Individuals with more extensive white matter hyperintensities (WMH) show poorer functional outcomes after stroke, but the basis for this relationship is unclear. Wilmskoetter, Marebwa et al. report that WMH are preferentially associated with damage to long-range white matter fibres and this disruption leads to more severe post-stroke aphasia.
Keywords: white matter, brain connectomics, stroke, aphasia, magnetic resonance imaging
Abstract
We sought to determine the underlying pathophysiology relating white matter hyperintensities to chronic aphasia severity. We hypothesized that: (i) white matter hyperintensities are associated with damage to fibres of any length, but to a higher percentage of long-range compared to mid- and short-range intracerebral white matter fibres; and (ii) the number of long-range fibres mediates the relationship between white matter hyperintensities and chronic post-stroke aphasia severity. We measured the severity of periventricular and deep white matter hyperintensities and calculated the number and percentages of short-, mid- and long-range white matter fibres in 48 individuals with chronic post-stroke aphasia. Correlation and mediation analyses were performed to assess the relationship between white matter hyperintensities, connectome fibre-length measures and aphasia severity as measured with the aphasia quotient of the Western Aphasia Battery-Revised (WAB-AQ). We found that more severe periventricular and deep white matter hyperintensities correlated with a lower proportion of long-range fibres (r = −0.423, P = 0.003 and r = −0.315, P = 0.029, respectively), counterbalanced by a higher proportion of short-range fibres (r = 0.427, P = 0.002 and r = 0.285, P = 0.050, respectively). More severe periventricular white matter hyperintensities also correlated with a lower proportion of mid-range fibres (r = −0.334, P = 0.020), while deep white matter hyperintensities did not correlate with mid-range fibres (r = −0.169, P = 0.250). Mediation analyses revealed: (i) a significant total effect of periventricular white matter hyperintensities on WAB-AQ (standardized beta = −0.348, P = 0.008); (ii) a non-significant direct effect of periventricular white matter hyperintensities on WAB-AQ (P > 0.05); (iii) significant indirect effects of more severe periventricular white matter hyperintensities on worse aphasia severity mediated in parallel by fewer long-range fibres (effect = −6.23, bootstrapping: standard error = 2.64, 95%CI: −11.82 to −1.56) and more short-range fibres (effect = 4.50, bootstrapping: standard error = 2.59, 95%CI: 0.16 to 10.29). We conclude that small vessel brain disease seems to affect chronic aphasia severity through a change of the proportions of long- and short-range fibres. This observation provides insight into the pathophysiology of small vessel brain disease, and its relationship with brain health and chronic aphasia severity.
Introduction
Besides microbleeds and lacunar infarcts, white matter hyperintensities (WMH), also referred to as leukoaraiosis, are one of the most important markers for small vessel brain disease (Wardlaw et al., 2013). WMH are usually identified as periventricular and/or deep white matter signal abnormalities on T2-weighted, or fluid-attenuated inversion recovery (FLAIR) MRI imaging. WMH are commonly observed in older individuals (King et al., 2014), affecting up to 95% of individuals age 60 or older (de Leeuw et al., 2001; Grueter and Schulz, 2012). Cardiovascular diseases, such as new or recurrent strokes and dementia, (Henon et al., 2003; Prins et al., 2004; Wen and Sachdev, 2004; Arsava et al., 2011; Kim et al., 2014), and cerebro- and cardiovascular risk factors, such as hypoperfusion, hypertension, diabetes mellitus, reduced renal function, are strongly associated with WMH (Liao et al., 1996; Bisschops et al., 2004; ten Dam et al., 2007; King et al., 2014). WMH are linked to cognitive decline (De Groot et al., 2002) and worse outcomes after stroke (Liou et al., 2010), such as persistent language impairments (naming and fluency) (Wright et al., 2018), swallowing impairments (Moon et al., 2017), physical and cognitive impairments (Kang et al., 2013), as well as general functional deficits (Arsava et al., 2009; Liou et al., 2010).
While several studies have indicated the relationship between WMH and compromised stroke outcome, little is known about the underlying pathophysiology that mediates this relationship. Revealing the mechanisms of WMH is crucial to identify patients at risk for decreased functional outcomes, potentially predict recovery, plan rehabilitation, and develop future prevention and treatment strategies. More specifically, a better understanding of how WMH relate to white matter integrity at a neural network level could provide important information regarding the relationship between small vessel brain disease and neurological function.
Within white matter, it is now increasingly recognized that long-range axonal fibres connecting grey matter regions provide integration between distant areas, which are locally segregated by shorter range fibres (Bullmore and Sporns, 2009; Sporns, 2014). Even though long-range neurons are crucial for the maintenance of network organization and efficiency, they are less numerous and require more energy compared to short-range ones (Buzsaki, 2006; Ju et al., 2016). Postulated reasons for higher energy consumption in long-range axons are higher costs of axial currents and sodium influx to promote the conduction of action potentials across long axons (Ju et al., 2016). The higher energy demand might explain why long-range fibres are especially susceptible to microangiopathic changes as found in a 3-year longitudinal study. In this study, WMH grew over time with faster growth within long association tracts, such as the superior and inferior longitudinal fasciculi, fronto-occipital fasciculus, and arcuate fasciculus (Lambert et al., 2016).
Thus, small vessel brain disease (evidenced partially by WMH) may lead to a gradual decline especially in long white matter fibre tract architecture, which in turn predisposes individuals to a worse impact of additional brain damage, such as stroke. Language is a complex cognitive function that requires knowledge association, information binding, and semantical, syntactical, morphological as well as phonological mapping. As long-range fibres are necessary for multi-modal integration, and are likely more susceptible to ischaemia, we speculated that the damage to long-range fibres may be a mechanistic mediator between WMH and chronic post-stroke aphasia severity.
The objective of this study was to assess the relationships between WMH, axonal fibre damage, and chronic aphasia severity. We hypothesized that: (i) WMH are associated with damage to fibres of any length, but to a higher percentage of intracerebral long-range white matter fibres compared to medium- and short-range fibres; and (ii) the decrease in the number of long-range fibres mediates the relationship between WMH and worse chronic post-stroke aphasia severity.
Materials and methods
Participants
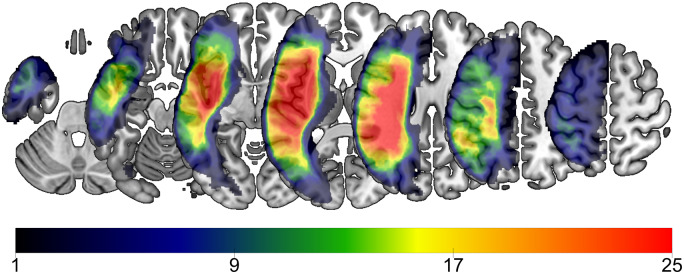
Participants’ data were leveraged retrospectively from an ongoing, prospective, multi-site study identifying factors predictive of treated aphasia recovery in individuals with chronic aphasia. Over a time span of 21 months, participants were recruited in the prospective study if they had experienced an ischaemic or haemorrhagic stroke to the left hemisphere, were at least 12 months post-stroke, had a diagnosis of aphasia according to the Western Aphasia Battery-Revised (WAB-R) (Kertesz, 2007a), and were between 21 and 80 years of age. Participants were excluded if they had severely limited verbal severely limited verbal output (i.e. a score ≤1 on the spontaneous speech scale), limited auditory comprehension (i.e. a WAB-R comprehension score ≤1), bilateral stroke, stroke affecting the right hemisphere, or other neurological illness/injury affecting the brain. In this study, we excluded two participants because one did not have a diagnosis of aphasia per WAB-R cut-off, and another one did not have a diffusion tensor imaging (DTI) scan. In total, 48 participants (32 males, 16 females) were included in this study. Participants were 54.44 months post-stroke [standard deviation (SD) = 53.64, range = 12–245], and mean age at testing was 60.44 (SD = 11.96, range = 29–76). Forty-four participants (92%) were premorbid right-handed and 37 (77%) had an ischaemic stroke. Table 1 presents participant characteristics and Fig. 1 shows the lesion overlay of all participants. Data were collected from the University of South Carolina and Medical University of South Carolina. Institutional Review Boards at each University approved all study procedures, and participants provided informed consent to participate.
Table 1.
Demographic and medical information of study participants (n = 48)
| Race, n (%) | 11 (23) Black/African American, 37 (77) White |
| Ethnicity, n (%) | 48 (100) Not Hispanic or Latino |
| Sex, n (%) | 16 (33) females, 32 (67) males |
| Education, years, mean (SD) [range] | 15.49 (2.45) [12–20] |
| Age at test, years, mean (SD) [range] | 60.44 (11.96) [29–76] |
| Age at stroke, years, mean (SD) [range] | 55.92 (12.39) [27–75] |
| Time post-stroke, months, mean (SD) [range] | 54.44 (53.64) [12–245] |
| Stroke type, n (%) | |
| Ischaemic | 37 (77 |
| Haemorrhagic | 9 (19) |
| Unknown | 2 (4) |
| Number of strokes before enrollment, n (%) | |
| One | 43 (90) |
| Two | 3 (6) |
| Three | 1 (2) |
| Four | 1 (2) |
| Lesion volume, ml, mean (SD) [range] | 133.93 (98.80) [4.93–467.46] |
| WAB-AQ; mean (SD) [range] | 59.06 (22.13) [20.10–93.10] |
| PVH score, median [range]; mean (SD) | 1 [0–3]; 1.46 (1.15) |
| 0 (absence), n (%) | 13 (27) |
| 1 (caps or pencil-thin lining), n (%) | 12 (25) |
| 2 (smooth halo), n (%) | 11 (23) |
| 3 (PVH extending into surrounding deep white matter), n (%) | 12 (25) |
| Deep WMH score, median [range]; mean (SD) | 1 [0–3]; 1.19 (0.76) |
| 0 (absence), n (%) | 6 (12) |
| 1 (punctate foci), n (%) | 31 (65) |
| 2 (beginning of confluent foci), n (%) | 7 (15) |
| 3 (confluent areas), n (%) | 4 (8) |
Figure 1.
Lesion overlay of all participants (n = 48). Colours represent the number of patients with a lesion in that area, with warmer colours indicating greater regions of overlap.
White matter hyperintensity scoring
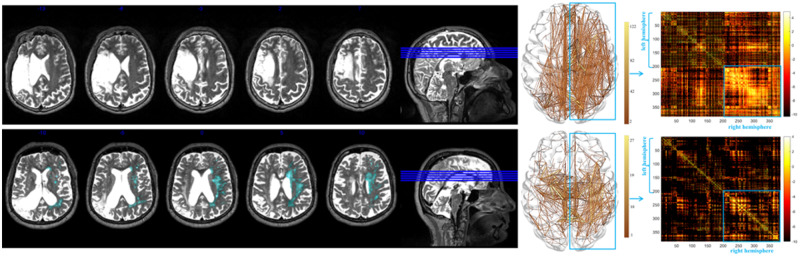
We used a rating scale developed by Fazekas et al. (1987) (the Fazekas scale) to rate the presence and extent of WMH in the right, contralesional hemisphere (Henon et al., 2003; Wright et al., 2018). WMH were rated in the right hemisphere only to avoid bias from the left hemisphere stroke lesion, as the goal of this study was to assess the effects of small vessel disease, and not the white matter damage that would have occurred as a consequence of the large vessel occlusion that caused the aphasia. Because the extent of WMH is generally assumed symmetrical across hemispheres and because it is impossible to fully ascertain the pre-morbid status prior to the large vessel occlusion, it is reasonable to use the right hemisphere as a surrogate for the left hemisphere (Pantoni, 2008). WMH were rated separately for the periventricular space (periventricular hyperintensities; PVH) and in the deep white matter (deep WMH), each rating measured on a 4-point scale, ranging from 0 (absence of WMH) to 3 (confluent WMH). Ratings were made on T2-weighted MRI scans. As per Fazekas et al. (1987), WMH in the periventricular area (PVH) were defined as ‘caps or pencil-thin lining’ (PVH score of 1), ‘smooth halo’ (PVH score of 2), and periventricular hyperintensities that extended into surrounding deep white matter (PVH score of 3). Deep WMH were defined as punctate foci (deep WMH score of 1), the beginning of confluent foci (deep WMH score of 2), and confluent areas (deep WMH score of 3) (Fazekas et al., 1987). Thus, participants with PVH and deep WMH ratings of 0 (absence of WMH) served as a control group with a stroke lesion but without WMH, compared to patients with ratings > 0 who had a stroke and WMH. The Fazekas scale scores for the participants included in this study are presented in Table 1. Figure 2 presents examples of PVH and deep WMH ratings from selected participants in this study sample.
Figure 2.
T2-weighted MRI images from two patients of the study sample. Examples of PVH and deep WMH ratings (left: WMH are highlighted in light blue), as well as the corresponding fibre tracking and structural connectome matrix (right: x- and y-axes correspond to the AICHA region of interest numbers, warmer colours represent higher connectivity between regions of interest). Patient 1 (top row) did not present with WMH; Patient 2 (bottom row) presented with the most severe scores (3 for PVH and 3 for deep WMH). The connectome matrices show that the more severe WMH scores for Patient 2 coincided with fewer connections, particularly in brain areas with long range projections such as the frontal lobe, compared to Patient 1.
WMH ratings were based on a consensus between the primary rater (A.B.) and secondary raters (B.S. and L.J.), who each rated a subset (50% each) of MRIs. Raters were blind to all participant demographics and test scores.
Image acquisition
Imaging was acquired on a Siemens Prisma 3 T scanner equipped with a 20-element head/neck (16/4) coil at the University of South Carolina or Medical University of South Carolina. Images were generally acquired within 2 days of behavioural testing. This study used whole-brain T1-weighted, T2-weighted, and diffusion echo planar imaging (EPI) images collected from each participant. Parameters were as follows:
T1-weighted image utilizing an MP-RAGE sequence with 1 mm isotropic voxels, a 256 × 256 matrix size, a 9-degree flip angle, and a 192 slice sequence with repetition time = 2250 ms, inversion time = 925 ms, echo time = 4.11 ms with parallel imaging (GRAPPA = 2, 80 reference lines).
T2-weighted image utilizing a sampling perfection with application optimized contrasts using a different flip angle evolution (3D-SPACE) sequence. This 3D turbo spin echo (TSE) scan uses a repletion time of 3200 ms, an echo time of 567 ms, variable flip angle, 256 × 256 matrix scan with 176 slices (1-mm thick), using parallel imaging (GRAPPA = 80 reference lines).
Diffusion mono-polar EPI scan that uses 43 volumes sampling 36 directions with b = 1000 s/mm2 (with seven volumes b = 0), repetition time = 5250 ms, echo time = 80 ms, 140 × 140 matrix, 90-degree flip angle, 210 × 210 mm field of view, with parallel imaging GRAPPA = 2, 80 contiguous 1.5 mm slices. This sequence was acquired twice, with phase encoding polarity reversed for the second series.
Image processing
Lesion mapping
The chronic post-stroke lesions were drawn by a stroke neurologist (L.B.) or by a researcher with extensive experience with brain imaging in stroke populations; both were blinded to behavioural scores at time of lesion drawing. Lesions were manually drawn using the MRIcron software.
Using SPM12 and MATLAB scripts developed in-house, the stroke lesion maps were spatially normalized to standard space through the following steps: (i) The T2 scan was co-registered with the individual’s T1 scan with the transforms used to resliced the lesion into native T1 space; (ii) the resliced lesion maps were smoothed with a 3 mm full-width at half-maximum Gaussian kernel to remove jagged edges associated with manual drawing; (iii) an enantiomorphic normalization (Nachev et al., 2008) approach using SPM12’s unified segmentation-normalization (Ashburner and Friston, 2005) was applied to normalize the T1-weighted images onto the standard space, using a chimeric T1-weighted image where the area corresponding to the stroke lesion was replaced by the mirrored equivalent region in the intact (right) hemisphere; and (iv) the lesion mask was then binarized, and only voxels with a probability >50% were maintained in the final normalized lesion mask.
Once the lesion masks were placed in standard space, each image was divided into anatomical grey matter regions based on the Atlas of Intrinsic Connectivity of Homotopic Areas (AICHA) (Joliot et al., 2015) brain atlas to determine the overall lesion size.
Structural connectome
Each participant’s individual connectome was built from the neuroimaging data using steps defined in our previous publication (Fridriksson et al., 2018). Briefly, (i) T1-weighted images were segmented into probabilistic grey and white matter maps using SPM12’s unified segmentation-normalization; (ii) each individual’s grey matter map was divided into 384 regions using the AICHA brain atlas (Joliot et al., 2015); (iii) the grey matter parcellation maps were non-linearly registered into the DTI space; (iv) pairwise probabilistic DTI fibre tracking was computed for all possible pairs of grey matter regions; (v) the weight of each pairwise connectivity link was determined based on the number of probabilistic streamlines connecting the grey matter region pair, corrected by distance travelled by each streamline and by the total volume of the connected regions; and (vi) a weighted adjacency matrix M of size 384 × 384 was constructed for each participant with Mi,j representing the weighted link between regions of interest i and j.
Diffusion images were undistorted using TOPUP (Andersson et al., 2003) and Eddy (Andersson and Sotiropoulos, 2016). Tractography was estimated using FSL’s FMRIB’s Diffusion Toolbox (FDT) probabilistic method (Behrens et al., 2007) with FDT’s accelerated BEDPOST (Hernandez et al., 2013) being used to assess default distributions of diffusion parameters at each voxel, and probabilistic tractography was performed using FDT’s probtrackX (parameters: 5000 individual pathways drawn through the probability distributions on principal fibre direction, curvature threshold set at 0.2, 200 maximum steps, step length 0.5 mm, and distance correction). The waypoint mask was set as the white matter probabilistic map excluding the stroke lesion. The weighted connectivity between the regions i and j was defined as the number of probabilistic streamlines arriving at j region of interest when i was seeded, averaged with the number of probabilistic streamlines arriving at i region of interest when j was seeded. The connection weight was corrected based on the distance travelled by the streamlines connecting i and j (probtrackX’s ‘distance correction’). The number of streamlines connecting each pair of regions of interest was further divided by the sum of the volumes of these regions of interest to compensate for the unequal size of grey matter regions of interest. In summary, each individual connectome was represented by a 384 × 384 matrix, where the nodes corresponded to the AICHA anatomical regions of interest and the edges to the structural connectivity between the nodes.
White matter fibre length
To determine the number of short-, mid- and long-range white matter connections, we calculated the Euclidean distance between each pair of region of interest centroids in standard MRI space. The connections were then grouped into whether they connected regions of interest whose distance was within the first quartile (lowest 25%) as ‘short distance’ fibres, and all fibres within the fourth quartile (75% and above) as ‘long distance’ fibres. Mid-range fibres had lengths within the second and third quartiles (25–75%). We calculated the total number of connections and determined the percentage of all existing connections in each connectome that were either short-, mid-, or long-range fibres.
Lesion volume
To account for the influence of the lesion in the left hemisphere on WAB-AQ scores, we controlled for lesion volume in statistical analyses. The stroke lesion maps were normalized into standard space and co-registered to the MNI 152 1 mm atlas. Stroke lesion volume (in millilitres) was equal to the number of lesioned voxels in cubic millimetres divided by 1000, because each voxel had a size of 1 mm × 1 mm × 1 mm.
Assessment of aphasia: WAB-AQ
The WAB-R (Kertesz, 2007b) is a commonly used clinical assessment of aphasia that evaluates the presence, type, and severity of aphasia on a 0–100 scale (scores <93.8 are indicative of aphasia). The WAB-R broadly assesses the domains of expression and comprehension, yielding summary scores for the following four domains: spontaneous speech, auditory verbal comprehension, repetition, and naming and word finding. The Aphasia Quotient (AQ), the weighted composite of these four scores, was used as the dependent (behavioural) variable of interest in this study and is indicative of the overall severity of the individual’s aphasia.
Statistical analyses
IBM SPSS Statistics for Windows (version 24, released 2016, IBM Corp., Armonk, N.Y., USA) was used for all analyses. P-values ≤ 0.05 were considered statistically significant.
To determine the relationship between WMH and fibre length, we performed correlation analyses on PVH, deep WMH and the number and percentage of short-, mid-, and long-range fibres. We used one-tailed statistical tests for the number of fibres, because we hypothesized WMH are associated with a general decrease and not gain in the number of fibres independently of fibre length, but we used two-tailed statistical tests for percentage of fibres, because we hypothesized a proportional change for each fibre length group. Based on visual inspection of the data and the Shapiro-Wilk test for normality, we found that all variables were not normally distributed, thus, we used non-parametric, bivariate Spearman correlations. The strength of correlations was interpreted as weak for |r| < 0.3, moderate for 0.3 ≤ |r| < 0.5, and strong for |r| ≥ 0.5 (Cohen, 1988).
To assess whether WMH and fibre length types (short-, mid-, long-range fibres) had an independent or combined effect on the distribution of percentages of fibre length types, we performed a two-way ANOVA for unbalanced designs, because there were no equal sized groups across the factors.
Mediation analysis
To determine if the relationship between WMH and aphasia severity is related to fibre length, we performed a statistical, parallel mediation analysis by using the PROCESS macro (Hayes, 2018), a validated, freely-available computational tool. We conducted multivariable regression modelling for the mediation analysis and used stroke lesion volume as a control variable to account for the impact of the stroke lesion in the left hemisphere on communicative abilities.
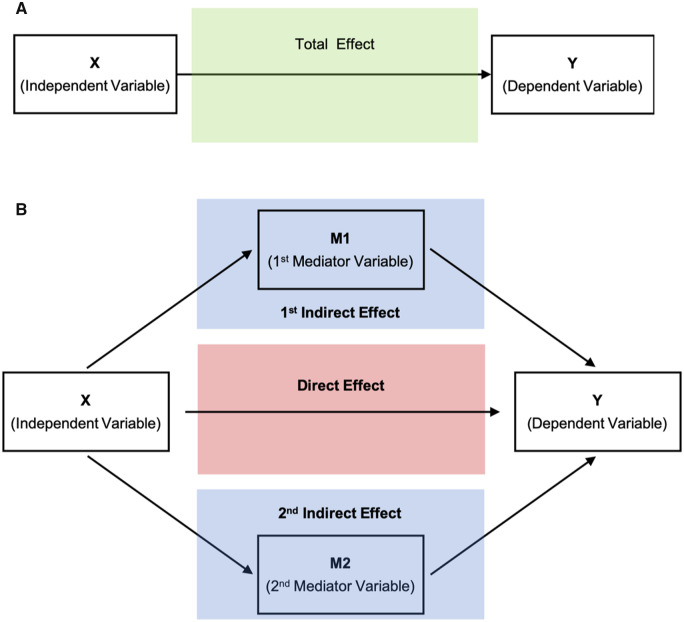
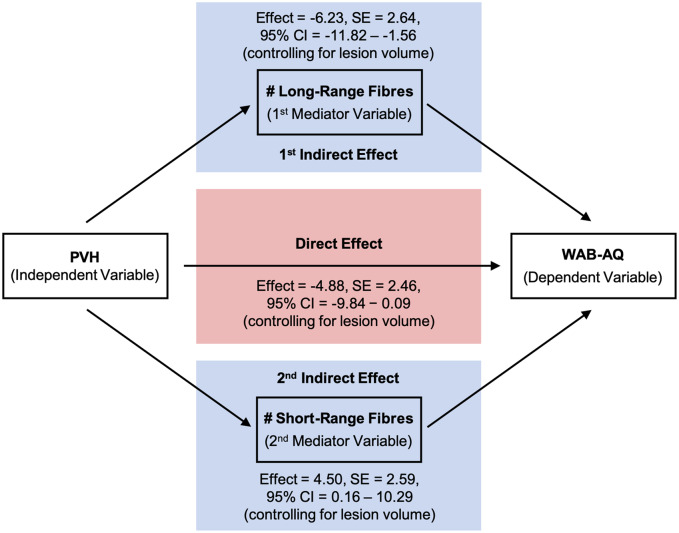
As shown in Fig. 3A and B, steps for a parallel mediation analysis with two mediators include the determination of (i) the total effect of the independent variable (X) on the dependent variable, (Y); (ii) the direct effect of X on Y when accounting for mediating variables (in our study two mediators: M1 and M2); and (iii) the two indirect effects of X on Y through M1 and of X on Y through M2. Using parallel mediation we can test each mediator’s contribution while holding another mediator constant (Kane and Ashbaugh, 2017).
Figure 3.
Simplified schematic representation of the parallel mediation analysis with two mediators. The total effect (green box) of the independent variable (X) on the dependent variable (Y) (A) is the predictive power of X on Y without taking mediators into account. The direct effect (red box) is the predictive power of X on Y while controlling for M1 and M2 (B). The indirect effects (blue box) of X on Y are the processes of the impact of X on M (M1, M2) and M on Y (B). In the parallel mediation model with two mediators, there are two different indirect effects: (i) X on M1 and M1 on Y; and (ii) X on M2 and M2 on Y. Thus, the total effect is the sum of the indirect effects (blue box) and the direct effect (red box) of X on Y while accounting for mediating variables (M1 and M2) (Kane and Ashbaugh, 2017).
In this study we assessed the total, direct and indirect effects of WMH (X) on WAB-AQ (Y). Based on our hypothesis that damage to a higher proportion of long-range fibres counterbalanced by damage to a lower proportion of short-range fibres mediates the relationship between WMH and worse chronic post-stroke aphasia severity, we assessed the indirect effect of WMH on WAB-AQ through the number of long-range (M1) and number of short-range fibres (M2). Using model 4 in the PROCESS macro, we modelled two indirect effects of WMH on WAB-AQ mediated by the number of short- and long-range fibres, and we modelled the direct effect of WMH on WAB-AQ. To determine the indirect effects we performed two regression models for each mediator. First, we assessed the impact of WMH on the number of long- or short-range fibres while controlling for the other mediator (Models 1 and 2 in Table 3); and second, we assessed the impact of the number of long- or short-range fibres while controlling for the other mediator and WMH (Model 3 in Table 3). The last regression model (Model 3 in Table 3), was also used to determine the direct effect of WMH on WAB-AQ while controlling for both mediators.
Table 3.
Mediation analysis for the effect of PVH on WAB-AQ mediated in parallel by the number of long-range fibres and number of short-range fibres, while controlling for lesion volume
| Unstandardized coefficients β (SE) | Standardized coefficients β | t | P | |
|---|---|---|---|---|
| Model 1 Dependent variable: number of long-range fibres in whole brain | ||||
| Model (r2 = 0.24, P = 0.0022) | ||||
| Constant* | 17055.41 (1900.69) | – | 8.97 | <0.0001 |
| PVH* | −2725.69 (854.38) | −0.43 | −3.19 | 0.0026 |
| Lesion volume | −13.39 (10.01) | −0.18 | −1.34 | 0.1879 |
| Model 2 Dependent variable: number of short-range fibres in whole brain | ||||
| Model (r2 = 0.23, P = 0.0031) | ||||
| Constant* | 31422.45 (1505.18) | – | 20.88 | <0.0001 |
| PVH* | −1593.58 (676.59) | −0.32 | −2.36 | 0.0230 |
| Lesion volume* | −17.97 (7.93) | −0.31 | −2.27 | 0.0283 |
| Model 3 Dependent variable: WAB-AQ | ||||
| Model (r2 = 0.45, P < 0.0001) | ||||
| Constant | 130.81 (20.79) | – | 6.29 | <0.0001 |
| PVH | −4.88 (2.46) | −0.26 | −1.98 | 0.0540 |
| n of long-range fibres* | 0.002 (0.001) | 0.77 | 2.99 | 0.0046 |
| n of short-range fibres* | −0.003 (0.001) | −0.75 | −2.93 | 0.0054 |
| Lesion volume* | −0.11 (0.03) | −0.49 | −3.91 | 0.0003 |
The first indirect effect of PVH on WAB-AQ mediated by the number of long-range fibres (first indirect effect in Figs 3 and 5) is assessed with Models 1 and 3. Model 1 determines the impact of PVH on the number of long-range fibres, and Model 3 determines the impact of the number long-range fibres on WAB-AQ. The second indirect effect of PVH on WAB-AQ mediated by the number of short-range fibres (second indirect effect in Figs 3 and 5) is assessed with Models 2 and 3 same as for the first indirect effect. The direct effect of PVH on WAB-AQ (direct effect in Figs 3 and 5) is assessed with Model 3.
*Variable is a significant predictor at the 0.05 level.
We used bias corrected bootstrapping with 5000 samples and 95% confidence intervals (CI) to evaluate our hypothesis of indirect effects of WMH on WAB-AQ. We rejected our null hypothesis (no indirect effect present) if the confidence interval did not include zero.
We chose the number instead of percentage of fibre types to avoid multicollinearity in the regression models, because the variables percentage of short-, mid-, and long-range fibres were interdependent. We tested all regression models for multicollinearity by calculating the variance of inflation factor (VIF) and considered VIF > 6 as evidence for multicollinearity (Keith, 2006). All variables in all performed regression models had VIF < 6 and thus, we assumed that multicollinearity was absent or within acceptable means.
Results
Relationship between WMH and axonal fibre damage
As expected, the two subscales PVH and deep WMH significantly correlated with each other (r = 0.454, P = 0.001). The correlation size was moderate, confirming that PVH and deep WMH measure inter-related, but distinct, phenomena.
Relationship with fibre length
We assessed the association between WMH (PVH and deep WMH) and the absolute (count) and relative (percentage) number of short-, mid- and long-range fibres in the right hemisphere. Regarding the absolute fibre count, Spearman correlations were statistically significant for PVH and deep WMH scores and all three fibre length types (Table 2, see Supplementary Fig. 1 for scatterplots). The higher (more severe) the PVH or deep WMH scores, the lower the number of all three fibre length groups. From the lowest, least severe PVH score of ‘0’ to the highest, most severe score of ‘3’, there was a 15% decrease (median) in the absolute number of short-, 35% decrease in mid-, and 47% decrease in long-range fibres. For deep WMH there was a 19% decrease in the absolute number of short-, 36% decrease in mid-, and 51% decrease in long-range fibres.
Table 2.
Correlations (Spearman’s rho) between WMH scores and connectome measures of the right hemisphere (n = 48)
| Short-range fibres | Mid-range fibres | Long-range fibres | |
|---|---|---|---|
| r/P-value | r/P-value | r/P-value | |
| Absolute number of fibres (count) | |||
| PVH | −0.334*/0.010 | −0.335*/0.010 | −0.318*/0.014 |
| Deep WMH | −0.262*/0.036 | −0.270*/0.032 | −0.293*/0.022 |
| Relative number of fibres (percentage) | |||
| PVH | 0.298*/0.040 | −0.264/0.070 | −0.296*/0.041 |
| Deep WMH | 0.299*/0.039 | −0.140/0.340 | −0.319*/0.027 |
*Correlation is significant at the 0.05 level (one-tailed for absolute number of fibres; two-tailed for relative number of fibres).
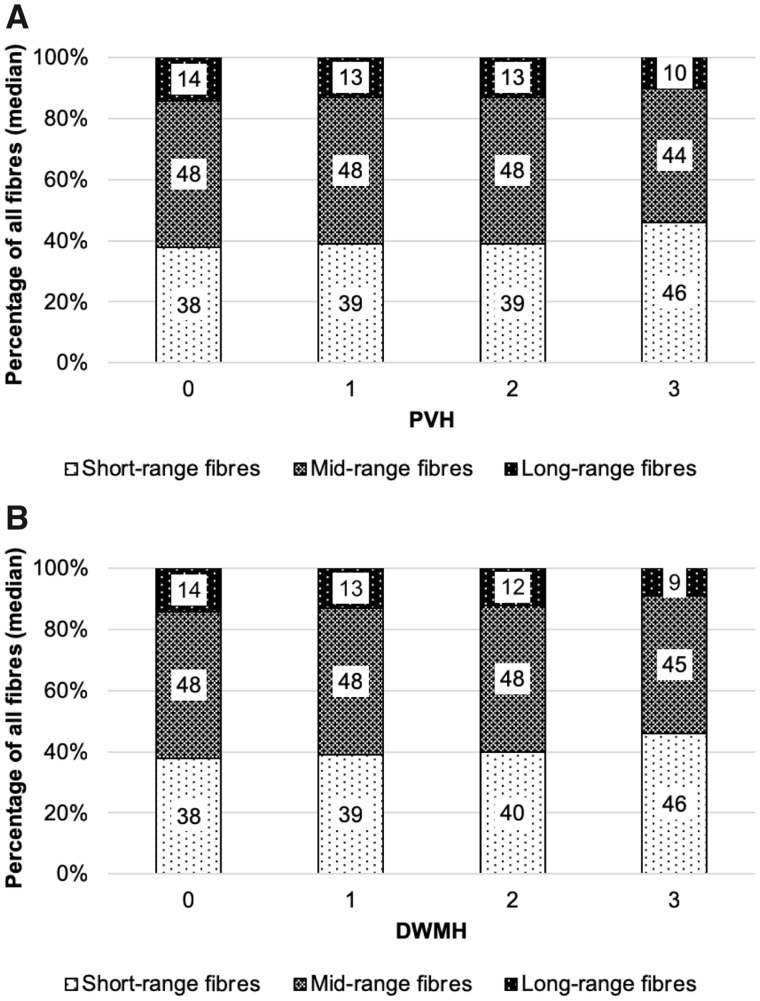
PVH and deep WMH scores were significantly correlated with the percentage of short- and long-range fibres (Table 2). Higher (more severe) PVH and deep WMH scores were associated with a significantly lower percentage of long-range (r = −0.296, P = 0.041, and r = −0.319, P = 0.027, respectively), but significantly higher percentage of short-range fibres (r = 0.298, P = 0.040, and r = 0.299, P = 0.039, respectively) (reflecting a disproportionate damage to long-range fibres, compared with short-range fibres) (Fig. 4). Correlations between PVH scores and axonal fibre damage were weak to moderate in size.
Figure 4.
Median percentage of short-, mid- and long-range fibres in the right hemisphere for patients (n = 48) with different PVH (A) and deep WMH (DWMH) (B) scores.
Using a two-way ANOVA, there were no significant interaction effects for PVH and fibre length types [F(6,135) = 1.95, P = 0.07], and for deep WMH and fibre length type [F(6,135) = 1.41, P = 0.2168]. Assessing the distribution of WMH scores across the fibre length types, the score of 1 showed the largest variability (widest range) of percentages for each fibre type. When we excluded the score of 1 from the WMH scores and only included scores of 0, 2 and 3, we found significant interaction effects for both PVH and fibre length types [F(4,99) = 6.67, P = 0.0001], and for deep WMH and fibre length types [F(4,42) = 3.23, P = 0.0213], indicating that the effect of fibre length types was dependent on these WMH scores.
In post hoc analyses, we assessed whether the relationship between WMH and fibre types in the right hemisphere was confounded by the stroke lesion in the left hemisphere, as the lesion may have indirect effects on right hemisphere white matter. We performed multivariable linear regression modelling with the number or percentage of fibre types as the dependent variable, PVH or deep WMH as the main independent variable, and lesion volume as the control variable. We confirmed the significant relationships (P ≤ 0.05) between higher (more severe) PVH/deep WMH scores and a lower number of mid- and long-range fibres, and higher (more severe) deep WMH scores and a lower percentage of long-range fibres. Higher (more severe) PVH scores showed a strong trend towards significance for a decrease in the percentage of long-range fibres (P = 0.054). These post hoc analyses indicate an effect of WMH on fibre length groups independent of the stroke lesion, with damage to a higher percentage of long-range compared to short- and mid-range fibres.
Relationship between WMH, axonal fibre damage, and chronic aphasia severity
We found a significant total effect of PVH on WAB-AQ with higher (more severe) PVH scores linked to lower (more severe) WAB-AQ scores (unstandardized beta = −6.607, standard error = 2.386, standardized beta = −0.348, P = 0.008) when controlling for stroke lesion volume. There was no effect of deep WMH on WAB-AQ (P > 0.05), thus, we explored direct and indirect effects further, for PVH only.
We performed mediation modelling with PVH as the independent variable, WAB-AQ as the dependent variable, and number of long-range and short-range fibres in the whole brain as the two mediator variables, and lesion volume as the control variable. Contrary to the first objective where we assessed only the right hemisphere, we chose to specify the number of long- and short-range fibres in the whole brain instead of the right hemisphere only, because (i) of the importance of the left hemisphere for aphasia severity; and (ii) WMH are usually symmetric, thus the extent of WMH should be similar between both hemispheres.
Results from the mediation analysis are presented in Table 3 and Fig. 5. The results indicated that there was a (marginally failed) non-significant direct effect of PVH on WAB-AQ [effect = −4.8803, standard error (SE) = 2.4617, 95% CI: −9.8483 to 0.0877, P = 0.0540], but there were significant indirect effects of PVH on WAB-AQ mediated by the number of long-range fibres (effect = −6.2273, bootstrapping: SE = 2.6426, 95% CI: −11.8243 to −1.5599) and the number of short-range fibres (effect = 4.5006, bootstrapping: SE = 2.588195% CI: 0.1631 to 10.2897). More severe PVH scores were associated with a lower number of long-range fibres, and in turn a lower number of long-range fibres were associated with more severe WAB-AQ scores. Further, more severe PVH scores were associated with a lower number of short-range fibres, and in turn a lower number of short-range fibres were associated with less severe WAB-AQ scores. Thus, the total effect of PVH on WAB-AQ that we had found initially was mainly based on indirect effects and not direct effects of PVH on WAB-AQ.
Figure 5.
Direct and indirect (mediated) effects of PVH on WAB-AQ estimated through regression modelling. The direct effect was non-significant. The two indirect effects through the mediating variables—number of long-range and number of short-range fibres—were significant (bootstrapping 95% CI did not include zero).
Discussion
Most individuals 60 years or older show evidence of cerebral WMH on neuroimaging (de Leeuw et al., 2001; Grueter and Schulz, 2012). Increasing severity of WMH has been linked to decline and worse recovery of physical, functional and cognitive abilities in stroke survivors (Arsava et al., 2009; Liou et al., 2010; Kang et al., 2013; Moon et al., 2017; Wright et al., 2018). The underlying neurophysiological correlates are poorly understood, hampering the strategic development of treatment approaches for stroke survivors that could take the neurophysiological changes resulting from WMH into account. In the study presented here, we sought to investigate the relationship between WMH, structural brain network integrity, and post-stroke aphasia severity.
Relationship between WMH and axonal fibre damage
In the first part of the study, we assessed the relationship between WMH (PVH and deep WMH) and structural brain connectivity measured by axonal damage of white matter fibres with different lengths. Our findings indicate that WMH are associated with damage to a higher percentage of long-range fibres compared to mid- and short-range fibres.
Axonal damage as a microstructural correlate of WMH has been described before (Pantoni and Garcia, 1997; Gouw et al., 2011). Recent explorative research further suggests that long-range fibres are more susceptible to WMH-related damage than short-range fibres (Lambert et al., 2016). Our study supports and expands on these findings by using sophisticated fibre tracking methods. We found that while the absolute number (count) of all fibres—independent of length—decreased with more severe WMH, the relative number (proportion/percentage) of long-range fibres decreased more than twice as much as short-range fibres.
Reasons for this heterogeneous decline are not fully understood. Compared to grey matter, white matter is in general susceptible to damage when lesioned, because of its lower blood flow (Lo et al., 2003). Long-range fibres are particularly susceptible to damage. This may be due to their higher metabolic demands (Buzsaki, 2006; Ju et al., 2016), or due to the fact that long range fibres are perfused by a larger number of blood vessels and obstructions in any perfusion territory may lead to fibre damage.
In general, it is possible that our findings on the relationship between WMH and axonal fibre damage can assist in the development of therapeutic interventions for stroke survivors that target the neurophysiological correlates of WMH. Although not tested here, this information could be used to track if interventions such as close control of blood glucose, blood pressure, cholesterol, diet, and exercise lead to preservation of long-range fibres and improve neurorehabilitation outcomes. Likewise, preservation of long-range fibres could be used as a marker of interventional efficacy.
Relationship between WMH, axonal fibre damage, and chronic aphasia severity
In the second part of the study, we assessed the impact of WMH and axonal fibre damage on language performance in stroke survivors. We found that PVH were associated with more severe aphasia, mediated by the number of long-range and short-range white matter fibres. Thus, while PVH marginally failed the statistical significance level to suggest a direct impact on language abilities, PVH did significantly impact language abilities indirectly. With more severe PVH, the number of long-range fibres was lower, and aphasia more severe. Additionally, with more severe PVH, the number of short-range fibres was lower, which in turn led to less severe aphasia. While the latter association—fewer short-range fibres led to milder aphasia—seems counter-intuitive at first, it is a result of the disproportional decline of long- and short-range fibres. As we have shown in our first objective, WMH are related to a decline in the number of fibres of any length (long- as well as short-range fibres); however, the decline in long-range fibres is proportionally greater than that of short-range fibres. Thus, the opposite predictive value of long- and short-range fibres is a reflection of their disproportional decline with more severe WMH.
Moreover, these opposite indirect effects also emphasize the importance of long-range fibres for aphasia severity. Our results suggest that patients whose residual neural network consisted of a higher proportion of long-range fibres (counterbalanced by a lower proportion of short-range fibres), were more likely to show milder aphasia compared to patients with a lower proportion of long-range fibres (counterbalanced by a higher proportion of short-range fibres). These findings are in line with our previous research, showing that the severity and treatment response of post-stroke aphasia depend on the preservation of residual neural networks (Bonilha et al., 2015; Marebwa et al., 2017).
In this study we determined the number and proportion of long-range and short-range fibres in both hemispheres, and WMH in the contralesional (right) hemisphere, assuming that WMH will be symmetrical in both hemispheres. The exact role of the right hemisphere for aphasia severity and recovery is controversial, but a contribution to some degree is likely, at least in individuals with very large left hemisphere lesions (Schlaug, 2018; Hartwigsen and Saur, 2019). Thus, the deteriorating effects of WMH on aphasia severity might stem from compromised white matter networks in the left, right or both hemispheres.
Further, it remains speculative whether the link between WMH and aphasia severity is the result of WMH damaging white matter tracts belonging to language-specific brain networks or to domain-general networks or both. The interaction between language-specific and domain-general networks contributes to the severity and recovery of aphasia after stroke (Geranmayeh et al., 2016). It is possible that WMH affect primarily domain-general abilities, thus, they predispose individuals to more severe aphasia when a stroke might affects language-specific abilities.
In the future, dedicated studies are needed to shed light on precise WMH locations with the highest impact on aphasia severity and their role in language processing. Such studies may also want to disentangle if specific language modalities or tasks are more associated with long-range fibres than others. For example, we like to speculate that spontaneous speech or even word finding engages more long-range fibres than simple repetition tasks. Further, while we have focused on language abilities, future studies should focus on the effect of WMH on other cognitive abilities.
In summary, our findings suggest that after a patient suffered a stroke resulting in aphasia, WMH predispose individuals to lower chances of recovery. While WMH did not cause aphasia in the absence of stroke, WMH imply more severe chronic aphasia resulting from the stroke. We postulate that, compared to residual neural networks not affected by WMH, networks that are affected by WMH cannot compensate as well for acute brain injury. It is possible that the residual networks had already been affected before the stroke by pre-existing WMH, and it is also possible that the WMH affecting the residual networks worsened or newly developed after the stroke. This was not tested in our study.
Limitations
We recruited participants with an ischaemic or haemorrhagic stroke. Mixing participants with different stroke types might be problematic, as the prevalence of WMH could be different between groups. We accounted for this potential bias, by quantifying WMH severity independently of the stroke type and only in the contralesional (right) hemisphere to avoid confounders from haemorrhagic or cascading effects of the large vessel occlusion. Further, we did not measure microbleeds or lacunar infarcts, which are other markers of small vessel disease. Future studies may want to measure these together with WMH to provide a more detailed picture of structural brain changes associated with the composition white matter fibres, and their impact on chronic aphasia severity. We chose to measure WMH with a published ordinal visual rating scale, because of its widespread and easy use. However, the scale does not take exact brain locations into account, which could be important information to understand neurophysiological and functional correlates. Further, we conducted a cross-sectional study; however, a longitudinal study is warranted to assess possible changes in white matter hyperintensities and their long-term effects on aphasia severity. While our study provides evidence for the neurophysiological correlates of WMH and their impact on aphasia severity, we did not assess the generalizability of these findings. Thus, future studies may want to conduct an out-of-sample analysis to validate the generalizability and determine the clinical applicability of our findings.
Conclusions
Our findings indicate that (i) WMH are related to damage to long-range white matter fibres; and (ii) WMH lead to worse aphasia in chronic stroke by affecting a higher number of long-range fibres counterbalanced by affecting a lower number of short-range fibres. Thus, small vessel brain disease predisposes stroke survivors to worse chronic aphasia because of a compromised balance of long-range and short-range white matter fibres. As such, therapeutic and preventative interventions, e.g. aggressive management of modifiable cardiovascular risk factors, for individuals with stroke or high risk for stroke could target the damage to long-range fibres in order to preserve brain health and foster better outcomes in individuals with small vessel brain disease.
Funding
This study was supported by research grants from the National Institutes of Health / National Institute on Deafness and Other Communication Disorders (NIDCD): DC014021 (PI: L.B.), DC011739 (PI: J.F.), DC014664 (PI: J.F.), DC05375 (PI: A.E.H.), T32 DC0014435 (Trainee: A.B.), and from the American Heart Association: SFDRN26030003 (PI: L.B.).
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
Abbreviations
- PVH
periventricular hyperintensities
- WAB-AQ
aphasia quotient of the Western Aphasia Battery-Revised
- WMH
white matter hyperintensities
References
- Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003; 20: 870–88. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016; 125: 1063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsava EM, Bayrlee A, Vangel M, Rost NS, Rosand J, Furie KLet al. Severity of leukoaraiosis determines clinical phenotype after brain infarction. Neurology 2011; 77: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NSet al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 2009; 72: 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage 2005; 26: 839–51. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage 2007; 34: 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisschops RH, van der Graaf Y, Mali WP, van der Grond J. High total cerebral blood flow is associated with a decrease of white matter lesions. J Neurol 2004; 251: 1481–5. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabil Neural Repair 2015; 30: 266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009; 10: 186–98. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles Jet al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002; 52: 335–41. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer Ret al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987; 149: 351–6. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, den Ouden D-B, Hillis AE, Hickok G, Rorden C, Basilakos Aet al. Anatomy of aphasia revisited. Brain 2018; 141: 848–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Leech R, Wise RJS. Network dysfunction predicts speech production after left hemisphere stroke. Neurology 2016; 86: 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens Pet al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011; 82: 126–35. [DOI] [PubMed] [Google Scholar]
- Grueter BE, Schulz UG. Age-related cerebral white matter disease (leukoaraiosis): a review. Postgrad Med J 2012; 88: 79–87. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Saur D. Neuroimaging of stroke recovery from aphasia – Insights into plasticity of the human language network. Neuroimage 2019; 190: 14–31. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based perspective. 2nd edn. New York, NY: The Guilford Press; 2018. [Google Scholar]
- Henon H, Vroylandt P, Durieu I, Pasquier F, Leys D. Leukoaraiosis more than dementia is a predictor of stroke recurrence. Stroke 2003; 34: 2935–40. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Guerrero GD, Cecilia JM, Garcia JM, Inuggi A, Jbabdi Set al. Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PLoS One 2013; 8: e61892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot M, Jobard G, Naveau M, Delcroix N, Petit L, Zago Let al. AICHA: An atlas of intrinsic connectivity of homotopic areas. J Neurosci Methods 2015; 254: 46–59. [DOI] [PubMed] [Google Scholar]
- Ju H, Hines ML, Yu Y. Cable energy function of cortical axons. Sci Rep 2016; 6: 29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane L, Ashbaugh AR. Simple and parallel mediation: a tutorial exploring anxiety sensitivity, sensation seeking, and gender. Quant Methods Psychol 2017; 13: 148–65. [Google Scholar]
- Kang HJ, Stewart R, Park MS, Bae KY, Kim SW, Kim JMet al. White matter hyperintensities and functional outcomes at 2 weeks and 1 year after stroke. Cerebrovasc Dis 2013; 35: 138–45. [DOI] [PubMed] [Google Scholar]
- Keith TZ. Multiple regression and beyond. Upper Saddle River, NJ: Pearson Education; 2006. [Google Scholar]
- Kertesz A. The Western Aphasia Battery - Revised. New York: Grune & Stratton; 2007a. [Google Scholar]
- Kertesz A. Western Aphasia Battery-Revised. San Antionio, TX: Pearson; 2007b. [Google Scholar]
- Kim GM, Park KY, Avery R, Helenius J, Rost N, Rosand Jet al. Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke 2014; 45: 479–85. [DOI] [PubMed] [Google Scholar]
- King KS, Peshock RM, Rossetti HC, McColl RW, Ayers CR, Hulsey KMet al. Effect of normal aging versus hypertension, abnormal body mass index, and diabetes mellitus on white matter hyperintensity volume. Stroke 2014; 45: 255–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Benjamin P, Zeestraten E, Lawrence AJ, Barrick TR, Markus HS. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain 2016; 139 (Pt 4): 1136–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RGet al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke 1996; 27: 2262–70. [DOI] [PubMed] [Google Scholar]
- Liou LM, Chen CF, Guo YC, Cheng HL, Lee HL, Hsu JSet al. Cerebral white matter hyperintensities predict functional stroke outcome. Cerebrovasc Dis 2010; 29: 22–7. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003; 4: 399–415. [DOI] [PubMed] [Google Scholar]
- Marebwa BK, Fridriksson J, Yourganov G, Feenaughty L, Rorden C, Bonilha L. Chronic post-stroke aphasia severity is determined by fragmentation of residual white matter networks. Sci Rep 2017; 7: 8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HI, Nam JS, Leem MJ, Kim KH. Periventricular white matter lesions as a prognostic factor of swallowing function in older patients with mild stroke. Dysphagia 2017; 32: 480–6. [DOI] [PubMed] [Google Scholar]
- Nachev P, Coulthard E, Jager HR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. Neuroimage 2008; 39: 1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Leukoaraiosis: from an ancient term to an actual marker of poor prognosis. Stroke 2008; 39: 1401–3. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997; 28: 652–9. [DOI] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk Met al. Cerebral white matter lesions and the risk of dementia. Arch Neurol 2004; 61: 1531–4. [DOI] [PubMed] [Google Scholar]
- Schlaug G. Even when right is all that’s left: There are still more options for recovery from aphasia. Ann Neurol 2018; 83: 661–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nat Neurosci 2014; 17: 652. [DOI] [PubMed] [Google Scholar]
- ten Dam VH, van den Heuvel DM, de Craen AJ, Bollen EL, Murray HM, Westendorp RGet al. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology 2007; 243: 198–203. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne Ret al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Sachdev PS. Extent and distribution of white matter hyperintensities in stroke patients: the Sydney Stroke Study. Stroke 2004; 35: 2813–9. [DOI] [PubMed] [Google Scholar]
- Wright A, Tippett D, Saxena S, Sebastian R, Breining B, Faria Aet al. Leukoaraiosis is independently associated with naming outcome in poststroke aphasia. Neurology 2018; 91: e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.