Abstract
Background:
Parkinson’s disease (PD) and essential tremor (ET) are commonly encountered movement disorders. Pathophysiologic processes that localize to the cerebellum are described in both. There are limited studies investigating cerebellar structural changes in these conditions, largely because of inherent challenges in the efficiency of segmentation.
Methods:
We applied a novel multiatlas cerebellar segmentation method to T1-weighted images in 282 PD and 111 essential tremor patients to define 26 cerebellar lobule volumes. The severity of postural and resting tremor in both populations and gait and postural instability in PD patients were defined using subscores of the UPDRS and Washington Heights-Inwood Genetic Study motor scales. These clinical measurements were related to lobule volume size. Multiple comparisons were controlled using a false discovery rate method.
Results:
Group differences were identified between ET and PD patients, with reductions in deep cerebellar nucleus volume in ET versus reduced lobule VI volume in PD. In ET patients, lobule VIII was negatively correlated with the severity of postural tremor. In PD patients, lobule IV was positively correlated with resting tremor and total tremor severity. We observed differences in cerebellar structure that localized to sensorimotor lobules of the cerebellum. Lobule volumes appeared to differentially relate to clinical symptoms, suggesting important clinicopathologic distinctions between these conditions. These results emphasize the role of the cerebellum in tremor symptoms and should foster future clinical and pathologic investigations of the sensorimotor lobules of the cerebellum.
Keywords: cerebellum, essential tremor, Parkinson’s disease
Disease-related changes to cerebellar structure and function1 are reported in Parkinson’s disease (PD) and essential tremor (ET). Although patients may share similar clinical features of tremor, the clinical presentation, underlying pathologic process, and treatment options differ. Tremor phenomena in PD patients are often noted at rest, but patients may have concomitant postural and kinetic tremor symptoms.2 ET patients, in contrast, present with an action tremor and may have varying degrees of postural and kinetic tremor.3–5 Previous studies have localized changes to the cerebellum and related networks in ET.6,7 However, there is a paucity of investigations that have assessed cerebellar size and structure in ET and PD patients, especially investigations that link clinical symptoms to the cerebellar structure.8,9
There are substantive reasons to hypothesize that group differences in cerebellar structure exist (ie, ET versus PD). Pathologic changes to the dentate nucleus are noted in postmortem studies of ET, yet this is not typical in PD.1 In addition, previous neuroimaging studies report structural cerebellar changes in ET,10,11 noting that clinical symptoms appear to localize to this region.12,13 Further, previous studies reported altered gray matter in the cerebellum,14 and outflow tracts.15–19 Given these findings, we hypothesize that tremor symptoms may relate to cerebellar regions that govern sensorimotor function.20,21
The somatotopic organization of the cerebellum is such that peripheral lobules control movements of the hands and legs, whereas midline cerebellum structures such as the vermis mediate gait and balance.5,22 Imaging studies emphasize duplicate representation in the anterior (lobules IV and V) and posterior (lobules VI and VIII) lobes, involved in fine-motor control.20,22–26 Sensorimotor networks travel through the dentate nucleus of the cerebellum via afferent projections along the corticopontocerebellar and efferent cerebellar-thalamocortical tracts.23,27–30
There exist several inherent challenges to imaging investigations of the cerebellum, including inadequate cerebellar segmentation algorithms and difficulty accounting for motion artifact in patients with tremor (especially head tremor). Although cerebellar automation techniques can improve the efficiencies of cerebellar segmentation, many algorithms poorly delineate cerebellar lobules.31 One method that overcomes these segmentation challenges is multiatlas segmentation, an automated tool that applies multiple cerebellar atlases to identify 26 lobules of the cerebellum and overcomes atlas bias by applying various statistical methods to delineate these structures.32
The overarching goal of this study was to determine if cerebellar volume differed between PD and ET patients and to investigate if clinical symptomatology (tremor type, tremor severity, and gait symptoms) relates to cerebellar volume of lobules that control sensorimotor function. The main methodological approach we employed was a multiatlas segmentation atlas in a large convenience cohort of ET and PD patients. All participants underwent a standardized clinical assessments and magnetic resonance imaging (MRI) of the brain under general anesthesia, as part of standard procedures for deep brain stimulation (DBS) planning.7 We hypothesized that cerebellar volumes would be reduced in ET patients, specifically in the deep cerebellar nuclei and sensorimotor lobules. Also, given the clinical localization of action tremor, cerebellar-outflow networks, and postural instability, we hypothesized that the volumes of sensorimotor nuclei and vermal lobules would relate to the severity of tremor (ET) and gait (PD) symptoms.
Methods
Demographics
This convenience cohort included 393 participants with a diagnosis of PD or ET. All were evaluated at Vanderbilt University Medical Center between 2011 and 2017. The diagnosis of PD or ET was made by a movement disorder neurologist according to established criteria.33,34 Motor severity was assessed by a single rater using the United Parkinson’s Disease Rating Scale (UPDRS) for PD patients and the Washington Heights-Inwood Genetic Study (WHIGET) for ET patients. Postural tremor for the UPDRS and WHIGET were assessed with the patient extending his or her arms supinated for 10 seconds. For resting tremor, the participants were instructed to place their arms in a supinated fashion on their laps for 10 seconds. All participants were assessed in an off-medication state, which was defined as overnight withdrawal of therapies prescribed for motor symptoms. All participants provided informed written consent, and the study was approved by the Vanderbilt University Institutional Review Board.
Image Acquisition
Brain imaging was performed using a 3.0T MRI scanner (Philips Achieva, Best, the Netherlands) with phased-array SENSE 8–channel reception and body coil transmission. T1-weighted images were acquired using a 3-D magnetization-prepared rapid-gradient-echo sequence with TR/TE = 7.92/3.65 milliseconds and 1.0 mm isotropic spatial resolution. General anesthesia was administered for the duration of the MRI imaging. This procedure was part of the standard-of-care protocol for stereotactic planning for DBS surgery.7
Cerebellar Segmentation
MRI scans were deidentified and defaced, then automatically processed using a multiatlas plug-in that segments the cerebellum into 26 lobules.35,36 These lobules include the left and right lobule, III, IV, V, VI, VII, VIIIa, VIIIb, IX, Crus I, Crus II, and vermis VI, VII, VIIIa, IX, X, and deep cerebellar nuclei (Fig. 1). This cerebellar segmentation technique has been described previously and is optimal for identification of cerebellar structures.35 All scans were individually inspected for correct lobule identification and noncerebellar segmentation, with the reviewers blinded to disease state. The verification protocol established by Bogovic et al. was applied to ensure accurate labeling and segmentation of cerebellar lobules.37
FIG. 1.
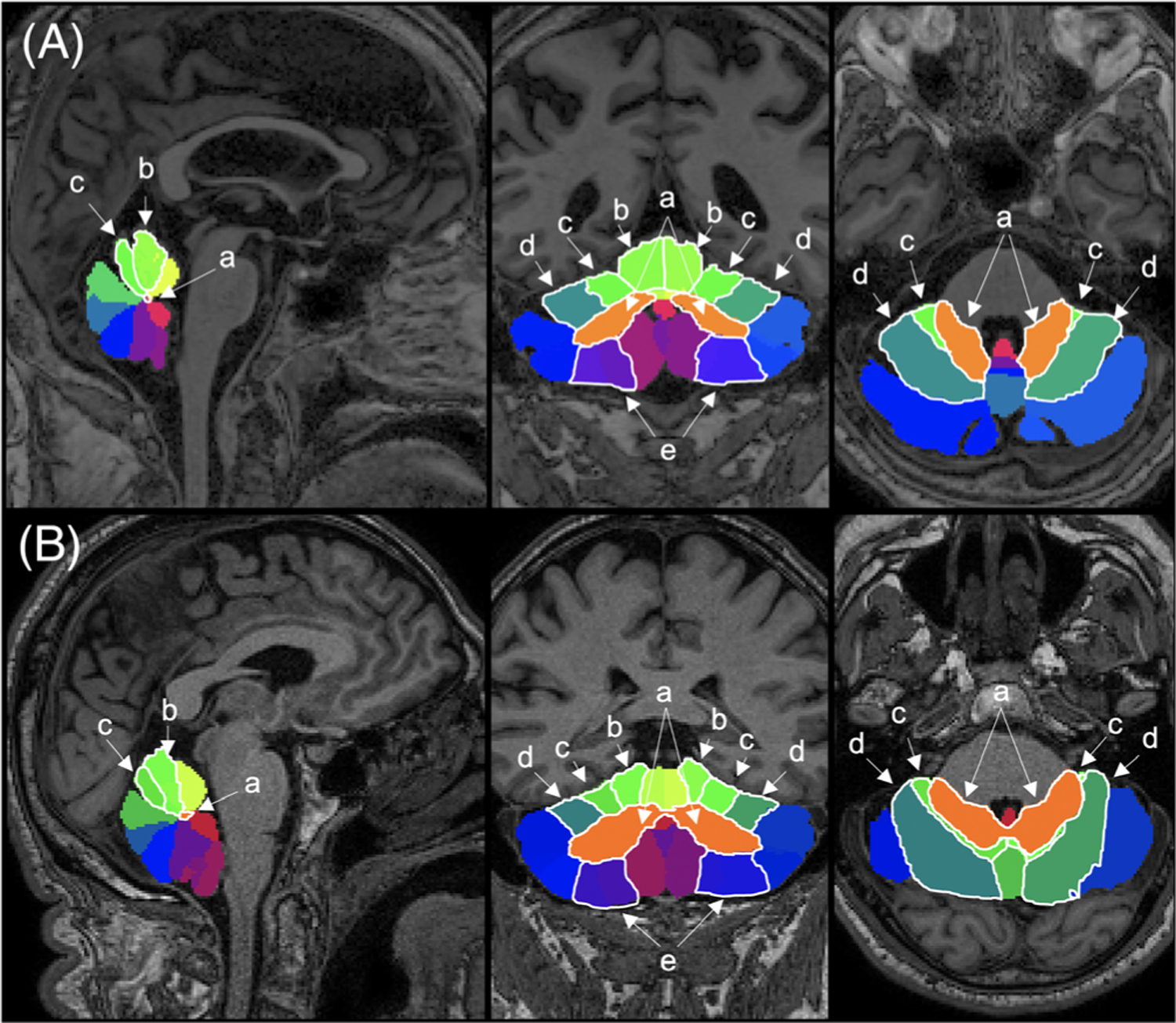
Multiatlas cerebellar segmentation. Sagittal (left), coronal (center), and axial views for (A) representative ET patient (male, 68 years) and (B) PD patient (male, 64 years). Specific lobules: (a) deep cerebellar nuclei, (b) lobule IV, (c) lobule V, (d) lobule VI, and (e) lobule VIII are outlined in white. [Color figure can be viewed at wileyonlinelibrary.com]
Calculation of Lobule and Lobar Volumes
Segmentation editing was performed using FSLeyes editing tool (0.22.6; FMRIB, Oxford, UK, https://git.fmrib.ox.ac.uk/fsl/fsleyes/fsleyes/), particularly in cases with erroneous identification of the neck muscles or the occipital lobe. This method allowed for subtraction of extracerebellar voxels and adjustments of lobule segmentation. In total, 393 cerebellar segmentations passed quality assessments as described by Bogovic et al. Lobule volumes were calculated using Matlab (Release 2018a; The MathWorks, Inc., Natick, MA) by counting voxels in a given lobule and multiplying by the voxel resolution, thus resulting in a cubic millimeter measurement. Left and right lobar volumes were summed, as well as lobules VIIIa and VIIIb, which subserve similar sensory motor function.22 Therefore, 16 lobule volumes were used for the statistical analyses. To account for differences in head size, volumes were standardized by posterior fossa volume size.38
Clinical Cohort and Motor Assessments
Demographic information including sex, age at imaging study, age at clinical diagnosis, disease duration, and motor severity (UPDRS-off medication/WHIGET) were collected. Disease duration was ascertained from the date of first reported motor symptom.39,40
Subscores of the UPDRS and WHIGET, which assessed resting, postural, or action tremor, were summed and standardized to a common tremor ratio based on a total possible score of 56 on the UPDRS and 52 on the WHIGET. Using these subscores, we calculated 3 tremor scores: postural tremor, resting tremor, and total tremor score. To score resting or postural tremor, the UPDRS uses amplitude, frequency of tremor, and if it is apparent at rest or during a motor task. It is rated 0–4, with higher scores indicating more severity. For resting and postural tremor, the WHIGET uses amplitude, persistence at rest or action, and oscillatory movement and is assessed on a 0–3 scale, increasing with severity. Resting and postural tremors of all extremities were summed for a total tremor score (Q10 and Q21 in the UPDRS and Q1 and Q2 of the WHIGET). Gait severity was based on Q29 and Q30 of the UPDRS, which assesses the severity of gait, festination, propulsion, and need for assistance, and the patient’s ability to recover from a fall after being pulled backward from a standing position. These scores were summed for a total gait/posture score in PD patients, in which the WHIGET does not include gait or posture assessments.
Statistical Analysis
Group differences in demographic and clinical parameters present in both ET and PD patients were evaluated using a t test or chi-square test (sex). General linear regression model (GLM) analyses were used to analyze group differences in cerebellar lobule volumes between PD and ET, covarying for age at scan and sex.41–43 To evaluate if the volume of the sensorimotor lobules (ie, lobules IV, V, VI, and VIII)22,23,25,44–46 is related to motor severity, we performed GLM analyses with cerebellar volume as dependent variables, motor scores as the independent variable, and age and sex as covariates. In addition, an interaction model was performed to identify if sensorimotor cerebellar lobules relate to tremors differently between ET and PD patients while correcting for age and sex. To evaluate if cerebellar vermis volume is related to gait in PD patients, we performed GLM analyses with vermal volume as a dependent variable, gait score as an independent variable, and age and sex as covariates. Finally, to investigate if there are disease-specific cerebellar networks characterizing motor scores across groups, we performed backward selection analysis with the motor score as the response, the 16 cerebellar volumes as predictors, and age, sex, and group as covariates (supplementary material).
Statistical analyses were performed using R (version 3.5.3). To account for multiple comparisons, the results were considered significant at the level of false discovery rate (FDR) of 0.05.
Results
Patient Demographics and Clinical Characteristics
Our cohort consisted of 393 individuals, 282 with PD and 111 with ET (Table 1). ET patients were older and reported earlier onset of tremor symptoms with longer disease duration. For PD patients, mean UPDRS off score was 42.35, and for ET individuals, their average WHIGET score was 28.40.
TABLE 1.
Demographic and clinical data
| Parkinson’s disease (n = 282) | Essential tremor (n = 111) | P | |
|---|---|---|---|
| Sex (M:F) | 184:98 | 50:61 | < 0.001 |
| Age at scan (years) | 62.14 (8.47) | 66.16 (9.65) | < 0.001 |
| Age at diagnosis (years) | 52.74 (8.89) | 43.95 (17.7) | < 0.001 |
| Disease duration (years) | 8.98 (4.45) | 21.77 (15.53) | < 0.001 |
| UPDRS OFF total | 42.35 (11.98) | — | — |
| UPDRS (resting) | 3 (2.51) | — | — |
| UPDRS (postural) | 2 (1.43) | — | — |
| UPDRS (gait) | 3 (1.62) | — | — |
| WHIGET OFF total | — | 28.40 (9.19) | — |
| WHIGET (resting) | — | 2 (1.72) | — |
| WHIGET (postural) | — | 3 (1.45) | — |
Data are shown as mean (standard deviation); UPDRS and WHIGET subscores as median (standard deviation).
UPDRS OFF, United Parkinson’s Disease Rating Scale off dopamine medication; UPDRS resting and postural, 8 points max; UPDRS gait, 4 points max; WHIGET OFF, Washington Heights-Inwood Genetic Study of Essential Tremor off essential tremor medication; WHIGET resting and postural, 6 points max.
Cerebellar Differences Between Parkinson’s Disease and Essential Tremor
Figure 1 illustrates cerebellar segmentation results in an ET and PD patient. Group differences between PD and ET are of interest in 2 cerebellar regions, the deep cerebellar nuclei and VI. The direction of these differences differed depending on the clinical diagnosis. Compared with PD patients, ET patients had lower volumes of the deep cerebellar nuclei (P = 0.083, corrected). On the other hand, compared with ET patients, lobule VI was smaller in PD patients (P = 0.083, corrected; Fig. 2).
FIG. 2.
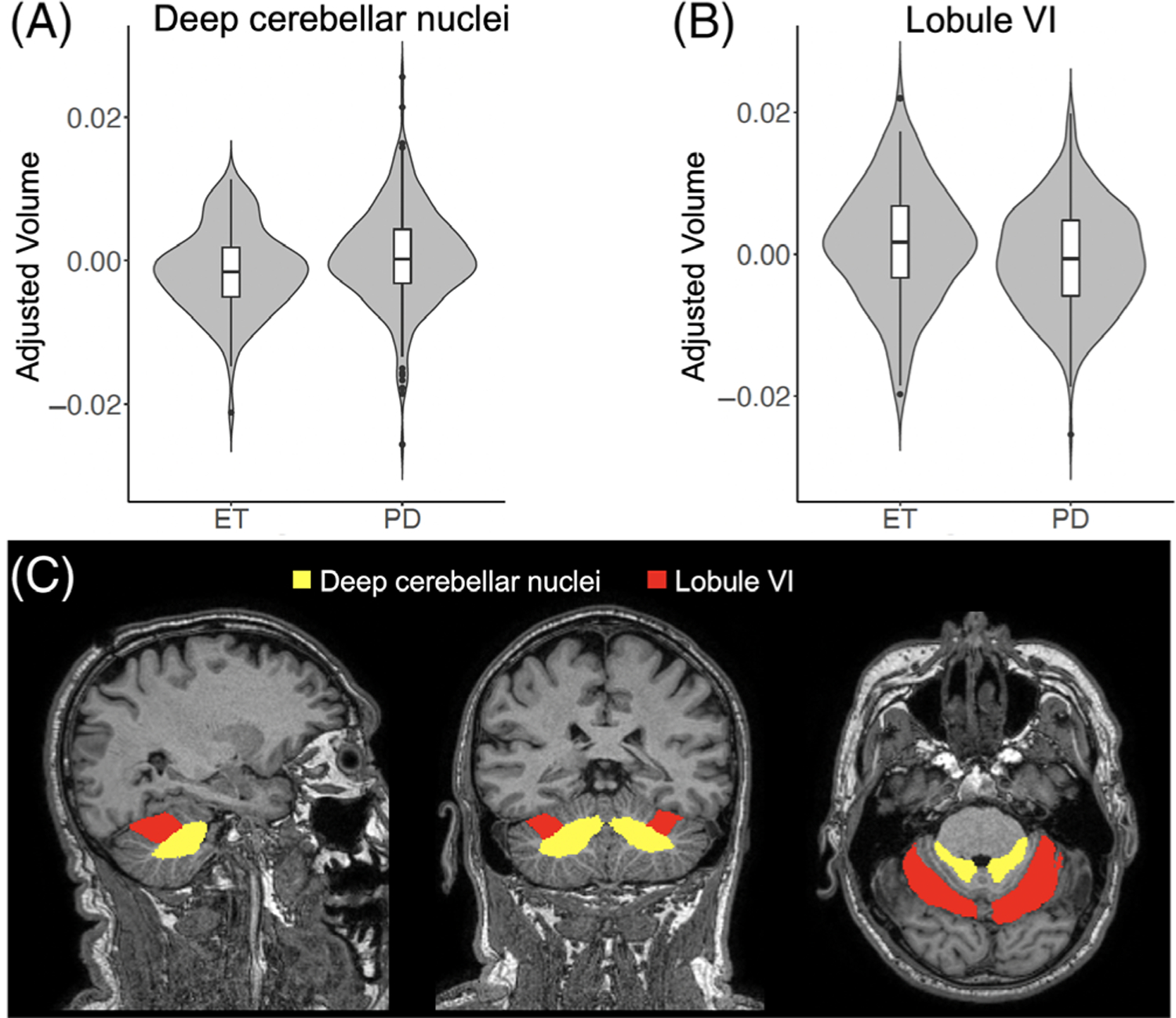
Cerebellar volume differences between ET and PD patients. (A–B) Violin plot showing the distribution of age- and sex-adjusted cerebellar volumes for deep cerebellar nuclei (A) and lobule VI (B) and for ET and PD patients. (C) Location of the ROIs in the deep cerebellar nuclei (yellow) and lobule VI (red) in sagittal (left), coronal (center), and axial (right) views. [Color figure can be viewed at wileyonlinelibrary.com]
Lobule Volume, Tremor Type, and Tremor Severity
To determine if clinical relevance of tremor type and severity with the volume of the sensorimotor lobules (IV, V, VI, and VIII) exists, we assessed the relationship between motor severity and lobule volume. This was based on an a priori definition of tremor type (ie, postural tremor, resting tremor, and total tremor). For postural tremor, tremor severity and lobule VIII volume were negatively correlated in ET patients (P = 0.016, corrected). In contrast, there was no relationship between postural tremor severity and lobule volumes in PD patients. However, a positive correlation between lobule IV volume and severity of resting tremor (P = 0.088, corrected) and total tremor score (P = 0.092, corrected) was noted in PD patients.
We tested for an interaction between cerebellar volume, tremor severity, and group diagnosis to identify if sensorimotor cerebellar lobules relate to tremor scores differently in ET and PD patients. An interesting group interaction was noted with postural tremor. Specifically, the relationship between lobule VIII and postural tremor was different between the ET and PD groups (P = 0.080, corrected; Fig. 3). No significant differences were identified in the relationship between resting tremor and cerebellar volumes between the 2 groups.
FIG. 3.
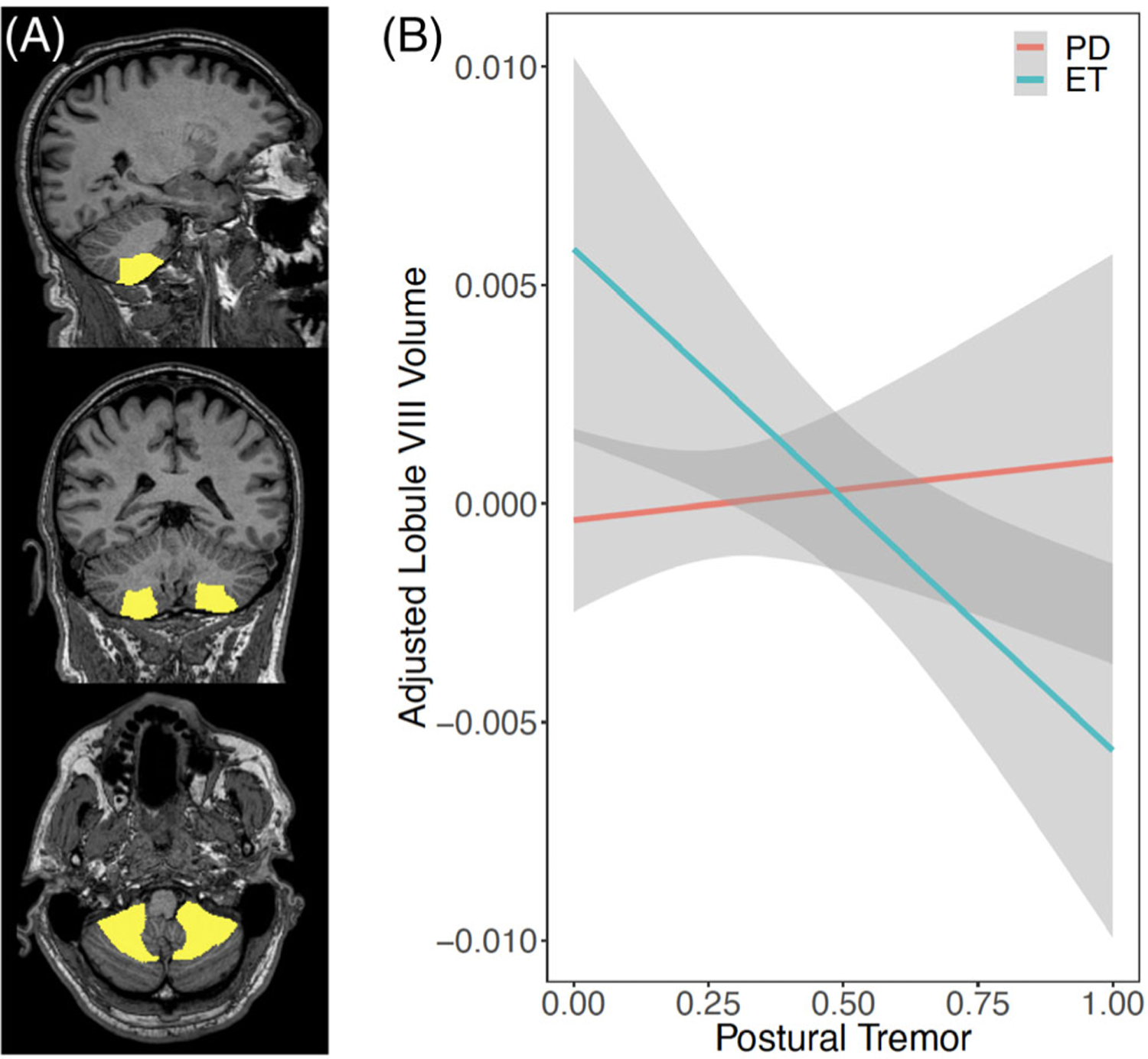
Relationship between lobule VIII volume and upper extremity postural tremor score. (A) Location of lobule VIII in sagittal (top), coronal (center), and axial (bottom) views. (B) Linear regression using age and sex as a covariate relating lobule VIII volume and postural tremor score. Higher postural tremor scores indicate greater impact of motor symptoms. The interaction model showed that the relationship between lobule VIII and postural tremor was different between ET and PD groups (FDR-corrected P = 0.080). [Color figure can be viewed at wileyonlinelibrary.com]
Vermis Volume and Gait
Based on our a priori hypothesis that lower volumes of the cerebellar vermis relate to the severity of gait symptoms in PD, we assessed the relationship between the severity of gait symptoms, with all 5 vermal lobules. This analysis revealed that lower lobule VIIIa volume is negatively correlated with the severity of gait symptoms in PD patients, but this finding did not pass FDR correction (P = 0.133, corrected).
Discussion
We observed differences in cerebellar lobule size between the ET and PD groups and describe interesting relationships between cerebellar structure and the nature and severity of tremor (postural and resting tremor) in patients. These findings link the sensorimotor cerebellum to disease-specific tremor symptoms. In ET patients, worsening postural tremor severity is negatively associated with lobule VIII volume, whereas in PD, worsening resting tremor scores appear to be positively correlated with lobule IV volume. We propose that pathologic and cerebellar-based network alterations in respect to each disease state underpin the clinical relationships between cerebellar lobule size and tremor symptomatology.
Cerebellar Lobule and Relationships to Motor Symptoms
Although postural and resting tremors are recognized clinically as distinct entities, our findings suggest evidence of within-group differences in cerebellar lobule volume and the presence of postural and resting tremor. Consistent with the somatotopic organization of the cerebellum, we note important relationships between lobule VIII size and postural tremor. In ET, the negative relationship between postural tremor score and lobule VIII volume is supported by previous studies linking this region to upper extremity somatomotor function.22 Upper extremity motor movement has traditionally been thought to solely involve the anterior lobe; our findings support that the posterior lobule is also relevant to motor function, supporting the conserved role of the anterior and posterior lobes.46
The positive correlation between increased lobule IV volume and worsening resting and total tremor scores in PD was unexpected but may support the concept of cerebellar compensation in PD,47 which may be driven by medication or disease-related effects on somatomotor cerebellar networks. Previous fMRI studies suggest that increased connectivity of cerebellar-thalamocortical networks are necessary to compensate for progressive motor dysfunction in PD.48,49 In patients assessed off l-dopa therapy, increased connectivity between bilateral lobule IV predicted faster Grooved Pegboard completion, a standardized assessment of upper extremity dexterity.50 In addition, bilateral activation of lobule IV was noted in PD patients performing simple motor tasks.50 We hypothesize the increased connectivity between the putamen and lobule IV can result in relative hypertrophy of this lobule.51 The relationship with total tremor is most likely driven by resting tremor because total tremor is a composite score of resting and postural tremor. Increased activity in lobule IV may function to reduce the amplitude and severity of resting tremor.52,53 Prior studies suggest that lobule V is also involved in compensatory mechanisms of the cerebellum in PD through an anterior cerebellar lobe network51,54; however, we did not see a relationship between postural and resting tremor scores in our study. This may be a limitation of the structural assessment.
In this study, we began with an a priori hypothesis assessing the relationship between sensorimotor lobules IV, V, VI, and VIII and tremor severity and vermal volumes and gait severity. It is possible that disease-specific cerebellar networks may include other regions and impact motor scores differentially in different groups. Unbiased multivariate backward selection analyses (supplementary material) confirmed that sensorimotor lobules IV and V are relevant to group-related tremor symptoms, but also emphasized the potential role of Crus I and lobule IX. Traditionally, Crus I is associated with nonmotor function such as speech and memory,21,46 whereas lobule IX is involved with visual guidance.21 Crus I and lobule IX’s role in motor movement is not well understood and deserves further investigation, especially as visually guided networks may differ in ET patients.55
Group Differences Between ET and PD
ET and PD patients can have overlapping clinical characteristics, sometimes making a definitive diagnosis difficult. However, we noted 2 regions of the cerebellum that differed between ET and PD groups. After age- and sex-adjusted cerebellar lobule volume comparisons, deep cerebellar nuclei regions were smaller in ET compared with PD patients (P = 0.083, corrected). Deep cerebellar nuclei contain 4 nuclei; one is the dentate nucleus. The dentate nucleus has been identified as the possible etiology of tremor in ET, and pathophysiologic changes to this region may occur in ET.56,57
Although lobule VI was smaller in PD patients (P = 0.083, corrected), the somatotopic organization of this region is complex. Lobule VI has been linked to upper extremity somatomotor function, topographic face recognition, and cognitive function.21 We assessed various clinical motor scores, such as upper extremity total tremor, postural tremor, resting tremor, head tremor, and bradykinesia scores, yet these variables did not indicate a relationship with lobule VI. Lobule VI is functionally coupled to associative regions in the cerebellar cortex during cognitive and emotional tasks, known to be impacted in PD.58,59 Other neuroimaging studies have identified gray matter atrophy in cerebellar lobules such as Crus I.22,60 Our results appear to differ from that previously reported by Chen et al.60 These differences may result from distinctions in clinical demographics, such as years of education or cognitive function. Future studies should assess the role of lobule VI and other posterior lobules of the cerebellum in cognitive function in PD.
Clinical Implications of Cerebellar Changes in ET
The classification of ET as a neurodegenerative process is largely debated. We observed reduced volume of deep cerebellar nuclei in ET patients compared with PD patients, suggesting that ET symptoms are associated with a “degenerating” cerebellum. In previous studies using magnetic resonance spectroscopy, reductions in N-acetylaspartate/total creatine ratio, which is as an indicator for neuronal degeneration, were only found in the cerebellum and in no other brain regions in ET.10,61 In ET, the dentate can undergo a period of increased hypertrophy followed by neuronal loss, depending on the stage of disease.62,63 The cause of this degeneration may be because of atrophy of GABAergic purkinje cells projecting to the dentate.64,65 Postmortem studies in ET noted a loss of dendritic spines as well as dendritic arborization compared with healthy volunteers and individuals with PD.66 As ET progresses, reduced concentrations of GABA receptors in the deep cerebellar nuclei were also noted.67 Because our cohort was moderate to severe in nature (ie, patients needing DBS), this may account for the volume differences we noted in this region.
Importantly, our findings support our previous work linking white-matter changes seen in the cerebellothalamo-cortical tract in ET.7 This network involves the superior cerebellar peduncle, which receives input from deep cerebellar nuclei and the dentate and projects to the thalamus. Given the projections from the medial and dorsal accessory olivary nucleus to lobule VIII and cerebellar nuclei, future studies investigating changes to inputs to the dentate could clarify putative extracerebellar pathologic changes in ET.21
Limitations and Future Directions
Although this multiatlas approach was useful to delineating lobule volumes, it is possible that a degenerating lobule could artificially stretch adjacent lobules, making them appear larger volumetrically. We are unware of established methods to study and adjust for this phenomenon. Importantly, we did not appreciate an abnormal shape of the lobules during manual review of segmentation results. To better understand changes over time, a natural history of cerebellar lobule volumes should be performed, inspecting adjacent volume size and relationships with tremor symptoms. Also, we did not include a nontremor control population in this study. This is primarily because of possible anesthesia effects on structural MRI findings, yet the comparison between PD and ET was intended to highlight group and symptoms differences.68
Given our results, postmortem studies investigating sensorimotor lobules and deep cerebellar nuclei may be useful in identifying different pathologic processes in ET and PD patients. Tractography and network investigations of the sensorimotor networks could better elucidate connections between specific lobules of the cerebellum and subcortical and cortical structures. In addition, further studies assessing interhemispheric cerebellar changes in individuals with unilateral or bilateral tremor is warranted. This information may be critical in understanding clinical presentation, guiding disease-modifying treatments, as well as differences between other movement disorders.
Supplementary Material
Acknowledgments:
Vanderbilt Medical Scholars Program (to A.L.), R01 NS0997783 (to D.C.), R01 NS097618 (to D.E.), UL1 RR 024975 (to A.L.), and R01 NS095291 (to B.D.).
Footnotes
Relevant conflicts of interest/financial disclosures: None to report.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Helmich RC, Toni I, Deuschl G, Bloem BR. The pathophysiology of essential tremor and Parkinson’s tremor. Curr Neurol Neurosci Rep 2013;13(9):378. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008;79(4):368–376. [DOI] [PubMed] [Google Scholar]
- 3.Algarni M, Fasano A. The overlap between essential tremor and Parkinson disease. Parkinsonism Relat Disord 2018;46:S101–S104. [DOI] [PubMed] [Google Scholar]
- 4.Schwindt G, Rezmovitz J. Essential tremor. CMAJ 2017;189(44): E1364–E1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann CR. Epidemiology, diagnosis and differential diagnosis in Parkinson’s disease tremor. Parkinsonism Relat Disord. 2012;18: S90–S92. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED. Linking essential tremor to the cerebellum: neuropathological evidence. The Cerebellum 2016;15(3):235–242. [DOI] [PubMed] [Google Scholar]
- 7.Juttukonda MR, Franco G, Englot DJ, et al. White matter differences between essential tremor and Parkinson disease. Neurology 2019;92(1):e30–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Hopfner F, Becktepe JS, Deuschl G. Rest tremor revisited: Parkinson’s disease and other disorders. Transl Neurodegener 2017; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmahmann JD, Pandya DN. The cerebrocerebellar system. In: Schmahmann JD, ed. International Review of Neurobiology. Vol 41. International Review of Neurobiology. Academic Press; 1997: 31–60. [DOI] [PubMed] [Google Scholar]
- 10.Passamonti L, Cerasa A, Quattrone A. Neuroimaging of essential tremor: what is the evidence for cerebellar involvement? Tremor Other Hyperkinet Mov (N Y) 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis ED, Huang CC, Dyke JP, Long Z, Dydak U. Neuroimaging studies of essential tremor: how well do these studies support/refute the neurodegenerative hypothesis? Tremor Other Hyperkinet Mov (N Y) 2014;4:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark LN, Louis ED. Essential tremor. Handb Clin Neurol 2018; 147:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis ED. Essential tremor: a common disorder of Purkinje neurons? Neuroscientist 2016;22(2):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyke JP, Cameron E, Hernandez N, Dydak U, Louis ED. Gray matter density loss in essential tremor: a lobule by lobule analysis of the cerebellum. Cerebellum Ataxias 2017;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govert F, Becktepe JS, Deuschl G. Current concepts of essential tremor. Rev Neurol (Paris) 2016;172(8–9):416–422. [DOI] [PubMed] [Google Scholar]
- 16.Hopfner F, Deuschl G. Is essential tremor a single entity? Eur J Neurol 2018;25(1):71–82. [DOI] [PubMed] [Google Scholar]
- 17.Bareš M, Husárová I, Lungu OV. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov (N Y). 2012;2:tre-02–93-653–1. [PMC free article] [PubMed] [Google Scholar]
- 18.Louis ED, Rabinowitz D, Choe M, et al. Mapping purkinje cell placement along the Purkinje cell layer: an analysis of postmortem tissue from essential tremor patients vs. controls. Cerebellum 2016; 15(6):726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly G, Tate W, Faust P, Louis E. Dentate nucleus neuronal density: a postmortem study of essential tremor vs. control brains (P3.013). Neurology 2017;88(16 Supplement):P3.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 2009;44(2):489–501. [DOI] [PubMed] [Google Scholar]
- 21.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010;46(7):831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 2012;59(2):1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis MM, Slagle CG, Smith AB, et al. Task specific influences of Parkinson’s disease on the striato-thalamo-cortical and cerebellothalamo-cortical motor circuitries. Neuroscience 2007;147(1): 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharifi S, Nederveen AJ, Booij J, van Rootselaar A-F. Neuroimaging essentials in essential tremor: a systematic review. Neuroimage Clin 2014;5:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broersma M, van der Stouwe AMM, Buijink AWG, et al. Bilateral cerebellar activation in unilaterally challenged essential tremor. Neuroimage Clin 2015;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diedrichsen J, King M, Hernandez-Castillo C, Sereno M, Ivry RB. Universal transform or multiple functionality? Understanding the contribution of the human cerebellum across task domains. Neuron 2019;102(5):918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palesi F, Tournier J-D, Calamante F, et al. Contralateral cerebellothalamo-cortical pathways with prominent involvement of associative areas in humans in vivo. Brain Struct Funct 2015;220(6): 3369–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coenen VA, Mädler B, Allert N, Paus S, Kronenbürger M, Urbach H. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery 2014;75(6):657–670. [DOI] [PubMed] [Google Scholar]
- 29.Benito-León J, Serrano JI, Louis ED, et al. Essential tremor severity and anatomical changes in brain areas controlling movement sequencing. Ann Clin Transl Neurol 2019;6(1):83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoodley CJ, Schmahmann JD. Functional topography of the human cerebellum. Handb Clin Neurol 2018;154:59–70. [DOI] [PubMed] [Google Scholar]
- 31.Cerasa A, Messina D, Nicoletti G, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. Am J Neuroradiol 2009;30(6):1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plassard AJ, Yang Z, Rane S, Prince JL, Claassen DO, Landman BA. Improving cerebellar segmentation with statistical fusion. Proc SPIE Int Soc Opt Eng 2016;9784:97842R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol 1993;50(2): 140–148. [DOI] [PubMed] [Google Scholar]
- 34.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998;13(Suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 35.Carass A, Cuzzocreo JL, Han S, et al. Comparing fully automated state-of-the-art cerebellum parcellation from magnetic resonance images. Neuroimage 2018;183:150–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plassard AJ, D’Haese PF, Pallavaram S, et al. Multi-modal and targeted imaging improves automated mid-brain segmentation. Proc SPIE Int Soc Opt Eng 2017;10133:101330J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogovic JA, Jedynak B, Rigg R, et al. Approaching expert results using a hierarchical cerebellum parcellation protocol for multiple inexpert human raters. Neuroimage 2013;64:616–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huo Y, Asman AJ, Plassard AJ, Landman BA. Simultaneous total intracranial volume and posterior fossa volume estimation using multi-atlas label fusion. Hum Brain Mapp 2017;38(2):599–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claassen DO, McDonell KE, Donahue M, et al. Cortical asymmetry in Parkinson’s disease: early susceptibility of the left hemisphere. Brain Behav 2016;6(12):e00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniel O Claassen David A. Isaacs, Olivia C. et al. Linear and curvilinear trajectories of cortical loss with advancing age and disease duration in Parkinson’s disease. Aging Dis 2016;7(3): 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oguro H, Okada K, Yamaguchi S, Kobayashi S. Sex differences in morphology of the brain stem and cerebellum with normal ageing. Neuroradiology 1998;40(12):788–792. [DOI] [PubMed] [Google Scholar]
- 42.Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage 2010; 49(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol 2001;22(6):1161–1167. [PMC free article] [PubMed] [Google Scholar]
- 44.Stoodley C, Schmahmann J. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 2009;44(2):489–501. [DOI] [PubMed] [Google Scholar]
- 45.Guell X, Schmahmann JD, Gabrieli JDE, Ghosh SS. Functional gradients of the cerebellum. Elife 2018;7:e36652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci 2018;42: 337–364. [DOI] [PubMed] [Google Scholar]
- 47.Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain 2013;136(3):696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Appel-Cresswell S, de la Fuente-Fernandez R, Galley S, McKeown MJ. Imaging of compensatory mechanisms in Parkinson’s disease. Curr Opin Neurol 2010;23(4):407. [DOI] [PubMed] [Google Scholar]
- 49.Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg & Psychiatry 2008;79(7):760–766. [DOI] [PubMed] [Google Scholar]
- 50.Festini SB, Bernard JA, Kwak Y, et al. Altered cerebellar connectivity in Parkinson’s patients ON and OFF L-DOPA medication. Front Hum Neurosci 2015;9:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simioni AC, Dagher A, Fellows LK. Compensatory striatal–cerebellar connectivity in mild–moderate Parkinson’s disease. Neuroimage Clin 2016;10:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer SJ, Li J, Wang ZJ, McKeown MJ. Joint amplitude and connectivity compensatory mechanisms in Parkinson’s disease. Neuroscience 2010;166(4):1110–1118. [DOI] [PubMed] [Google Scholar]
- 53.Helmich RC, Janssen MJR, Oyen WJG, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 2011;69(2):269–281. [DOI] [PubMed] [Google Scholar]
- 54.Gao L, Zhang J, Hou Y, Hallett M, Chan P, Wu T. The cerebellum in dual-task performance in Parkinson’s disease. Sci Rep 2017;7:45662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Archer DB, Coombes SA, Chu WT, et al. A widespread visually-sensitive functional network relates to symptoms in essential tremor. Brain 2017;141(2):472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Santaniello S. Role of cerebellar GABAergic dysfunctions in the origins of essential tremor. Proc Natl Acad Sci U S A 2019; 116(27):13592–13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louis ED, Lenka A. The olivary hypothesis of essential tremor: time to lay this model to rest? Tremor Other Hyperkinet Mov (N Y) 2017;7:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 2013;17(5): 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braak H, Rüb U, Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson’s disease. J Neurol Sci 2006; 248(1–2):255–258. [DOI] [PubMed] [Google Scholar]
- 60.Lin C-H, Chen C-M, Lu M-K, et al. VBM reveals brain volume differences between Parkinson’s disease and essential tremor patients. Front Hum Neurosci 2013;7:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett 2002;333(1):17–20. [DOI] [PubMed] [Google Scholar]
- 62.Louis ED, Babij R, Lee M, Cortés E, Vonsattel J-PG. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord 2013;28(13): 1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuo S-H, Erickson-Davis C, Gillman A, Faust PL, Vonsattel J-PG, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry 2011;82(9): 1038–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louis ED. Essential tremor and the cerebellum. Handb Clin Neurol 2018;155:245–258. [DOI] [PubMed] [Google Scholar]
- 65.Louis ED, Hernandez N, Dyke JP, Ma RE, Dydak U. In vivo dentate nucleus gamma-aminobutyric acid concentration in essential tremor vs. controls. Cerebellum 2018;17(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Louis ED, Vonsattel JPG, Honig LS, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol 2006; 63(8):1189–1193. [DOI] [PubMed] [Google Scholar]
- 67.Paris-Robidas S, Brochu E, Sintes M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain 2011;135(1): 105–116. [DOI] [PubMed] [Google Scholar]
- 68.White JC, Verlot M, Selverstone B, Beecjer HK. Changes in brain volume during anesthesia: the effects of anoxia and hypercapnia. JAMA Surg 1942;44(1):1–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


