SUMMARY
How do neurons in orofacial motor cortex (MCtx) orchestrate behaviors? We show that focal activation of MCtx corticobulbar neurons evokes behaviorally-relevant concurrent movements of the forelimb, jaw, nose, and vibrissae. The projections from different locations in MCtx form gradients of boutons across premotor nuclei spinal trigeminal pars oralis (SpVO) and interpolaris rostralis (SpVIr). Further, retrograde viral tracing from muscles that control orofacial actions show that these premotor nuclei segregate their outputs. In the most dramatic case, both SpVO and SpVIr are premotor to forelimb and vibrissa muscles, while only SpVO is premotor to jaw muscles. Functional confirmation of the superimposed control by MCtx was obtained through selective optogenetic activation of corticobulbar neurons on the basis of their preferential projections to SpVO versus SpVIr. We conclude that neighboring projection neurons in orofacial MCtx form parallel pathways to two distinct pools of trigeminal premotor neurons that coordinate motor actions into a behavior.
eTOC blurb
[40] How are orofacial behaviors formed from motor actions? Mercer Lindsay et al. delimit the control of forelimb, jaw, nose, and vibrissa movements. Neighboring neurons in motor cortex are shown to form projections that coordinate these movements via trigeminal premotor neurons.
The motor cortex orchestrates complex voluntary movements through its connections with an array of cortical and subcortical targets (Alloway et al., 2010; Graziano, 2016; Graziano et al., 2002; Hattox et al., 2002; Jeong et al., 2016; Mao et al., 2011; Oswald et al., 2013; Sreenivasan et al., 2015). In rodents, the bulk of the descending, corticolbulbar projections from motor cortex (MCtx) transmit signals through an array of collaterals that target many premotor nuclei in the brainstem (Alloway et al., 2010; Economo et al., 2018; Jeong et al., 2016; Kita and Kita, 2012). Yet despite these broad patterns of downstream connectivity, activation of neurons in MCtx can evoke clearly defined movements (Harrison et al., 2012; Hira et al., 2015). These past data suggest the existence of specific patterns of connectivity from corticobulbar to premotor neurons.
Like the case of neurons in MCtx, individual premotor neurons send broad collateral projections to other premotor, brainstem, and thalamic structures (Bellavance et al., 2017; Stanek 4th et al., 2014; Yoshida et al., 1994). Further, neighboring neurons in a premotor nucleus can broadly target multiple motor nuclei (Amri et al., 1990; Cunningham Jr. and Sawchenko, 2000; Dong et al., 2011; Fay and Norgren, 1997a, b, c; Li et al., 1995; Pinganaud et al., 1999; Takatoh et al., 2013), with some evidence that individual premotor neurons can target pairs of motor nuclei to potentially enact concurrent movements (Amri et al., 1990; Dong et al., 2011; Li et al., 1993; Stanek 4th et al., 2014; Yoshida et al., 1994).
Here we investigate the nature of connectivity from corticobulbar to premotor neurons for orofacial pathways. Of particular interest are the inputs to secondary sensory neurons in the trigeminal complex that originate from motor cortex; these are known to have premotor connections (Jeong et al., 2016; Stanek 4th et al., 2014; Takatoh et al., 2013; Yoshida et al., 1994). These sensory neurons are, in fact, also premotor neurons that are uniquely positioned to receive both unprocessed sensory information and high-level motor commands (Jacquin and Rhoades, 1990; Jacquin et al., 1986; Matthews et al., 2015; McElvain et al., 2018). Classic tracing, along with modern monosynaptic rabies-EnvA tracing, show that the oralis (SpVO) and rostral interpolaris (SpVIr) subnuclei of the spinal trigeminal nucleus project to motoneurons for the vibrissae (Erzurumlu and Killackey, 1979; Nguyen and Kleinfeld, 2005; Pinganaud et al., 1999; Takatoh et al., 2013), jaw (Li et al., 1995; Olsson and Westberg, 1991; Stanek 4th et al., 2014; Westberg et al., 1998; Yoshida et al., 1994), tongue (Borke et al., 1983; Pinganaud et al., 1999; Stanek 4th et al., 2014), eyelid (Gonzalez-Joekes and Schreurs, 2012; Hiraoka and Shimamura, 1977; May et al., 2012; van Ham and Yeo, 1996), nose (Kurnikova et al., 2019a), and forelimb (Esposito et al., 2014). The results of these prior studies motivate the present study to delimit the involvement of SpVO and SpVIr in coordinating movements of the forelimbs, jaw, nose, and vibrissae.
The coordination of multiple motor actions into a clearly defined movement may utilize downstream premotor nuclei through labeled line or diverging projections. To discriminate among these possibilities, we ask: (i) What orofacial movements are elicited by stimulating layer 5 pyramidal neurons in discrete locations of orofacial MCtx? (ii) What then is the distribution of projections from orofacial MCtx to pools of premotor neurons in SpVO, SpVIr, and other trigeminal nuclei? Further, how do these pools map onto muscles for different orofacial motor actions? (iii) Does activation of SpVO- versus SpVIr-projecting neurons in MCtx evoke motor actions from a single orofacial appendage, consistent with signaling along a muscle-selective pathway that is utilized in different motor actions or does this cortical activation coordinate multiple appendages in an action, consistent with an action-selective pathway? (iv) Lastly, does the distinction between SpVO- and SpVIr-projecting activation show parallel signals transmitting from MCtx through different pools of premotor neurons to enact different motor actions? We address these questions in awake, head-fixed mice using optogenetic-driven stimulation of genetically or virally labeled neurons coupled with electromyographic and videographic recording, along with anatomical tract tracing.
Background
The mammalian MCtx is defined by three types of maps: a cytoarchitectural map (Brecht et al., 2004; Brodmann and Gary, 2006; Donoghue and Wise, 1982), a muscle twitch map (Ferezou et al., 2007; Fritsch and Hitzig, 2009; Tennant et al., 2011), and a map of coherent movements (Graziano and Aflalo, 2007; Graziano et al., 2002; Hira et al., 2015). One of the first described features of the MCtx was that it was agranular, i.e., lacking an identifiable layer 4 (Brodmann and Gary, 2006; Donoghue and Wise, 1982). In rodents, the cytoarchitecture map of MCtx is composed of two distinct regions, the agranular medial (AGm) and lateral (AGl) areas (Donoghue and Wise, 1982; Tennant et al., 2011). Agranular medial cortex has a dense layer 2 with a pale layer 3. In contrast, layers 2 and 3 in AGl cortex are largely indistinguishable (Donoghue and Wise, 1982; Tennant et al., 2011).
The muscle twitch map is a result of lowering the amplitude of electrical pulses applied to MCtx until only a dominant MCtx to muscle path dominates. Twitches of single muscles in the jaw, neck, and vibrissa are located more rostral while those of the body and trunk are located more caudal (Ferezou et al., 2007; Hollis 2nd et al., 2016; Tennant et al., 2011).
While brief pulses of electrical stimulation can evoke muscle twitches, excitatory neurons in MCtx tend to be active throughout a movement (Churchland et al., 2012; Georgopoulos et al., 1986; Graziano et al., 2002; Peters et al., 2014). When electrical- or channelrhodopsin-based stimulations mimic these longer durations of activity in primates (Graziano and Aflalo, 2007; Graziano et al., 2002; Overduin et al., 2012) and rodents (Bonazzi et al., 2013; Harrison et al., 2012; Hira et al., 2015), complete motor acts are observed.
While the muscle twitch map is a consequence of the minimum electrical stimulus required to evoke a twitch, at normal activity levels in behaving animals the representative region for a muscle would be larger and overlap with other muscles. Thus, the movement map is likely an expression of overlap of the many muscles required for movements. The synergy of muscle twitch and movement maps has begun to be elucidated by electrophysiological recordings of MCtx neurons (Kakei et al., 1999) and electromyographic recordings during intracranial microstimulation (Kakei et al., 1999; Overduin et al., 2012). These past results motivate the need to determine the relation of maps in MCtx with those in premotor SpVO and SpVIr.
RESULTS
Orofacial motor cortex muscle mapping
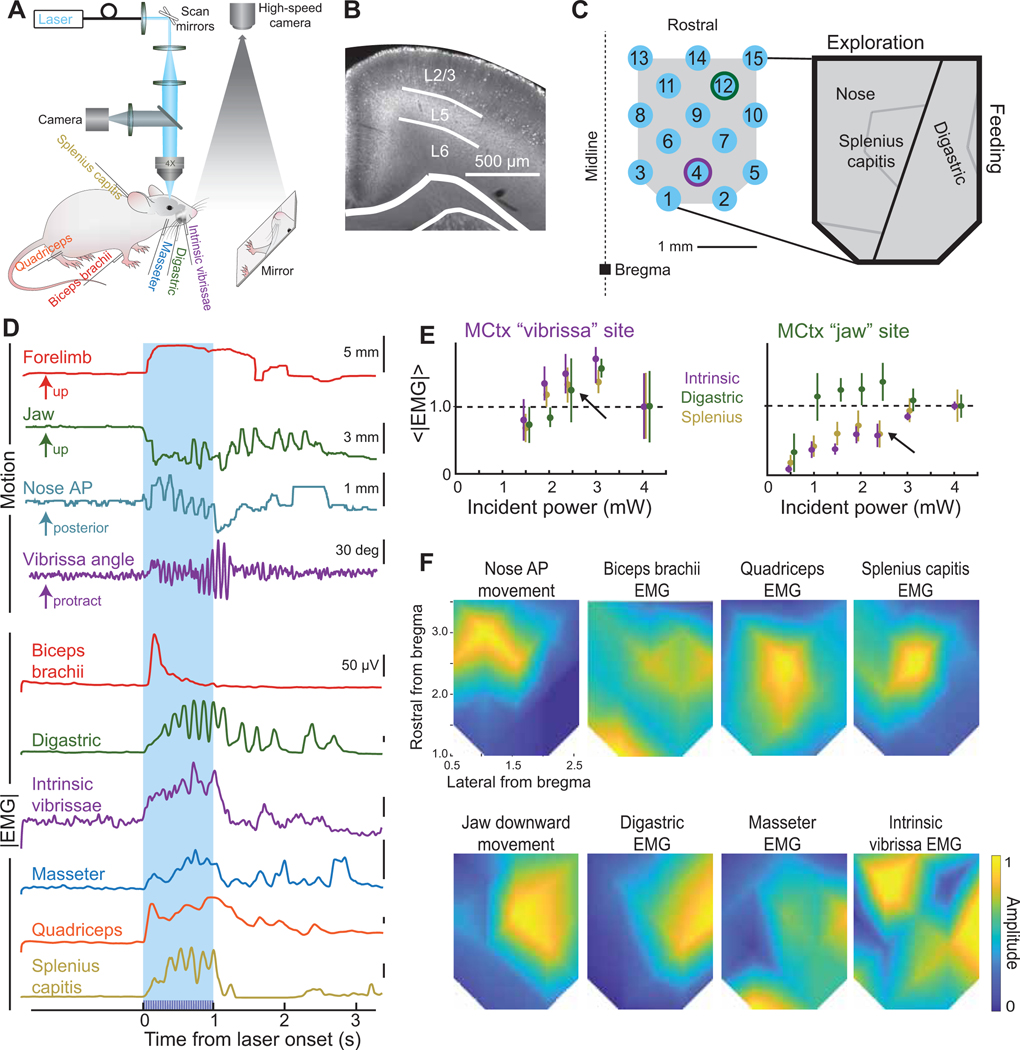
Inspired by past work with primates (Graziano and Aflalo, 2007; Graziano et al., 2002), we first mapped behaviorally relevant orofacial movements evoked from MCtx. To characterize many orofacial appendages, we recorded the electromyograms (EMGs) of the biceps brachii, digastric, intrinsic vibrissa, masseter, quadriceps, and splenius capitis muscles, as well as videographed concurrent motion of the forelimb, jaw, nose, and vibrissae during optical stimulation of MCtx in Thy1-ChR2 mice (Figs. 1A–D and 2A–C, laser diameter 35 μm).
Figure 1. Orofacial motor cortex map of induced muscle activity, as inferred from movement and EMGs.
(A) Schematic of laser activation of Thy1-ChR2-YFP mice with EMG recordings and high-speed videographs from above for the vibrissa and nose movements and using a mirror to reflect the forelimb and jaw movements up to the camera.
(B) Coronal section of Thy1-ChR2 mouse through MCtx with dense YFP labeling in layer 5.
(C) Schematic of positions targeted by a blue laser in motor cortex for trials of one second of blue light. Jaw and vibrissa motor cortex stimulus locations for panel E are identified by a green and purple outline of the laser spot, respectively. The expanded view is a summary of our observation that MCtx nominally partitions into a medial exploratory region and a lateral feeding division.
(D) Single trial examples of forelimb, jaw, nose, and vibrissa movements and biceps brachii, digastric, intrinsic vibrissa, masseter, quadriceps, and splenius capitis EMG envelopes. The blue box indicates the time the light train is ongoing. Examples are from a variety of stimulus locations, as labeled.
(E) Amplitude of the rectified EMG for the digastric, intrinsic vibrissa, and splenius capitis muscles from classic vibrissa motor cortex (AP + 1.5 mm, ML 1.5 mm; purple outline in Fig. 2C) compared with jaw motor cortex (AP + 3 mm, ML 2 mm; green outline in Fig. 2C) at different light intensities.
(F) Composite amplitude maps (five mice across a total of 15 days, interpolated between light points) for the biceps brachii, digastric, intrinsic vibrissa, quadriceps, and splenius capitis EMG envelope recordings and anterior-posterior nose and up-down jaw movements.
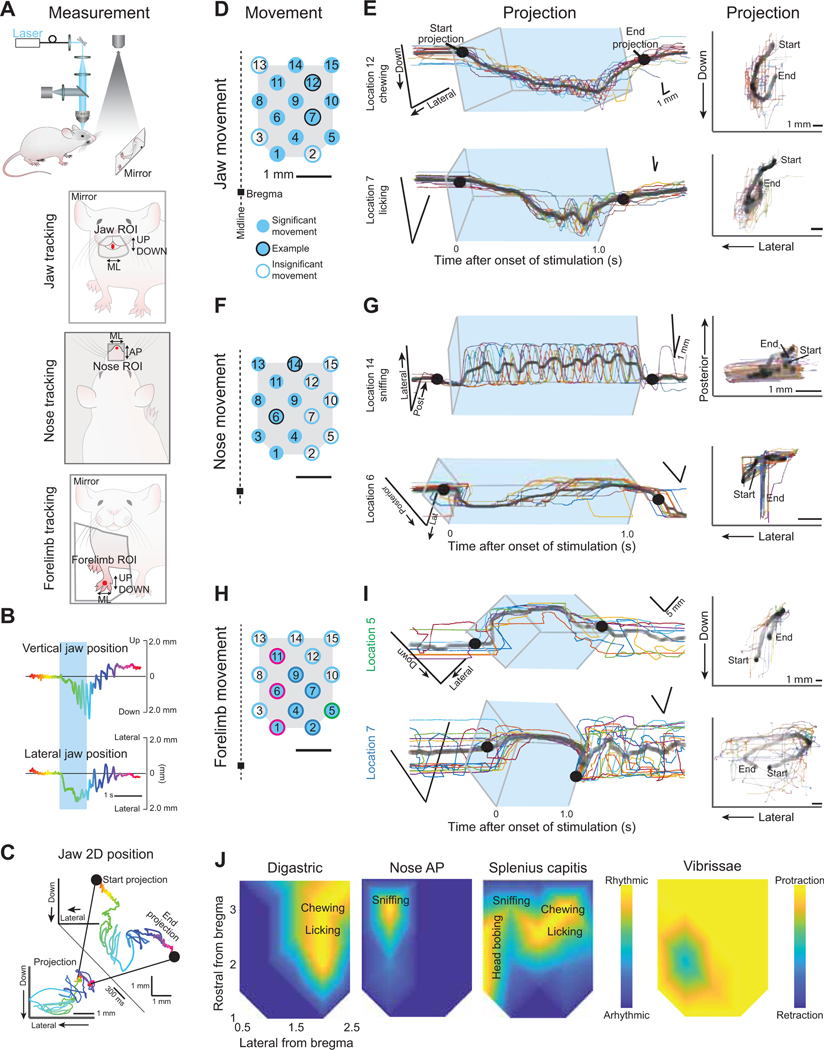
Figure 2. Mapping orofacial motor cortex-evoked forelimb, jaw, and nose movements, as inferred from videography.
(A) Schematic of the videographic recording set up with the mirror image shown for tracking the jaw (left) and forelimb (right) and for the videography from above as done for the nose (middle).
(B) Single trial example of vertical (top) and lateral (bottom) jaw position.
(C) Conversion of single dimension positions in Figure 2B to two-dimensional projection. Black dots illustrate the start and end of the projection (insert) that shows the movement path as observed looking on directly in the dimension of the mirror (forelimb and jaw) or from above (nose).
(D) Motor cortex stimulation points as seen in Figure 1C. Filled blue circles indicate locations where visible jaw movements were observed; black outlined circles indicate location from which jaw tracking examples are shown in Figure 2E. Movements were similarly observed in all five mice.
(E) Jaw movements in 2-D over time (left) with projected trajectory (right). All trials are shown (colored lines) with the average (black). In the projection, darker lines indicate more time was spent at those coordinates. The blue box indicates the stimulation duration.
(F) Same as Figure 2D but here filled blue circles indicate locations where visible nose movements were observed and black outlined circles indicate the example locations for traces in Figure 2G. Movements were similarly observed in three mice.
(G) Nose movements in two dimensions over time (left) and projected trajectory (right). All trials are shown (colored lines) with the average (black). In the projection, darker lines indicate more time was spent at those coordinates. The blue box indicates the stimulation duration.
(H) Similar to Fig. 2D and F, here filled blue circles indicate locations where forelimb movements were tracked and recorded. Colored outlines indicate which movement type (clusters 1, 2, or 3 as seen in Fig. 3A,B) was evoked from laser activation at that location. Movements were similarly observed in three mice.
(I) Forelimb movements in 2-D over time (left) with projected trajectory (right). All trials are shown (colored lines) with the average (black). In the projection, darker lines indicate more time was spent at those coordinates. The blue box indicates the stimulation duration. An example each for clusters 1 and 2 are shown with the example for cluster 3 in Fig. S2D.
(J) Composite maps of locations with rhythmic activity of the digastric (top) and splenius capitis (bottom) muscles along with rhythmic nose movements (middle). Movement descriptions are overlaid. The vibrissa map shows net movement; details are in Figure S1.
We modulated the incident laser power, while recording EMGs, at two of the intended fifteen stimulation sites (Fig. 1C, jaw and vibrissa MCtx stimulation sites outlined in green and purple, respectively) to determine the optimal power for mapping. Activation of motion either saturated or peaked at a power of 3.0 mW (Fig. 1E). The final power was chosen to be below this value, i.e., 2.4 mW (Fig. 1E, arrows). As a means to map motor actions from many locations in orofacial MCtx (Fig. 1C), we chose a 1 s, 30 Hz stimulus train (Fig. 1D).
Composite maps of evoked amplitude were made for each muscle EMG and for nose and jaw movements across all stimulation sites. Significant activation or suppression of all EMG channels was found at all locations (five mice; K-S test with p < 0.001). Some muscles, like the digastric and splenius capitis, exhibited “hot spots” of stronger activation from specific regions of MCtx, with smaller, yet still significant modulation from other stimulation locations (Fig. 1F). The broad, low-level activation of all muscles is consistent with control of posture by MCtx, in addition to coordination of specific motor actions (Amundsen Huffmaster et al., 2017; Mimica et al., 2018; Overduin et al., 2012).
Examination of the composite maps for the jaw and neck muscles and jaw and nose movement show a division between the medial and lateral aspects of MCtx. The medial division has strong activation of the nose and splenius capitis muscles while the lateral shows activation of the digastric and masseter muscles (insert in Fig. 1C). This division likely reflects a predominant, albeit incomplete, segregation of exploratory movements to medial orofacial MCtx and feeding movements to lateral orofacial MCtx.
The biceps brachii, intrinsic vibrissa, and quadriceps muscles transcended the division of exploratory versus feeding areas (Fig. 1F). We found that many stimulus sites evoked moderate activation of the intrinsic vibrissa muscle (Fig. 1F), which is responsible for protraction of the vibrissae. Surprisingly, this muscle was strongly activated along the lateral part of orofacial MCtx (Fig. 1F), a location that was not identified in mapping experiments performed with the animal under anesthesia (Ferezou et al., 2007; Haiss and Schwarz, 2005; Tennant et al., 2011). Consistent with previous work (Ferezou et al., 2007), we found only one stimulus location that evoked retraction of the vibrissa (Fig. S1A,B). This site directly overlaps with large amplitude activation of the splenius capitis neck muscle (Fig. 1F), a muscle most strongly active during deep (90° - 130°) head turns (Richmond et al., 1992; Roucoux et al., 1989). Further, this location is in close proximity to the location where head turning movements have been evoked in freely moving mice (Barthas and Kwan, 2017). The combination of vibrissa retraction and large amplitude activation of the splenius capitis suggests this area in MCtx is specialized for head turning (Fig. S1A–C).
Taken together, the amplitude maps show a patchwork of partially overlapping hot spots that tile across the motor cortex and represent unique patterns of muscle activity that cluster according to exploratory versus feeding movements (insert in Fig. 1C).
Orofacial motor cortex movement trajectory mapping
To advance from maps of individual muscle activation to movement, we measured entire trajectories of the forelimb, jaw, and nose (same five mice as in Figure 1) (Figs. 2, 3, and S2A) using videography (Fig. 2A–C). Consistent with the activation of the digastric and masseter muscles (Fig. 1F), jaw movements were evoked from most stimulus sites (filled blue circles in Fig. 2D). Jaw trajectories were downward and contralateral (Fig. 2E). Small movements of the jaw could be detected that corresponded to low amplitude contractions of the digastric (location 8 in Fig. S2A). Rhythmic jaw movements, i.e., chewing and licking, were evoked from a subset of locations that had large amplitude digastric activity (Figs. 2E,J and S2A–C) and were characterized by rhythmic activation of the digastric (Fig. S2B,C). As found previously with primates (Huang et al., 1989) and mice (Kobayashi et al., 2002), cortical activation of rhythmic jaw movements can differ slightly from natural chewing behaviors in that the masseter is often not rhythmically active and, when it is, the masseter and digastric rhythmic bouts overlap (Fig. S2C) as occurs pathologically during bruxism (Taylor et al., 2017). Lastly, in addition to rhythmic jaw activation, coordinated jaw motor actions were resolved at some stimulation sites. One such movement involved concurrent rhythmic jaw movement with rhythmic protrusion of the tongue (Fig. S2A) at a site that was close in location to the previously identified cortical licking region (Komiyama et al., 2010).
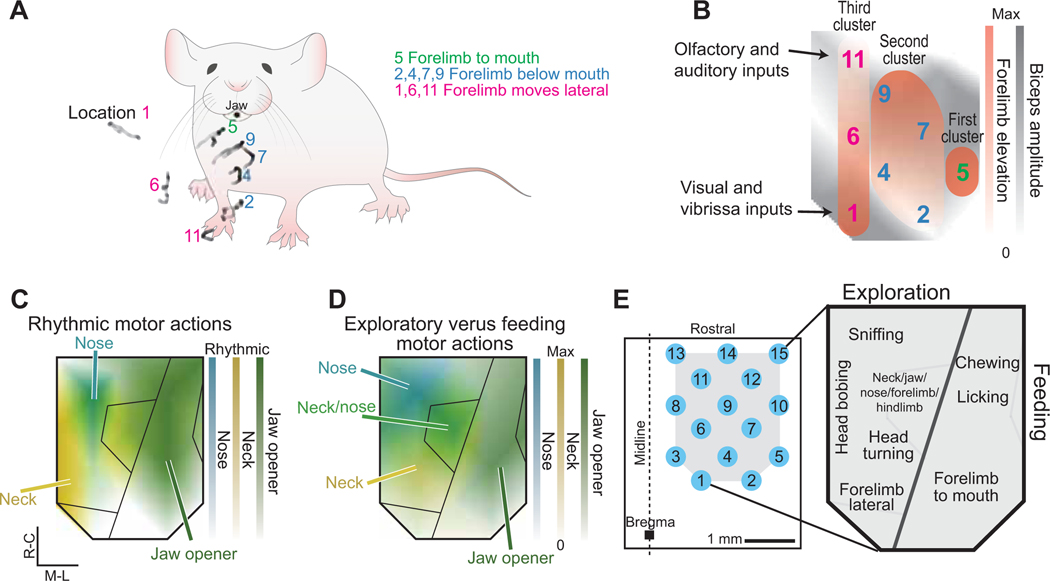
Figure 3. Ethological orofacial motor cortex map drawn from evoked rhythmic and ballistic movements.
(A) All evoked forelimb targets, i.e., the black average trace of the projected movements in Figures 2I and S2D, are shown overlaid on an example mouse. The movement from the first cluster is described in green while the movements from the second and third clusters are shown described in blue and pink respectively. Location number refers to Figure 3B.
(B) Overlay of composite amplitude map of the biceps brachii map (grey, Fig. 2F) with the three described clusters of forelimb elevation (red) illustrating that the darker grey locations in motor cortex (larger evoked amplitude of biceps brachii) coincide with the darker red stimulation sites in motor cortex (higher elevation of forelimb).
(C,D) Overlaid composite maps illustrating the division between exploratory and feeding movements based on rhythmic (panel C) and ballistic (panel D) motor actions.
(E) Ethological map of orofacial movements based on Figures 1 – 3.
Nose movements were elicited by activation of the medial region of MCtx (filled blue circles in Fig. 2F). Evoked nose movements were along both the anterior-posterior (Fig. 2G top) and medial-lateral directions (Fig. 2G bottom). Rhythmic nose movements moved the nose contralaterally and were evoked at only a single stimulus site within the broader region that evoked nose movements (Fig. 2G,J). The rhythmic activity from the nose was distinct and segregated from that of the jaw (Fig. 2J), consistent with known frequencies of chewing, licking, and sniffing in mice as well as their different functions in exploration and feeding (Fig. S3A) (Kobayashi et al., 2002; Kurnikova et al., 2017).
It is known that the neck is rhythmically activated during natural chewing (Giannakopoulos et al., 2013; Satoh et al., 2011), sniffing (Kurnikova et al., 2017), and head shakes and bobs (Kobayashi et al., 2002; Kurnikova et al., 2017). Here we found rhythmic activation of the splenius capitis neck muscle at three distinct frequencies, in different regions of orofacial motor cortex (Figs. 2J and S3A–C). The splenius was found to have a similar frequency at locations of digastric rhythmicity to the digastric (Fig. S3B green) and a different frequency that was similar to rhythmic nose movements at locations with nose rhythmicity (Fig. S3C teal). There was a third, slow frequency in medial orofacial motor cortex that is independent of other muscles and movements we measured and could, based on frequency, potentially be attributed to head bobbing (gold spectra in S3A–C).
The final piece of our cortical map of orofacial movements was to identify evoked forelimb movements (Fig. 2H–J). While the forelimb is not an orofacial appendage, forelimb movements are an essential aspect of orofacial behaviors like feeding and grooming. Here, activation of the biceps brachii muscle was induced from all stimulation sites in orofacial MCtx (Fig. 1F); however, forelimb movements could only be tracked from stimulation at a subset of sites (filled blue circles in Fig. 2H). Three categories of movements were evoked (green, blue, and pink in Figs. 2H, I, and S2D).
The first forelimb movement type (location 5 in Fig. 2H; first cluster in Fig. 3A,B) corresponds to a forelimb trajectory to the mouth with the paw supinated. The elevation of the paw during this evoked movement was higher than for any other evoked movement and was the most closely associated with the mouth (Fig. 3A). This stimulation location overlapped with large amplitude activation of the digastric and large tracked movements of the jaw (Fig. 1F). Supination to the mouth was evoked most laterally, within the “feeding” division of orofacial MCtx (Fig. 3D).
The second type of forelimb movement (locations 2, 4, 7, and 9 in Fig. 2I,J; second cluster of stimulation sites in Fig. 3A,B) evoked forelimb grasping below the mouth. These four stimulation sites overlap with the region of strong activation of the splenius capitis and quadriceps muscles (Fig. 1F). From the rostral locations of this 2nd cluster (Fig. 3A,B), evoked forelimb movements that came closer to the face, i.e., more elevated, corresponded with locations that evoked larger jaw opening (Fig. 1F), episodically with rhythmic jaw movements (Fig. 2H), and larger amplitude responses of the biceps brachii (Figs. 1F and 3B).
In the third type of forelimb movement (locations 1, 6, and 11 in Figs. 2I and S2D; third cluster of stimulation sites in Fig. 3A,B) the pronated forelimb moved away from the body into the surrounding space (Fig. 3A). Less elevated trajectories of this lateral movement were evoked from rostral stimulation sites (Fig. 3A,B). Activation of the biceps brachii muscle was greatest at locations with larger forelimb elevations (Fig. 3B).
We constructed an ethological map of orofacial movements based on overlapping patterns of cortically-evoked chewing, sniffing, forelimb to mouth movements, head bobbing, head turning, lateral forelimb movements, and licking (Fig. 3E). The resulting movements and known cortical inputs support the notion that the medial region of orofacial MCtx is responsible for distinct types of exploratory movements while more lateral regions are responsible for feeding movements (Figs. 3C,D and S3D). The central region leads to the onset of vibrissa movement (Figs. 2J and S1).
Premotor distribution in spinal trigeminal subnuclei SpVO and SpVIr
While MCtx can evoke coordinated movements, it remains unknown if downstream premotor circuits contribute to coordination of these movements. We next determined if trigeminal premotor nuclei have overlapping premotor neuron pools for forelimb, jaw, and vibrissa muscles that could contribute to coordinated movements.
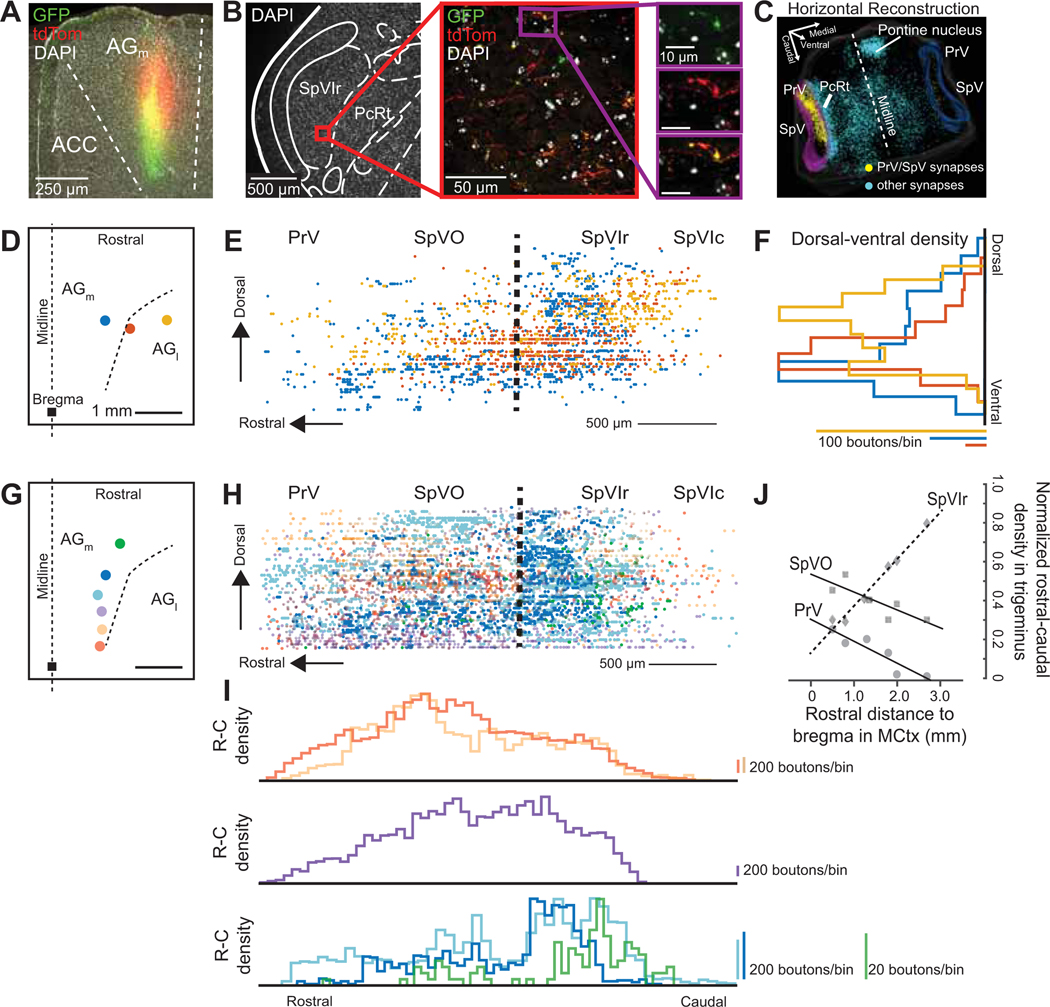
To map the premotor neuron input from muscles of the face and forelimb, we used the transsynaptic retrograde virus pseudorabies at a time point that labeled back to premotor neurons (Matthews et al., 2015). Pseudorabies was chosen for its high efficiency in transporting transsynaptically from muscle in adult animals. To explore coordination of different muscle groups, we chose one muscle from each muscle group of interest: the biceps brachii forelimb muscle, the digastric jaw muscle, and the intrinsic vibrissa muscle, (nine mice with three mice per muscle) (Fig. 4A). Pseudorabies labeled neurons were found in both SpVO and SpVIr in all nine mice; example sections for each muscle are shown in Figure 4B. The border between the spinal trigeminal nucleus and the parvocellular reticular formation (PcRt) was determined from the intensity of cytochrome oxidase staining (Furuta et al., 2006) (Fig. 4B). Premotor neurons were also found in the adjacent reticular formation and other known premotor regions (Fig. 4B).
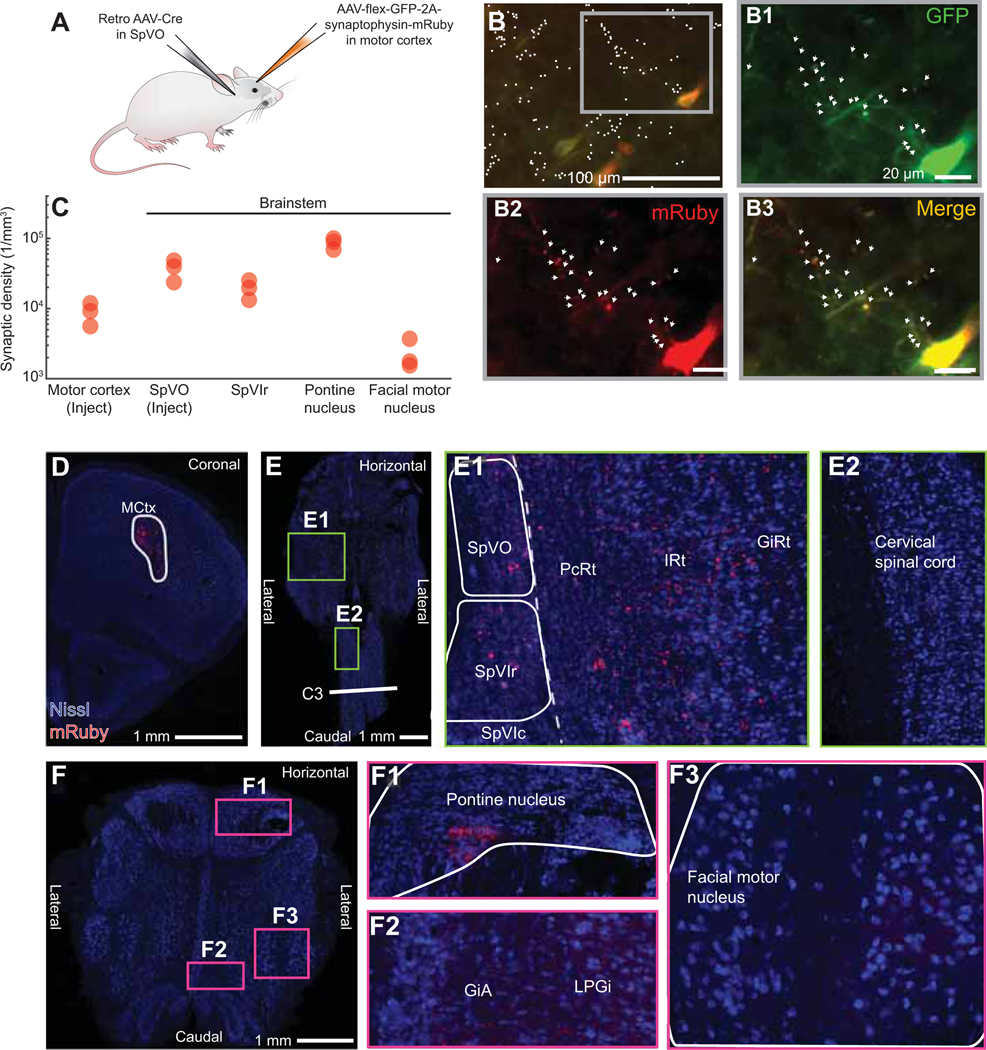
Figure 4. SpVO and SpVIr have two distinct premotor neuron clusters.
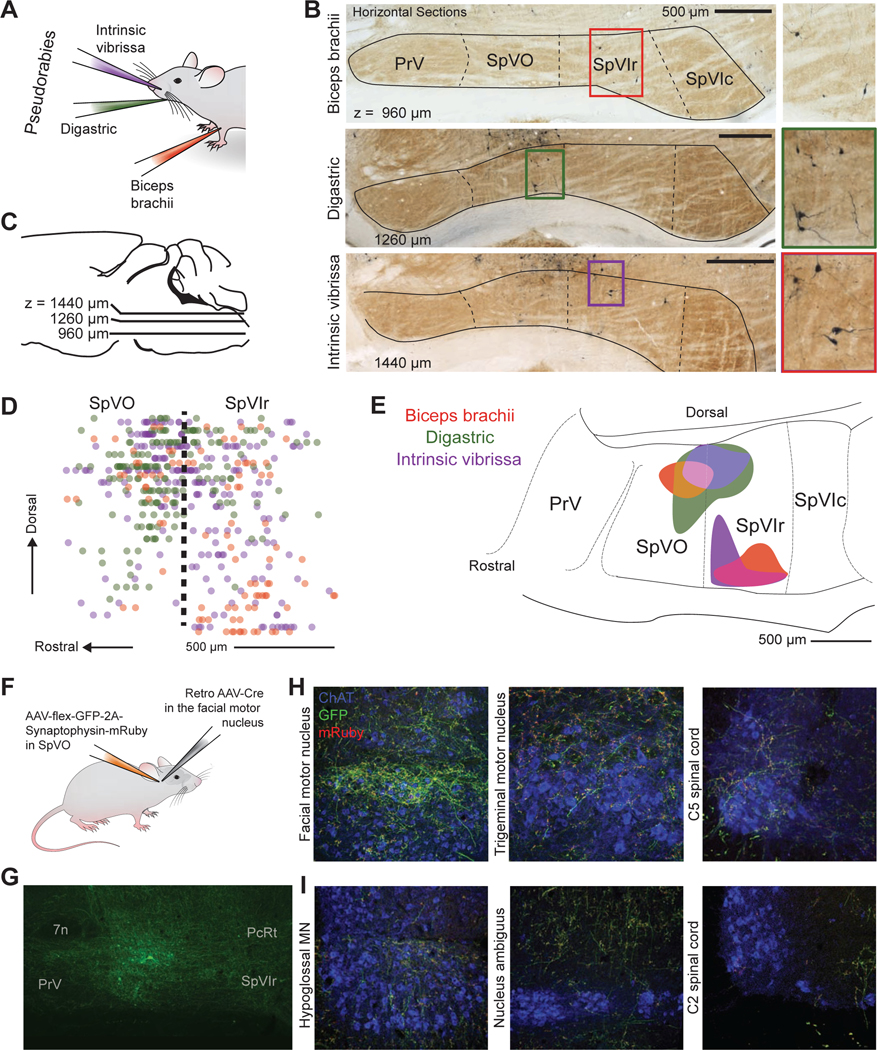
(A) Schematic illustration of muscle injections of Pseudorabies-GFP into the biceps brachii, digastric, and intrinsic vibrissa muscles.
(B,C) Example of horizontal sections from an injection in the biceps brachii, digastric, and intrinsic vibrissa muscles, oriented as in panel C. Green fluorescent protein was converted to dark product and sections were counterstained with cytochrome oxidase.
(D) Sagittal reconstruction of all premotor labeled neurons in SpVO and SpVIr.
(E) Two clusters found using the 50 % densest labeling of digastric and biceps premotor neurons and 42 % of intrinsic premotor neurons in SpV are overlaid on a sagittal atlas section (Paxinos and Franklin, 2008), see Methods. (SpVO cluster has 14 biceps, 88 digastric, and 57 intrinsic vibrissa premotor neurons. SpVIr cluster has 33 biceps, one digastric, and 34 intrinsic vibrissa neurons. Total premotor neurons was 94 biceps, 178 digastric, and 218 intrinsic vibrissa premotor neurons across nine mice).
(F) Schematic illustration of the injection scheme for a Cre-dependent AAV with somatic GFP and synaptophysin-mRuby into SpVO and a retrograde AAV-Cre into the facial motor nucleus.
(G)Image of GFP labeling at the anterograde injection site in SpVO.
(H) Images of axon and terminal labeling in the retrograde injection site of the facial motor nucleus (left) and of collateral axons and terminals in the trigeminal motor nucleus (middle) and the lower cervical spinal cord (right).
(I) Example section of terminal labeling in the hypoglossal motor nucleus (left) and lack of labeling in the region of motor neurons in the nucleus ambiguus (middle) and the upper cervical spinal cord (right).
Consistent with previous literature, more premotor neurons were found in PcRt than in SpVO and SpVIr (Fig. S4A left, supplemental data) (Stanek 4th et al., 2014; Takatoh et al., 2013). Across many animals, a group of neurons that crossed between the PcRt and SpVO was consistently observed (Fig. S4B). PcRt neurons appeared to be smaller and denser while SpVO premotor neurons were bigger with more elaborate dendrites (Fig. S4B). Prior literature also suggested PrV as an important premotor structure for jaw rhythmicity (Tsuboi et al., 2003). With pseudorabies labeling, we found that SpVO and SpVIr had more labeled premotor neurons than PrV (Fig. S4A middle panel). The tropism of pseudorabies is unknown, however, in a subset of mice, very dense labeling was found in nociceptive related substructures in SpVC (Fig. S4A right panel), leading to large variability in the number of pseudorabies-labeled neurons present there (Fig. S4C). While there are many premotor neurons in the reticular formation, the somatotopy, distinct subdivisions, and raw sensory input make the trigeminal complex an ideal location to examine for coordination of motor control (three additional mice were included in this Supplementary material).
We reconstructed the labeled neurons from SpVO and SpVIr in three dimensions and projected them onto the sagittal plane (Fig. 4D). The top 50 % densest labeled neurons (Fig. 4E) formed two clusters of premotor neurons. The first cluster is in the dorsal part of SpVO and the second is in the ventral part of SpVIr. The dorsal cluster in SpVO receives somatosensory information for the jaw, inside the mouth, and the teeth (Jacquin and Rhoades, 1990; Yoshida et al., 1994) and was found to have premotor neurons for all three muscles: the biceps brachii digastric, and intrinsic vibrissa muscles. The ventral premotor cluster in SpVIr receives sensory input from the face around the eyes, the nose, and the vibrissae (Jacquin et al., 1986) and has a more selective premotor neuron population for only the biceps brachii and intrinsic vibrissa muscles. The distinction between the premotor clusters and the known sensory topography suggests that these clusters are part of separate circuits (Fig. 4D,E).
To further understand the nature of the pathway from MCtx to muscles, we sought to determine if individual premotor neurons in the trigeminus send collateral axons to motoneurons in different cranial nuclei and the spinal cord. We injected a Cre-dependent virus that labeled the axons with GFP and the pre-synaptic terminals with mRuby into SpVO (Figs. 4F,G and S5A), while injecting a retrograde AAV-Cre virus into the facial motor nucleus (left panel in Fig. 4H and Fig. S5A), the location of vibrissa, nose, and other face motoneurons (five mice). Collateral terminals were observed in the trigeminal motor nucleus, the location for most jaw opening and closing motoneurons (masseter and anterior digastric motoneurons; middle panel in Fig. 4H and Fig. S5B), and in the ventral horn motor neurons in the lower cervical spinal cord, (right panel in Fig. 4H and Fig. S5B), at approximately the level where forelimb motor neurons are found. Across animals, more terminals were observed within the facial motor nucleus than the trigeminal motor nucleus or the ventral horn of the spinal cord (Fig. S5B). However, the effective strength of individual connections remains unknown.
These data provide anatomical evidence for functional coordination of motor actions by the face, forelimb, and jaw via single premotor neurons in SpVO. Additional collateral terminals were observed in the hypoglossal motor nucleus while mostly avoiding the nucleus ambiguus and the ventral horn in the upper cervical spinal cord (Fig. 4I), which suggests that while some overlap among premotor pathways is present, the pathways are targeting a distinct subset of motoneuron pools. Together, these data show that overlapping pathways from premotor to motoneurons can be through the same premotor neurons. They build on prior claims that individual premotor neurons contact motoneurons in different cranial nuclei and within the spinal cord (Li et al., 1993; Stanek 4th et al., 2014; Yoshida et al., 1994).
A final point concerns the range of input from orofacial MCtx to SpVO and SpVIr. We used a retrograde lentivirus-Cit (Fig. S6A) to label cortical neurons that projected to either dorsal SpVO (Fig. S6B top) or ventral SpVIr (Fig. S6B bottom panel). A three-dimensional reconstruction (Fig. S6D) shows that, for each viral injection in either dorsal SpVO or ventral SpVIr, neurons in MCtx were identified from the rostral pole to bregma and the entire mediolateral extent of AGm and AGl cortex (Fig. S6D). Similar results were found in all retrogradely labeled animals (eleven mice contributed to this Supplementary material). In addition, labeling from both dorsal SpVO and ventral SpVIr was seen across primary sensory somatosensory cortex (Fig. S6D).
Density analysis of motor cortex inputs
The density of axonal boutons from MCtx to premotor regions is roughly constant for injection sites across MCtx (Alloway et al., 2010; Jeong et al., 2016). Yet the distribution of inputs within the trigeminal subnuclei has not been quantified (Alloway et al., 2010). To form such a map, we first injected an anterograde lentivirus-synaptophysin-GFP virus that specifically labels presynaptic endings (Fig. 5A–C) in three locations across the mediolateral axis of MCtx (Fig. 5D; three mice). The distribution of boutons was reconstructed in three dimensions and then projected in the sagittal plane (Fig. 5E). The medial injections projected most densely to ventral SpVO and SpVIr (Fig. 5F). As the injections progressed towards the AGl cortex, the density shifted to the dorsal region of SpVO and SpVIr (Fig. 5E,F). This distinction in density suggests that the MCtx differentially targets the dorsal, jaw sensory region and the ventral, vibrissa/nose sensory region of SpVO and SpVIr depending on the mediolateral location in MCtx, further supporting the medial-exploratory and lateral-feeding division suggested from Figures 1–3.
Figure 5. Density of inputs from motor cortex biases within the spinal trigeminal complex dependent on cortex location.
(A) Lentivirus-CAG-synaptophysin-eGFP coinjected with AAV-hSyn-tdTomato in the AGm region of motor cortex. No spill was found in anterior cingulate cortex (ACC).
(B) Synapses (green and red) and axons (red only) labeled in SpVIr from panel A.
(C) 3-D reconstruction of GFP-labeled boutons of the hindbrain viewed from above.
(D) Schematic of motor cortex injections in AGm and lateral to AGl, the square represents bregma. Each colored circle corresponds to one injection in one mouse.
(E) Sagittal projection of synapses labeled rostrally from PrV through SpVIc caudally color coded by injection site in panel D (4,848 synapses across three mice).
(F) Overlaid histograms of dorsal – ventral density in PrV through SpVIc for injections in AGm and AGl, scaling information shown below.
(G) Schematic of motor cortex injections in AGm that run along the rostral – caudal axis. Each dot corresponds to one mouse.
(H) Sagittal projection of synapses labeled rostrally from PrV through SpVIc caudally color coded by injection site in panel F (17,226 synapses across six mice). Some data sets were down sampled for clarity.
(I) Histograms of rostral – caudal density from color coded injection sites in panel G, scaling information shown on the right.
(J) Plot of percent synaptic density in PrV, SpVO, and SpVIr out of all trigeminal synapses (PrV + SpVO + SpVIr + SpVIc) as the injections move rostral from bregma.
The caudal part of the AGm cortex is known to be innervated by primary visual and somatosensory cortices (Barthas and Kwan, 2017; Hoffer et al., 2003; Wang and Burkhalter, 2007), whereas the more rostral region is innervated by the auditory cortex (Donoghue and Parham, 1983; Reep et al., 1987). We next tested if there was a bias in the density of projections from these different parts of AGm through a series of injections on the rostral-caudal axis (Fig. 5G; one of the three original plus five additional mice). Here we found that injections into rostral AGm projected most densely to SpVIr and, as the injections were more caudal, the projections shift toward SpVO (Fig. 5H–J). This finding is further confirmed by examining the rostral-caudal terminal density from the medial-lateral MCtx injections (Fig. 5D–F); all injections were made at approximately the same rostral-caudal coordinates. We find that all the resulting terminal densities in the trigeminus have their major peak in the same approximate rostral-caudal location in SpVIr (Fig. S6E).
In toto, we observe two distinct gradients formed from MCtx inputs to SpVO and SpVIr. Medial-lateral injections shift the inputs ventro-laterally (Fig. 5F) while rostral-caudal injections shift the inputs caudal-rostrally (Fig. 5I,J). This data suggests that while MCtx projects broadly to these nuclei, one mechanism of movement specialization could be determined by gradients of cortical inputs.
Motor cortex corticofugal neurons are known to have broad axonal collateralization across the brainstem (Economo et al., 2018; Kita and Kita, 2012). As such, we asked if SpVO and SpVIr receive collaterals from the same neurons in MCtx. We injected a Cre-dependent AAV into MCtx and a retrograde AAV-Cre into SpVO (Fig. 6A). We observed boutons in SpVIr (Fig. 6B,C) in all animals [eight mice, five from vibrissa MCtx (AP +1.5, ML 1.5) and three from jaw MCtx (AP +3.0, ML 2.0)]. This shows that the same corticobulbar cell projects to two distinct regions of the spinal trigeminal nucleus, although likely not with the same density (Fig. 5).
Figure 6. Motor cortex collaterals broadly target brainstem premotor nuclei.
(A) Injection scheme for collateral labeling with a retro AAV-Cre injected in SpVO and AAV-flex-GFP-2A-Synaptophysin-mRuby in motor cortex.
(B) Pyramidal neurons (red and green) and labeled axons (green) and synapses (red and green) labeled by AAV-flex-mGFP-2A-synaptophysin-mRuby. White dots (left top image) indicate counted synapses with arrows (three enlarged images) showing co-labeling at synapses. Motor cortex injection coordinates: Bregma + 3.0, 2.0 lateral.
(C) Quantification of synaptic density in motor cortex, pontine nucleus, facial motor nucleus, SpVO, and SpVIr (50,473 boutons and 135 cells across 3 mice). See white outlines of motor cortex injection, SpVO, SpVIr, the facial motor nucleus, and the pontine nucleus (panels D-F) for areas used for density calculation.
(D) Large view of motor cortex injection site described in panel A with white outline used for density calculation.
(E) Retrograde injection into SpVO shown with collaterals found in SpVIr (panel E1), the medullary reticular formation (panel E1), and the cervical spinal cord (panel E2). White outlines for SpVO and SpVIr used in density calculation.
(F) Collaterals of SpVO-projecting motor cortex neurons in the pontine nucleus (panel F1), the alpha part of the giganticellular reticular formation (panel F2), the lateral paragiganticellular reticular formation (panel F2), and the facial motor nucleus (panel F3).
Given that the distinction between the SpVO and SpVIr premotor clusters concerns their projections to jaw motoneurons, we analyzed the projections from MCtx neurons that were located where rhythmic jaw movements were consistently observed (AP +3.0, ML 2.0; Fig. 3B). We determined the density of collateral inputs to both SpVO and SpVIr premotor neuron populations as well as local collaterals within MCtx (Fig. 6B,C). We labeled pyramidal neurons in MCtx by the intersection of a Cre-depended AAV injected into MCtx and retrograde AAV-Cre into SpVO (Fig. 6A); pre-synaptic terminals were labeled with mRuby and expressed somatic GFP (Figs. 6B–F and S7A–E). Distinct brainstem collateral projections were observed in the pontine nucleus, superior colliculus, periaqueductal gray, spinal cord, and reticular formation, i.e., parvicellular reticular formation (PcRt), intermediate reticular formation (IRt), gigantocellular reticular formation (GiRt), the alpha part of the gigantocellular reticular formation (GiA), the lateral paragigantocellularis (LPGi), and midbrain reticular formation (Figs. 6E,F and S7C–E). Fibers were seen consistently in the VM and CM thalamic nuclei (Fig. S7B) and sparse fibers were seen in primary somatosensory cortex (Fig. S7B) and the facial motor nucleus (Fig. 6F). To quantify the strength of the relative projections, we counted the number of boutons formed by axon collaterals of SpVO-projecting MCtx neurons in a known dense target, i.e. the pontine nucleus, a known weak target, i.e., the facial motor nucleus (Grinevich et al., 2005), the retrograde injection site SpVO, SpVIr, and within the anterograde injection site in MCtx (Fig. 6D–F). The density was estimated based on labeled outlines for MCtx, SpVO, SpVIr, pontine nucleus, and facial motor nucleus (Fig. 6D–F). We confirm a strong collateral projection to the pontine nucleus, with 7-times the density seen in MCtx, and a weak collateral to the facial motor nucleus, with 0.2-times of MCtx (Alloway et al., 2010; Grinevich et al., 2005; Kita and Kita, 2012) (Fig. 6C). With regard to collateral activation of neighboring cortical neurons near the injection site in MCtx, we found that the density of boutons is four-times higher in SpVO than within MCtx (Fig. 6C).
Taken together, the data from our anatomical studies (Figs. 4–6) confirm that SpVO and SpVIr are both premotor and receive input from motor cortex. The patterns of connectivity are found as gradients of inputs from cortex to the trigeminal nuclei along two directions (Fig. 5J) and both SpVO and SpVIr receive collaterals from the same corticobulbar neurons (Fig. 6C,E). Notably, both the MCtx neurons that project to the trigeminal complex (Fig. 6) and the premotor neurons from SpV to the facial motor nucleus (Fig. 4F–I) have many collaterals that project to arrays of motor nuclei. This creates a parallel set of connections that may be co-activated by cortical neurons.
Optogenetic activation of SpVO- and SpVIr-projecting MCtx neurons
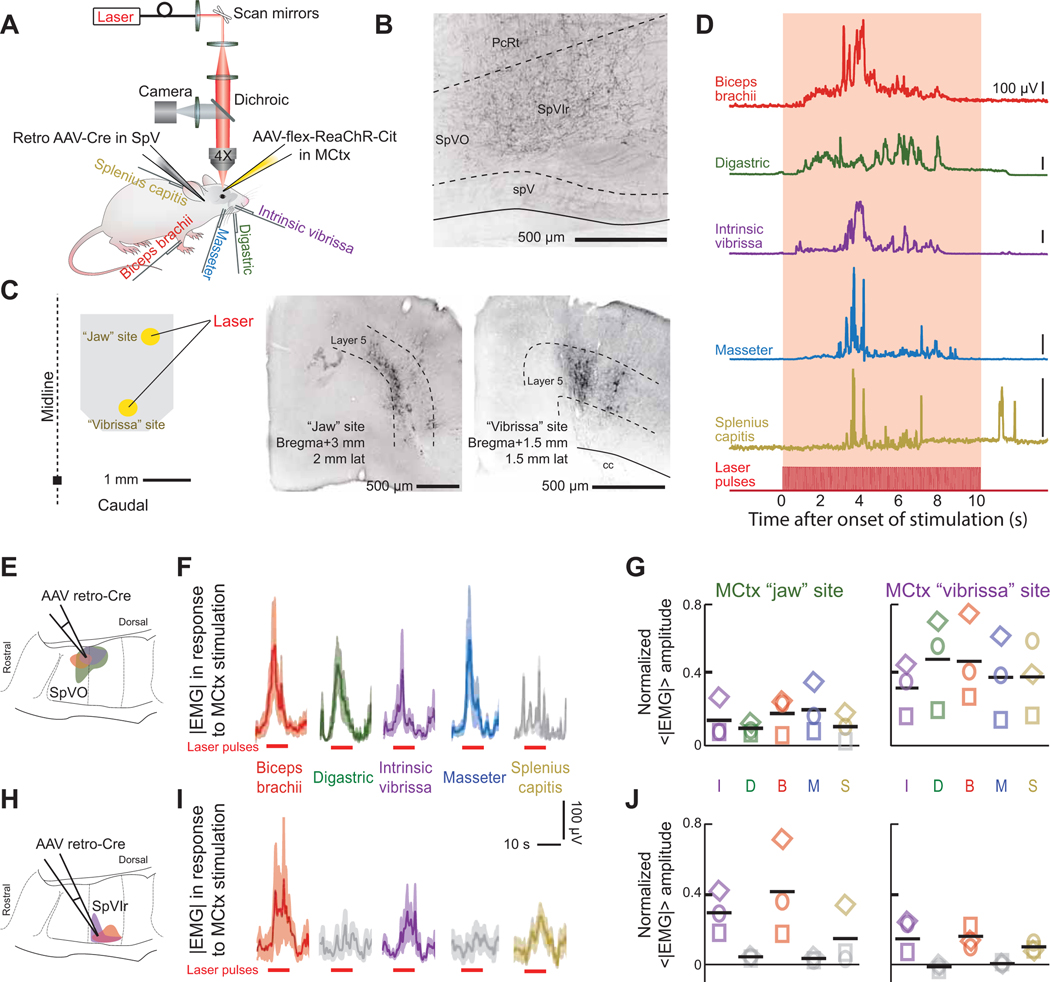
We now address how an isolated corticobulbar pathway contributes to coordination of motor actions. We used a transectional virus strategy to label SpVO- or SpVIr-projecting MCtx neurons with a red-shifted channelrhodopsin (Lin et al., 2013). We then tested if stimulation of SpVO- and SpVIr-projecting MCtx neurons activated distinct sets of muscles, consistent with their respective, distinct populations of premotor neurons (Fig. 4E). AAV retro-Cre was injected into either SpVO or SpVIr (Figs. 7A,B and S7F,G) while AAV-flex-ReaChR-Cit was injected into two cortical locations (Fig. 7C), one that in Thy1-ChR2 mice evoked rhythmic jaw movements and a second in vibrissa MCtx (Fig. 2J). After the viruses expressed, mice were head-fixed and a scanning laser system used to selectively stimulate a discrete region within each injection site in motor cortex with a 10 Hz, 10 s long train of pulses of red light (Fig. 7A–C). We concurrently recorded EMGs from the biceps brachii, digastric, intrinsic vibrissa, masseter, and splenius capitis muscles and observed robust single-trial responses (Fig. 7A,D).
Figure 7. SpVO- and SpVIr-projecting motor cortex neurons drive different networks that activate muscles reflecting their respective premotor clusters.
(A) Schematic illustrating the injection scheme as well as the later used red laser and recorded EMGs in the bicep brachii, digastric, intrinsic vibrissa, masseter, and splenius capitis muscles.
(B) Axon terminals in SpVIr, an example of a retrograde AAV-Cre injection site.
(C) Schematic of injection site and corresponding laser size (left). Cortex injection “jaw” site coordinates: bregma + 3.0, 2.0 lateral with. Cortex injection “vibrissa” site coordinates: bregma + 1.5, 1.5 lateral. Virus-labeled pyramidal neurons from a jaw MCtx and a vibrissa MCtx injection (middle and right respectively).
(D) Envelopes of the EMG signal for the biceps brachii, digastric, intrinsic vibrissa, masseter, and splenius capitis muscles for a single trial. Note that the intertrial interval was 50 s.
(E-G) Activation of EMGs using AAV retro-Cre targeted to the SpVO premotor cluster (panel E). Stimulus-triggered averages from stimulation the MCtx “jaw” site (panel F) and averages across mice (panel G) from jaw motor cortex (left) and vibrissa motor cortex (right) (10 – 20 measurements per muscle per cortex location across three mice). Grey traces in panel F or symbols in panel G show insignificant responses (Student’s t-test, p < 0.05).
(H-J) Activation of EMGs using AAV retro-Cre targeted to the SpVIr premotor cluster. Data are formatted as in panels E to G (10 – 20 measurements per muscle per cortex location across three mice).
Prolonged, localized stimulation of cortex in mice with labeled SpVO-projecting MCtx neurons led to concurrent activation of the biceps brachii, digastric, intrinsic vibrissa, and masseter muscles (Fig. 7E,F). Averaged across animals (three mice), there was statistically equal activation across all muscle groups for stimulation at a given site (ANOVA, p > 0.1) (Fig. 7G, colors indicate significant responses from baseline while grey indicate insignificant responses).
In contrast to the result for SpVO-projecting MCtx neurons, stimulating SpVIr-projecting MCtx neurons only reliably activated the biceps brachii and intrinsic vibrissa muscles (Fig. 7H,I). Critically, there was a significant and notable lack of evoked activity in either jaw muscle, a result that held across all animals (three mice) and from both stimulus sites (Fig. 7J). A final point is that SpVO-projecting MCtx neurons from the “vibrissa” site evoked larger amplitude movements than from the “jaw” site (Fig. 7G), while SpVIr-projecting MCtx neurons in the “jaw” site evoked larger movements than from the “vibrissa” site (Fig. 7J). This is consistent with the result that SpVO receives a denser cortical input from more caudal MCtx locations and SpVIr receives a denser cortical input from more rostral MCtx locations (Fig. 5J).
The reduced expression of channelrhodopsin with viral labeling versus expression in Thy1-ChR2 mice necessitated the use of prolonged stimulation. Rhythmic and ballistic movements have been shown to be continuously evoked throughout a cortical stimulation for durations up to 10 s (Graziano and Aflalo, 2007; Huang et al., 1989; Isogai et al., 2012; Lund et al., 1984). Nonetheless, we compared the response for blue light with Thy1-ChR2 animals versus red light with virus-expressed ReaChR animals using 10 s stimuli at the same stimulation location in MCtx (Fig. S7H). Qualitatively similar EMG responses are seen for the vibrissa intrinsic and digastric muscles, with the exception that stimulation of SpVIr-projecting MCtx neurons do not drive the digastric muscle (Fig. 7J,K).
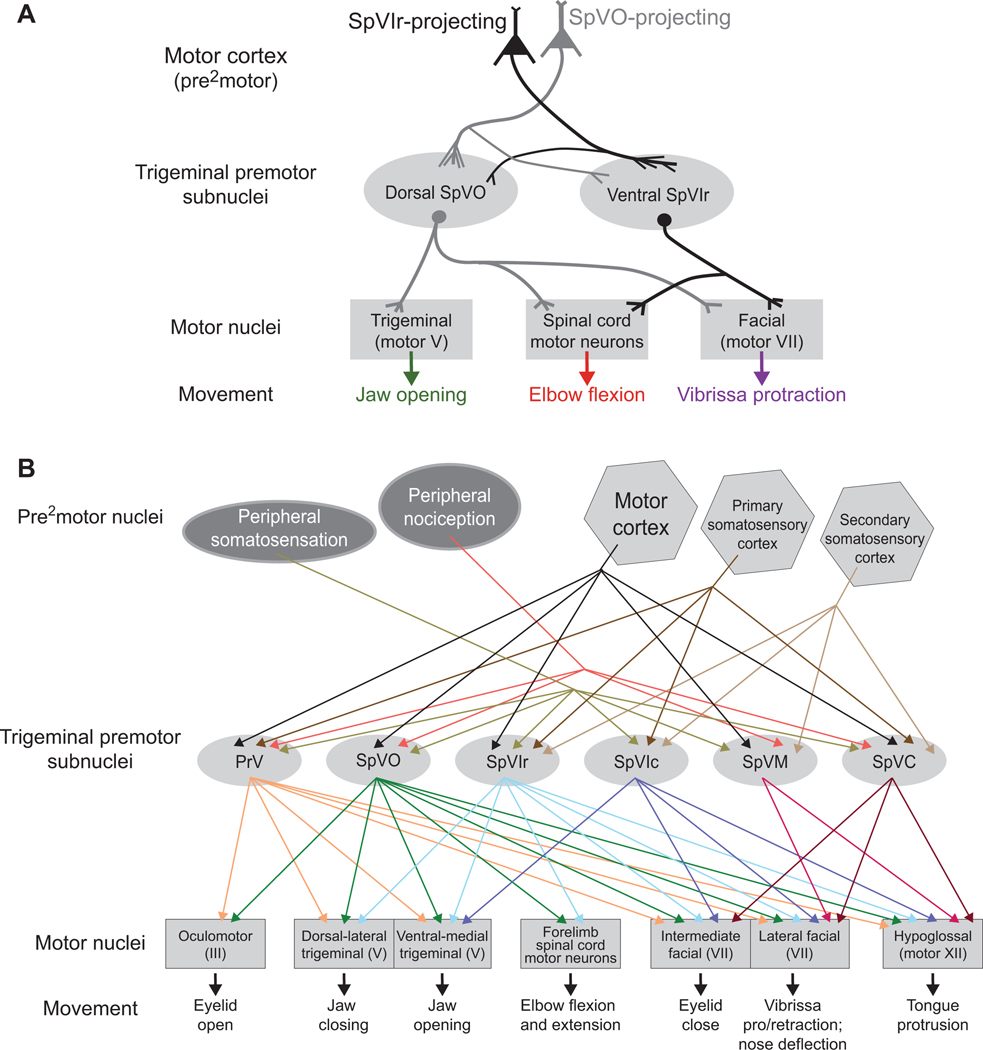
These stimulation data, in combination with the mapping data of trigeminal subnuclei to different motoneurons pools (Fig. 4E,H), highlight that motor control is in part set by premotor neurons and not strictly by neurons in MCtx (Fig. 8A). Taken altogether, our data shows that MCtx corticobulbar pathways have specific contacts at the premotor level with premotor neurons in control of certain aspects of movement and thus coordination of orofacial movements is controlled at the premotor level as well as the MCtx (Fig. 8A,B).
Figure 8. Model and summary.
(A) Model summarizing the activation with the anatomical data from Figures 5–7. There are two distinct premotor clusters, one in SpVO and another in SpVIr. When activating cortical projection neurons targeting either SpVO or SpVIr, resulting muscle activation reflects the premotor neurons in the targeted cluster.
(B) Summary of published data, as well as data from this manuscript, on the connectivity from premotor areas, including peripheral (Bereiter et al., 2000; Capra and Dessem, 1992; Furuta et al., 2006; Ren and Dubner, 2011; Sabino et al., 2002; Sakurai et al., 2013; Yoshida et al., 1994) and cortical (Smith et al., 2015; Sreenivasan et al., 2015) areas, through all trigeminal premotor areas to distinct motor neurons (Borke et al., 1983; Erzurumlu and Killackey, 1979; Esposito et al., 2014; Kurnikova et al., 2019b; Li et al., 1995; May et al., 2012; Pinganaud et al., 1999; Stanek 4th et al., 2014; Takatoh et al., 2013; van Ham and Yeo, 1996). See tab for “Figure 8B” in Supporting Data for a link of publications to specific pathways.
DISCUSSION
We observed that ethologically relevant movements were tiled across orofacial motor cortex (Figs. 1–3). The combined results from anatomical (Figs. 4 - 6) and cortical activation (Fig. 7) studies support the presence of two distinct circuits that originate from the same locations in cortex and act, in part, through identified clusters of premotor neurons in trigeminal subnuclei SpVO and SpVIr. Activation of either pathway drove the forelimb and vibrissa muscles (Fig. 7E–J), while jaw movements only occurred from activation of SpVO-projecting MCtx neurons (Fig. 7E–G). These different patterns occur even though both SpVO and SpVIr receive collateral input from the same MCtx neurons (Fig. 6C,E). We thus conjecture that neighboring corticobulbar projections neurons from motor cortex form synapses on specific subsets of neurons in each trigeminal target. These feedforward circuits then drive specific combinations of motor actions that involve the forelimb, head, jaw, and vibrissa (Figs. 3G and 8A).
The corticobulbar inputs to the trigeminus are broadly distributed in the form of spatial gradients (Fig. 5). The data on projections from localized regions in motor cortex to the trigeminus (Fig. 5) reveal a newfound organization of the cortical activation of trigeminal premotor nuclei. When we activated one of two distinct locations in motor cortex that selectively targets SpVO versus SpVIr (Fig. 7), the strength of the evoked amplitudes of muscle activity reflects the gradients of input, i.e., larger amplitude responses occurred for regions of denser corticobulbar connections (Figs. 5J and 7G,J).
One caveat to this study is the reliance on viral methods. While viruses show very clear labeling of axons and can be used to encode opsins, they, like traditional tracers, can spread beyond the intended injection target. As such, we purposely show injection sites for example data and include measures of the spread of the injected virus (Figs. S5A and S7F). Further, electrophysiological recording was used to identify these target structures for injections. For anterograde injections into SpVO (Figs. 4G and S5A), all neurons were identified to be within SpVO. For retrograde injection sites into SpVO and SpVIr (Fig. 6A,E), it was difficult to identify the spread as there are collateral axons in many of the neighboring structures. It is possible that some spill occurred, most likely into the PcRt as the spinal trigeminal nucleus is most narrow in the medial-lateral dimension.
Altogether, our data highlight the distributed nature of the coordination of motor actions into behaviors along both the motor cortex to trigeminus projections and the trigeminus to motoneuron projections (Fig. 8A). While we focused on two subpathways from motor cortex, we also confirmed the previously reported existence of broad collateralization (Fig. 6). It is important to note that all the stimulation experiments (Fig. 7) were simultaneously activating the trigeminal nucleus targeted with the AAV-retro Cre, i.e., SpVO or SpVIr, as well as the collateral targets of those corticobulbar neurons (Fig. 6). Further, the complexity of activation continued as the premotor neurons acted on all their collaterals (Fig. 4G,H). Notably however, in both the stimulation of the Thy1-ChR2 mice (Figs. 1–3) and virus-encoded ReaChR mice (Fig. 7), the activity patterns were consistent across short, i.e., 1 s, versus long, i.e., 10 s stimulation (Figs. 2 and 7). This result indicates that corticobulbar neurons engage entire networks and that multiple corticobulbar and corticospinal networks work together to piece different movements into behaviors.
One feature of the trigeminus is the convergence of peripheral sensory input, including input related to self-motion of the sensors (Nguyen and Kleinfeld, 2005; Bellavance et al., 2017) as well as nociception (Capra and Dessem, 1992; Sabino et al., 2002), with input from higher order cortical centers (Kleinfeld et al.,1999; Bosman et al., 2011; McElvain et al., 2017). This includes input from motor cortex along with primary and secondary somatosensory cortices (Smith et al., 2015; Sreenivasan et al., 2015) to premotor neurons (Fig. 8B). While rodent motor cortex targets many premotor nuclei, the trigeminal complex is unique in receiving an abundance of somatosensory information in addition to motor information. The pattern of connectivity from premotor neurons in the trigeminal complex to muscle groups that span different motor nuclei has the form of a highly divergent feed-forward network. Going forward, the more far reaching goal would be to identify the specific role of different collaterals from corticobulbar and corticospinal neurons. Conditional expression of Cre transynaptic from neurons in motor cortex, a potential emerging technology (Lo and Anderson, 2011), should enable us to parse these connections. At the intersection of motor and sensory, the trigeminal nuclei have the potential to be an ideal location to begin this process (Fig. 8B).
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed and will be fulfilled by the Lead Contact, David Kleinfeld (dk@physics.ucsd.edu). This study did not generate new unique reagents.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice.
50 female C57BL/6 mice and 5 female Thy1-ChR2 mice (JAX strain B6.Cg-Tg (Thy1-COP4/EYFP)18Gfn/J) (Arenkiel et al., 2007) age 5 – 18 weeks contributed to this study. All experimental procedures followed the Guide for the Care and Use of Laboratory Animals and has been approved by the Institutional Animal Care and Use Committee at the University of California, San Diego.
METHOD DETAILS
Muscle injections.
Nine C57BL/6 mice were anesthetized in a box with 2 % (v/v) isoflurane with oxygen until they did not respond to a toe pinch. A single injection of 500 nL of pseudorabies was injected into either the whisker pad, the anterior belly of the digastric muscle, or the biceps brachii muscle. Seventy-five to eighty hours later the mice were deeply anesthetized with pentobarbitol before being transcardially perfused with a 0.01 mM phosphate buffered salt solution (PBS) followed by 4 % (w/v) paraformaldehyde in PBS.
Brain injections.
Each animal was anesthetized with 2 % (v/v) isoflurane with oxygen until they did not respond to a toe pinch. Body temperature was maintained at 37°C (no. 40–90-8; FHC Inc.) and isoflurane decreased to 1.5 % (v/v) once placed in the stereotaxic frame (Kopf). The fur above the skull was cleaned with betadine before being cut to open access to the skull. Small holes were drilled over the motor cortex and/or the spinal trigeminal nucleus (EXL-M40, Osada, CA, USA). Injections were made using a Nanojet (Drummond). Anterior-posterior (AP) and medial-lateral (ML) coordinates are relative to Bregma, while dorsal-vental coordinates are relative to the surface of the brain, as follows: jaw motor cortex = AP +3 mm, ML 2 mm, DV 0.8 mm; dorsal SpVO = AP –5.4 mm, ML 2.0 mm lateral, DV 3.9 – 4.1 mm; SpVIr = AP – 5.7 mm, ML 2.0 mm, DV 4.2–4.5 mm; facial nucleus = AP –5.0 mm, ML 1.5 mm, DV 6.0 mm. Coordinates for SpVO and SpVIr were confirmed by electrophysiology recordings probing somatosensory responses prior to injecting. Coordinates for the facial nucleus were determined from intercranial microstimulation. For virus details, see Supplementary Table 1.
Headbar placement.
Six C57BL/6 mice and nine Thy1-ChR2 mice were anesthetized and placed in a stereotaxic apparatus as described above. The fur over the skin was cleaned with betadine before a straight anterior posterior cut was opened from the nasal to the intraparietal bone. A 4 mm diameter cranial window was made over the frontal bone with a centroid over the spot AP +2 mm, ML 2 mm. A thin layer of ACSF was applied before a 4 mm diameter glass coverslip (Fisher Brand) was gently placed over the brain surface. A small amount of cyanoacrylate glue was used to seal the bone to the glass. Once the glue was dry, the remaining exposed skull was cleaned and layered with cyanoacrylate glue. Once dry, a metal headbar was attached to the skull via cyanoacrylate glue. Last, a layer of dental cement covered the headbar and skull.
Optogenetic stimulation experiments.
The scan maps and specific positions of the laser were made using a scan system of Murphy and colleagues (Lim et al., 2012) (Fig. 2A). Either a blue 446 nm laser (Cube; Coherent Inc.) or a red 637 nm laser (Obis; Coherent Inc.) was scanned. The scan objective, effective NA of 0.01, was used to create a focal spot of 35 μm in diameter
Electrodes for EMGs were made of 50 μm diameter insulated tungsten wire (AM systems 795500). The tip of the wire for recording was stripped 1 mm before threaded through a 30-gauge needle and hooked. EMGs were inserted at the beginning of each recording session while the mice were under light (0.5 – 1.0 %) isoflurane. Two EMGs electrodes were inserted in each muscle, i.e., biceps brachii, digastric, intrinsic vibrissa, masseter, quadriceps, or splenius capitis. Three to five muscles were recorded per session. EMG recordings were taken using individual amplifiers for each muscle (DAM 80, World Precision Instruments) at 10 kHz. The raw signals were processed for extraction of the EMG envelope by using an 8th order Butterworth filter between 250 Hz and 2.5 kHz in the forward and reverse direction, then rectified by taking the absolute value, followed by a low pass 2nd-order Butterworth filter at 50 Hz in the forward and reverse directions. Last, a median filter was applied.
Videography were used to track the forelimb, jaw, nose and vibrissae. Nose and vibrissa videographs were taken from above. The forelimb and jaw utilized two mirrors, one in front and one to the side, that reflected the front image of the mouse up to the camera above. A high speed, 1000 by 1000-pixel camera was used with a frame rate of 200 frames per second (no. A504K; Basler Vision Technologies). For vibrissa tracking, one vibrissa was painted (Tulip dimensional fabric paint, 65101) for light contrast imaging with a mask for live tracking. For nose and jaw tracking, a dot of paint was placed on the top of the nose or the center bottom of the jaw. Forelimb tracking focused bright light on the paws.
Histology.
After perfusion, brains were left in 4 % (w/v) paraformaldehyde in PBS between 4 and 24 hours and then cryoprotected in 30 % (w/v) sucrose in PBS. Sections were collected on a sliding microtome maintained between −21 and −24°C. For synapse reconstructions, sections were cut horizontally at 16 μm and mounted on slides prior to immunostaining. For cell reconstructions, sections were cut at 60 μm and every other section was immunostained free floating. The synapse and cell labeling was converted to dark product using rabbit anti GFP (Novus; 1:1000 (v/v) in a 2 % (v/v) goat block solution in PBS), biotinylated anti-rabbit secondary antibodies (1:200 (v/v) in goat block), amplified with ABC kit (Vector Labs) and converted to dark product with the SG kit (Vector Labs). Slides were processed with increasing ethanol concentrations, followed by xylines, before being coverslipped with DPX (Sigma). All slides with dark product were counterstained with cytochrome oxidase. Slides or free-floating sections were incubated in cytochrome oxidase (37.5 mL PBS + 1.5 g sucrose + 33 mg DAB tetrahydrochloride (Sigma D5637–5G) + 15 mg cytochrome C (Sigma C-2506)) and maintained at 37° C for 1 – 3 hours. All slides were scanned on a Hammamatsu Nanozoomer and loaded into Neurolucida and then MATLAB for three-dimensional reconstruction. Fluorescent sections were cut between 30 and 50 μm. Citrine containing sections were amplified with rabbit anti GFP (Novus; 1:1000 in goat block) and Alexa™ 488 conjugated goat anti rabbit (Invitrogen; 1:1000 (v/v) in goat block) and coverslipped with Fluoromount (Southern Biotech). 16-bit images were collected on a fluorescent microscope and analyzed in ImageJ.
Computational analyses.
All code was written in Matlab (The Mathworks). For the spectral analysis we used the Chronux package (Mitra and Bokil, 2008). Composite maps in Figure 2F were created by taking the average amplitude within the trial, then averaging all trials at a given location across all animals (20 trials in each of five mice) and interpolating linearly between locations. Clusters identified in Figure 4E were found using a 2-D density plot of data in Figure 4D.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical tests.
Statistical tests are identified in the Results section. To identify if EMG signals were significantly different from baseline in Figure 1 we used a K-S test with p < 0.001. To compare normalized muscle activation across muscles in Figure 7 we used an ANOVA test with p > 0.1. To compare activation of muscle EMG from baseline in Figure 7 we used a student’s ttest with p < 0.05. In Figure 7F,G,I and J, greyed out traces and graph markers indicate that the result was not significant. All activation analysis used all data taken during the stimulus period and compared it with an equal period of time during the baseline period.
Quantification.
All quantification of synapses and neurons in Neurolucida or FIJI. For 3-D reconstructions in Figures 4, 5, and S5, Neurolucida was used to both count and identify coordinate location of each synapse or neuron. To create sagittal projections and histograms in Figures 5 and S5, Neurolucida data was exported to Matlab. For density quantification in Figure 4E, neurons were counted within the peak density region of the 2-D histogram as described in the figure legend. For density quantification for Figures 4J and 6C, 5 – 8 sections were taken at even intervals through a structure. An area outline was created around the structure in FIJI based on histological markers except for motor cortex in which the outline was create around the injection site as marked by the presence of neurons and their prominent dendrites (white outlines in Figs. 4,6, and S6). All presynaptic terminals were identified by colabeling of mRuby and GFP within the white outlines. Volume of a structure in one image was calculated by multiplying the area of the structure, as identified by the white outline, by the thickness of the section. For each section, the number of synapses were divided by the volume and final density was calculated by averaging across sections (Table S2).
DATA AND CODE AVAILABILITY
The datasets generated during this study are available in Table S2.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti GFP (made in goat) | Novus | NB600-308, RRID: AB_1048879 |
| Biotinylated anti rabbit IgG (made in goat) | Vector | BA-1000, RRID: AB_2336201 |
| Goat anti rabbit conjugated with Alexa 488 | Invitrogen | A-11034, RRID: AB_221544 |
| DAB tetrahydrochloride | Sigma | D5637-5G |
| Cytochrome C | Sigma | C-2506 |
| Bacterial and viral strains | ||
| Pseudorabies-GFP (strain 152) | Enquist lab | |
| Lentivirus-CAG-synaptophysin-eGFP | Adrian Lozada | |
| Retrograde lentivirus-hsyn-Cit | Daniel Gibbs | |
| AAV2.5-hsyn-flex-Cit | John Lin (Lin et al., 2013) | |
| AAV2.5-hsyn-flex-ReaChR-Cit | Salk Virus Core | Special preparation |
| AAV retro-hsyn-Cre | UPenn Virus Core | Special preparation |
| AAV-DJ-hsyn-flex-mGFP-2a-mRuby | Stanford Virus Core | GVVC-AAV-100 |
| Retrograde lentivirus-hsyn-Cre | Fan Wang | |
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| Critical commercial assays | ||
| Deposited data | ||
| Raw data | This paper | |
| Experimental models: Cell lines | ||
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | Jackson Laboratories | #000664, RRID: MGI:5657312 |
| Thy1-ChR2-YFP alias B6.Cg-Tg(Thy1-COP4/EYFP)18Gfng/J | Jackson Laboratories (Arenkiel et al., 2007; Wang et al., 2007) |
#007612, RRID: IMSR_JAX:007615 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and algorithms | ||
| MATLAB | Mathworks | 2007b to 2017b |
| Chronux | Chronux.org | http://chronux.org |
| FIJI | NIH | http://imagej.net/Fiji |
Highlights.
[83] Focal activation of corticobulbar neurons in motor cortex evokes orofacial movements.
[83] Corticobulbar neurons form orderly projections across multiple trigeminal subnuclei.
[82] Trigeminal subnuclei, premotor for motor actions, project to multiple motor nuclei.
[82] The cortico-trigemino-motoneuron network coordinates motor actions into a behavior.
Acknowledgements:
We thank Martin Deschênes, Beth Friedman, Josh Huang, Lauren McElvain, Takaki Komiyama, and Fan Wang for discussions, Grégory Scherrer for the use of reagents, Agnieszka Brzozowska-Prechtl for assistance with the histology, and Beth Friedman for assistance with the manuscript. This work was supported by NIH grants NS058668, NS0905905, and NS097265. The Neuroscience Microscopy Imaging Center was supported by NIH grant NS047101 and gift funds through the UC San Diego School of Medicine.
Footnotes
Declaration of interests: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alloway KD, Smith JB, and Beauchemin KJ (2010). Quantitative analysis of the bilateral brainstem projections from the whisker and forepaw regions in rat primary motor cortex. Journal of Comparative Neurology 518, 4546–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri M, Car A, and Roman C. (1990). Axonal branching of medullary swallowing neurons projecting on the trigeminal and hypoglossal motor nuclei: Demonstration by electrophysiological and fluorescent double labeling techniques. Experimental Brain Research 81, 384–390. [DOI] [PubMed] [Google Scholar]
- Amundsen Huffmaster SL, Van Acker GM 3rd, Luchies CW, and Cheney PD (2017). Muscle synergies obtained from comprehensive mapping of the primary motor cortex forelimb representation using high-frequency, long-duration ICMS. Journal of Neurophysiology 118, 455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, and Feng G. (2007). In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthas F, and Kwan AC (2017). Secondary motor cortex: Where ‘sensory’ meets ‘motor’ in the rodent rrontal cortex. Trends in Neuroscience 40, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance M-A, Takatoh J, Lu J, Demers M, Kleinfeld D, Wang F, and Deschênes M. (2017). Parallel inhibitory and excitatory trigemino-facial feedback circuitry for reflexive vibrissa movement. Neuron 95, 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter DA, Hirata H, and Hu JW (2000). Trigeminal subnucleus caudalis: Beyond homologies with the spinal dorsal horn. Pain 88, 221–224. [DOI] [PubMed] [Google Scholar]
- Bonazzi L, Viaro R, Lodi E, Canto R, Bonifazzi C, and Franchi G. (2013). Complex movement topography and extrinsic space representation in the rat forelimb motor cortex as defined by long-duration intracortical microstimulation. Journal of Neuroscience 33, 2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borke RC, Nau ME, and Ringler RL (1983). Brain-stem afferents of hypoglossal neurons in the rat. Brain Research 269, 47–55. [DOI] [PubMed] [Google Scholar]
- Brecht M, Krauss A, Muhammad S, Sinai-Esfahani L, Bellanca S, and Margrie TW (2004). Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. Journal of Comparative Neurology 479, 360–373. [DOI] [PubMed] [Google Scholar]
- Brodmann K, and Gary LJ (2006). Brodmann’s Localisation in the Cerebral Cortex: The Principles of Comparative Localisation in the Cerebral Cortex Based on Cytoarchitectonics (New York: Springer; ). [Google Scholar]
- Capra NF, and Dessem D. (1992). Central connections of trigeminal primary afferent neurons: topographical and functional considerations. Critical Reviews in Oral Biology & Medicine 4, 1–52. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, and Shenoy KV (2012). Neural population dynamics during reaching. Nature 487, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET Jr., and Sawchenko PE (2000). Dorsal medullary pathways subserving oromotor reflexes in the rat: Implications for the central neural control of swallowing. Jounal of Comparative Neurology 417, 448–466. [PubMed] [Google Scholar]
- Dong Y, Li J, Zhang F, and Li Y. (2011). Nociceptive afferents to the premotor neurons that send axons simultaneously to the facial and hypoglossal motoneurons by means of axon collaterals. Pubic Library of Science ONE 6, e25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, and Parham C. (1983). Afferent connections of the lateral agranular field of the rat motor cortex. Journal of Comparative Neurology 217, 390–404. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, and Wise SP (1982). The motor cortex of the rat: Cytoarchitecture and microstimulation mapping. Journal of Comparative Neurology 212, 76–88. [DOI] [PubMed] [Google Scholar]
- Economo MN, Viswanathan S, Tasic B, Bas E, Winnubst J, Menon V, Graybuck LT, Nguyen TN, Smith KA, Yao Z, et al. (2018). Distinct descending motor cortex pathways and their roles in movement. Nature 563, 79–84. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, and Killackey HP (1979). Efferent connections of the brainstem trigeminal complex with the facial nucleus of the rat. Journal of Comparative Neurology 188, 75–86. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Capelli P, and Arber S. (2014). Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature 508, 351–356. [DOI] [PubMed] [Google Scholar]
- Fay RA, and Norgren R. (1997a). Identification of rat brainstem multisynaptic connections to the oral motor nuclei in the rat using pseudorabies virus. II. Facial muscle motor systems. Brain Research Brain Research Reviews 25, 276–290. [DOI] [PubMed] [Google Scholar]
- Fay RA, and Norgren R. (1997b). Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. I. Masticatory muscle motor systems. Brain Research Brain Research Reviews 25, 255–275. [DOI] [PubMed] [Google Scholar]
- Fay RA, and Norgren R. (1997c). Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Research Brain Research Reviews 25, 291–311. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, and Petersen CCH (2007). Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 56, 907–923. [DOI] [PubMed] [Google Scholar]
- Fritsch G, and Hitzig E. (2009). Electric excitability of the cerebrum (Uber die elektrische Erregbarkeit des Grosshirns). Epilepsy and Behavior 15, 123–130. [DOI] [PubMed] [Google Scholar]
- Furuta T, Nakamura K, and Deschenes M. (2006). Angular tuning bias of vibrissa-responsive cells in the paralemniscal pathway. Journal of Neuroscience 26, 10548–10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, and Kettner RE (1986). Neuronal population coding of movement direction. Science 233, 1416–1419. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos NN, Hellman D, Schmitter M, Kruger B, Hauser T, and Schindler HJ (2013). Neuromuscular interaction of jaw and neck muscles during jaw clenching. Journal of Orofacial Pain 27, 61–71. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Joekes J, and Schreurs BG (2012). Anatomical characterization of a rabbit cerebellar eyeblink premotor pathway using pseudorabies and identification of a local modulatory network in anterior interpositus. Journal of Neuroscience 32, 12472–12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS, and Aflalo TN (2007). Mapping behavioral repertoire onto the cortex. Neuron 56, 239–251. [DOI] [PubMed] [Google Scholar]
- Graziano MSA (2016). Ethological action maps: A paradigm shift for the motor cortex. Trends in Cognitve Neuroscience 20, 121–132. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, and Moore T. (2002). Complex movements evoked by microstimulation of precentral cortex. Neuron 34, 841–851. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Brecht M, and Osten P. (2005). Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. Journal of Neuroscience 25, 8250–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiss F, and Schwarz C. (2005). Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. Journal of Neuroscience 25, 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TC, Ayling OG, and Murphy TH (2012). Distinct cortical circuit mechanisms for complex forelimb movement and motor map topography. Neuron 74, 397–409. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Priest CA, and Keller A. (2002). Functional circuitry involved in the regulation of whisker movements. Journal of Comparative Neurology 442, 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hira R, Terada S, Kondo M, and Matsuzaki M. (2015). Distinct functional modules for discrete and rhythmic forelimb movements in the mouse motor cortex. Journal of Neuroscience, 13311–13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M, and Shimamura M. (1977). Neural mechanisms of the corneal blinking reflex in cats. Brain Research 125, 265–275. [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Hoover JE, and Alloway KD (2003). Sensorimotor corticocortical projections from rat barrel cortex have an anisotropic organization that facilitates integration of inputs from whiskers in the same row. Journal of Comparative Neurology 466, 525–544. [DOI] [PubMed] [Google Scholar]
- Hollis ER 2nd, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, Richman A, Tury A, Wang SH, Pessian M, et al. (2016). Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nature Neuroscience 19, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Hiraba H, Murray GM, and Sessle BJ (1989). Topographical distribution and functional properties of cortically induced rhythmical jaw movements in the monkey (Macaca fascicularis). Journal of Neurophysiology 61, 635–650. [DOI] [PubMed] [Google Scholar]
- Isogai F, Kato T, Fujimoto M, Toi S, Oka A, Adachi T, Maeda Y, Morimoto T, Yoshida A, and Masuda Y. (2012). Cortical area inducing chewing-like rhythmical jaw movements and its connections with thalamic nuclei in guinea pigs. Neuroscience Research 74, 239–247. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, and Rhoades RW (1990). Cell structure and response properties in the trigeminal subnucleus oralis. Somatosensory and Motor Research 7, 265–288. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Woerner D, Szczepanik AM, Riecker V, Mooney RD, and Rhoades RW (1986). Structure-function relationships in rat brainstem subnucleus interpolaris. I. Vibrissa primary afferents. Journal of Comparative Neurology 243, 266–279. [DOI] [PubMed] [Google Scholar]
- Jeong M, Kim Y, Kim J, Ferrante DD, Mitra PP, Osten P, and Kim D. (2016). Comparative three-dimensional connectome map of motor cortical projections in the mouse brain. Science Reports 6, 20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, and Strick PL (1999). Muscle and movement representations in the primary motor cortex. Science 285, 2136–2139. [DOI] [PubMed] [Google Scholar]
- Kita T, and Kita H. (2012). The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: A single-axon tracing study in the rat. Journal of Neuroscience 32, 5990–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Masuda Y, Fujimoto Y, Matsuya T, Yamamura K, Yamada Y, Maeda N, and Morimoto T. (2002). Electrophysiological analysis of rhythmic jaw movements in the freely moving mouse. Physiology and Behavior 75, 377–385. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O’Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, and Svoboda K. (2010). Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464, 1182–1186. [DOI] [PubMed] [Google Scholar]
- Kurnikova A, Deschenes M, and Kleinfeld D. (2019a). Functional brain stem circuits for control of nose motion. J Neurophysiol 121, 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnikova A, Deschênes M, and Kleinfeld D. (2019b). Functional brainstem circuits for control of nose motion. Journal of Neurophysiology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnikova A, Moore JD, Liao S-M, Deschênes M, and Kleinfeld D. (2017). Coordination of orofacial motor actions into exploratory behavior by rat. Current Biology 27, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-Q, Takada M, Kaneko T, and Mizuno N. (1995). Premotor neurons for trigeminal motor nucleus neurons innervating the jaw-closing and jaw-opening muscles: Differential distribution in the lower brainstem of the rat. Journal of Comparative Neurology 12, 563–579. [DOI] [PubMed] [Google Scholar]
- Li Y-Q, Takada M, and Mizuno N. (1993). Premotor neurons projecting simultaneously to two ororfacial motor nuclei by sending their branched axons. A study with a fluorescent retrograde double-labeling technique in the rat. Neuroscience Letters 152, 29–32. [DOI] [PubMed] [Google Scholar]
- Lim DH, Mohajerani MH, LeDue J, Boyd J, Chen S, and Murphy TH (2012). In vivo large-scale cortical mapping using channelrhodopsin-2 stimulation in transgenic mice reveals asymmetric and reciprocal relationships between cortical areas. Frontiers in Neural Circuits 6, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Knutsen PM, Muller A, Kleinfeld D, and Tsien RY (2013). ReaChR: A red-shifted variant of channelrhodopsin enables neuronal activation through the intact skull. Nature Neuroscience 16, 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L, and Anderson DJ (2011). A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron, 938–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JP, Sasamoto K, Murakami T, and Olsson KA (1984). Analysis of rhythmic jaw movements produced by electrical stimulation of motor-sensory cortex of rabbits. Journal of Neurophysiology 52, 1014–1029. [DOI] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, and Svoboda K. (2011). Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DW, Deschênes M, Furuta T, Moore JD, Wang F, Karten HJ, and Kleinfeld D. (2015). Feedback in the brainstem: An excitatory disynaptic pathway for control of whisking. Journal of Comparative Neurology 523, 921–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Vidal PP, Baker H, and Baker R. (2012). Physiological and anatomical evidence for an inhibitory trigemino-oculomotor pathway in the cat. Jounal of Comparative Neurology 520, 2218–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElvain LE, Friedman B, Karten HJ, Svoboda K, Wang F, Deschênes M, and Kleinfeld D. (2018). Circuits in the rodent brainstem that control whisking in concert with other orofacial motor actions. Neuroscience 368, 152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimica B, Dunn BA, Tombaz T, Bojja V, and Whitlock JR (2018). Efficient cortical coding of 3D posture in freely behaving rats. Science 362, 584–589. [DOI] [PubMed] [Google Scholar]
- Mitra PP, and Bokil HS (2008). Observed Brain Dynamics (New York: Oxford University Press; ). [Google Scholar]
- Nguyen Q-T, and Kleinfeld D. (2005). Positive feedback in a brainstem tactile sensorimotor loop. Neuron 45, 447–457. [DOI] [PubMed] [Google Scholar]
- Olsson KA, and Westberg KG (1991). Integration in trigeminal premotor interneurones in the cat. 2. Functional characteristics of neurones in the subnucleus-gamma of the oral nucleus of the spinal trigeminal tract with a projection to the digastric motoneurone subnucleus. Experimental Brain Research 84, 115–124. [DOI] [PubMed] [Google Scholar]
- Oswald MJ, Tantirigama ML, Sonntag I, Hughes SM, and Empson RM (2013). Diversity of layer 5 projection neurons in the mouse motor cortex. Frontiers of Cellular Neuroscience 7, e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, d’Avella A, Carmena JM, and Bizzi E. (2012). Microstimulation activates a handful of muscle synergies. Neuron 76, 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, and Franklin KB (2008). The Mouse Brain in Stereotaxic Coordinates (Amsterdam: Academic Press; ). [Google Scholar]
- Peters AJ, Chen SX, and Komiyama T. (2014). Emergence of reproducible spatiotemporal activity during motor learning. Nature 510, 263–267. [DOI] [PubMed] [Google Scholar]
- Pinganaud G, Bernat I, Buisseret P, and Buisseret-Delmas C. (1999). Trigeminal projections to hypoglossal and facial motor nuclei in the rat. Journal of Comparative Neurology 415, 91–104. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, and Watson RT (1987). Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Research Bullitrn 19, 203–221. [DOI] [PubMed] [Google Scholar]
- Ren K, and Dubner R. (2011). The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int Rev Neurobiol 97, 207–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond FJ, Thomson DB, and Loeb GE (1992). Electromyographic studies of neck muscles in the intact cat. I. Patterns of recruitment underlying posture and movement during natural behaviors. Experimental Brain Research 88, 41–58. [DOI] [PubMed] [Google Scholar]
- Roucoux A, Crommelinck M, and Decostre MF (1989). Afferent Control of Posture and Locomotion. In Neck muscle activity in eye-head coordinated movements, pp. 351–362. [DOI] [PubMed] [Google Scholar]
- Sabino MA, Honore P, Rogers SD, Mach DB, Luger NM, and Mantyh PW (2002). Tooth extraction-induced internalization of the substance P receptor in trigeminal nucleus and spinal cord neurons: Imaging the neurochemistry of dental pain. Pain 95, 175–186. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Akiyama M, Cai B, Scott A, Han B-X, Takatoh J, Sigrist M, Arber S, and Wang F. (2013). The organization of submodality-specific touch afferent inputs in the vibrissa column. Cell Reports 5, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y, Yajima E, Nagamine Y, Ishizuka K, and Murakami T. (2011). Effects of neck muscle activities during rhythmic jaw movements by stimulation of the medial vestibular nucleus in rats. Brain Research Bulliten 86, 447–453. [DOI] [PubMed] [Google Scholar]
- Smith JB, Watson GD, Alloway KD, Schwarz C, and Chakrabarti S. (2015). Corticofugal projection patterns of whisker sensorimotor cortex to the sensory trigeminal nuclei. Frontiers of Neural Circuits 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan V, Karmakar K, Rijli FM, and Petersen CC (2015). Parallel pathways from motor and somatosensory cortex for controlling whisker movements in mice. European Journal of Neuroscience 41, 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek E 4th, Cheng S, Takatoh J, Han BX, and Wang F. (2014). Monosynaptic premotor circuit tracing reveals neural substrates for oro-motor coordination. eLife 30, e02511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatoh J, Nelson A, Zhou X, Bolton MM, Ehlers MD, Arenkiel BR, Mooney R, and Wang F. (2013). New modules are added to vibrissal premotor circuitry with the emergence of exploratory whisking. Neuron 77, 346–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JE, Wall JD, and Welch DB (2017). Masticatory and brux-like motor patterns in the freely behaving rat: Electromyography and phase analysis. Transactions of the Illinois State Academy of Science 110, 1–7. [Google Scholar]
- Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, and Jones TA (2011). The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cerebral Cortex 21, 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A, Kolta A, Chen CC, and Lund JP (2003). Neurons of the trigeminal main sensory nucleus participate in the generation of rhythmic motor patterns. Eur J Neurosci 17, 229–238. [DOI] [PubMed] [Google Scholar]
- van Ham JJ, and Yeo CH (1996). Trigeminal inputs to eyeblink motoneurons in the rabbit. Experimental Neurology 142, 244–257. [DOI] [PubMed] [Google Scholar]
- Wang H, Peca J, Matsuzaki M, Matsuzaki K, Noguchi J, Qiu L, Wang D, Zhang F, Boyden E,K,D, et al. (2007). High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proceedings of the National Academy of Sciences USA 104, 8143–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, and Burkhalter A. (2007). Area map of mouse visual cortex. Journal Comparatiive Neurology 502, 339–357. [DOI] [PubMed] [Google Scholar]
- Westberg K, Clavelou P, Sandström G, and Lund JP (1998). Evidence that trigeminal brainstem interneurons form subpopulations to produce different forms of mastication in the rabbit. Journal of Neuroscience 18, 6466–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Yasuda K, Dostrovsky JO, Bae YC, Takemura M, Shigenaga Y, and Sessle BJ (1994). Two major types of premotoneurons in the feline trigeminal nucleus oralis as demonstrated by intracellular staining with horseradish peroxidase. Journal Comparatiive Neurology 417, 448–466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during this study are available in Table S2.