ABSTRACT
Salmonella enterica is one of the most common pathogens associated with produce outbreaks worldwide; nonetheless, the mechanisms uncovering their interaction with plants are elusive. Previous reports demonstrate that S. enterica ser. Typhimurium (STm), similar to the phytopathogen Pseudomonas syringae pv. tomato (Pst) DC3000, triggers a transient stomatal closure suggesting its ability to overcome this plant defense and colonize the leaf apoplast. In order to discover new molecular players that function in the stomatal reopening by STm and Pst DC3000, we performed an Arabidopsis mutant screening using thermal imaging. Further stomatal bioassay confirmed that the mutant plants exo70h4-3, sce1-3, bbe8, stp1, and lsu2 have smaller stomatal aperture widths than the wild type Col-0 in response to STm 14028s. The mutants bbe8, stp1 and lsu2 have impaired stomatal movement in response to Pst DC3000. These findings indicate that EXO70H4 and SCE1 are involved in bacterial-specific responses, while BBE8, STP1, and LSU2 may be required for stomatal response to a broad range of bacteria. The identification of new molecular components of the guard cell movement induced by bacteria will enable a better understanding of the initial stages of plant colonization and facilitate targeted prevention of leaf contamination with harmful pathogens.
Keywords: phytopathogen, enterobacteria, foodborne illness, stomatal movement, Arabidopsis mutant screening
S. enterica and P. syringae activate alternative pathways in the guard cells to induce stomatal movement, although common players are also involved.
INTRODUCTION
Stomata are natural pores on the plant leaf surface surrounded by a pair of guard cells. These pores are important to promote gas exchange between the aerial parts of the plant and the atmosphere, controlling water losses during transpiration. Plants regulate stomatal movement in order to maximize fitness and survive under diverse environmental challenges, including pathogen attack (Martínez-Sánchez et al.2011; Panchal and Melotto 2017). Microbe-associated molecular patterns (MAMPs), such as the bacterial motor protein flagellin (Gómez‐Gómez and Boller 2000), can induce plant basal defenses known as MAMP-triggered immunity (MTI) (Zipfel et al.2004). As part of the MTI, guard cells surrounding the stomatal pores can sense bacterial flagellin and trigger stomatal closure, a process known as stomatal defense (Melotto et al.2006; Zeng and He 2010). Interestingly, several plant bacterial pathogens are able to overcome the stomatal defense through the secretion of different virulence factors. For instance, Pseudomonas syringae pv. tomato (Pst) strain DC3000 produces the phytotoxin coronatine that represses MTI-triggered stomatal closure, resulting in stomatal reopening. Suppression of stomatal immunity facilitates the entrance of bacterium in the leaf apoplast and subsequent colonization of internal leaf tissue, leading to the development of disease (reviewed by Melotto et al.2017).
The human pathogen Salmonella enterica serovar Typhimurium (STm) also induces strong stomatal closure in plants, including Arabidopsis thaliana, lettuce (Lactuca sativa L.), spinach (Spinacia oleracea), basil (Ocimum basilicum) and cilantro (Coriandrum sativum) (Kroupitski et al.2009; Roy et al.2013; Roy and Melotto 2019). STm shows chemotaxis and tropism toward the leaf stomata (Kroupitski et al.2009; 2011), suggesting that the stomatal pore is an important entry site for this bacterium. Interestingly, similar to the phytopathogen Pst DC3000, STm is capable of inducing stomatal reopening (Kroupitski et al.2009; Roy et al.2013). Indeed, STm colonization and survival in the plant leaf apoplast has been observed for several plant species (Dong et al.2003; Iniguez et al.2005; Schikora et al.2008; Kroupitski et al.2009; Gu et al.2011; Park et al.2013; Roy and Melotto 2019). Inside the leaf tissue, STm remains protected against environmental stressors such as drought and the sun light, as well as against commonly applied sanitation treatments of edible leaves (Golberg et al.2011; Ziuzina et al.2014).
The mechanisms by which STm induces stomatal reopening are largely unknown. To gain knowledge on this process, we have performed a thermal image-based screening of Arabidopsis mutants. The screening is a non-destructive method useful for rapid assays in plants (reviewed by Ishimwe et al. 2014), which enabled us to find novel components of the guard cell signaling involved in both Pst DC3000- and STm 14028s-mediated stomatal movement. Importantly, we discovered common and unique proteins involved in this process, suggesting that STm 14028s and Pst DC3000 may activate distinct pathways in the guard cells to induce stomatal movement. This study may help elucidate mechanisms targeting the protection of plants from bacterial contamination and disease development.
MATERIALS AND METHODS
Plant material and growth conditions
Arabidopsis thaliana (L. Heyhn.) mutant lines and wild type plants were used in this study. The seeds of wild type ecotype Columbia (Col-0, ABRC stock CS60000) and derived mutants were obtained through The Arabidopsis Information Resource (TAIR; https://www.arabidopsis.org/). For the genetic screening, we obtained a mutant collection (ABRC stock CS27942) that contains 3,980 individual mutant lines created by T-DNA insertion (TAIR). Additionally, we have tested mutant lines for specific genes as follows: exo70h4-3 (SALK_003200C), sce1-3 (SALK_05 8401), bbe8 (SALK_117810C), stp1 (SALK_048848C), and lsu2 (SALK_031648C). Seeds were sown in a 1:1:1 v:v:v mixture of growing medium (Redi-earth plug and seedling mix, Sun Gro, Sacramento, CA, USA), fine vermiculite and perlite, and grown in controlled environmental chambers at 22 ± 2°C, 60 ± 10% relative humidity, and a 12-hour photoperiod under light intensity of 100 μmol.m−2.s−1. Plants were maintained under these conditions until the completion of all experiments.
Bacterial strains and culturing conditions
Bacterial cells of Pst strain DC3000 and Salmonella enterica subsp. enterica serovar Typhimurium (STm) strain 14028s were streaked on solid low-salt Luria-Bertani (LSLB; 10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl, pH = 7.0) medium from frozen glycerol stocks. Plates were kept at 28°C until the appearance of single colonies. For inoculum preparation, a single colony was transferred to liquid LSLB medium and kept at 28°C in an orbital shaker-incubator overnight. Medium was supplemented with rifampin (100 µg/mL) to grow Pst DC3000.
Thermal imaging screening
A thermal imaging-based approach (Merlot et al.2002) was used to access whether bacterial inoculation differentially modulates stomatal responses between each Arabidopsis mutant and the wild type Col-0. In this study, we screened ∼1,000 single mutant lines that were picked randomly from the collection CS27942. Col-0 plants were included in every batch of plants for direct comparison with the mutant plants. Three- to four-week-old plants were dip-inoculated with 2 × 108 CFU.mL−1 STm supplemented with 0.03% Silwet L-77 (Lehle Seeds Co., Round Rock, TX, USA) at 3 hours after dawn following the procedure described by Katagiri et al. (2002). To avoid changes in leaf temperature due to environmental conditions, experiments were conducted inside a walk-in growth chamber set at 25°C, 60 ± 10% relative humidity, and light intensity of 100 μmol.m−2.s−1. At 4 hours post inoculation (hpi), pictures were taken from the rosettes using an FLIR® infrared camera (FLIR-T600). Pictures were analyzed using the FLIR Tools software, in which all pictures were set at constant range of temperature based on pictures of Col-0. Differential temperature of the center of the rosette was used to identify mutants with potential role in stomatal susceptibility to STm.
Genotyping of arabidopsis mutants
To confirm the presence of the T-DNA insertion in selected Arabidopsis mutants (Table 1), fresh leaf samples (5 mg) were grounded in 200 µL of Edwards Solution (Edwards et al. 1991) and used as gDNA template in PCR reactions. Primers were designed using the T-DNA Primer Design tool of the T-DNA Express website from the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu/cgi-bin/tdnaexpress). PCR reaction (25 µL) was performed with 1 U DNA polymerase Gotaq® (Promega, WI, USA), 1× enzyme buffer, 1.5 mM MgCl2, 200 µM dNTP, 1 µL of gDNA template, and 400 nM of reverse and forward specific primers (Table 1). Reaction cycling parameters were: 1 cycle of 95°C for 5 min and 40 cycles of 95°C for 30 sec, 52°C for 30 sec, and 72°C for 1 min, with final extension of 72°C for 10 min. Amplicons were visualized after gel electrophoresis using the UV light on a C300 imaging system (Azure Biosystems, CA, USA).
Table 1.
List of Arabidopsis mutants and primer sequences used to validate the mutation.
| Locus number | Gene | ABRC mutant seed stock | Primer sequences |
|---|---|---|---|
| AT3G09520 | EXO70H4 | SALK_003200C (exo70h4-3) | LP—AACAAACCTGAAGCCATGATG |
| RP—GTTGCTGTAGTCAGCGAGGAG | |||
| AT3G57870 | SCE1 | SALK_05 8401 (sce1-3) | LP—TGGTGATGATGTATCCGTCTG |
| RP—GTGCAATGCCACACCATTAG | |||
| AT1G30700 | BBE8 | SALK_117810C (bbe8) | LP—ATATCCTCCCCATCTCACCAC |
| RP—GAAACCCTTCCTCATAATCGC | |||
| AT1G11260 | STP1 | SALK_048848C (stp1) | LP—ACACTCCCAATTCAATGATCG |
| RP—TCATATGCATAATTTCTTCATGTGG | |||
| AT5G24660 | LSU2 | SALK_031648C (lsu2) | LP—CAGCTCATTGGGGCTATAATG |
| RP—TCGCAAAAATCAATCGGTAAC | |||
| T-DNA-LBb1.3–ATTTTGCCGATTTCGGAAC |
Stomatal bioassay
To examine the stomatal reopening of mutant plants in response to Pst DC3000 and STm 14028s, stomatal bioassays were conducted as previously described (Montano and Melotto 2017). Briefly, three detached whole leaves of four- to five-week old plants were floated on water or 1 × 108 CFU.mL−1 of either Pst DC3000 or STm 14028s. Floating leaves were kept at 25°C and light intensity of 100 μmol.m−2.s−1 for the duration of the experiment (4 hours). Stomatal images and aperture width measurements were obtained with a Nikon Eclipse 80i fluorescent microscope (Nikon Corporations, Shinagawa-ku, Tokyo, Japan), equipped with long-distance objectives. Data points represent the mean of two independent biological replicates (n = 120) ± standard error (SE). Student's t-test statistical analysis was performed by comparing the stomatal aperture width of each mutant to that of the wild type Col-0 for each treatment.
RESULTS
Arabidopsis mutants screening reveals proteins involved in bacterium-induced stomatal reopening
Thermal-image screening was established >17 years ago (Merlot et al.2002) and has been previously used in a number of screening assays (reviewed by Fiorani and Schurr 2013; Osakabe et al.2016). Taking advantage of this technology, we first determined whether it would be suitable for our experimental setup. Indeed, Col-0 plants show an overall hotter leaf surface temperature at 2 hpi with STm when compared to the mock-inoculated plants (Fig. 1A), indicating an overall decrease of stomatal pore size . Furthermore, Col-0 rosettes became cold at 4 hpi with STm, indicating that stomatal pores reopened (Fig. 1A). This observation provided an opportunity to screen Arabidopsis mutants for compromised stomatal reopening at 4 hpi.
Figure 1.
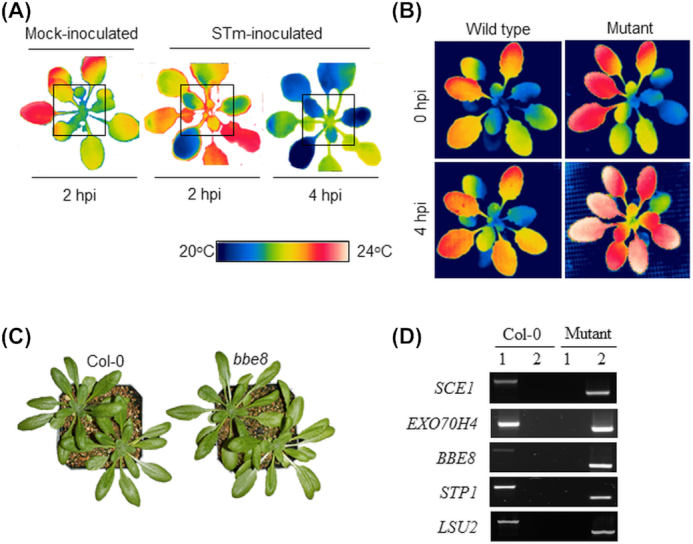
Arabidopsis mutant phenotyping and genotyping. (A) Representative thermal images of inoculated Col-0 plants. Three- to four-week-old plants were mock- or dip-inoculated with STm (2 × 108 CFU.mL−1). The thermal images were taken 2 and 4 hpi using an infrared camera (FLIR T600). Images were analyzed with the FLIR Tools Thermal Analysis and Reporting software (https://www.flir.com), in which the temperature in the center of the rosette (square) was used to assess the change in leaf temperature in bacterium-inoculated plants. Note that exposure to STm increases and decreases the temperature of the inner rosette at 2 hpi and 4 hpi, respectively. (B) Representative thermal images used to select plant mutants during the genetic screening. Images were taken before inoculation (0 hpi) and at 4 hpi with STm. Note that the center of the mutant rosette is hotter than the wild type rosette at 4 hpi. (C) Representative photographs of Arabidopsis plants used for mutant genotyping. Note that the plant mutant bbe8 (as all other mutants reported in this study) does not have obvious defect in plant growth or development as compared to the wild type Col-0. (D) Genomic DNA for each T-DNA insertion mutant plant and the wild type Col-0 was used as a template in PCR amplification with gene-specific primers listed in Table 1. Reactions loaded onto lane 1 contained the LP (left primer) and RP (right primer) set to amplify the wild type allele in Col-0, whereas reactions loaded onto lane 2 contained a T-DNA specific primer and the RP primer to amplify the mutant allele.
Moving toward a mechanistic understanding of plant molecular responses to human pathogens, we set up a medium throughput assay and phenotyped ∼1,000 mutants for hotter leaf surface temperature at 4 hpi with STm by comparing with that of the wild type control. Representative images used to select mutant plants are shown in Fig. 1B. From this screening, we selected five plant mutants for further analysis. Each of these plant lines has a mutation in the following genes: EXO70H4 (EXOCYST SUBUNIT EXO70 FAMILY PROTEIN H4) and SCE1 (SUMO CONJUGATING ENZYME 1) involved in callose deposition (Kulich et al.2015; 2018) and protein SUMOylation (Saracco et al.2007), respectively; the BBE8 (BERBERINE BRIDGE ENZYME 8) that is a novel protein; the hexose symporter STP1 (SUGAR TRANSPORTER 1) (Shearson et al.2000); and the stress mediator LSU2 (RESPONSE TO LOW SULFUR 2) (Sirko et al.2015). These mutants had no obvious growth and developmental defects under the conditions used for the experiment (representative images shown in Fig. 1C). Homozygous mutation in the expected gene for each of the five Arabidopsis mutants was confirmed by PCR (Fig. 1D), and mutant plants were subjected to stomatal bioassays, which comprised of direct measurements of stomatal aperture width after treatment.
STm 14028s and Pst DC3000 may activate distinct pathways to induce stomatal reopening
It is well known that Pst DC3000 can reopen MTI-closed stomata due to the action of its phytotoxin coronatine (Melotto et al.2006). STm is also capable of inducing stomatal reopening on both Arabidopsis and lettuce leaves by an unknown mechanism (Kroupitski et al. 2009; Roy et al.2013). In order to shed light on the molecular mechanism involved in the stomatal movement evoked by these bacteria, we evaluated the stomatal movement in leaves of the five pre-selected Arabidopsis mutants (exo70h4–3, sce1–3, bbe8, stp1 and lsu2) identified in the screen (Fig. 1) after 4 hours of exposure to either Pst DC3000 or STm 14028s.
All five mutants showed no defect in stomatal movement when their leaves were incubated with the water as a control for 4 hours, as the stomatal aperture width was similar between mutants and Col-0 grown and analyzed side-by-side (Fig. 2A). When interacting with the plant pathogen Pst DC3000, the mutants exo70h4-3 with T-DNA insertion in the coding sequence of the EXO70H4 gene (Kulich et al.2015), and the sce1-3 that has a T-DNA insertion upstream of the 5′ UTR of the SCE1 (Saracco et al.2007), showed no differential stomatal response in comparison to Col-0 inoculated leaves (Fig. 2B). However, the plant mutants bbe8 with a T-DNA insertion in the first intron of the BBE8 gene (Peng et al.2014), stp1 containing T-DNA insertion at the fourth exon of the STP1 gene (Yamada et al.2016), and lsu2 that has a T-DNA insertion at the 5′ UTR region of the LSU2 gene (Sirko et al.2015) showed narrower stomatal aperture widths than Col-0 after incubation with the plant pathogen Pst DC3000 (Fig. 2B). Confirming the genetic screening data, we observed that the mean stomata aperture width in all mutants were smaller than the wild type Col-0 when leaves were incubated with STm 14028s (Fig. 2C). These results suggest that although both Pst DC3000 and STm 14028s induce a transient stomatal closure in Arabidopsis, these bacteria may do so by activating distinct plant signaling processes.
Figure 2.

Arabidopsis genes involved in bacterium-mediated stomatal susceptibility. Stomatal aperture width at 4 hpi with (A) water, (B)Pst DC3000, (C) STm 14028s. Leaves of Arabidopsis wild type Col-0 and mutants were floated on water (control) or bacterial inoculum (1 × 108 CFU.mL−1Pst DC3000 or STm 14028s). Results are shown as mean of two independent experiments (n = 120 ± SE). Statistical significance between means of each mutant and Col-0 inoculated with the same bacterium was detected using two-tailed Student's t-test (*** = P < 0.001; ns = non-significant). The pictures are representative of closed and open stomata. Red bars in the stomatal pore indicate the stomatal aperture width that was measured to obtain the graphs.
DISCUSSION
Contamination of fresh produce with human pathogens has a strong social and economic impact due to frequent foodborne disease outbreaks (Bennett et al.2018). Contrary to the widely studied Arabidopsis-Pst DC3000 system, useful to understand the molecular mechanisms underlying plant disease (reviewed by Xin and He 2013), very little is known about how bacterial pathogens of human are able to survive in the phyllosphere until they reach their natural host. Two bacterial species, the phytopathogen Pst DC3000 and the enteric STm, modulate the stomatal pore size thereby facilitating their colonization of the leaf interior (Melotto et al.2006; Kroupitski et al.2009; Roy et al.2013). However, whether this process is controlled by different mechanisms in a bacterium-dependent manner is unknown. Therefore, we sought to identify novel components of the guard cell molecular signaling that allow STm to overcome stomatal defense and establish correlations with the model system Arabidopsis-Pst DC3000. We first explored the guard cells movement of a collection of Arabidopsis mutants in response to STm, specifically in the context of bacterium-induced stomatal opening (i.e. at 4 hpi; Fig. 1). We were able to verify that five Arabidopsis mutants have impaired stomatal movement in response to STm 14028s and/or Pst DC3000.
A smaller stomatal aperture width was observed in the bbe8 mutant after inoculation with Pst DC3000 and STm 14028s as compared to the wild type Col-0 (Fig. 2). Arabidopsis has 28 members of the BBE family and functional redundancy is expected among the family members; however, this is yet to be fully determined (Locci et al.2019). Members of this family are known to promote the folding of the flavoenzyme vanillyl alcohol-oxidase (Daniel et al.2016). Recently, four members of the BBE-like family were shown to be oligogalacturonides (OGs) oxidases, which may reduce the activation of plant immune responses (Benedetti et al.2018). Furthermore, flavoproteins act as oxidoreductases to control redox burst processes (reviewed by Macheroux et al. 2011). Reactive oxygen species (ROS) burst is a hallmark of plant defense activated by microbes and function as secondary messenger to promote Pst-triggered stomatal closure (reviewed by Melotto et al.2017). Thus, BBE8 may act on the oxidation of cell wall-derived oligosaccharides in the guard cells to facilitate stomatal opening after Pst DC3000 or STm inoculation.
The hexose symporter SPT1 and the sulfur-responsive protein LSU2 were also found to modulate stomatal movement in response to both Pst DC3000 and STm 14028s (Fig. 2). Guard cells require carbon sources to generate malate, which function as an osmotically active solute to induce influx of water and promote stomatal opening (reviewed by Santelia and Lawson 2016). Although malate can be imported by the guard cell (Lee et al.2008) or synthetized through the degradation of starch and/or CO2 fixation by the chloroplast, the constant supply of extracellular sugar into the guard cell is required for its proper functioning (reviewed by Santelia and Lawson 2016). In fact, STP1 was proposed to have a role in the osmoregulation of the guard cells during the day (Stadler et al.2003). Therefore, deficiency in the guard cell osmoregulation in the spt1 mutant may inhibit bacterial triggered stomatal opening. Interestingly, LSU2 was previously shown to be involved in response to several stresses, including carbon starvation and sugar treatment (Usadel et al.2008). Moreover, LSU2 is induced during oxidative stress (Davletova et al.2005), its expression is required for ROS production in guard cell chloroplasts and subsequent stomatal closure during sulfur stress (Garcia-Molina et al.2017), and it also participates in the apoplastic defense against Pst DC3000 (Mukhtar et al.2011). Hence, it is possible that LSU2 functions as a hub required for proper guard cell signaling in response to bacteria. These findings suggest that STP1 and LSU2 act in guard cells in response to a broad range of bacteria, as they may participate in core processes of stomatal movement.
The mutants exo70h4-3 and sce1-3 showed narrower stomatal aperture width in comparison to Col-0 after 4 hours of exposure to STm 14028s, but not Pst DC3000 (Fig. 2). The EXO70H4 protein acts on secondary cell wall formation and callose deposition (Kulich et al.2015; 2018). Callose is a highly dynamic glucose polymer that is rapidly synthetized or degraded in response to diverse environmental stimuli or during plant development (Stone and Clarke 1992). Changes in guard cell turgor, which allows stomatal movement, require a dynamic cell wall. In the fern Asplenium nidus for instance, inhibition of callose synthesis reduces the stomatal ability to open (Apostolakos et al.2010). SCE1 is a protein that catalyzes the attachment of the SUMO peptide to target proteins. SUMOylation is a post-translational modification that modulates protein sub-cellular localization, activity, and protein–protein interactions. Protein SUMOylation plays a major role in plant development, as well as in responses to abiotic and biotic stresses (reviewed by Verma et al. 2018). In fact, SCE1 interacts with FLS2 (FLAGELLIN-SENSING 2) and TPR1 (TOPLESS-RELATED 1), two proteins that activate plant immune responses, suggesting that SUMOylation can directly participate on the control of plant responses to microbes (Orosa et al.2018; Niu et al.2019). Therefore, our results suggest that callose deposition and protein SUMOylation in the guard cells are required for STm 14028s to overcome stomatal immunity and induce stomatal opening.
In this study, we have found novel components of guard cell signaling pathway that function in the response to two bacteria. The results indicate that EXO70H4 and SCE1 may have a greater importance in the guard cell response to human pathogens than to plant pathogens. As callose deposition and ROS burst are common MTI responses, the distinctions observed in this study are dependent on specific interaction with each pathogen. Interestingly, the novel protein BBE8, the sugar import by STP1, and LSU2-mediated stress responses in the guard cell were common players in response to both human and plant pathogens. Altogether, the results indicate that human and plant pathogens activate alternative pathways in the guard cells to induce stomatal movement, although common players are also involved. This study provides new perspectives to assist the control of human and plant pathogen colonization of plants and improve our knowledge on stomatal movement controlled by bacteria.
ACKNOWLEDGEMENTS
We would like to thank the undergraduate students Cristie Le, Samantha Thanh Trinh, Ahn Quang Le, Jennifer La, Karen Truong, Davina Sassoon, Mark Torres, and Roshni Kharadi for adjusting the protocol and conducting parts of the plant mutant screen.
FUNDING
This work was supported in part by a grant from the U.S. National Institute of Allergy and Infectious Disease (5R01AI068718), the U.S. Department of Agriculture—National Institute of Food and Agriculture (USDA-NIFA; 2015-67017-23360 and 2017-67017-26180), and NIFA Hatch (CA-D-PLS-2327-H) to MM.
Conflict of Interest. None declared.
REFERENCES
- Apostolakos P, Livanos P, Nikolakopoulou TLet al. Callose implication in stomatal opening and closure in the fern Asplenium nidus. New Phytol. 2010;186:623–35. [DOI] [PubMed] [Google Scholar]
- Bennett SD, Sodha SV, Ayers TLet al. Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiol Infect. 2018;146:1397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti M, Verrascina I, Pontiggia Det al. Four Arabidopsis berberine bridge enzyme-like proteins are specific oxidases that inactivate the elicitor-active oligogalacturonides. Plant J. 2018;94:260–73. [DOI] [PubMed] [Google Scholar]
- Daniel B, Wallner S, Steiner Bet al. Structure of a berberine bridge enzyme-like enzyme with an active site specific to the plant family Brassicaceae. PLoS One. 2016;11:e0156892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Schlauch K, Coutu Jet al. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005;139:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Iniguez AL, Ahmer BMet al. Kinetics and strain specificity of rhizosphere and endophytic colonization by enteric bacteria on seedlings of Medicago sativa and Medicago truncatula. Appl Environ Microb. 2003;69:1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991;19:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorani F, Schurr U. Future scenarios for plant phenotyping. Annu Rev Plant Biol. 2013;64:267–91. [DOI] [PubMed] [Google Scholar]
- Garcia-Molina A, Altmann M, Alkofer Aet al. LSU network hubs integrate abiotic and biotic stress responses via interaction with the superoxide dismutase FSD2. J Exp Bot. 2017;68:1185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golberg D, Kroupitski Y, Belausov Eet al. Salmonella Typhimurium internalization is variable in leafy vegetables and fresh herbs. Int J Food Microbiol. 2011;145:250–7. [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. FLS2: an LRR receptor–like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–11. [DOI] [PubMed] [Google Scholar]
- Gu G, Hu J, Cevallos-Cevallos JMet al. Internal colonization of Salmonella enterica serovar Typhimurium in tomato plants. PLoS One. 2011;6:e27340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez AL, Dong Y, Carter HDet al. Regulation of enteric endophytic bacterial colonization by plant defenses. Mol Plant Microbe In. 2005;18:169–78. [DOI] [PubMed] [Google Scholar]
- Ishimwe R, Abutaleb K, Ahmed F. Applications of thermal imaging in agriculture—a review. ARS. 2014;03:128–40. [Google Scholar]
- Katagiri F, Thilmony R, He SY. The Arabidopsis thaliana–Pseudomonas syringae interaction. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupitski Y, Golberg D, Belausov Eet al. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microb. 2009;75:6076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupitski Y, Pinto R, Belausov Eet al. Distribution of Salmonella typhimurium in romaine lettuce leaves. Food Microbiol. 2011;28:990–7. [DOI] [PubMed] [Google Scholar]
- Kulich I, Vojtíková Z, Glanc Met al. Cell wall maturation of Arabidopsis trichomes is dependent on exocyst subunit EXO70H4 and involves callose deposition. Plant Physiol. 2015;168:120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich I, Vojtíková Z, Sabol Pet al. Exocyst subunit EXO70H4 has a specific role in callose synthase secretion and silica accumulation. Plant Physiol. 2018;176:2040–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Choi Y, Burla Bet al. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat Cell Biol. 2008;10:1217. [DOI] [PubMed] [Google Scholar]
- Locci F, Benedetti M, Pontiggia Det al. An Arabidopsis berberine bridge enzyme-like protein specifically oxidizes cellulose oligomers and plays a role in immunity. Plant J. 2019;98:540–54. [DOI] [PubMed] [Google Scholar]
- Macheroux P, Kappes B, Ealick SE. Flavogenomics–a genomic and structural view of flavin-dependent proteins. FEBS J. 2011;278:2625–34. [DOI] [PubMed] [Google Scholar]
- Martínez-Sánchez A, Tudela JA, Luna Cet al. Low oxygen levels and light exposure affect quality of fresh-cut Romaine lettuce. Postharvest Biol Tec. 2011;59:34–42. [Google Scholar]
- Melotto M, Underwood W, Koczan Jet al. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–80. [DOI] [PubMed] [Google Scholar]
- Melotto M, Zhang L, Oblessuc PRet al. Stomatal defense a decade later. Plant Physiol. 2017;174:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Mustilli AC, Genty Bet al. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 2002;30:601–9. [DOI] [PubMed] [Google Scholar]
- Montano J, Melotto M. Stomatal bioassay to characterize bacterial-stimulated PTI at the pre-invasion phase of infection. Plant Pattern Recognition Receptors. New York, NY: Humana Press, 2017, 233–41. [DOI] [PubMed] [Google Scholar]
- Mukhtar MS, Carvunis AR, Dreze Met al. Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science. 2011;333:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu D, Lin XL, Kong Xet al. SIZ1-mediated SUMOylation of TPR1 suppresses plant immunity in arabidopsis. Mol Plant. 2019;12:215–28. [DOI] [PubMed] [Google Scholar]
- Orosa B, Yates G, Verma Vet al. SUMO conjugation to the pattern recognition receptor FLS2 triggers intracellular signalling in plant innate immunity. Nat Commun. 2018;9:5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Watanabe T, Sugano SSet al. Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci Rep. 2016;6:26685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S, Melotto M. Stomate-based defense and environmental cues. Plant Signal Behav. 2017;12:e1362517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Park JW, Wikle HC IIIet al. Evaluation of phage-based magnetoelastic biosensors for direct detection of Salmonella Typhimurium on spinach leaves. Sensor Actuat B-Chem. 2013;176:1134–40. [Google Scholar]
- Peng J, Li Z, Wen Xet al. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet. 2014;10:e1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D, Panchal S, Rosa BAet al. Escherichia coli O157: H7 induces stronger plant immunity than Salmonella enterica Typhimurium SL1344. Phytopathology. 2013;103:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D, Melotto M. Stomatal response and human pathogen persistence in leafy greens under preharvest and postharvest environmental conditions. Postharvest Biol Tec. 2019;148:76–82. [Google Scholar]
- Santelia D, Lawson T. Rethinking guard cell metabolism. Plant Physiol. 2016;172:1371–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracco SA, Miller MJ, Kurepa Jet al. Genetic analysis of SUMOylation in Arabidopsis: conjugation of SUMO1 and SUMO2 to nuclear proteins is essential. Plant Physiol. 2007;145:119–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A, Carreri A, Charpentier Eet al. The dark side of the salad: Salmonella typhimurium overcomes the innate immune response of Arabidopsis thaliana and shows an endopathogenic lifestyle. PLoS One. 2008;3:e2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearson SM, Hemmann G, Wallace Get al. Monosaccharide/proton symporter AtSTP1 plays a major role in uptake and response of Arabidopsis seeds and seedlings to sugars. Plant J. 2000;24:849–57. [DOI] [PubMed] [Google Scholar]
- Sirko A, Wawrzyńska A, Rodríguez MCet al. The family of LSU-like proteins. Front Plant Sci. 2015;5:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Büttner M, Ache Pet al. Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol. 2003;133:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BA, Clarke AE. Chemistry and physiology of higher plant 1, 3-β-glucans (callose). In: Stone BA, Clarke AE(eds). Chemistry and Biology of (1, 3)- β-glucans. Bundoora: La Trobe University Press, 1992, 365–429. [Google Scholar]
- Usadel B, Bläsing OE, Gibon Yet al. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 2008;146:1834–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Croley F, Sadanandom A. Fifty shades of SUMO: its role in immunity and at the fulcrum of the growth–defence balance. Mol Plant Pathol. 2018;19:1537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin XF, He SY. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol. 2013;51:473–98. [DOI] [PubMed] [Google Scholar]
- Yamada K, Saijo Y, Nakagami Het al. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science. 2016;354:1427–30. [DOI] [PubMed] [Google Scholar]
- Zeng W, He SY. A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 2010;153:1188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro Let al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764. [DOI] [PubMed] [Google Scholar]
- Ziuzina D, Patil S, Cullen PJet al. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 2014;42: 109–16. [DOI] [PubMed] [Google Scholar]


