Abstract
Insulin-like growth factors (IGFs) are known for their involvement in endocrine and paracrine regulation of ovarian function. Although IGF2 is the predominant circulating and intraovarian form of IGFs in primate species, the stage-specific follicular expression, action, and regulation of IGF2 are not well defined. Therefore, experiments were conducted to investigate the follicular IGF production in response to steroid hormone regulation and the direct IGF actions on follicular development and function in vitro. Preantral follicles were isolated from rhesus macaque ovaries and cultured to the antral stage in media supplemented with follicle-stimulating hormone and insulin. Follicles were randomly assigned to treatment groups: (a) control, (b) trilostane (a steroid synthesis inhibitor), (c) trilostane + estradiol, (d) trilostane + progesterone, and (e) trilostane + dihydrotestosterone. Media was analyzed for IGF concentrations, which were correlated to follicle growth. Follicles produced IGF2, but not IGF1, at the antral stage. Steroid depletion decreased, whereas steroid replacement increased, IGF2 production by antral follicles. Media IGF2 levels correlated positively with antral follicle diameters. Macaque preantral follicles and granulosa cells were subsequently cultured without (control) and with recombinant human IGF2 supplementation. Follicle survival, growth, and paracrine factor production, as well as granulosa cell proliferation and gonadotropin receptor gene expression, were assessed. IGF2 addition increased follicle survival rates, diameters and inhibin B production, as well as granulosa cell proliferation. These data demonstrate that IGF2 produced by antral follicles, in response to steroid hormone regulation, could act as a paracrine factor that positively impacts preantral follicle development and function in primates.
Keywords: insulin-like growth factor, preantral follicle, antral follicle, primate, follicle culture
Insulin-like growth factor 2, but not insulin-like growth factor 1, is produced by antral follicles in response to steroid hormone regulation in nonhuman primates, and promotes preantral follicle growth with increased paracrine factor production.
Introduction
Insulin-like growth factors (IGFs), IGF1 and IGF2 mainly produced by the liver, are known for their involvement in endocrine and paracrine regulation of ovarian function [1, 2]. Different from rodents (polyovulatory species) that have trace amounts of circulating IGF2, mono-ovulatory species, including humans, has 2–3-fold higher levels of IGF2 than IGF1 in the circulation [3]. Circulating IGF1 does not appear to be crucial for human follicular development [4]. The species-specific aspect of IGF activity is further demonstrated by follicular expression of the ligands as paracrine factors in the ovary [3]. In rodents, IGF1 is the predominant form produced by granulosa cells (GCs) of growing preantral and antral follicles, whereas follicular IGF2 expression is very limited [5, 6]. In contrast, IGF1 is weakly expressed in the ovine, bovine, and human ovaries [7–9]. Rather, significant IGF2 production is observed in theca and GCs of growing antral follicles [7–9]. Therefore, the endocrine and paracrine actions of IGFs in the ovary could be studied according to the ligand availability during different stages of follicular development in relevant species.
To date, the production of IGFs regulated by endocrine or local factors is not well understood, especially in ovarian follicles. Gonadotropins, particularly follicle-stimulating hormone (FSH), appear to increase follicular IGF1 production, as suggested by studies in goats and humans [10, 11]. Androgen-stimulated IGF1 expression was reported in rhesus macaques [12]. In contrast to IGF1, data regarding FSH-regulated IGF2 production are inconsistent between humans and nonprimate species. Although FSH promoted IGF2 expression in cultured human cumulus cells [13], no similar effect was observed in goats [10]. Estradiol and progesterone-regulated IGF2 production was suggested in the rat hippocampus and the bovine endometrium, respectively [14, 15]. However, relevant data are lacking in the ovary. Thus, further investigations are needed to elucidate the regulation of follicular IGF production, particularly in primate species.
The type I IGF receptor mediates most of the somatomedin-like biological actions of IGF1 and IGF2, and is expressed in GCs of both preantral and antral follicles in the human ovary [1, 2]. IGF1 treatment increased proliferation rates of cultured human GCs [16, 17]. Although IGF2 appears to be the major endocrine and paracrine factor in human ovarian function regulation [3, 9], IGF2 effects on follicular development are not well documented. Because IGF2 is predominantly produced by antral follicles [7–9], it has been mainly studied in cultured human granulosa and theca cells from antral follicles [18, 19]. Studies are needed to determine the direct IGF actions during the specific stages of follicular development, especially the preantral stage.
Individual follicle culture techniques have been developed to investigate follicular growth, function, and regulation at specific stages of development by maintaining follicle integrity and avoiding interfollicular interactions [20, 21]. Therefore, the present study was designed to determine follicular IGF1 and IGF2 production, as well as their regulation by steroid hormones, in a nonhuman primate model. Experiments were also performed to examine the direct IGF actions on follicular development and function in vitro.
Methods
Animal care and ovary collection
The general care of rhesus macaques (Macaca mulatta) was provided by the Division of Comparative Medicine, Oregon National Primate Research Center (ONPRC), Oregon Health & Science University, as previously described [20]. Briefly, animals were pair-caged in a temperature-controlled (22°C), light-regulated (12 L:12D) room. Diet consisted of Purina monkey chow (Ralston-Purina) twice a day, supplemented with fresh fruit or vegetables once a day and water ad libitum. Animals were treated according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Protocols were approved by the ONPRC Institutional Animal Care and Use Committee.
Ovaries were collected from adult female animals through the ONPRC Pathology Services Unit tissue distribution program. Euthanasia was due to reasons unrelated to reproductive health, e.g., chronic colitis and reactive arthritis. Ovaries were transferred to the laboratory in HEPES-buffered holding media (Cooper Surgical, Inc.) supplemented with 2% (v/v) human serum protein supplement (SPS; CooperSurgical, Inc.) at 37°C within 30 min [20].
Follicular IGF production under steroid ablation and estradiol/progesterone replacement
Encapsulated three-dimensional follicle culture was used to determine follicular IGF production and its regulation by GC-derived steroid hormones i.e., estradiol and progesterone. Ovaries were collected from six animals (7–13 years old) for follicle isolation, encapsulation, and culture as reported previously [20]. Briefly, preantral follicles (diameter = 125–250 μm) were mechanically isolated, encapsulated in 0.25% (w/v) sodium alginate gel matrix (FMC BioPolymers)-phosphate-buffered saline, and cultured individually in 300 μl media in 48-well plates at 37°C and 5% O2. Culture media contained alpha minimum essential medium (Thermo Fisher Scientific Inc.) supplemented with 3 ng/ml recombinant human FSH (NV Organon), 6% (v/v) SPS, 0.5 mg/ml bovine fetuin, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, and 10 μg/ml gentamicin (Sigma–Aldrich). Follicles from each of the six animals were randomly assigned to four groups (24 follicles/animal/group) for 5 weeks of culture: (a) control (containing ethanol vehicle at 0.01% v/v), (b) 250 ng/ml trilostane (TRL, a 3β-hydroxysteroid dehydrogenase inhibitor to generate a steroid-hormone depleted milieu, dissolved in 100% ethanol; Dechra Veterinary Products; dose based on a previous dose–response study in macaque GC culture [22]), (c) 250 ng/ml TRL + 1 ng/ml 17β estradiol (E2, dissolved in 100% ethanol; PerkinElmer; dose based on a previous dose–response study in macaque follicle culture [23]), and (d) 250 ng/ml TRL + 100 ng/ml R5020 (P, a nonaromatizable progesterone without converting to estrogen or androgen, dissolved in 100% ethanol; PerkinElmer; dose based on a previous dose–response study in macaque follicle culture [23]).
Follicle survival, antrum formation, and growth (diameter) were assessed weekly by microscopy as previously described [20]. Follicles were considered to undergo atresia if the oocyte was degenerated or extruded from the follicle. Follicle diameters were measured weekly using Image J software (National Institutes of Health). Media (150 μl) was changed every other day. Media samples from week 1 (preantral stage), 3 (antrum formation), and 5 (antral stage) were assayed for concentrations of IGF1 using a Human IGF-I/IGF-1 Quantikine ELISA Kit (R&D Systems, Inc.) and IGF2 using a RayBio Rhesus Monkey IGF-2 ELISA Kit (RayBiotech, Inc.) based on the manufacturers’ instructions by the Endocrine Technologies Core at ONPRC. ELISA kits were validated for macaque follicle culture media, and measurement from blank media without culture was used for background subtraction. The assay sensitivity was 0.06 ng/ml for IGF1 and 0.2 ng/ml for IGF2, and the standard curve of the assay ranged 0.1–6 ng/ml for IGF1 and 0.2–50 ng/ml for IGF2.
Follicular IGF production under steroid ablation and androgen replacement
Encapsulated three-dimensional follicle culture was used to determine follicular IGF production and its regulation by theca cell-derived steroid hormone, androgen. Ovaries were collected from four animals (8–13 years old) for follicle isolation, encapsulation, and culture as described above. Preantral follicles (diameter = 125–225 μm) from each of the four animals were randomly assigned to three groups (24 follicles/animal/group) for 5 weeks of culture: (a) control (containing ethanol vehicle at 0.01% v/v), (b) 250 ng/ml TRL (dissolved in 100% ethanol), and (c) 250 ng/ml TRL + 50 ng/ml dihydrotestosterone (DHT, a nonaromatizable testosterone without converting to estrogen, dissolved in 100% ethanol; PerkinElmer; dose based on a previous dose–response study in macaque follicle culture [24]). Follicle survival, antrum formation, and growth assessment, as well as media assays for IGF1 and IGF2, were conducted as described above.
Follicular growth and function with IGF2 supplementation
Guided by results obtained from the above experiments, an additional experiment was performed to examine the direct actions of IGF2 on the development and function of preantral follicles using matrix-free three-dimensional follicle culture. Ovaries from three animals (6–9 years old) were collected for follicle isolation, as described above, and culture as reported previously [21]. Briefly, mechanically isolated preantral follicles (diameter = 140–180 μm) were distributed individually into wells of the 96-well ultralow attachment microplates (Corning Inc.) and cultured in 200 μl media, as described above, at 37°C and 5% CO2. Follicles from each of the three animals were randomly assigned to two groups (12 follicles/animal/group) for 2 weeks of culture: (a) control and (b) 50 ng/ml recombinant human IGF2 (292-G2; R&D Systems; dose based on a preliminary study in macaque follicle culture).
Follicle survival and growth were assessed as described above. Media (100 μl) was changed every other day. To evaluate follicular paracrine factor production, media samples from week 2 (preantral stage) were assayed for anti-Müllerian hormone (AMH) concentrations using an AMH ELISA kit (AL-105; AnshLabs) as previously described [20]. Media inhibin B concentrations were assayed using an Inhibin B ELISA Test kit (DSL-10-84100; Diagnostic Systems Laboratories, Inc.) based on the manufacturers’ instructions by the Endocrine Technologies Core at ONPRC [25]. Inhibin B ELISA kit was validated for macaque follicle culture media, and measurement from blank media without culture was used for background subtraction. The assay sensitivity was 10 pg/ml, and the standard curve of the assay ranged 15–1000 pg/ml.
GC proliferation and gonadotropin receptor expression with IGF2 supplementation
The direct action of IGF2 on follicular cell proliferation was further examined in GC culture, as previously described [26]. Briefly, GCs were collected via follicle aspiration from 3 animals by the Assisted Reproductive Technologies Core at ONPRC, and were pelleted by centrifugation at 170 g. Cells were resuspended in Ham F10 medium containing 25 mM HEPES and 0.1% (w/v) BSA (pH 7.4). Red blood cell contaminates were removed by Percoll (Sigma–Aldrich) gradient centrifugation at 470 g for 30 min. The GC fraction was isolated and diluted at 1:5 (v/v) in Ham F-10/0.1% BSA medium to remove contaminating Percoll. A centrifugation was performed at 170 g to pellet GCs, and the cell viability was assessed by trypan blue (Sigma–Aldrich) dye exclusion. Cells were cultured in fibronectin-coated 24-well plates (70 000–100 000 cells per well; 12 wells/animal) with DMEM/F12 medium containing 15 mM HEPES, 100 U penicillin/100 μg/ml streptomycin, and 10% (v/v) fetal bovine serum (Heat-inactivated; R&D systems) at 37°C and 5% CO2 for 6 days. Media was changed every 24 h. GCs were then deluteinized to recapitulate periovulatory outcomes as previously described [27]. Briefly, GCs were cultured in serum-free media at 37°C and 5% CO2 for 72 h. DMEM/F12 medium without fetal bovine serum was supplemented with 1 × insulin/transferrin/sodium selenite (ITS) liquid media supplement (Sigma–Aldrich), 0.028 mg/ml low-density lipoprotein, 0.002 mg/ml aprotonin, and 2.5 ng/ml FSH. GCs from each of the three animals were then randomly assigned to four groups (three wells/animal/group) for 72 h of IGF2 dose–response culture: (a) 0 ng/ml (control), (b) 10 ng/ml, (c) 50 ng/ml, and (d) 250 ng/ml recombinant human IGF2. Media was changed every 24 h.
GC images were obtained for cell proliferation analysis. GCs were counted using ImageJ software as previously described [28]. GCs were then harvest and total RNA was extracted using a ReliaPrep RNA Cell Miniprep System (Promega Corporation). RNA was reverse-transcribed into cDNA using a GoScript Reverse Transcription System (Promega Corporation). Quantitative real-time PCR was performed using the TaqMan Gene Expression Assays and Applied Biosystems 7900HT Fast Real-time PCR System (Thermo Fisher Scientific) for genes that are responsible for gonadotropin signaling (FSH receptor, FSHR: Rh01026045_m1; luteinizing hormone/choriogonadotropin receptor, LHCGR: Hs00174885_m1), as previously described [29]. Mitochondrial ribosomal protein S10 (MRPS10) served as the internal control.
Statistical analysis
Statistical analysis was performed using SPSS Version 22 software (IBM). Due to the skewed distributions, a mixed model was used for analyses of follicle survival, growth, and media paracrine factor concentrations with rank-based analysis or logarithmic transformation applied. One-way ANOVA followed by the Student–Newman–Keuls post hoc test was performed to compare media IGF2 concentrations over time or between culture groups, as well as GC culture data. Differences are considered significant at P < 0.05 and values are presented as the mean ± SEM. Correlation between IGF2 production and antral follicle growth were analyzed using Pearson correlation coefficient.
Results
IGF1 is not produced by macaque follicles developed in vitro
For follicles that survived the 5 weeks of culture, three distinct cohorts were observed in every culture group, in spite of steroid treatment, based on antrum formation and follicle diameters as previously reported [20]. The cohort that remained at the preantral stage with diameter < 250 μm at week 5 was termed “no-grow” follicles. Another cohort formed an antrum at week 3 with diameter reaching 250–500 μm at week 5 and was termed “slow-grow” follicles. The third cohort increased diameter markedly to >500 μm at week 5 after antrum formation at week 3 and was termed “fast-grow” follicles (Figure 1).
Figure 1.
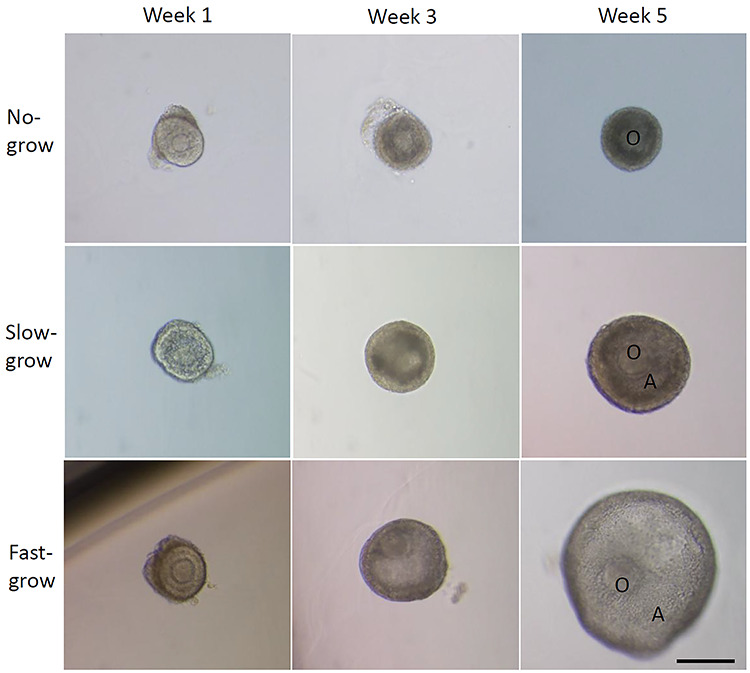
Three distinct follicle cohorts during 5 weeks of control culture. O, oocyte; A, antrum. Scale bar = 200 μm.
Media samples from culture week 1, 3, and 5 were analyzed for IGF1 concentrations produced by no-, slow-, and fast-grow follicles from the two control groups of steroid replacement experiments (19–23 follicles from 10 animals for each follicle cohort). IGF1 production was undetectable in the media regardless of culture weeks or follicle cohorts.
IGF2 production by macaque follicles developed in vitro is regulated by steroids
Media samples from culture week 1, 3, and 5 of the same no-, slow-, and fast-grow follicles from the two control groups of steroid replacement experiments were also analyzed for IGF2 concentrations. IGF2 production was undetectable in the media from no-grow follicles regardless of culture weeks. Although IGF2 production was undetectable in the media from slow-grow follicles at week 1 (preantral stage) and 3 (antrum formation), it became evident at week 5 (antral stage) (Figure 2A). IGF2 produced by fast-grow follicles was undetectable in the media at week 1 (preantral stage), became evident at week 3 (antrum formation), and further increased (P < 0.05) at week 5 (antral stage) (Figure 2B). At week 5, media IGF2 concentrations were higher (P < 0.05) in fast-grow follicles than those of slow-grow follicles (Figure 2A).
Figure 2.
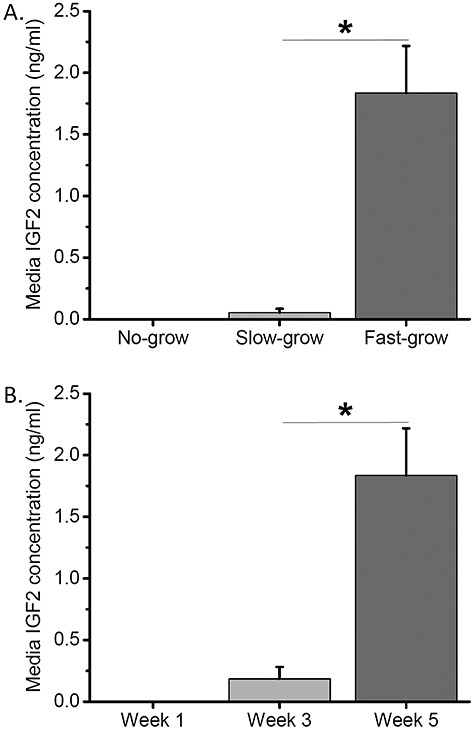
Insulin-like growth factor 2 (IGF2) production by follicles cultured under control conditions. (A) Media IGF2 concentrations at culture week 5 were plotted for no-grow (n = 19), slow-grow (n = 21), and fast-grow (n = 23) follicles. (B) Media IGF2 concentrations at culture week 1, 3, and 5 were plotted for fast-grow follicles (n = 23). *, significant difference between follicle cohorts (A) or culture weeks (B), P < 0.05. Data from 10 animals are presented as the mean ± SEM. One-way ANOVA followed by the Student–Newman–Keuls post hoc test was performed to compare media IGF2 concentrations between follicle cohorts (A) or over time (B).
Media samples from culture week 5 (antral stage) were analyzed for IGF2 concentrations produced by fast-grow follicles with steroid depletion by TRL followed by E2 or P replacement. Media IGF2 concentrations were lower (P < 0.05) in TRL-treated follicles than those of the control follicles (Figure 3A). Although E2 addition restored media IGF2 to the control levels, the addition of P increased (P < 0.05) media IGF2 concentrations compared with those of the control group (Figure 3A). Media samples from culture week 5 were also analyzed for IGF2 concentrations produced by fast-grow follicles with steroid depletion by TRL followed by DHT replacement. Similar to E2/P replacement experiment, TRL treatment decreased (P < 0.05) media IGF2 concentrations relative to those of the control group (Figure 3B). DHT addition restored media IGF2 to the control levels, which were higher (P < 0.05) than those of the TRL group (Figure 3B).
Figure 3.
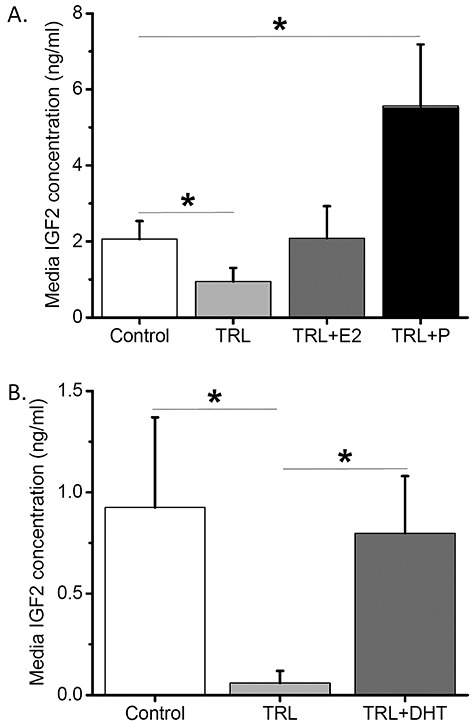
Steroid-regulated insulin-like growth factor 2 (IGF2) production by fast-grow follicles at culture week 5. (A) Media IGF2 concentrations were plotted for the control (n = 12), steroid depletion (TRL, trilostane; n = 15), and estradiol (E2; n = 8)/progesterone (P; n = 10) replacement culture groups. (B) Media IGF2 concentrations were plotted for the control (n = 11), steroid depletion (n = 6), and androgen (DHT, dihydrotestosterone; n = 9) replacement culture groups. *, significant difference between culture groups (A, B), P < 0.05. Data from six (A) or four (B) animals are presented as the mean ± SEM. One-way ANOVA followed by the Student–Newman–Keuls post hoc test was performed to compare media IGF2 concentrations between culture groups (A, B).
IGF2 promotes macaque follicle development and GC proliferation in vitro
Because antral follicles express the type I IGF receptor which mediates IGF2 actions [1, 2], and cultured follicles secreted IGF2 at the antral stage, the association between endogenous IGF2 production levels and antral follicle growth was assessed. Media IGF2 concentrations produced by fast-grow follicles from the two control groups of steroid replacement experiments were analyzed for their relationship with follicle diameters at culture week 5 (antral stage). A significant positive correlation (r = 0.58, P < 0.005) was observed between media IGF2 concentrations and antral follicle diameters (Figure 4).
Figure 4.
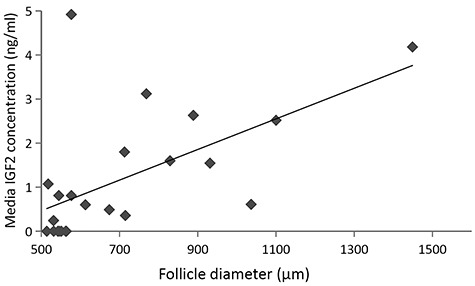
Correlation between insulin-like growth factor 2 (IGF2) production and antral follicle growth in the control culture. Media IGF2 concentrations and follicle diameters at culture week 5 were plotted for fast-grow follicles (n = 23). Data from 10 animals are presented. Correlation between IGF2 production and antral follicle growth were analyzed using Pearson correlation coefficient.
Preantral follicles also express the type I IGF receptor, which could respond to IGF2 [1, 2]. Because cultured follicles did not secrete IGF2 at the preantral stage, IGF2 actions on preantral follicle viability, growth, and specific paracrine factor production were assessed by administration of bioactive IGF2 protein. The percentages of follicles that survived at culture week 2, when follicles were still at the preantral stage, was 25% greater (P < 0.05) in the presence of IGF2 than those of the control group (85 ± 6 versus 68 ± 3%). Diameters of surviving follicles increased (P < 0.05) at week 2 relative to those of week 1 regardless of culture conditions (Figure 5A). Although follicles exhibited equivalent sizes at week 1 (preantral stage), growing follicles attained larger (P < 0.05) diameters at week 2 in the IGF2 group compared with those cultured in the control conditions (Figure 5A). Surviving preantral follicles produced appreciable amounts of paracrine factors, including AMH and inhibin B, into culture media as reported previously [20, 25]. IGF2 supplementation did not altered media AMH concentrations produced by follicles compared with those cultured in the control conditions at week 2 (Figure 5B). In contrast, media inhibin B concentrations produced by follicles were higher (P < 0.05) in the IGF2 group than those of the control group at week 2 (Figure 5B).
Figure 5.
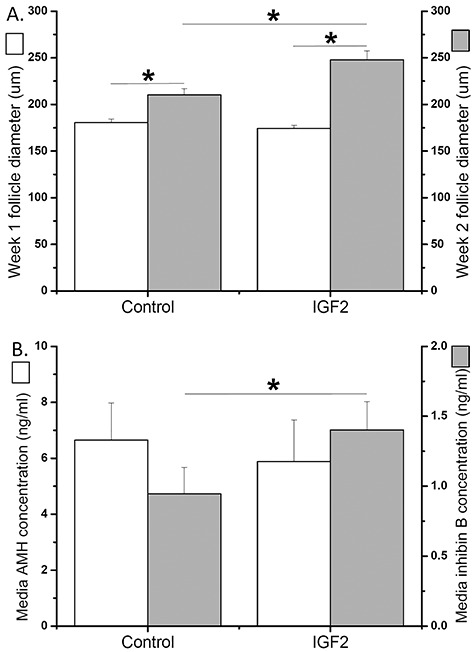
Insulin-like growth factor 2 (IGF2) effects during preantral follicle culture. (A) Follicle diameters at culture week 1 and 2 were plotted for follicles cultured without (control; n = 24) and with (n = 28) IGF2 addition. (B) Media anti-Müllerian hormone (AMH) and inhibin B concentrations at culture week 2 were plotted for follicles cultured without (n = 24) or with (n = 28) IGF2 addition. *, significant difference between culture weeks (A) or culture groups (A, B), P < 0.05. Data from three animals are presented as the mean ± SEM. A mixed model was used for analyses of follicle growth and media paracrine factor concentrations with logarithmic transformation applied.
In the presence of FSH, the number of cultured GCs was greater (P < 0.05) with IGF2 supplementation at 50 ng/ml and 250 ng/ml, but not at 10 ng/ml, compared with that of the control group (0 ng/ml) (Figure 6A). The mRNA levels of neither FSHR nor LHCGR were different between culture groups regardless of IGF2 doses (Figure 6B and6C).
Figure 6.
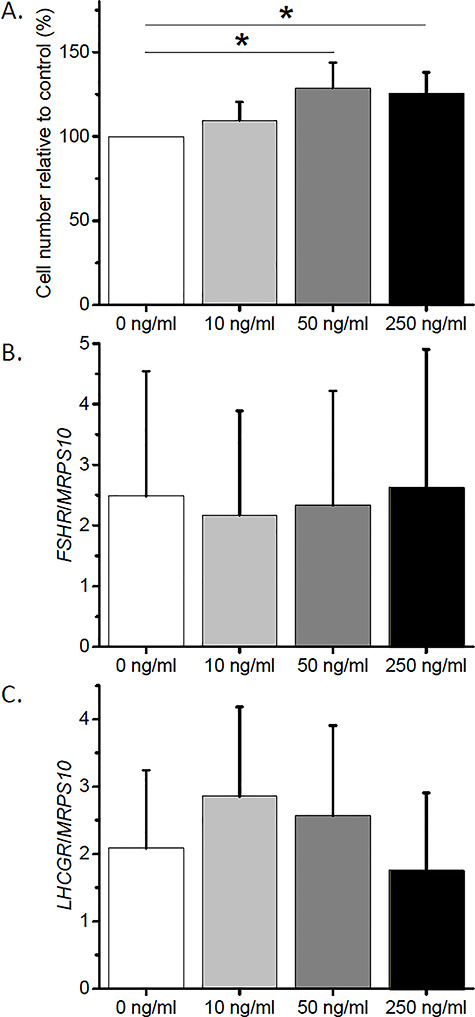
Dose–response effect of insulin-like growth factor 2 (IGF2) on proliferation and gene expression of cultured granulosa cells (GCs). (A) GC proliferation was assessed by cell counting. (B and C) GC follicle-stimulating hormone receptor (FSHR) and luteinizing hormone/choriogonadotropin receptor (LHCGR) mRNA levels were determined by real-time PCR. Mitochondrial ribosomal protein S10 (MRPS10) served as the internal control. *, significant difference between culture groups, P < 0.05. Data from three animals are presented as the mean ± SEM. One-way ANOVA followed by the Student–Newman–Keuls post hoc test was performed to compare GC proliferation and gene expression between culture groups.
Discussion
The present study investigated the IGF production patterns in developing macaque follicles. The results provide the first evidence that IGF2 is the major IGF ligand produced dynamically by growing primate follicles, which appears to depend on or be regulated by ovarian steroid hormones. For the first time, IGF2-associated follicular growth and function were assessed in individual follicles at specific developmental stages. Data suggest that IGF2 plays a stimulatory role during primate preantral follicle development, and antral follicles produce IGF2 as a function of developmental progress.
IGF1 production was not evident in primate follicles developed in vitro in the presence of FSH. Neither preantral nor antral follicles secreted detectable levels of IGF1 into the media over 5 weeks of culture. Data are consistent with previous observations in in vivo-developed follicles of ovarian tissue collected from reproductive-age women without endocrinological or ovarian pathology [9, 30]. Microarray analysis did not detect IGF1 gene expression in preantral follicles isolated from human ovarian tissue [30]. Although IGF1 mRNA was localized in small antral follicles by in situ hybridization, IGF1 protein was not expressed in either small or large antral follicles in the human ovary as indicated by immunohistochemistry [9]. Similarly, in situ hybridization showed very scarce IGF1 gene expression in the ovary of normal-cycling female rhesus macaques [12], while data on IGF1 protein translation was not reported. Therefore, locally produced IGF1 is not likely to play a major role in the primate ovary. Nevertheless, effects from circulating IGF1 on the ovary cannot be ruled out, though IGF1 is not the major circulating IGF ligand in primate species [3].
In contrast to IGF1, in vitro-developed primate follicles exhibited stage-dependent IGF2 production in the presence of FSH. Preantral follicles did not secret detectable levels of IGF2 into the media, including no-grow follicles over 5 weeks of culture and slow−/fast-grow follicles at culture week 1 (preantral stage). IGF2 production became evident after antral formation and increased as antral follicles grew larger, which was correlated positively with follicle diameters, as shown in slow- and fast-grow follicles at culture week 3 (antral formation) and 5 (antral stage). Consistent with the present findings, though IGF2 mRNA was detected in in vivo-developed preantral and antral follicles in the human ovary [9, 30], IGF2 protein expression was only localized in theca and GCs of small and large antral follicles, respectively, with its corresponding mRNA [9]. Furthermore, a positive correlation was observed between IGF2 levels in the follicular fluid and diameters of antral follicles in healthy reproductive-age women [31], though follicular fluid IGF2 could be of both ovarian and systemic origin. Thus, locally produced IGF2 may act as a paracrine/autocrine factor in regulating follicular development and function in the primate ovary.
IGF2 production by primate follicles appeared to be associated with ovarian steroid hormones. In a steroid-depleted milieu, IGF2 levels secreted by in vitro-developed antral follicles decreased significantly. Follicular IGF2 production could be restored to the control levels by either estrogen or androgen supplementation, and be markedly increased by progesterone treatment. The positive correlations between steroid hormone and IGF2 production are supported by findings of previous studies using rhesus macaque follicle culture. Similar to the IGF2 production pattern, estradiol, progesterone, and androstenedione production increased as antral follicles grew larger in vitro with peak levels achieved at culture week 5 [20]. Although there is a lack of information regarding steroid-regulated, especially androgen-regulated, IGF2 production in the ovary, igf2 mRNA levels increased in the white muscle of female fish following in vivo estradiol treatment and in a rat pituitary cell line cultured with estradiol as determined by real-time PCR [32, 14]. In adult female rats, igf2 gene expression decreased in the hippocampus following ovariectomy, which was restored to levels of the intact animals by subcutaneous estradiol administration [14]. Intravaginal administration of progesterone increased IGF2 protein levels in the endometrium of cycling heifers as shown by immunohistochemistry [15]. Collectively, these data suggest that IGF2 production is likely steroid-dependent or regulated by steroid hormones in the ovary. More in-depth studies are needed to further unravel the interaction between steroid hormone actions and follicular IGF2 production in the ovary, especially the relatively potent stimulatory effects from progesterone.
IGF2 exhibited stimulatory actions at the early stage of follicular development in primates. For in vitro-developed preantral follicles, the addition of IGF2 enhanced follicle viability as indicated by improved survival rates. IGF2 treatment also promoted follicle growth as suggested by increased follicle diameters and production of inhibin B. Inhibin B is a paracrine factor secreted by preantral follicles, which is a sensitive marker for GC proliferation in early stage follicles developed in vitro as shown in individually cultured human and mouse follicles [25, 33]. Although the direct actions of IGF2 on follicular development and function are poorly understood, particularly in preantral follicles, the existing data are consistent with the present findings. During long-term culture of ovarian cortical tissue, follicle death was prevented by IGF2 supplementation, suggesting that IGF2 could act as a survival factor for early stage follicles [34]. Preantral follicles with actively proliferating GCs were identified in IGF2-treated ovarian tissue in culture [34]. IGF2 was also reported to promote follicular cell proliferation in fish [35]. Because GCs of primate preantral follicles express type I IGF receptor [1, 3], which mediates biological activities of IGF2, IGF2 originating from the circulation and antral follicles could generate regulatory effects on preantral follicles as an endocrine and paracrine factor, respectively.
Because IGF2 is produced by antral follicles, which may complicate exogenous IGF2 treatment results, futures studies could be performed to determine the direct IGF2 action on antral follicle development and function by IGF2-knockdwon and replacement. Nevertheless, when cultured GCs were treated with IGF2 in the presence of FSH, the cell proliferation was enhanced in a dose-dependent manner. Data are consistent with previous evidence that IGF2 synergized with FSH to increase cell proliferation during human GC culture [13]. IGF2-induced cell proliferation, as determined by increased DNA replication during the cell cycle, was observed in cultured GCs from adult female possum ovaries during the breeding season [36]. IGF2 was also suggested to act alongside FSH to promote gonadotropin-supported cell viability and function by increasing FSHR and LHCGR expression in cultured human GCs [37]. However, differences in FSHR and LHCGR mRNA levels were not identified after IGF2 treatment in the present study, which could be due to the individual differences between animals. Future studies with increased sample size and protein level assessment are needed to further investigate the molecular mechanism of IGF2 action in stimulating GC proliferation, as well as follicular development and function.
In summary, the present study demonstrates the stage-dependent IGF2 secretion by primate ovarian follicles, as well as its association with and direct actions on follicular development. Locally produced IGF2 may act on preantral follicles in a paracrine fashion, and on antral follicles in an autocrine manner, to promote follicle growth and function. Further studies are warranted to identify the cell origin of IGF2 production in the antral follicle, IGF2-mediated steroid actions on antral follicle growth and oocyte maturation, as well as ovarian IGF2 signaling regulation involving IGF receptors, binding proteins, and degrading enzymes [1]. Because IGF system component expression and actions are different between polyovulatory and mono-ovulatory species [3, 4], the nonhuman primate model serves as an adequate surrogate for understanding IGF-regulated follicular development and ovarian function in women.
Acknowledgments
We are grateful for the assistance provided by members of the Division of Comparative Medicine, the Pathology Services Unit, and the Assisted Reproductive Technologies Core, the Endocrine Technologies Core, and the Molecular Technologies Core at the Oregon National Primate Research Center, Oregon Health & Science University.
Grant support: This research was supported by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) P50 HD071836 (National Center for Translational Research in Reproduction and Infertility, NCTRI), NIH/NICHD R01HD082208, NIH Office of Research on Women's Health/NICHD K12HD043488 (Building Interdisciplinary Research Careers in Women's Health, BIRCWH), and NIH Office of the Director P51OD011092. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Olena Y Tkachenko, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, OR, USA.
Shally Wolf, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, OR, USA.
Maralee S Lawson, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, OR, USA.
Alison Y Ting, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, OR, USA.
Jhenifer K Rodrigues, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, OR, USA.
Fuhua Xu, Department of Obstetrics and Gynecology, School of Medicine, Oregon Health & Science University, OR, USA.
Cecily V Bishop, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, OR, USA; Department of Animal and Rangeland Sciences, College of Agriculture, Oregon State University, OR, USA.
Richard L Stouffer, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, OR, USA; Department of Obstetrics and Gynecology, School of Medicine, Oregon Health & Science University, OR, USA.
Jing Xu, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, OR, USA; Department of Obstetrics and Gynecology, School of Medicine, Oregon Health & Science University, OR, USA.
Conflict of interest
The authors do not have any potential or actual conflicts of interest with respect to the work reported in the article.
Authors’ contributions
J.X. and R.S. contributed to experimental design and data interpretation; O.T., S.W., M.L., A.T., J.R., F.X., and C.B. performed experiments. O.T., C.B, and J.X. performed data analysis. O.T. and J. X. wrote the manuscript. All authors contributed to critical manuscript revising for important intellectual content and approved the final version of the manuscript to be submitted for publication.
References
- 1. Gougeon A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr Rev 1996; 17:121–155. [DOI] [PubMed] [Google Scholar]
- 2. O'Dell SD, Day IN. Molecules in focus insulin-like growth factor II (IGF-II). Int J Biochem Cell Biol 1998; 30:767–771. [DOI] [PubMed] [Google Scholar]
- 3. Monget P, Bondy C. Importance of the IGF system in early folliculogenesis. Mol Cell Endocrinol 2000; 163:89–93. [DOI] [PubMed] [Google Scholar]
- 4. Dor J, Ben-Shlomo I, Lunenfeld B, Pariente C, Levran D, Karasik A, Seppälä M, Mashiach S. Insulin-like growth factor-I (IGF-I) may not be essential for ovarian follicular development: Evidence from IGF-I deficiency. J Clin Endocrinol Metab 1992; 74:539–542. [DOI] [PubMed] [Google Scholar]
- 5. Wandji SA, Wood TL, Crawford J, Levison SW, Hammond JM. Expression of mouse ovarian insulin growth factor system components during follicular development and atresia. Endocrinology 1998; 139:5205–5214. [DOI] [PubMed] [Google Scholar]
- 6. Oliver JE, Aitman TJ, PowellL JF, Wilson CA, Clayton RN. Insulin-like growth factor I gene expression in the rat ovary is confined to the granulosa cells of developing follicles. Endocrinology 1989; 124:2671–2679. [DOI] [PubMed] [Google Scholar]
- 7. Perks CM, Denning-Kendall PA, Gilmour RS, Wathes DC. Localization of messenger ribonucleic acids for insulin-like growth factor I (IGF-I), IGF-II, and the type I receptor in the ovine ovary throughout the estrous cycle. Endocrinology 1995; 136:5266–5273. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong DG, Gutierrez CG, Baxter G, Glazyrin AL, Mann GE, Woad KJ, Hogg CO, Webb R. Expression of mRNA encoding IGF-I, IGF-II and type 1 IGF receptor in bovine ovarian follicles. J Endocrinol 2000; 165:101–113. [DOI] [PubMed] [Google Scholar]
- 9. el-Roeiy A, Chen X, Roberts VJ, LeRoith D, Roberts CT, Jr.,Yen SS. Expression of insulin-like growth factor-I (IGF-I) and IGF-II and the IGF-I, IGF-II, and insulin receptor genes and localization of the gene products in the human ovary. J Clin Endocrinol Metab 1993, 77:1411–1418. [DOI] [PubMed] [Google Scholar]
- 10. Yu Y, LiW HZ, Luo M, Chang Z, Tan J. The effect of follicle-stimulating hormone on follicular development, granulosa cell apoptosis and steroidogenesis and its mediation by insulin-like growth factor-I in the goat ovary. Theriogenology 2003; 60:1691–1704. [DOI] [PubMed] [Google Scholar]
- 11. Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator-activated receptor-gamma mediates bisphenol a inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect 2010; 118:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod 1999; 14:2328–2332. [DOI] [PubMed] [Google Scholar]
- 13. Baumgarten SC, Convissar SM, Zamah AM, Fierro MA, Winston NJ, Scoccia B, Stocco CFSH. Regulates IGF-2 expression in human granulosa cells in an AKT-dependent manner. J Clin Endocrinol Metab 2015; 100:E1046–E1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takeo C, Ikeda K, Horie-Inoue K, Inoue S. Identification of Igf2, Igfbp2 and Enpp2 as estrogen-responsive genes in rat hippocampus. Endocr J 2009; 56:113–120. [DOI] [PubMed] [Google Scholar]
- 15. McCarthy SD, Roche JF, Forde N. Temporal changes in endometrial gene expression and protein localization of members of the IGF family in cattle: effects of progesterone and pregnancy. Physiol Genomics 2012; 44:130–140. [DOI] [PubMed] [Google Scholar]
- 16. Yong EL, Baird DT, Yates R, Reichert LE Jr, Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab 1992; 74:842–849. [DOI] [PubMed] [Google Scholar]
- 17. Bergh C, Olsson JH, Hillensjo T. Effect of insulin-like growth factor I on steroidogenesis in cultured human granulosa cells. Acta Endocrinol 1991; 125:177–185. [DOI] [PubMed] [Google Scholar]
- 18. Kubota T, Kamada S, Ohara M, Taguchi M, Sakamoto S, Shimizu Y, Aso T. Insulin-like growth factor II in follicular fluid of the patients with in vitro fertilization and embryo transfer. Fertil Steril 1993; 59:844–849. [DOI] [PubMed] [Google Scholar]
- 19. Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod 1995; 10:75–81. [DOI] [PubMed] [Google Scholar]
- 20. Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod 2011; 26:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J, Xu F, Lawson MS, Tkachenko OY, Ting AY, Kahl CA, Park BS, Stouffer RR, Bishop CV. Anti-Müllerian hormone is a survival factor and promotes the growth of rhesus macaque preantral follicles during matrix-free culture. Biol Reprod 2018, 98:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duffy DM, Molskness TA, Stouffer RL. Progesterone receptor messenger ribonucleic acid and protein in luteinized granulosa cells of rhesus monkeys are regulated in vitro by gonadotropins and steroids. Biol Reprod 1996; 54:888–895. [DOI] [PubMed] [Google Scholar]
- 23. Ting AY, Xu J, Stouffer RL. Differential effects of estrogen and progesterone on development of primate secondary follicles in a steroid-depleted milieu in vitro. Hum Reprod 2015; 30:1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodrigues JK, Navarro PA, Zelinski MB, Stouffer RL, Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Müllerian hormone by individual macaque follicles during three-dimensional culture. Hum Reprod 2015; 30:664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009; 24:2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bishop CV, Hennebold JD, Kahl C, Stouffer RL. Knockdown of progesterone receptor (PGR) in macaque granulosa cells disrupts ovulation and progesterone production. Biol Reprod 2016; 94:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hannon PR, Duffy DM, Rosewell KL, Brannstrom M, Akin JW, Curry Jr. TE. Ovulatory induction of SCG2 in human, nonhuman primate, and rodent granulosa cells stimulates ovarian angiogenesis. Endocrinology 2018, 159:2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Venter C, Niesler CU. Rapid quantification of cellular proliferation and migration using ImageJ. Biotechniques 2019; 66:99–102. [DOI] [PubMed] [Google Scholar]
- 29. Xu J, Lawson MS, Xu F, Du Y, Tkachenko OY, Bishop CV, Pejovic-Nezhat L, Seifer DB, Hennebold JD. Vitamin D3 regulates follicular development and intrafollicular vitamin D biosynthesis and signaling in the primate ovary. Front Physiol 2018; 9:1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kristensen SG, Ebbesen P, Andersen CY. Transcriptional profiling of five isolated size-matched stages of human preantral follicles. Mol Cell Endocrinol 2015; 401:189–201. [DOI] [PubMed] [Google Scholar]
- 31. Thierry van Dessel HJ, Chandrasekher Y, Yap OW, Lee PD, Hintz RL, Faessen GH, Braat DD, Fauser BC, Giudice LC. Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab 1996; 81:1224–1231. [DOI] [PubMed] [Google Scholar]
- 32. Cleveland BM, Weber GM. Effects of sex steroids on expression of genes regulating growth-related mechanisms in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 2015; 216:103–115. [DOI] [PubMed] [Google Scholar]
- 33. Smitz J, Cortvrindt R. Inhibin A and B secretion in mouse preantral follicle culture. Hum Reprod 1998; 13:927–935. [DOI] [PubMed] [Google Scholar]
- 34. Louhio H, Hovatta O, Sjöberg J, Tuuri T. The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long-term culture. Mol Hum Reprod 2000; 6:694–698. [DOI] [PubMed] [Google Scholar]
- 35. Baroiller JF, D'Cotta H, Shved N, Berishvili G, Toguyeni A, Fostier A, Eppler E, Reinecke M. Oestrogen and insulin-like growth factors during the reproduction and growth of the tilapia Oreochromis niloticus and their interactions. Gen Comp Endocrinol 2014; 205:142–150. [DOI] [PubMed] [Google Scholar]
- 36. Juengel JL, Haydon LJ, Mester B, Thomson BP, Beaumont M, Eckery DC. The role of IGFs in the regulation of ovarian follicular growth in the brushtail possum (Trichosurus Vulpecula). Reproduction 2010; 140:295–303. [DOI] [PubMed] [Google Scholar]
- 37. Hensen K, Pook M, Sikut A, Org T, Maimets T, Salumets A, Kurg A. Utilising FGF2, IGF2 and FSH in serum-free protocol for long-term in vitro cultivation of primary human granulosa cells. Mol Cell Endocrinol 2020; 510:110816. [DOI] [PubMed] [Google Scholar]


