Abstract
Simple Summary
Angiogenesis and apoptosis play a pivotal role in the pathogenesis and clinical course not only of nasopharyngeal cancer (NPC), but also of other subgroups of head and neck cancer (HNC), such as laryngeal cancer. Thus, the aim of this study was to investigate the clinical significance of genetic polymorphisms in four pivotal angiogenesis- and apoptosis-related genes (VEGFA, FAS, EDNRA and NBS1) in HNC patients. Thirty-four genetic variants located in the studied genes were assessed. Two of them (VEGFA rs13207351 and FAS rs2234768) were associated with overall survival for patients with laryngeal cancer and NPC, respectively, with VEGFA rs13207351 showing the most promise for its prognostic value in the subgroup of laryngeal cancer patients. This study suggests that genetic variations in angiogenesis- and apoptosis-related genes may be useful in the management of HNC patients.
Abstract
Head and neck cancer (HNC) is a significantly heterogeneous disease and includes malignancies arising from different anatomical sites, such as nasopharyngeal cancer (NPC) and laryngeal cancer (LC). In the current study, polymorphisms located in angiogenesis- and apoptosis-related genes (VEGFA, FAS, EDNRA and NBS1) were evaluated regarding their clinical significance in HNC patients. In total, 333 HNC patients were enrolled in this study and 34 variants located on the aforementioned genes were genotyped via Sanger sequencing. LC patients, homozygous A for VEGFA rs13207351, had shorter overall survival (OS) as opposed to homozygous G (Hazard ratio (HR) = 2.06, Wald’s p = 0.017) upon adjustment for age, disease stage, and surgery. Following the dominant model, LC patients carrying the A allele had a marginally significantly higher risk for death (HR = 1.72, p = 0.059). NPC patients heterozygous (CT) for FAS rs2234768 had a marginal but significantly higher risk of death compared to those with homozygosity for the T allele (HR = 2.22, p = 0.056). In conclusion, rs13207351 (VEGFA) and rs2234768 (FAS) polymorphisms seem to have prognostic significance in HNC, with VEGFA rs13207351 showing the most promise in this subgroup of LC patients.
Keywords: head and neck cancer, nasopharyngeal cancer, VEGFA, FAS, endothelin, NBS1, SNPs
1. Introduction
Head and neck cancer (HNC) is the seventh most frequent cancer type worldwide [1]. According to Global Cancer Observatory (GLOBOCAN) 2020, cancers of the lips, oral cavity, larynx, and pharynx account for 4.6% of all new cases and deaths globally [2]. In the USA, the estimated new cases and deaths due to malignancies in the oral cavity, pharynx and larynx during the current year will be 66,630 and 14,620, respectively [3]. In Europe, approximately 140,000 cases and 63,500 deaths due to HNC were reported in 2012 [4]. HNC is associated with an increased consumption of alcohol and/or the use of tobacco [5], whereas some specific subtypes are closely related to human papillomavirus (HPV) [6] and Epstein–Barr virus (EBV) infection [7].
HNC is characterized by significant heterogeneity, as it includes cancers arising from different anatomical sites in the head and neck. In addition, this group of carcinomas is also heterogeneous regarding the molecular mechanisms involved in the pathophysiology of the disease, as well as their pathological characteristics and their embryological origin. The majority (more than 90%) of HNCs present with squamous cell histology [8]. Nasopharyngeal carcinoma (NPC), a type of HNC, also has squamous histology and is categorized into three histological subtypes according to World Health Organization (WHO) classification: keratinizing (20–25%), non-keratinizing undifferentiated type (60–65%), and non-keratinizing differentiated type (10–15%) [9]. NPC mainly develops in the nasopharyngeal portion of the pharynx, which is derived from the neural crest of the ectodermal leaflet, while laryngeal cancer (LC), the main non-NPC carcinoma in the current study, is derived from the larynx, which arises from the 4th and 6th pharyngeal arches in combination with an endodermal inner surface lining [10,11]. NPC is rare in Caucasians from Western countries, with the annual incidence ranging between 0.1–1.1/100,000 people, while it reaches 1/100,000 inhabitants in Southeast Europe [12,13]. In contrast, NPC is deemed to be endemic in China, Southeast Asia, and Africa [14].
The role of EBV infection in NPC pathogenesis and progression is well documented, and due to this direct etiological implication, NPC has been characterized as a virus-related malignancy [7]. In addition, EBV infection has also been associated with the undifferentiated histological subtype of NPC, which is the most common subtype in endemic countries. In the USA and North European countries, differentiated and non-keratinizing squamous cell carcinoma is the predominant histology of NPC [15].
A pivotal cancer hallmark, aberrant in NPC as well as in HNC, is angiogenesis [16,17]. One of the most essential angiogenesis-related molecules is vascular endothelial growth factor A (VEGFA), which has attracted the interest of the scientific community regarding its role in both NPC and HNC [18]. Amongst the most interesting issues about angiogenesis is the clinical value of genetic variations in the VEGFA gene, a topic that has been studied in many cancer types from our as well as other research groups [19,20,21,22,23,24].
Furthermore, the endothelin-1 (END1)/endothelin A receptor (EDNRA) axis also plays an essential role in angiogenesis [25]. In particular, EDNRA has been reported as an independent prognostic value for distant metastases in patients with NPC, while almost three-quarters of NPCs overexpress EDNRA [26]. Similar to VEGFA, variants of EDNRA have also been associated with the prognosis of patients with locoregionally advanced NPC [27], as well as with reduced post radiotherapy xerostomia [28].
A significant role has also been attributed to apoptosis in HNC, which is particularly deregulated, leading to the bypass of apoptotic signals. One of the major regulators of apoptosis is the Fas cell surface death receptor (FAS) protein, a cell surface receptor that interacts with FAS ligand (FASL), leading to apoptosis [29]. Genetic variants of the FAS gene have been documented to interrupt apoptosis-related signal transduction pathways, destabilizing the balance between cell death and proliferation [30]. In particular, a meta-analysis of 52 studies by Xu et al., showed that genetic variations of FAS are associated with a decreased or increased risk of different types of cancer [31]. Additionally, FAS rs1800682 (-670A>G) has been associated with susceptibility to prostate cancer, esophageal cancer, hepatocellular carcinoma [32], and papillary thyroid cancer [33], but not with cervical cancer [34].
Furthermore, defects in DNA repair mechanisms have also been observed in HNC [35]. A central molecule in cellular response to double-strand DNA breaks is nibrin (NBS1), which participates in the MRN complex (MRE11(Meiotic Recombination 11 Homolog 1)/RAD50 (RAD50 Double Strand Break Repair Protein)/NBS1(Nibrin)), promoting DNA repair and chromosome integrity [36,37]. Genetic polymorphisms in the NBS1 gene may impair the mechanism of responding to DNA damage, creating a predisposition for cancer, whereas mutations in the same gene have been related to Nijmegen breakage syndrome (NBS) [38].
In this study, we evaluated the clinical significance of 34 genetic polymorphisms located in the VEGFA, EDNRA, FAS and NBS1 genes in patients with confirmed NPC or non-NPC HNC.
2. Results
2.1. Patient Characteristics
Among the 333 patients included in this study, 144 (43.2%) had NPC and the rest (56.8%) were diagnosed with non-NPC disease. The majority of the patients with non-NPC had squamous cell carcinomas (96.6%), tumors located in the larynx (85.6%), and had undergone surgery (81.3%). More than half of the NPC patients had undifferentiated carcinomas (58.1%) and were mostly stage III (38.6%). Selected patient and tumor characteristics for the entire cohort are presented in Table 1 by tumor type.
Table 1.
Patient and tumor characteristics by tumor type.
| Parameter | Total (N = 333) | Non-NPC (N = 189) | NPC (N = 144) |
|---|---|---|---|
| Age (N = 314) | |||
| Median (min, max) | 60.2 (14.3, 88.3) | 64.9 (37.1, 88.3) | 51.6 (14.3, 81.5) |
| N (%) | N (%) | N (%) | |
| Sex (N = 333) | |||
| Female | 52 (15.6) | 16 (8.5) | 36 (24.8) |
| Male | 282 (84.4) | 173 (91.5) | 109 (75.2) |
| Alcohol Abuse (N = 292) | |||
| No | 145 (49.7) | 47 (26.6) | 98 (85.2) |
| Yes | 147 (50.3) | 130 (73.4) | 17 (14.8) |
| Smoking (N = 293) | |||
| No | 60 (20.5) | 19 (10.8) | 41 (35.0) |
| Yes | 233 (79.5) | 157 (89.2) | 76 (65.0) |
| Histological Type (N = 307) | |||
| Non-Keratinizing Carcinoma | 37 (12.1) | 0 (0.0) | 37 (28.7) |
| Squamous Cell Carcinoma | 189 (61.6) | 172 (96.6) | 17 (13.2) |
| Undifferentiated Carcinoma | 77 (25.1) | 2 (1.1) | 75 (58.1) |
| Other | 4 (1.3) | 4 (2.2) | 0 (0.0) |
| Primary Tumor Location (N = 318) | |||
| Hypopharynx | 1 (0.3) | 1 (0.57) | 0 (0.0) |
| Larynx | 149 (46.9) | 149 (85.6) | 0 (0.0) |
| Major Salivary Glands | 2 (0.6) | 2 (1.1) | 0 (0.0) |
| Nasopharynx | 144 (45.3) | 0 (0.0) | 144 (100.0) |
| Oral Cavity | 11 (3.5) | 11 (6.3) | 0 (0.0) |
| Oropharynx | 7 (2.2) | 7 (4.0) | 0 (0.0) |
| Paranasal Sinuses | 4 (1.3) | 4 (2.3) | 0 (0.0) |
| T (N = 272) | |||
| T1 | 45 (16.5) | 19 (11.3) | 26 (25.0) |
| T2 | 79 (29.0) | 34 (20.2) | 45 (43.3) |
| T3 | 94 (34.6) | 77 (45.8) | 17 (16.3) |
| T4 | 54 (19.9) | 38 (22.6) | 16 (15.4) |
| N (N = 275) | |||
| N0 | 126 (45.8) | 114 (67.1) | 12 (11.4) |
| N1 | 42 (15.3) | 14 (8.2) | 28 (26.7) |
| N2 | 80 (29.1) | 33 (19.4) | 47 (44.8) |
| N3 | 23 (8.4) | 5 (2.9) | 18 (17.1) |
| Nx | 4 (1.5) | 4 (2.4) | 0 (0.0) |
| Stage (N = 281) | |||
| I | 19 (6.8) | 15 (9.0) | 4 (3.5) |
| II | 52 (18.5) | 23 (13.8) | 29 (25.4) |
| III | 105 (37.4) | 61 (36.5) | 44 (38.6) |
| IV | 105 (37.4) | 68 (40.7) | 37 (32.5) |
| Surgery (N = 176) | |||
| No | 33 (18.8) | 33 (18.8) | - |
| Yes | 143 (81.3) | 143 (81.3) | - |
| Type of Surgery (N = 142) | |||
| Chordectomy | 8 (5.6) | 8 (5.6) | - |
| Hemiglossectomy | 5 (3.5) | 5 (3.5) | - |
| Hemiglossectomy and Laryngectomy | 2 (1.4) | 2 (1.4) | - |
| Laryngectomy | 113 (79.6) | 113 (79.6) | - |
| Other | 14 (9.9) | 14 (9.9) | - |
| Treatment Received (N = 311) | |||
| No | 44 (14.1) | 43 (23.9) | 1 (0.8) |
| Yes | 267 (85.9) | 137 (76.1) | 130 (99.2) |
| Type of First Treatment Received (N = 267) | |||
| Induction Chemotherapy | 10 (3.8) | 0 (0.0) | 10 (7.7) |
| Primary concomitant CT and RT | 179 (67.0) | 65 (47.4) | 114 (87.7) |
| Primary RT | 34 (12.7) | 33 (24.1) | 1 (0.8) |
| RT after First Progression | 1 (0.4) | 1 (0.7) | 0 (0.0) |
| Adjuvant | 32 (12.0) | 29 (21.2) | 3 (2.3) |
| First-Line | 11 (4.1) | 9 (6.6) | 2 (1.5) |
The percentages for the type of surgery and the type of primary treatment were calculated from the total number of patients with available data that had undergone surgery and had received treatment, respectively.
2.2. Variant Distribution
The frequency distribution of the examined variants for the entire cohort and separately for patients with NPC and non-NPC is presented in Table 2. In the non-NPC group of patients, analysis focused on patients with tumors located in the larynx (n = 149), thus leading to a total of 293 patients. Genotyping of VEGFA polymorphisms revealed that all patients were homozygous G for rs12664104 and rs112005313, homozygous T for rs34376996 and rs111933757, homozygous A for rs28357093 and for rs187429037, and homozygous C for rs79469752, rs59260042, rs149179279 and rs112256643. All non-NPC patients were homozygous C for rs149983590, whereas only one of the NPC patients (0.7%) had a heterozygous CA genotype. The observed genotypes for the aforementioned VEGFA single nucleotide polymorphisms (SNPs) in our study, including rs833062, are expected for low frequency or rare SNPs and are in accordance with the respective minor allele frequency (MAF) values in the global and/or European population, as reported in the Single Nucleotide Polymorphism Database (dbSNP) database according to 1000 Genomes Project (1000G), Exome Aggregation Consortium (ExAC), Genome Aggregation Database (gnomAD) or Allele Frequency Aggregator project (ALFA) projects (https://www.ncbi.nlm.nih.gov/snp/, accessed on 10 October 2020). In the case of common VEGFA polymorphisms, most patients were heterozygous AC for rs699947 (49% of non-NPC and 46% of NPC patients), heterozygous for the rs144854329 18 bp indel (49% of non-NPC and 53.6% of NPC), heterozygous for the 1 bp insertion rs35864111 (49% of non-NPC and 53.6% of NPC) and heterozygous CT for rs833061 (referred to as −1498 herein) (50.3% for non-NPC and 48.8% for NPC). These findings are in line with previous haplotype analysis studies, at least for rs699947 and rs144854329 or −1498, which showed strong linkage disequilibrium among the two polymorphisms in both cases [20,39,40]. Additionally, both non-NPC and NPC patients were mostly homozygous A for rs13207351 (46.3% for non-NPC and 47.9% for NPC) and homozygous C for +936 (70.3% for non-NPC and 70.1% for NPC). Finally, most non-NPC patients were homozygous A for −1154 (40.3%), whereas most NPC patients were homozygous G for −1154 (43.6%). The observed frequencies of the common VEGFA polymorphisms described above are comparable to the minor allele frequencies (MAFs) reported for the global and/or European population in dbSNP. The genotyping of EDNRA variants revealed that none of the patients harbored p.L322V (all were homozygous C). Most of the patients were homozygous T for p.H323H (56.8% of non-NPC and 55.3% of NPC patients) and homozygous G for p.E335E (56.8% of non-NPC and 56.1% of NPC patients), consistent with the reported dbSNP MAFs for these common SNPs. Concerning rare EDNRA rs10305924 and rs112710542 SNPs, all non-NPC patients were homozygous G for both, whereas only one NPC patient (0.7%) was heterozygous AG for rs10305924 and six NPC patients (4.3%) were heterozygous AG for rs112710542. For FAS, more than half of the patients were heterozygous AG for −670 among both NPC (63.8%) and non-NPC patients (50.3%). The majority of non-NPC (81.2%) and NPC patients (82.3%) were homozygous T for −690, whereas all patients were homozygous T for rs34995925 and homozygous A for rs150130637. In all instances, the observed genotype frequencies for FAS promoter SNPs in our study were in agreement with their reported MAFs in National Center for Biotechnology Information (NCBI) dbSNP.
Table 2.
Frequency distribution of the examined variants in the total cohort and by tumor type.
| Parameter | Total (N = 293) | Non-NPC (N = 149) | NPC (N = 144) |
|---|---|---|---|
| VEGFA rs699947 (−2578) (N = 286) | |||
| A | 40 (14.0) | 20 (13.4) | 20 (14.6) |
| AC | 136 (47.6) | 73 (49.0) | 63 (46.0) |
| C | 110 (38.5) | 56 (37.6) | 54 (39.4) |
| VEGFA rs12664104 (N = 285) | |||
| G | 285 (100.0) | 149 (100.0) | 136 (100.0) |
| VEGFA rs34376996 (N = 258) | |||
| T | 258 (100.0) | 149 (100.0) | 109 (100.0) |
| VEGFA rs144854329 (N = 289) | |||
| het del18bp | 148 (51.2) | 73 (49.0) | 75 (53.6) |
| hom del18bp | 101 (34.9) | 56 (37.6) | 45 (32.1) |
| reference | 40 (13.8) | 20 (13.4) | 20 (14.3) |
| VEGFA rs35864111 (N = 289) | |||
| het ins1bp | 148 (51.2) | 73 (49.0) | 75 (53.6) |
| hom ins1bp | 101 (34.9) | 56 (37.6) | 45 (32.1) |
| reference | 40 (13.8) | 20 (13.4) | 20 (14.3) |
| VEGFA rs833061 (-1498) (N = 272) | |||
| C | 55 (20.2) | 31 (20.8) | 24 (19.5) |
| CT | 135 (49.6) | 75 (50.3) | 60 (48.8) |
| T | 82 (30.1) | 43 (28.9) | 39 (31.7) |
| VEGFA rs149983590 (N = 288) | |||
| C | 287 (99.7) | 149 (100.0) | 138 (99.3) |
| CA | 1 (0.35) | 0 (0.0) | 1 (0.72) |
| VEGFA rs833062 (N = 276) | |||
| CT | 8 (2.9) | 7 (4.7) | 1 (0.79) |
| T | 268 (97.1) | 142 (95.3) | 126 (99.2) |
| VEGFA rs1570360 (−1154) (N = 289) | |||
| A | 91 (31.5) | 60 (40.3) | 31 (22.1) |
| G | 97 (33.6) | 36 (24.2) | 61 (43.6) |
| GA | 101 (34.9) | 53 (35.6) | 48 (34.3) |
| VEGFA rs28357093 (N = 289) | |||
| A | 289 (100.0) | 149 (100.0) | 140 (100.0) |
| VEGFA rs13207351 (−1190) (N = 289) |
|||
| A | 136 (47.1) | 69 (46.3) | 67 (47.9) |
| AG | 64 (22.1) | 34 (22.8) | 30 (21.4) |
| G | 89 (30.8) | 46 (30.9) | 43 (30.7) |
| VEGFA rs79469752 (N = 289) | |||
| C | 289 (100.0) | 149 (100.0) | 140 (100.0) |
| VEGFA rs59260042 (N = 289) | |||
| C | 289 (100.0) | 149 (100.0) | 140 (100.0) |
| VEGFA rs3025039 (+936) (N = 285) | |||
| C | 200 (70.2) | 104 (70.3) | 96 (70.1) |
| CT | 71 (24.9) | 34 (23.0) | 37 (27.0) |
| T | 14 (4.9) | 10 (6.8) | 4 (2.9) |
| VEGFA rs149179279 (N = 285) | |||
| C | 285 (100.0) | 148 (100.0) | 137 (100.0) |
| VEGFA rs112256643 (N = 285) | |||
| C | 285 (100.0) | 148 (100.0) | 137 (100.0) |
| VEGFA rs112005313 (N = 285) | |||
| G | 285 (100.0) | 148 (100.0) | 137 (100.0) |
| VEGFA rs187429037 (N = 283) | |||
| A | 283 (100.0) | 148 (100.0) | 135 (100.0) |
| VEGFA rs111933757 (N = 280) | |||
| T | 280 (100.0) | 148 (100.0) | 132 (100.0) |
| EDNRA rs5333 (p.H323H) (N = 289) | |||
| C | 13 (4.5) | 6 (4.1) | 7 (5.0) |
| CT | 114 (39.4) | 58 (39.2) | 56 (39.7) |
| T | 162 (56.1) | 84 (56.8) | 78 (55.3) |
| EDNRA rs5334 (p.E335E) (N = 287) | |||
| A | 12 (4.2) | 6 (4.1) | 6 (4.3) |
| AG | 113 (39.4) | 58 (39.2) | 55 (39.6) |
| G | 162 (56.4) | 84 (56.8) | 78 (56.1) |
| EDNRA rs10305924 (N = 285) | |||
| AG | 1 (0.35) | 0 (0.0) | 1 (0.73) |
| G | 284 (99.6) | 148 (100.0) | 136 (99.3) |
| EDNRA rs17856670 (p.L322V) (N = 289) | |||
| C | 289 (100.0) | 148 (100.0) | 141 (100.0) |
| EDNRA rs112710542 (N = 288) | |||
| AG | 6 (2.1) | 0 (0.0) | 6 (4.3) |
| G | 282 (97.9) | 147 (100.0) | 135 (95.7) |
| FAS rs1800682 (-670) (N = 290) | |||
| A | 74 (25.5) | 40 (26.8) | 34 (24.1) |
| AG | 165 (56.9) | 75 (50.3) | 90 (63.8) |
| G | 51 (17.6) | 34 (22.8) | 17 (12.1) |
| FAS rs34995925 (N = 290) | |||
| T | 290 (100.0) | 149 (100.0) | 141 (100.0) |
| FAS rs2234768 (−690) (N = 290) | |||
| C | 1 (0.34) | 1 (0.67) | 0 (0.0) |
| CT | 52 (17.9) | 27 (18.1) | 25 (17.7) |
| T | 237 (81.7) | 121 (81.2) | 116 (82.3) |
| FAS rs150130637 (N = 286) | |||
| A | 286 (100.0) | 149 (100.0) | 137 (100.0) |
| NBS1 rs1805794 (p.E185Q) (N = 291) | |||
| C | 37 (12.7) | 21 (14.1) | 16 (11.3) |
| G | 109 (37.5) | 61 (40.9) | 48 (33.8) |
| GC | 145 (49.8) | 67 (45.0) | 78 (54.9) |
| NBS1 rs192240705 (N = 291) | |||
| T | 291 (100.0) | 149 (100.0) | 142 (100.0) |
| NBS1 rs780661058 (p.A183A) (N = 291) | |||
| A | 291 (100.0) | 149 (100.0) | 142 (100.0) |
| NBS1 rs151070415 (p.A183T) (N = 291) | |||
| G | 291 (100.0) | 149 (100.0) | 142 (100.0) |
| NBS1 rs61754966 (p.I171V) (N = 291) | |||
| A | 289 (99.3) | 148 (99.3) | 141 (99.3) |
| AG | 2 (0.7) | 1 (0.7) | 1 (0.7) |
| NBS1 rs182756889 (p.R169C) (N = 291) | |||
| C | 291 (100.0) | 149 (100.0) | 142 (100.0) |
Regarding NBS1 rare SNPs, all patients were homozygous T for rs192240705 and homozygous A for p.A183A. None of the patients carried p.A183T (homozygous G) or p.R169C (homozygous C), whereas only two patients (0.7%) were heterozygous AG for p.I171V—one with non-NPC and one with NPC. Finally, GC heterozygosity was observed in most non-NPC (45%) and NPC (54.9%) patients for NBS1 p.E185Q.
2.3. Association of Variants with Clinicopathological Characteristics
A significant association was found between VEGFA +936 and age at diagnosis (Wilcoxon rank-sum p = 0.010) in NPC patients. NPC patients who were heterozygous CT for VEGFA +936 were diagnosed at an older age compared to those who were homozygous T (median age 59.4 vs. 47.7 years, Wilcoxon rank-sum p = 0.033) and patients who were homozygous C (median age 59.4 vs. 47.6, Wilcoxon rank-sum p = 0.004) (Figure 1). Among non-NPC patients, FAS rs2234768 (−690) was significantly associated with alcohol abuse (Fisher’s p = 0.033). More specifically, alcohol abuse was more frequent in patients with homozygous T FAS rs2234768 (−690) compared to those with heterozygous CT (81% vs. 59.3%).
Figure 1.
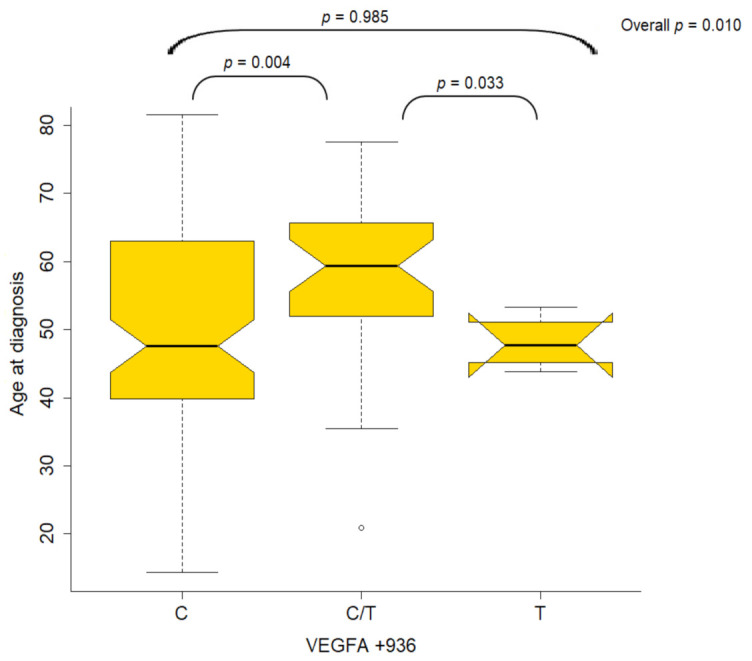
Boxplots of the distribution of age for VEGFA +936 genotypes in NPC patients.
2.4. Improved Overall Survival for NPC Patients
Survival data were available for 272 of 293 patients (92.8%); overall, 148/149 LC (99.3%) and 124 of 144 NPC patients (86.1%). At a median follow-up of 119.4 months [95% confidence interval (CI): 70–130.5], 32 NPC patients had died (25.8%), while the median OS had not been reached at the time of analysis. Patients with LC were followed-up for a median of 95.6 months (95% CI: 74.0–124.8), and during this period, 71 deaths (48%) were reported in LC patients. The median OS for LC patients was 88.6 months (95% CI: 62.2–135.5).
2.5. FAS −690 and Overall Survival in NPC Patients
NPC patients with heterozygous CT for FAS −690 were at a marginal but significantly higher risk of death compared to those with homozygous T (Hazard Ratio (HR) = 2.22, 95% CI: 0.98–5.00, Wald’s p = 0.056, Figure 2); the rest of the variants were not found to be prognostic for OS among patients with NPC (Table S1). Upon adjustment for age and stage, FAS −690 was not found to be a significant prognosticator for OS (p = 0.66) among NPC patients, even though the hazard ratio for patients with heterozygous CT was in the same direction as the one obtained by the univariate analysis (HR = 1.23, 95% CI: 0.48–3.17). We further assessed the potential prognostic significance of variants among NPC patients treated with primary concomitant chemotherapy and radiotherapy (N = 114) since this subgroup accounted for the vast majority of NPC patients. As in the entire cohort of NPC patients, heterozygous CT for FAS −690 as compared to homozygous T showed a marginal but significantly higher risk of death univariately in the subpopulation of NPC patients treated with primary concomitant chemotherapy and radiotherapy (HR = 2.27, 95% CI: 1.00–5.18, p = 0.051), which was not retained upon adjustment for age and stage (HR = 1.32, 95% CI: 0.50–3.44, p = 0.58).
Figure 2.
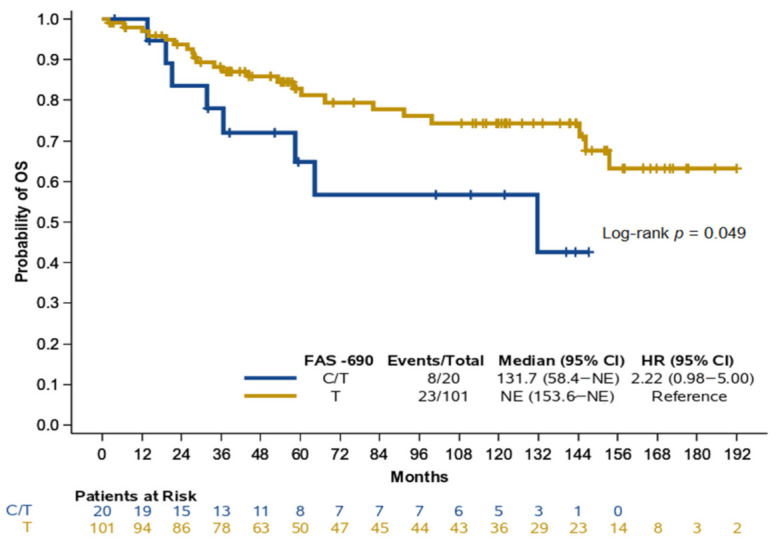
Kaplan–Meier curves for OS according to FAS −690 genotypes in NPC patients. HR: hazard ratio, CI: confidence interval, NE: not estimable.
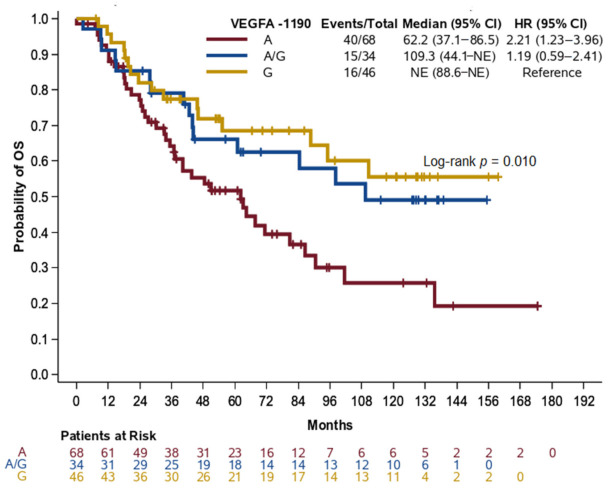
2.6. LC Patients Carrying the A Allele of VEGFA rs13207351 Had a Higher Risk of Death and Poorer Outcomes
LC patients with homozygous A for VEGFA rs13207351 (referred to as −1190 herein) had shorter OS compared to LC patients with homozygous G (HR = 2.21, 95% CI: 1.23–3.96, p = 0.008, Figure 3). Homozygosity A for VEGFA −1190 retained its unfavorable prognostic significance for OS over homozygosity G in LC patients (HR = 2.06, 95% CI: 1.14–3.72, p = 0.017) after adjustment for age (p = 0.69), disease stage (p = 0.10), and surgery (p = 0.87), thus underlining the independent prognostic significance of VEGFA −1190. We further examined the dominant and recessive genetic models for VEGFA −1190 in LC patients. Assuming that A is the risk allele, the dominant genetic model confirmed that carriers of the VEGFA −1190 A allele (A+AG) had a higher risk of death compared to homozygotes for the G allele univariately (HR = 1.79, 95% CI: 1.02–3.12, p = 0.042), while marginal significance was retained after adjustment for age, stage, and surgery (HR = 1.72, 95% CI: 0.98–3.03, p = 0.059, Table 3).
Figure 3.
Kaplan–Meier curves for OS according to VEGFA −1190 genotypes among non-NPC patients. HR: hazard ratio, CI: confidence interval, NE: not estimable.
Table 3.
Hazard ratios and 95% CIs estimated by Cox regression analysis under additive, dominant, and recessive genetic models for VEGFA rs13207351 (−1190) in non-NPC patients.
| Parameter | Univariate | p-Value | Multivariate * | p-Value | ||
|---|---|---|---|---|---|---|
| Event/Total | HR (95% CI) | Event/Total | HR (95% CI) | |||
| VEGFA rs13207351 (−1190) | ||||||
| Additive model | 0.012 | 0.031 | ||||
| A | 40/68 | 2.21 (1.23–3.96) | 0.008 | 38/64 | 2.06 (1.14–3.72) | 0.017 |
| AG | 15/34 | 1.19 (0.59–2.41) | 0.624 | 14/33 | 1.19 (0.57–2.45) | 0.645 |
| G | 16/46 | Reference | 1 | 16/46 | Reference | - |
| Dominant model | ||||||
| A+AG | 55/102 | 1.79 (1.02–3.12) | 0.042 | 52/97 | 1.72 (0.98–3.03) | 0.059 |
| G | 16/46 | Reference | - | 16/46 | Reference | - |
| Recessive model | ||||||
| A | 40/68 | 2.04 (1.27–3.28) | 0.003 | 38/64 | 1.91 (1.17–3.10) | 0.009 |
| AG+G | 31/80 | Reference | - | 30/79 | Reference | - |
* Adjusted for age (continuous) and stage (I–II, III–IV). HR, hazard ratio; CI, confidence interval. Significant p-values are shown in bold italics.
3. Discussion
Despite the advances in the management of HNC during the last few decades, the survival outcome of patients with HNC remains very poor, with the exception of some specific subgroups that are characterized by better clinical course [1]. HNC is a heterogeneous disease not only regarding the clinical manifestations and course, but also in the genetic variations present [41]. Therefore, the study of these genetic variations not only on sheds light on the underlying mechanisms, but also uncovers clinically useful biomarkers, which can contribute to the personalization of treatment. In the present study, 34 variants in the VEGFA, EDNRA, NBS1 and FAS genes were assessed in HNC patients (NPC vs. non-NPC), and the results from genotyping were correlated with clinical and pathological characteristics, as well as with survival outcome. The most interesting finding in our study was the association of the common VEGFA rs13207351 (−1190) SNP with survival outcome. In LC patients, carriers of the A allele had an increased risk of death during the observation period and poorer outcome. This SNP, which is located in the promoter region of the VEGFA gene in a region marked by H3K27Ac and H3K4me3, has a high possibility of being a transcription factor binding site (Figure S1) [42,43] and has been extensively studied in the context of diabetes mellitus. For instance, homozygotes for the minor allele of this SNP have been associated with an increased risk of developing diabetic retinopathy in Chinese patients with type 2 diabetes mellitus [44], but not with the risk for diabetic foot ulcers in the Chinese Han population [45]. Limited data regarding the possible role of rs13207351 in cancer come from a study by our group in patients with metastatic breast cancer that were treated with weekly docetaxel. In that study, homozygotes for the rs13207351 G allele had shorter Progression-free Survival (PFS) compared to carriers of the A allele (combined patients with AG and AA) [46]. Although these studies did not have the same endpoint, as the current study evaluated the prognostic value whereas the previous study evaluated the predictive value of this SNP, this difference may reflect the distinct molecular backgrounds of metastatic breast cancer and HNC, as well as the differences in management and the systematic regimens administered.
Our study further revealed an association of the FAS −690 SNP with OS in NPC patients. The clinical significance of this SNP has not been studied in cancer. However, this SNP is located in a regulatory genomic region (positive strand) marked by H3K27Ac and H3K4me3, and has a high possibility of being a transcription factor binding site (Figure S2) [42,43]. Its location at the promoter of the FAS gene, combined with the significance of the same gene in NPC, leads us to speculate that this SNP may influence underlying pathophysiological mechanisms. This notion is further supported by the findings of Ho et al., who have shown that Fas ligand expression in NPC patients was associated with poorer disease-free survival, as well as poorer overall survival [47]. In addition, other SNPs close to FAS −690 SNP have also been implicated in the clinical outcome of patients with NPC. Bel Hadj Jrad et al., have shown that G allele carriers of the FAS −670 A>G SNP had a significantly increased risk for NPC [48]. Additionally, the G allele of the same SNP has been associated with an increased risk for lymph node infiltration, as well as distant metastases, in a cohort of Chinese patients with NPC [49]. Two other functional SNPs of the FAS/FASL axis (FAS −1377G/A and FASL −844T/C) have also been associated with the development of NPC [50]. Interestingly, the FAS −1377 A allele changes the binding site of the Sp1 transcription factor, decreasing FAS gene expression [51,52]. Thus, further evaluation in a larger cohort of patients is needed to clarify the potential role of this SNP in NPC.
We have to acknowledge some limitations in the current study. First, a major limitation of our study is the number of included patients, which did not permit potent significant associations to be uncovered. Moreover, the heterogeneity of cohort regarding histological identity, as well as treatment management, are limitations of this study. Furthermore, a two-phase design would have been more informative, providing vali-dated results. Assessment of the same SNPs in healthy controls could provide more information regarding their value as risk factors for the development of HNC.
In conclusion, two SNPs in the promoter region of the FAS (rs2234768) and VEGFA (rs13207351) genes seem to be associated with the clinical outcome of patients with HNC, with VEGFA rs13207351 being the most promising. Although these findings need further validation in larger cohorts of NPC and LC patients, this study suggests that genetic variations in angiogenesis- and apoptosis-related genes may be useful in the management of HNC patients.
4. Patients and Methods
4.1. Study Design, Population and Data Collection
The present study was performed upon the approval by the Bioethics Committee of the Aristotle University of Thessaloniki, School of Health Sciences, Faculty of Medicine (#2/23 March 2016) following the Helsinki Declaration (2013) on ethical guidelines [53].
Written informed consent was obtained from all participants unless the Committee had granted a waiver. Patients with histologically confirmed HNC, exclusively of Greek origin, were retrieved from the electronic database of the Hellenic Cooperative Oncology Group (HeCOG). Available germline DNA samples for patients under study were retrieved from the HeCOG Tumor Repository. Genotyping was performed in the Laboratory of Molecular Oncology (MOL) by the Hellenic Foundation for Cancer Research/Aristotle University of Thessaloniki, Thessaloniki, Greece.
4.2. Genetic Variant Selection
We selected known VEGFA, EDNRA, FAS and NBS1 SNPs relevant to nasopharyngeal and other cancers based on previous studies by our group and others, as well as on adequate MAF data and a high rate of heterozygosity in the general population according to dbSNP, 1000G, ExAC and GnomAD databases [19,20,21,23,31,39,46,54,55,56,57,58]. Notably, our genotype analysis by Sanger sequencing allowed us to examine nearby genetic variations in addition to each interrogated SNP, resulting in the examination of 34 variants in the four genes [46].
For VEGFA, we assessed 19 polymorphisms, with 13 located within the 5′ regulatory promoter region, including six common polymorphisms, four SNPs (rs699947 (known as −2578C>A), rs833061 (−1498 or −460C>T), rs13207351 (−1190 or −152G>A), and rs1570360 (known as −1154G>A)), two indels (rs144854329 (-2549) and rs35864111)), and seven SNPs with low (rs34376996, rs833062 (−1455T>C), rs59260042 (−1210 or −172C>A), rs79469752 (−1203 or −165C>T), rs28357093 (−1179 or −141A>C)) or rare (rs149983590 and rs12664104) frequencies. The other six VEGFA polymorphisms resided within the 3′ untranslated region and represented one common (rs3025039 (known as +936C>T)) and five rare (rs111933757, rs187429037, rs112005313, rs112256643, and rs149179279) SNPs. For FAS, we analyzed two common (rs1800682 (known as −670A>G) and rs2234768 (known as −690T>C)) and two rare (rs34995925 (−663T>C) and rs150130637) SNPs in the promoter region. In the case of EDNRA, we examined five variants, three found within coding exon 5 (CCDS 3769.1) representing two common synonymous SNPs (rs5333 (p.H323H) and rs5334 (p.E335E)) and a missense rare variant, considered a mutation, with no reported MAF (rs17856670 (p.L322V), as well as two rare SNPs (rs112710542 (c.901−50G>A) and rs10305924 (c.1034+19G>A)) located in intron 4 and 5, respectively. Finally, for NBS1, we evaluated six variants, five located within coding exon 5 (CCDS 6249.1) including one non-synonymous common SNP (rs1805794 (known as p.E185Q)), a rare synonymous SNP (rs780661058 (p.A183A)), three missense mutations (rs182756889 (p.R169C), rs61754966 (p.I171V), and rs151070415 (p.A183T)) with no reported MAF or global MAF (GMAF) < 0.1%, as well as a rare SNP within intron 5 (rs192240705 (c.584+32T>C)). In the case of VEGFA and FAS, the (−) and (+) symbols denote nucleotides before and after translation start and end, accordingly, with SNP nomenclature according to the dbSNP database and previous cited literature. For EDNRA and NBS1, the reference sequences for numbering annotations are NM_001957.3 and NM_002485.5.
4.3. Variant Genotyping
Germline DNA was extracted from the peripheral blood samples of patients using a standard desalting method, whereas genotypic analysis was performed by Sanger sequencing, as previously described in detail [19,46]. Concerning VEGFA, the primers used for variant detection were (sense and antisense): 5′-AGGATGGGGCTGACTAGGTAA-3′ and 5′-CCCCCTTTTCCTCCAACTCTC-3′ (detects rs699947, rs34376996, rs12664104, rs144854329, and rs35864111); 5′-GCCCATTCCCTCTTTAGCCA-3′ and 5′-AGTGAGGTTAC-GTGCGGACAG-3′ (detects rs833061, rs149983590, and rs833062); 5′-CTGCTCCCTCCTCGCCAATGC-3′ and 5′-CCAAGCCTCCGCGATCCT-3′ (detects rs59260042, rs79469752, rs13207351, rs28357093, and rs1570360); and 5′-TCACCAGGAAAGACTGATACA-3′ and 5′-GGTGGGTGTGTCTACAGGA-3′ (detects rs111933757, rs187429037, rs112005313, rs112256643, rs149179279, and rs3025039). A single primer pair was used for the detection of EDNRA variants (5′-ATTCTTTCTCTGGTGTCTGC-3′ and 5′-GAAAATCTGAGAAACTCCAAT-3′); FAS variants (5′-TGCGATTTGGCTTAAGTTGT-3′ and 5′-GGCTTCTGCTGTAGTTCAACC-3′) and NBS1 variants (5′-AGAGAGATGAAAGGGAAA-3′ and 5′-ATTACATCCTGAAACAAGCAT-3′).
All aforementioned primers were M13-coupled for genotyping. This involved PCR amplification on a GeneAmp PCR system, followed by sense and antisense sequencing with M13 forward and reverse primers and the Big Dye Terminator kit v.1.1 (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. Samples were purified by ethanol/Ethylene Diamine Tetraacetic Acid (EDTA) precipitation and loaded on an Applied Biosystems (ABI) 3130xl Genetic Analyzer. Sequences were visualized upon capillary electrophoresis and called with the Sequencing Analysis software v5.2 (Applied Biosystems/Biosolutions, Athens, Greece).
4.4. Statistical Considerations
Descriptive statistics, including counts with the corresponding percentages (for categorical variables) and medians with the respective ranges (for continuous variables), were used to summarize patient/tumor characteristics and the distribution of the examined variants for the entire cohort by tumor type (NPC vs. non-NPC). The associations of the selected clinicopathological variables with the variants of interest were assessed by the chi-square/Fisher’s exact test and the Wilcoxon rank-sum test.
Overall survival (OS) was defined as the time (in months) from diagnosis to death from any cause. Patients alive and those lost to follow-up were censored at the date of last follow-up. Survival curves were estimated with the Kaplan–Meier product limit method and compared among groups with the log-rank test. The corresponding 95% confidence intervals for the median values were calculated by the complementary log–log transformation. The prognostic significance of variants was estimated by Cox proportional hazard regression analyses under the additive genetic models univariately and after adjustment for already established prognostic covariates including age, stage, and surgery (only for non-NPC patients) for variants that were (marginally) significant univariately. Departures from the proportional hazards assumption for all Cox models was assessed using time-dependent covariates. All tests were two-sided at an alpha 5% level of significance. Analyses were conducted using the SAS (version 9.3) software (SAS Institute Inc., Cary, NC, USA).
5. Conclusions
In conclusion, two SNPs in the FAS (rs2234768) and VEGFA (rs13207351) genes seem to have an association with clinical outcome for patients with HNC, with VEGFA rs13207351 being the most promising. These findings need further validation in a bigger cohort of NPC and non-NPC patients for definitive results.
Acknowledgments
The authors are indebted to the patients and their families for their trust and participation in the present registry. The authors wish to thank Emily Daskalaki for technical assistance, Eneida Jaupaj for the collection of biological material, and Maria Moschoni for coordination of data management.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/5/1163/s1, Table S1: Hazard ratios and 95% CIs estimated by univariate Cox regression analysis under additive genetic models for all examined variants in NPC and non-NPC patients, Figure S1: Analysis of regulatory element markers as well as transcription factor binding sites in loci associated with VEGFA rs13207351 from the USCS Genome Browser (http://ge-nome.ucsc.edu, accessed on 15 February 2021) and ENCODE (https://genome.ucsc.edu/cgi-bin/hgTracks?db=hg19&lastVirtMode-Type=default&lastVirtModeExtraState=&virtMode-Type=default&virtMode=0&nonVirtPosi-tion=&position=chr6%3A43737744%2D43737844&hgsid=1030849587_t7VZPWaPfJsrlzTTaw-dln7DOIGoT, accessed on 15 February 2021, Figure S2: Analysis of regulatory element markers as well as transcription factor binding sites in genomic loci associated with FAS rs2234768 from the USCS Genome Browser (http://ge-nome.ucsc.edu) and ENCODE (https://genome.ucsc.edu/cgi-bin/hgTracks?db=hg19&lastVirtModeType=default&lastVirtModeExtraState=&virtModeType=default&virtMode=0&nonVirtPosi-tion=&posi-tion=chr10%3A90748194%2D90753813&hgsid=1030946669_QZu9OaqVBNaI1d5rluV4CLcRdd5q, accessed on 15 February 2021).
Author Contributions
Conceptualization, A.K., V.K. and G.F.; Methodology, F.-I.D., G.-A.K., K.P., G.F. and A.K.; Formal Analysis, G.-A.K.; Investigation, V.K., K.P.; Resources, F.I.-D., K.M., K.V., N.A., I.K., A.N., A.P., A.V., P.K., G.F., A.K.; Writing—Original Draft Preparation, F.-I.D., G.-A.K., K.P., G.F., A.K., Writing—Review and Editing, V.K., K.M., K.V., N.A., I.K., A.N., A.P., A.V., P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an internal HeCOG research grant.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was performed upon approval by the Bioethics Committee of the Aristotle University of Thessaloniki, School of Health Sciences, Faculty of Medicine (#2/23 March 2016).
Informed Consent Statement
Written informed consent was obtained from all participants unless the Committee had granted a waiver.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to ethical and legal issues.
Conflicts of Interest
AP: Consultation Fees: Amgen, Merck Serono, Roche, BMS, Astra Zeneca, MSD. Honoraria: Amgen, Merck Serono, Roche, BMS, Astra Zeneca, MSD, Research funds: BMS, Kura; PK: Honoraria: Novartis, MSD, Pfizer. Travel: Pfizer, MSD, Genesis; GF: Advisory Board: Pfizer, Sanofi and Roche. Honoraria: Astra Zeneca. Stock ownership: Ariad, Genprex, Daiichi Sankyo, ARIAD, RFL Holdings, Formycon; AK: Consulting or advisory role: Novartis, Roche, Genesis, Astra-Zeneca. Speaker’s bureau: GSK. Travel/Accommodations: Sanofi-Aventis, Astellas, Genesis, Amgen, BMS, Merck Serono. The rest of the authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chow L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 4.Gatta G., Botta L., Sánchez M.J., Anderson L.A., Pierannunzio D., Licitra L., Hackl M., Zielonke N., Oberaigner W., Van Eycken E., et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer. 2015;51:2130–2143. doi: 10.1016/j.ejca.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 5.Lubin J.H., Purdue M., Kelsey K., Zhang Z.-F., Winn D., Wei Q., Talamini R., Szeszenia-Dabrowska N., Sturgis E.M., Smith E., et al. Total Exposure and Exposure Rate Effects for Alcohol and Smoking and Risk of Head and Neck Cancer: A Pooled Analysis of Case-Control Studies. Am. J. Epidemiol. 2009;170:937–947. doi: 10.1093/aje/kwp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mork J., Lie A.K., Glattre E., Clark S., Hallmans G., Jellum E., Koskela P., Møller B., Pukkala E., Schiller J.T., et al. Human Papillomavirus Infection as a Risk Factor for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y. EBV based cancer prevention and therapy in nasopharyngeal carcinoma. NPJ Precis. Oncol. 2017;1:10. doi: 10.1038/s41698-017-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai S.I., Westra W.H. Molecular pathology of head and neck cancer: Implications for diagnosis, prognosis, and treatment. Annu. Rev. Pathol. Mech. Dis. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y.P., Chan A.T.C., Le Q.T., Blanchard P., Sun Y., Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 10.Tobias P.V. The nasopharynx: Review of structure and development, with notes on speech, pharyngeal hypophysis, chordoma and the dens. J. Dent. Assoc. S. Afr. 1981;36:765–778. [PubMed] [Google Scholar]
- 11.White S., Danowitz M., Solounias N. Embryology and evolutionary history of the respiratory tract. Edorium J. Anat. Embryol. 2016;3:54–62. [Google Scholar]
- 12.Chou J., Lin Y.C., Kim J., You L., Xu Z., He B., Jablons D.M. Nasopharyngeal carcinoma—Review of the molecular mechanisms of tumorigenesis. Head Neck. 2008;30:946–963. doi: 10.1002/hed.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musil M. Serological differences between some isolates of bean yellow mosaic virus. Acta Virol. 1975;19:473–480. [PubMed] [Google Scholar]
- 14.Petersson F. Nasopharyngeal carcinoma: A review. Semin Diagn. Pathol. 2015;32:54–73. doi: 10.1053/j.semdp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Rottey S., Madani I., Deron P., Van Belle S. Modern treatment for nasopharyngeal carcinoma: Current status and prospects. Curr. Opin. Oncol. 2011;23:254–258. doi: 10.1097/CCO.0b013e328344f527. [DOI] [PubMed] [Google Scholar]
- 16.Kim T.J., Lee Y.S., Kang J.H., Kim Y.S., Kang C.S. Prognostic significance of expression of VEGF and Cox-2 in nasopharyngeal carcinoma and its association with expression of C-erbB2 and EGFR. J. Surg. Oncol. 2011;103:46–52. doi: 10.1002/jso.21767. [DOI] [PubMed] [Google Scholar]
- 17.Saaristo A., Partanen T.A., Arola J., Jussila L., Hytönen M., Mäkitie A., Vento S., Kaipainen A., Malmberg H., Alitalo K. Vascular Endothelial Growth Factor-C and Its Receptor VEGFR-3 in the Nasal Mucosa and in Nasopharyngeal Tumors. Am. J. Pathol. 2000;157:7–14. doi: 10.1016/S0002-9440(10)64510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacks D., Baxter B., Campbell B.C., Carpenter J.S., Cognard C., Dippel D., Eesa M., Fischer U., Hausegger K., Hirsch J.A. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke. 2018;13:612–632. doi: 10.1016/j.jvir.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Psoma E., Koliou G.A., Dimitrakopoulos F.I., Papadopoulou K., Rontogianni D., Bobos M., Visvikis A., Kosmidis P.A., Fountzilas G., Constantinidis J., et al. Genetic Variations of VEGFA Gene Are Associated with Infiltration of Adjacent Tissues and the Clinical Outcome of Patients with Nasopharyngeal Carcinoma. Anticancer Res. 2020;40:677–688. doi: 10.21873/anticanres.13997. [DOI] [PubMed] [Google Scholar]
- 20.Koutras A.K., Antonacopoulou A.G., Eleftheraki A.G., Dimitrakopoulos F.I., Koumarianou A., Varthalitis I., Fostira F., Sgouros J., Briasoulis E., Bournakis E., et al. Vascular endothelial growth factor polymorphisms and clinical outcome in colorectal cancer patients treated with irinotecan-based chemotherapy and bevacizumab. Pharm. J. 2012;12:468–475. doi: 10.1038/tpj.2011.37. [DOI] [PubMed] [Google Scholar]
- 21.Antonacopoulou A.G., Kottorou A.E., Dimitrakopoulos F.-I.D., Triantafyllia V., Marousi S., Koutras A., Kalofonos H.P. VEGF polymorphisms may be associated with susceptibility to colorectal cancer: A case-control study. Cancer Biomark. 2011;10:213–217. doi: 10.3233/CBM-2012-0249. [DOI] [PubMed] [Google Scholar]
- 22.Jain L., Vargo C.A., Danesi R., Sissung T.M., Price D.K., Venzon D., Venitz J., Figg W.D. The role of vascular endothelial growth factor SNPs as predictive and prognostic markers for major solid tumors. Mol. Cancer Ther. 2009;8:2496–2508. doi: 10.1158/1535-7163.MCT-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutras A., Kotoula V., Fountzilas G. Prognostic and predictive role of vascular endothelial growth factor polymorphisms in breast cancer. Pharmacogenomics. 2015;16:79–94. doi: 10.2217/pgs.14.148. [DOI] [PubMed] [Google Scholar]
- 24.Makni L., Stayoussef M., Ghazouani E., Mezlini A., Almawi W.Y., BesmaYacoubi L. Distinct association of VEGF-A polymorphisms with laryngeal and nasopharyngeal cancer. Meta Gene. 2016;10:90–94. doi: 10.1016/j.mgene.2016.02.003. [DOI] [Google Scholar]
- 25.Irani S., Salajegheh A., Smith R.A., Lam A.K.-Y. A review of the profile of endothelin axis in cancer and its management. Crit. Rev. Oncol. 2014;89:314–321. doi: 10.1016/j.critrevonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Mai H.-Q., Zeng Z.-Y., Zhang H.-Z., Hou J.-H., Mo H.-Y., Guo X., Min H.-Q., Hong M.-H. [Correlation of endothelin A receptor expression to prognosis of nasopharyngeal carcinoma] Ai Zheng. 2005;24:611–615. [PubMed] [Google Scholar]
- 27.Wen Y.-F., Qi B., Liu H., Mo H.-Y., Chen Q.-Y., Li J., Huang P.-Y., Ye Y.-F., Zhang Y., Deng M.-Q., et al. Polymorphisms in the Endothelin-1 and Endothelin A Receptor Genes and Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma. Clin. Cancer Res. 2011;17:2451–2458. doi: 10.1158/1078-0432.CCR-10-2264. [DOI] [PubMed] [Google Scholar]
- 28.Ma W.-L., Liu R., Huang L.-H., Zou C., Huang J., Wang J., Chen S.-J., Meng X.-G., Yang J.-K., Li H., et al. Impact of polymorphisms in angiogenesis-related genes on clinical outcomes of radiotherapy in patients with nasopharyngeal carcinoma. Clin. Exp. Pharmacol. Physiol. 2017;44:539–548. doi: 10.1111/1440-1681.12738. [DOI] [PubMed] [Google Scholar]
- 29.Peter M.E., Hadji A., Murmann A.E., Brockway S., Putzbach W., Pattanayak A., Ceppi P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015;22:549–559. doi: 10.1038/cdd.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villa-Morales M., Fernández-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16:85–101. doi: 10.1517/14728222.2011.628937. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y., He B., Li R., Pan Y., Gao T., Deng Q., Sun H., Song G., Wang S. Association of the polymorphisms in the Fas/FasL promoter regions with cancer susceptibility: A systematic review and meta-analysis of 52 studies. PLoS ONE. 2014;9:e90090. doi: 10.1371/journal.pone.0090090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T., Zuo L., Li L., Yin L., Liang K., Yu H., Ren H., Zhou W., Jing H., Liu Y., et al. Significant association among the Fas -670 A/G (rs1800682) polymorphism and esophageal cancer, hepatocellular carcinoma, and prostate cancer susceptibility: A meta-analysis. Tumor Biol. 2014;35:10911–10918. doi: 10.1007/s13277-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 33.Eun Y.G., Lee Y.C., Kim S.K., Chung J.-H., Kwon K.H., Park I.S. Single nucleotide polymorphisms of the Fas gene are associated with papillary thyroid cancer. Auris Nasus Larynx. 2015;42:326–331. doi: 10.1016/j.anl.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Tong S., Guan L., Na F., Zhao W., Wei L. CD95 rs1800682 polymorphism and cervical cancer risk: Evidence from a meta-analysis. Tumor Biol. 2014;35:1785–1790. doi: 10.1007/s13277-013-1237-6. [DOI] [PubMed] [Google Scholar]
- 35.Friedlander P.L. Genomic instability in head and neck cancer patients. Head Neck. 2001;23:683–691. doi: 10.1002/hed.1096. [DOI] [PubMed] [Google Scholar]
- 36.Gollin S.M. Mechanisms leading to chromosomal instability. Semin. Cancer Biol. 2005;15:33–42. doi: 10.1016/j.semcancer.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Berardinelli F., Di Masi A., Antoccia A. NBN Gene Polymorphisms and Cancer Susceptibility: A Systemic Review. Curr. Genom. 2013;14:425–440. doi: 10.2174/13892029113146660012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chrzanowska K.H., Gregorek H., Dembowska-Bagińska B., Kalina M.A., Digweed M. Nijmegen breakage syndrome (NBS) Orphanet J. Rare Dis. 2012;7:13. doi: 10.1186/1750-1172-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong S.Y., Park J.W., Lee J.A., Park J.E., Park K.W., Hong E.K., Kim C.M. Association between vascular endothelial growth factor gene polymorphisms and survival in hepatocellular carcinoma patients. Hepatology. 2007;46:446–455. doi: 10.1002/hep.21720. [DOI] [PubMed] [Google Scholar]
- 40.Drobkova H., Jurečeková J., Sivoňová M.K., Mazuchová J., Škorvanová M., Šarlinová M., Halašová E., Kliment J. Associations Between Gene Polymorphisms of Vascular Endothelial Growth Factor and Prostate Cancer. Anticancer Res. 2019;39:2903–2909. doi: 10.21873/anticanres.13419. [DOI] [PubMed] [Google Scholar]
- 41.Gingerich M.A., Smith J.D., Michmerhuizen N.L., Ludwig M., Devenport S., Matovina C., Brenner C., Chinn S.B. Comprehensive review of genetic factors contributing to head and neck squamous cell carcinoma development in low-risk, nontraditional patients. Head Neck. 2018;40:943–954. doi: 10.1002/hed.25057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent W.J., Sugnet C.W., Furey T.S., Roskin K.M., Pringle T.H., Zahler A.M., Haussler A.D. The Human Genome Browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Consortium E.P. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X., Deng Y., Gu H., Lim A., Altankhuyag A., Jia W., Ma K., Xu J., Zou Y., Snellingen T., et al. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol. Vis. 2011;17:3088–3096. [PMC free article] [PubMed] [Google Scholar]
- 45.Li X., Lu Y., Wei P. Association between VEGF genetic variants and diabetic foot ulcer in Chinese Han population: A case-control study. Medicine. 2018;97:e10672. doi: 10.1097/MD.0000000000010672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koutras A.K., Kotoula V., Papadimitriou C., Dionysopoulos D., Zagouri F., Kalofonos H.P., Kourea H.P., Skarlos D.V., Samantas E., Papadopoulou K., et al. Vascular endothelial growth factor polymorphisms and clinical outcome in patients with metastatic breast cancer treated with weekly docetaxel. Pharm. J. 2014;14:248–255. doi: 10.1038/tpj.2013.36. [DOI] [PubMed] [Google Scholar]
- 47.Ho S.-Y., Guo H.-R., Chen H.H.W., Hsiao J.-R., Jin Y.-T., Tsai S.-T. Prognostic implications of Fas-ligand expression in nasopharyngeal carcinoma. Head Neck. 2004;26:977–983. doi: 10.1002/hed.20090. [DOI] [PubMed] [Google Scholar]
- 48.Jrad B.B., Mahfouth W., Bouaouina N., Gabbouj S., Ahmed S.B., Ltaïef M., Jalbout M., Chouchane L. A polymorphism in FAS gene promoter associated with increased risk of nasopharyngeal carcinoma and correlated with anti-nuclear autoantibodies induction. Cancer Lett. 2006;233:21–27. doi: 10.1016/j.canlet.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Q., Wang T., Ren J., Hu K., Liu W., Wu G. FAS-670A/G polymorphism: A biomarker for the metastasis of nasopharyngeal carcinoma in a Chinese population. Clin. Chim. Acta. 2010;411:179–183. doi: 10.1016/j.cca.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y., Miao X.-P., Huang M.-Y., Deng L., Lin D.-X., Zeng Y.-X., Shao J.-Y. Polymorphisms of death pathway genes FAS and FASL and risk of nasopharyngeal carcinoma. Mol. Carcinog. 2010;49:944–950. doi: 10.1002/mc.20676. [DOI] [PubMed] [Google Scholar]
- 51.Huang Q.R., Morris D., Manolios N. Identification and characterisation of polymorphisms in the promoter region of the human Apo-1/Fas (CD95) gene. Mol. Immunol. 1997;34:577–582. doi: 10.1016/S0161-5890(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 52.Sibley K., Rollinson S., Allan J.M., Smith A.G., Law G.R., Roddam P.L., Skibola C.F., Smith M.T., Morgan G.J. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 2003;63:4327–4330. [PubMed] [Google Scholar]
- 53.World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 54.Ricceri F., Matullo G., Vineis P. Is there evidence of involvement of DNA repair polymorphisms in human cancer? Mutat. Res. Mol. Mech. Mutagen. 2012;736:117–121. doi: 10.1016/j.mrfmmm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Adjadj E., Schlumberger M., de Vathaire F. Germ-line DNA polymorphisms and susceptibility to differentiated thyroid cancer. Lancet Oncol. 2009;10:181–190. doi: 10.1016/S1470-2045(09)70020-8. [DOI] [PubMed] [Google Scholar]
- 56.Tan J., Jiang L., Cheng X., Wang C., Chen J., Huang X., Xie P., Xia D., Wang R., Zhang Y. Association between VEGF-460T/C gene polymorphism and clinical outcomes of nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Onco Targets Ther. 2017;10:909–918. doi: 10.2147/OTT.S126159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ungerbäck J., Elander N., Dimberg J., Söderkvist P. Analysis of VEGF polymorphisms, tumor expression of VEGF mRNA and colorectal cancer susceptibility in a Swedish population. Mol. Med. Rep. 2009;2:435–439. doi: 10.3892/mmr_00000118. [DOI] [PubMed] [Google Scholar]
- 58.Ho T., Li G., Zhao C., Zheng R., Wei Q., Sturgis E.M. Fas single nucleotide polymorphisms and risk of thyroid and salivary gland carcinomas: A case-control analysis. Head Neck. 2008;30:297–305. doi: 10.1002/hed.20699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to ethical and legal issues.