Abstract
Background
Disrupted sleep quality is one of the proposed mechanisms through which chronic stress may lead to depression. However, there exist significant individual differences in sleep reactivity, which is the extent to which one experiences sleep disturbances in response to stress.
Purpose
The aim of the current study was to investigate whether low high-frequency heart rate variability (HRV), as a psychophysiological marker of poor emotional and physiological arousal regulation, predicts stress-related sleep disturbances associated with greater risk of depression symptoms.
Methods
Using a chronic caregiving stress model, 125 mothers of adolescents with developmental disorders and 97 mothers of typically developing adolescents had their resting HRV and HRV reactivity recorded and completed a measure of depressive symptoms, as well as a 7 day sleep diary to assess their sleep quality. A moderated mediation model tested whether sleep quality mediated the association between chronic stress exposure and depressive symptoms and whether HRV moderated this mediation.
Results
After controlling for participant age, body mass index, ethnicity, socioeconomic status, and employment status, poor sleep quality mediated the association between chronic stress and depressive symptoms. Resting HRV moderated this indirect effect such that individuals with lower HRV were more likely to report poorer sleep quality in the context of chronic stressor exposure, which, in turn, was related to greater depressive symptoms.
Conclusions
Lower HRV, a potential biomarker of increased sleep reactivity to stress, is associated with greater vulnerability to stress-related sleep disturbances, which, in turn, increases the risk for elevated depressive symptoms in response to chronic stress.
Keywords: Chronic stress, Depression, Sleep reactivity, High-frequency heart rate variability, Respiratory sinus arrhythmia
Individuals with lower heart rate variability, a marker of poor parasympathetic functioning, are more likely to experience poorer sleep quality in the context of a chronic stressor, putting them at an increased risk for depressive symptoms as compared to individuals with higher heart rate variability.
Chronic stress is a well-established risk factor for depression. Empirical evidence supports a causal relationship between chronic stressor exposure and the development of depressive symptoms [1]. Disrupted sleep quality is one of the key mechanisms that have been posited to explain how stress leads to depression [2–4]. However, there exist important individual differences in vulnerability to stress-related sleep disturbances, a construct known as sleep reactivity [5].
When considering the effects of transient sleep disturbances, experimental studies demonstrate how acute sleep deprivation negatively impacts daily mood amongst healthy individuals [6–8]. Transient reductions in sleep quality have also been associated with increased negative mood in response to daily stressors [9]. Moreover, increased insomnia severity prospectively predicts the onset, maintenance, and risk of recurrence of major depressive disorder [10], and interventions targeting insomnia symptoms also yield improvements in depressive symptoms [11]. Thus, sleep disturbances appear to play a role in both the diathesis and maintenance of depressive symptoms over time.
Cross-sectional, longitudinal, and experimental studies suggest that stressor exposure is a precipitating factor for the development of sleep disturbances [12, 13]. The association between stressor exposure and increased subjective and objective measures of sleep quality has been shown across a range of psychosocial stressors [14–20]. Daily diary studies also indicate that perceived stress on a given day predicts lower sleep efficiency and sleep satisfaction that night [21, 22]. Longitudinal studies highlight that chronic stressor exposure is a robust predictor of disrupted sleep quality over time [23–25]. However, not all individuals will develop sleep disturbances in response to stressor exposure [5].
Sleep reactivity to stress appears to be a stable characteristic that is influenced by both genetic and environmental factors [26, 27]. In particular, individual differences in physiological and emotional arousal have been proposed to underlie vulnerability to stress-related sleep disturbances [28, 29]. Within this context, high-frequency heart rate variability (HRV), as a marker of vagally mediated parasympathetic activity, has recently been identified as a putative marker of vulnerability to sleep reactivity [26, 30, 31]. HRV refers to the fluctuation in time intervals between consecutive heartbeats. At rest, both branches of the autonomic nervous system influence cardiac activity; sympathetic activation accelerates heart rate, while parasympathetic activation is primarily responsible for its deceleration. While the low-frequency component of HRV reflects the influence of both the sympathetic and parasympathetic nervous system, the high-frequency component is predominantly modulated by parasympathetic output to the heart through the vagal nerve. More specifically, HRV is regulated by the integration of afferent projections from the periphery to the brain via the vagal nerve and efferent projections connecting the prefrontal cortex with the amygdala and the brain stem where parasympathetic output to the sinoatrial node of the heart is gated [32]. Poorer physiological and emotional arousal regulation, indexed by lower resting HRV, has been associated with increased physiological and emotion arousal in response to stress [33, 34].
HRV is also associated with individual differences in objective and subjective assessments of sleep quality. Some studies suggest that both nocturnal and diurnal HRV differs between good sleepers and individuals with insomnia, but these results have not been replicated in all studies [35]. In some studies, diurnal HRV was more strongly associated with objective and self-reported measures of sleep quality than nocturnal HRV [36]. Higher HRV during resting wakefulness has been associated with higher actigraphy-based assessments of sleep efficiency and sleep duration [37, 38] and negatively correlated with self-reported sleep onset latency and sleep disturbances [39].
There is emerging evidence that individual differences in diurnal HRV may also moderate the association between stressor exposure and sleep quality. In a study examining predictors of sleep reactivity, resting HRV predicted differences in sleep quality in response to a range of situational stressors [26]. Furthermore, individual differences in HRV reactivity, changes in HRV in response to a laboratory stressor, were associated with actigraphy-based and self-report measures of sleep quality [40, 41] and moderated the association between psychosocial risk factors and the development of sleep disturbances [42, 43]. In longitudinal studies of academic stress, greater HRV reactivity predicted changes in self-reported sleep disturbances as participants transitioned from periods of lower stress to higher stress [30, 31]. These studies provide preliminary evidence that HRV may be a psychophysiological marker of sleep reactivity. However, prior studies have focused on HRV within the context of laboratory stressors or ecologically valid stressors that were time limited and relatively predictable. Whether HRV is associated with increased sleep reactivity in response to more chronic and uncontrollable stressors has yet to be explicitly tested.
Prior studies indicate that sleep reactivity is an independent predictor of both depressive symptoms and of insomnia symptoms, which then further increases the risk of major depressive disorder [2, 4]. In parallel, HRV has been associated with both elevated depressive symptoms and insomnia severity [44, 45] and emerging evidence indicates that it may be a biomarker of sleep reactivity. This suggests that HRV may be associated with higher depression risk through greater stress-related sleep disturbances.
The goal of the current study was thus to test whether HRV moderates the impact of chronic stressor exposure on sleep quality and the mediating effect of poor sleep quality on the association between chronic stressor exposure and depressive symptoms using a moderated mediation model. Using a chronic parenting stress model, an ecologically valid model of chronic stressor exposure that is both ongoing and largely uncontrollable [46], this study compared mothers of adolescents with developmental disorders and mothers of typically developing adolescents. Parents of children with developmental disorders tend to report poorer sleep quality and more depressive symptoms than other parenting populations [47, 48] and, therefore, provide a unique opportunity to study the interplay between chronic stressor exposure, sleep quality, and depression.
It was hypothesized that the chronic caregiving stress group would report poorer sleep quality and greater depressive symptoms as compared to the control group. Furthermore, it was hypothesized that sleep quality would mediate the association between chronic stressor exposure and depressive symptoms and that HRV would moderate the association between chronic stressor exposure and sleep quality. Specifically, it was predicted that individuals with lower resting HRV and increased HRV reactivity would be particularly vulnerable to experiencing poorer sleep quality in the context of chronic stressor exposure, putting them at increased risk of developing depressive symptoms.
Method
Participants
The total sample (N = 222) comprised of two distinct groups, the chronic caregiving stress group (n = 125) comprising mothers of adolescents with an autism spectrum disorder (n = 94) or intellectual disability (n = 31) and the comparison group of mothers of typically developing adolescents (n = 97). Participants were the biological or legal mothers cohabiting with their child and were in the process of transitioning their child in or out of the high school system. The exclusion criteria included being pregnant or nursing, having a chronic medical condition or acute infection, regular use of anti-inflammatory medication, or major mental illness (e.g., schizophrenia, bipolar disorder, or substance abuse). Participants were recruited via posters and meetings at schools, community groups and social service centers, and ads in the local newspapers in the Greater Montreal Area. Participants were compensated $60 CAD for their participation in the study.
Procedure
Participants first completed an online questionnaire assessing sociodemographic information and depressive symptoms over the past week. For seven consecutive days prior to the study visit, participants completed a sleep diary assessing their sleep quality. Specifically, participants reported on their previous night’s sleep and functioning throughout the day before going to bed each night. Participants then attended a home or laboratory session to measure their HRV using a digital interbeat interval recorder (Polar RS800CX; Finland: Kempele). To obtain a measure of resting HRV, participants were seated and instructed to breathe normally and to try to relax as much as possible for 5 min. Following the resting period, the experimenter administered a worry induction task. Specifically, participants were asked to identify a topic they tended to worry about the most and then worry about that topic for the 5 min period [49]. This task elicited significant HRV reactivity in prior studies [30, 31, 50]. Anthropometric measurements were also taken to calculate body mass index (BMI). To account for diurnal variations in HRV, testing sessions were completed in the morning before noon while participants were fasting [51].
Measures
Heart rate variability
At the beginning of the laboratory session, participants were fitted with a digital interbeat interval recorder (Polar RS800CX; Finland: Kempele). This device recorded the timing between consecutive R-peaks of QRS complexes (interbeat intervals) at a sampling rate of 1,000 Hz. Interbeat intervals were visually screened for recording artifacts and manually corrected in CardioEdit. HRV values were calculated with CardioBatch from corrected interbeat interval (IBI) files according to the Porges–Bohrer method [52]. First, a moving polynomial is applied to the IBI time series to remove HRV associated with slower periodic or aperiodic processes. Then, a bandpass filter is applied to the detrended time series to isolate HRV attributable to the typical respiratory cycle frequency in adults (between 0.12 and 0.40 Hz) to assess respiratory sinus arrhythmia. Variance scores were calculated from 30 s epochs of the detrended and filtered IBI time series. These HRV values were log-transformed and averaged across the different epochs within each task. HRV reactivity was computed as the difference between participant’s HRV during the resting period and the 5 min worry induction task.
Depressive Symptoms
The Center for Epidemiologic Studies Depression Scale (CES-D; 53) is a 20-item self-report scale that was used to assess the frequency of depressive symptoms during the past week. Participants were asked to rate how many days during the past week they felt or behaved a certain way on a four-point Likert scale (0 = less than 1 day and 3 = 5–7 days). Responses on each item were summed, with higher scores indicating more depressive symptoms. Scores range from 0 to 60; any score above 16 is considered to be a clinically significant level of depressive symptoms [53]. The sample specific Cronbach’s α was .91.
Sleep Quality
Sleep quality was evaluated over seven consecutive days using sleep diaries adapted from the Consensus Sleep Diary [54]. Specifically, the sleep diary assessed total time spent in bed, total sleep time, sleep onset latency, wake time after initial sleep onset, and early morning awakenings. Participants also rated their sleep satisfaction on a four-point Likert scale (1 = Very bad and 4 = Very good) and were asked to rate their level of fatigue throughout the day (0 = Not at all fatigued and 5 = As fatigued as I could be; reverse coded). Sleep efficiency was calculated as a ratio of total sleep time to total time spent in bed, with a higher ratio indicative of better sleep efficiency [55]. Total time spent in bed was calculated by adding the time between when the participant went to bed at night and got out of bed in the morning. Total sleep time was calculated by subtracting sleep onset latency, nocturnal awakenings, and early morning awakenings from the total time in bed. Sleep efficiency, sleep duration (i.e., total sleep time), sleep satisfaction, and fatigue were recorded for each participant on each day. A mean value across the entire sleep diary period was also computed. A global sleep quality composite was then calculated by averaging the standardized means of all four indices such that higher scores represented better sleep quality. This provided a unified measure of sleep quality that accounts for multiple dimensions of subjective sleep quality, including waking variables, important to both good sleepers and individuals with insomnia [56] and that is consistent with sleep health recommendations [57]. The Cronbach’s α for this composite was .75.
Statistical Analysis
A moderated mediation model was used to test the main study hypothesis that sleep quality mediates the association between chronic stressor exposure and depressive symptoms and that HRV moderates the association between chronic stressor exposure (coded as −1 for participants in the comparison group and +1 for participants in the chronic caregiving stress group) and sleep quality. This model simultaneously tests the effect of chronic stressor exposure on sleep quality (path a) and sleep quality on depressive symptoms (path b), as well as the total (path c), direct (path c’), and indirect effect of chronic stressor exposure on depressive symptoms (path ab) using a series of regression models. Further, it tests the interaction of chronic stressor exposure and HRV in predicting sleep quality and the conditional indirect effect of chronic stressor exposure on depressive symptoms. This model also computes an index of the moderated mediation. Using bootstrapping procedures, a 5,000 bootstrapped sample was used to compute 95% bias corrected confidence intervals (CIs) for each effect. Data was analyzed using the PROCESS macro (Model 7; [58]) in SPSS version 25.
Participant socioeconomic status (SES) was calculated as a composite of the standardized means of their education level and reported annual household income. This SES variable was included along with participant age, BMI, ethnicity (coded as 0 for those who self-identified as White and 1 for those who did not self-identify as White), and employment status (coded as 1 for employed and 0 for unemployed) as covariates in the model. These covariates were selected given their known associations with HRV and sleep or depressive symptoms in prior studies [59–61]. Inclusion of these covariates did not change the pattern of results for the moderated mediation models; therefore, only fully adjusted models are presented in the manuscript.
Two outliers, defined as a score 3 standard deviations (SDs) away from the sample mean, were identified on the sleep duration variable; these scores were then winsorized to 3 SDs above the sample mean. One participant had missing data for sleep efficiency and sleep duration, so only their self-reported fatigue and sleep satisfaction were used to compute their sleep quality score. For the main statistical analyses, scores on the CES-D were log-transformed to correct for positive skewness in this variable. An alpha level of .05 was used in the current study.
Results
Descriptive Statistics and Intercorrelations
Descriptive statistics and group comparisons of participants’ sociodemographic characteristics are presented in Table 1. On average, participants completed 6.05 out of 7 daily diaries (SD = 1.44). In total, 35.6% of participants self-reported difficulty falling sleep, staying asleep, or early morning awakenings lasting longer than 30 min at least three times during the sleep diary period, which is suggestive of clinically significant insomnia symptoms. Further, 28.2% of the sample reported an average sleep efficiency ratio of less than .85. In terms of depressive symptoms, 41.0% of the sample had a score on the CES-D above the clinical cutoff suggesting the presence of clinically significant depressive symptoms.
Table 1.
Comparison of participants’ sociodemographic characteristics and the main study variables as a function of group
| Chronic stress group | Comparison group | t (d) | |
|---|---|---|---|
| M (SD) | M (SD) | ||
| Participant age (years) | 47.71 (6.15) | 45.69 (5.69) | −2.51* (0.34) |
| Child age (years) | 16.45 (2.82) | 15.16 (1.80) | −3.91** (0.56) |
| BMI | 28.01 (5.86) | 27.58 (6.71) | −0.50 (0.07) |
| Income (CAN$) | 64,919.31 (41,667.45) | 66,822.91 (42,414.76) | 0.33 (0.05) |
| HRV ln(ms2) | 5.55 (1.30) | 5.77 (1.31) | 1.26 (0.17) |
| HRV reactivity ln(ms2) | −0.23 (.72) | −0.16 (0.55) | 0.82 (0.11) |
| Depressive symptoms | 17.89 (10.93) | 14.40 (10.44) | −2.40* (0.33) |
| Sleep quality | −0.14 (0.77) | 0.18 (0.68) | 3.13** (0.43) |
| Sleep efficiency | 0.86 (0.10) | 0.89 (0.08) | 1.92’ (0.35) |
| Sleep duration (hours) | 6.72 (1.02) | 7.04 (0.89) | 2.46* (0.33) |
| Sleep satisfaction | 2.88 (0.55) | 2.95 (0.46) | 1.12 (0.14) |
| Fatigue | 2.61 (0.88) | 2.16 (0.83) | −3.93** (0.53) |
| % | % | χ 2 | |
| Relationship status (% in a current romantic relationship) | 75.2 | 77.3 | 0.14 |
| Ethnicity (% self-identified as White) | 80.8 | 74.2 | 1.37 |
| Employment status (% employed) | 72.8 | 76.3 | 0.35 |
| Education level (% graduated from university) | 46.4 | 47.4 | 8.76 |
BMI body mass index; HRV heart rate variability; ln logarithmic transformation of power spectral measures; SD standard deviation.
**p < .01, *p < .05, ’p < .10.
Spearman’s rho correlations were computed amongst the main study variables. There was a marginally significant negative correlation between resting HRV and depressive symptoms r(220) = −.13, p = .06. Frequency of depressive symptoms was significantly negatively correlated with sleep quality, r(220) = −.42, p < .001, including shorter sleep duration, r(218) = −.15, p = .03, lower sleep efficiency, r(220) = −.30, p < .001, lower sleep satisfaction, r(219) = −.44, p < .001, and greater daytime fatigue, r(220) = −.41, p < .001. HRV reactivity was not significantly correlated with depressive symptoms or any of the sleep quality parameters.
Group Comparisons Amongst the Main Study Variables
As reported in Table 1, independent samples t-tests were run to test group differences on the main study variables. Overall, the chronic caregiving stress group was older, t(220) = 2.51, p = .013, d = 0.34, and had older children, t(219) = 3.91, p < .01, d = 0.56, than the comparison group. They also reported greater depressive symptoms, t(220) = 2.40, p = .017, d = 0.33, and poorer overall sleep quality, t(220) = −3.13, p < .001, d = 0.43. Specifically, the chronic caregiving stress group slept less hours, t(218) = −2.46, p = .015, d = 0.35, and were more fatigued, t(220) = 3.93 p < .01, d = 0.53, than the comparison group. Group differences in sleep efficiency were marginally significant, with the chronic stress group reporting poorer sleep efficiency, t(218) = −1.92, p = .056, d = 0.33. Sleep satisfaction did not differ as a function of group, t(219) = 1.12, p = −.266, d = 0.14, and neither did resting HRV, t(220) = −1.26, p = .209, d = 0.17 or HRV reactivity, t(214) = −.82, p = .412, d = 0.11.
Moderated Mediation Analyses
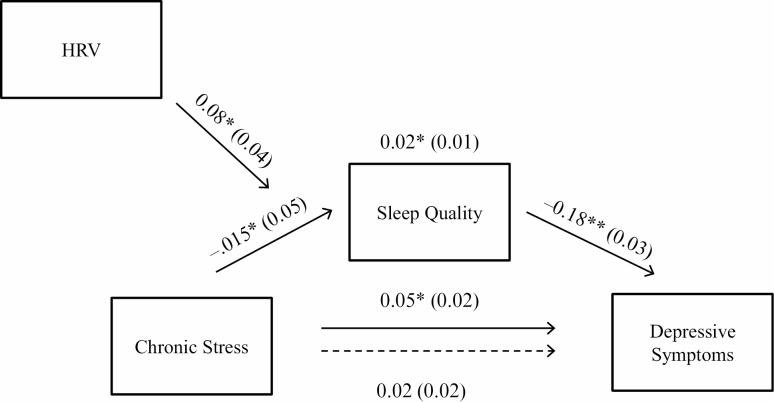
As illustrated in Fig. 1, a moderated mediation model was used to the test the main study hypothesis. When controlling for participant age (β = −.001, standard error [SE] = .004, p = .752), BMI (β = .002, SE = .004, p = .604), ethnicity (β = 0.08, SE = 0.05, p = .156), SES (β = −0.02, SE = 0.05, p = .420), employment status (β = −0.06, SE = 0.06, p = .274), and HRV (β = −0.03, SE = 0.02, p = .112), the total effect of chronic stressor exposure (i.e., having an adolescent with an autism spectrum disorder or intellectual disability) on depressive symptoms was statistically significant (path c in Fig. 1; β = 0.05, SE = 0.02, p = .047, 95% CI [0.001, 0.09]) such that chronic stressor exposure was associated with greater depressive symptoms.
Fig. 1.
Visual depiction of the moderated mediation model tested. Beta coefficients and corresponding standard errors (in parentheses) for each effect are also presented. The dotted arrow refers to the direct effect of chronic stress predicting depressive symptoms when controlling for the effect of sleep quality. **p < .01, *p < .05. HRV = resting high-frequency heart rate variability.
When adjusting for the effects of participant age (β = −0.008, SE = 0.01, p = .337), BMI (β = −0.01, SE = 0.008, p = .073), ethnicity (β = −0.35, SE = 0.11, p = .002), SES (β = −0.04, SE = 0.06, p = .548), and employment status (β = 0.16, SE = 0.11, p = .164), the effect of chronic stress on sleep quality was statistically significant such that chronic stressor exposure predicted poorer sleep quality (path a; β = −0.15, SE = 0.05, p = .002, 95% CI [−0.25, −0.06]). Further, when controlling for participant age (β = −0.001, SE = 0.004, p = .717), BMI (β = 0.002, SE = 0.004, p = .948), ethnicity (β = 0.002, SE = 0.05, p = .961), SES (β = −0.03, SE = 0.03, p = .305), and employment status (β = −0.04, SE = 0.05, p = .417), the effect of sleep quality on depressive symptoms was also statistically significant, whereby poorer sleep quality was associated with greater depressive symptoms (path b; β = −0.18, SE = 0.03, p < .01,95% CI [−0.24, −0.12]).
After controlling for sleep quality, the direct effect of chronic stressor exposure on depressive symptoms was no longer statistically significant and reduced compared to the total effect (path c'; β = 0.02, SE = 0.02, p = .369, 95% CI [−0.02, 0.06]). The indirect effect of chronic stressor exposure on depressive symptoms was statistically significant (path ab; β = 0.02, SE = 0.01, 95% CI [0.01, 0.04]). Taken together, this pattern of results suggests that sleep quality partially mediated the association between chronic stressor exposure and frequency of depressive symptoms. This model accounted for 18.18% of the variance in depressive symptoms.
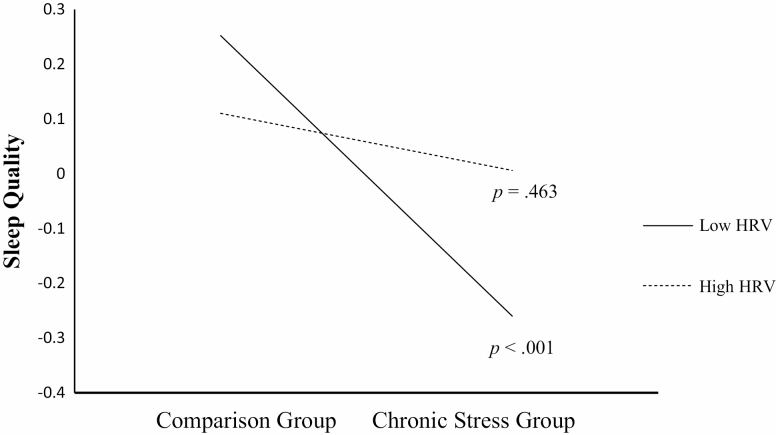
As part of the moderated mediation hypothesis, the interaction between chronic stressor exposure and resting HRV in predicting sleep quality was tested. There was a statistically significant interaction between chronic stressor exposure and resting HRV in predicting sleep quality (β = 0.08, SE = 0.04, p = .036, 95% CI [0.005, 0.15], R2= .02). As illustrated in Fig. 2, there was a significant association between chronic stressor exposure and poor sleep quality at lower resting HRV levels (effect = −0.26, SE = 0.07, p < .001, 95% CI [−0.39, −0.12]) but not at higher resting HRV levels (effect = −0.05, SE = 0.07, p = .450, 95% CI [−0.17, 0.11]). Further, the 95% CI around the index of moderated mediation suggests that resting HRV moderated the indirect effect of sleep quality on the association between chronic stressor exposure and depressive symptoms (index = −0.01, boot SE = 0.01, 95% CI [−0.03, −0.001]). Specifically, the conditional indirect effect of chronic stress on depressive symptoms was statistically significant at lower resting HRV (effect = 0.05, boot SE = 0.01, 95% CI [0.02, 0.08]) but not higher resting HRV (effect = 0.009, boot SE = 0.01, 95% CI [−0.01, 0.04]). Given that the CES-D included a sleep-related item ("My sleep was restless"), analyses were repeated while omitting this item. The pattern of results remained the same such that the moderated mediation remained statistically significant (index = −0.01, boot SE = 0.006, 95% CI [−0.03, −0.003]). As excessive fatigue is a symptom of depression, analyses were also repeated using a sleep quality variable excluding the daily fatigue component. Again, the pattern of results remained the same; the moderated mediation remained statistically significant, index = −0.01, boot SE = 0.006, 95% CI [−0.03, −0.001].
Fig. 2.
Interaction between chronic stress and HRV in predicting sleep quality, plotted as a function of ± 1 standard deviation around the mean of HRV. HRV = resting high-frequency heart rate variability. p values refer to the statistical significance of the simple slopes.
In contrast, HRV reactivity did not moderate the association between chronic stressor exposure and sleep quality, β = −0.05, SE = 0.07, p = .494, 95% CI [−0.19, 0.09], R2 = .002. The index of moderated mediation was also not significant for HRV reactivity (index = 0.009, boot SE = 0.01, 95% CI [−0.01, 0.04]). These analyses were repeated using residualized change scores as an alternative way to calculate HRV reactivity; the pattern of results remained the same; the moderated mediation was not statistically significant; index = −0.005, boot SE = 0.01, 95% CI [−0.03, 0.02].
Discussion
The goals of the current study were to investigate whether sleep quality mediated the association between chronic stressor exposure and depressive symptoms and whether HRV moderated this association. As hypothesized, chronic stressor exposure was associated with decreased sleep quality, which then predicted greater depressive symptoms. Moreover, this mediation effect was moderated by HRV. Specifically, individuals with lower resting HRV were more likely to report poorer sleep quality in the context of chronic stressor exposure than their counterparts with higher HRV. These findings suggest that lower HRV may be a biomarker of sleep reactivity to stress and that vulnerability to stress-related sleep disturbances may be a key pathway through which individuals with lower HRV are at greater risk for stress-related depressive symptoms.
In the current study, individuals with lower resting HRV were more susceptible to experiencing poorer sleep quality in the context of chronic stressor exposure than individuals with higher HRV. Contemporary models of insomnia posit that emotional and physiological hyperarousal are key factors involved in the development and maintenance of sleep disturbances [29]. It is well known that stress increases arousal, and individuals with lower HRV are more likely to exhibit increased arousal in response to stress. Specifically, in addition to increased emotional arousal [33, 50], lower HRV is also associated with increased physiological arousal as indicated by increased blood pressure and cortisol [34]. This stress-induced hyperarousal may interfere with sleep onset and maintenance among individuals with lower resting HRV.
Sleep reactivity, the tendency to experience sleep disturbances in response to stress exposure, has been associated with greater risk of depression, over and above the effects of insomnia severity [2, 4]. Prior work indicates that lower HRV is associated with greater sleep disturbances in response to acute laboratory challenges, as well as predictable and time-limited naturalistic stressors [26, 30, 31]. The present findings provide further evidence that lower resting HRV may be a biomarker of sleep reactivity. Specifically, the current study extended previous findings by examining the link between HRV and sleep reactivity in the context of a chronic stressor.
Sleep quality mediated the association between chronic stressor exposure and depressive symptoms in the current study. This is consistent with previous research that highlights disrupted sleep quality as a key mechanism linking stress and depression [2, 3, 62]. The present findings suggest that this greater sleep reactivity may be one of the pathways through which individuals with lower HRV are at greater risk of developing depressive symptoms in response to chronic stressor exposure. The literature on the link between HRV and depressive symptoms has previously been characterized by modest and heterogeneous effect sizes [44]. Recent findings suggest that accounting for sleep disturbances may explain some of the heterogeneity in the association between low HRV and depression [41, 63, 64].
However, contrary to what was hypothesized, the moderated mediation effect with HRV reactivity was not significant. This is consistent with a recent daily diary study that demonstrated that individual differences in resting HRV, but not HRV reactivity, moderated the association between sleep quality and depressive symptoms amongst individuals with a prior history of depression [63]. Furthermore, while resting levels of HRV is a reliable predictor of psychopathology, the predictive utility of HRV reactivity has been limited by methodological challenges. Specifically, findings from a recent meta-analysis suggest that null findings can partially be attributed to systematic differences in the baseline and reactivity procedures used, as well as participants’ sex [65]. The current study included only females and used a worry induction task as the reactivity procedure. Future studies should include both sexes and test whether different reactivity procedures modify the association between HRV reactivity and stress-related sleep reactivity.
The chronic caregiving stress model used in the present study included mothers of adolescents with an autism spectrum disorder or intellectual disability. Although mothers of children with special needs report more depressive symptoms than mothers of typically developing children [66], prior research indicates that mothers of children with autism report more parenting stress than mothers of children with other developmental disabilities [67]. In the present study, additional follow-up analyses indicated that mothers of adolescents with autism spectrum disorder (ASD) showed the highest levels of depressive symptoms and poorest sleep quality, followed by mothers of adolescents with intellectual disabilities and then mothers of typically developing adolescents. However, significant group differences in depressive symptoms, t(189) = 2.25, p = .025, d = 0.33, and sleep quality, t(189) = −3.36, p = .001, d = 0.49, were observed between mothers of adolescents with ASD and mothers of typically developing adolescents but not between mothers of adolescents with ASD and mothers of adolescents with other developmental disabilities (depressive symptoms, t(123) = −0.085, p = .932, d = 0.02; sleep quality, t(123) = 1.06, p = .294, d = 0.22). Of note, although the chronic caregiving stress group displayed worst overall sleep quality, the overall differences were of small magnitude. These results dovetail with prior studies highlighting individual differences in sleep reactivity to stress [5]. Furthermore, while it is often assumed that caregivers and noncaregiving populations differ on health outcomes, the association between caregiver status and health is heterogeneous and influenced by other individual differences [68]. Low resting HRV may be one interindividual factor that places certain caregivers at an increased risk for stress-related sleep disturbances.
One of the key limitations of this study is its cross-sectional design, precluding inferences of directionality among the associations observed. Depressive symptoms may have preceded sleep disturbances [69]. An alternative model wherein lower HRV is related to increased vulnerability to the effects of sleep deprivation on mood is also possible [63]. The cognitive energy model [70] posits that sleep loss deplete the cognitive resources required for adaptive self-regulation, which may be enhanced among individuals with lower HRV who may have less self-regulatory capacity [33]. Although alternative moderated mediation models wherein depressive symptoms was the mediator or HRV moderated the association between sleep quality and depressive symptoms were not significant (data not shown), these alternative conceptualizations cannot be ruled out in this cross-sectional study. As such, longitudinal studies are required to better comment on the directionality of the observed associations. Furthermore, chronic stressor exposure was inferred by caregiver status; future studies should include continuous measure of stress severity (e.g., child’s symptoms severity) rather than relying on a dichotomous caregiving variable [71]. This is important to consider given that different individuals with the same diagnosis of ASD or intellectual disability may exhibit a wide range of functioning that is directly impacting the caregiving stress experienced by the mother. Moreover, the generalizability of the sample is limited such that future studies should include men as sex and gender differences have been found in relation to HRV [72], insomnia [73], and depression [74]. In the current study, participant age also differed across groups and may represent an important confound as age is independently associated with both HRV and sleep disturbances [59, 75]. However, the associations observed in the current study were found over and above the effects of age. Moreover, a longer assessment of the sleep quality variables (e.g., 2 weeks instead of 1 week) would better capture the instability of sleep quality characteristic of poor sleepers [76]. Furthermore, the inclusion of objective measures of sleep quality is also warranted.
Meta-analytic studies suggest that lower HRV is associated with elevated depressive symptoms [44, 77]. The present findings indicate that individuals with lower HRV are more likely to experience stress-related impairment in sleep quality and, in turn, poor sleep explains part of the association between chronic stressor exposure and depressive symptoms for these individuals. This suggests that sleep-focused treatment could play an important role in preventing depressive symptoms in the context of chronic stressor exposure, particularly among individuals with lower HRV. Cognitive behavioral therapy for insomnia is the current gold standard treatment for chronic insomnia [78]. Even when it includes only sleep-related interventions, CBT for insomnia also reduces depression [79]. This intervention strategy, which has already been successfully adapted to caregiving populations, may be particularly helpful for individuals who are facing exposure to uncontrollable and unpredictable stressors [80], such as parents of children with developmental disabilities. Individuals with lower HRV may reap larger benefits from such sleep-focused intervention. However, at this time, a clinical HRV threshold value has not been identified to select individuals who would benefit most from such treatment. Future studies should investigate the clinical utility of HRV measures in tailoring treatments for individuals facing chronic stressor exposure. Furthermore, there is also research indicating that successful CBT for depression is associated with increases in HRV [81, 82] and that HRV biofeedback enhances the efficacy of CBT for depression [83]. It is possible that interventions that increase HRV may then reduce sleep reactivity. Further empirical work is needed to test this hypothesis.
Taken together, the results from the current study suggest that low resting HRV moderates the impact of chronic stressor exposure on sleep quality. Specifically, low HRV appears to be a psychophysiological marker of sleep reactivity that is associated with risk for elevated depressive symptoms in response to chronic stressor exposure. Future studies should replicate these findings in longitudinal studies and evaluate whether difficulties with physiological and emotional arousal regulation explain the risk for poor sleep and depression associated with lower HRV.
Funding
This study was funded by grants from the Social Sciences and Humanities Research Council of Canada and the Canadian Institutes for Health Research (PI: Gouin).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards All authors have no actual or potential conflicts of interest to report.
Authors’ Contributions J.P.G., J.M., and L.B. designed the study. J.P.G. and C.d.E. analyzed and interpreted the data. C.d.E. wrote the first draft of the manuscript. All authors edited subsequent versions of the manuscript and approved the final manuscript.
Ethical Approval All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. [DOI] [PubMed] [Google Scholar]
- 2. Drake CL, Pillai V, Roth T. Stress and sleep reactivity: A prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riemann D, Krone LB, Wulff K, Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology. 2020;45:74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vargas I, Friedman NP, Drake CL. Vulnerability to stress-related sleep disturbance and insomnia: Investigating the link with comorbid depressive symptoms. Transl Issues Psychol Sci. 2015;1:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–291. [DOI] [PubMed] [Google Scholar]
- 6. Babson KA, Trainor CD, Feldner MT, Blumenthal H. A test of the effects of acute sleep deprivation on general and specific self-reported anxiety and depressive symptoms: An experimental extension. J Behav Ther Exp Psychiatry. 2010;41:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minkel JD, Banks S, Htaik O, et al. Sleep deprivation and stressors: Evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12:1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scott JP, McNaughton LR, Polman RC. Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiol Behav. 2006;87:396–408. [DOI] [PubMed] [Google Scholar]
- 9. da Estrela C, Barker ET, Lantagne S, Gouin JP. Chronic parenting stress and mood reactivity: The role of sleep quality. Stress Health. 2018;34:296–305. [DOI] [PubMed] [Google Scholar]
- 10. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–19. [DOI] [PubMed] [Google Scholar]
- 11. Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: A systematic review. Int Rev Psychiatry. 2014;26:205–213. [DOI] [PubMed] [Google Scholar]
- 12. Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: An overview. Depress Anxiety. 2003;18:163–176. [DOI] [PubMed] [Google Scholar]
- 13. Pillai V, Roth T, Mullins HM, Drake CL. Moderators and mediators of the relationship between stress and insomnia: Stressor chronicity, cognitive intrusion, and coping. Sleep. 2014;37:1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akerstedt T. Psychosocial stress and impaired sleep. Scand J Work Environ Health. 2006;32:493–501. [PubMed] [Google Scholar]
- 15. Hall M, Buysse DJ, Nofzinger EA, et al. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biol Psychol. 2008;77:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5:178–193. [DOI] [PubMed] [Google Scholar]
- 17. Kalimo R, Tenkanen L, Härmä M, Poppius E, Heinsalmi P. Job stress and sleep disorders: Findings from the Helsinki Heart Study. Stress Med. 2000;16:65–75. [Google Scholar]
- 18. Kim EJ, Dimsdale JE. The effect of psychosocial stress on sleep: A review of polysomnographic evidence. Behav Sleep Med. 2007;5:256–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor DJ, Pruiksma KE, Hale WJ, et al. ; STRONG STAR Consortium . Prevalence, correlates, and predictors of insomnia in the US army prior to deployment. Sleep. 2016;39:1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verlander LA, Benedict JO, Hanson DP. Stress and sleep patterns of college students. Percept Mot Skills. 1999;88:893–898. [DOI] [PubMed] [Google Scholar]
- 21. Åkerstedt T, Orsini N, Petersen H, Axelsson J, Lekander M, Kecklund G. Predicting sleep quality from stress and prior sleep—A study of day-to-day covariation across six weeks. Sleep Med. 2012;13:674–679. [DOI] [PubMed] [Google Scholar]
- 22. Winzeler K, Voellmin A, Schäfer V, et al. Daily stress, presleep arousal, and sleep in healthy young women: A daily life computerized sleep diary and actigraphy study. Sleep Med. 2014;15:359–366. [DOI] [PubMed] [Google Scholar]
- 23. de Lange AH, Kompier MA, Taris TW, et al. A hard day’s night: A longitudinal study on the relationships among job demands and job control, sleep quality and fatigue. J Sleep Res. 2009;18:374–383. [DOI] [PubMed] [Google Scholar]
- 24. Hall MH, Casement MD, Troxel WM, et al. Chronic stress is prospectively associated with sleep in midlife women: The SWAN sleep study. Sleep. 2015;38:1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linton SJ. Does work stress predict insomnia? A prospective study. Br J Health Psychol. 2004;9:127–136. [DOI] [PubMed] [Google Scholar]
- 26. Bonnet MH, Arand DL. Situational insomnia: Consistency, predictors, and outcomes. Sleep. 2003;26:1029–1036. [DOI] [PubMed] [Google Scholar]
- 27. Drake CL, Friedman NP, WrightKP, Jr, Roth T. Sleep reactivity and insomnia: Genetic and environmental influences. Sleep. 2011;34:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalmbach DA, Anderson JR, Drake CL. The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res. 2018;6:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. [DOI] [PubMed] [Google Scholar]
- 30. Gouin JP, Wenzel K, Boucetta S, O’Byrne J, Salimi A, Dang-Vu TT. High-frequency heart rate variability during worry predicts stress-related increases in sleep disturbances. Sleep Med. 2015;16:659–664. [DOI] [PubMed] [Google Scholar]
- 31. MacNeil S, Deschênes SS, Caldwell W, Brouillard M, Dang-Vu TT, Gouin JP. High-frequency heart rate variability reactivity and trait worry interact to predict the development of sleep disturbances in response to a naturalistic stressor. Ann Behav Med. 2017;51:912–924. [DOI] [PubMed] [Google Scholar]
- 32. Berntson GG, BiggerJT, Jr, Eckberg DL, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. [DOI] [PubMed] [Google Scholar]
- 33. Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. [DOI] [PubMed] [Google Scholar]
- 34. Weber CS, Thayer JF, Rudat M, et al. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol. 2010;109:201–211. [DOI] [PubMed] [Google Scholar]
- 35. Dodds KL, Miller CB, Kyle SD, Marshall NS, Gordon CJ. Heart rate variability in insomnia patients: A critical review of the literature. Sleep Med Rev. 2017;33:88–100. [DOI] [PubMed] [Google Scholar]
- 36. Werner GG, Ford BQ, Mauss IB, Schabus M, Blechert J, Wilhelm FH. High cardiac vagal control is related to better subjective and objective sleep quality. Biol Psychol. 2015;106:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elmore-Staton L, El-Sheikh M, Vaughn B, Arsiwalla DD. Preschoolers’ daytime respiratory sinus arrhythmia and nighttime sleep. Physiol Behav. 2012;107:414–417. [DOI] [PubMed] [Google Scholar]
- 38. Castro-Diehl C, Diez Roux AV, Redline S, et al. Sleep duration and quality in relation to autonomic nervous system measures: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2016;39:1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hovland A, Pallesen S, Hammar A, et al. Subjective sleep quality in relation to inhibition and heart rate variability in patients with panic disorder. J Affect Disord. 2013;150:152–155. [DOI] [PubMed] [Google Scholar]
- 40. El-Sheikh M, Erath SA, Bagley EJ. Parasympathetic nervous system activity and children’s sleep. J Sleep Res. 2013;22:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, Rottenberg J. Respiratory sinus arrhythmia reactivity in current and remitted major depressive disorder. Psychosom Med. 2014;76:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. El-Sheikh M, Hinnant JB, Erath SA. Vi. Marital conflict, vagal regulation, and children’s sleep: A longitudinal investigation. Monogr Soc Res Child Dev. 2015;80:89–106. [DOI] [PubMed] [Google Scholar]
- 43. Keller PS, Kouros CD, Erath SA, Dahl RE, El-Sheikh M. Longitudinal relations between maternal depressive symptoms and child sleep problems: The role of parasympathetic nervous system reactivity. J Child Psychol Psychiatry. 2014;55:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koch C, Wilhelm M, Salzmann S, Rief W, Euteneuer F. A meta-analysis of heart rate variability in major depression. Psychol Med. 2019;49:1948–1957. [DOI] [PubMed] [Google Scholar]
- 45. Schiweck C, Piette D, Berckmans D, Claes S, Vrieze E. Heart rate and high frequency heart rate variability during stress as biomarker for clinical depression. A systematic review. Psychol Med. 2019;49:200–211. [DOI] [PubMed] [Google Scholar]
- 46. Quittner AL, Glueckauf RL, Jackson DN. Chronic parenting stress: Moderating versus mediating effects of social support. J Pers Soc Psychol. 1990;59:1266–1278. [DOI] [PubMed] [Google Scholar]
- 47. Karst JS, Van Hecke AV. Parent and family impact of autism spectrum disorders: A review and proposed model for intervention evaluation. Clin Child Fam Psychol Rev. 2012;15:247–277. [DOI] [PubMed] [Google Scholar]
- 48. Lopez-Wagner MC, Hoffman CD, Sweeney DP, Hodge D, Gilliam JE. Sleep problems of parents of typically developing children and parents of children with autism. J Genet Psychol. 2008;169:245–259. [DOI] [PubMed] [Google Scholar]
- 49. Hofmann SG, Schulz SM, Heering S, Muench F, Bufka LF. Psychophysiological correlates of generalized anxiety disorder with or without comorbid depression. Int J Psychophysiol. 2010;78:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gouin JP, Deschênes SS, Dugas MJ. Respiratory sinus arrhythmia during worry forecasts stress-related increases in psychological distress. Stress. 2014;17:416–422. [DOI] [PubMed] [Google Scholar]
- 51. Bonnemeier H, Richardt G, Potratz J, et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: Differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol. 2003;14:791–799. [DOI] [PubMed] [Google Scholar]
- 52. Porges SW, Bohrer RE, Cheung MN, Drasgow F, McCabe PM, Keren G. New time-series statistic for detecting rhythmic co-occurrence in the frequency domain: The weighted coherence and its application to psychophysiological research. Psychol Bull. 1980;88:580–587. [PubMed] [Google Scholar]
- 53. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 54. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. [DOI] [PubMed] [Google Scholar]
- 56. Harvey AG, Stinson K, Whitaker KL, Moskovitz D, Virk H. The subjective meaning of sleep quality: A comparison of individuals with and without insomnia. Sleep. 2008;31:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Buysse DJ. Sleep health: Can we define it? Does it matter? Sleep. 2014;37:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hayes AF. Methodology in the Social Sciences. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 59. Sloan RP, Huang MH, McCreath H, et al. Cardiac autonomic control and the effects of age, race, and sex: The CARDIA study. Auton Neurosci. 2008;139:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosom Med. 2008;70:1020–1027. [DOI] [PubMed] [Google Scholar]
- 61. Grandner MA, Patel NP, Gehrman PR, et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010;11:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harvey CJ. Sleep and circadian functioning: Critical mechanisms in the mood disorders? Annu Rev Clin Psychol. 2011;7:297–319. [DOI] [PubMed] [Google Scholar]
- 63. Hamilton JL, Stange JP, Burke TA, Franzen PL, Alloy LB. Sleep disturbance and physiological regulation among young adults with prior depression. J Psychiatr Res. 2019;115:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Werner GG, Ford BQ, Mauss IB, Schabus M, Blechert J, Wilhelm FH. Cardiac vagal control and depressive symptoms: The moderating role of sleep quality. Behav Sleep Med. 2017;15:451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beauchaine TP, Bell Z, Knapton E, McDonough-Caplan H, Shader T, Zisner A. Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology. 2019;56:e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Olsson MB, Hwang CP. Depression in mothers and fathers of children with intellectual disability. J Intellect Disabil Res. 2001;45:535–543. [DOI] [PubMed] [Google Scholar]
- 67. Hayes SA, Watson SL. The impact of parenting stress: A meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord. 2013;43:629–642. [DOI] [PubMed] [Google Scholar]
- 68. Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. [DOI] [PubMed] [Google Scholar]
- 69. Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res. 2008;64:443–449. [DOI] [PubMed] [Google Scholar]
- 70. Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: A cognitive-energy model. Sleep. 2005;28:47–54. [DOI] [PubMed] [Google Scholar]
- 71. Pastor-Cerezuela G, Fernández-Andrés MI, Tárraga-Mínguez R, Navarro-Peña JM. Parental stress and ASD: Relationship with autism symptom severity, IQ, and resilience. Focus Autism Other Dev Disabl. 2016;31:300–311. [Google Scholar]
- 72. Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310. [DOI] [PubMed] [Google Scholar]
- 73. Zhang B, Wing YK. Sex differences in insomnia: A meta-analysis. Sleep. 2006;29:85–93. [DOI] [PubMed] [Google Scholar]
- 74. Nolen-Hoeksema S. Gender differences in depression. Curr Dir Psychol Sci. 2001;10:173–176. [Google Scholar]
- 75. Martin J, Shochat T, Ancoli-Israel S. Assessment and treatment of sleep disturbances in older adults. Clin Psychol Rev. 2000;20:783–805. [DOI] [PubMed] [Google Scholar]
- 76. Wohlgemuth WK, Edinger JD, Fins AI, Sullivan RJ. How many nights are enough? The short‐term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology. 1999;36:233–244. [PubMed] [Google Scholar]
- 77. Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol. 2015;98:338–350. [DOI] [PubMed] [Google Scholar]
- 78. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA. 2009;301:2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cunningham JEA, Shapiro CM. Cognitive behavioural therapy for insomnia (CBT-I) to treat depression: A systematic review. J Psychosom Res. 2018;106:1–12. [DOI] [PubMed] [Google Scholar]
- 80. Swanson LM, Flynn H, Adams-Mundy JD, Armitage R, Arnedt JT. An open pilot of cognitive-behavioral therapy for insomnia in women with postpartum depression. Behav Sleep Med. 2013;11:297–307. [DOI] [PubMed] [Google Scholar]
- 81. Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000;62:639–647. [DOI] [PubMed] [Google Scholar]
- 82. Taylor CB, Conrad A, Wilhelm FH, et al. Does improving mood in depressed patients alter factors that may affect cardiovascular disease risk? J Psychiatr Res. 2009;43:1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Caldwell YT, Steffen PR. Adding HRV biofeedback to psychotherapy increases heart rate variability and improves the treatment of major depressive disorder. Int J Psychophysiol. 2018;131:96–101. [DOI] [PubMed] [Google Scholar]