Abstract
BACKGROUND
The risk for cardiovascular events increases within hours of near-roadway exposures. We aimed to determine the traffic-related air pollution (TRAP) and biological mechanisms involved and if reducing particulate matter <2.5 µm (PM2.5) inhalation is protective.
METHODS
Fifty healthy-adults underwent multiple 2-hour near-roadway exposures (Tuesdays to Fridays) in Ann Arbor during 2 separate weeks (randomized to wear an N95 respirator during 1 week). Monday both weeks, participants rested 2 hours in an exam room (once wearing an N95 respirator). Brachial blood pressure, aortic hemodynamics, and heart rate variability were repeatedly measured during exposures. Endothelial function (reactive hyperemia index [RHI]) was measured post-exposures (Thursdays). Black carbon (BC), total particle count (PC), PM2.5, noise and temperature were measured throughout exposures.
RESULTS
PM2.5 (9.3 ± 7.7 µg/m3), BC (1.3 ± 0.6 µg/m3), PC (8,375 ± 4,930 particles/cm3) and noise (69.2 ± 4.2 dB) were higher (P values <0.01) and aortic hemodynamic parameters trended worse while near-roadway (P values<0.15 vs. exam room). Other outcomes were unchanged. Aortic hemodynamics trended towards improvements with N95 respirator usage while near-roadway (P values<0.15 vs. no-use), whereas other outcomes remained unaffected. Higher near-roadway PC and BC exposures were associated with increases in aortic augmentation pressures (P values<0.05) and trends toward lower RHI (P values <0.2). N95 respirator usage did not mitigate these adverse responses (nonsignificant pollutant–respirator interactions). Near-roadway outdoor-temperature and noise were also associated with cardiovascular changes.
CONCLUSIONS
Exposure to real-world combustion-derived particulates in TRAP, even at relatively low concentrations, acutely worsened aortic hemodynamics. Our mixed findings regarding the health benefits of wearing N95 respirators support that further studies are needed to validate if they adequately protect against TRAP given their growing worldwide usage.
Keywords: air pollution, arterial compliance, blood pressure, endothelial function, heart rate variability, hypertension
Fine particulate matter <2.5 µm (PM2.5) air pollution is a leading cause of global morbidity and mortality.1–3 The largest portion of deaths is from cardiovascular disease (CVD).4,5 Although air quality has improved across the United States, billions of people living in developing nations (e.g., China, India) continue to face extraordinarily high PM2.5 levels.1–3 Even in North America many individuals remain highly exposed, with traffic-related air pollution (TRAP) being one of the major sources.3,6,7 The average American spends nearly an hour every day driving and roughly one-fifth of households are located near a high-volume roadway.6–9
Residing in close proximity to roadways promotes CVD events and chronic cardio-metabolic conditions (e.g., hypertension).7,10,11 Even brief exposure to traffic dramatically (~3-fold) increases the risk for CVD events within a few hours.10–13 Traffic exposure is the single most important trigger of acute myocardial infarctions from a global public health standpoint.14 Although TRAP is likely involved, the specific pollutants responsible, such as fresh combustion-derived particulates including black carbon (BC) and total particle count (PC) (driven mostly by ultrafine nanoparticle concentrations), vs. other factors (e.g., noises) remain to be clarified.15–21 Several biological mechanisms have been described (e.g., vascular dysfunction, autonomic imbalance)5,20–25; however, the relevant physiological perturbations that occur during the actual exposure period have yet to be fully elucidated. We and others have identified pollution-induced elevations in blood pressure (BP) and worsened aortic hemodynamic indices as prime candidates.4,5,16,17,20–25 Thus far, most studies have evaluated health responses only after exposures to TRAP and/or were limited by confounding activity (e.g., walking) or the stress of driving/riding in traffic.26–36 Finally, given the fact that billions of people are exposed to TRAP on a daily basis; practical approaches to reduce the associated CVD risk are critically important.37 One feasible intervention is to wear an N95 respirator. Their use has shown to help mitigate adverse health responses due to air pollution, but as of yet only in heavily polluted locations (e.g., China).38–41 No study has tested their efficacy in near-roadway settings typical for North America, nor used them as a strategy to assess the importance of particulate vs. other near-roadway factors in mediating adverse health responses. Here, we aimed to address each of these issues.
METHODS
The protocol was designed to evaluate the effect of N95 respirator use vs. no-use during near-roadway exposures on brachial systolic BP level (primary outcome). The study was approved by the institutional review board of the University of Michigan and all participants signed a written informed consent during screening visit. Inclusion criteria were nonsmoking adults living in nonsmoking households aged 18–65 years without CVD or risk factors including prior diagnosis of hypertension (and with a screening visit BP <140/90 mm), diabetes mellitus or treated hyperlipidemia. Body mass index was calculated from height and weight. Subjects were excluded if taking routine medication that affects BP or could impact responsivity to exposure (e.g., cholesterol or BP-lowering drug, fish oil, anti-oxidant, folate).
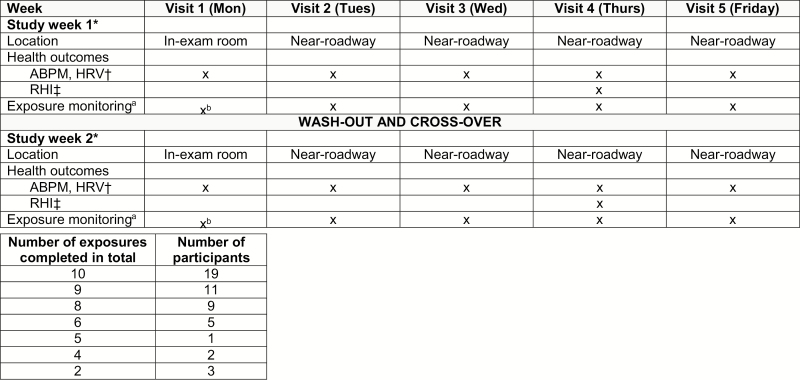
Fifty participants enrolled into the randomized single-blind crossover study (Figure 1). Briefly, each participant underwent up to 5 study visits (Monday to Friday) on 2 separate weeks. During the first study week, participants were randomized to wear (active) vs. not-wear (control) an N95 respirator during each visit and they crossed-over to the alternate intervention during the second week. On Monday from 8 to 10 am of both weeks (visit 1), participants rested seated 2 hours in a clean indoor exam room (wearing an N95 respirator during one of the weeks). On Tuesdays through Fridays (visits 2–5), scripted 2-hour (8–10 am) near-roadway exposures (<25 m from US-23 in Ann Arbor, Michigan) were conducted. Study health outcomes were measured in the same manner throughout all visits 1–5 while participants remained seated quietly with no activity. After completing the first week, participants crossed-over to repeat visits 1–5 with the alternative intervention (active vs. control). There were up to 2 clean-room and 8 near-roadway exposure visits (up to 10 study visits in total) per participant (Figure 1).
Figure 1.
Study protocol flow diagram. The figure is a flow diagram of the randomized single-blind crossover protocol design. The study was conducted during warm months (May to October) 2017–2018 to avoid exposures to excessively cold temperatures. Visits were canceled on case-by-case basis due to inclement weather (excessive cold, storms) at discretion of investigators. Participants were fasting ≥8 hours prior to visits which each lasted 2-hours (8 to 10 am). Participants remained seated in a chair without talking or activity throughout all visits. The near-roadway site was a commuter parking lot (https://www.theride.org/Services/Commuter-Services/Park-Ride/Plymouth-Rd-Park-Ride-Lot) located near to over-pass of Plymouth Road (4-lane roadway) and US-23 (4-lane freeway) in Northeast Ann Arbor, Michigan. The geocode is: 42°18′18.1′′N 83°41′16.7′′W (42.305060, −83.687976). During scripted near-roadway exposures (visits 2–5) participants sat at the south-west corner of the lot within 25 m of south-bound US-23. The study exam room was in the Preventive Cardiology outpatient clinics of Michigan Medicine located at Domino’s Farms Lobby A (<0.5 km from the near-roadway site). aAt the near-roadway exposure site (n = 312), we performed continuous measurements (8–10 am) of fine particulate matter (PM2.5) mass, particle count (PC), black carbon (BC), outdoor ambient temperature and noise intensity (dB). PM2.5 mass and ambient temperature were measured by a personal particulate monitor (Thermo Scientific, pDR-1500). PC was measured by a portable condensation particle counter (TSI, P-TRAK, http://www.tsi.com/p-trak-ultrafine-particle-counter-8525/). Noise intensity (20–140 dB range) was determined by an Optimus sound level meter (OHD, 161B https://afcintl.com/wp-content/uploads/docs/OHD%20pdfs/optimusred.pdf). BC was measured by a micro-aethalometer (MicroAeth, AE51, https://aethlabs.com/). bExposures were measured on several representative days (n = 13) in the exam room due to the stability of parameters and lack of need to repeat monitoring given the indoor clean air patient exam room environment. *Participants were randomized to open-label usage of wearing vs. not-wearing an N95 respirator during all visits (1–5) of each week. For example, if participant #1 did not wear the respirator week 1 then they would crossover to wear it during week 2. We enrolled 2 participants to be exposed concomitantly during each week. Participants were block randomized in the opposite intervention ordering. One participant wore the respirator whereas the other participant undergoing concomitant exposures did not wear it. They would then crossover together to the alternate intervention type. This assured that exposures were nearly identical for the wearing vs. not-wearing the respirator periods. Minor nonsignificant exposure differences occurred due to small unbalances in the enrollment when only one participant missed visits during a study week. Typically, the study was completed over 2 weeks in a row (separated by a weekend washout period). Rarely, there was a 1- to 2-week washout between study weeks for logistical reasons. †ABPM (ambulatory blood pressure monitoring) was performed using the OSCAR-2 with SphygmoCor-inside (Atcor Medical, Sydney; https://atcormedical.com/). The ABPM monitor was positioned on the left upper arm of each participant and measured brachial BP and central aortic hemodynamics from 8 to 10 am every 10-minutes (average of 9 readings per participant each visit). HRV (heart rate variability) metrics were analyzed from continuous electrocardiogram monitoring data using a Spacelabs evo Holter system. Standard deviation of normal-to-normal intervals (SDNN), high frequency (HF), and low (LF) frequency HRV metrics were analyzed using the Pathfinder software system (Spacelabs Healthcare, Snoqualmie, WA). ‡RHI (reactive hyperemia index) was performed using the EndoPAT2000 system (http://www.itamar-medical.com/endopat-main/). Standard protocol was followed per manufacturer directions.
A sham facemask was not used as a control intervention because they reduce particle inhalation by an unreliable and inconsistent amount and can be easily differentiated from a real N95 respirator. To assess for a possible effect of wearing the N95 respirator per se on study outcomes, we evaluated for favorable (placebo) or unfavorable (nocebo) responses in each participant. This was accomplished in our design by including visit 1 during each study week. We planned to account for each participant’s visit-1 health responses (i.e., health outcome differences between wearing vs. not-wearing the respirator while seated in an indoor clean exam room) in statistical models; however, no significant effects were found (online supplement, Supplementary Table 1). There were no differences in results when included in analyses, thus these responses were not included in our final models.
Cardiovascular outcomes
Brachial BP, aortic hemodynamics, and heart rate variability metrics were measured throughout all visits. BP and aortic hemodynamic parameters (aortic systolic BP, pulse pressure, augmentation pressure (AP) and augmentation index at a heart rate of 75 [AIx@75]) were measured every 10 minutes using an OSCAR-2 BP monitor with SphygmoCor-inside and the XCEL PWA system (Atcor Medical, Sydney). Continuous electrocardiogram monitoring was performed using a Spacelabs evo Holter system. The heart rate variability metrics including standard deviation of normal-to-normal intervals, high and low frequency domains were analyzed by the Pathfinder system (Spacelabs Healthcare, Snoqualmie WA). Immediately post-exposures on Thursdays (visit 4), seated microvascular endothelial-dependent vasodilatation (reactive hyperemia index [RHI]) was measured on the right index finger using the commercially available EndoPAT system using the standardized methodology described by the manufacturer (Itamar Medical, Caesarea, Israel). RHI was calculated automatically by the device and also manually measured from 90 to 120 seconds after reactive hyperemia (RHI90–120).
Environmental exposures
BC, PC ranging from 20 to 1,000 nm in diameter, PM2.5 mass, noise intensity and ambient temperature levels were measured at the near-roadway site throughout all 2-hour exposures. They were also measured in the exam room on representative occasions. Methodological details are provided in Supplementary Figure 1.
N95-respirator
We used a commercially available N95 respirator validated by the National Institutes of Occupational Safety and health (NIOSH). Participants wore a new Dettol SiTi shield Protect Plus Smart Mask (https://www.dettolsitishield.co.in/products/smart-mask/) of the correct facial size throughout each 2-hour exposure period as designated by the study protocol (RB, Slough, UK). The respirator has a one-way exhalation valve and a micro-fan, which was active throughout exposures to lessen the buildup of heat, moister, and CO2 inside the respirator in the breathing zone. The participants were instructed prior to exposures on the proper protocol for wearing the respirator with an air-tight facial seal.
Statistical methods
Summary statistics were computed for continuous measures as mean ± standard deviation, as well as minimum and maximum values, and for categorical variables as frequency and proportion (%). The ranges of continuous measures were reported as well. We analyzed the associations of longitudinal health measurements (e.g., systolic BP) repetitively obtained and exposure parameters (e.g., categorical near-roadway site, BC) using mixed-effect models. Random effects were included to account for within subject correlations. Models were adjusted for a priori determined covariates (patient age, sex, and body mass index). Model diagnostics revealed reasonable model fits in most cases. Details regarding the individual models are provided in the footnotes of each table. The primary outcome of the study was the difference in brachial systolic BP during near-roadway exposures between the active and control intervention limbs given a crossover study design. A priori estimations based upon our prior studies supported that our trial had 90% power to detect a group difference of between 2.0 and 3.0 mm Hg systolic BP (depending upon repeated BP correlations). All other outcomes were considered secondary endpoints. Statistical significance was defined as P values <0.05. We defined trends in statistical results as P values <0.15 in order to highlight certain responses that followed cohesive physiological patterns. Analyses were performed using the statistical software package R (version 3.2.5).
RESULTS
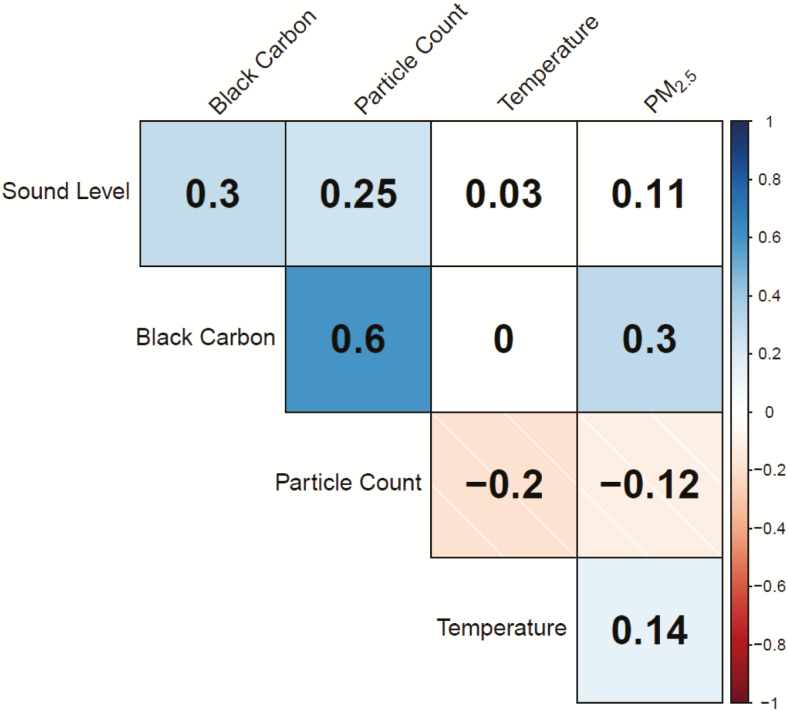
Study participants were young (mean age: 36 ± 14 years), healthy and normotensive (mean BP: 120.0/75.9 ± 13.4/9.4 mm Hg) (Table 1). Environmental exposures, except for ambient temperature, were significantly higher near-roadway (all visits 2–5) compared to in the exam room (visit 1 periods) (Table 2). There were no differences in exposure levels between intervention weeks (i.e., N95 respirator use vs. no-use) while near-roadway. Several exposure conditions and TRAP components, notably fresh combustion-derived particulates (PC and BC), were modestly inter-correlated while near-roadway (Figure 2).
Table 1.
Participant characteristicsa
| Mean (SD) | Min | Max | |
|---|---|---|---|
| Demographics (N = 50) | |||
| Age (years) | 36 (14) | 19 | 64 |
| Female (%) | 36 (72) | ||
| BMI (kg/m2) | 26.1 (4.7) | 19.1 | 39.7 |
| Blood pressures (N = 50) | |||
| SBP (mm Hg) | 120.0 (13.4) | 96 | 158 |
| Diastolic BP (mm Hg) | 75.9 (9.4) | 56 | 104 |
| Heart rate (beats/minute) | 67.6 (10.2) | 49 | 96 |
| Central aortic blood pressures (N = 49) | |||
| AorticsBP (mm Hg) | 111.4 (12.6) | 89 | 145 |
| AorticPP (mm Hg) | 34.0 (5.8) | 23 | 56 |
| AorticAP (mm Hg) | 11.2 (6.1) | 0 | 36 |
| AIx@75 (%) | 27.1 (12.2) | −4 | 58 |
| Heart rate variability (N = 50) | |||
| SDNN (ms) | 89.0 (43.0) | 41.9 | 247.7 |
| LF (ms2) | 3,007 (5,312) | 0.02 | 24,877 |
| HF (ms2) | 1,444 (2,124) | 0.04 | 9,129 |
| LF/HF | 2.6 (1.7) | 0.42 | 7.9 |
| Endothelial function (N = 35)b | |||
| RHI (%) | 0.5 (0.4) | 0.01 | 1.6 |
| RHI90–120 (%) | 2.2 (1.2) | 0.34 | 6.0 |
Abbreviations: AIx@75, augmentation index at a heart rate of 75 beats/minute; AP, augmentation pressure; BMI, body mass index; BP, blood pressure; HF, high frequency power; LF, low frequency power; max, maximum value; min, minimum value; PP, pulse pressure; RHI, reactive hyperemia index; RHI90–120, reactive hyperemia index from 90 to 120 seconds after cuff release; SBP, systolic blood pressure; SD, standard deviation; SDNN, standard deviation of normal-to-normal intervals.
aValues calculated from visit 1 data while not wearing a respirator.
bRHI summary statistics are provided for visit 4-without use of N95 respirator.
Table 2.
Environmental exposures
| Near-roadway exposures (visits 2–5) | Exam room (visit 1) | |||||
|---|---|---|---|---|---|---|
| Number of observations | 156 | 156 | 312 | 13 | ||
| Exposure variables | N95 respirator Mean (SD) [min–max] |
No respirator Mean (SD) [min–max] |
P value* | All visits Mean (SD) [min–max] |
All visits Mean (SD) [min–max] |
P value** |
| Temperature (°C) | 22.4 (4.9) [7.5–33.2] |
22.4 (4.9) [7.5–33.2] |
0.98 | 22.4 (4.9) [7.5–33.2] |
23.3 (2.0) [19.7–25.4] |
0.63 |
| PM2.5 (µg/m3) | 9.2 (7.6) [0–42.1] |
9.3 (7.8) [0–42.1] |
0.94 | 9.3 (7.7) [0–42.1] |
1.5 (1.2) [0–3.3] |
0.003 |
| Black carbon (µg/m3) | 1.3 (0.6) [0.3–3.2] |
1.3 (0.6) [0.3–3.2] |
0.99 | 1.3 (0.6) [0.3–3.2] |
0.2 (0.1) [0.1–0.6] |
<0.001 |
| Particle count (particles/cm3) | 8,365 (4,895) [1,256–24,353] |
8,384 (4,979) [1,214–24,353] |
0.86 | 8,375 (4,930) [1,214–24,353] |
1,597 (1,663) [458–6,021] |
<0.001 |
| Sound level (dB) | 69.2 (4.2) [56.7–74.3] |
69.2 (4.2) [56.7–74.3] |
0.97 | 69.2 (4.2) [56.7–74.3] |
54.0 (5.4) [50.3–71.4] |
<0.001 |
| Relative humidity (%) | 54.1 (15.0) [8.0–84.7] |
54.3 (14.8) [8.0–84.7] |
0.97 | 54.3 (14.8) [8.0–84.7] |
36.3 (10.3) [23.6–47.4] |
0.008 |
Abbreviations: max, maximum value; min, minimum value; PM2.5, fine particulate matter < 2.5 µm; SD, standard deviation.
*Comparison between wearing vs. not wearing N95 respirator during roadway exposure blocks (visits 2–5) evaluated using linear mixed model with a patient-level random intercept when needed to account for within-patient correlation of environmental exposures (e.g., correlation due to day or season of observation) and with linear model otherwise; P value represents that of the N95 respirator main effect.
**Comparison between exam room (visit 1) vs. all roadway exposures days (visits 2–5) evaluated using linear mixed model with a patient-level random intercept when needed to account for within-patient correlation of environmental exposures (e.g., correlation due to day or season of observation) and with linear model otherwise; P value represents that of the roadway exposure main effect.
Figure 2.
Correlations of environmental exposure parameters while near-roadway. The estimated correlation coefficient for each pairwise correlation is provided. Blue shading represents statistically significant positive correlations (P < 0.05); red shading represents statistically significant negative correlations (P < 0.05). White boxes represent correlations that are not statistically significant.
The near-roadway period (all visits 2–5) was not associated with significant changes in brachial BP or other cardiovascular outcomes when compared categorically to the visits when participants rested in the exam room (visit 1 periods) (Table 3). However, aorticAP and aorticpp levels trended higher during near-roadway exposures. Table 4 demonstrates the associations between exposure aspects while near-roadway (all visits 2–5) and changes in health outcomes. Higher BC exposures were associated with significant increases in aorticAP and AIx@75 levels. Higher PC exposures were associated with significant increases in aorticAP. Higher BC and PC exposures showed trends towards lower RHI values. Louder noise levels trended to be associated with changes in several outcomes; while colder temperatures were associated with a worsening of many outcomes.
Table 3.
Effect of exposure to near-roadway conditions (all visits 2–5) vs. being in the exam room (both visit 1 periods) on cardiovascular outcomesa
| Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood pressuresb | Aortic blood pressuresb | Heart rate variabilityb | |||||||||
| Systolic BP (mm Hg) |
Diastolic BP (mm Hg) |
Heart rate (beats/minute) |
AorticSBP (mm Hg) |
AorticPP (mm Hg) |
AorticAP (mm Hg) |
AIX@75 (%) |
SDNN (ms) |
LF (ms2) |
HF (ms2) |
LF/HF | |
| Covariates | |||||||||||
| Near-roadway exposuresc | 0.48 (−2.32, 3.3) P = 0.74 |
−0.81 (−3.05, 1.42) P = 0.48 |
0.63 (−2.12, 3.38) P = 0.65 |
0.54 (−2.39, 3.48) P = 0.72 |
1.55
(−0.33, 3.43) P = 0.11 |
1.41∗ (−0.23, 3.05)
P = 0.09 |
2.66 (−1.64, 6.96) P = 0.23 |
−5.7 (−16.3, 4.8) P = 0.29 |
680 (−1,844, 3,203) P = 0.60 |
139 (−1,004, 1,281) P = 0.81 |
0.2 (−0.7, 1.1) P = 0.65 |
| N95 respirator | 0.07 (−0.94, 1.08) P = 0.89 |
−0.26 (−1.06, 0.55) P = 0.53 |
0.15 (−0.84, 1.14) P = 0.76 |
−0.30 (−1.39, 0.79) P = 0.59 |
0.16 (−0.54, 0.85) P = 0.66 |
−0.41 (−1.02, 0.20) P = 0.18 |
−1.07 (−2.67, 0.53) P = 0.19 |
2.9 (−0.9, 6.7) P = 0.14 |
−217 (−1,143, 709) P = 0.65 |
351∗ (−65, 768) P = 0.10 |
−0.3∗ (−0.6, 0.03) P = 0.08 |
| Age (per year) | 0.06 (−0.12, 0.25) P = 0.49 |
0.01 (−0.14, 0.16) P = 0.85 |
−0.05 (−0.25, 0.15) P = 0.63 |
0.14 (−0.03, 0.30) P = 0.11 |
0.12∗ (0.04, 0.21) P = 0.07 |
0.15∗∗∗ (0.08, 0.22) P<0.001 | 0.31∗∗∗ (0.10, 0.53) P = 0.005 |
−1.1∗∗∗ (−1.9, −0.3) P = 0.006 |
−93∗∗ (−165, −20) P = 0.013 |
−57∗∗∗ (−95, −18) P = 0.004 |
0.02 (−0.01, 0.04) P = 0.17 |
| Female | −4.99∗ (−10.6, 0.64) P = 0.08 |
−3.29 (−7.92, 1.34) P = 0.16 |
0.55 (−5.73, 6.80) P = 0.86 |
−3.24 (−8.38, 1.90) P = 0.22 |
0.17 (−2.54, 2.89) P = 0.90 |
1.82 (−0.43, 4.06) P = 0.11 |
5.30 (−1.35, 11.95) P = 0.12 |
−0.5 (−25.3, 24.4) P = 0.97 |
48 (−2,218, 2,313) P = 0.97 |
197 (−997, 1,390) P = 0.75 |
−1.2∗∗∗ (−1.9, −0.4) P = 0.003 |
| BMI (Per 1 kg/m2) | 1.21∗∗∗ (0.70, 1.71) P<0.001 | 0.65∗∗∗ (0.24, 1.07) P = 0.003 |
0.22 (−0.34, 0.79) P = 0.44 |
1.14∗∗∗ (0.68, 1.60) P < 0.001 | 0.45∗∗∗ (0.21, 0.69) P < 0.001 | 0.22∗∗ (0.02, 0.42) P = 0.030 |
0.35 (−0.25, 0.95) P = 0.25 |
−0.8 (−3.1, 1.4) P = 0.47 |
−2 (−204, 199) P = 0.98 |
43 (−63, 150) P = 0.43 |
−0.01 (−0.07, 0.06) P = 0.86 |
| Temperature (per 1 °C) | −0.51∗∗∗ (−0.64, −0.37) P < 0.001 | −0.33∗∗∗ (−0.44, −0.23) P < 0.001 | 0.53∗∗∗ (0.40, 0.66) P < 0.001 | −0.37∗∗∗ (−0.51, −0.23) P < 0.001 | −0.09∗ (−0.18, 0.003) P = 0.06 |
0.20∗∗∗ (0.13, 0.28) P < 0.001 | 0.76∗∗∗ (0.55, 0.96) P < 0.001 | −1.6∗∗∗ (−2.1, −1.1) P <0.001 | 17 (−96, 131) P = 0.77 |
−30 (−82, 23) P = 0.27 |
0.1∗∗∗ (0.05, 0.1) P<0.001 |
Data presented as effect estimate, (95% confidence interval), P value. Main effect of roadway exposure on cardiovascular outcomes trending toward significant changes (P values <0.15) are in bold. Abbreviations: AIx@75, augmentation index at a heart rate of 75 beats/minute; AP , augmentation pressure; BMI, body mass index; BP, blood pressure; HF, high frequency power; LF, low frequency power; PP, pulse pressure; RHI, reactive hyperemia index; RHI90–120, reactive hyperemia index from 90 to 120 seconds after cuff release; SBP, systolic blood pressure; SDNN, standard deviation of normal-to-normal intervals.
aAll visits regardless of N95 respirator use are combined in this model.
bResults represent main effect estimates (i.e., β-coefficients) of the association of roadway exposure on cardiac outcomes, adjusting for N95 respirator use, demographics, and temperature, evaluated using linear mixed models with patient-level random intercept to account for within-patient correlation of outcomes; with exception of the constant term, effect estimates for all covariates included in the models are provided.
cMain outcome. Effect estimates represent the change in cardiac outcomes associated with roadway exposure (i.e., all visits 2–5) vs. during exam room (visit 1), controlling for demographics, temperature, and N95 respirator use.
*P < 0.1, **P < 0.05, ***P < 0.01.
Table 4.
Associations of environmental exposures during near-roadway conditions (all visits 2–5) with changes in cardiovascular outcomesa
| Blood pressuresb | Aortic blood pressuresb | Heart rate variabilityb | RHI outcomesc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes Covariates |
Systolic BP (mm Hg) |
Diastolic BP (mm Hg) |
Heart rate (beats/minute) |
AorticSBP (mm Hg) |
AorticPP (mm Hg) |
AorticAP (mm Hg) |
AIx@75 (%) |
SDNN (ms) |
LF (ms2) |
HF (ms2) |
LF/HF | RHI (%) |
RHI90–120 (%) |
| Temperature (per 1 °C) | −0.51*** (−0.64, −0.37) P < 0.001 |
−0.33*** (−0.44, −0.22) P < 0.001 |
0.53*** (0.40, 0.66) P < 0.001 | −0.37*** (−0.51, −0.22) P < 0.001 |
−0.09* (−0.18, 0.01) P = 0.06 |
0.21*** (0.13, 0.28) P < 0.001 | 0.76*** (0.56, 0.97) P < 0.001 | −1.5*** (−2.0, −1.1) P < 0.001 |
17 (−99, 133) P = 0.77 |
−31 (−84, 23) P = 0.26 |
0.1*** (0.056, 0.130) P < 0.001 |
0.001 (−0.02, 0.02) P = 0.91 |
−0.04 (−0.13, 0.05) P = 0.40 |
| Log (PM2.5) (per 2.71- fold increase) |
−0.84**
(−1.59, −0.08) P = 0.031 |
−0.36 (−0.96, 0.25) P = 0.25 |
−0.002 (−0.75, 0.75) P = 0.99 |
−0.56 (−1.38, 0.26) P = 0.18 |
−0.43 (−0.96, 0.10) P = 0.11 |
0.25 (−0.21, 0.71) P = 0.29 |
1.04* (−0.16, 2.24) P = 0.09 |
0.1 (−2.6, 2.8) P = 0.95 |
52 (−640, 744) P = 0.88 |
−34 (−348, 280) P=0.83 |
0.1 (−0.1, 0.3) P=0.27 |
0.01 (−0.10, 0.12) P = 0.92 |
−0.03 (−0.49, 0.42) P = 0.89 |
| Black carbon (per 1 µg/m3) | −0.76 (−1.70, 0.18) P = 0.11 |
−0.64* (−1.39, 0.11) P = 0.09 |
0.32 (−0.61, 1.24) P = 0.50 |
−0.48 (−1.51, 0.55) P = 0.36 |
0.13 (−0.53, 0.80) P = 0.70 |
0.88*** (0.32, 1.45) P = 0.003 | 1.89** (0.40, 3.38) P = 0.013 | −1.5 (−4.8, 1.8) P = 0.38 |
−286 (−1,148, 575) P = 0.52 |
−127 (−516, 263) P=0.52 |
0.2 (−0.1, 0.4) P = 0.16 |
−0.1 (−0.2, 0.04) P = 0.16 |
−0.42 (−0.98, 0.14) P = 0.15 |
| Particle count (per 5,000 particles/cm3) | −0.08 (−0.74, 0.59) P = 0.83 |
−0.24 (−0.76, 0.29) P = 0.38 |
0.00 (−0.65, 0.65) P = 1.00 |
0.02 (−0.68, 0.72) P = 0.96 |
0.30 (−0.15, 0.74) P = 0.19 |
0.61*** (0.23, 0.99)
P = 0.002 |
0.90* (−0.12, 1.93) P = 0.09 |
0.79 (−1.57, 3.15) P = 0.52 |
−87 (−665, 491) P = 0.77 |
48 (−217, 313) P = 0.73 |
0.02 (−0.16, 0.21) P = 0.81 |
−0.08 (−0.19, 0.04) P = 0.19 |
−0.34 (−0.81, 0.14) P = 0.18 |
| Sound level (per 10 dB) | 1.10 (−0.26, 2.45) P = 0.11 |
0.20 (−0.89, 1.29) P = 0.72 |
−0.14 (−1.48, 1.21) P = 0.85 |
1.32* (−0.12, 2.77) P = 0.08 |
0.69 (−0.24, 1.62) P = 0.15 |
0.71* (−0.10, 1.52) P = 0.09 |
0.97 (−1.16, 3.10) P = 0.38 |
−0.29 (−5.20, 4.63) P = 0.91 |
−292 (−1,555, 971) P = 0.66 |
102 (−470, 675) P = 0.73 |
−0.36* (−0.76, 0.04) P = 0.08 |
−0.06 (−0.23, 0.12) P = 0.52 |
0.11 (−0.63, 0.85) P = 0.78 |
Data presented as effect estimate, (95% confidence interval), P value. Cardiovascular outcomes that significantly changed (P values <0.05) in association with exposures to components of TRAP are in bold. Abbreviations: AIx@75, augmentation index at a heart rate of 75 beats/minute; AP, augmentation pressure; BP, blood pressure; HF, high frequency power; LF, low frequency power; PP, pulse pressure; RHI, reactive hyperemia index; RHI90–120, reactive hyperemia index from 90 to 120 seconds after cuff release; SBP, systolic blood pressure; SDNN, standard deviation of normal-to-normal intervals.
aAll visits 2–5 regardless of N95 respirator use are combined in this model.
bResults represent effect estimate (i.e., β-coefficient) of per-unit change in exposure covariate on cardiovascular outcomes, adjusting for N95 respirator use, demographics and temperature, which is evaluated using linear mixed models with patient-level random intercept to account for within-patient correlation of outcomes.
cPerformed using linear model, adjusting for respirator use, demographics and temperature, as RHI outcomes were collected only at visit 4.
*P < 0.1, **P < 0.05, ***P < 0.01.
Wearing an N95 respirator while in the exam room did not induce changes in the health outcomes evaluated in our study, indicating a lack of a significant placebo or nocebo effect (Supplementary Table 1). The effects of wearing an N95 respirator on brachial BP and cardiovascular outcomes during near-roadway exposures are shown in Table 5. There were no significant differences; however, aorticAP and AIx@75 levels trended lower. Given this finding and the fact that higher BC and PC exposures were both associated with worse aortic hemodynamics (i.e., the most likely responsible TRAP components) (Table 3), we further evaluated if wearing an N95 respirator modifies their adverse effects (i.e., supports a protective action). Supplementary Table 2 shows that there was no evidence for effect modification (nonsignificant interaction terms) of wearing an N95 respirator while near-roadway on the associations of BC and PC exposures with aortic hemodynamic changes. There was also no effect modification of the categorical impact of near-roadway exposures compared to being in the exam room on outcomes by wearing N95 respirators while near-roadway (Supplementary Table 3).
Table 5.
Effect of wearing vs. not wearing an N95 respirator during near-roadway exposures (visits 2–5) on cardiovascular outcomes
| Blood pressuresa | Aortic blood pressuresa | Heart rate variabilitya | RHI outcomesb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes Covariates |
Systolic BP (mm Hg) |
Diastolic BP (mm Hg) |
Heart rate (beats/minute) |
AorticSBP (mm Hg) |
AorticPP (mm Hg) |
AorticAP (mm Hg) |
AIx@75 (%) |
SDNN (ms) |
LF (ms2) |
HF (ms2) |
LF/HF | RHI (%) |
RHI90–120 (%) |
| N95 respiratorc | 0.04 (−1.00, 1.08) P = 0.94 |
−0.33 (−1.16, 0.50) P = 0.43 |
0.15 (−0.87, 1.17) P = 0.77 |
−0.39 (−1.52, 0.73) P = 0.49 |
0.14 (−0.58, 0.87) P = 0.70 |
−0.47
(−1.10, 0.16) P = 0.14 |
−1.28
(−2.93, 0.37) P = 0.13 |
2.3 (−1.4, 5.9) P = 0.22 |
−170 (−1,135, 796) P = 0.73 |
343
(−91, 778) P = 0.12 |
−0.2 (−0.5, 0.1) P = 0.16 |
0.1 (−0.1, 0.2) P = 0.44 |
0.4 (−0.3, 1.1) P = 0.26 |
| Age (per year) | 0.06 (−0.12, 0.24) P = 0.50 |
0.02 (−0.14, 0.17) P = 0.85 |
−0.05 (−0.26, 0.15) P = 0.61 |
0.14 (−0.03, 0.30) P = 0.11 |
0.12∗∗∗ (0.03, 0.21) P = 0.008 |
0.15∗∗∗ (0.072, 0.22) P<0.001 | 0.30∗∗∗ (0.09, 0.52) P = 0.006 |
−1.1∗∗∗ (−2.0, −0.4) P = 0.005 |
−92∗∗ (−165, −19) P = 0.014 |
−57∗∗∗ (−94, −19) P = 0.004 |
0.02 (−0.01, 0.04) P = 0.15 |
0.01∗∗ (0.00, 0.01) P = 0.030 |
0.02 (−0.01, 0.04) P = 0.19 |
| Female | −4.94∗ (−10.6, 0.72) P = 0.09 |
−3.34 (−7.99, 1.30) P = 0.16 |
0.70 (−5.60, 6.99) P = 0.83 |
−3.21 (−8.37, 1.94) P = 0.22 |
0.31 (−2.42, 3.04) P = 0.82 |
1.94∗ (−0.33, 4.21) P = 0.10 |
5.62 (−1.09, 12.33) P = 0.10 |
0.4 (−24.3, 25.2) P = 0.97 |
23 (−2,265, 2,311) P = 0.98 |
185 (−1,000, 1,369) P = 0.76 |
−1.2∗∗∗ (−2.0, −0.5) P = 0.002 |
−0.01 (−0.2, 0.2) P = 0.93 |
0.3 (−0.5, 1.1) P = 0.49 |
| BMI (per 1 kg/m2) | 1.22∗∗∗ (0.72, 1.73) P<0.001 | 0.66∗∗∗ (0.24, 1.08) P = 0.002 |
0.24 (−0.33, 0.80) P = 0.41 |
1.16∗∗∗ (0.69, 1.62) P < 0.001 | 0.46∗∗∗ (0.21, 0.70) P < 0.001 | 0.24∗∗ (0.03, 0.44) P = 0.024 |
0.38 (−0.22, 0.99) P = 0.21 |
−0.8 (−3.1, 1.4) P = 0.46 |
−7 (−211, 196) P = 0.94 |
45 (−60, 151) P = 0.40 |
−0.02 (−0.08, 0.05) P = 0.66 |
−0.01 (−0.02, 0.01) P = 0.50 |
−0.04 (−0.1, 0.03) P = 0.30 |
| Temperature (per 1 °C) |
−0.51∗∗∗ (−0.64, −0.37) P<0.001 |
−0.33∗∗∗ (−0.44, −0.22) P<0.001 | 0.53∗∗∗ (0.40, 0.66) P<0.001 | −0.37∗∗∗ (−0.51, −0.22) P < 0.001 | −0.09∗ (−0.18, 0.01) P = 0.06 |
0.21∗∗∗ (0.13, 0.28) P<0.001 | 0.76∗∗∗ (0.56, 0.97) P<0.001 | −1.5∗∗∗ (−2.0, −1.1) P<0.001 | 17 (−99, 133) P = 0.77 |
−31 (−84, 23) P = 0.26 |
0.1∗∗∗ (0.06, 0.1) P<0.001 | 0.00 (−0.02, 0.02) P = 0.91 |
−0.04 (−0.1, 0.05) P = 0.40 |
Data presented as effect estimate, (95% confidence interval), P value. Main effect of use of N95 respirator on cardiovascular outcomes trending toward significant changes (P values <0.15) during near-roadway exposures in bold. Abbreviations: AIx@75, augmentation index at a heart rate of 75 beats/minute; AP, augmentation pressure; BMI, body mass index; BP, blood pressure; HF, high frequency power; LF, low frequency power; PP, pulse pressure; RHI, reactive hyperemia index; RHI90–120, reactive hyperemia index from 90 to 120 seconds after cuff release; SBP, systolic blood pressure; SDNN, standard deviation of normal-to-normal intervals.
aResults represent main effect estimates (i.e., β-coefficients) of association of respirator use on cardiac outcomes, controlling for demographics and temperature, evaluated using linear mixed models with patient-level random intercept to account for within-patient correlation of outcomes; with exception of the constant term, effect estimates for all covariates included in the models are provided. We did not include visit and the visit × N95 respirator interaction in models because no visit-effect was observed—and we expect the environmental exposure(s) to capture variation of interest. We did not include a variable for the “Visit 1, N95 respirator placebo vs. nocebo effect” in the models as we found no associations with any outcome across any model (all P’s > 0.10) and any variation due to the N95 respirator should be captured in the N95 respirator main effect.
bResults represent main effect estimates (i.e., β-coefficients) of association of respirator use on cardiac outcomes, controlling for demographics and temperature, evaluated using linear model as RHI outcomes were collected only at visit 4.
cMain outcome in Table 4. Effect estimates represent the change in cardiac outcomes associated with use of the N95 respirator during roadway exposures (visits 2–5).
*P < 0.1, **P < 0.05, ***P < 0.01.
DISCUSSION
Exposure to traffic increases the risk for CVD events over the ensuing few hours.10–14 Several factors including TRAP, noise, and temperature extremes could be responsible.15–21 We investigated for the first time the effects of each of these exposures on relevant cardiovascular outcomes while resting near-roadway as well as the protective action of wearing an N95 respirator. The sum effect of being in a near-roadway environment did not worsen brachial BP or other cardiovascular outcomes. This may be due to the fact that TRAP levels were relatively low, albeit in a typical range for North American near-roadway sites.7,10,11,28,29,32–36 It is possible that if BC and PC concentrations were higher, BP elevations would have occurred as we previously reported.23–25 The participants were healthy and may have been resistant to the health effects of TRAP. In addition, opposing actions of warmer outdoor ambient temperatures during the predominately summer months of this study may have countered the effects of TRAP.42 Nevertheless, we observed that higher exposures to PC and BC were independently associated with the worsening of several aortic hemodynamic parameters. Finally, we observed mixed results regarding the protection afforded by N95 respirators. Although aortic hemodynamics trended better with their usage while near-roadway, the adverse effects of BC and PC exposures did not appear to be mitigated.
Clinical relevance
Traffic accounts for the triggering of more CVD events than any other factor worldwide.14 Our results corroborate that air pollutants (e.g., diesel exhaust) can increase aortic BP levels.43–48 We previously observed that exposure to ambient BC at high levels in Beijing is associated with an acute worsening of aortic hemodynamics.48 Several mechanisms may be responsible including arterial stiffening or heightened reflected pressure waves due arteriole vasoconstriction (albeit not robust enough to raise brachial BP in this study). Systemic inflammation induced by the inhalation of nanoparticles (BC and PC) leading to “functional” arterial stiffening is a plausible culprit.43 Although vascular dysfunction and sympathetic activation could elicit similar responses, no changes in heart rate variability and only trends to lower RHI were herein observed—making these pathways less likely. Regardless of the underlying mechanism(s), our results support that “real-world” combustion-derived TRAP constituents (BC and PC), even at relatively low levels typical for North America, can acutely impact aortic hemodynamics in a manner linked to a worse CVD prognosis.49,50 Indeed, these changes could increase myocardial strain/oxygen demand or trigger instability of atherosclerotic plaques and thus prompt ischemic events in susceptible individuals.
Near-roadway factors responsible for eliciting cardiovascular effects
Several environmental factors including TRAP, noise, and temperature extremes could be responsible for the triggering of CVD events.16–21 We showed that colder ambient temperatures promote a host of adverse responses (as is well-known).16 It is thus important to control for temperature levels in studies (as we herein did); however, cold itself cannot entirely explain the numerous epidemiological associations between traffic and CVD.5–7,12,14 A growing science is also attempting to disentangle the mutual health effects of TRAP from noise.17–21,51,52 Although our results only showed trends for effects, they were generally supportive that roadway noise promotes adverse responses. The study was not designed to evaluate for independent health effects of noise and was thus likely underpowered in this regard.
A growing literature shows that fresh combustion-derived particulates are harmful to cardiovascular health.15,53–55 These particles are mostly in the nanometer size range (<100 nm) and tend to be enriched in surface chemicals (e.g., metals, hydrocarbons) capable of provoking oxidative stress and inflammation upon inhalation. Both BC and PC are markers of combustion-derived particulates and their levels are often interrelated near-roadways as they are determined largely by ultrafine (nanoparticle) concentrations and derived from similar sources (e.g., diesel emissions).7 Hence, our findings add further evidence supporting a pathological role for combustion-derived nano-particulates within TRAP in promoting adverse cardiovascular responses. Conversely, we did not observe health effects associated with PM2.5 mass. This is not surprising given our study design. PM2.5 mass is less indicative of fresh roadway pollutants and more affected by secondary aerosols from regional sources given that it primarily reflects larger particles 1–2.5 µm in diameter. Finally, we were limited by a lack of on-site gaseous pollutant (e.g., NOx) measurements, thus a potential for missing their independent effects cannot be ruled out. However, prior human studies support that most of the acute cardiovascular effects of TRAP are due to the particulate phase.55,56 Exposures to high levels of NO2 as well as to gaseous diesel exhaust (without particles) did not trigger the adverse responses observed when particulates were present.57 Although animal studies suggest that some gases might pose CVD risk,57 our findings support that nanoparticles are likely principally implicated. More studies are warranted to determine the independent (or additive/synergist) cardiovascular effects gaseous pollutants (e.g., NOx).
N95-respirators and personal-approaches to reduce exposures
Billions of people are exposed to TRAP every day. We have made emphatic calls for studying the benefits of personal-interventions against air pollutants.5,23,37 N95 respirators are one viable strategy—particularly for highly polluted locations. Several studies in China have shown that they can mitigate adverse cardiovascular responses to ambient air pollution exposure.38–41 However, this is the first study to test their efficacy in a near-roadway environment typical for North America (less severely polluted than Asia) and faced by tens of millions of people daily.
Our findings regarding the protective effects of N95 respirators are mixed. AorticAP and AIx@75 showed trends toward improvements while wearing them near-roadway. Conversely, higher BC and PC exposures remained associated with adverse aortic hemodynamic changes regardless of N95 respirator usage. We believe caution is warranted in interpreting these findings given that they were secondary outcomes. Our study was powered to determine differences in brachial BP while wearing vs. not-wearing N95 respirators near-roadway. Unexpectedly, our exposure setting did not induce an acute increase brachial BP (for reasons previously outlined). This explains the lack of any potential benefit of respirator use on the primary outcome. Nevertheless, our findings do raise the possibility that even respirators certified at the N95 level might not fully protect against fresh combustion-derived nanoparticles. N95 respirators are validated for occupational settings (not public use) by NIOSH to prevent the penetration of ≥95% of PM2.5 mass.58 Their filtering effectiveness focuses on particles 0.3 µm in diameter (the most difficult size to capture) and it is generally believed that they are even more effective against both larger and smaller particles. However, laboratory procedures typically use artificial NaCl particles.58 A recent study,40 as well as our own unpublished data, suggest that N95 respirators may be less efficacious in preventing the penetration of real-world combustion nanoparticles. Although still beneficial, the filter material was shown to only reduce the transmission of roughly 50%–75% of the total number of these nanoparticles.40 This efficacy also assumes an ideal air-tight facial fit—which was assured on an individual basis in our trial protocol. However, we cannot rule out subtle facial seal leaks that might have allowed for the inhalation of nanoparticles at a more than expected concentration. Either imperfect filtering of the respirator material or seal leaks could explain the persistent associations of PC and BC levels with worsened aortic hemodynamics in our study despite use of a validated N95 respirator. Either way, our study methods followed an ideal usage scenario using a validated N95 respirator. Given the vast commercial market for facemasks, particularly across Asia (with varied reliability and filtering efficacy) and the high likelihood for less-than-perfect usage in the real-world,41 our results highlight the importance of further studies to validate their protection against TRAP in a variety of settings among actual patients.
It is important to note that our results do not challenge nor invalidate the findings of prior studies showing potential health benefits of using N95 respirators.38–41 Previous studies focused on reducing exposure to high levels of urban or regional background PM2.5 rather than specifically attempting to mitigate the health effects of TRAP—which is dominated by nanoparticles. We believe that if worn properly N95 respirators are likely to be an effective personal protection device when aiming to prevent exposure to larger particles (e.g., >0.1 µm) common within prevailing PM2.5 air pollution. Further investigation is warranted to determine if these respirators are indeed less effective in reducing the inhalation of real-world nanoparticles concentrated in near-roadway settings compared to other common sources of PM2.5.
Finally, it is possible that we may have observed different BP and/or more robust aortic hemodynamic changes along with other biological responses if each near-roadway exposure duration was longer than 2 hours. However, several of our own prior experiments have shown that controlled exposures to higher levels of particulate air pollutants for only 2 hours is capable of raising BP within this brief time period.59,60 Therefore, a 2-hour exposure period has a sound scientific rationale and it was also thought to be experimentally feasible. Future analyses will explore if the measured health responses were augmented or showed trends to greater changes as the duration of exposure increased within each individual visit day.
Acute exposure to fresh combustion-derived particles in TRAP, even at relatively low concentrations in healthy people, can worsen central aortic hemodynamics. This could be an important biological mechanism underlying the linkage between traffic exposure and heightened CVD risk. The degree of protection offered by N95 respirators against real-world TRAP remains uncertain and more studies are warranted.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Khalid Ijaz, DVM, MSc (Hons), PhD, for his critical review of the manuscript. We also would like to thank Ryan Crane, BS, and Zachary Klaver, MSE, of Michigan State University Exposure Science Laboratory for their sample preparation and data analyses. This project was supported by the National Institutes of Environmental Health (2R01 ES015146) and an investigator-initiated grant from RB.
REFERENCES
- 1. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA III, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017; 389:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA III, Apte JS, Brauer M, Cohen A, Weichenthal S, Coggins J, Di Q, Brunekreef B, Frostad J, Lim SS, Kan H, Walker KD, Thurston GD, Hayes RB, Lim CC, Turner MC, Jerrett M, Krewski D, Gapstur SM, Diver WR, Ostro B, Goldberg D, Crouse DL, Martin RV, Peters P, Pinault L, Tjepkema M, van Donkelaar A, Villeneuve PJ, Miller AB, Yin P, Zhou M, Wang L, Janssen NAH, Marra M, Atkinson RW, Tsang H, Quoc Thach T, Cannon JB, Allen RT, Hart JE, Laden F, Cesaroni G, Forastiere F, Weinmayr G, Jaensch A, Nagel G, Concin H, Spadaro JV. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A 2018; 115:9592–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015; 525:367–371. [DOI] [PubMed] [Google Scholar]
- 4. Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010; 121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 5. Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2018; 72:2054–2070. [DOI] [PubMed] [Google Scholar]
- 6.AAA Foundation for Traffic Safety. American Driving Survey, 2014–2017. https://aaafoundation.org/american-driving-survey-2014-2017/
- 7. HEI Panel on the Health Effects of Traffic Related Air Pollution. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. In Book Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. Health Effects Institute. Special Report 17, 2010. [Google Scholar]
- 8.Boehmer TK, Foster SL, Henry JR, Woghiren-Akinnifesi EL, Yip FY. Residential Proximity to Major Highways—United States, 2010. https://www.cdc.gov/MMWr/preview/mmwrhtml/su6203a8.htm [PubMed]
- 9. Rowangould GM. A census of the US near-roadway population: public health and environmental justice considerations. Transportation Research Part D: Transport and the Environment 2013; 25: 59–67. [Google Scholar]
- 10. Kirwa K, Eliot MN, Wang Y, Adams MA, Morgan CG, Kerr J, Norman GJ, Eaton CB, Allison MA, Wellenius GA. Residential proximity to major roadways and prevalent hypertension among postmenopausal women: results from the Women’s Health Initiative San Diego Cohort. J Am Heart Assoc 2014; 3:e000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ward-Caviness CK, Kraus WE, Blach C, Haynes CS, Dowdy E, Miranda ML, Devlin R, Diaz-Sanchez D, Cascio WE, Mukerjee S, Stallings C, Smith LA, Gregory SG, Shah SH, Neas LM, Hauser ER. Associations between residential proximity to traffic and vascular disease in a cardiac catheterization cohort. Arterioscler Thromb Vasc Biol 2018; 38:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wichmann HE, Löwel H; Cooperative Health Research in the Region of Augsburg Study Group . Exposure to traffic and the onset of myocardial infarction. N Engl J Med 2004; 351:1721–1730. [DOI] [PubMed] [Google Scholar]
- 13. Peters A, von Klot S, Mittleman MA, Meisinger C, Hörmann A, Kuch B, Wichmann HE. Triggering of acute myocardial infarction by different means of transportation. Eur J Prev Cardiol 2013; 20:750–758. [DOI] [PubMed] [Google Scholar]
- 14. Nawrot TS, Perez L, Künzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet 2011; 377:732–740. [DOI] [PubMed] [Google Scholar]
- 15. von Klot S, Cyrys J, Hoek G, Kühnel B, Pitz M, Kuhn U, Kuch B, Meisinger C, Hörmann A, Wichmann HE, Peters A. Estimated personal soot exposure is associated with acute myocardial infarction onset in a case-crossover study. Prog Cardiovasc Dis 2011; 53:361–368. [DOI] [PubMed] [Google Scholar]
- 16. Sharma P, Brook RD. Echoes from Gaea, Poseidon, Hephaestus, and Prometheus: environmental risk factors for high blood pressure. J Hum Hypertens 2018; 32:594–607. [DOI] [PubMed] [Google Scholar]
- 17. Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res 2017; 121:162–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riggs DW, Yeager RA, Bhatnagar A. Defining the human envirome: an omics approach for assessing the environmental risk of cardiovascular disease. Circ Res 2018; 122:1259–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Münzel T, Sørensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017; 38:550–556. [DOI] [PubMed] [Google Scholar]
- 20. Münzel T, Sørensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017; 38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Münzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sørensen M. Environmental noise and the cardiovascular system. J Am Coll Cardiol 2018; 71:688–697. [DOI] [PubMed] [Google Scholar]
- 22. Cai Y, Zhang B, Ke W, Feng B, Lin H, Xiao J, Zeng W, Li X, Tao J, Yang Z, Ma W, Liu T. Associations of short-term and long-term exposure to ambient air pollutants with hypertension: a systematic review and meta-analysis. Hypertension 2016; 68:62–70. [DOI] [PubMed] [Google Scholar]
- 23. Brook RD, Newby DE, Rajagopalan S. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am J Hypertens 2017; 31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang W, Wang L, Li J, Liu M, Xu H, Liu S, Chen J, Zhang Y, Morishita M, Bard RL, Harkema JR, Rajagopalan S, Brook RD. Short-Term blood pressure responses to ambient fine particulate matter exposures at the extremes of global air pollution concentrations. Am J Hypertens 2018; 31:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao X, Sun Z, Ruan Y, Yan J, Mukherjee B, Yang F, Duan F, Sun L, Liang R, Lian H, Zhang S, Fang Q, Gu D, Brook JR, Sun Q, Brook RD, Rajagopalan S, Fan Z. Personal black carbon exposure influences ambulatory blood pressure: air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension 2014; 63:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinharay R, Gong J, Barratt B, Ohman-Strickland P, Ernst S, Kelly FJ, Zhang JJ, Collins P, Cullinan P, Chung KF. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: a randomised, crossover study. Lancet 2018; 391:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cole CA, Carlsten C, Koehle M, Brauer M. Particulate matter exposure and health impacts of urban cyclists: a randomized crossover study. Environ Health 2018; 17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weichenthal S, Hatzopoulou M, Goldberg MS. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: a cross-over study. Part Fibre Toxicol 2014; 11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dales R, Liu L, Szyszkowicz M, Dalipaj M, Willey J, Kulka R, Ruddy TD. Particulate air pollution and vascular reactivity: the bus stop study. Int Arch Occup Environ Health 2007; 81:159–164. [DOI] [PubMed] [Google Scholar]
- 30. Hemmingsen JG, Rissler J, Lykkesfeldt J, Sallsten G, Kristiansen J, Møller P P, Loft S. Controlled exposure to particulate matter from urban street air is associated with decreased vasodilation and heart rate variability in overweight and older adults. Part Fibre Toxicol 2015; 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bräuner EV, Møller P, Barregard L, Dragsted LO, Glasius M, Wåhlin P, Vinzents P, Raaschou-Nielsen O, Loft S. Exposure to ambient concentrations of particulate air pollution does not influence vascular function or inflammatory pathways in young healthy individuals. Part Fibre Toxicol 2008; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giles LV, Tebbutt SJ, Carlsten C, Koehle MS. The effect of low and high-intensity cycling in diesel exhaust on flow-mediated dilation, circulating NOx, endothelin-1 and blood pressure. PLoS One 2018; 13:e0192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Breitner S, Peters A, Zareba W, Hampel R, Oakes D, Wiltshire J, Frampton MW, Hopke PK, Cyrys J, Utell MJ, Kane C, Schneider A, Rich DQ. Ambient and controlled exposures to particulate air pollution and acute changes in heart rate variability and repolarization. Sci Rep 2019; 9:1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang X, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM, Hasheminassab S, Pakbin P, Longhurst J, Sioutas C, Delfino RJ. Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ Health 2016; 15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laumbach RJ, Rich DQ, Gandhi S, Amorosa L, Schneider S, Zhang J, Ohman-Strickland P, Gong J, Lelyanov O, Kipen HM. Acute changes in heart rate variability in subjects with diabetes following a highway traffic exposure. J Occup Environ Med 2010; 52:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarnat JA, Golan R, Greenwald R, Raysoni AU, Kewada P, Winquist A, Sarnat SE, Dana Flanders W, Mirabelli MC, Zora JE, Bergin MH, Yip F. Exposure to traffic pollution, acute inflammation and autonomic response in a panel of car commuters. Environ Res 2014; 133:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morishita M, Thompson KC, Brook RD. Understanding air pollution and cardiovascular diseases: is it preventable? Curr Cardiovasc Risk Rep 2015; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Langrish JP, Li X, Wang S, Lee MM, Barnes GD, Miller MR, Cassee FR, Boon NA, Donaldson K, Li J, Li L, Mills NL, Newby DE, Jiang L. Reducing personal exposure to particulate air pollution improves cardiovascular health in patients with coronary heart disease. Environ Health Perspect 2012; 120:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi J, Lin Z, Chen R, Wang C, Yang C, Cai J, Lin J, Xu X, Ross JA, Zhao Z, Kan H. Cardiovascular benefits of wearing particulate-filtering respirators: a randomized crossover trial. Environ Health Perspect 2017; 125:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guan T, Hu S, Han Y, Wang R, Zhu Q, Hu Y, Fan H, Zhu T. The effects of facemasks on airway inflammation and endothelial dysfunction in healthy young adults: a double-blind, randomized, controlled crossover study. Part Fibre Toxicol 2018; 15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cherrie JW, Apsley A, Cowie H, Steinle S, Mueller W, Lin C, Horwell CJ, Sleeuwenhoek A, Loh M. Effectiveness of face masks used to protect Beijing residents against particulate air pollution. Occup Environ Med 2018; 75:446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giorgini P, Rubenfire M, Das R, Gracik T, Wang L, Morishita M, Bard RL, Jackson EA, Fitzner CA, Ferri C, Brook RD. Particulate matter air pollution and ambient temperature: opposing effects on blood pressure in high-risk cardiac patients. J Hypertens 2015; 33:2032–2038. [DOI] [PubMed] [Google Scholar]
- 43. Zanoli L, Lentini P, Granata A, Gaudio A, Fatuzzo P, Serafino L, Rastelli S, Fiore V, D’Anca A, Signorelli SS, Castellino P. A systematic review of arterial stiffness, wave reflection and air pollution. Mol Med Rep 2017; 15:3425–3429. [DOI] [PubMed] [Google Scholar]
- 44. Ljungman PLS, Li W, Rice MB, Wilker EH, Schwartz J, Gold DR, Koutrakis P, Benjamin EJ, Vasan RS, Mitchell GF, Hamburg NM, Mittleman MA. Long- and short-term air pollution exposure and measures of arterial stiffness in the Framingham Heart Study. Environ Int 2018; 121:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baumgartner J, Carter E, Schauer JJ, Ezzati M, Daskalopoulou SS, Valois MF, Shan M, Yang X. Household air pollution and measures of blood pressure, arterial stiffness and central haemodynamics. Heart 2018; 104:1515–1521. [DOI] [PubMed] [Google Scholar]
- 46. Adamopoulos D, Vyssoulis G, Karpanou E, Kyvelou SM, Argacha JF, Cokkinos D, Stefanadis C, van de Borne P. Environmental determinants of blood pressure, arterial stiffness, and central hemodynamics. J Hypertens 2010; 28:903–909. [DOI] [PubMed] [Google Scholar]
- 47. Lundbäck M, Mills NL, Lucking A, Barath S, Donaldson K, Newby DE, Sandström T, Blomberg A. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part Fibre Toxicol 2009; 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu S, Brook RD, Huang W, Fan Z, Xu H, Wu R, Sun Z, Zhao X, Ruan Y, Yan J, Sun L, Liang R, Lian H, Gu D, Rajagopalan S. Extreme levels of ambient air pollution adversely impact cardiac and central aortic hemodynamics: the AIRCMD-China study. J Am Soc Hypertens 2017; 11:754–761.e3. [DOI] [PubMed] [Google Scholar]
- 49. Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010; 31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 50. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 51. Héritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, de Hoogh K, Thiesse L, Rudzik F, Habermacher M, Köpfli M, Pieren R, Brink M, Cajochen C, Wunderli JM, Probst-Hensch N, Röösli M. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: a nationwide cohort study in Switzerland. Eur Heart J 2019; 40:598–603. [DOI] [PubMed] [Google Scholar]
- 52. Zijlema W, Cai Y, Doiron D, Mbatchou S, Fortier I, Gulliver J, de Hoogh K, Morley D, Hodgson S, Elliott P, Key T, Kongsgard H, Hveem K, Gaye A, Burton P, Hansell A, Stolk R, Rosmalen J. Road traffic noise, blood pressure and heart rate: pooled analyses of harmonized data from 88,336 participants. Environ Res 2016; 151:804–813. [DOI] [PubMed] [Google Scholar]
- 53. Ohlwein S, Kappeler R, Kutlar Joss M, Künzli N, Hoffmann B. Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence. Int J Public Health 2019; 64:547–559. [DOI] [PubMed] [Google Scholar]
- 54. Downward GS, van Nunen EJHM, Kerckhoffs J, Vineis P, Brunekreef B, Boer JMA, Messier KP, Roy A, Verschuren WMM, van der Schouw YT, Sluijs I, Gulliver J, Hoek G, Vermeulen R. Long-Term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a Dutch Cohort. Environ Health Perspect 2018; 126:127007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lucking AJ, Lundbäck M, Barath SL, Mills NL, Sidhu MK, Langrish JP, Boon NA, Pourazar J, Badimon JJ, Gerlofs-Nijland ME, Cassee FR, Boman C, Donaldson K, Sandstrom T, Newby DE, Blomberg A. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation 2011; 123:1721–1728. [DOI] [PubMed] [Google Scholar]
- 56. Langrish JP, Lundbäck M, Barath S, Söderberg S, Mills NL, Newby DE, Sandström T, Blomberg A. Exposure to nitrogen dioxide is not associated with vascular dysfunction in man. Inhal Toxicol 2010; 22:192–198. [DOI] [PubMed] [Google Scholar]
- 57. Münzel T, Gori T, Al-Kindi S, Deanfield J, Lelieveld J, Daiber A, Rajagopalan S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J 2018; 39:3543–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.The National Personal Protective Technology Laboratory (NPPTL). Standard Respirator Testing Procedures. https://www.cdc.gov/niosh/npptl/stps/respirator_testing.html
- 59. Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 2009; 54:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Byrd JB, Morishita M, Bard RL, Das R, Wang L, Sun Z, Spino C, Harkema J, Dvonch JT, Rajagopalan S, Brook RD. Acute increase in blood pressure during inhalation of coarse particulate matter air pollution from an urban location. J Am Soc Hypertens 2016; 10:133–139.e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.