Abstract
Aims
The European Society of Cardiology (ESC) European Observational Research Programme (EORP) Cardiomyopathy Registry is a prospective multinational registry of consecutive patients with cardiomyopathies. The objective of this report is to describe the short-term outcomes of adult patients (≥18 years old).
Methods and results
Out of 3208 patients recruited, follow-up data at 1 year were obtained in 2713 patients (84.6%) [1420 with hypertrophic (HCM); 1105 dilated (DCM); 128 arrhythmogenic right ventricular (ARVC); and 60 restrictive (RCM) cardiomyopathies]. Improvement of symptoms (dyspnoea, chest pain, and palpitations) was globally observed over time (P < 0.05 for each). Additional invasive procedures were performed: prophylactic implantation of implantable cardioverter-defibrillator (ICD) (5.2%), pacemaker (1.2%), heart transplant (1.1%), ablation for atrial or ventricular arrhythmia (0.5% and 0.1%). Patients with atrial fibrillation increased from 28.7% to 32.2% of the cohort. Ventricular arrhythmias (VF/ventricular tachycardias) in ICD carriers (primary prevention) at 1 year were more frequent in ARVC, then in DCM, HCM, and RCM (10.3%, 8.2%, 7.5%, and 0%, respectively). Major cardiovascular events (MACE) occurred in 29.3% of RCM, 10.5% of DCM, 5.3% of HCM, and 3.9% of ARVC (P < 0.001). MACE were more frequent in index patients compared to relatives (10.8% vs. 4.4%, P < 0.001), more frequent in East Europe centres (13.1%) and least common in South Europe (5.3%) (P < 0.001). Subtype of cardiomyopathy, geographical region, and proband were predictors of MACE on multivariable analysis.
Conclusions
Despite symptomatic improvement, patients with cardiomyopathies remain prone to major clinical events in the short term. Outcomes were different not only according to cardiomyopathy subtypes but also in relatives vs. index patients, and according to European regions.
Keywords: Cardiomyopathy, Registry, Prognosis, MACE
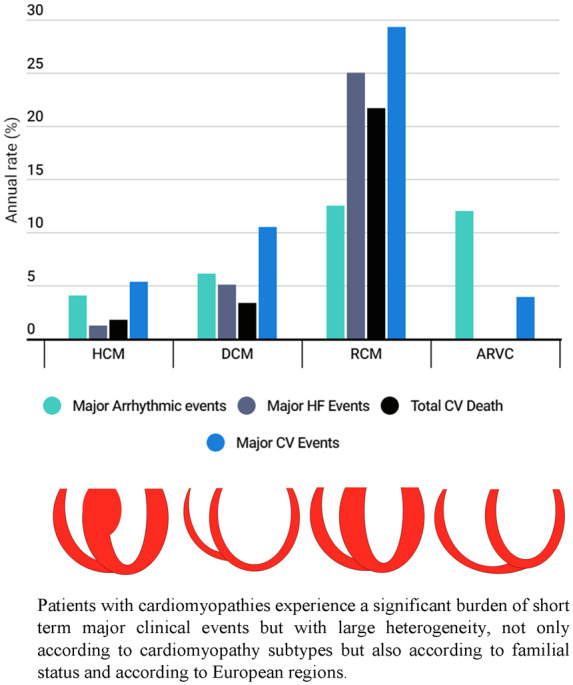
Graphical Abstract
Graphical Abstract.
Introduction
Cardiomyopathies are a heterogeneous group of disorders characterized by structural and functional abnormalities of the myocardium that are unexplained solely by coronary artery disease or abnormal loading conditions.1 Individually, the various subtypes of cardiomyopathy are relatively uncommon, but collectively they represent a major health burden for the European population.2–8 All cardiomyopathies can cause premature death from arrhythmia and progressive heart failure.2,4–9
To date, most information about the presentation and natural history of cardiomyopathies in adults has come from retrospective cohort studies in a few centres and without considering all cardiomyopathy subtypes together. The European Society of Cardiology (ESC) launched the European Observational Research Programme (EORP) in 2009 with the explicit aim of improving the understanding of medical practice through prospective collection of observational data in patients with heart muscle disease recruited in centres across Europe9 (https://www.escardio.org/Research/Registries-&-surveys/Observational-research-programme/Cardiomyopathy-and-Myocarditis-Registry). The baseline data on the adult population have been published.3 This second report describes clinical work-up and outcomes at 1-year follow-up of patients enrolled in the registry.
The primary aims of the follow-up phase of the registry were (i) to record the current practices for diagnostic workup and clinical follow-up of patients; (ii) to describe the therapeutic approaches implemented during the follow-up; and (iii) to report the major clinical events or complications during the follow-up.
Methods
Registry design and patients
Participating centres in each country were selected using pre-specified inclusion and exclusion criteria.3,9 Four major phenotypes of cardiomyopathy were eligible for inclusion: hypertrophic (HCM), dilated (DCM), arrhythmogenic right ventricular (ARVC), and restrictive (RCM) cardiomyopathies. Age at enrolment had to be ≥18 years old. Each centre was asked to enter about 40 consecutively assessed patients over a 12-month period. The study was approved by each local Ethical Committee according to the local rules. Written informed consent was obtained from all participants before data collection. All diagnostic or therapeutic procedures were left to the discretion of the attending physician. The registry was conducted by an Executive Committee and managed by the EORP department of the ESC which also performed statistical analyses. Definitions used for analyses of subgroups (including definition of regions) were previously detailed.3,9
A total of 3208 adult patients with a cardiomyopathy were enrolled in the pilot and long-term phases of the registry by 69 centres in 18 countries. There were two periods of inclusion: from 12 December to 13 November and from 14 June to 16 December.9 The cardiomyopathy subtypes were: HCM (n = 1739); DCM (n = 1260); ARVC (n = 143); and RCM (n = 66). Median age at enrolment was 55 (interquartile range 43–64).3,9
A follow-up at 1 year was planned by EORP, without additional follow-up period in this registry. Information was taken from clinical visits or clinical records.
Combined endpoints for outcomes were defined as:
Major arrhythmic event: sudden death or resuscitated ventricular fibrillation/cardiac arrest or sustained ventricular tachycardia (VT).
Major heart failure event: heart failure death or heart transplant or ventricular assist device implantation.
Vascular death: death due to acute myocardial infarction, ischaemic stroke, haemorrhagic stroke, pulmonary, or peripheral embolism.
Cardiovascular death: death due to arrhythmia, heart failure, or any cardiovascular cause (resuscitated cardiac arrest, other life-threatening arrhythmia, and transplants were excluded).
Major cardiovascular events (MACE): any type of cardiovascular death or hospital urgent admission for cardiac reason (combined endpoint n°4 + urgent cardiac admissions).
Statistical analysis
Univariable analysis was applied to both continuous and categorical variables. Continuous variables were reported as mean ± standard deviation. Among-group comparisons were made using a non-parametric test (Kruskal–Wallis test). For the comparisons of repeated measures, the sign test was used. Categorical variables were reported as counts and percentages. Among-group comparisons 2 × 2 were made using a χ2 test or Fisher’s exact test if any expected cell count was less than five. For the comparisons of repeated measures, the McNemar’s test was used.
Univariate Cox regression analysis and plots of Kaplan–Meier curves for the combined events were performed. Cox proportional hazards model was used for survival estimates reporting hazard ratios and 95% confidence intervals (95% CI’s).
A stepwise multivariable logistic regression analysis was performed to establish the relationship between the patient characteristics and the MACE including into the model all the candidate variables (P < 0.10 in univariate). A significance level of 0.05 was required to allow a variable into the model (SLENTRY = 0.05) and a significance level of 0.05 was required to stay in the model (SLSTAY = 0.05). No interaction was tested. A Hosmer and Lemeshow Goodness-of-Fit test was used to verify that the model was optimal.
Annual rates together with their 95% CI’s were estimated.
A two-sided P-value of <0.05 was considered as statistically significant. All analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Data collection
Follow-up data at 1-year [median 376 (363–438) days] were obtained in 2713 patients (84.6%), including 1420 (52.3%) with HCM, 1105 (40.7%) DCM, 128 (4.7%) ARVC, and 60 (2.2%) RCM. A total of 1050 (38.7%) patients were enrolled in the pilot and 1663 (61.3%) in the long-term phase. A total of 1543 (82.0% of those reported) were probands and 339 (18.0%) were relatives. A total of 1056 (39.6%) were incident (new cases) and 1607 (60.4%) prevalent cases. Regarding geographical areas, there were 533 (19.6%) from East, 512 (18.9%) North, 1193 (44.0%) South, and 452 (16.7%) West Europe. There were also 23 patients included from North Africa (0.85%).
Symptoms during follow-up
Overall the proportion of patients in New York Heart Association (NYHA) functional class III–IV decreased from 25.7% to 16.7% (P < 0.001) during follow-up compared to baseline. NYHA status improved in HCM, DCM, and ARVC but not in patients with RCM (Table 1). The proportion of patients with chest pain decreased from 26.7% to 12.5% during follow-up (P < 0.001). Suspected cardiogenic syncope was reported in 2.4% of patients during follow-up which was higher in ARVC (4.9%) and lower in RCM (1.9%).
Table 1.
Symptoms and examinations at 1-year follow-up for each type of cardiomyopathy
| HCM |
DCM |
RCM |
ARVC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Baseline (N = 1420) | FU (N = 1420) | P-value | Baseline (N = 1105) | FU (N = 1105) | P-value | Baseline (N = 60) | FU (N = 60) | P-value | Baseline (N = 128) | FU (N = 128) | P-value |
| Dyspnoea | ||||||||||||
| NYHA I | 401/1127 (35.58%) | 435/1127 (38.60%) |
0.041 |
172/845 (20.36%) | 255/845 (30.18%) |
<0.001 |
10/50 (20.00%) | 8/50 (16.00%) |
0.017 |
49/83 (59.04%) | 52/83 (62.65%) |
0.815 |
| NYHA II | 543/1127 (48.18%) | 529/1127 (46.94%) | 363/845 (42.96%) | 390/845 (46.15%) | 20/50 (40.00%) | 15/50 (30.00%) | 32/83 (38.55%) | 28/83 (33.73%) | ||||
| NYHA III | 175/1127 (15.53%) | 150/1127 (13.31%) | 245/845 (28.99%) | 168/845 (19.88%) | 19/50 (38.00%) | 18/50 (36.00%) | 2/83 (2.41%) | 3/83 (3.61%) | ||||
| NYHA IV | 8/1127 (0.71%) | 13/1127 (1.15%) | 65/845 (7.69%) | 32/845 (3.79%) | 1/50 (2.00%) | 9/50 (18.00%) | 0/83 (0.00%) | 0/83 (0.00%) | ||||
| Chest pain | 414/1217 (34.02%) | 185/1217 (15.20%) | <0.001 | 200/992 (20.16%) | 108/992 (10.89%) | <0.001 | 6/54 (11.11%) | 10/54 (18.52%) | 0.157 | 15/107 (14.02%) | 10/107 (9.35%) | 0.275 |
| Palpitations | 440/1217 (36.15%) | 298/1217 (24.49%) | <0.001 | 371/992 (37.40%) | 217/992 (21.88%) | <0.001 | 10/54 (18.52%) | 9/54 (16.67%) | 0.739 | 65/107 (60.75%) | 41/107 (38.32%) | <0.001 |
| Syncope (suspected arrhythmic/ cardiogenic) | 146/1172 (12.46%) | 27/1172 (2.30%) | <0.001 | 79/913 (8.65%) | 24/913 (2.63%) | <0.001 | 6/52 (11.54%) | 1/52 (1.92%) | 0.059 | 34/103 (33.01%) | 5/103 (4.85%) | <0.001 |
| Atrial fibrillation | 377/1372 (27.48%) | 48/995 (4.82%) | — | 323/1075 (30.05%) | 41/752 (5.45%) | — | 24/53 (45.28%) | 3/29 (10.34%) | — | 18/124 (14.52%) | 3/106 (2.83%) | — |
| Examinations | ||||||||||||
| ECG | 1357/1403 (96.72%) | 980/1403 (69.85%) | <0.001 | 1079/1092 (98.81%) | 743/1092 (68.04%) | <0.001 | 56/56 (100.00%) | 34/56 (60.71%) | — | 127/128 (99.22%) | 98/128 (76.56%) | <0.001 |
| Echocardiography | 1339/1403 (95.44%) | 855/1403 (60.94%) | <0.001 | 1060/1091 (97.16%) | 666/1091 (61.04%) | <0.001 | 53/56 (94.64%) | 22/56 (39.29%) | <0.001 | 122/128 (95.31%) | 74/128 (57.81%) | <0.001 |
| Holter ECG | 950/1404 (67.66%) | 477/1404 (33.97%) | <0.001 | 397/1092 (36.36%) | 156/1092 (14.29%) | <0.001 | 19/56 (33.93%) | 8/56 (14.29%) | 0.008 | 90/128 (70.31%) | 51/128 (39.84%) | <0.001 |
| Exercise test | 612/1404 (43.59%) | 235/1404 (16.74%) | <0.001 | 324/1092 (29.67%) | 125/1092 (11.45%) | <0.001 | 5/56 (8.93%) | 5/56 (8.93%) | 1.000 | 66/128 (51.56%) | 20/128 (15.63%) | <0.001 |
| MRI scan | 495/1404 (35.26%) | 89/1404 (6.34%) | <0.001 | 211/1092 (19.32%) | 31/1092 (2.84%) | <0.001 | 19/56 (33.93%) | 3/56 (5.36%) | <0.001 | 69/128 (53.91%) | 7/128 (5.47%) | <0.001 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; FU, follow-up; HCM, hypertrophic cardiomyopathy; RCM, restrictive cardiomyopathy.
Use of diagnostic tests
The utilization of cardiac investigations is summarized in Table 1. The majority of patients had an electrocardiogram (ECG) or echocardiogram performed at 1-year follow-up (69.2% and 60.4%, respectively). Ambulatory ECG monitoring was performed in a smaller proportion of patients (25.8%) and was reported more frequently in ARVC and HCM groups (39.8% and 34.0%, respectively) and less in DCM and RCM (14.3% and 14.3%, respectively) (P < 0.001). Use of cardiac magnetic resonance imaging (MRI) was significantly lower during follow-up compared to baseline evaluation (4.9% vs. 30.1%, P < 0.001). Invasive procedures like biopsy were performed only in 20 patients (0.7%) during follow-up.
Medication
Distribution and proportion of medication at 1 year were generally similar to baseline profile (Supplementary material online, Table S1). There was a significant increase in the global use of anticoagulants in HCM patients, whereas a decrease in the proportion of DCM patients on angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and diuretics was seen.
Non-pharmacological therapeutic procedures
One hundred and nine (5.2%) patients underwent implantable cardioverter-defibrillator (ICD) implantation for primary and 23 (1.0%) for secondary prevention during follow-up (Table 2). The proportion of new prophylactic ICD implantations was highest in ARVC (8.0%) then DCM (5.8%), HCM (4.9%), and RCM (0.0%).
Table 2.
Non-pharmacological therapies at baseline and at 1-year follow-up for each type of cardiomyopathy
| HCM |
DCM |
RCM |
ARVC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Baseline (N = 1420) | FU (N = 1420) | P-value | Baseline (N = 1105) | FU (N = 1105) | P-value | Baseline (N = 60) | FU (N = 60) | P-value | Baseline (N = 128) | FU (N = 128) | P-value |
| Pacemaker implanted | 97/1383 (7.01%) | 14/1286 (1.09%) | — | 46/1079 (4.26%) | 4/1033 (0.39%) | — | 8/54 (14.81%) | 0/46 (0.00%) | — | 3/125 (2.40%) | 0/122 (0.00%) | — |
| CRT | 8/1368 (0.58%) | 2/1360 (0.15%) | — | 113/1073 (10.53%) | 9/960 (0.94%) | — | 0/53 (0.00%) | 0/53 (0.00%) | — | 0/125 (0.00%) | 0/125 (0.00%) | — |
| ICD primary prophylaxis | 260/1401 (18.56%) | 56/1141 (4.91%) | — | 294/1091 (26.95%) | 46/797 (5.77%) | — | 1/56 (1.79%) | 0/55 (0.00%) | — | 39/127 (30.71%) | 7/88 (7.95%) | — |
| ICD secondary prophylaxis | 38/1328 (2.86%) | 0/1290 (0.00%) | — | 52/1028 (5.06%) | 0/976 (0.00%) | — | 3/56 (5.36%) | 0/53 (0.00%) | — | 31/119 (26.05%) | 0/88 (0.00%) | — |
| Ablation for atrial fibrillation | 33/1403 (2.35%) | 6/1370 (0.44%) | — | 14/1091 (1.28%) | 6/1077 (0.56%) | — | 1/56 (1.79%) | 1/55 (1.82%) | — | 1/127 (0.79%) | 0/126 (0.00%) | — |
| Ablation for ventricular tachycardia | 0/1403 (0.00%) | 1/1403 (0.07%) | — | 15/1091 (1.37%) | 1/1076 (0.09%) | — | 0/56 (0.00%) | 0/56 (0.00%) | — | 11/127 (8.66%) | 1/116 (0.86%) | — |
| Ventricular assist device | 0/777 (0.00%) | 1/777 (0.13%) | — | 18/778 (2.31%) | 15/760 (1.97%) | — | 0/32 (0.00%) | 0/32 (0.00%) | — | 0/73 (0.00%) | 0/73 (0.00%) | — |
| Heart Transplant | 0/1403 (0.00%) | 3/1403 (0.21%) | — | 6/1090 (0.55%) | 25/1084 (2.31%) | — | 0/56 (0.00%) | 2/56 (3.57%) | — | 0/127 (0.00%) | 0/127 (0.00%) | — |
Significance not applicable as differences in denominators from baseline to follow-up.
ARVC, arrhythmogenic right ventricular cardiomyopathy; CRT, cardiac resynchronization therapy; DCM, dilated cardiomyopathy; FU, follow-up; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter-defibrillator; RCM, restrictive cardiomyopathy.
The number of patients with a pacemaker implanted during follow-up was 18 (0.7%); 11 (0.4%) patients (9 DCM and 2 HCM) underwent cardiac resynchronization therapy device implantation.
Thirteen (0.49%) patients underwent atrial fibrillation (AF) ablation and 3 (0.11%) VT ablation procedures during follow-up (1.8% and 1.0% at baseline, respectively).
Sixteen patients (0.6%) underwent a ventricular assist device implantation for advance heart failure (15 DCM and 1 HCM patients).
Thirty-three (2.5%) patients with HCM underwent septal reduction procedures, including 23 surgical myectomies and 10 alcohol septal ablation procedures during follow-up.
Death and complications
There were 93 (3.4%) deaths, including 38 (40.9%) heart failure-related deaths, 24 (25.8%) sudden cardiac deaths (SCDs), 3 (3.2%) stroke-related deaths, 1 (1.1%) arrhythmic (non-sudden), 1 acute myocardial infarction, and 3 (3.2%) other cardiovascular deaths. Six (6.4%) deaths were procedure related. There were 7 (7.5%) unrelated with the disease and 10 (10.8%) unknown causes of death.
Thirty patients (1.1%) underwent heart transplantation, 39 (1.5%) were resuscitated from cardiac arrest and 40 (1.5%) had non-fatal stroke. There were 68 (10.6%) patients with ICD devices who developed ventricular arrhythmias (sustained VT/ventricular arrhythmia) (47/539 primary and 21/105 secondary prophylaxis).
The proportion of patients with new onset AF during follow-up was 5.1% (28.7% at baseline). New AF proportion tended to be higher in RCM 10.3% followed by 5.5% in DCM, 4.8% in HCM, and 2.8% in ARVC.
Ventricular arrhythmias (ventricular fibrillation or sustained VT) at 1 year in patients with ICD for primary prevention were more frequent in ARVC than in DCM, HCM, and RCM (10.3%, 8.2%, 7.5%, 0% respectively, NS). Similarly, rates of ventricular arrhythmias in ICD carriers for secondary prevention were higher in ARVC and DCM followed by HCM (32.3%, 15.1%, 5.1%, respectively, P = 0.015). There were only three patients with RCM who had history of cardiac arrest with an ICD implanted.
The various pre-defined combined endpoints for each cardiomyopathy subtype are detailed in Supplementary material online, Table S2 and Figure 1. MACE occurred in 29.3% of RCM, 10.5% of DCM, 5.3% of HCM, and 3.9% of ARVC (P < 0.001). RCM showed the highest annual rates for most of the combined events. ARVC showed a high proportion of major arrhythmic events, similar to RCM.
Figure 1.
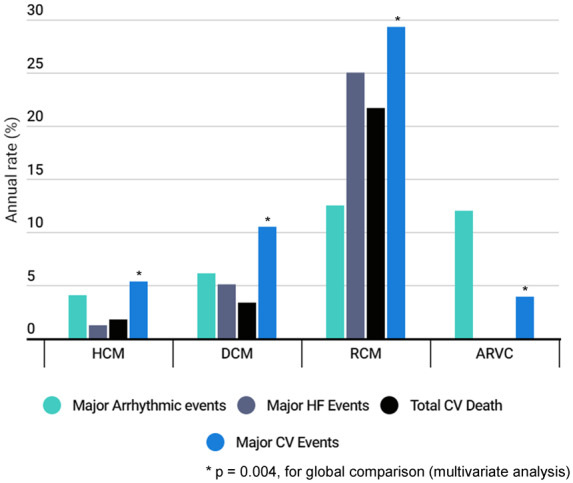
Rate of the combined events at 1-year follow-up for each subtype of cardiomyopathy. Combined endpoints as defined in methods. *P = 0.004, for global comparison (multivariate analysis).
Using Cox survival analysis, RCM [odds ratio (OR): 20.17; CI: 9.63; 42.27, P < 0.001] and DCM (OR: 4.27; CI: 2.48; 7.37, P < 0.001) showed an increased risk of reaching the heart failure combined event compared to HCM. DCM patients were at increased risk of combined arrhythmic event compared to HCM (OR: 2.37; CI: 1.05; 5.36, P = 0.039). Regarding total cardiovascular death, RCM (OR: 12.37; CI: 5.88; 26.01, P < 0.001) and DCM (OR: 1.95; CI: 1.15; 3.30, P = 0.014) were at highest risk as compared to HCM. Consistently, the OR for combined MACE was higher in RCM (5.68; CI: 3.20; 10.06, P < 0.001) and then in DCM (OR: 2.03; CI: 1.51; 2.73, P < 0.001) as compared to HCM. MACE in ARVC was not significantly different from HCM.
Comparison of probands and relatives
Outcomes in 1543 probands were compared to those of 339 relatives. Probands were significantly older at inclusion [median 55.0 (44.0; 64.0) years old] than relatives [47.0 (34.0; 58.0) years old, P < 0.001], and there were more males (67.0%) compared to relatives (54.6%), P < 0.001. During follow-up probands had a higher proportion of dyspnoea III–IV (19.7%) compared to relatives (7.9%), (P < 0.001) but a similar proportion of cardiogenic syncope (2.7% vs. 2.7%). All types of diagnostic tests during follow-up were more frequently performed in relatives compared to probands (ECG: 76.6% vs. 69.0%; echocardiogram: 68.3% vs. 60.3%, exercise test: 20.7% vs. 15.0% and Holter: 36.3% vs. 25.9%, all P ≤ 0.01). MRI was the only test with similar proportion of use during follow-up in probands and relatives (5.5% vs. 6.5%, P = 0.443).
Use of medication (beta-blockers, ACE inhibitors, angiotensin II receptor blockers, spironolactone, and anticoagulation) was significantly higher in probands compared to relatives (all P ≤ 0.05). Major arrhythmic events (5.7% vs. 3.6%, P = 0.119) were similar in probands and relatives. Major heart failure events (3.7% vs. 0.9%, P = 0.008), cardiovascular death (3.2% vs. 0.9%, P = 0.018), and combined MACE (10.8% vs. 4.4%, P < 0.001) were more common in probands (Figure 2).
Figure 2.
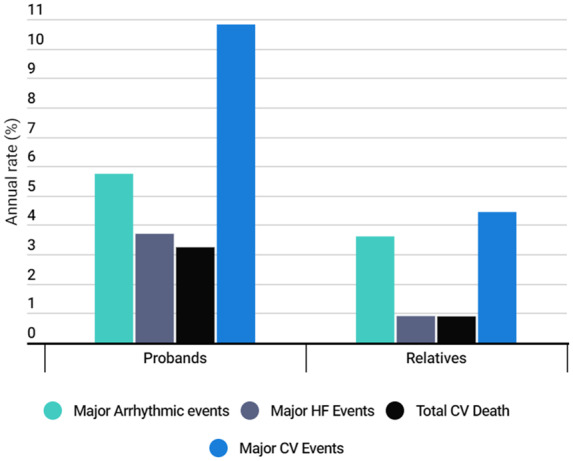
Rate of the combined events at 1-year follow-up regarding probands vs. relatives. Combined endpoints as defined in methods.
Geographical differences across Europe
Clinical characteristics and follow-up of patients regarding geographical areas are described in Supplementary material online, Table S3. There was a difference in age and in the proportion of probands between the five regions (P = 0.011, P < 0.0001, respectively). Patients were younger and there were more probands in the East.
Proportion of patients with dyspnoea (NYHA III/IV) and syncope during follow-up were different between areas (P < 0.001, P = 0.017). The rate of new implantation of devices was globally low in all geographical areas with no significant differences.
When considering combined MACE there were significant differences between areas with the highest rate in East Europe (13.1%) and lowest in South Europe (5.4%) (P < 0.001 for univariate analysis) (Figure 3).
Figure 3.
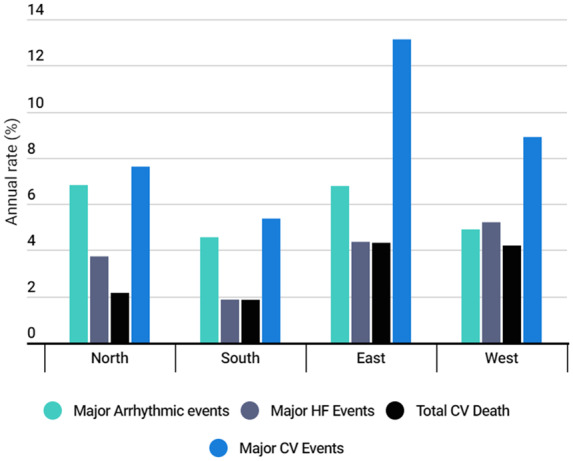
Rate of the combined events at 1-year follow-up regarding geographical areas. Combined endpoints as defined in methods.
The rate of sudden death and major arrhythmic events during follow-up was similar across Europe. Major heart failure events, as well as heart transplants, were higher in West and lowest in South area (P < 0.001, P < 0.001). Cardiovascular death was globally low during follow-up, being higher in the West and East compared to the North and South (P < 0.010) (Figure 3).
Multivariable analysis of variable associated with MACE
Variables with P < 0.10 in univariate were included in the multivariable analysis for predictors of MACE at 1 year, including cardiomyopathy subtype, proband or relative, and geographical region (Supplementary material online, Table S4).
Compared to the RCM group, ARVC, DCM, and HCM had better survival [OR: 0.126 (0.037–0.435), P < 0.001, OR: 0.313 (0.148–0.660), P = 0.002 and OR: 0.179 (0.084–0.381), P < 0.001, respectively]. Compared to South, East, and North European centres had a higher rate of MACE [OR: 2.057 (1.385–3.054), P < 0.001 and OR: 1.629 (1.022–2.597), P = 0.040]. Probands compared to relatives had an increased rate of MACE [OR: 1.828 (1.044–3.203), P = 0.035].
Discussion
The present work reports unique data regarding the management, follow-up and outcomes of adult patients with cardiomyopathy across a broad range of centres in Europe.
There is no similar study with observational data collected prospectively on consecutive adult patients on the various cardiomyopathy subtypes across Europe. In contrast, some information on the burden of cardiomyopathies has been reported previously in the paediatric population.10–12
Our study provides real-world contemporary data on adult patients and we show a significant global burden of major clinical events at short term. The under-recognized burden of cardiomyopathies is consistent with recent epidemiological data.13 We also show differences in outcome not only according to cardiomyopathy subtypes but also in relatives vs. index patients, and according to European regions.
Diagnostic/prognostic workup
Interestingly, clinical improvement was observed during follow-up in patients with HCM, DCM and ARVC, but not in RCM. This finding suggests effective therapeutic management of symptoms in the short term in most cardiomyopathy subtypes but also confirms the particularly adverse outcome in RCM patients.
First-line tests like echocardiogram and ECG were performed in two-thirds of patients during follow-up, which is consistent with recommendations from guidelines.8 The use of Holter ECG monitoring was however unexpectedly low (one-third) and far from recommendations.8,14 The low percentage of some cardiac examination during follow-up might be hypothesized as playing a role in the adverse event but cannot be affirmed.
The results from this registry highlight the need for implemented guidelines on the recommended tests for the evaluation and follow-up of patients with cardiomyopathies. Apart from HCM in which the periodicity of the cardiac tests is specifically recorded in the 2014 ESC guidelines, for other cardiomyopathies, the recommendations are scarce and available only from expert consensus documents.14,15 A summary of the recommendations for periodical tests is included in Supplementary material online, Table S5.
Medical therapy
The proportion and distribution of medication was generally similar during 1-year follow-up compared to baseline. There was however a significant increase of anticoagulation in HCM, probably related to occurrence of new onset AF and new stroke, whereas a decrease of diuretics, beta-blockers and ACE inhibitors was surprisingly observed in DCM patients, possibly related to the introduction of new agents (sacubitril/valsartan).
Non-pharmacological therapies
A small but significant proportion of patients required ICD implantation during follow-up (mostly for primary prevention), in keeping with the arrhythmic nature of these cardiac conditions.16–18 The proportion was particularly high for ARVC, with two out of three patients carrying an ICD.
A relatively low proportion of HCM patients required invasive septal reduction procedures, with an observed unexpected predominance of myectomy over ablation, likely reflecting preferences of referral centres.9
Clinical outcomes
The direct comparison between subtypes of cardiomyopathies regarding prognosis is one of the original contributions of the registry (Figure 1). MACE were relatively low in HCM and DCM but very important in ARVC and RCM.
The risk of reaching combined arrhythmic, heart failure, or cardiovascular major events was consistently higher (OR ∼2) for DCM as compared with HCM patients, which is consistent with the literature.10,12,14,19 Indeed, annual rates of events (sudden death and heart failure) in DCM series varies from 2% to 6% per year respectively.14 For HCM, sudden death is the major cause of death and the annual rate in the larger cohorts published is around 1%.19 In contrast, outcome was most severe in RCM patients (OR for MACE ∼5 and OR for cardiovascular death ∼12 as compared to HCM) and medication/devices do not seem to prevent the progression of the disease.
Despite the development of new risk stratification scores for HCM, ARVC20,21 and the results from large registries in DCM,22 patients with cardiomyopathy continue to die suddenly (annual rate 1.0%), and about two-thirds of those with sudden or arrhythmic death had a diagnosis of DCM. These results highlight the limitations of current SCD risk stratification strategies in cardiomyopathies, particularly in DCM in which indications for ICD are still somewhat controversial.22 Emerging new data show that the role of genetic background might be broader than previously estimated and that some genes (such as LMNA, FLNC, RBM20) that are underdiagnosed in routine practice are associated with a high risk of SCD.14 To progress towards a better prognosis of patients with cardiomyopathies may therefore require more detailed aetiology work-up, refined risk stratification including recent data on MRI and genetics, and may suggest more pro-active use of available therapeutics. Another way is probably to promote the development of ‘Cardiomyopathies multidisciplinary teams’ and not only ‘Heart failure teams’ in order to manage the various aspects of these diseases including aetiology-oriented management.
Probands and relatives
The higher proportion of diagnostic tests performed during follow-up in relatives compared to probands was not expected but might be related to the date of diagnosis and a higher proportion of incident vs. prevalent cases within relatives as compared to probands.
Despite the fact that probands were older, more symptomatic and required more medication than relatives, rates of combined arrhythmic and heart failure major events were not different in both groups of patients. However, probands reached a significantly higher rate of MACE, including urgent admissions, which was double that in relatives.
Geographical differences across Europe
One of the main goals of the Cardiomyopathy registry was to report on standards of diagnosis and management across Europe, to show adherence to guidelines and to provide important information on provision of care. Geographical differences in the type of patients seen, incident or prevalent, probands and relatives, sporadic or familial, and the use of specific diagnostic tests or therapies have been demonstrated in earlier publications from this cohort.3,9
In this follow-up analysis, differences in outcomes have arisen. While these differences may represent real variation in accessibility to expert teams and to some advanced therapies like heart transplantation, they might be also related to differences in the cohorts. In particular, East Europe had a higher proportion of probands, which are known to have a higher risk of events. In contrast, the South had the higher percentage of patients with early diagnosis through family screening. However, multivariable analyses still confirmed the regional differences in MACE across Europe. Therefore, improvement in access to optimal care and global equity across Europe should be promoted.
Limitations
Follow-up information was not available in 495/3208 (15.4%) of the initial cohort but patients with missing data were similar to patients with available follow-up for most variables (Supplementary material online, Table S6) (including age at enrolment, age of diagnosis, gender distribution, NYHA status) but had fewer ICD, were most frequently probands and incident cases. There were also differences across Europe, with more patients with missing follow-up information from East Europe, followed by South and then North and West regions. Finally, the follow-up period was limited to 1 year, without planned extension, and our results may not apply to a longer follow-up period.
Conclusions
The present work reports unique data regarding the management, follow-up and outcomes of adult patients with various cardiomyopathy subtypes across a range of centres in Europe. We observed that a significant number of diagnostic and prognostic tests are required during follow-up, for management of these patients. Despite a significant symptomatic improvement after the first year of medication and invasive therapies, arrhythmic and heart failure complications occurred frequently, demonstrating a significant global burden of major clinical events at short term. Outcomes were different not only according to cardiomyopathy subtypes but also in relatives vs. index patients and according to European regions.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Supplementary Material
Acknowledgements
EORP Oversight Committee, the Registry Executive Committee of the EURObservational Research Programme (EORP). Data collection was conducted by the EORP department from the ESC by Rachid Mir Hassaine as Clinical Project Manager, Emanuela Fiorucci, Myriam Glemot, and Patti-Ann McNeill as Project Officers, Marème Konté and Sebastien Authier as Data Managers. Statistical analyses were performed by Cécile Laroche. Overall activities were coordinated and supervised by Dr Aldo P. Maggioni (EORP Scientific Coordinator). All investigators are listed in the Supplementary material online, Appendix S1.
Funding
Since the start of EORP, the following companies have supported the programme: Abbott Vascular Int. (2011–2021), Amgen Cardiovascular (2009–2018), AstraZeneca (2014–2021), Bayer AG (2009–2018), Boehringer Ingelheim (2009–2019), Boston Scientific (2009–2012), the Bristol Myers Squibb and Pfizer Alliance (2011–2019), Daiichi Sankyo Europe GmbH (2011–2020), the Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–2017), Edwards (2016–2019), Gedeon Richter Plc. (2014–2016), Menarini Int. Op. (2009–2012), MSD-Merck & Co. (2011–2014), Novartis Pharma AG (2014–2020), ResMed (2014–2016), Sanofi (2009–2011), SERVIER (2009–2021), and Vifor (2019–2022).
Conflict of interest L.T. reports personal fees from Servier, CVIE Therapeutics outside the submitted work; M.T. reports personal fees from Bayer, Cadila Pharmacceuticals, Janssen-Cilag, Kowa, OncoArendi, PERFUSE Group, Servier, UCB Pharmaceuticals outside the submitted work; J.P.K. reports grants from the British Heart Foundation, non-financial support from NIHR GOSH BRC outside the submitted work; R.B.-V. reports grants and other from Sanofi-Genzyme, other from Pfizer outside the submitted work. P.S. reports Medtronic honorarium for lecture, Abbott honorarium for lecture, Servier honorarium for lecture, Astra Zeneca honorarium for lecture, Respicardia honorarium for lecture, Boehringer Ingelheim consultancy agreement and honorarium for lecture, Novartis consultancy agreement and honorarium for lecture. A.H. reports grants and personal fees from Sanofi-Genzyme, personal fees from Pfizer, Gilead, Myokardia outside the submitted work. A.P.M. reports personal fees from Bayer, Fresenius, Novartis outside the submitted work; P.H.C. reports personal fees from Amicus, Pfizer, grants from Sanofi, Shire outside the submitted work. All other authors have declared no conflict of interest.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study.
References
- 1. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2007;29:270–276. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D et al. ; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 3. Charron P, Elliott PM, Gimeno JR, Caforio ALP, Kaski JP, Tavazzi L et al. ; EORP Cardiomyopathy Registry Investigators. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: baseline data and contemporary management of adult patients with cardiomyopathies. Eur Heart J 2018;39:1784–1793. [DOI] [PubMed] [Google Scholar]
- 4. McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circ Res 2017;121:722–730. [DOI] [PubMed] [Google Scholar]
- 5. Lund LH, Edwards LB, Dipchand AI, Goldfarb S, Kucheryavaya AY, Levvey BJ et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third Adult Heart Transplantation Report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35:1158–1169. [DOI] [PubMed] [Google Scholar]
- 6. Seferović PM, Polovina M, Bauersachs J, Arad M, Gal TB, Lund LH et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:553–576. [DOI] [PubMed] [Google Scholar]
- 7. Bagnall RD, Weintraub RG, Ingles J, Duflou J, Yeates L, Lam L et al. A prospective study of sudden cardiac death among children and young adults. N Engl J Med 2016;374:2441–2452. [DOI] [PubMed] [Google Scholar]
- 8. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 9. Elliott P, Charron P, Blanes JR, Tavazzi L, Tendera M, Konté M et al. ; EORP Cardiomyopathy Registry Pilot Investigators. European Cardiomyopathy Pilot Registry: EURObservational Research Programme of the European Society of Cardiology. Eur Heart J 2016;37:164–173. [DOI] [PubMed] [Google Scholar]
- 10. Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med 2003;348:1647–1655. [DOI] [PubMed] [Google Scholar]
- 11. Nugent AW, Daubeney PE, Chondros P, Carlin JB, Cheung M, Wilkinson LC et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med 2003;348:1639–1646. [DOI] [PubMed] [Google Scholar]
- 12. Wilkinson JD, Westphal JA, Bansal N, Czachor JD, Razoky H, Lipshultz SE. Lessons learned from the Pediatric Cardiomyopathy Registry (PCMR) study group. Cardiol Young 2015;25:140–153. [DOI] [PubMed] [Google Scholar]
- 13. Lannou S, Mansencal N, Couchoud C, Lassalle M, Dubourg O, Stengel B et al. The public health burden of cardiomyopathies: insights from a nationwide inpatient study. J Clin Med 2020;9:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2016;37:1850–1858. [DOI] [PubMed] [Google Scholar]
- 15. Haugaa KH, Basso C, Badano LP, Bucciarelli-Ducci C, Cardim N, Gaemperli O et al. ; EACVI Scientific Documents Committee, EACVI Board members and external reviewers; EACVI Scientific Documents Committee, EACVI Board members and external reviewers. Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy-an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:237–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pinamonti B, Dragos AM, Pyxaras SA, Merlo M, Pivetta A, Barbati G et al. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: results from a 10-year registry. Eur Heart J 2011;32:1105–1113. [DOI] [PubMed] [Google Scholar]
- 18. Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J 2015;36:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elliott PM, Gimeno JR, Thaman R, Shah J, Ward D, Dickie S et al. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart 2005;92:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C et al. ; for the Hypertrophic Cardiomyopathy Outcomes Investigators. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014;35:2010–2020. [DOI] [PubMed] [Google Scholar]
- 21. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019;40:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolff G, Lin Y, Karathanos A, Brockmeyer M, Wolters S, Nowak B et al. Implantable cardioverter/defibrillators for primary prevention in dilated cardiomyopathy post-DANISH: an updated meta-analysis and systematic review of randomized controlled trials. Clin Res Cardiol 2017;106:501–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study.


