Abstract
Among a wide diversity of sexually reproducing species, male ejaculates coagulate to form what has been termed a copulatory plug. A number of functions have been attributed to copulatory plugs, including the inhibition of female remating and the promotion of ejaculate movement. Here we demonstrate that copulatory plugs also influence the likelihood of implantation, which occurs roughly 4 days after copulation in mice. Using a bead transfer method to control for differences in ejaculate retention and fertilization rates, we show that implantation rates significantly drop among females mated to genetically engineered males incapable of forming plugs (because they lack functional transglutaminase 4, the main enzyme responsible for its formation). Surprisingly, this result does not correlate with differences in circulating progesterone levels among females, an important hormone involved in implantation. We discuss three models that connect male-derived copulatory plugs to implantation success, including the hypothesis that plugs contribute to a threshold amount of stimulation required for females to become receptive to implantation.
Keywords: pseudopregnancy, bead transfer method, implantation, copulatory plug, progesterone
Male-derived copulatory plugs influence implantation success among their female mates.
Introduction
Across a huge diversity of sexually reproducing species, male ejaculates coagulate to various degrees. In some cases, ejaculates form a solid structure referred to as a “copulatory plug.” Biologists have long sought to understand the molecular basis, functional role, and evolutionary significance of copulatory plugs (reviewed in [1]). Multiple lines of evidence suggest copulatory plugs evolved as a means for one male to inhibit remating by females [2–8]. However, copulatory plugs also play important roles in non-competitive mating, including retention of the ejaculate and sperm survival [9–22].
Genetic engineering experiments have enabled powerful experimental approaches aimed at understanding the function of the copulatory plug. Such experiments have demonstrated that transglutaminase 4 (TGM4) and seminal vesicle secretory protein 2 (SVS2) are required for copulatory plug formation, with contributions from the protein prostate and testis expressed 4 (PATE4) [9, 10, 22]. TGM4 is a catalytic enzyme that crosslinks SVS2 and is required to form the copulatory plug in rodents [23, 24]. In a previous study, we showed that TGM4 knockout (hereafter, TGM4−/−) males were unable to form a copulatory plug and were significantly less likely to sire a litter after ~ 2 weeks of being paired with a female [10]. In the absence of a plug, their ejaculates were quickly lost from the female’s reproductive tract. However, TGM4−/− males still fertilized what seemed to be a normal number of embryos each mating [10], leaving the reduction in litter success unexplained.
The overall goal of the present study was to understand which post-copulatory reproductive stages are defective for TGM4−/− males. We focus on whether copulatory plugs influence the probability that females enter a physiological state receptive for implantation, which in mice occurs approximately four and a half days after copulation. In many mammalian species (including mice), copulatory stimulation is required for implantation to proceed normally [17, 25–35]. This stimulation can even be applied artificially; Diamond [36] showed that the number, duration, and intervals between vaginal insertions of an artificial stimulator differentially affected the probability of “behavioral pseudopregnancy,” an indication that the female is primed for implantation. Interestingly, the parameters that most closely mimicked natural mating behaviors of male mice were also the most successful at artificially inducing pseudopregnancy, suggesting that females require a species-specific set of copulatory cues to allow implantation. Consistent with the idea that some threshold level of copulatory stimulation must be achieved prior to future reproductive effort, female rodents demonstrate a behavior referred to as “paced mating,” where females are more likely to return for more copulation bouts with a male if copulation is interrupted [37–42]. Lastly, it is well known that artificial insemination in female mice almost always has to be accompanied by mating with vasectomized males if implantation is to succeed [43]. The molecular mechanisms that link copulatory stimulation with downstream reproductive events are not well understood. However, it is known that in mice, copulation induces differential gene expression and neurogenesis in female brains [44, 45].
Given the relationship between copulatory stimulation and subsequent pregnancy, we hypothesized that the lack of a copulatory plug leads to reduced implantation success. Using a bead transfer approach, we demonstrate that implantation success was significantly reduced among females mated to TGM4−/− males. Surprisingly, this implantation defect occurred even though females mated to TGM4−/− males showed the characteristic surge in progesterone, the main hormone required to initiate implantation and sustain pregnancy. Our study expands the functional roles of copulatory plugs, suggesting they influence implantation several days after copulation, but leave the molecular mechanism of this effect unknown. We discuss several models that could link copulatory plugs with implantation success, including that they contribute to copulatory stimulation required to shift female physiology toward a state receptive for implantation.
Materials and methods
Ethics statement
All experiments, animal husbandry, and personnel were approved by the University of Southern California’s Institute of Animal Care and Use Committee under the protocols #11777 and #11394. The goal of the four main experiments below was to evaluate which aspects of reproduction were compromised among TGM4−/− males.
Animals
Mice were housed on a 14:10 h light:dark cycle with ad libidum access to water and food. Three different strains of mice were used throughout. All female mice were derived from the FVB/NJ strain (hereafter, FVB). Males were either wild type C57BL/6N (TGM4+/+) or homozygous TGM4tm1a(KOMP)Wtsi (TGM4−/−), a knockout for the prostate-specific protein TGM4. The TGM4−/− model was constructed by the multi-institutional Knockout Mouse Project [46] and consists of a ~ 7 kb “knockout first” cassette inserted into the C57BL/6N genetic background (project #CSD30105). Alternative crossing to Cre and/or FLP mice allows for further genetic modification of the knockout allele but was unnecessary in the present study. All male mice were essentially genetically identical except for this ~ 7 kb “knockout first” cassette that spans exons 2–3 of TGM4.
Seminal vesicle secretory protein 2 (SVS2) and PATE4 are also involved in making functional copulatory plugs [11, 22], and there are available knockout models for both. However, SVS2 has additional off-target effects, including the influence of sperm capacitation [11, 47, 48] and protection of sperm from the uterine environment [9, 49], which could confound our studies of plug function. PATE4 does not completely eliminate the copulatory plug [22], making it a less ideal model to study the role of the plug. Knocking out TGM4 fully prevents formation of the copulatory plug, and TGM4−/− males show no defects in sperm count, sperm motility, anatomy, or behavior [10]. Several additional features suggest that knocking TGM4 has minimal off-target effects: TGM4 is only expressed in the male prostate [50–52], and the only annotated domains are related to the cross-linking reaction necessary for plug formation. Importantly, TGM4 has accumulated multiple loss-of-function mutations in some species that do not form a plug [53], which is not predicted if this gene has important functions outside the context of plug formation.
To produce experimental mice, we paired a single breeder male and single female together for 2 weeks, and then separated them so that the dam could give birth in isolation. Pups were weaned at 21–28 days of age and placed in cages of up to three siblings segregated by sex. All experimental females were between 8–9 weeks old at time of experiment. Experimental males were caged individually to avoid dominance interactions at the time of the experiment [54, 55].
Vasectomies
Most of our experiments detailed below required males that were incapable of fertilization. We vasectomized males at approximately 10 weeks of age using the scrotal entry method [43]. Males were anesthetized by inhalation of 1.5–2% isoflurane (FLURISO™) with pure oxygen and injected with 5 mg/kg ketoprofen (KETOFEN®) as an analgesic. We applied eye lubricant, and then shaved the scrotum followed by three alternative washes of betadine and 20% isopropyl alcohol. A small incision was made in the scrotal region, and we carefully lifted each vas deferens out to cut and remove a segment. We applied a Chromic Gut 3/0 simple suture to the incision, followed by 3 M Vetbond glue. A vasectomy usually took ~ 30 min, and animals generally woke and became active 5–10 min after cessation of isoflurane. Animals recovered for at least 2 weeks prior to experiments.
Inducing ovulation
In most of our experiments detailed below, we artificially induced estrus in females between 8–9 weeks of age. We intraperitoneally injected females with 5 IU Pregnant Mare Serum Gonadotropin (BioVendor #RP1782725000) approximately 60 h before copulation and then injected with 5 IU Human Chorionic Gonadotropin (hCG; Millipore Sigma #230734) approximately 8–9 h before copulation and 7–8 h before the start of their dark cycle. As much as possible, we controlled batch effects by assigning sibling females to different conditions. For example, with three full sisters in a litter, one would mate to a TGM4+/+ male, another would mate to a TGM4−/− male, and the last would be used as a control. Of course, this was not always possible and depended on the number of females weaned per litter.
Identifying ejaculation based only on behavior
Successful ejaculation is often identified by observing copulatory plugs [43], but TGM4−/− males do not produce a copulatory plug. Therefore, we scored ejaculation success only through behavioral observations and not by the presence of copulatory plugs, so that females mated to TGM4+/+ and TGM4−/− could be scored with the same method. We continuously observed males and females for a maximum of 4 h after pairing. Pre-copulatory behaviors include chasing, mounting, intromissions, and bouts of thrusting without ejaculation [56, 57]. Ejaculation was identified as sustained thrusting that culminated in male and female freezing in position, often followed by the pair collapsing to their side [58]. After ejaculation, males and females often appear relatively uninterested in each other, occupying separate areas of the cage while grooming themselves. All behavioral observations were done blind to genotype and all females were checked for copulatory plugs for later quantification of our accuracy in behavioral scoring of ejaculation.
Experiment 1: 24-hour fertility (non-vasectomized males)
We induced ovulation in FVB females, then individually paired them with either a TGM4+/+ or TGM4−/− male, and then euthanized the females 24 h after ejaculation. We dissected the oviducts, removed eggs from the ampullae, treated them with hyaluronidase (Sigma #H4272) to remove the cumulus cells, and then transferred eggs to a customized depression slide. Using a compound microscope, all eggs were scored as having 2 pronuclei or in some stage of later cell division. Eggs that were undergoing apoptosis or lacked a zona pellucida were considered damaged and excluded from analysis. The remaining eggs were considered unfertilized. All scoring was done blind to treatment. We used a generalized linear model with binomial variance (logit link) to test whether the number of fertilized (two pronuclei stage or beyond) vs. unfertilized eggs varied according to male genotype, using the glm function in R [59]. In an additional analysis of only fertilized eggs, we used the same approach to test whether the number of fertilized eggs reaching the two-cell stage varied according to male genotype. Significance was determined using a χ2test.
Experiment 2: implantation rates (vasectomized males)
We induced ovulation in females then split them into three groups: mated to vasectomized TGM4+/+ males, mated to vasectomized TGM4−/− males, or unpaired but otherwise treated identically. We observed pairs until ejaculation, and then removed females within 20 min, or in the case of unpaired controls moved them to a new cage.
To test whether females mated to TGM4−/− males experienced reduced implantation success, we required a method that was independent of fertilization success. We modified a technique referred to as nonsurgical embryo transfer (NSET; Paratechs #60010) [60–62], whereby embryos are trans-cervically transferred into the uterus using a specialized pipette tip. Instead of embryos, we transferred Concanavalin A Sepharose (Con-A) beads (Sigma #C9017), a well-established method for assessing the decidual response in the mouse uterus [63–66]. Compared to natural implantation, mothers who implant Con-A beads show no detectable difference in uterine gene expression [67], suggesting they are a good proxy for testing whether the uterus is receptive to implantation.
Females were trans-cervically injected with 12–18 Con-A beads in 1.8 μL 2% bovine serum albumin phosphate-buffered solution 2.5 days after ejaculation, using the NSET semi-flexible pipette tip fitted to a P-2.5 μL pipettor [63–65]. Implantation occurs approximately 4.5 days after ejaculation in female mice [43, 68]. Four days after injecting beads (6.5 days after ejaculation), we injected 100 mL of 1% Chicago Blue Dye into one of the female’s two lateral tail veins, via a 27-gauge needle [43, 69]. Circulating dye collects at implantation sites, enhancing detectability. Approximately 3 min later, females were euthanized, and their uteri dissected for scoring under a dissection microscope. To be scored as an implantation site, the observer had to see the localization of dye as well as general decidualization of the uterus [65]. Scoring was done blind to treatment. When implantation occurred, it was normally a small number of beads (see Results); therefore, we scored implantations as successful or not rather than by the number of beads implanted.
Among females receiving an ejaculate, we tested whether implantation success varied according to male genotype (TGM4+/+ vs. TGM4−/−) using a modified χ2 approach. To account for the non-independence that arises because most males were used in multiple crosses, we permuted genotype among males 10,000 times to build an empirical null distribution of chi-squared values. To make the test one-tailed (because we predicted females mated to TGM4−/− males would display reduced implantation success), we multiplied permuted chi-squared values by −1 if the permutation showed that females mated to TGM4−/− males had higher implantation rates. In other words, our empirical null distribution was centered onzero.
Experiment 3: progesterone assays (vasectomized and non-vasectomized males)
Progesterone is an essential reproductive hormone that regulates the initiation and timing of implantation success in mammals, spiking in female mice at roughly 4.5 day post-ejaculation and remaining high for at least 5 days afterward in female mice [70, 71]. For a subset of females from Experiment 2, we measured serum progesterone through enzyme-linked immunosorbent assays (ELISA).
At approximately 6.5 days after ejaculation, a subset of females was injected with Chicago Blue dye, anesthetized with 1.5–2% isoflurane (FLURISO™) in pure oxygen and exsanguinated via cardiac puncture with a 22 G needle [43]. This procedure typically yielded 600–900 μL of blood. Blood was allowed to settle for a minimum of 20 min before being spun down for 12–15 min at 4 degrees Celsius at 2000 x g. Spun-down blood separated into two distinct layers; the top serum layer was collected via pipet. Serum was aliquoted and frozen for later use in Cayman Chemical’s Progesterone ELISA Kit (Product #582601).
Samples were run on 96-well plates, with at least 8 wells of each plate consisting solely of positive and negative controls as well as 16 wells to draw a standard curve following the manufacturer’s recommendations (two replicates each of 1000, 500, 250, 125, 62.5, 31.3, 15.6, and 7.8 pg/mL standards). Each sample was diluted to three different concentrations. During the course of our experiment, we discovered that variability in absorbance readings was related to both the progesterone level of the sample and the dilutions applied during the ELISA assays. For example, replicates with low progesterone and high dilutions—or high progesterone with low dilution—tended to yield unstable estimates of progesterone. Therefore, we diluted serum of mated females 200X, 400X, and 500X, and unmated females 25X, 50X, and 100X. Each set of dilutions was run in triplicate, for a total of nine absorbance reads per female. Raw absorbance reads were transformed into pg/mL according to the manufacturer’s protocols and customized R scripts. Within individual blood samples, some dilution series were more variable than others. We therefore calculated a weighted mean progesterone level per individual female, with the contribution of each dilution factor weighted by the reciprocal of its coefficient of variation (standard deviation divided by mean). All statistics are based on this weightedmean.
Experiment 4: differential abortion (non-vasectomized males)
To test whether the subfertility of TGM4−/− males was due to increased rates of abortion among their mates rather than reduced implantation, we compared placental scars to the number of pups born across a number of crosses. Placental scars are melanized tissue left at implantation sites for several months after birth [72]. If females abort their litters after implantation but before birth, we would observe more placental scars than pupsborn.
In this experiment, females were not induced to ovulate, but rather paired with a non-vasectomized TGM4+/+ or TGM4−/− male for 2 weeks, then separated. Females were allowed to give birth, then euthanized a week later. We dissected out the uterus, pinned it onto to a petri dish and submerged it in hydrogen peroxide for 2 h to bleach the tissue and highlight placental scars.
Results
TGM4−/− males sired fewer, smaller litters that were less likely to reach weaning age
In a previous study, we showed that TGM4−/− males sired significantly fewer litters. However, TGM4−/− males’ successful litters did not differ in the number of offspring, nor did they differ in the likelihood that litters reached weaning age. This previous study was based on almost 200 crosses (Table 2 of reference [[10]]). Here we included a greater volume of colony records spanning from 2012 through June 2018, under the assumption that larger sample sizes would provide additional power.
After being paired with a female for between 10 and 18 days, TGM4−/− males sired litters in 141/499 (28.2%) of crosses, compared to 575/740 (77.8%) by TGM4+/+ males (χ2 = 296.7, df = 1, P < 10−15). We previously reported that if a litter was born, there was no difference in the likelihood that a litter reached weaning age or litter size [10]. With the larger dataset compiled here, litters sired by TGM4−/− males were significantly less likely to reach weaning age compared to litters sired by TGM4+/+ males (TGM4−/− =104/141 [73.0%] vs. TGM4+/+ =496/575 [86.3%] litters reached weaning age, χ2 = 12.1, df = 1, P = 0.0005). The low success of crosses producing weaned litters is not unusual in C57BL/6 backgrounds, where less than 50% of all crosses produced pups that survived to weaning [73, 74]. Among those litters successfully reaching weaning age, TGM4−/− males sired a median 6 offspring, slightly but significantly fewer than the median 7 offspring sired by TGM4+/+ males (Wilcoxon rank sum test, P = 0.004). There was no difference in the variance of litter size (F495,103 = 1.13, P = 0.46). In sum, TGM4−/− males sired fewer, smaller litters, that were less likely to survive to weaning. The reduced probability of weaning may indicate that females mated to TGM4−/− males are more likely to neglect their litters.
Behavioral scoring of ejaculations is reliable
Ejaculation success was scored only via behavioral observations so that all crosses could be treated the same. Plug presence/absence offered a means to assess our accuracy in behavioral scoring. We observed a plug in 71 of 72 crosses to TGM4+/+ males whom we judged ended in ejaculation. Of 48 crosses to TGM4+/+ males whom we judged did not end in successful ejaculation, we observed a plug in 13 of them. Thus, we estimate our accuracy in scoring successful ejaculation was 71/72 (99%), and in unsuccessful ejaculation as 35/48 (73%). Our main inferences below (i.e., Figure 1) are based on crosses that ended in successful ejaculation, where our accuracy washigh.
Figure 1.
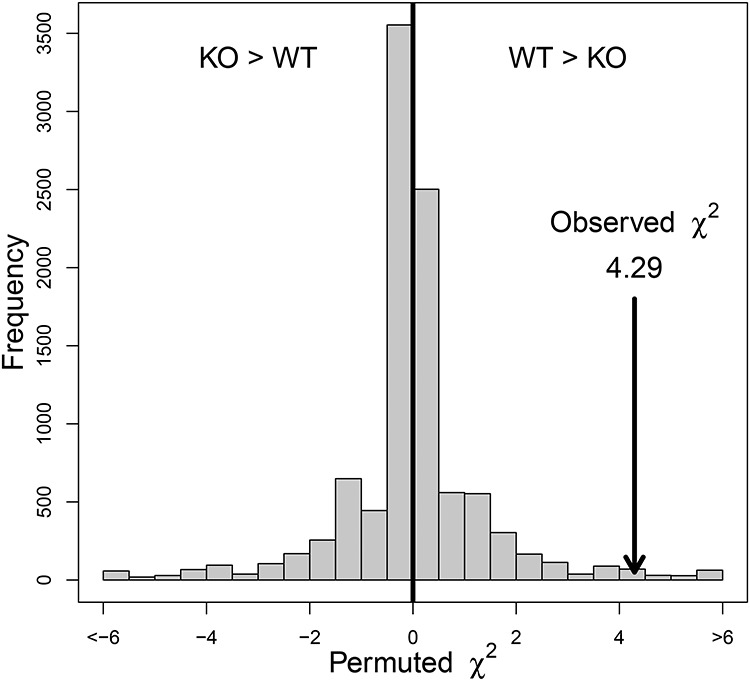
Permutation based χ2 test demonstrates that females receiving an ejaculate from TGM4−/− (KO) males are significantly less likely to implant than females mating to TGM4+/+ (WT) males.
Experiment 1: TGM4−/− males had reduced fertilization rate 24 h after ejaculation
We scored 642 eggs from 52 crosses to TGM4−/− males (N = 30 crosses) and TGM4+/+ males (N = 22 crosses). Females mated to TGM4−/− males had significantly fewer eggs fertilized 24 h after ejaculation (number of fertilized eggs: TGM4+/+ 9.4 ± 5.4, range = 0–18; TGM4−/− 3.4 ± 3.7, range = 0–13) (χ2 = 75.4, df = 1, P < 10−15, Table 1, Supplementary Data S1). There was no difference in the total number of eggs between treatments (total number of eggs: TGM4+/+ 16.4 ± 8.1, range = 2–28; TGM4−/− 14.3 ± 8.0, range = 3–35) (two-tailed t-test = 0.93, df = 45.39, P = 0.36). These results were consistent with our previous study, but here we carefully controlled time since ejaculation [10].
Table 1.
Distribution of developmental stages 24 h after copulation
| Male | N females | Total eggs | Unfertilized (%) | 2 pronuclei (%) | ≥2 Cells (%) |
|---|---|---|---|---|---|
| TGM4+/+ | 22 | 317 | 111 (35.0) | 36 (11.4) | 170 (53.6) |
| TGM4−/− | 30 | 325 | 224 (68.9) | 43 (13.2) | 58 (17.8) |
We scored 307 fertilized eggs from 44 females (21 to TGM4+/+ males, 23 to TGM4−/− males). Among fertilized eggs, significantly more eggs reached the two-cell stage in females mated to TGM4+/+ males (χ2 = 21.5, df = 1, P < 10−5, Table 1, Supplementary Data S1). There were fewer total females in this second analysis because eight females had zero fertilized eggs, of whom seven were mated to TGM4−/− males. Mating to TGM4−/− males resulted in slower fertilization rates that could have resulted in their fertilized eggs not reaching as advanced of a developmental stage as females mated to TGM4+/+ males. This overall difference in fertilization and developmental rate would confound any test of implantation success, further justifying our use of the bead transfer experiments of Experiment 2.
Experiment 2: females mated to TGM4−/− males were less receptive to implantation
Of the 50 control females never exposed to males, none showed implanted Con-A beads, as expected because female mice are not receptive to implantation without copulation [43] (Table 2). Of 72 females who received an ejaculate from vasectomized TGM4+/+ males, 42 (58.3%) successfully implanted beads (Table 2). Of 64 females who received an ejaculate from vasectomized TGM4−/− males, 25 (39.1%) implanted beads (Table 2), a significant reduction in implantation success compared to TGM4+/+ males (χ2 = 4.29, a value greater than 9,858/10,000 permutations, P-value = 0.014, Figure 1). A total of 50 unique males were used in these 135 crosses, each male copulated with a mean of 2.72 (standard deviation [SD] = 2.58, range = 1–11) females. Because all males for this experiment were vasectomized, differences in implantation could not be due to differences in fertilization rate. In addition, all females received a similar number of beads for each injection (number of beads injected: TGM4−/− 16.0 ± 1.7, range = 13–18; TGM4+/+ 15.3 ± 1.8, range = 12–18, controls 14.6 ± 2.1, range = 11–18) (one-way analysis of variance [ANOVA]: F2, 276 = 1.75, P = 0.18), so differences could not be due to variation in the number of beads transferred (number of implanted beads: TGM4−/− 2.2 ± 1.40 range = 1–5; TGM4+/+ 2.8 ± 1.9, range = 1–8; Welch two-sample t-test =1.53, df = 62.3, P = 0.13).
Table 2.
The number of females implanting Con-A beads according to category. The total number of unique males was 31 TGM4+/+ and 31 TGM4−/− individuals
| Genotype | Ejaculation | Implanted | Not implanted | % implanted | ||||
|---|---|---|---|---|---|---|---|---|
| Plug | No plug | Unique males | Plug | No plug | Unique males | |||
| Alone | No | 0 | 0 | 0 | 0 | 58 | 0 | 0 |
| TGM4+/+ | Yes | 42 | 0 | 21 | 29 | 1 | 15 | 58.33 |
| TGM4−/− | Yes | - | 25 | 18 | - | 39 | 15 | 39.06 |
| TGM4+/+ | No | 8 | 0 | 8 | 5 | 35 | 20 | 16.67 |
| TGM4−/− | No | - | 3 | 3 | - | 28 | 18 | 9.67 |
Females who were paired with males but did not receive an ejaculation (scored behaviorally) had low implantation success (16.67% for TGM4+/+ males and 9.67% for TGM4−/− males, Table 2) (χ2 = 0.3, df = 1, P = 0.59). For females whom we scored as having not received an ejaculation from TGM4+/+ males, it is interesting to note that all eight successful implantations were incorrectly scored based on the presence of a copulatory plug. Furthermore, among all 13 females who had a plug even though they were mis-scored as not receiving an ejaculation, implantation success (8/13 = 61.55%) was very similar to the 58.33% implantation rate identified from females receiving an ejaculate from TGM4+/+ males. These results indicate that the presence of a male alone, often accompanied by copulatory attempts, is not sufficient to induce implantation. Rather, females receiving an ejaculate are much more likely to be receptive for implantation, especially in cases where the ejaculate forms a copulatory plug (Table 2, Supplementary Data S2).
Experiment 3: progesterone is equally elevated among females who receive an ejaculate
Across 42 plates’ worth of ELISA assays (Supplementary Data S3), the r2 of the standard curve averaged 0.918 (SD = 0.085) (Supplementary Figure S1), indicating we could reliably construct standard curves across plates. From serum samples taken from 98 females, we made a total of 333 dilutions in triplicate. Across 333 dilution sets, the median coefficient of variation was 0.11, with 147 of these less than 0.1. These coefficients of variation are reasonably low, indicating good repeatability in spite of the known noisiness associated with ELISA assays.
A total of 49 females tested received an ejaculate from either vasectomized TGM4+/+ (N = 20) or vasectomized TGM4−/− (N = 29) males, of whom 11 or 12 females, respectively, implanted beads successfully. Among these 49 females, there was no significant difference in serum progesterone level based on mate genotype or implantation success (two -way ANOVA, male genotype: F1,46 = 0.043, P = 0.84; implantation success: F1,46 = 0.96, P = 0.33). However, across all five possible mating outcomes (females paired and mated to either TGM4+/+ or TGM4−/− males, females paired but unmated to either TGM4+/+ or TGM4−/− males, control females) progesterone levels varied (ANOVA F4,70 = 16.6, P < 10−8, Figure 2). Tukey honest significant differences revealed that females receiving an ejaculate had higher progesterone expression when compared to the other mating outcomes (Figure 2), consistent with previous studies showing that mated females have elevated progesterone [75, 76]. Control females showed uniformly low levels of progesterone expression (Figure 2). In sum, females receiving an ejaculate showed an upwards shift in circulating progesterone approximately 6.5 days after ejaculation, regardless of their implantation success or the genotype of their mates.
Figure 2.
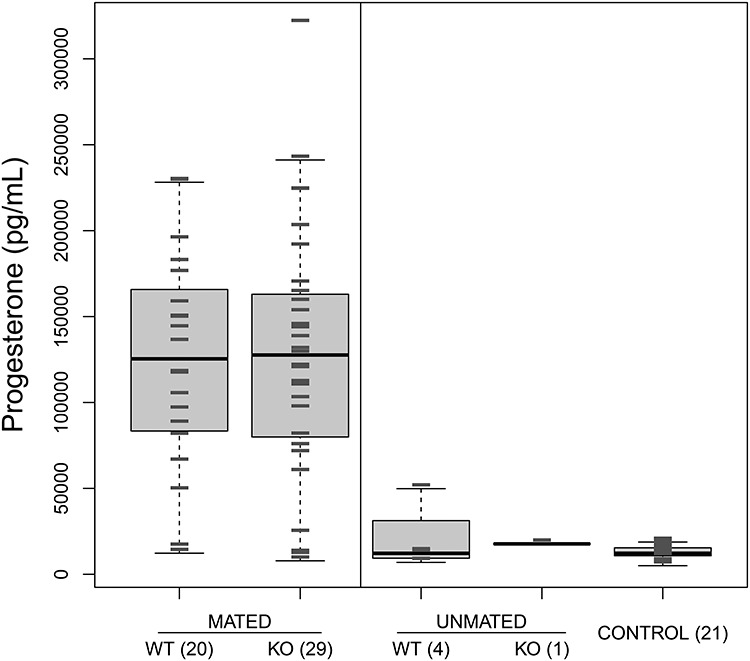
Boxplot of progesterone levels estimated across 98 females from five different groups; Mated vs. Unmated indicates whether ejaculation was scored within 4 h of pairing. All male mates were vasectomized. WT = females paired with a TGM4+/+ male, KO = females paired with a TGM4−/− males. Control = females never paired with males. Numbers in parentheses indicate number of females per group. Gray hash marks indicate individual observations. Vertical line separates the two different groups (homogeneous within each group) identified with Tukey HSD tests.
Although tangential to our main line of investigation, we were interested in how these results would change if we used non-vasectomized (intact) males. We repeated the experiment with 42 females who received an ejaculate from a non-vasectomized TGM4+/+ (N = 21) or TGM4−/− (N = 21) male, of whom 12 or 2 females, respectively, showed implanted embryos. Among these 42 females, there was no significant difference in progesterone level based on male genotype or implantation success (two -way ANOVA, male genotype: F1,39 = 2.8, P = 0.1; implantation success: F1,39 = 0.46, P = 0.5). However, progesterone levels varied across all five possible mating treatments (one-way ANOVA F4,64 = 14.9, P < 10−7). Tukey honest significant differences revealed that females receiving an ejaculate had high progesterone compared to the other three groups (Supplementary Figure S2). Interestingly, however, the progesterone levels were higher among females mated to intact males vs. vasectomized males (compare Supplementary Figure S2 vs. Figure 2, Supplementary Data S3).
Experiment 4: females mated to TGM4−/− males did not have increased rates of abortion
We found no evidence for increased abortion among females mated to TGM4−/− males (Supplementary Data S4). Nine of 10 females mated to TGM4+/+ males produced litters with an average size of 8.1 pups and an average of 9.0 scars. Thus, there were generally more placental scars than pups born, indicating at least some offspring died during gestation. Only one out of eight females mated to TGM4−/− males produced a litter, and she had a single pup with a single placental scar; none of the other females had placental scars, suggesting that their litters failed prior to implantation.
Discussion
Male-derived copulatory plugs promote implantation 4 days post-copulation. Females who copulated with TGM4−/− males, which cannot form a copulatory plug, suffered reduced implantation success compared to females mated to TGM4+/+ males. Their reduced implantation success cannot be solely attributed to reduced volume of ejaculate in the female reproductive tract or reduced fertilization (Table 1), because our bead transfer experiments (Table 2, Figure 1) used vasectomized males (so all males were sterile) and all females received the same amount of Con-A beads (which mimic embryos). We now discuss three different models that could link male-derived copulatory plugs to downstream implantation events.
Model 1: differences in ejaculate composition or time in situ may influence implantation success
Because ejaculates from TGM4−/− males do not coagulate, their composition must differ from TGM4+/+ ejaculates in multiple ways. The TGM4 protein normally crosslinks its main target protein, SVS2, causing it to precipitate into the copulatory plug [52, 77]. Since TGM4−/− males lack functional TGM4 protein, their ejaculates will contain an unusually high amount of soluble SVS2. SVS2 is required for copulatory plug formation [9], but it has additional functions including regulation of sperm capacitation [11–13], and protection from cytotoxic challenges [9, 14].
Seminal fluid proteins have been shown to impact female reproductive biology. Some seminal fluid proteins induce inflammatory responses and cytokine expression in the uterus [78–80]. Cytokines and prostaglandins from male seminal fluid bind to receptors in the female reproductive tract, and induce shifts in gene expression that, among other functions, promote implantation [81, 82]. Seminal fluid also impacts reproductive events in other species. Ovulation-inducing factor/ß nerve growth factor (OIF/NGF) [83–86] is a neuropeptide in seminal fluid that triggers ovulation and upregulation of reproductive hormones such as prolactin in induced ovulators (e.g., camelids, rabbits) [86–92] and is present in the semen of some (e.g., bovids and cervids) but not all (horses, pigs) spontaneous ovulators [85, 90, 93, 94]. Prepubertal female mice can be induced to ovulate via injections with OIF/NGF, with similar rates of success to hCG, but OIF/NGF has not been tested for its effects on downstream reproductive events [95]. In the spontaneous ovulator Bos taurus, OIF/NGF is associated with increased plasma progesterone and rates of pregnancy [94].
Although not all molecules discussed here are part of the copulatory plug itself, the stoichiometry of ejaculated protein molecules will likely differ when males cannot form a copulatory plug, which could in turn differentially influence implantation success. In addition to different stoichiometries, all seminal fluid from a TGM4−/− male will spend less time in the female reproductive tract (uterus and oviducts), as ejaculates essentially “leak out” in the absence of plugs [10]. It is therefore possible that molecular interactions simply have less time to manifest themselves.
Model 2: copulatory plugs may act as chemical/hormone delivery systems
It is possible that copulatory plugs themselves store and deliver chemical or hormonal signals to females via diffusion into the surrounding epithelial tissue. In mice, some male-derived hormones enter the female’s bloodstream after copulation [96–98]. Under a model where copulatory plugs represent delivery mechanisms for such hormones, females mated to TGM4−/− males may not receive an adequately timed dosage of chemical signals to continue with implantation.
Model 3: copulatory plugs may contribute to the threshold level of stimulation required to facilitate implantation
Many studies have linked copulatory stimulation to reproductive success [99, 100]. In mice, the copulatory plug is very large and prominent, and glues into the cervix and vaginal canal for 24–48 h after ejaculation [101]. Since it is often visible externally, the copulatory plug in mice is likely to induce considerable mechanical stretch reception. The copulatory plug may be an important component of the stimulation required to shifts females toward implantation receptivity.
Several follow-up experiments could more precisely test this physical stimulation model. One could insert artificial plugs and determine whether implantation occurs with stretch reception itself. Yang et al. [44] demonstrated that gene expression in female brain regions shifts in response to copulation. Our study predicts that such changes will be muted when females mate to TGM4−/− males, or more generally, to males that provide inadequate copulatory stimulation. Another prediction is that experimentally preventing copulatory stimulation, through either application of anesthetics or ablation of critical nerves [102, 103], could mimic the reduced implantation we observe here. Recently, it has been shown that female mice with a history of reproduction show relatively early aging and decrease in late-life reproductive potential compared to females housed with sterile males [104]. It would be interesting to test whether these shifts in life history occur when males cannot form a copulatoryplug.
If this third model is correct, then the copulatory plug may be one of many traits assessed during female choice, which is simply the phenomenon by which females nonrandomly invest in reproduction with particular males [105–108]. Both copulatory plug size and resident time vary among mouse strains, and copulatory plugs derived from males who have recently mated are smaller [101, 109], indicating genetic and environmental variance that could potentially covary with implantation success.
Progesterone levels are only slightly correlated with defects in implantation
Under normal reproduction, progesterone shows a predictable cycle in the events leading up to implantation and pregnancy in mice [75]. It begins at low physiological levels during early pregnancy, peaking between 5 and 9 days after copulation to promote decidualization and early gestation [110]. Low levels of progesterone are associated with an inability to sustain pregnancies past 11 days [110].
Contrary to expectation, females receiving an ejaculate showed this characteristic post-copulatory increase in progesterone, regardless of the male’s genotype, vasectomy status, or implantation success. Perhaps other hormones not measured here, like prolactin, better explain for the differences in implantation. Females experience twice daily surges in prolactin that maintain corpora lutea via increased expression of estradiol and luteinizing hormone receptors, thus controlling female progesterone levels through pregnancy [104, 111–113]. One possibility is that prolactin surges are winding down prematurely in females mated to TGM4−/− males. If prolactin levels in early pregnancy are too low, postpartum maternal investment decreases because prolactin normally drives maternal neurogenesis [45, 114, 115]. If copulatory plugs are affecting prolactin levels, then it could explain both the reduced implantation and increased litter death of females mated to TGM4−/− males. Prolactin in female rats has also been shown to increase the accumulation of uterine fluid [116, 117], and it is possible that increased uterine or oviductal fluid dynamics contributes to signaling related to implantation. Female mating history also influences levels of prolactin [104].
Our results indicate that even though many females mated to TGM4−/− males showed what were considered normal levels of progesterone, their uteri were not always receptive to implantation. It is also possible that progesterone defects occur at stages other than those observedhere.
Conclusions
Our study expands upon a growing list of functional roles of copulatory plugs. By using a bead transfer approach that bypasses differences in ejaculate retention and embryonic development, we demonstrate that females are less receptive to implantation, and less likely to wean litters that are born, if their mates cannot form a copulatory plug. We present three models to explain this phenomenon, including that copulatory plugs contribute to a threshold level of stimulation required to trigger female physiology toward a state of implantation. Perhaps most surprisingly, plug formation and implantation success is separated by about 4 days in mice, implying long-term effects of copulatory plugs.
Supplementary Material
Acknowledgments
We thank Shelby Chickman and Juanna Xie for their assistance with data collection and colony maintenance and the members of the Dean laboratory for their helpful feedback. Ian Ehrenreich and Rachel Schell provided access to a plate reader. Three anonymous reviewers and an associate editor greatly improved the manuscript.
Contributor Information
Michael Lough-Stevens, Molecular and Computational Biology, Department of Biological Sciences, University of Southern California, Los Angeles, CA, USA.
Caleb R Ghione, Molecular and Computational Biology, Department of Biological Sciences, University of Southern California, Los Angeles, CA, USA.
Matthew Urness, Molecular and Computational Biology, Department of Biological Sciences, University of Southern California, Los Angeles, CA, USA.
Adelaide Hobbs, Molecular and Computational Biology, Department of Biological Sciences, University of Southern California, Los Angeles, CA, USA.
Colleen M Sweeney, Molecular and Computational Biology, Department of Biological Sciences, University of Southern California, Los Angeles, CA, USA.
Matthew D Dean, Molecular and Computational Biology, Department of Biological Sciences, University of Southern California, Los Angeles, CA, USA.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author contributions
M.L.S. and M.D. conceived and designed the research; M.L.S., C.G., M.U., A.H., and C.S. performed the research; M.L.S. and M.D. conducted the analysis; and M.L.S. and M.D. wrote the paper.
References
- 1. Schneider MR, Mangels R, Dean MD. The molecular basis and reproductive function(s) of copulatory plugs. Mol Reprod Dev 2016; 83:755–767. [DOI] [PubMed] [Google Scholar]
- 2. Voss R. Male accessory glands and the evolution of copulatory plugs in rodents. Occas Pap Mus Zool Univ Mich 1979; 689:1–27. [Google Scholar]
- 3. Martan J, Shepherd BA. The role of the copulatory plug in reproduction of the guinea pig. J Exp Zool 1976; 196:79–83. [DOI] [PubMed] [Google Scholar]
- 4. Mangels R, Tsung K, Kwan K, Dean MD. Copulatory plugs inhibit the reproductive success of rival males. J Evol Biol 2016; 29:2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dixson AL, Anderson MJ. Sexual selection, seminal coagulation and copulatory plug formation in primates. Folia Primatol 2002; 73:63–69. [DOI] [PubMed] [Google Scholar]
- 6. Lemaître J-F, Ramm SA, Hurst JL, Stockley P. Sperm competition roles and ejaculate investment in a promiscuous mammal. J Evol Biol 2012; 25:1216–1225. [DOI] [PubMed] [Google Scholar]
- 7. Edward DA, Stockley P, Hosken DJ. Sexual conflict and sperm competition. Cold Spring Harb Perspect Biol 2015; 7:a017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lemaître J-F, Ramm SA, Hurst JL, Stockley P. Social cues of sperm competition influence accessory reproductive gland size in a promiscuous mammal. Proc. R. Soc. B 2011; 278:1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawano N, Araki N, Yoshida K, Hibino T, Ohnami N, Makino M, Kanai S, Hasuwa H, Yoshida M, Miyado K, Umezawa A. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc Natl Acad Sci 2014; 111:4145–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dean MD. Genetic disruption of the copulatory plug in mice leads to severely reduced fertility. PLoS Genet 2013; 9:e1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawano N, Yoshida M. Semen-coagulating protein, SVS2, in mouse seminal plasma controls sperm fertility. Biol Reprod 2007; 76:353–361. [DOI] [PubMed] [Google Scholar]
- 12. Carballada R, Esponda P. Binding of seminal vesicle proteins to the plasma membrane of rat spermatozoa in vivo and in vitro. Int J Androl 1998; 21:19–28. [DOI] [PubMed] [Google Scholar]
- 13. Carballada R, Esponda P. Effect of antibodies against seminal vesicle secretion on fertility in the rat. Zygote 1999; 7:223–231. [DOI] [PubMed] [Google Scholar]
- 14. Metafora S, Esposito C, Caputo I, Lepretti M, Cassese D, Dicitore A, Ferranti P, Stiuso P. Seminal vesicle protein IV and its derived active peptides: a possible physiological role in seminal clotting. Semin Thromb Hemost 2007; 33:53–59. [DOI] [PubMed] [Google Scholar]
- 15. Toner JP, Attas AI, Adler NT. Transcervical sperm transport in the rat: the roles of pre-ejaculatory behavior and copulatory plug fit. Physiol Behav 1987; 39:371–375. [DOI] [PubMed] [Google Scholar]
- 16. Cukierski MA, Sina JL, Prahalada S, Robertson RT. Effects of seminal vesicle and coagulating gland ablation on fertility in rats. Reprod Toxicol 1991; 5:347–352. [DOI] [PubMed] [Google Scholar]
- 17. Matthews MK Jr, Adler NT. Systematic interrelationship of mating, vaginal plug position, and sperm transport in the rat. Physiol Behav 1978; 20:303–309. [DOI] [PubMed] [Google Scholar]
- 18. Carballada R, Esponda P. Role of fluid from seminal vesicles and coagulating glands in sperm transport into the uterus and fertility in rats. J Reprod Fertil 1992; 95:639–648. [DOI] [PubMed] [Google Scholar]
- 19. Blandau RJ. Is the copulation plug necessary for the en masse transport of spermatozoa into the uterine cornua of the albino rat? Anat Rec 1945; 91:266–267. [Google Scholar]
- 20. Blandau RJ. On the factors involved in sperm transport through the cervix uteri of the albino rat. Am J Anat 1945; 77:253–272. [Google Scholar]
- 21. Ramm SA, Oliver PL, Ponting CP, Stockley P, Emes RD. Sexual selection and the adaptive evolution of mammalian ejaculate proteins. Mol Biol Evol 2007; 25:207–219. [DOI] [PubMed] [Google Scholar]
- 22. Noda T, Fujihara Y, Matsumura T, Oura S, Kobayashi S, Ikawa M. Seminal vesicle secretory protein 7, PATE4, is not required for sperm function but for copulatory plug formation to ensure fecundity†. Biol Reprod 2019; 100:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Notides AC, Williams-Ashman H. The basic protein responsible for the clotting of guinea pig semen. Proc Natl Acad Sci U S A 1967; 58:1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams-Ashman H, Notides A, Pabalan S, Lorand L. Transamidase reactions involved in the enzymic coagulation of semen: isolation of γ-glutamyl-ε-lysine dipeptide from clotted secretion protein of guinea pig seminal vesicle. Proc Natl Acad Sci 1972; 69:2322–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lanier DL, Estep DQ, Dewsbury DA. Copulatory behavior of golden hamsters: effects on pregnancy. Physiol Behav 1975; 15:209–212. [DOI] [PubMed] [Google Scholar]
- 26. Lanier DL, Dewsbury DA. Studies of copulatory behaviour in northern grasshopper mice (Onychomys leucogaster). Anim Behav 1977; 25:185–192. [Google Scholar]
- 27. Lanier DL, Estep DQ, Dewsbury DA. Role of prolonged copulatory behavior in facilitating reproductive success in a competitive mating situation in laboratory rats. J Comp Physiol Psychol 1979; 93:781–792. [Google Scholar]
- 28. Adler NT. Effects of the male's copulatory behavior on successful pregnancy of the female rat. J Comp Physiol Psychol 1969; 69:613–622. [DOI] [PubMed] [Google Scholar]
- 29. Coolen LM, Peters HJPW, Veening JG. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res 1996; 738:67–82. [DOI] [PubMed] [Google Scholar]
- 30. Long JA, Evans HM. The Oestrus Cycle in the Rat and Its Associated Phenomena. Memoirs of the University of California, vol. 6. 1922: 1–148. University of California Press. [Google Scholar]
- 31. Ball J. Demonstration of a quantitative relation between stimulus and response in pseudopregnancy in the rat. Am J Physiol 1934; 107:698–703. [Google Scholar]
- 32. Diamond M, Yanagimachi R. Induction of pseudopregnancy in the golden hamster. Reproduction 1968; 17:165–168. [DOI] [PubMed] [Google Scholar]
- 33. Carlson R, DeFeo V. Comparative effectiveness of physiological, mechanical, and pharmacological means of inducing pseudopregnancy in rat, mouse, and hamster. In: Anatomical Record, vol. 145. New York, NY: Wiley-Liss Div John Wiley & Sons Inc; 1963: 312. [Google Scholar]
- 34. DeFeo VJ. Vaginal-cervical vibration: a simple and effective method for the induction of pseudopregnancy in the rat. Endocrinology 1966; 79:440–442. [DOI] [PubMed] [Google Scholar]
- 35. Wynne-Edwards K, Huck U, Lisk R. Influence of pre-and post-copulatory pair contact on pregnancy success in Djungarian hamsters, Phodopus campbelli. Reproduction 1987; 80:241–249. [DOI] [PubMed] [Google Scholar]
- 36. Diamond M. Intromission pattern and species vaginal code in relation to induction of pseudopregnancy. Science 1970; 169:995–997. [DOI] [PubMed] [Google Scholar]
- 37. Johansen JA, Clemens LG, Nunez AA. Characterization of copulatory behavior in female mice: evidence for paced mating. Physiol Behav 2008; 95:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martínez I, Paredes RG. Only self-paced mating is rewarding in rats of both sexes. Horm Behav 2001; 40:510–517. [DOI] [PubMed] [Google Scholar]
- 39. Paredes RG, Vazquez B. What do female rats like about sex? Paced mating. Behav Brain Res 1999; 105:117–127. [DOI] [PubMed] [Google Scholar]
- 40. Erskine MS, Hanrahan SB. Effects of paced mating on c-fos gene expression in the female rat Brain. J Neuroendocrinol 1997; 9:903–912. [DOI] [PubMed] [Google Scholar]
- 41. Coopersmith C, Erskine MS. Influence of paced mating and number of intromissions on fertility in the laboratory rat. Reproduction 1994; 102:451–458. [DOI] [PubMed] [Google Scholar]
- 42. Zipse LR, Brandling-Bennett EM, Clark AS. Paced mating behavior in the naturally cycling and the hormone-treated female rat. Physiol Behav 2000; 70:205–209. [DOI] [PubMed] [Google Scholar]
- 43. Nagy A. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 44. Yang JJ, Larsen CM, Grattan DR, Erskine MS. Mating-induced neuroendocrine responses during pseudopregnancy in the female mouse. J Neuroendocrinol 2008; 21:30–39. [DOI] [PubMed] [Google Scholar]
- 45. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun 2012; 26:201–209. [DOI] [PubMed] [Google Scholar]
- 46. Austin CP, Battey JF, Bradley A, Bucan M, Capecchi M, Collins FS, Dove WF, Duyk G, Dymecki S, Eppig JT, Grieder FB, Heintz N et al. The knockout mouse project. Nat Genet 2004; 36:921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Araki N, Kawano N, Kang W, Miyado K, Yoshida K, Yoshida M. Seminal vesicle proteins SVS3 and SVS4 facilitate SVS2 effect on sperm capacitation. Reproduction 2016; 152:313. [DOI] [PubMed] [Google Scholar]
- 48. Kawano N, Yoshida K, Iwamoto T, Yoshida M. Ganglioside GM1 mediates decapacitation effects of SVS2 on murine Spermatozoa1. Biol Reprod 2008; 79:1153–1159. [DOI] [PubMed] [Google Scholar]
- 49. Shindo M, Inui M, Kang W, Tamano M, Tingwei C, Takada S, Hibino T, Yoshida M, Yoshida K, Okada H, Iwamoto T, Miyado K et al. Deletion of a seminal gene cluster reinforces a crucial role of SVS2 in male fertility. Int J Mol Sci 2019; 20:4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Engle ET. A morphological and experimental study of the proximal lobes of the prostate of the guinea-pig, Cavia cobaya. Anat Rec 1926; 34:75–90. [Google Scholar]
- 51. Dean MD, Clark NL, Findlay GD, Karn RC, Yi X, Swanson WJ, MacCoss MJ, Nachman MW. Proteomics and comparative genomic investigations reveal heterogeneity in evolutionary rate of male reproductive proteins in mice (Mus domesticus). Mol Biol Evol 2009; 26:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dubbink HJ, de Waal L, van Haperen R, Verkaik NS, Trapman J, Romijn JC. The human prostate-specific transglutaminase gene (TGM4): genomic organization, tissue-specific expression, and promoter characterization. Genomics 1998; 51:434–444. [DOI] [PubMed] [Google Scholar]
- 53. Carnahan SJ, Jensen-Seaman MI. Hominoid seminal protein evolution and ancestral mating behavior. Am J Primatol 2008; 70:939–948. [DOI] [PubMed] [Google Scholar]
- 54. D'Amato FR. Effects of male social status on reproductive success and on behavior in mice (Mus musculus). J Comp Psychol 1988; 102:146–151. [DOI] [PubMed] [Google Scholar]
- 55. Snyder RL. Fertility and reproductive performance of grouped male mice. In: Benirschke K (ed.), Comparative Aspects of Reproductive Failure. New York: Springer-Verlag; 1967: 458–472. [Google Scholar]
- 56. McGill TE. Sexual behavior in three inbred strains of mice. Behaviour 1962; 19:341–350. [Google Scholar]
- 57. Mosig DW, Dewsbury DA. Studies of the copulatory behavior of house mice (Mus musculus). Behav Biol 1976; 16:463–473. [DOI] [PubMed] [Google Scholar]
- 58. McGill TE. Induction of luteal activity in female house mice. Horm Behav 1970; 1:211–222. [Google Scholar]
- 59. R Core Team . R: a language and environment for statistical computing. In. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/. 2020. Accessed 1 February 2020. [Google Scholar]
- 60. Steele KH, Hester JM, Stone BJ, Carrico KM, Spear BT, Fath-Goodin A. Nonsurgical embryo transfer device compared with surgery for embryo transfer in mice. J Am Assoc Lab Anim Sci 2013; 52:17–21. [PMC free article] [PubMed] [Google Scholar]
- 61. Ali RB, van der Ahé F, Braumuller TM, Pritchard C, Krimpenfort P, Berns A, Huijbers IJ. Improved pregnancy and birth rates with routine application of nonsurgical embryo transfer. Transgenic Res 2014; 23:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Green MA, Bass S, Spear BT. Transcervical transfer of mouse embryos using the non-surgical embryo transfer (NSETTM) device. Biotechniques 2009; 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sakoff JA, Murdoch RN. Alterations in uterine calcium ions during induction of the decidual cell reaction in pseudopregnant mice. J Reprod Fertil 1994; 101:97–102. [DOI] [PubMed] [Google Scholar]
- 64. Bany BM. Pseudopregnant bead-induced mouse deciduoma model. In: Croy A, Yamada AT, DeMayo FJ, Adamson SL (eds.), The Guide of Investigation of Mouse Pregnancy. London: Academic Press; 2014. [Google Scholar]
- 65. Barrette VF, Adams MA, Croy BA. Endometrial decidualization does not trigger the blood pressure decline of normal early pregnancy in mice. Biol Reprod 2012; 86:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Albaghdadi AJ, Kan FW. Endometrial receptivity defects and impaired implantation in diabetic NOD mice. Biol Reprod 2012; 87:31–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McConaha ME, Eckstrum K, An J, Steinle JJ, Bany BM. Microarray assessment of the influence of the conceptus on gene expression in the mouse uterus during decidualization. Reproduction 2011; 141:511–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McLaren A, Michie D. Studies on the transfer of fertilized mouse eggs to uterine Foster-mothers. J Exp Biol 1956; 33:394. [Google Scholar]
- 69. Dey SK. Visualizing Early Embryo Implantation Sites by Dye Injection. Cold Spring Harbor Protocols 2006; 2006:pdb. prot4361. [DOI] [PubMed]
- 70. Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006; 7:185–199. [DOI] [PubMed] [Google Scholar]
- 71. Namiki T, Ito J, Kashiwazaki N. Molecular mechanisms of embryonic implantation in mammals: lessons from the gene manipulation of mice. Reproductive Medicine and Biology 2018; 17:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Conaway CH. Embryo resorption and placental scar formation in the rat. J Mammal 1955; 36:516–532. [Google Scholar]
- 73. Inglis CA, Campbell ER, Auciello SL, Sarawar SR. Effects of enrichment devices on stress-related problems in mouse breeding. Johns Hopkins Center for Alternatives to Animal Testing: Final report for the Animal Welfare Enhancement Award 2004.
- 74. Morello GM, Hultgren J, Capas-Peneda S, Wiltshire M, Thomas A, Wardle-Jones H, Brajon S, Gilbert C, Olsson IAS. High laboratory mouse pre-weaning mortality associated with litter overlap, advanced dam age, small and large litters. Plos One 2020; 15:e0236290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Virgo BB, Bellward GD. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology 1974; 95:1486–1490. [DOI] [PubMed] [Google Scholar]
- 76. Holinka CF, Tseng Y-C, Finch CE. Reproductive aging in C57BL/6J mice: plasma progesterone, viable embryos and resorption frequency throughout pregnancy. Biol Reprod 1979; 20:1201–1211. [DOI] [PubMed] [Google Scholar]
- 77. Dubbink HJ, Verkaik NS, Faber PW, Trapman J, Schröder FH, Romijn JC. Tissue-specific and androgen-regulated expression of human prostate-specific transglutaminase. Biochem J 1996; 315:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Robertson SA, Mau V, Tremellen K, Seamark R. Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J Reprod Fertil 1996; 107:265–277. [DOI] [PubMed] [Google Scholar]
- 79. Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod 1998; 58:1217–1225. [DOI] [PubMed] [Google Scholar]
- 80. Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci 2014; 111:2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res 2005; 322:43–52. [DOI] [PubMed] [Google Scholar]
- 82. Robertson SA. Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs1. J Anim Sci 2007; 85:E36–E44. [DOI] [PubMed] [Google Scholar]
- 83. Adams GP, Ratto MH, Silva M, Carrasco R. Ovulation-inducing factor (OIF/NGF) in seminal plasma: a review and update. Reprod Domest Anim 2016; 51:4–17. [DOI] [PubMed] [Google Scholar]
- 84. Adams GP, Ratto MH. Ovulation-inducing factor in seminal plasma: a review. Anim Reprod Sci 2013; 136:148–156. [DOI] [PubMed] [Google Scholar]
- 85. Ratto MH, Leduc YA, Valderrama XP, van Straaten KE, Delbaere LTJ, Pierson RA, Adams GP. The nerve of ovulation-inducing factor in semen. Proc Natl Acad Sci 2012; 109:15042–15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. El Allali K, El Bousmaki N, Ainani H, Simonneaux V. Effect of the Camelid’s seminal plasma ovulation-inducing factor/-NGF: a Kisspeptin target hypothesis. Front Veter Sci 2017; 4:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maranesi M, Petrucci L, Leonardi L, Piro F, Rebollar PG, Millán P, Cocci P, Vullo C, Parillo F, Moura A, Mariscal GG, Boiti C et al. New insights on a NGF-mediated pathway to induce ovulation in rabbits (Oryctolagus cuniculus)†. Biol Reprod 2018; 98:634–643. [DOI] [PubMed] [Google Scholar]
- 88. Silva M, Niño A, Guerra M, Letelier C, Valderrama XP, Adams GP, Ratto MH. Is an ovulation-inducing factor (OIF) present in the seminal plasma of rabbits? Anim Reprod Sci 2011; 127:213–221. [DOI] [PubMed] [Google Scholar]
- 89. Ratto MH, Delbaere LTJ, Leduc YA, Pierson RA, Adams GP. Biochemical isolation and purification of ovulation-inducing factor (OIF) in seminal plasma of llamas. Reprod Biol Endocrinol 2011; 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ratto MH, Huanca W, Singh J, Adams GP. Comparison of the effect of ovulation-inducing factor (OIF) in the seminal plasma of llamas, alpacas and bulls. Theriogenology 2006; 66:1102–1106. [DOI] [PubMed] [Google Scholar]
- 91. Chen B, Yuen Z, Pan G. Semen-induced ovulation in the Bactrian camel (Camelus bactrianus). Reproduction 1985; 74:335–339. [DOI] [PubMed] [Google Scholar]
- 92. Kershaw-Young CM, Druart X, Vaughan J, WMC M. β-Nerve growth factor is a major component of alpaca seminal plasma and induces ovulation in female alpacas. Reprod Fertil Dev 2012; 24:1093–1097. [DOI] [PubMed] [Google Scholar]
- 93. Bogle OA, Carrasco RA, Ratto MH, Singh J, Adams GP. Source and localization of ovulation-inducing factor/nerve growth factor in male reproductive tissues among mammalian species†. Biol Reprod 2018; 99:1194–1204. [DOI] [PubMed] [Google Scholar]
- 94. Stewart JL, Mercadante VRG, Dias NW, Canisso IF, Yau P, Imai B, Lima FS. Nerve growth factor-beta, purified from bull seminal plasma, enhances corpus luteum formation and conceptus development in Bos taurus cows. Theriogenology 2018; 106:30–38. [DOI] [PubMed] [Google Scholar]
- 95. Bogle OA, Ratto MH, Adams GP. Evidence for the conservation of biological activity of ovulation-inducing factor in seminal plasma. Reproduction 2011; 142:277–283. [DOI] [PubMed] [Google Scholar]
- 96. DeCatanzaro D, Wyngaarden P, Griffiths J, Ham M, Hancox J, Brain D. Interactions of contact, odor cues, and androgens in strange-male-induced early pregnancy disruptions in mice (Mus musculus). J Comp Psychol 1995; 109:115. [DOI] [PubMed] [Google Scholar]
- 97. deCatanzaro D, Pollock T. Absorption and distribution of estradiol from male seminal emissions during mating. J Endocrinol 2016; 231:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. deCatanzaro D. Duration of mating relates to fertility in mice. Physiol Behav 1991; 50:393–395. [DOI] [PubMed] [Google Scholar]
- 99. Lariviere S, Ferguson SH. On the evolution of the mammalian baculum: vaginal friction, prolonged intromission or induced ovulation? Mamm. Rev 2002; 32:83–294. [Google Scholar]
- 100. André GI, Firman RC, Simmons LW. Baculum shape and paternity success in house mice: evidence for genital coevolution. Phil. Trans. R. Soc. B 2002; 375:20200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mangels R, Young B, Keeble S, Ardekani R, Meslin C, Ferreira Z, Clark NL, Good JM, Dean MD. Genetic and phenotypic influences on copulatory plug survival in mice. Heredity 2015; 115:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Levine JE. Chapter 26 - Neuroendocrine control of the ovarian cycle of the rat. In: Plant TM, Zeleznik AJ (eds.), Knobil and Neill's Physiology of Reproduction, Fourth ed. San Diego: Academic Press; 2015: 1199–1257. [Google Scholar]
- 103. Peters LC, Kristal MB, Komisaruk BR. Sensory innervation of the external and internal genitalia of the female rat. Brain Res 1987; 408:199–204. [DOI] [PubMed] [Google Scholar]
- 104. Garratt M, Try H, Smiley KO, Grattan DR, Brooks RC. Mating in the absence of fertilization promotes a growth-reproduction versus lifespan trade-off in female mice. Proc Natl Acad Sci U S A 2020; 117:15748–15754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Eberhard WG. Female Control: Sexual Selection by Cryptic Female Choice. Series: Monographs in Behavior and Ecology, vol. 69, 1996. Princeton University Press. [Google Scholar]
- 106. Eberhard WG. Postcopulatory sexual selection: Darwin's omission and its consequences. Proc Natl Acad Sci 2009; 106:10025–10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Eberhard WG, Cordero C. Sexual selection by cryptic female choice on male seminal products – a new bridge between sexual selection and reproductive physiology. Trends Ecol Evol 1995; 10:493–495. [DOI] [PubMed] [Google Scholar]
- 108. Puniamoorthy N, Kotrba M, Meier R. Unlocking the "black box": internal female genitalia in Sepsidae (Diptera) evolve fast and are species-specific. BMC Evol Biol 2010; 10:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sutter A, Simmons LW, Lindholm AK, Firman RC. Function of copulatory plugs in house mice: mating behavior and paternity outcomes of rival males. Behavioral Ecology 2015; 27:185–195. [Google Scholar]
- 110. Milligan SR, Finn CA. Minimal progesterone support required for the maintenance of pregnancy in mice. Hum Reprod 1997; 12:602–607. [DOI] [PubMed] [Google Scholar]
- 111. Bachelot A, Binart N. Corpus luteum development: lessons from genetic models in mice. In: Current Topics in Developmental Biology, vol. 68, 2005. Cambridge, Massachusetts: Academic Press; 49–84. [DOI] [PubMed] [Google Scholar]
- 112. Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 1975; 96:219–226. [DOI] [PubMed] [Google Scholar]
- 113. Schjenken JE, Robertson SA. The female response to seminal fluid. Physiol Rev 2020; 100:1077–1117. [DOI] [PubMed] [Google Scholar]
- 114. Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 2003; 299:117. [DOI] [PubMed] [Google Scholar]
- 115. Larsen CM, Kokay IC, Grattan DR. Male pheromones initiate prolactin-induced neurogenesis and advance maternal behavior in female mice. Horm Behav 2008; 53:509–517. [DOI] [PubMed] [Google Scholar]
- 116. Neill JD, Freeman ME, Tillson SA. Control of the Proestrus surge of prolactin and luteinizing hormone secretion by Estrogens in the Rat. Endocrinology 1971; 89:1448–1453. [DOI] [PubMed] [Google Scholar]
- 117. Kennedy TG, Armstrong DT. Extra-ovarian action of prolactin in the regulation of uterine lumen fluid accumulation in Rats. Endocrinology 1972; 90:1503–1509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


