Abstract
Background
Self-monitoring is a key component of behavioral weight loss (BWL) interventions. Past research suggests that individuals may avoid self-monitoring in certain contexts (e.g., skipping self-weighing after higher-than-usual calorie intake). However, no studies have attempted to quantify individuals’ inclination to avoid information about their weight control (“weight-related information avoidance”; WIA) or prospectively examined its implications for treatment engagement and outcomes in BWL programs.
Purpose
Characterize WIA using a validated questionnaire among adults enrolled in BWL treatment and examine whether WIA prospectively predicts self-monitoring adherence, session attendance, treatment discontinuation, or weight loss.
Methods
Participants (N = 87; MBMI = 34.9 kg/m2, 83% female) completed a measure of WIA prior to starting a 12 week, group-based BWL intervention. Participants were given digital self-monitoring tools and instructed to self-monitor their food intake daily, physical activity daily, and body weight weekly (Weeks 1–10) and then daily (Weeks 11–12). Session attendance and treatment discontinuation were recorded. Weight was measured in-clinic pretreatment and posttreatment.
Results
While mean WIA was low (M = 2.23, standard deviation [SD] = 0.95; potential scale range: 1–7), greater WIA predicted poorer attendance (r = −.23; p = .03) and poorer self-monitoring of physical activity (r = −.28; p = .009) and body weight (r = −.32; p = .003). WIA did not predict food monitoring (p = .08), treatment discontinuation (p = .09), or 12 week weight loss (p = .91).
Conclusions
Greater WIA, as assessed via a brief questionnaire, may place individuals at risk for poorer self-monitoring and treatment engagement during BWL. Further research on the implications of WIA in the context of weight management is warranted, including evaluation of correlates, moderators, and mechanisms of action of WIA.
Clinical Trial Registration
Keywords: Self-monitoring, Avoidance, Lifestyle modification, Obesity, Adherence
Adults in a behavioral weight loss program who scored higher on a pre-treatment questionnaire assessing their inclination to avoid information about their weight control attended fewer treatment sessions, self-weighed less often, and self-monitored physical activity less frequently during a 12-week intervention.
Consistent self-monitoring of eating behavior, physical activity, and body weight is integral to success in behavioral weight loss (BWL) programs [1, 2], yet individuals in these programs may struggle to consistently self-monitor [3, 4]. Numerous factors likely contribute to this difficulty, including logistical barriers and finding self-monitoring to be burdensome and boring [5]. Digital self-monitoring tools (e.g., passive physical activity monitors, electronic food diaries, and “smart” scales) have greatly reduced the time and effort required for self-monitoring, helping to counter some of these barriers and improving adherence [3, 5–7]. However, clinical wisdom and a small body of empirical work suggests that individuals may also at times choose not to self-monitor in order to avoid obtaining information about their weight management that may be experienced as unwanted or unpleasant (e.g., avoiding self-weighing when weight gain is anticipated) [8, 9]. While information avoidance, defined as any behavior that is designed to prevent or delay the acquisition of available but potentially unwanted information [10], may thus interfere with self-monitoring during BWL treatment and could negatively impact treatment outcomes, few studies have examined information avoidant attitudes and their implications in the context of weight management.
A key function of self-monitoring during BWL is to help individuals to monitor their progress with eating, physical activity, and weight loss goals [11]. Monitoring of goal progress is an effective behavior change and goal attainment strategy [12, 13] that involves tracking behaviors or outcomes and comparing them to a reference value [14]. Goal progress monitoring is theorized to facilitate behavior change partially by indicating whether or not there is a discrepancy between one’s current and desired state, thus providing information about what action(s) one should take in the future [15, 16]. When one is successfully achieving their desired target (no discrepancy), monitoring can provide positive reinforcement (e.g., through a sense of accomplishment) for behavioral changes [16]. Per operant conditioning principles, this positive reinforcement should make the behaviors that led to goal attainment—and self-monitoring itself—more likely to recur. When one is not meeting their desired target (discrepancy), monitoring can signal that additional effort or alternative approaches are needed to reach one’s goal [15, 16]. While monitoring of goal progress can, therefore, provide valuable data for informing future behavior when a goal has not been met, individuals may also experience uncomfortable thoughts or feelings (e.g., disappointment) when seeing this discrepancy. Individuals may sometimes choose to avoid this information—a phenomenon that some have termed “the ostrich problem” [17]. This avoidance may become a negatively reinforced behavior; by preventing one from experiencing aversive thoughts and feelings, the likelihood of avoiding self-monitoring again in similar future situations increases. Information avoidance may thus interfere with both short-term and long-term goal progress.
Research across disciplines (e.g., psychology and communications) suggests that people may avoid information for three main reasons: (a) it may cause unpleasant emotions, (b) it may demand undesired action, and (c) it may demand a change in beliefs (for a review, see Sweeney et al. [10]). Integrating and extending on these reasons for general information avoidance, Webb et al. proposed a model of the motives underlying avoidance of goal progress monitoring in particular [17]. They posit that the extent of one’s goal progress monitoring is determined by an interaction among four motives: self-assessment (i.e., the desire to obtain accurate knowledge about goal progress), self-improvement (i.e., the desire to better the self), self-enhancement (i.e., the desire to maintain a positive view of the self), and self-verification (i.e., the desire to maintain a coherent self-representation). When conflict between these motives arise (e.g., the self-assessment motive argues for stepping on the scale to obtain accurate information about weight loss progress, while the self-enhancement motive argues against self-weighing when one suspects weight gain), a dilemma may arise and individuals may opt to avoid information. Acceptance-based therapies, which have successfully been applied to BWL, have posited similar notions; that is, that individuals may avoid behaviors like self-monitoring in order to avoid or reduce uncomfortable thoughts and feelings [18, 19]. Factors such as goal importance, self-regulatory strength, self-efficacy, coping style, and psychological acceptance (i.e., openness to and willingness to experience uncomfortable thoughts and feelings) may influence whether one ultimately decides to self-monitor in such situations [10, 17–20].
Several studies suggest that individuals engaged in BWL may exhibit information avoidance. For example, one study found that when individuals consumed more calories on a given day than was typical for them, they were less likely to weigh themselves the following day [8]. Qualitative research comparing the behaviors of individuals who successfully maintain weight loss and those who regain weight has also indicated that those who regain, but not those who maintain, tend to avoid self-weighing when they suspect they have gained weight [9]. As these latter findings highlight, if individuals avoid progress monitoring, it can make it difficult to know when and how to act, thus hindering effective self-regulation and goal pursuit [15, 17]. Indeed, less frequent self-monitoring is consistently associated with less weight loss in BWL [1, 21, 22]. In summary, information avoidance appears relevant to self-monitoring during BWL [8] and qualitative data suggest that information avoidance may impair long-term outcomes [9]. However, no studies have attempted to quantify individuals’ inclination to avoid information about their weight control or prospectively examined its implications for BWL treatment.
Recently, a self-report measure of individuals’ propensity to avoid learning information was developed (the Information Avoidance Scale) [23]. This questionnaire assesses individuals’ attitudes toward learning information about a particular topic (e.g., “When it comes to ___, sometimes ignorance is bliss”) and their reported tendency to avoid learning information (e.g., “I would avoid learning information about ___”). This scale thus both identifies individual differences in information avoidance tendencies and may serve as a domain-specific predictor of avoidance behaviors [23]. This measure may be a useful tool for assessing individuals’ information avoidance attitudes and tendencies with regard to their weight control, hereafter referred to as “weight-related information avoidance” (WIA). Although the construct of information avoidance has similarities to several other constructs previously investigated in the health behavior literature, such as experiential avoidance (i.e., a desire or attempt to avoid or minimize unwanted internal experiences, including thoughts, feelings, memories, and physical sensations) [24]; anticipated regret; and instrumental attitudes (i.e., evaluations or beliefs about how beneficial vs. harmful a particular behavior is) [25], it also differs from previous constructs in important ways. At the broadest level, while these other constructs all relate to potential reasons or motivations for why individuals may avoid information, questionnaires related to these other constructs do not explicitly assess individuals’ desire to avoid information itself nor behavioral tendencies to avoid learning information (see Sweeny et al. and Howell and Shepperd for further discussion) [10, 23]. Thus, the Information Avoidance Scale represents a brief, focused tool for evaluating attitudes and tendencies to avoid information about a topic of interest—here, weight management—regardless of the exact reasons for such avoidance. Further examination of WIA among adults engaged in BWL treatment is needed to determine whether WIA, as assessed via self-report, may represent a risk factor for poorer outcomes in BWL in terms of poorer treatment engagement or response.
The present study aimed to: (a) characterize WIA among individuals enrolled in a 12 week, group-based BWL program that used digital self-monitoring tools and (b) examine whether WIA, as measured via self-report prior to the start of treatment, predicts self-monitoring adherence, session attendance, treatment discontinuation, or weight loss during the 12 week program. Reported reasons for (or for not) self-monitoring, attending sessions, and completing the program were not directly assessed; instead, we conceptualized these variables as potential markers of behavioral avoidance. We hypothesized that higher WIA would predict less frequent self-monitoring, poorer attendance, greater likelihood of discontinuation, and less weight loss.
Methods
Participants
The present project was a substudy conducted with participants originally recruited for a clinical trial (R21 DK112741) focused on weight loss maintenance [26]. Participants were eligible for the parent study if they were 18–70 years old, had a body mass index between 25.0 and 45.0 kg/m2, were able to engage in physical activity, owned a smartphone, and had access to wireless internet in their home. Participants were excluded if they were diagnosed with a medical or psychiatric condition that would contraindicate study participation, recently began or changed a medication that is known to affect weight, had undergone bariatric surgery, had lost ≥5% of their body weight in the past 3 months, or were nursing, pregnant, or planning to become pregnant during the course of the trial. All participants provided informed consent.
Procedure
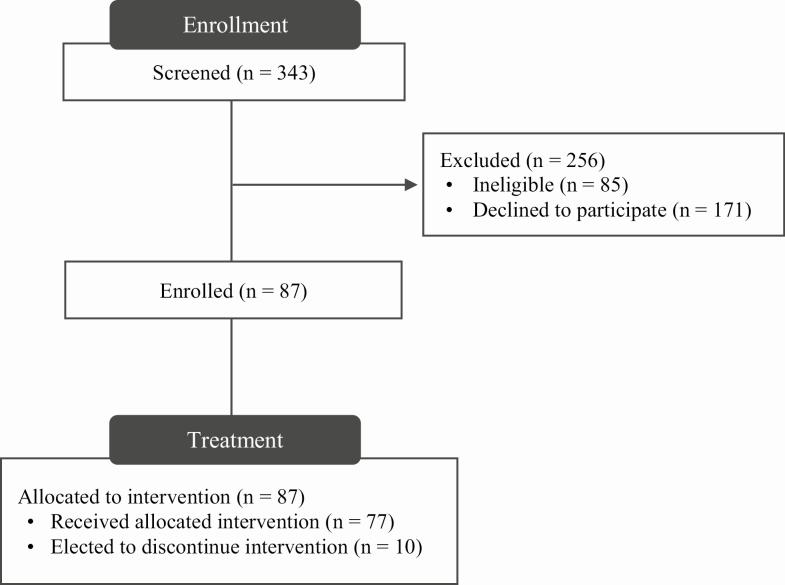
The parent study was approved by the institutional review board and was registered at www.clinicaltrials.gov (NCT03337139). Potential participants were recruited for the parent study from the community using media and print advertisements. Individuals who expressed interest initially completed a brief phone screening to assess eligibility and, then, attended a group informational session to learn more about the study. Figure 1 displays information about the study flow.
Figure 1.
Study flow.
All participants (N = 87) attended one orientation session that was intended to orient them to program procedures and tools, followed by 12 sessions of weekly BWL treatment. The present study analyzed data from this 12 week period only, which was meant to induce initial weight loss of 10% of baseline weight and was considered to be the active weight loss phase of treatment. Consistent with the parent study’s focus on weight loss maintenance, participants were randomized to different weight loss maintenance conditions after completing this initial 12 week period. All participants thus received identical intervention for the time period of interest (Weeks 1–12). Treatment during the 12 weeks consisted of in-person, group treatment based on the content from the Diabetes Prevention Program and Look AHEAD [27, 28]. Groups were led by masters- or PhD-level clinicians. Participants were encouraged to strive for a weight loss goal of 10%. To achieve this goal, participants were taught dietary self-monitoring principles and encouraged to meet a daily calorie goal of 1,200–1,500 for those with a starting weight <250 lbs. and 1,500–1,800 for those ≥250 lbs. Weekly physical activity goals were set progressively such that participants engaged in greater physical activity every week to a final goal of 250 min/week. Prior to each group session, participants were weighed individually by an interventionist and provided with brief, personalized feedback. If participants could not attend a scheduled group, they were expected to meet individually with an interventionist to review the missed content.
All participants were instructed to use several digital self-monitoring devices during treatment, which were provided to them at the orientation session. Specifically, participants received a digital Yunmai scale to track their weight change and a Fitbit Flex to track their physical activity, and the Fitbit food recording app was downloaded onto each participant’s personal smartphone for monitoring of dietary intake. With regard to self-monitoring prescriptions, participants were instructed to weigh themselves weekly for the first 10 weeks and daily thereafter (Weeks 11–12). This self-weighing prescription was meant to align with traditional recommendations to self-monitor weight weekly during active weight loss and daily during weight loss maintenance [1]. Although the study’s weight loss maintenance period did not formally begin until Week 13, a daily self-weighing prescription was introduced during Weeks 11 and 12 to help participants transition to the maintenance weighing prescription while still having the support of the BWL group and interventionists. Participants were instructed to monitor their food intake and physical activity using the Fitbit app and Fitbit Flex device, respectively, on a daily basis throughout the 12 week intervention. Data from the three self-monitoring devices (Fitbit Flex, wireless scale, and food recording app) were automatically uploaded into a personalized Fitbit account that could be accessed via smartphone and online. Participants were encouraged to view their data online or on the smartphone app throughout the 12 week period to assess progress. An online research portal also automatically captured data from participants’ self-monitoring devices; participants provided permission at treatment start for study staff to access and download this information for research purposes.
Measures
Sociodemographic characteristics and BMI
Participants self-reported their age, gender, race, and ethnicity on a questionnaire. Participants’ height and weight were measured by research staff using a Tanita model WB-3000 digital scale and its built-in height rod. Participants were weighed in lightweight clothes without shoes. Percentage of weight loss was calculated such that negative values indicate weight loss.
Weight-related information avoidance
A modified version of the Information Avoidance Scale [23] was used to assess WIA. This eight-item scale was designed to be used flexibly such that content added to each item stem is tailored to assess information avoidance attitudes and tendencies specific to a particular topic area (e.g., weight management). Table 1 shows the scale items, with original item stems bolded. For four of the original eight items, we completed the stem with “information about my weight control (e.g., my weight, calorie intake, or level of physical activity).” For two of the original stems, we opted to assess information avoidance for weight, calorie intake, and level of physical activity separately, resulting in the use of three items for each original stem. To avoid further increasing scale length, we then opted to remove two stems that appeared less applicable to weight control and/or redundant (i.e., “I want to know ___ immediately” and “I want to know ___”). Participants responded to each item using a seven-point Likert-type scale ranging from 1 (strongly disagree) to 7 (strongly agree) and a mean score was computed. Cronbach’s alpha for the present study was good (∝ = .85).
Table 1.
Weight-related information avoidance measure
| Item | M (SD) |
|---|---|
| I would rather not know my weight. | 2.21 (1.48) |
| I would rather not know my calorie intake. | 2.17 (1.34) |
| I would rather not know my level of physical activity. | 1.83 (1.05) |
| I would avoid learning information about my weight control (e.g., my weight, calorie intake, or level of physical activity). | 1.94 (1.36) |
| Even if it will upset me, I want to know information about my weight control (e.g., my weight, calorie intake, or level of physical activity; R). | 1.90 (1.16) |
| When it comes to information about my weight control (e.g., my weight, calorie intake, or level of physical activity), sometimes ignorance is bliss. | 2.58 (1.76) |
| I can think of situations in which I would rather not know information about my weight control (e.g., my weight, calorie intake, or level of physical activity). | 3.17 (1.99) |
| It is important to know my weight (R). | 2.10 (1.45) |
| It is important to know my calorie intake (R). | 2.20 (1.37) |
| It is important to know my level of physical activity (R). | 1.90 (1.01) |
Bolded text added to indicate stem from original Information Avoidance Scale [23].
R reverse scored; SD standard deviation.
Self-monitoring adherence
Adherence to the self-weighing prescription was determined via a multistep process, given that the frequency of prescribed self-weighing changed during the intervention (i.e., weekly during Weeks 1–10 and daily thereafter). We first calculated adherence to the self-weighing prescription at the weekly level (i.e., Weeks 1–10: ≥1 weigh-in = 100% adherence for that week; Weeks 11 and 12: adherence = (days self-weighed/7) × 100%). For Weeks 11 and 12, we opted to calculate the percentage of days that participants self-weighed continuously instead of dichotomously coding weeks as adherent (i.e., weighed on all 7 days) or nonadherent (i.e., weighed <7 days) in order to better capture variability in adherence to the daily self-weighing prescription in these latter weeks and to better detect potential behavioral avoidance of self-weighing. We then computed an average of these week-level adherence values to summarize overall adherence to the self-weighing prescription across the 12 weeks.
Consistent with prior studies, adherence to physical activity monitoring was assessed by computing the percentage of days that participants wore and logged at least 500 steps on their Fitbit Flex [29–31]. This threshold was selected as it avoids counting ambient movement of the device and days on which participants likely wore the device for very little time while minimizing the amount of discarded data that would be considered valid with a lower threshold (e.g., any steps) [32]. However, as a sensitivity analysis, we also computed and analyzed the percentage of days that any steps were logged by the Fitbit Flex [33].
Past studies have used a variety of thresholds for defining adherence to food logging (e.g., ≥50% of daily kilocalories goal logged; ≥500 or 800 kcal logged; any kilocalorie intake logged; ≥5 items logged; ≥2 meals logged; see Turney-McGrievy et al. [34]). We opted to operationalize adherence based on the percentage of days with ≥800 kcal recorded. We choose this threshold because it emerged as the threshold that best predicted weight loss among individuals using a food tracking app in a recent study that evaluated several potential logging thresholds [34], 800 kcal is generally considered adequate to deem a day of dietary intake plausible [35, 36], and it has been used as a marker of adherence in several prior studies [37, 38].
Session attendance
Research staff recorded whether participants attended each of the 12 sessions. While individuals were asked to complete an individual makeup session if unable to attend one of the group meetings, these makeup sessions were not included in attendance calculations to provide a more conservative estimate of attendance. Session attendance was expressed as a percentage of the 12 sessions attended.
Treatment discontinuation
Research staff recorded if a participant elected to formally discontinue participation during the 12 week treatment period. Treatment discontinuation was coded such that 1 = elected to discontinue treatment and 0 = remained enrolled in treatment.
Statistical Approach
Data were analyzed in SPSS version 25 and statistical level was set at ∝ = .05. All data were screened for outliers, normality, and missingness. The distributions for self-monitoring adherence and session attendance variables were nonnormally distributed. Specifically, these distributions were negatively skewed, with the degree of skewness ranging from −1.41 (food logging) to −2.61 (Fitbit Flex wear). For ease of interpretation and given that most parametric tests are quite robust to skewness [39], we opted to use parametric models. Importantly, however, the results remained unchanged when conducting sensitivity analyses using both nonparametric models (e.g., Spearman correlation) and Pearson correlation with bootstrapping (1,000 samples, 95% confidence intervals). WIA was characterized with descriptive statistics. Associations between WIA and sociodemographic characteristics were examined using t-tests (for categorical variables) and Pearson correlation (for continuous variables). Participant race was dichotomized for analyses (White = 1, non-White = 0). Sensitivity analyses were conducted that controlled for any sociodemographic characteristics that were significantly related to WIA scores. The prospective associations of WIA with self-monitoring adherence and treatment-related outcomes were assessed with Pearson correlations (for continuous variables) and logistic regression (for predicting treatment discontinuation). If participants elected to discontinue treatment, data from their self-monitoring devices were retained until the time that they discontinued treatment, after which point values were entered that assumed nonadherence. Only completers were used in analyses assessing the relationship between WIA and 12 week weight loss. One participant was missing self-monitoring data and was excluded from these analyses.
Results
Participant Sample Characteristics and Associations Between Sociodemographic and Anthropometric Characteristics With WIA
Table 2 displays sociodemographic and anthropometric characteristics for the sample. As shown, participants (N = 87) on average had a BMI in the obese range and were middle aged. Most participants identified as female and non-Hispanic/Latina, and approximately half (51.72%) of the sample identified as White. WIA did not significantly differ based on gender (t = −1.63, p = .11), race (t = −1.13, p = .26), or ethnicity (t = 0.21, p = .83). WIA also was not significantly related to BMI, r = .15, p = .16, or age, r = −.18, p = .10.
Table 2.
Sociodemographic/anthropometric characteristics
| M (SD) or n (%) | |
|---|---|
| BMI (kg/m2) | 34.92 (4.87) |
| Age (years) | 50.02 (13.14) |
| Gender (n, % female) | 72 (82.76%) |
| Race | |
| American Indian/Native Alaskan | 1 (1.15%) |
| Asian | 3 (3.45%) |
| Native Hawaiian or other Pacific Islander | 0 (0%) |
| Black/African American | 31 (35.63%) |
| White/Caucasian | 45 (51.72%) |
| Other or more than one race | 7 (8.05%) |
| Ethnicity | |
| Hispanic/Latina | 5 (5.75%) |
| Not Hispanic/Latina | 82 (94.25%) |
BMI body mass index
Characterizing WIA
On average, reported WIA was low, with a mean ± standard deviation (SD) of 2.23 ± 0.95 and an observed range of 1.00–4.70 (potential scale range: 1.00–7.00). A majority (88.50%) of the sample reported WIA values below the scale midpoint.
WIA as a Prospective Predictor of Self-Monitoring, Session Attendance, Treatment Discontinuation, and Weight Loss
Mean adherence to self-monitoring recommendations was high: self-weighing at the prescribed frequency = 80.54 ± 24.56%, percentage of days of Fitbit wear with ≥500 steps = 88.67 ± 21.21%, and percentage of days logging ≥800 kcal = 76.64 ± 24.68%. WIA was significantly correlated with adherence to the prescribed self-weighing frequency, r = −.32, p = .003 and percentage of days wearing the Fitbit with ≥500 recorded steps, r = −.28, p = .009. Sensitivity analyses indicated that the relation between WIA and Fitbit wear remained significant when using a threshold of ≥1 recorded step/day, r = .28, p = .008 (M ± SD = 89.78 ± 21.00%). WIA was not significantly related to percentage of days logging ≥800 kcal, r = −.19, p = .08.
Attendance at group sessions across the 87 enrolled participants was high, with participants attending an average of 81.6 ± 22.61% of group sessions. WIA significantly predicted attendance, r = −.23, p = .03. Ten (11.49%) participants elected to discontinue their participation during the 12 week treatment period. Logistic regression revealed that WIA did not significantly predict treatment discontinuation, χ 2 (1, N = 87) = 2.90, p = .09. Mean 12 week percentage of weight loss was −6.05 ± 4.19%, with a range of −19.70% to +2.07%. WIA was not related to percentage of weight loss, r = −.01, p = .91.
Discussion
This study is the first to our knowledge to characterize weight-related information avoidance attitudes and tendencies (i.e., WIA) among adults enrolled in a BWL program and to examine WIA as a prospective predictor of treatment engagement and outcomes. WIA was on average quite low. This may have partially resulted from self-selection of participants enrolling in this study; as participants were told during the enrollment process that the study placed a strong emphasis on monitoring and would require them to regularly self-monitor with digital tools, to have their weight measured by staff, and to report on their progress during group sessions, participants with high levels of WIA may have elected not to participate. Despite being low, however, greater pretreatment WIA predicted poorer self-monitoring of body weight and physical activity, as well as poorer attendance. WIA was not significantly related to self-monitoring of food intake (p = .08; small to medium effect size) or weight loss. Overall, these findings suggest that the brief measure of WIA used in this study may be a useful tool for identifying individuals who are at risk for poorer treatment engagement. Findings also warrant further research on WIA in the context of weight management.
While we did not directly assess factors that led to days without self-monitoring or session absences and thus cannot ascertain the extent to which WIA directly impacted these variables, our findings suggest that higher WIA predicts more frequent behavioral avoidance during BWL. This is consistent with the conceptualization of the Information Avoidance Scale, which was partially developed to serve as a proxy for avoidance behaviors and has been shown to have good predictive validity [23]. Still, there is appeal in being able to administer a brief self-report measure to participants at the start of BWL treatment to identify those who may be more prone to difficulties with self-monitoring adherence and attendance. Our failure to detect a significant relation between WIA and self-monitoring of eating may have been influenced by limited power, a more nuanced relation between WIA and eating self-monitoring than our threshold could detect (e.g., avoiding recording only when kilocalorie intake exceeds one’s daily target) or the greater complexity of food logging. Accurate kilocalorie estimation and food logging require some knowledge and skill and eating must be logged repeatedly throughout the day. Factors other than WIA (e.g., nutritional literacy and motivation) may thus have influenced apparent adherence to eating self-monitoring [40, 41] and minimized the predictive effect of WIA. In contrast, self-weighing and wearing a FitBit are comparatively brief and less cognitively taxing; thus, failure to perform these behaviors may be more likely to be indicative of behavioral avoidance and more strongly predicted by a measure of WIA [17].
WIA did not predict percentage of weight loss during treatment. This is interesting given that greater WIA, on average, related to poorer self-monitoring, and poorer self-monitoring consistently predicts less weight loss [1, 2, 21]. There are a number of potential explanations for why WIA would predict poorer self-monitoring yet not negatively affect weight loss. First, adherence to the self-monitoring prescriptions in the present study was very good, and WIA was not strongly related to self-monitoring of eating. Even those with greater WIA and relatively poorer self-monitoring adherence may have engaged in self-monitoring at levels sufficient for achieving weight loss. Second, the effect of WIA on weight loss may be small and/or complex (e.g., see Kangovi and Asch’s conceptual paper on a potential behavioral phenotype around avoiding failure) [42]. Consequently, the effect of WIA on weight loss may be difficult to detect in the context of the many other powerful factors that influence weight loss, including biological, environmental, and psychosocial variables [43]. Additional research is warranted to elucidate whether and how WIA impacts weight loss.
This study has several strengths and limitations. We objectively assessed self-monitoring adherence in three domains with digital tools rather than relying on self-reports of adherence; we assessed several aspects of WIA using a validated measure; and our sample was racially diverse. Regarding limitations, this study was conducted using a self-selected sample of adults enrolled in a BWL program that emphasized self-monitoring with digital tools. Results may not generalize to other samples and replication with larger samples is needed. Second, while participants were encouraged to regularly view their data, we could not be certain that participants complied with this request (e.g., participants may have worn the FitBit but not viewed their activity data). Future studies assess how WIA relates to data recording and viewing. Additionally, we did not assess potential psychosocial correlates of WIA, mechanisms of action of the relation between WIA and treatment engagement/outcomes, or moderators of this relation. It will be critical for future research to (a) assess process measures of potential reasons why people who score higher on WIA exhibit lesser self-monitoring and attendance (e.g., anticipated negative affect) and (b) better isolate the effects of WIA on treatment engagement by controlling for potential confounds (e.g., motivation). The self-motive model outlined by Webb et al. [17], as well as the broader literature on information avoidance [10], can guide such work. Lastly, examination of the impact of WIA over longer time periods is needed, particularly as WIA may negatively affect weight loss maintenance (e.g., by hindering periodic evaluations of one’s progress that could alert one to needed action early on).
In conclusion, this study indicated that WIA, as assessed with a brief questionnaire, predicted self-monitoring adherence of physical activity and weight and attendance in a BWL treatment that used digital self-monitoring tools. Additional research is warranted that further examines the implications of WIA for both short- and long-term self-monitoring and weight loss success, as is research that clarifies who scores higher on WIA and the processes by which WIA impacts weight management.
Acknowledgments
We would also like to thank the study participants and study staff for their contributions to the present study.
Funding
The authors would like to acknowledge their funding sources: National Institute of Diabetes and Digestive and Kidney Diseases (R21 DK112741; PI: M.L.B.) and National Heart, Lung, and Blood Institute (T32 HL076134; L.M.S.).
Compliance With Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions M.L.B. and E.M.F. designed the study and oversaw data collection and management. L.M.S. analyzed data, and L.M.S., M.K.M., and A.D.C. drafted the manuscript. All authors reviewed and substantively edited the manuscript and approved the final version.
Ethical Approval This study and its procedures were approved by the Drexel University Institutional Review Board.
Informed Consent All individuals provided informed consent to participate in this study.
References
- 1. Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: A systematic review of the literature. J Am Diet Assoc. 2011;111:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self‐weighing in weight management: A systematic literature review. Obesity. 2015;23(2):256–265. [DOI] [PubMed] [Google Scholar]
- 3. Burke LE, Styn MA, Sereika SM, et al. Using mHealth technology to enhance self-monitoring for weight loss: A randomized trial. Am J Prev Med. 2012;43:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krukowski RA, Harvey-Berino J, Bursac Z, Ashikaga T, West DS. Patterns of success: Online self-monitoring in a web-based behavioral weight control program. Health Psychol. 2013;32:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burke LE, Swigart V, Warziski Turk M, Derro N, Ewing LJ. Experiences of self-monitoring: Successes and struggles during treatment for weight loss. Qual Health Res. 2009;19:815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spring B, Duncan JM, Janke EA, et al. Integrating technology into standard weight loss treatment: A randomized controlled trial. JAMA Intern Med. 2013;173:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas J, Raynor H, Bond D, et al. Weight loss and frequency of body‐weight self‐monitoring in an online commercial weight management program with and without a cellular‐connected “smart” scale: A randomized pilot study. Obesity Sci Pract. 2017;3(4):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanenbaum ML, Ross KM, Wing RR. Overeat today, skip the scale tomorrow: An examination of caloric intake predicting nonadherence to daily self‐weighing. Obesity. 2016;24(11):2341–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reyes NR, Oliver TL, Klotz AA, et al. Similarities and differences between weight loss maintainers and regainers: A qualitative analysis. J Acad Nutr Diet. 2012;112:499–505. [DOI] [PubMed] [Google Scholar]
- 10. Sweeny K, Melnyk D, Miller W, Shepperd JA. Information avoidance: Who, what, when, and why. Rev Gen Psychol. 2010;14(4):340–353. [Google Scholar]
- 11. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34(4):841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harkin B, Webb TL, Chang BP, et al. Does monitoring goal progress promote goal attainment? A meta-analysis of the experimental evidence. Psychol Bull. 2016;142:198–229. [DOI] [PubMed] [Google Scholar]
- 13. Dombrowski SU, Sniehotta FF, Avenell A, Johnston M, MacLennan G, Araújo-Soares V. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: A systematic review. Health Psychol Rev. 2012;6(1):7–32. [Google Scholar]
- 14. Carver CS, Scheier MF. Control theory: A useful conceptual framework for personality-social, clinical, and health psychology. Psychol Bull. 1982;92:111–135. [PubMed] [Google Scholar]
- 15. Myrseth KOR, Fishbach A. Self-control: A function of knowing when and how to exercise restraint. Curr Dir Psychol Sci. 2009;18(4):247–252. [Google Scholar]
- 16. Carver CS, Scheier MF. Origins and functions of positive and negative affect: A control-process view. Psychol Rev. 1990;97(1):19. [Google Scholar]
- 17. Webb TL, Chang BP, Benn Y.“The Ostrich Problem”: Motivated avoidance or rejection of information about goal progress. Soc Pers Psychol Compass. 2013;7(11):794–807. [Google Scholar]
- 18. Lillis J, Kendra KE. Acceptance and Commitment Therapy for weight control: Model, evidence, and future directions. J Contextual Behav Sci. 2014;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forman EM, Butryn ML. A new look at the science of weight control: How acceptance and commitment strategies can address the challenge of self-regulation. Appetite. 2015;84:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang BP, Webb TL, Benn Y. Why do people act like the proverbial ostrich? Investigating the reasons that people provide for not monitoring their goal progress. Front Psychol. 2017;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang J, Sereika SM, Chasens ER, Ewing LJ, Matthews JT, Burke LE. Effect of adherence to self-monitoring of diet and physical activity on weight loss in a technology-supported behavioral intervention. Patient Prefer Adherence. 2012;6:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harvey J, Krukowski R, Priest J, West D. Log often, lose more: Electronic dietary self‐monitoring for weight loss. Obesity. 2019;27(3):380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Howell JL, Shepperd JA. Establishing an Information Avoidance Scale. Psychol Assess. 2016;28:1695–1708. [DOI] [PubMed] [Google Scholar]
- 24. Hayes SC, Strosahl K, Wilson KGet al. Measuring experiential avoidance: A preliminary test of a working model. Psychol Rec. 2004;54(4):553–578. [Google Scholar]
- 25. Rhodes RE, Courneya KS. Investigating multiple components of attitude, subjective norm, and perceived control: An examination of the theory of planned behaviour in the exercise domain. Br J Soc Psychol. 2003;42:129–146. [DOI] [PubMed] [Google Scholar]
- 26. Butryn ML, Godfrey KM, Martinelli MK, Roberts SR, Forman EM, Zhang F. Digital self‐monitoring: Does adherence or association with outcomes differ by self‐monitoring target? Obes Sci Pract. 2020;6:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Group LAR. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Group DPPR. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korinek EV, Phatak SS, Martin CA, et al. Adaptive step goals and rewards: A longitudinal growth model of daily steps for a smartphone-based walking intervention. J Behav Med. 2018;41:74–86. [DOI] [PubMed] [Google Scholar]
- 30. Meyer J, Wasmann M, Heuten W, El Ali A, Boll SC. Identification and classification of usage patterns in long-term activity tracking. Paper presented at: Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems; May 6–11, 2017; Denver, CO.
- 31. Meyer J, Heuten W, Boll S. No effects but useful? Long term use of smart health devices. Paper presented at: Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing: Adjunct; September 12–16, 2016; Heidelberg, Germany.
- 32. Tang LM, Meyer J, Epstein DA, et al. Defining adherence: Making sense of physical activity tracker data. Proc ACM Interact Mob Wearable Ubiquitous Technol. 2018;2(1):1–22. [Google Scholar]
- 33. Tang LM, Kay J. Harnessing long term physical activity data—How long-term trackers use data and how an adherence-based interface supports new insights. Proc ACM Interact Mob Wearable Ubiquitous Technol. 2017;1(2):1–28. [Google Scholar]
- 34. Turner-McGrievy GM, Dunn CG, Wilcox S, et al. Defining adherence to mobile dietary self-monitoring and assessing tracking over time: Tracking at least two eating occasions per day is best marker of adherence within two different mobile health randomized weight loss interventions. J Acad Nutr Diet. 2019;119:1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The eating at America’s table study. Am J Epidemiol. 2001;154:1089–1099. [DOI] [PubMed] [Google Scholar]
- 36. Mendez MA, Popkin BM, Buckland G, et al. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am J Epidemiol. 2011;173:448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shay LE, Seibert D, Watts D, Sbrocco T, Pagliara C. Adherence and weight loss outcomes associated with food-exercise diary preference in a military weight management program. Eat Behav. 2009;10:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel ML, Hopkins CM, Brooks TL, Bennett GG. Comparing self-monitoring strategies for weight loss in a smartphone app: Randomized controlled trial. JMIR Mhealth Uhealth. 2019;7:e12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Field A. Discovering Statistics Using IBM SPSS Statistics. Los Angeles, CA: Sage; 2013. [Google Scholar]
- 40. Rosenbaum DL, Clark MH, Convertino AD, Call CC, Forman EM, Butryn ML. Examination of nutrition literacy and quality of self-monitoring in behavioral weight loss. Ann Behav Med. 2018;52:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Webber KH, Tate DF, Ward DS, Bowling JM. Motivation and its relationship to adherence to self-monitoring and weight loss in a 16-week Internet behavioral weight loss intervention. J Nutr Educ Behav. 2010;42:161–167. [DOI] [PubMed] [Google Scholar]
- 42. Kangovi S, Asch DA. Behavioral phenotyping in health promotion: Embracing or avoiding failure. JAMA. 2018;319:2075–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faith MS, Kral TV. Social environmental and genetic influences on obesity and obesity-promoting behaviors: Fostering research integration. In: Hernandez LM, Blazer DG, eds. Genes, Behavior, and the Social Environment: Moving Beyond the Nature/Nurture Debate. Washington, DC: National Academies Press; 2006:236–280. [PubMed] [Google Scholar]