To the Editor—Sekaggya-Wiltshire et al demonstrated in an important observational study in patients coinfected with tuberculosis and human immunodeficiency virus (N = 268) that time to sputum culture conversion was longer in patients with low drug concentrations [1]. In a similar high-tuberculosis- burden setting in East Africa, we have also found such pharmacokinetic variability to be common and peak concentrations for key drugs to be well below the expected range [2].
In the accompanying editorial, Pasipanodya and Gumbo highlighted the central role of pharmacokinetic variability in determining treatment response, justifying that therapeutic drug monitoring (TDM) is necessary in tuberculosis- endemic settings, and recommended implementation of TDM to detect patients at risk for suboptimal drug exposure [3].
While the first opus of TDM for management of tuberculosis was penned by Peloquin in 2002 [4], few settings routinely employ it in 2018. Clearly, more needs to be done to deliver TDM to patients in most need. We suggest 3 practical steps.
First, it is a logistic and financial challenge to be able to process multiple blood samples to determine serum exposure within a dosing interval using current assays [1] in a programmatic setting. Rapid turnaround time of TDM results conveyed to the bedside is required to make a difference in treatment. Such barriers to care can be overcome with multianalyte assays combining the analysis of drugs in a single test using modern mass spectrometry while developing protocols to streamline specimen collection and delivery to regional laboratories such as the use of dried blood spots [5]. Additionally, some essential drugs [6] are heat stable; for example, levofloxacin was stable in serum at 50°C for >10 days (data on file, JWC Alffenaar), which would facilitate TDM. We acknowledge that such a patient-centered expansion of services in tuberculosis-endemic settings will require a significant infusion of funding. A “Global TDM Implementation Fund,” we argue, would be in line with the World Health Organization’s (WHO) current End TB Strategy.
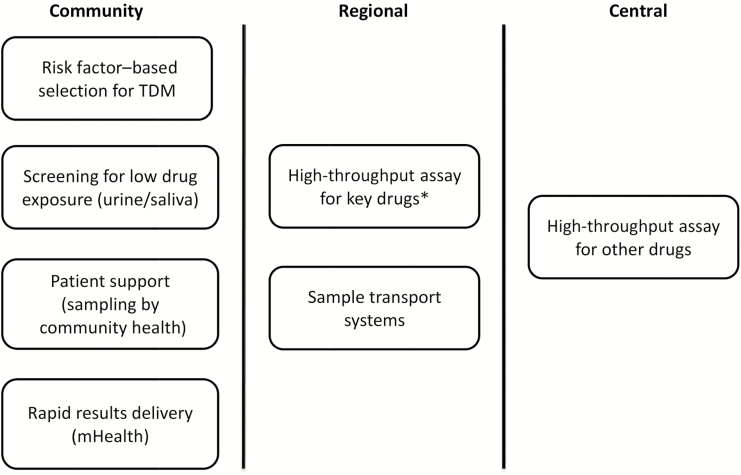
Second, just as not all people with tuberculosis are alike, programs will need to prioritize aspects of TDM most fitting to their setting and their patients’ needs (Figure 1). For instance, we believe that development of robust semiquantitative point-of-care tests to distinguish between patients with low or normal/high drug exposure may reduce the need to perform high-performance liquid chromatography with ultraviolet detection or mass spectrometry. Promising platforms include colorimetric testing of urine and saliva [7, 8].
Figure 1.
Levels of programmatic therapeutic drug monitoring (TDM). Screening for low drug exposure using semiquantative assays can be done at the bedside of the patient in the local healthcare facility. Patients with low drug exposure will qualify for a dose increase and TDM of the key drugs by measuring the concentrations in plasma/serum/dried blood spot. These more advanced analytical assays should be made available at a regional level. In addition, some patients may qualify immediately for TDM based on risk factors [9]. For patients with specific needs, TDM for other drugs should be made available at a central level. To reduce sample shipment costs, dried blood spot is the preferred sample material unless the drug has proven to be stable at high temperatures for a prolonged time. *Key drugs include: isoniazid, rifampicin for drug susceptible tuberculosis and levofloxacin/moxifloxacin, linezolid for multidrug resistant tuberculosis.
Third, technical and clinical guidance is urgently needed [5]. Although indications for TDM are clearly mentioned in some guidelines [9], practice has varied where, for instance, at least one state program in the United States routinely performs TDM for all people with tuberculosis and diabetes, human immunodeficiency virus, or those prescribed second-line drugs [10]. The WHO established a task force in 2017 to optimize dosing of antituberculosis drugs based on pharmacokinetic/pharmacodynamic (PK/PD) science [11], and ongoing clinical trials (eg, NCT01918397) will further validate in vitro models, refine existing PK/PD science, and determine optimal drug concentration targets. Yet in the interim, we urge the WHO to issue guidance on the use of TDM to facilitate uptake and implementation study in tuberculosis-endemic settings.
Note
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sekaggya-Wiltshire C, von Braun A, Lamorde M, et al. Delayed sputum culture conversion in tuberculosis-human immunodeficiency virus-coinfected patients with low isoniazid and rifampicin concentrations. Clin Infect Dis 2018; 67:708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heysell SK, Mtabho C, Mpagama S, et al. Plasma drug activity assay for treatment optimization in tuberculosis patients. Antimicrob Agents Chemother 2011; 55:5819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasipanodya JG, Gumbo T. Individualizing tuberculosis (TB) treatment: are TB programs in high burden settings ready for prime time therapeutic drug monitoring?Clin Infect Dis 2018; 67:717–8. [DOI] [PubMed] [Google Scholar]
- 4. Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 2002; 62:2169–83. [DOI] [PubMed] [Google Scholar]
- 5. Alffenaar JC, Tiberi S, Verbeeck RK, Heysell SK, Grobusch MP. Therapeutic drug monitoring in tuberculosis: practical application for physicians. Clin Infect Dis 2017; 64:104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). Geneva, Switzerland: WHO, 2018.
- 7. Zentner I, Schlecht HP, Khensouvann L, et al. Urine colorimetry to detect low rifampin exposure during tuberculosis therapy: a proof-of-concept study. BMC Infect Dis 2016; 16:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van den Elsen SHJ, Oostenbrink LM, Heysell SK, et al. Systematic review of salivary versus blood concentrations of antituberculosis drugs and their potential for salivary therapeutic drug monitoring. Ther Drug Monit 2018; 40:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63:e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alkabab Y, Keller S, Dodge D, Houpt E, Staley D, Heysell S. Early interventions for diabetes related tuberculosis associate with hastened sputum microbiological clearance in Virginia, USA. BMC Infect Dis 2017; 17:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Technical report on the pharmacokinetics and pharmacodynamics (PK/PD) of medicines used in the treatment of drug-resistant tuberculosis. 2018. Available at: http://apps.who.int/iris/bitstream/handle/10665/ 260440/WHO-CDS-TB-2018.6-eng.pdf. Accessed 23 August 2018.