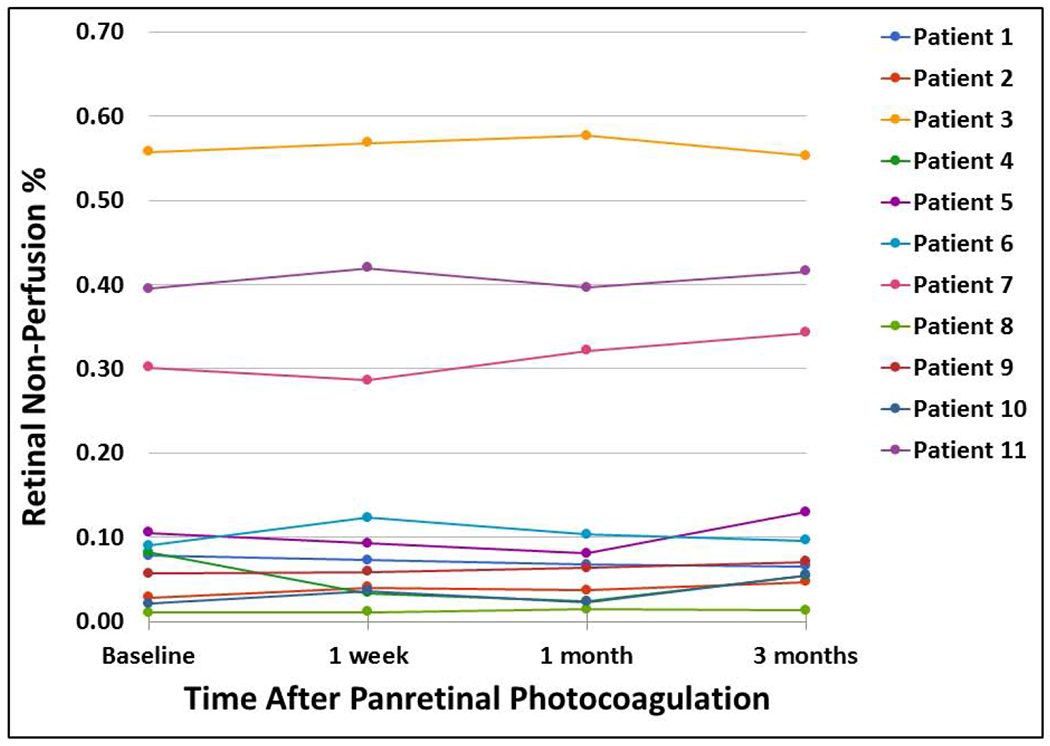
RNP% was calculated by masked, two-grader, consensus grading of RNP areas on WF SS-OCTA 12x12mm total retinal vasculature images obtained at baseline and 1 week, 1 month, and 3 months after PRP. There were statistically significant differences in RNP% between patients, but there were no statistically significant differences in RNP% over time. See text for details.
DISCUSSION
Wide-field SS-OCTA imaging at baseline and 3 months after PRP was comparable to UWF FA in its ability to identify and monitor RNP in eyes with treatment-naive PDR when similar regions of the posterior pole were compared. WF SS-OCTA demonstrated reproducible and stable areas of RNP throughout the posterior pole for up to 1 year after PRP. Small changes in capillary and large-vessel dropout occurred in a few eyes with severe macular ischemia, but these changes were not statistically significant. In total, our findings demonstrate the correspondence and benefits of WF SS-OCTA over UWF FA for the longitudinal evaluation of RNP in PDR and provide important insights into the stability of retinal ischemia after PRP.
A principal finding of this work was that RNP does not appear to change in the immediate post-PRP period and appears stable for up to 1 year after PRP. The possibility that PRP (and/or anti-VEGF agents) can cause acute vision loss from rapid retinal ischemic progression has been proposed 19–21 and reported anecdotally22–23 but has never been studied rigorously. To our knowledge, our study18 is the earliest angiographic evaluation after PRP in a cohort of PDR eyes. We did not find any statistically significant changes in the areas of RNP at the 1 week visit in our cohort of eyes. Moreover, there were no eyes that were outliers with markedly increased RNP at the 1 week visit, even those eyes with severe macular ischemia at baseline. There were also no significant decreases in BCVA at the 1 week visit or at any visit through 3 months.18 Over the 3-month to 1 year time-frame of this study, we did observe some small changes in the dropout of capillaries and large-caliber vessels in a few eyes with severe macular ischemia. The large-caliber vessel dropout occurred in areas that completely lacked adjacent capillary perfusion, so we hypothesize that dropout of these large-caliber vessels is unlikely to have affected visual function. Furthermore, it is not clear whether these vascular changes were a result of PRP or simply reflected the natural history of retinal ischemia in PDR. Taken together, our data indicate that even in severely ischemic eyes, PRP is unlikely to induce rapid retinal ischemic progression with consequent vision loss.
Some physicians perform PRP in multiple sessions or only treat portions of the fundus at each visit, with one justification being to avoid retinal ischemic progression. This is sometimes advocated particularly for eyes with pre-existing ischemic maculopathy,19,20 and some practitioners may forgo PRP altogether in these eyes. In our study, we performed a single session of 360-degree PRP even in severely ischemic eyes and did not observe retinal ischemic progression. Our results suggest that staging PRP is not necessary even for severely ischemic eyes. This is an important finding since patients with PDR are at very high risk of loss to follow up24,25 and PRP is an effective and durable treatment.2,25 For these reasons, in PDR patients deemed to be high risk for loss to follow up that do not have significant baseline subfoveal DME, even if there is severe ischemia on angiography, we perform a session of full rather than staged PRP at the first opportunity. In the presence of baseline DME, it is probably optimal to either simultaneously treat with anti-VEGF drugs at the time of PRP or pre-treat with anti-VEGF agents prior to PRP.26 Patient tolerance and/or baseline macular edema may be additional considerations in deciding to perform staged rather than single-session PRP.
Our study was not designed to assess whether anti-VEGF drugs affect retinal ischemia. A prospective, randomized study of intravitreal bevacizumab versus macular laser for DME found no worsening of macular ischemia using FA at 4 months after either treatment.27 Similarly, another study that used serial FA over 3 years found that intravitreal ranibizumab treatment for DME was not associated with worsening of macular ischemia.28 Recent prospective studies using UWF FA have suggested a possible improvement in RNP with anti-VEGF treatment.12,13 A number of small retrospective studies have investigated this possibility of improvement using OCTA, which appears to be a more reliable modality for examining RNP than FA.14 The data remain mixed, but the preponderance of evidence to date using OCTA suggest that there is no or minimal effects of anti-VEGF drugs on RNP.14,16
While it is well established that RNP leads to vision loss via retinal atrophy, DME, and NV, the clinical utility of delineating areas of RNP in everyday practice is less clear. Extensive RNP could be used to justify PRP, though high-risk PDR characteristics are the classic indications to initiate PRP.2 Another possible clinical use for determining the areas of RNP is to target PRP to these regions, though this approach has not been proven beneficial to date.29 Progression of RNP areas is the likely cause of peripheral visual field constriction in eyes with PDR that are observed without treatment or that are treated with anti-VEGF agents rather than PRP.3 However, even areas of RNP may have some preservation of photoreceptor function and intact visual field since the outer retina receives its blood supply from the choriocapillaris.30
In contrast to RNP, the clinical utility of identifying and monitoring NV in the management of PDR is well known, and we have previously shown that WF SS-OCTA is adequate for the detection and monitoring of NV.18,31 Since WF SS-OCTA also reveals microaneurysms, intraretinal microvascular abnormalities, and DME, which in combination with RNP and NV encompass all the information a clinician needs to decide on treatment in DR, we believe our studies indicate that WF SS-OCTA can serve as a stand-alone imaging modality for the management of both proliferative and non-proliferative forms of DR. The ability of WF SS-OCTA to identify and monitor RNP and NV in PDR up to 1 year as demonstrated by our studies suggest that WF SS-OCTA can be used for diagnosis and long-term management of other retinal conditions that feature retinal ischemia and NV, such as retinal vascular occlusions, radiation retinopathy, sickle cell retinopathy, and more.
The current study was not designed to quantitatively compare areas of RNP documented by WF SS-OCTA and UWF FA performed on the same visit. Such quantitative comparisons have been made previously.14 However, our qualitative observations were that RNP areas on WF SS-OCTA closely corresponded to RNP areas on UWF FA. In our experience, WF SS-OCTA enables better delineation of RNP areas by visualizing actual capillary flow rather than areas of diffuse hyperfluorescence on FA that are presumed to result from capillary perfusion. Moreover, defining RNP areas on UWF FA can be confounded by large vessel leakage or staining of PRP scars, but neither of these phenomena affect interpretation of RNP on WF SS-OCTA images. WF SS-OCTA is also fast, safe, non-invasive, easily repeatable, and easier to interpret since a transit eye does not have to be selected as with FA, so similar data are collected for both eyes during a single imaging session. The 12x12mm SS-OCTA images easily capture the entire macula, and WF SS-OCTA montages encompass the entire posterior pole. While UWF FA does capture the far peripheral retina beyond the field of view of current SS-OCTA instruments, we do not believe this far peripheral retina significantly contributes to the diagnosis and management of these patients.31 FA does outperform WF SS-OCTA in eyes with severe media opacity and in patients unable to fixate.
The limitations of our study included heterogeneity in PRP parameters,18 a small sample size (20 eyes), and short follow-up (between 3 months and 1 year). There was subjectivity inherent in RNP area grading, though we utilized a two-grader consensus-based protocol with adjudication. Instead of subjective grading of RNP areas, some groups have utilized automated calculations of vessel density metrics.9–11,15,16 In our experience, automated vessel density calculations are more variable between visits because of differential image gain and/or artefacts from media opacity.32,33 In any case, utilizing either RNP grading or automated vessel density calculations, it is becoming evident that changes in blood pressure, time of day, and other incidental factors can affect OCTA detection of vascular perfusion.32–35 RNP may also be a dynamic process, confounding interpretation of data from static time-points. To definitively answer the question of whether PRP and/or anti-VEGF drugs affect RNP, a large, prospective clinical trial utilizing standardized WF SS-OCTA acquisition protocols is needed.
In summary, WF SS-OCTA was comparable to UWF FA for the longitudinal evaluation of RNP in treatment-naïve PDR eyes after PRP, and single-session PRP did not significantly worsen retinal ischemia up to 1 year in severely ischemic eyes. We have previously demonstrated that WF SS-OCTA is likely adequate for the identification of diabetic NV in nearly every case of PDR31 and that it provides critical benefits over UWF FA for identification and monitoring of NV.18 By demonstrating here the ability of WF SS-OCTA to longitudinally evaluate areas of RNP, we provide additional justification for utilizing WF SS-OCTA as a potential stand-alone imaging modality for the management of PDR.
ACKNOWLEDGMENTS
a. Funding/support: Research supported by grants from Carl Zeiss Meditec, Inc. (Dublin, CA), the Salah Foundation, an unrestricted grant from the Research to Prevent Blindness, Inc. (New York, NY), and the National Eye Institute Center Core Grant (P30EY014801) to the Department of Ophthalmology, University of Miami Miller School of Medicine. The funding organizations had no role in the design or conduct of this research.
b. Financial disclosures: Dr. Gregori and Dr. Rosenfeld received research support from Carl Zeiss Meditec, Inc. Dr. Gregori and the University of Miami co-own a patent that is licensed to Carl Zeiss Meditec, Inc. Dr. Rosenfeld also received additional research support from Stealth BioTherapeutics and Boehringer Ingelheim; consultancy for Apellis, Boehringer-Ingelheim, Carl Zeiss Meditec, Chengdu Kanghong Biotech, Ocudyne, Ocunexus Therapeutics, and Unity Biotechnology; equity interests in Apellis, Verana Health, and Ocudyne. The other authors have no disclosures.
Abbreviations/Acronyms:
- WF
wide-field
- SS
swept-source
- OCT
optical coherence tomography
- OCTA
OCT angiography
- PRP
panretinal photocoagulation
- UWF
ultrawide-field
- FA
fluorescein angiography
- RNP
retinal non-perfusion
- ANOVA
analysis of variance
- DME
diabetic macular edema
- NV
neovascularization
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1).Zhang X, Saaddine JB, Chou CF, et al. 2010. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010, 304: 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Diabetic Retinopathy Study (DRS) Research Group. 1978. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of the Diabetic Retinopathy Study findings. Ophthalmology 1978, 85: 82–106 [DOI] [PubMed] [Google Scholar]
- 3).Gross JG, Glassman AR, Liu D, et al. 2018. Five-year outcomes of panretinal photocoagulation vs intravenous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol, 136(10): 1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Grunwald JE, Riva CE, Brucker AJ et al. Effect of panretinal photocoagulation on retinal blood flow in proliferative diabetic retinopathy. Ophthalmology, 1986, 93(5): 590–595 [DOI] [PubMed] [Google Scholar]
- 5).Grunwald JE, Brucker AJ, Petrig BL, et al. Retinal blood flow regulation and the clinical response to panretinal photocoagulation in proliferative diabetic retinopathy. Ophthalmology, 1989, 96 (1): 1518–1522 [DOI] [PubMed] [Google Scholar]
- 6).Yamada Y, Suzuma K, Onizuka N, et al. Evaluation of retinal blood flow before and after panretinal photocoagulation using pattern scan laser for diabetic retinopathy. Curr Eye Res, 2017, 42 (12): 1707–1712 [DOI] [PubMed] [Google Scholar]
- 7).Song Y, Tani T, Omae T, et al. Retinal blood flow reduction after panretinal photocoagulation in type 2 diabetes mellitus: Doppler optical coherence tomography flowmeter pilot study. PLoS ONE 13 (11): e0207288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Lee JC, Wong BJ, Tan O, et al. Pilot study of Doppler optical coherence tomography of retinal blood flow following laser photocoagulation in poorly controlled diabetic patients. Invest Ophthalmol Vis Sci 2013, 54 (9): 6104–6111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Lorusso M, Milano V, Nikolopoulou E, et al. Panretinal photocoagulation does not change macular perfusion in eyes with proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina, 2019, 50 (3): 174–178 [DOI] [PubMed] [Google Scholar]
- 10).Fawzi AA, Fayed AE, Linsenmeier RA, et al. Improved macular capillary flow on optical coherence tomography angiography after panretinal photocoagulation for proliferative diabetic retinopathy. Am J Ophthalmol, 2019, 10.1016/j.ajo.2019.04.032 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Mirshahi A, Ghassemi F, Fadakar K, et al. Effects of panretinal photocoagulation on retinal vasculature and foveal avascular zone in diabetic retinopathy using optical coherence tomography: a pilot study. J Curr Ophthalmol, 2019, 31 (3): 287–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Wykoff CC, Nittala MG, Zhou B, et al. Intravitreal aflibercept for retinal non-perfusion in proliferative diabetic retinopathy: outcomes from the RECOVERY randomized trial. Ophthalmol Retina, 2019, 10.1016/j.oret.2019.07.011 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 13).Wykoff CC, Shah C, Dhoot D, et al. Longitudinal retinal perfusion status in eyes with diabetic macular edema receiving intravitreal aflibercept or laser in VISTA study. Ophthalmology, 2019, 126 (8): 1171–1180 [DOI] [PubMed] [Google Scholar]
- 14).Couturier A, Rey PA, Erginay A, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology, 2019, 10.1016/j.ophtha.2019.06.022 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 15).Sorour OA, Sabrosa AS, Yasin Alibhai A, et al. Optical coherence tomography angiography analysis of macular vessel density before and after anti-VEGF therapy in eyes with diabetic retinopathy. Int Ophthalmol, 2091, 10.1007/s10792-019-01076-x (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 16).Hsieh YT, Alam MN, Le D, et al. OCT angiography biomarkers for predicting visual outcomes after ranibizumab treatment for diabetic macular edema. Ophthalmol Retina, 2019, 10.1016/j.oret.2019.04.027 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).La Mantia A, Kurt RA, Mejor S, et al. Comparing fundus fluorescein angiography and swept-source optical coherence tomography angiography in the evaluation of diabetic macular perfusion. Retina 2019, 10.1097/IAE.0000000000002045 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 18).Russell JF, Shi Y, Hinkle JW, et al. Longitudinal wide-field swept-source OCT angiography of neovascularization in proliferative diabetic retinopathy after panretinal photocoagulation. Ophthalmol Retina, 2019, 3 (4): 350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Schachat AP, Wilkinson CP, Hinton DR, Sadda SR, Wiedemann P, eds. 2017. Ryan’s Retina, 6th edition. Edinburgh: Elsevier. P. 1334 [Google Scholar]
- 20).McDonald HR, Schatz H. Visual loss following panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 1985, 92 (3): 388–393 [DOI] [PubMed] [Google Scholar]
- 21).Tinley CG, Gray RH. Routine, single session, indirect laser for proliferative diabetic retinopathy. Eye (Lond.), 2009, 23 (9): 1819–1823 [DOI] [PubMed] [Google Scholar]
- 22).Lee SJ, Koh HJ. Enlargement of the foveal avascular zone in diabetic retinopathy after adjunctive intravitreal bevacizumab (avastin) with pars plana vitrectomy. J Ocul Pharmacol Ther, 2009, 25 (2): 173–174 [DOI] [PubMed] [Google Scholar]
- 23).Goel N, Kumar V, Ghosh B. Ischemic maculopathy following intravitreal bevacizumab for refractory diabetic macular edema. Int Ophthalmol, 2011, 31 (1): 39–42 [DOI] [PubMed] [Google Scholar]
- 24).Hinkle JW, Flynn HW, Banta JT. 2019. Patients presenting emergently with proliferative diabetic retinoptahy: follow-up and factors associated with compliance. Retina doi: 10.1097/IAE.00000000000002481 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Obeid A, Su D, Patel SN, et al. 2019. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology, 126 (3): 407–413 [DOI] [PubMed] [Google Scholar]
- 26).Figueira J, Fletcher E, Massin P, et al. 2018. Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS study). Ophthalmology 125: 691–700 [DOI] [PubMed] [Google Scholar]
- 27).Michaelides M, Fraser-Bell S, Hamilton R, et al. Macular perfusion determined by fundus fluorescein angiography at the 4-month time point in a prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (Bolt study): Report 1. Retina, 2010, 30 (5): 781–786 [DOI] [PubMed] [Google Scholar]
- 28).Karst SG, Deak GG, Gerendas BS, et al. Association of changes in macular perfusion with ranibizumab treatment for diabetic macular edema: a subanalysis of the RESTORE (extension) study. JAMA Ophthalmol, 2018, 136 (4): 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Brown DM, Ou WC, Wong TP, et al. Targeted retinal photocoagulation for diabetic macular edema with peripheral retinal nonperfusion: three-year randomized DAVE trial. Ophthalmology, 2018, 125 (5): 683–690 [DOI] [PubMed] [Google Scholar]
- 30).Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina. I. Ophthalmoscopic and fluorescein angiographic studies. Br J Ophthalmol, 1980, 64: 896–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Russell JF, Flynn HW Jr, Sridhar J, et al. Distribution of diabetic neovascularization on ultra-widefield fluorescein angiography and on simulated widefield OCT angiography. Am J Ophthalmol, 2019, 10.1016/j.ajo.2019.05.031 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 32).De Pretto LR, Moult EM, Alibhai AY, et al. Controlling for artifacts in widefield optical coherence tomography angiography measurements of non-perfusion area. Sci Rep, 2019, 9: 9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Yu JJ, Camino A, Liu L, et al. Signal strength reduction effects in OCT angiography. Ophthalmol Retina, 2019, 10.1016/j.oret.2019.04.029 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Sarwar S, Hassan M, Soliman MK, et al. Diurnal variation of choriocapillaris vessel flow density in normal subjects measured using optical coherence tomography angiography. Int J Retina Vitreous, 2018, 4: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Kim SV, Semoun O, Pedinielli A, et al. Optical coherence tomography angiography quantitative assessment of exercise-induced variations in retinal vascular plexa of healthy subjects. Invest Ophthalmol Vis Sci, 2019, 60, 1412–1419 [DOI] [PubMed] [Google Scholar]


