Abstract
Infectious disease management of Staphylococcus aureus bacteremia (SAB) was surveyed through the Emerging Infections Network. Although there were areas of consensus, we found substantial practice variation in diagnostic evaluation and management of adult patients with SAB. These findings highlight opportunities for further research and guidance to define best practices.
Keywords: Staphylococcus aureus, bacteremia, infectious disease physicians
Staphylococcus aureus bacteremia (SAB) is associated with high morbidity, mortality, and healthcare costs [1]. Infectious disease (ID) consultation for SAB has been associated with significant improvement in patient outcomes and increased adherence to best practices in SAB management such as follow-up blood cultures, echocardiography, source identification/control, and appropriate antibiotic therapy [2, 3]. However, little is known about practice patterns among ID physicians in scenarios where data are limited or inconclusive. We distributed a survey to members of the Emerging Infections Network (EIN) to assess physician practices in the management of SAB.
METHODS
The Infectious Diseases Society of America’s (IDSA) EIN is a network of practicing ID physicians in the United States and Canada, funded by the Centers for Disease Control and Prevention [4]. We developed an 11-question multiple-choice survey (Supplementary Materials) to assess adult ID specialist opinions and practice patterns in the management of SAB. The EIN distributed the survey via emailed weblink or facsimile on 5 January 2017. Two reminders at 1-week intervals were provided. Survey responses were analyzed using SAS version 9.4. A P value of < .05 was considered statistically significant.
RESULTS
Characteristics of Participants
Of 1286 active EIN physician members with an adult ID practice, 723 (56%) responded to this survey. Respondents (220/723 [30%]) were more likely than nonrespondents (117/563 [21%]) to have ≥25 years of ID experience (P < .0001). No other significant differences were identified. Baseline practice characteristics including clinical experience, practice type, and geographic location are provided in Supplementary Table 1. Fifty-four (7%) respondents did not manage SAB and opted out.
Diagnostic Evaluation of SAB
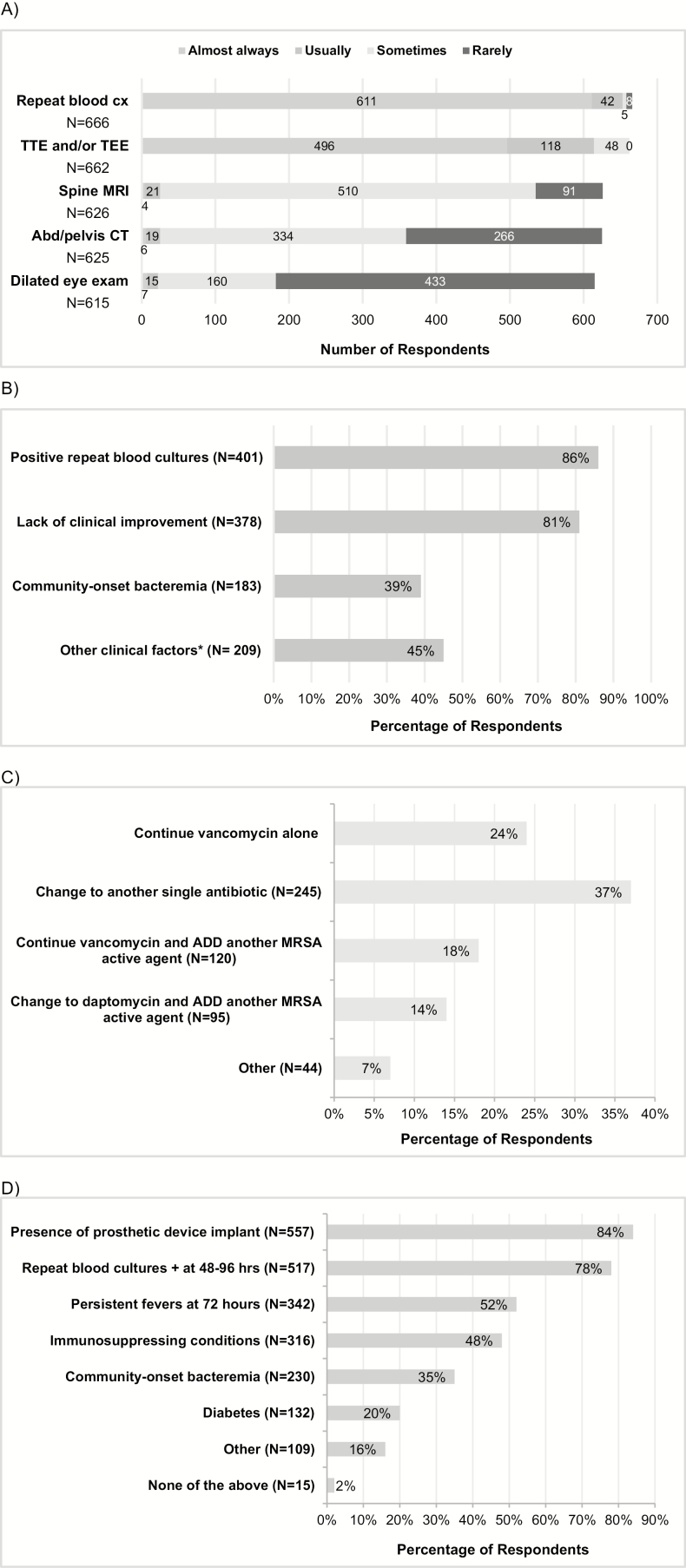
Repeat blood cultures and echocardiography are performed by the majority of respondents (Figure 1A). Most (599/667 [90%]) indicated they would always perform a transthoracic echocardiogram (TTE). Those with <15 years of experience were more likely to always do a TTE (93% vs 86%; P = .01). A transesophageal echocardiogram (TEE) would be performed on every patient with a negative TTE by 126 (19%) of respondents whereas 473 (71%) would only perform TEE under selected circumstances (Figure 1B). Those practicing in the Midwest, Northeast, or South [5] regions were more likely to always perform a TEE compared to those in the West or Canada/Puerto Rico (24% vs 11%; P = .009).
Figure 1.
Practice patterns among survey respondents on the diagnostic evaluation and management of Staphylococcus aureus bacteremia (SAB). A, Diagnostic workup routinely performed in the evaluation of a patient with SAB. B, Respondents indicating they would perform transthoracic echocardiogram (TTE) on every patient but only perform transesophageal echocardiogram (TEE) under these selected circumstances (n = 473). C, Management of patient with methicillin-resistant Staphylococcus aureus endocarditis and persistent SAB on day 6 of vancomycin, with therapeutic trough and vancomycin MIC of .5 mg/L. D, Factors influencing decision to extend duration of therapy from 2 weeks to 4–6 weeks assuming negative echocardiography (TTE and/or TEE). Abbreviations: Abd, abdominal; cx, culture; CT, computed tomography; MRI, magnetic resonance imaging; MRSA, methicillin-resistant Staphylococcus aureus; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram. *Among 222 respondents who commented on other clinical factors that would prompt TEE, common responses included presence of cardiac device or prosthetic valve (58 [26%]); clinical suspicion for infective endocarditis including embolic phenomenon or metastatic infection (43 [19%]); or TEE results would change management (eg, duration of therapy, surgical management) (36 [16%]).
Nafcillin or Cefazolin for Methicillin-susceptible Staphylococcus aureus Endocarditis
For treatment of left-sided methicillin-susceptible S. aureus (MSSA) endocarditis without CNS involvement, 32% chose cefazolin and 29% favored nafcillin, whereas 32% considered the 2 equivalent. Among the 215 who chose cefazolin, 207 provided a rationale, with most citing a combination of equal efficacy, less toxicity, dosing convenience, and cost. Among the 193 who selected nafcillin, 169 provided a rationale, with most citing nafcillin as the “gold standard,” whereas others favored it due to inoculum effect or better CNS penetration for clinically silent disease. Those with <5 (vs ≥5) years of experience were more likely to use cefazolin (39% vs 33%) whereas those with ≥15 (vs <15) years of experience were more likely to use nafcillin (37% vs 26%) (P = .048).
Management of Methicillin-resistant S. aureus Bacteremia and Endocarditis
When managing a patient with methicillin-resistant S. aureus (MRSA) bacteremia and a vancomycin minimum inhibitory concentration (MIC) of 2 mg/L, a majority (336/665 [51%]) of respondents would treat with vancomycin if clinically responding, whereas 248 (37%) favored daptomycin and 29 (4%) chose ceftaroline. Less than 1% of respondents selected linezolid, telavancin, trimethoprim-sulfamethoxazole, or daptomycin plus ceftaroline. Respondents in a university or teaching hospital were more likely to use vancomycin compared with those in a community or Veterans Affairs/Defense Department hospital (55% vs 43%; P = .03).
In a patient with MRSA endocarditis and persistent bacteremia on day 6 of vancomycin, most (504/668 [75%]) would modify therapy. Alternative monotherapy was selected by 245 (37%) respondents whereas 215 (32%) favored combination therapy (Figure 1C). Among those who chose another single agent, 193 (78%) selected daptomycin while 36 (15%) chose ceftaroline. The specific combination of daptomycin and ceftaroline was chosen by 66 (10%). Those practicing in the Midwest were most likely to choose alternative monotherapy (62%). Those in the Northeast and West were most likely to use combination therapy with daptomycin (28%; P = .04), and were more likely to use daptomycin and ceftaroline (15%) compared to those in the Midwest (5%) and South (7%) (P = .004).
The daptomycin dose used for MRSA bacteremia varied, with 38%, 43%, and 17% of respondents selecting 6 mg/kg, 8 mg/kg, and 10–12 mg/kg, respectively. Doses of 10–12 mg/kg were most likely to be used in the Northeast and West (21% and 20%, respectively), while 6–8 mg/kg was most likely to be used in the Midwest (91%) (P = .0002).
Duration of Therapy
Most respondents managed SAB with at least 14 days of intravenous (IV) antibiotics in several scenarios. In a patient with MRSA bacteremia and a skin and soft tissue source, rapid clearance of blood cultures, negative TTE, and no evidence of metastatic infection, most (491/669 [73%]) would treat with IV vancomycin for 14 days whereas 87 (13%) transitioned to oral antibiotics to complete a 14-day course. A minority (24/669 [4%]) would treat for 5–7 days with either oral or IV antibiotics while 47 (7%) favored a longer duration of 21–28 days.
In a patient with 1 of 2 blood cultures positive for MSSA, no obvious signs or symptoms of infection, a normal white blood cell count, negative repeat blood cultures, negative TTE, and no evidence of metastatic infection, most (445/664 [67%]) respondents would treat with IV antibiotics for 14 days whereas 51 (8%) would treat for 4–6 weeks. A minority (10%) would consider the cultures a contaminant and stop antibiotics.
Most respondents would extend treatment duration to 4–6 weeks in the setting of SAB and a negative echocardiogram for patients with a prosthetic device or positive repeat blood cultures (Figure 1D).
Management of Septic Thrombophlebitis
Most respondents (467/657 [71%]) recommended anticoagulation in the setting of SAB and peripherally inserted central catheter–associated deep vein thrombosis after catheter removal. Duration of antimicrobial therapy varied with 52% of respondents treating for 4 weeks while 19% chose 2 weeks and 25% chose 6 weeks.
DISCUSSION
SAB is a serious disease commonly managed by ID physicians. Most respondents performed repeat blood cultures and echocardiography, and treated with IV therapy for at least 14 days. There were some areas of consensus, but this survey highlights considerable practice variation among respondents representing a wide breadth of ID practitioners in North America, including differences by years of experience, geographic region, and practice environment.
The IDSA MRSA treatment guidelines recommend echocardiography in all patients with SAB, with TEE being the preferred modality due to its greater sensitivity [6]. Although the vast majority of respondents supported TTE as part of the diagnostic evaluation of SAB, only 19% of respondents indicated they would always perform a TEE. These findings are consistent with other studies that suggest routine use of TEE is infrequent [7]. TEE is an invasive procedure that has complication risks, is resource intensive, and may not be available at all centers. Some studies suggest that TEE may not be necessary for all cases of SAB and that clinical prediction rules may help with risk stratification, but these require external validation [8]. The lack of concordance between guideline recommendations and current practice indicates a need for further research and guidance on the role of TEE among patients with SAB.
There was lack of consensus regarding the treatment of MSSA endocarditis, with respondents almost evenly distributed among cefazolin, nafcillin, or use of either drug, suggesting the need for evidence-based guidelines to define optimal therapy. Those with fewer years of experience favored cefazolin whereas more experienced clinicians preferred nafcillin. These differences may reflect a growing body of literature suggesting similar clinical outcomes and fewer drug-related adverse events with the use of cefazolin for MSSA bacteremia [9]. However, early reports of cefazolin treatment failure in the setting of endocarditis have led others to caution its use in high-inocula infections [10].
Although the presence of prosthetic devices or positive repeat blood cultures would prompt most to extend therapy to 4–6 weeks, a smaller proportion of respondents were influenced by immunosuppression, diabetes, or community-onset bacteremia. Further guidance is needed to identify patients who are at increased risk of relapse or serious complications, in whom prolonged treatment duration may be warranted.
Consistent with guideline recommendations [6], clinical response influenced 51% of respondents to continue vancomycin in a patient with MRSA bacteremia and vancomycin MIC of 2 μg/mL, although a substantial portion would switch to daptomycin. Observational studies examining the role of daptomycin vs vancomycin in management of MRSA bacteremia with high vancomycin MICs have yielded mixed results [11, 12], and a randomized controlled trial designed to evaluate this issue was recently terminated due to slow accrual [11]. Although 8 mg/kg was the most commonly selected dose of daptomycin, a sizable minority chose the US Food and Drug Administration label dose of 6 mg/kg for management of MRSA bacteremia. The substantial differences in management of the above scenario and treatment of persistent bacteremia highlight the lack of high-quality evidence in these areas.
Our study has several limitations. As with all voluntary surveys, selection bias could yield results not generalizable to all ID specialists. Response bias is possible and survey answers may not accurately reflect clinical practice. Although the value of ID consultation in SAB management has been established by multiple studies, this survey demonstrates that there remains ample opportunity to further define best practices and optimize management of this complex disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all Infectious Diseases Society of America’s Emerging Infections Network members who participated in this survey.
Disclaimer. The findings and conclusions in the manuscript are those of the authors and do not necessarily represent the official views of the US Centers for Disease Control and Prevention or the Department of Health and Human Services.
Financial support. This publication was supported by the Centers for Disease Control and Prevention (cooperative agreement number 1 U50 CK000477).
Potential conflicts of interest. C. L. has served as a consultant for Theravance. H. F. C. has received grant support from Genentech and personal fees from Allergan. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit Care Med 2006; 34:2588–95. [DOI] [PubMed] [Google Scholar]
- 2. Paulsen J, Solligard E, Damas JK, DeWan A, Asvold BO, Bracken MB. The impact of infectious disease specialist consultation for Staphylococcus aureus bloodstream infections: a systematic review. Open Forum Infect Dis 2016; 3:ofw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vogel M, Schmitz RP, Hagel S, et al. Infectious disease consultation for Staphylococcus aureus bacteremia—a systematic review and meta-analysis. J Infect 2016; 72:19–28. [DOI] [PubMed] [Google Scholar]
- 4. Pillai SK, Beekmann SE, Santibanez S, Polgreen PM. The Infectious Diseases Society of America Emerging Infections Network: bridging the gap between clinical infectious diseases and public health. Clin Infect Dis 2014; 58:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. United States Census Bureau. Geographic terms and concepts. Available at: https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html. Accessed 12 October 2018.
- 6. Liu C, Bayer A, Cosgrove SE, et al. Infectious Diseases Society of America . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 7. Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3:ofw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bai AD, Agarwal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23:900–6. [DOI] [PubMed] [Google Scholar]
- 9. Loubet P, Burdet C, Vindrios W, et al. Cefazolin versus anti-staphylococcal penicillins for treatment of methicillin-susceptible Staphylococcus aureus bacteraemia: a narrative review. Clin Microbiol Infect 2018; 24:125–32. [DOI] [PubMed] [Google Scholar]
- 10. Miller WR, Seas C, Carvajal LP, et al. The cefazolin inoculum effect is associated with increased mortality in methicillin-susceptible Staphylococcus aureus bacteremia. Open Forum Infect Dis 2018; 5:ofy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalimuddin S, Chan YFZ, Phillips R, et al. A randomized phase 2B trial of vancomycin versus daptomycin for the treatment of methicillin-resistant Staphylococcus aureus bacteremia due to isolates with high vancomycin minimum inhibitory concentrations—results of a prematurely terminated study. Trials 2018; 19:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murray KP, Zhao JJ, Davis SL, et al. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: a matched cohort study. Clin Infect Dis 2013; 56:1562–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.