Abstract
This scientific commentary refers to ‘Evolutionary modifications in human brain connectivity associated with schizophrenia’ by van den Heuvel et al. (doi:10.1093/brain/awz330).
This scientific commentary refers to ‘Evolutionary modifications in human brain connectivity associated with schizophrenia’ by van den Heuvel et al. (doi:10.1093/brain/awz330).
All scientific knowledge relies on observation, but with complex phenomena there is often an awful lot to observe. Schizophrenia is a neuropsychiatric disorder characterized by a wide range of symptoms such as delusions, hallucinations, lack of motivation, cognitive difficulties, impaired speech and aberrant motor functioning. Beyond the behavioural level of symptoms, however, additional hallmarks of the disorder have been discovered across every level of neuroscientific investigation. Epidemiological studies have shown, for example, strong heritability, late-adolescent onset, and higher incidence in males than females. Neuroimaging studies have found characteristic alterations in both structural and functional brain connectivity. Pharmacological studies have uncovered disruptions in dopamine function. Histological studies have implicated parvalbumin-containing GABAergic neurons. Transcriptomic studies of post-mortem brain tissue have highlighted hundreds of differentially expressed genes, while genome-wide association studies have identified scores of risk loci in the genome. However, this rich, multi-scale description of ‘what’ the disorder is makes it ever more challenging to advance mechanistic hypotheses for ‘how’ these complex phenotypes come about. In this issue of Brain, van den Heuvel and co-workers cannily propose that we may get closer to understanding ‘how’ schizophrenia emerges by focusing first on the apparently harder question of ‘why’ it exists at all (van den Heuvel et al., 2019).
Schizophrenia has long been understood as a heritable—and therefore genetic—disorder. As such, its relatively high population-wide prevalence (∼1%) presents an evolutionary puzzle. As individuals with schizophrenia also have lower fertility rates, one might expect related genotypes to be naturally selected against. One popular theory is that alleles associated with schizophrenia also confer adaptive advantages to individuals not severely affected by disease symptoms. This viewpoint is supported by a number of observations. First, schizophrenia is a highly polygenic disease, with large numbers of genes each contributing in small ways to the overall genetic risk for the disorder. As such, differing genotypes in various combinations of risk genes are likely to generate evolutionarily useful individual phenotypes and advantageous diversity across the population. Second, schizotypal traits occur along a continuum in the population. Recent work by Shoval et al. (2012) highlights how such continuously varying traits are often an expression of competition between multiple selection pressures. One simple example of this is the linear change in molar size across 29 species of rodents, each optimized for different diets across herbivores, omnivores and faunivores. Similarly, an intriguing hypothesis is that genotypes resulting in more or fewer schizotypal traits may be optimally suited for different types of environments and selection pressures in humans. This could be driven by traits sharing a genetic origin with schizotypy, or alternatively schizotypal traits themselves could be adaptive in some circumstances and only become maladaptive in high doses and in certain situations. A third observation in support of evolutionary origins of schizophrenia is that it has emerged independently in humans across all societies but is absent, as far as we can tell, in other primates and all other species.
This striking cross-species observation also provides the starting point for van den Heuvel and colleagues’ comparative study of white matter ‘dysconnectivity’ in schizophrenia. They begin by reproducing prior results from their group and others, mapping schizophrenia-related changes in large-scale white matter tracts between cortical regions. Next, they use comparable in vivo neuroimaging data from chimpanzees and macaque monkeys to map evolutionary changes in white matter connectivity over the past 25 million years. By comparing these two maps, the authors show that the white matter tracts that tend to be compromised in schizophrenia also overlap with human-specific connections that first emerged about 5–10 million years ago, when humans and chimpanzees diverged from a common ancestor. Importantly, the authors also show that these results are relatively specific to schizophrenia, when compared to seven other psychiatric and neurological disorders—such as depression and obsessive-compulsive disorder—with less human-specific behavioural symptoms. In future work, it would be interesting to extend these findings to examine inter-individual variability in human-specific connections and how these relate to dimensional aspects of schizotypy, cognitive functions and genetic risk for schizophrenia.
Figure 1.
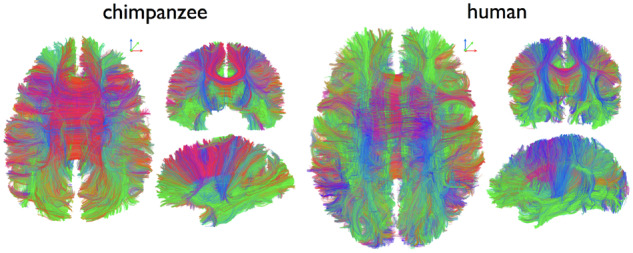
Example of connectome fibre tracking in a chimpanzee (left) and human (right). From van den Heuvel et al. (2019)..
One powerful aspect of adopting an evolutionary perspective is that the logic of natural selection reaches across biological scales, from genetic mutations, to brain phenotypes, and all the way to behavioural and cognitive phenotypes. Following this logical thread back to its source, the authors note the importance of ‘human accelerated regions’ (HAR) of the genome, which, despite being highly conserved across vertebrates, show striking human-specific differences. Previous work has demonstrated a strong link between these HAR regions and schizophrenia symptomatology, providing a compelling narrative to support the theory that schizophrenia may have been a relatively recent development in human evolution. The authors therefore ask whether the human-specific brain connections also arise from human-specific genetics. In particular, using open-access data from the Allen Human Brain Atlas (2010), they show that HAR genes are preferentially expressed in cortical regions with human-specific connectivity patterns.
Taken together, van den Heuvel and colleagues’ findings bolster and connect two prior seams of literature on how evolutionary trade-offs may have shaped the human brain. In 1997, Crow’s famous paper entitled ‘Is schizophrenia the price that Homo sapiens pays for language?’ was the first of a string of papers suggesting that schizophrenia may result from an evolutionary trade-off between the emergence of complex cognitive functions and increased vulnerability (Crow et al., 1997). Separately, in a 2012 paper entitled ‘The economy of brain network organization’, Bullmore and Sporns (2012) drew on a large body of literature dating back to Ramón y Cajal’s pioneering observations of neuronal morphology. They proposed that the key organizing principle for brain connectivity, across many scales and species, is an economic trade-off between minimizing connection costs and allowing the emergence of adaptively valuable features of anatomical or functional connectivity. For example, integrative features such as highly connected ‘hub’ regions or long-range connections are costly to develop and maintain in the brain, but have been shown to underpin complex and adaptively important cognitive functions. Despite this rich history of evolutionary thinking in the field of connectomics, the focus thus far has predominantly remained on cross-species commonalities in organizational principles. Here, focusing on human specificity, van den Heuvel and colleagues find that the increasingly sophisticated patterns of brain connectivity required for human cognition may come at the cost of both an energetic premium, and also the risk for psychosis.
Despite being grounded in evolutionary theory and supported by a decade of connectomics research, one limitation of this study is that it rests mainly on evidence derived from diffusion-weighted imaging, which is susceptible to acquisition and other methodological artefacts, and necessarily captures only a narrow phenotype. In this context it is worth noting that the symptoms of schizophrenia do not affect human-specific functions alone, so a simple-minded interpretation of schizophrenia resulting directly from the disruption of white-matter connections evolved in service of higher cognition is unlikely to be correct. More generally, as the authors themselves point out, many aspects of schizophrenia cannot be readily explained by such a simple connectomic theory. For example, one obvious point is that all humans possess superior cognitive functions to chimpanzees, but very few go on to develop schizophrenia. This suggests that the emergence of the disorder is only made possible by human specific connections, but requires additional triggers, perhaps during critical periods in development.
In addition, many symptoms of schizophrenia can be pharmacologically manipulated, suggesting that alterations in physical wiring are only one aspect of the disorder. For example, dopamine-receptor antagonists have antipsychotic effects in humans, while NMDA antagonists such as ketamine elicit psychotic and related symptoms in both humans and non-human primates. Similarly, it is unclear how an anatomical basis for schizophrenia dovetails with more functional theories. Specifically, the predictive coding theory of schizophrenia suggests that symptoms such as hallucinations or delusions could arise from disrupted Bayesian inference in the brain (Fletcher and Frith, 2009). In this context, it would be interesting to identify which aspects of predictive coding, if any, depend on human-specific white matter tracts.
Ultimately, combining descriptive knowledge from multiple scales of investigation into a unified theory of neuropsychiatric disease remains one of the most difficult and potentially transformative challenges in neuroscience. As this work reminds us, the human-specific nature of schizophrenia offers a unique opportunity to address this challenge with a footing in evolutionary theory. To fully seize this opportunity will require further comparative studies of phenotypes associated with the disorder across multiple biological scales.
Competing interests
The authors report no competing interests.
References
- Allen Institute for Brain Science. Allen Human Brain Atlas. 2010. human.brain-map.org.
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012; 13: 336–49. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Is schizophrenia the price that Homo sapiens pays for language? Schizophr Res 1997; 28: 127–41. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD. Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci 2009; 10: 48–58. [DOI] [PubMed] [Google Scholar]
- Shoval O, Sheftel H, Shinar G, Hart Y, Ramote O, Mayo A, et al. Science 2012; 336: 1157–60. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Scholtens LH, de lange SC, Pijnenburg R, Cahn W, van Haren NEM, et al. Evolutionary modifications of human brain connectivity associated with schizophrenia. Brain 2019; 142: 3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]


