The following was originally published in Volume 26, No. 1, pages 103–110, 2018 (doi: https://doi.org/10.3727/096504017X14934860122864). There was an error in Figure 4E in the Transwell assay. A corrected version of the figure is shown here and the figure has been replaced with the corrected version in the original published article in the online site (https://www.ingentaconnect.com/contentone/cog/or/2018/00000026/00000001/art00011). The original version of the figure did not affect the results or conclusion of the article.
Abstract
Glioma is the most common and lethal malignant intracranial tumor. Long noncoding RNAs (lncRNAs) have been identified as pivotal regulators in the tumorigenesis of glioma. However, the role of lncRNA urothelial carcinoma-associated 1 (UCA1) in glioma genesis is still unknown. The purpose of this study was to investigate the underlying function of UCA1 on glioma genesis. The results demonstrated that UCA1 was upregulated in glioma tissue and indicated a poor prognosis. UCA1 knockdown induced by si-UCA1 significantly suppressed the proliferative, migrative, and invasive activities of glioma cell lines (U87 and U251). Bioinformatics analysis and luciferase reporter assay verified the complementary binding within UCA1 and miR-122 at the 3′-UTR. Functional experiments revealed that UCA1 acted as an miR-122 “sponge” to modulate glioma cell proliferation, migration, and invasion via downregulation of miR-122. Overall, the present study demonstrated that lncRNA UCA1 acts as an endogenous sponge of miR-122 to promote glioma cell proliferation, migration, and invasion, which provides a novel insight and therapeutic target in the tumorigenesis of glioma.
Key words: Glioma, Long noncoding RNAs (lncRNAs), Urothelial carcinoma-associated 1 (UCA1), miR-122, Migration, Invasion
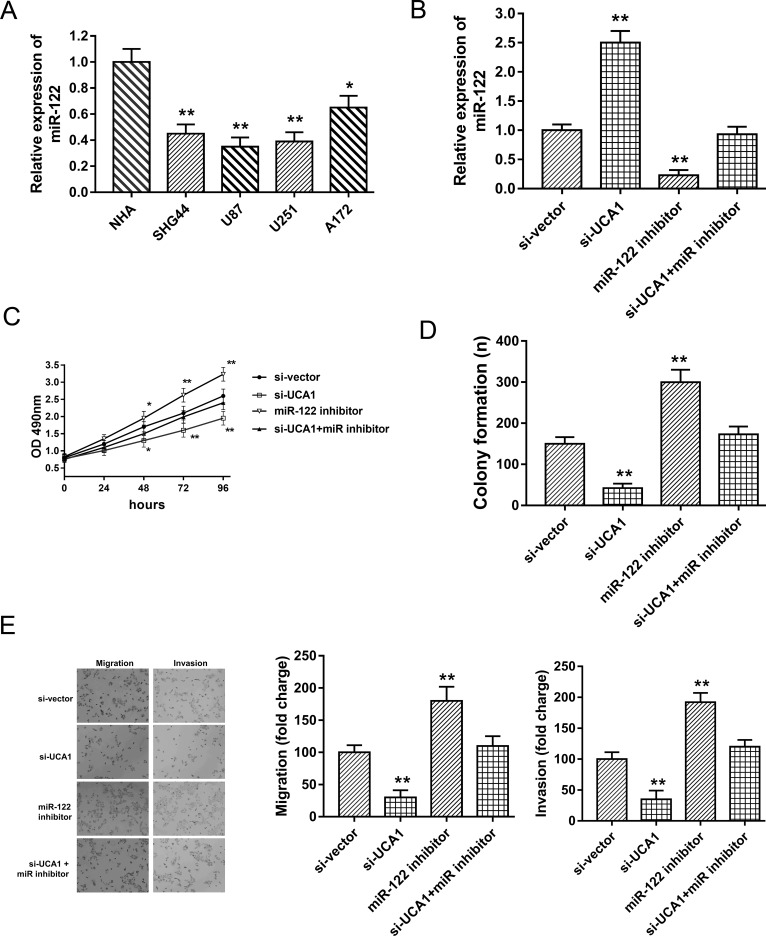
Figure 4.
UCA1 facilitated glioma proliferation, migration, and invasion partially targeting miR-122 in U87 cell lines. (A) The lower expressed miR-122 in glioma cell lines (SHG44, U87, U251, and A172) compared with NHAs. (B) Expression of miR-122 in U87 cell lines, respectively, transfected with si-UCA1 miR-122 inhibitor. (C) MTT assay showed proliferation ability. (D) Colony formation assay. (E) Transwell assay showed the migrative and invasive capabilities. Data are presented as mean ± SD. *p < 0.05, **p < 0.01 compared to the control group.