Abstract
Wnt molecules play crucial roles in development and adult homeostasis through their receptors Frizzled proteins (Fzds). Fzds mediate canonical β-catenin pathway and various noncanonical β-catenin-independent pathways. Aberrant Fzd signaling is involved in many diseases including cancer. Wnt/β-catenin is a well-established oncogenic pathway involved in almost every aspect of tumor development. However, Fzd-mediated noncanonical Wnt pathways function as both tumor promoters and tumor suppressors depending on cellular context. Fzd-targeted therapies have proven to be effective on cultured tumor cells, tumor cell xenografts, mouse tumor models, and patient-derived xenografts (PDX). Moreover, Fzd-targeted therapies synergize with chemotherapy in preclinical models. However, the occurrence of fragility fractures in patients treated with Fzd-targeted agents such as OMP-54F28 and OMP-18R5 limits the development of this combination. Along with new insights on signaling, roles, and modulation mechanisms of Fzds in human tumors, more Fzd-related therapeutic targets will be developed.
Key words: Wnt, Frizzled, β-Catenin, Tumor
INTRODUCTION
Frizzled proteins (Fzds) belong to the superfamily of G protein-coupled receptor (GPCR), with extracellular N-terminus, seven-transmembrane domain, and intracellular C-terminus. The extracellular N-terminus contains a cysteine-rich domain (CRD), which is highly conserved and can bind Wnt ligands. A flexible linker region connects the CRD to the transmembrane domain. The C-terminus contains several conserved motifs, which can bind the PDZ domain of Dishevelled (Dvl). The C-terminus also interacts with G proteins. Ten Fzds have been identified in humans. Phylogenetic analysis indicates that these Fzds can be divided into four or five subfamilies: Fzd1/2/7, Fzd3/6, Fzd5/8, Fzd4/9/10 or Fzd4 and Fzd9/101–3.
Fzd SIGNALOSOME
Canonical Signalosome
Canonical Wnt/β-catenin pathway is mediated by a signalosome consisting of Fzd receptors, Lrp5/6 coreceptors, and Dvl and Axin adapters. Upon binding Wnt ligands, Fzds and Lrp5/6 oligomerize to create a scaffold in which Dvl is phosphorylated and copolymerizes with Axin, leading to the dissociation of β-catenin destruction complex4. Notably, a recent study has shown that oligomerization of Fzds and Lrp5/6 can activate β-catenin pathway in the absence of Wnt ligands5.
Noncanonical Signalosome
Several Wnt ligands such as Wnt5a generally fail to induce Lrp6 phosphorylation and β-catenin pathway activation due to the lack of functional interaction with Lrp5/66–8. Binding of Wnt5a to Fzds results in Fzd oligomerization and Dvl phosphorylation independent of Lrp5/69,10. In some circumstances, Wnt5a signaling requires Ror1/2, which can form a receptor complex with Fzds8,11–13.
DOWNSTREAM SIGNALING OF Fzds IN TUMORS
β-Catenin Pathway
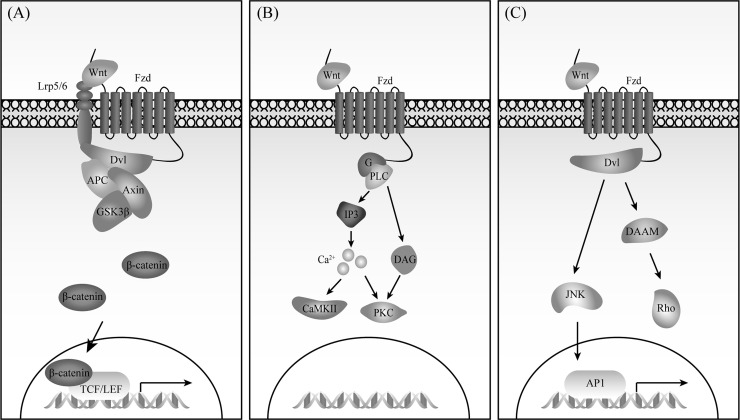
Activation of canonical Fzd signalosome causes β-catenin destruction complex dissociation. Therefore, β-catenin in the cytoplasm escapes from phosphorylation by GSK3β and degradation by proteasome. Accumulated β-catenin translocates into the nucleus, promoting the transcription of TCF/LEF target genes (Fig. 1A). MMTV/WNT1 transgenic mice that can develop mammary hyperplasia and adenocarcinoma clearly demonstrate the capacity of β-catenin pathway to induce malignant transformation14,15. In colorectal cancer, adenomatous polyposis coli (APC), a component in β-catenin destruction complex, is frequently mutated, leading to activation of β-catenin pathway16. Actually, activation of β-catenin pathway is involved in almost all aspects of human tumor initiation and progression, including stemness, growth, survival, drug resistance, angiogenesis, immune evasion, and metastasis17–19.
Figure 1.
Downstream signaling of Frizzled proteins (Fzds). (A) β-Catenin pathway. (B) G protein–Ca2+–PKC pathway. (C) PCP pathway.
G Protein-Ca2+-PKC Pathway
Binding of Wnt5a to several Fzds such as Fzd1, Fzd2, Fzd5, Fzd6, and Fzd9 has been shown to activate G protein signaling20–24. Consistently, Fzd-mediated β-catenin pathway seems not to require the involvement of heterotrimeric G proteins25. Although the structure of Fzds is distinct from that of other GPCRs26, Fzds still can mediate intracellular Ca2+ release and PKC activation through phospholipases C (PLC) signaling (Fig. 1B). Wnt5a–Fzd promotes melanoma metastasis via PKC27–29, and colitis-associated cancer via CaMKII30.
Planar Cell Polarity (PCP) Pathway
The Wnt/PCP pathway controls tissue polarity and cell movement through downstream signaling such as Rho GTPases and JNK31–33 (Fig. 1C). During development, Wnt5a/b and Wnt11 activate the PCP pathway through Fzd3/6 and Ror1/2, involving Vangl1/2 and Dvl33. In human tumors, the Wnt5a–Fzd/PCP pathway promotes the progression of chronic lymphocytic leukemia (CLL) and ovarian cancer34–37.
Stat3 Pathway
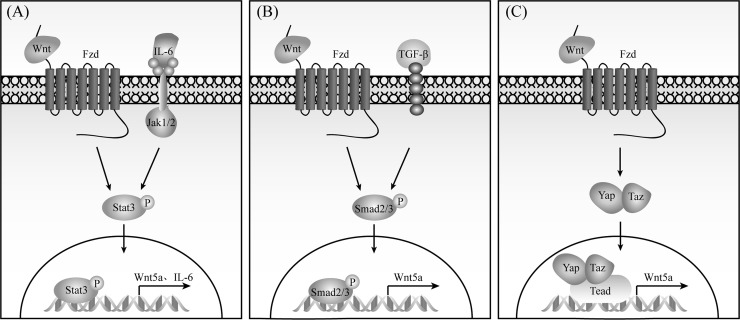
The oncogenic pathway interleukin-6 (IL-6)/Jak/Stat3 is involved in many types of tumors. Binding of Wnt5a/b to Fzd2 recruits and phosphorylates Stat3 in cancer cells, which seems not to be related to G proteins and Dvl38. In liver cancer cells, Fyn kinase but not IL-6/Jak is responsible for Stat3 phosphorylation. However, Wnt5a activates IL-6/Stat3 signaling in CLL and breast cancer39,40. Furthermore, Wnt5a and IL-6 form a positive feedback loop to activate Stat3 in melanoma41–43, and Stat3 transcriptional upregulates Wnt5a expression in CLL44. These studies indicate that Wnt5a–Fzd cross-talks with IL-6/Stat3 in some human tumors (Fig. 2A).
Figure 2.
Fzd signaling cross-talks with Stat3 (A), Yap/Taz (B), and TGF-β (C) pathways.
Yap/Taz Pathway
Activation of LATS1/2 in the Hippo pathway induces phosphorylation of Yap and Taz, two transcriptional coactivators, which are subsequently degraded by proteasome. When Yap and Taz are not phosphorylated, they enter into the nucleus to bind transcription factor Tead. Both Wnt5a/b and Wnt3a activate Yap/Taz–Tead signaling independent of Lrp5/6 and β-catenin; Fzd1, Fzd2, and Fzd5 all can mediate this signaling, but Ror1 is required to coordinate with Fzd2 and Fzd522. Yap/Taz–Tead signaling antagonizes β-catenin pathway, while it promotes Wnt5a transcription, thereby forming a positive feedback loop (Fig. 2B). Wnt5a/b-Fzd2/5 modulates Yap expression in a series of human malignant tumors including breast cancer, hepatocelluar carcinoma (HCC), melanoma, and squamous subtype pancreatic adenocarcinoma40,45–47.
Transforming Growth Factor-β (TGF-β) Pathway
Both TGF-β and Wnt5a play dual roles in human tumors. As strong inducers of epithelial–mesenchymal transition (EMT), they increase cell motility and promote tumor metastasis. On the other hand, they have the potential to suppress cell growth, functioning as tumor suppressors. Cross-talk between Wnt5a and TGF-β exits in breast cancer; TGF-β maintains Wnt5a expression, and inversely, Wnt5a stimulates Smad2 phosphorylation in a TGFBR1-dependent manner40,48,49. Moreover, Fzd8 forms a receptor complex with TGFBR1 to mediate both Wnt11 and TGF-β signaling in prostate cancer50. Wnt5a and TGF-β also coordinate in the pathogenesis of organ fibrosis51–53. TGF-β can induce Fzd expression in fibroblasts54,55. Together, these studies indicate that Fzds interact with TGF-β receptors to enhance TGF-β signaling, which further upregulates Wnt5a/Fzds (Fig. 2C).
TUMOR-PROMOTING ROLES OF Fzds
All Fzds except Fzd9 have been found to play tumor-promoting roles. Through both canonical and noncanonical Wnt pathways, Fzds contribute to tumor initiation, growth, chemoresistance, and metastasis (Table 1).
Table 1.
Tumor-Promoting Roles of Frizzled Proteins (Fzds)
| Fzds | Wnt Pathways | Malignant Tumors | Functions | References |
|---|---|---|---|---|
| Fzd1 | Canonical | NB, breast cancer, AML | Chemoresistance | 55–58 |
| Fzd2 | Noncanonical | Liver, breast, colon, lung cancers | EMT, metastasis, stemness | 38, 40, 45 |
| Fzd7 | Canonical | Liver, breast cancers | Growth, stemness | 59–62 |
| Fzd3 | Noncanonical | Melanoma | Stemness, metastasis | 69 |
| Fzd6 | Noncanonical | NB, TNBC | Stemness, metastasis, chemoresistance | 70, 71 |
| Fzd5 | Canonical | Endometrial, ovarian, pancreatic cancers | Growth | 72–74 |
| Noncanonical | Melanoma, cHL | Migration, invasion | 27, 75 | |
| Fzd8 | Canonical | HNSCC, TNBC | Stemness, metastasis, chemoresistance | 76, 77 |
| Noncanonical | Prostate cancer | EMT, metastasis | 50 | |
| Fzd4 | Canonical | GBM, prostate cancer | EMT, stemness | 78, 79 |
| Fzd10 | Canonical | Breast, ovarian cancers | EMT, chemoresistance | 80–82 |
Fzd1/2/7 Subfamily
Comparative studies between neuroblastoma (NB) cell lines and their chemoresistant counterparts revealed that Fzd1 contributes to NB chemoresistance56. Fzd1 expression in chemoresistant NB cells is associated with Wnt/β-catenin activity and multidrug resistance gene MDR1 expression. Fzd1 knockdown enhances the sensitivity of chemoresistant NB cells to chemical drugs. Moreover, Fzd1 expression is significantly upregulated in NB tumors from relapsed patients after chemotherapy. Fzd1 is also overexpressed in chemoresistant breast cancer and leukemic cell lines, and Fzd1 knockdown downregulates MDR1 expression, suppresses Wnt/β-catenin activity, and restores the sensitivity to chemotherapy57,58. These studies have clearly demonstrated that Fzd1 overexpression induces chemoresistance through activation of the Wnt/β-catenin–MDR1 pathway, potentially facilitating tumor recurrence.
Fzd2 is overexpressed in poorly differentiated, mesenchymal-type, and late-stage tumor samples of liver, lung, colon, and mammary gland38. In vitro studies further showed that Fzd2 overexpression induces EMT and cell migration via Stat3 signaling, and consistently, Fzd2 knockdown or treatment with an anti-Fzd2 antibody suppresses tumor metastasis in mouse xenograft models38. Both EMT and Stat3 signaling are related to cancer cell mesenchymal-like stemness. Accordingly, Fzd2 signaling stimulates stem-like properties in breast and liver cancer cells, and higher Fzd2 expression is correlated with shorter survival of these cancer patients40,45.
Fzd7 is overexpressed in HCC samples59. Transgenic HCC murine models showed that activation of Fzd7/β-catenin signaling occurs early during the progression to HCC, suggesting an oncogenic role of Fzd760. Wnt3 was identified as a ligand for Fzd7 in HCC, and interaction between Wnt3 and Fzd7 is responsible for β-catenin activation and cell proliferation61. Fzd7 is also overexpressed in triple-negative breast cancer (TNBC) samples and cell lines; Fzd7 knockdown suppresses the proliferation of in vitro cultured cells and the growth of in vivo xenograft tumors62. Of note, Fzd7 signaling is required for the expansion of embryonic stem cells, Lgr5+ intestinal stem cells, mammary stem cells, and breast cancer stem cells63–65.
Although Fzd1, Fzd2, and Fzd7 belong to the same subfamily, they seem to have distinct roles in human tumors. Fzd1 contributes to chemoresistance, Fzd2 promotes tumor metastasis, and Fzd7 induces carcinogenesis and tumor growth. Moreover, Fzd1 and Fzd7 act through the β-catenin pathway, whereas Fzd2 functions independent of β-catenin. Interestingly, both Fzd2 and Fzd7 are involved in cancer cell stemness even though via different mechanisms.
Fzd3/6 Subfamily
Both Fzd3 and Fzd6 have a key role in PCP during development66,67. Fzd3 as well as other PCP pathway components such as Vangl2 and Celsr1 are upregulated in B lymphocytes of patients with CLL; high Fzd3 level is correlated with unfavorable prognosis35. Fzd3 is also overexpressed in bone marrow cells of patients with acute lymphocytic leukemia (ALL) and myelodysplastic syndrome (MDS)68. However, the role of Fzd3 in these hematologic malignancies remains unknown. Recently, the regulatory function of Fzd3 in melanoma was explored69. Global gene expression analysis using melanoma patient-derived cells identified Fzd3 as a regulator of cell cycle progression. Fzd3 knockdown inhibits the proliferation of in vitro cultured cells, and the growth, initiation, and metastasis of xenograft tumors. In melanoma patients, Fzd3 expression is associated with disease progression and reduced survival.
Fzd6 maintains stem-like phenotype and chemoresistance of NB cells; xenograft tumors derived from Fzd6-positive NB cells grow faster than those from Fzd6-negative cells. Moreover, high Fzd6 expression is correlated with poor survival of NB patients70. Similarly, Fzd6 is associated with reduced distant relapse-free survival and has an independent prognostic significance in predicting distant TNBC relapse as shown by multivariate analysis. Fzd6 knockdown inhibits TNBC cell motility and invasion in vitro, and bone and liver metastasis in vivo71.
Fzd3 and Fzd6 seem to be less frequently involved in human tumors. Moreover, Fzd3 and Fzd6 signaling in these tumors is not related to β-catenin pathway.
Fzd5/8 Subfamily
Wnt7a and Wnt7b specifically bind to Fzd5 among the 10 Fzds2. Wnt7a–Fzd5 induces the proliferation of endometrial and ovarian cancer cells through the β-catenin pathway72,73. Wnt7b–Fzd5 promotes RNF43-mutant pancreatic ductal adenocarcinoma (PDAC) cell in vitro proliferation and in vivo growth74. Moreover, Fzd5 promotes the proliferation of a patient-derived PDAC cell line that harbors an RNF43 variant. Upon binding to Wnt5a, Fzd5 mediates noncanonical Wnt pathways. Wnt5a–Fzd5 increases melanoma cell motility and invasion through PKC27. This pair also stimulates classical Hodgkin lymphoma (cHL) cell migration through RhoA75.
Fzd8 was shown to be a downstream component of c-Met signaling and responsible for β-catenin activation in head and neck squamous cell carcinomas (HNSCC); ectopic expression of Fzd8 rescues c-Met inhibition-induced impairment of tumor incidence, growth, and metastasis76. Treatment with chemical agents upregulates Fzd8 expression in TNBC cells and tumors; Fzd8 knockdown in TNBC cells reduced β-catenin and survivin levels and increased the sensitivity to chemical agents, suggesting that canonical Fzd8 signaling mediates TNBC chemoresistance77. Similar to Fzd5, Fzd8 mediates β-catenin-independent pathway upon binding noncanonical Wnt ligands. As a receptor of Wnt11, Fzd8 forms a complex with the TGF-β receptor, thereby cross-talking with the TGF-β pathway and promoting EMT in prostate cancer cells and invasion in prostate cancer cell organotypic 3D cultures50.
Clearly, two members in this subfamily mediate both canonical and noncanonical Wnt pathways in human tumors depending on their binding ligands. They are involved in stemness, growth, chemoresistance, and metastasis of human tumors.
Fzd4/9/10 Subfamily
Fzd4 is upregulated in highly invasive glioblastoma (GBM) cells, maintaining stem cell properties through the β-catenin pathway, and EMT phenotype through SNAI178. Consistently, expression of Fzd4 and nuclear β-catenin was detected at the invasive front of primary GBM specimens. Fzd4 also induces EMT through the β-catenin pathway in ERG-positive prostate cancer cells79. These two studies suggest that Fzd4 is a prometastatic factor through induction of EMT.
BRMS1L suppresses breast cancer cell invasion and migration in vitro and metastasis in xenograft models; these inhibitory effects are mediated by inactivation of the Fzd10–β-catenin pathway80. Fzd10 knockdown inhibits the Wnt/β-catenin pathway in PARPi-resistant ovarian cancer cells and increases the sensitivity of these cells to PARP inhibitors Olaparib and Rucaparib; moreover, β-catenin inhibitor XAV939 synergizes with Olaparib in suppressing PARPi-resistant cells in vitro and in vivo81. Fzd10 is highly methylated and significantly downregulated in chemoresponsive ovarian cancer samples; Fzd10 knockdown synergizes with cisplatin to inhibit growth and induce apoptosis in ovarian cancer cell lines82.
Together, Fzd4 and Fzd10 are involved in tumor metastasis through activation of canonical Wnt pathway. Moreover, Fzd10 contributes to the chemoresistance of ovarian cancer.
TUMOR-SUPPRESSING ROLES OF Fzds
Fzd-mediated noncanonical pathways have been demonstrated to antagonize β-catenin activity, thereby functioning as tumor suppressor depending on cellular context (Table 2).
Table 2.
Tumor-Suppressing Roles of Fzds
| Fzds | Wnt Pathways | Malignant Tumors | Functions | References |
|---|---|---|---|---|
| Fzd1 | Noncanonical | FTC | Growth, invasion, migration | 83 |
| Fzd2 | Noncanonical | HCC | Growth, stemness | 85 |
| Fzd6 | Noncanonical | Gastric, prostate, breast cancers | Growth, migration, stemness | 87, 88 |
| Fzd5 | Noncanonical | Prostate cancer | Growth | 90, 91 |
| Fzd8 | Noncanonical | Pancreatic cancer, glioma | Stemness, growth | 92, 93 |
| Fzd9 | Noncanonical | NSCLC | EMT | 99, 100 |
Fzd1/2/7 Subfamily
Fzd1 functions as a putative tumor suppressor in follicular thyroid carcinoma (FTC). Fzd1 expression is downregulated in this type of tumor, and overexpression of Fzd1 reduces FTC cell proliferation, invasion, and migration83. The antitumor effect of Fzd1may be associated with its noncanonical Wnt ligand. Overexpression of Wnt5a in a thyroid tumor cell line reduces proliferation, migration, and invasion by antagonizing the β-catenin pathway84.
Wnt5a signaling inhibits the proliferation of normal hepatocytes and HCC cells85,86. The inhibitory effect of Wnt5a is mediated by Fzd2. Wnt5a–Fzd2 suppresses β-catenin–TCF activity in HCC cells, suggesting that this signaling may hinder tumor initiation and growth. However, as described above, Wnt5a–Fzd2 also functions as a prometastatic signaling, especially in the absence of β-catenin pathway activity.
Fzd3/6 Subfamily
Fzd6 signaling represses proliferation and migration of gastric cancer cells87 and stemness of prostate cancer cells by antagonizing the β-catenin pathway88, suggesting Fzd6 as a putative tumor suppressor in these two tumors. In 293T cells, Fzd6 signaling does not affect β-catenin stabilization and β-catenin/TCF4 complex formation, but impairs the binding of TCF/LEF factor to promoters of target genes89. In addition to antagonizing the β-catenin pathway, cross-talking with the TGF-β1 pathway is another mechanism underlying the tumor-suppressing roles of noncanonical Fzds. In breast cancer transgenic mouse models, Wnt5a dampens the expansion of tumor-initiating cells involving TGFR1/Smad2 and Fzd649. Therefore, similar to Wnt5a–Fzd2 in HCC, Wnt5a–Fzd6 may inhibit carcinogenesis but promote metastasis in breast cancer.
Fzd5/8 Subfamily
Similar to Fzd2 and Fzd6, Fzd5 exerts antiproliferative and proapoptotic effects on prostate cancer cells upon binding Wnt5a90,91. Fzd8, which is repressed by oncogenic K-Ras, blocks tumorigenicity by reducing β-catenin transcriptional activity92. Restoration of Fzd8 reduces tumor formation capacity of K-RasV12-transformed NIH/3T3 cells and K-Ras-possessing PDAC cells in xenograft models. Fzd8 expression is epigenetically downregulated during tumorigenesis of glioma in a mouse model, and Fzd8 negatively regulates tumor cell proliferation in vitro93. Both FZD5 and FZD8 are hypermethylated in a mouse model of AML, and the hypermethylation level increases with disease progression, suggesting their tumor-suppressing role in this tumor94. Notably, Wnt7a functions as a putative tumor suppressor in gastric cancer and HCC95,96. As the special receptor for Wnt7a, Fzd5 has the potential to transduce suppressive signaling in these two tumors.
Fzd4/9/10 Subfamily
Wnt7a maintains E-cadherin expression and inhibits EMT in non-small cell lung cancer (NSCLC) cell lines97,98. The antitumor effects of Wnt7a depend on Fzd998–100. Wnt7a–Fzd9 contributes to the inhibition of NSCLC cell growth and migration. FZD9 was frequently hypermethylated in human AML samples, and FZD9 methylation is an independent predictor of decreased survival for AML patients, suggesting that Fzd9 is a candidate tumor suppressor in AML101. FZD9 is also frequently hypermethylated in ER/PR+ breast cancer and GBM, but the role of FZD9 hypermethylation in these two tumors is unexplored102,103.
MODULATION OF Fzds IN TUMORS
Gene Mutation
Mutations in genes encoding Fzds are rare in tumors. Loss of heterozygosity (LOH) of FZD1 gene at 7q21.2 results in a reduced Fzd1 expression in FTC83. As Fzds play crucial roles in development, FZD mutations may lead to dysplasia. For instance, FZD2 mutation causes autosomal dominant omodysplasia and Robinow syndrome-like features104,105, and FZD4 mutation causes familial exudative vitreoretinopathy106.
Gene Promoter Hypermethylation
Hypermethylation of gene promoter is a common epigenetic mechanism leading to gene expression silence. In tumors, hypermethylation of FZDs mainly occurs in Fzd5/8 and Fzd4/9/10 subfamilies. Both FZD5 and FZD8 are hypermethylated in AML compared to granulocytes and CD34+ cells from healthy donors94. FZD8 is also hypermethylated in B-cell lymphoma but not in normal B cells107. FZD9 is hypermethylated in several types of tumors including AML, ER/PR+ breast cancer, and GBM101–103. Both FZD4 and FZD10 are hypermethylated in epithelial ovarian cancers82,108.
Histone Modification
Histone modifications alter the chromatin structure, thereby affecting gene transcription. Trimethylation of histone H3 at lysine 27 (H3K27me3) is induced by Polycomb repressive complex 2 (PRC2), in which EZH1/2 catalyzes methyltransferase activity in the presence of EED and SUZ12. H3K27me3 modification results in downregulation of gene expression in all cell types. FZD4 and FZD8 have been shown to be downregulated by H3K27me3 modification via EZH2 recruitment in gastric cancer and glioma, respectively93,109. FZD10 is downregulated by histone H3K9 deacetylation via HDAC1 recruitment in breast cancer80. Notably, histone deacetylation may promote subsequent H3K27me3 by PRC2110,111. Moreover, EZH2 can bind DNA methyltransferases DNMT1 and DNMT3A/B to modulate DNA methylation111.
M6A Modification
N6-methyladenosine (m6A) is the most prevalent modification of RNA, which is critical to almost every aspect of mRNA metabolism. The abundance of m6A is controlled by methyltransferases, RNA binding proteins, and demethylases. Reduced m6A demethylases FTO, and ALKBH5 increases m6A modification and stability of FZD10 mRNA in BRCA-mutated epithelial ovarian cancer cells, contributing to PARPi resistance by upregulating β-catenin pathway81.
Posttranscriptional Modulation by MicroRNAs
MicroRNAs (miRs) are short noncoding RNAs with a length of approximate 22 nucleotides and are involved in posttranscriptional regulation of gene expression, mainly leading to mRNA degradation or translation inhibition. Aberrant expression of miRs is implicated in many diseases. Most Fzds have been reported to be negatively modulated by miRs in various tumors112–119 (Table 3). These miRs act as tumor suppressors or promoters. Interestingly, miR-31 is tumor suppressing in breast cancer by targeting Fzd3, whereas it is tumor promoting in lung cancer by targeting Fzd9.
Table 3.
miRs Modulating Fzds in Tumors
| Fzds | miRs | Modulation | Tumors | Function of miRs | References |
|---|---|---|---|---|---|
| Fzd2 | miR-30a | Negative | HNSCC | Suppressor | 112 |
| Fzd3 | miR-31 | Negative | Breast cancer | Suppressor | 113 |
| Fzd4 | miR-493 | Negative | Bladder cancer | Suppressor | 114 |
| Fzd5 | miR-23a/24 | Negative | Pancreatic cancer | Suppressor | 115 |
| Fzd6 | miR-194 | Negative | HCC | Suppressor | 116 |
| Fzd7 | miR-23b | Negative | Colon cancer | Suppressor | 117 |
| Fzd8 | miR-1249 | Negative | Biliary tract cancer | Promoter | 118 |
| Fzd9 | miR-31 | Negative | Lung cancer | Promoter | 119 |
Protein Ubiquitination
E3 ubiquitin-protein ligase RNF43 ubiquitinates Fzds and exerts a negative effect on the Wnt/β-catenin pathway120. Inactivating RNF43 mutations are frequent in colorectal, endometrial, and pancreatic cancer, accompanied by increased β-catenin activity121,122. Using genome-wide CRISPR-Cas9 screening, Fzd5 was identified to mediate the activation of the β-catenin pathway and the growth of several RNF43-mutated PDAC cells74. Interestingly, Dvl is required for RNF43-mediated ubiquitination and degradation of Fzds. Canonical β-catenin pathway negatively regulates Fzds via RNF43 to ensure proper control of pathway activity122,123. Contrary to RNF43, ubiquitin-specific protease 6 (USP6) is a deubiquitylase; ectopic expression of USP6 increases cell surface abundance of Fzds and Lrp6 and activates β-catenin pathway in tumor cells124.
Fzd-TARGETED THERAPIES IN TUMORS
A number of small molecules, peptides, and blocking antibodies have been developed, targeting ligands, receptors, or key downstream molecules of the Wnt pathway125,126. This review only focused on Fzd-targeted therapies.
Fzd7
Fzd7 acts as a tumor promoter through mediating the canonical Wnt pathway, which involves Dvl. Therefore, disrupting the binding of Fzd7 to the PDZ domain of Dvl is considered a strategy to block the β-catenin pathway. Peptide and small-molecule inhibitor that impair Fzd7–Dvl binding exhibited an antitumor effect on liver and lung cancers, respectively; they were shown to suppress the growth of cultured tumor cells, tumor cell xenografts, and mouse tumor models127,128. Soluble receptors are often used to compete with membrane receptors for their ligands, leading to signaling blockade. Given the oncogenic role of Wnt3–Fzd7 in HCC, a soluble extracellular domain of Fzd7 was generated to bind HCC-derived Wnt3, accompanied by a reduction in HCC cell growth in vitro and in xenografts129. Moreover, antibodies against Fzd7 displayed an antigrowth effect on cultured Wilms’ tumor cells and TNBC cells130,131.
Fzd5/8
Antibodies against Fzd5 were shown to inhibit the growth of pancreatic cancer cells with RNF43 mutation in vitro and in xenografts, and the growth of tumor organoid cultures from colorectal cancer patients carrying RNF43 mutations74. In combination with various chemotherapy agents, soluble Fzd8 (OMP-54F28, ipafricept) exhibited an antitumor effect on patient-derived xenografts (PDXs) of pancreatic, ovarian, and breast cancers132. Consistently, patients with advanced solid tumors were not responsive to single OMP-54F28 in a first-in-human phase I study133, while patients with recurrent platinum-sensitive ovarian cancer were responsive to OMP-54F28 in combination with chemotherapy in a phase 1b study134. In the latter study, the overall response rate was 75.7%, slightly higher than historical data with chemotherapy alone on OCEANS (57%) and GOG 213 (56%); the median progression-free survival (PFS) was 10.3 months, similar to historical data on OCEANS (8.4 months) and GOG 213 (10.4 months)135,136. Actually, the occurrence of fragility fractures at doses associated with efficacy may limit further development of OMP-54F28.
Fzd1/2/7/5/8
OMP-18R5 (vantictumab) is a monoclonal antibody against the Fzd1/2/7 subfamily and Fzd5/8 subfamily, which has the capacity to block the β-catenin pathway induced by multiple Wnt molecules. OMP-18R5 was shown to suppress the development of pancreatic cancer and gastric cancer in mouse models137,138. Similar to OMP-54F28, OMP-18R5 was also shown to synergize with chemotherapy agents on several types of cancers in xenografts and PDXs132,139. A phase Ib study evaluating OMP-18R5 in combination with chemotherapy in patients with metastatic pancreatic cancer was terminated because of fragility fractures140.
Fzd1/2/7/5/8/4
By using combinatorial antibody engineering, a variant antibody of OMP-18R5 named F2.A was developed. In addition to against the Fzd1/2/7 subfamily and Fzd5/8 subfamily, F2.A also targets Fzd4. It was shown that F2.A was much more effective than OMP-18R5 in suppressing the growth of RNF43-mutant pancreatic cancer cells141.
Fzd10
Antibodies against Fzd10 displayed suppressive effects on the growth of synovial sarcoma cells in vitro and in xenografts142,143.
CONCLUSIONS AND PERSPECTIVES
Fzds mediate both canonical Wnt/β-catenin pathway and various noncanonical pathways. The Wnt/β-catenin pathway has been fully demonstrated to be oncogenic. However, in the presence of noncanonical Wnt ligands such as Wnt5a/b and Wnt11, Fzd signaling plays a dual role in tumors. Generally, noncanonical Wnt pathways promote tumor metastasis by inducing EMT, but potentially hinder tumor initiation and growth by antagonizing the β-catenin pathway. An apparent explanation for this duality is that noncanonical Wnts compete with canonical Wnts for Fzds, further preventing β-catenin translocation into the nucleus. Study on Fzd6 may provide an alternative mechanism that noncanonical Wnt pathways interfere with the binding of transcription factor TCF/LEF to promoters of target genes. Notably, Yap/Taz and TGF-β, downstream signaling of Fzds also has a dual role in human tumors, suggesting that in the absence of β-catenin activity, noncanonical Wnt pathway still can exert tumor-suppressing effects by cross-talking with this signaling.
At present, targeting Fzds has proven to be effective on cultured tumor cells, tumor cell xenografts, mouse tumor models, and PDXs. As chemotherapy increases Wnt pathway activity, which counteracts the antitumor effect, Fzd-targeted therapies can enhance the sensitivity of tumor cells to chemical agents. Actually, Fzd-targeted therapies seem to be effective upon combining with chemotherapy in preclinical models. However, the occurrence of fragility fractures in patients treated with Fzd-targeted agents such as OMP-54F28 and OMP-18R5 limits the development of this combination. Given the crucial role of the Wnt pathway in tissue homeostasis (the intestine, hematopoietic system, bone, and so on), adverse effects with regard to Fzd-targeted therapies are anticipated. Therefore, to obtain clinical efficacy and safety, aside from specific protection and prevention such as using vitamin D3 and calcium carbonate against fragility fractures, a fine balance is required between repressing tumors and maintaining homeostasis.
Because of the heterogeneity of tumors and the lack of specific biomarkers, the Wnt/Fzd expression profile should be evaluated in individual tumor. According to the expression pattern, one can more precisely determine Fzd-targeted strategies, thereby improving efficacy. The early phase compounds OMP-54F28 and OMP-18R5 block a variety of Wnts/Fzds. The excessive blocking may aggravate the side effects, especially in patients with tumors overexpressing fewer Fzds. Therefore, therapies targeting single Fzd or Fzd subfamily should also be developed.
As noncanonical Fzd signaling has antitumor activity, targeting Fzds should be cautious and tumor and patient specific. Recently, some novel downstream pathways and modification mechanisms of Fzds have been discovered. New insights on signaling, modulation mechanisms, and roles of Fzds in human tumors undoubtedly will contribute to identifying more Fzd-related therapeutic targets.
ACKNOWLEDGMENTS
This work was supported by grants from the Department of Science and Technology of Liaoning Province (2019-MS-363 and 2017225028).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Schenkelaars Q, Fierro-Constain L, Renard E, Hill AL, Borchiellini C. Insights into Frizzled evolution and new perspectives. Evol Dev. 2015;17(2):160–9. [DOI] [PubMed] [Google Scholar]
- 2. Voloshanenko O, Gmach P, Winter J, Kranz D, Boutros M. Mapping of Wnt–Frizzled interactions by multiplex CRISPR targeting of receptor gene families. FASEB J. 2017;31(11):4832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4(12):a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeBruine ZJ, Xu HE, Melcher K. Assembly and architecture of the Wnt/beta-catenin signalosome at the membrane. Br J Pharmacol. 2017;174(24):4564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hua Y, Yang Y, Li Q, He X, Zhu W, Wang J, Gan X. Oligomerization of Frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/beta-catenin pathway. J Biol Chem. 2018;293(51):19710–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu G, Bafico A, Aaronson SA. The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts. Mol Cell Biol. 2005;25(9):3475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bryja V, Andersson ER, Schambony A, Esner M, Bryjova L, Biris KK, Hall AC, Kraft B, Cajanek L, Yamaguchi TP, Buckingham M, Arenas E. The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo. Mol Biol Cell 2009;20(3):924–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24(22):2517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeBruine ZJ, Ke J, Harikumar KG, Gu X, Borowsky P, Williams BO, Xu W, Miller LJ, Xu HE, Melcher K. Wnt5a promotes Frizzled-4 signalosome assembly by stabilizing cysteine-rich domain dimerization. Genes Dev. 2017;31(9):916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AM. Wnt proteins induce Dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol Cell Biol. 2004;24(11):4757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010;29(1):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishita M, Itsukushima S, Nomachi A, Endo M, Wang Z, Inaba D, Qiao S, Takada S, Kikuchi A, Minami Y. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol. 2010;30(14):3610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, Kuruvilla R, Greenberg ME. Wnt5a–Ror–Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci USA 2012;109(11):4044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yin P, Wang W, Zhang Z, Bai Y, Gao J, Zhao C. Wnt signaling in human and mouse breast cancer: Focusing on Wnt ligands, receptors and antagonists. Cancer Sci. 2018;109(11):3368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM, Varmus HE. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA 2003;100(26):15853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487(7407):330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen VHL, Hough R, Bernaudo S, Peng C. Wnt/beta-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J Ovarian Res. 2019;12(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dittmer J. Breast cancer stem cells: Features, key drivers and treatment options. Semin Cancer Biol. 2018;53:59–74. [DOI] [PubMed] [Google Scholar]
- 19. Gajos-Michniewicz A, Czyz M. WNT signaling in melanoma. Int J Mol Sci. 2020;21(14):4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 1997;390(6658):410–3. [DOI] [PubMed] [Google Scholar]
- 21. Kilander MB, Petersen J, Andressen KW, Ganji RS, Levy FO, Schuster J, Dahl N, Bryja V, Schulte G. Disheveled regulates precoupling of heterotrimeric G proteins to Frizzled 6. FASEB J. 2014;28(5):2293–305. [DOI] [PubMed] [Google Scholar]
- 22. Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL. Alternative Wnt signaling activates YAP/TAZ. Cell 2015;162(4):780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramirez VT, Ramos-Fernandez E, Henriquez JP, Lorenzo A, Inestrosa NC. Wnt-5a/Frizzled9 receptor signaling through the Galphao–Gbetagamma complex regulates dendritic spine formation. J Biol Chem. 2016;291(36):19092–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright SC, Canizal MCA, Benkel T, Simon K, Le Gouill C, Matricon P, Namkung Y, Lukasheva V, Konig GM, Laporte SA, Carlsson J, Kostenis E, Bouvier M, Schulte G, Hoffmann C. FZD5 is a Galphaq-coupled receptor that exhibits the functional hallmarks of prototypical GPCRs. Sci Signal 2018;11(559):eaar5536. [DOI] [PubMed] [Google Scholar]
- 25. Bowin CF, Inoue A, Schulte G. WNT-3A-induced beta-catenin signaling does not require signaling through heterotrimeric G proteins. J Biol Chem. 2019;294(31):11677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang S, Wu Y, Xu TH, de Waal PW, He Y, Pu M, Chen Y, DeBruine ZJ, Zhang B, Zaidi SA, Popov P, Guo Y, Han GW, Lu Y, Suino-Powell K, Dong S, Harikumar KG, Miller LJ, Katritch V, Xu HE, Shui W, Stevens RC, Melcher K, Zhao S, Xu F. Crystal structure of the Frizzled 4 receptor in a ligand-free state. Nature 2018;560(7720):666–70. [DOI] [PubMed] [Google Scholar]
- 27. Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002;1(3):279–88. [DOI] [PubMed] [Google Scholar]
- 28. Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE, Jiang Y, Nickoloff BJ, Taub DD, Trent JM, Moon RT, Bittner M, Weeraratna AT. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282(23):17259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohapatra P, Yadav V, Toftdahl M, Andersson T. WNT5A-induced activation of the protein kinase C substrate MARCKS is required for melanoma cell invasion. Cancers (Basel) 2020;12(2):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma X, Meng Z, Jin L, Xiao Z, Wang X, Tsark WM, Ding L, Gu Y, Zhang J, Kim B, He M, Gan X, Shively JE, Yu H, Xu R, Huang W. CAMK2gamma in intestinal epithelial cells modulates colitis-associated colorectal carcinogenesis via enhancing STAT3 activation. Oncogene 2017;36(28):4060–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 2012;149(5):1084–97. [DOI] [PubMed] [Google Scholar]
- 32. Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Katoh M. WNT/PCP signaling pathway and human cancer (review). Oncol Rep. 2005;14(6):1583–8. [PubMed] [Google Scholar]
- 34. Janovska P, Poppova L, Plevova K, Plesingerova H, Behal M, Kaucka M, Ovesna P, Hlozkova M, Borsky M, Stehlikova O, Brychtova Y, Doubek M, Machalova M, Baskar S, Kozubik A, Pospisilova S, Pavlova S, Bryja V. Autocrine signaling by Wnt-5a deregulates chemotaxis of leukemic cells and predicts clinical outcome in chronic lymphocytic leukemia. Clin Cancer Res. 2016;22(2):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaucka M, Plevova K, Pavlova S, Janovska P, Mishra A, Verner J, Prochazkova J, Krejci P, Kotaskova J, Ovesna P, Tichy B, Brychtova Y, Doubek M, Kozubik A, Mayer J, Pospisilova S, Bryja V. The planar cell polarity pathway drives pathogenesis of chronic lymphocytic leukemia by the regulation of B-lymphocyte migration. Cancer Res. 2013;73(5):1491–501. [DOI] [PubMed] [Google Scholar]
- 36. Kotrbova A, Ovesna P, Gybel T, Radaszkiewicz T, Bednarikova M, Hausnerova J, Jandakova E, Minar L, Crha I, Weinberger V, Zavesky L, Bryja V, Pospichalova V. WNT signaling inducing activity in ascites predicts poor outcome in ovarian cancer. Theranostics 2020;10(2):537–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asad M, Wong MK, Tan TZ, Choolani M, Low J, Mori S, Virshup D, Thiery JP, Huang RY. FZD7 drives in vitro aggressiveness in Stem-A subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Cell Death Dis. 2014;5(7):e1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial–mesenchymal transition and metastasis. Cell 2014;159(4):844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Y, Chen L, Yu J, Ghia EM, Choi MY, Zhang L, Zhang S, Sanchez-Lopez E, Widhopf GF, 2nd, Messer K, Rassenti LZ, Jamieson C, Kipps TJ. Cirmtuzumab blocks Wnt5a/ROR1 stimulation of NF-kappaB to repress autocrine STAT3 activation in chronic lymphocytic leukemia. Blood 2019;134(13):1084–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yin P, Wang W, Gao J, Bai Y, Wang Z, Na L, Sun Y, Zhao C. Fzd2 contributes to breast cancer cell mesenchymal-like stemness and drug resistance. Oncol Res. 2020;28(3):273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dissanayake SK, Olkhanud PB, O’Connell MP, Carter A, French AD, Camilli TC, Emeche CD, Hewitt KJ, Rosenthal DT, Leotlela PD, Wade MS, Yang SW, Brant L, Nickoloff BJ, Messina JL, Biragyn A, Hoek KS, Taub DD, Longo DL, Sondak VK, Hewitt SM, Weeraratna AT. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68(24):10205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ekstrom EJ, Bergenfelz C, von Bulow V, Serifler F, Carlemalm E, Jonsson G, Andersson T, Leandersson K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol Cancer 2014;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Linnskog R, Jonsson G, Axelsson L, Prasad CP, Andersson T. Interleukin-6 drives melanoma cell motility through p38alpha-MAPK-dependent up-regulation of WNT5A expression. Mol Oncol. 2014;8(8):1365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rozovski U, Harris DM, Li P, Liu Z, Jain P, Ferrajoli A, Burger JA, Bose P, Thompson PA, Jain N, Wierda WG, Uziel O, Keating MJ, Estrov Z. STAT3-induced Wnt5a provides chronic lymphocytic leukemia cells with survival advantage. J Immunol. 2019;203(11):3078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ou H, Chen Z, Xiang L, Fang Y, Xu Y, Liu Q, Hu Z, Li X, Huang Y, Yang D. Frizzled 2-induced epithelial–mesenchymal transition correlates with vasculogenic mimicry, stemness, and Hippo signaling in hepatocellular carcinoma. Cancer Sci. 2019;110(4):1169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luo C, Balsa E, Perry EA, Liang J, Tavares CD, Vazquez F, Widlund HR, Puigserver P. H3K27me3-mediated PGC1alpha gene silencing promotes melanoma invasion through WNT5A and YAP. J Clin Invest. 2020;130(2):853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tu B, Yao J, Ferri-Borgogno S, Zhao J, Chen S, Wang Q, Yan L, Zhou X, Zhu C, Bang S, Chang Q, Bristow CA, Kang Y, Zheng H, Wang H, Fleming JB, Kim M, Heffernan TP, Draetta GF, Pan D, Maitra A, Yao W, Gupta S, Ying H. YAP1 oncogene is a context-specific driver for pancreatic ductal adenocarcinoma. JCI Insight 2019;4(21):e130811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roarty K, Baxley SE, Crowley MR, Frost AR, Serra R. Loss of TGF-beta or Wnt5a results in an increase in Wnt/beta-catenin activity and redirects mammary tumour phenotype. Breast Cancer Res. 2009;11(2):R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Borcherding N, Kusner D, Kolb R, Xie Q, Li W, Yuan F, Velez G, Askeland R, Weigel RJ, Zhang W. Paracrine WNT5A signaling inhibits expansion of tumor-initiating cells. Cancer Res. 2015;75(10):1972–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murillo-Garzon V, Gorrono-Etxebarria I, Akerfelt M, Puustinen MC, Sistonen L, Nees M, Carton J, Waxman J, Kypta RM. Frizzled-8 integrates Wnt-11 and transforming growth factor-beta signaling in prostate cancer. Nat Commun. 2018;9(1):1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Corbett L, Mann J, Mann DA. Non-canonical Wnt predominates in activated rat hepatic stellate cells, influencing HSC survival and paracrine stimulation of Kupffer cells. PLoS One 2015;10(11):e0142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Newman DR, Sills WS, Hanrahan K, Ziegler A, Tidd KM, Cook E, Sannes PL. Expression of WNT5A in idiopathic pulmonary fibrosis and its control by TGF-beta and WNT7B in human lung fibroblasts. J Histochem Cytochem. 2016;64(2):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Feng Y, Liang Y, Zhu X, Wang M, Gui Y, Lu Q, Gu M, Xue X, Sun X, He W, Yang J, Johnson RL, Dai C. The signaling protein Wnt5a promotes TGFbeta1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J Biol Chem. 2018;293(50):19290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guan S, Zhou J. Frizzled-7 mediates TGF-beta-induced pulmonary fibrosis by transmitting non-canonical Wnt signaling. Exp Cell Res. 2017;359(1):226–34. [DOI] [PubMed] [Google Scholar]
- 55. Beljaars L, Daliri S, Dijkhuizen C, Poelstra K, Gosens R. WNT-5A regulates TGF-beta-related activities in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G219–G27. [DOI] [PubMed] [Google Scholar]
- 56. Flahaut M, Meier R, Coulon A, Nardou KA, Niggli FK, Martinet D, Beckmann JS, Joseph JM, Muhlethaler-Mottet A, Gross N. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta-catenin pathway. Oncogene 2009;28(23):2245–56. [DOI] [PubMed] [Google Scholar]
- 57. Zhang H, Zhang X, Wu X, Li W, Su P, Cheng H, Xiang L, Gao P, Zhou G. Interference of Frizzled 1 (FZD1) reverses multidrug resistance in breast cancer cells through the Wnt/beta-catenin pathway. Cancer Lett. 2012;323(1):106–13. [DOI] [PubMed] [Google Scholar]
- 58. Wang YH, Imai Y, Shiseki M, Tanaka J, Motoji T. Knockdown of the Wnt receptor Frizzled-1 (FZD1) reduces MDR1/P-glycoprotein expression in multidrug resistant leukemic cells and inhibits leukemic cell proliferation. Leuk Res. 2018;67:99–108. [DOI] [PubMed] [Google Scholar]
- 59. Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology 2004;127(4):1110–22. [DOI] [PubMed] [Google Scholar]
- 60. Merle P, Kim M, Herrmann M, Gupte A, Lefrancois L, Califano S, Trepo C, Tanaka S, Vitvitski L, de la Monte S, Wands JR. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43(5):854–62. [DOI] [PubMed] [Google Scholar]
- 61. Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48(5):780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, Somlo G, Yen Y. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 2011;30(43):4437–46. [DOI] [PubMed] [Google Scholar]
- 63. Fernandez A, Huggins IJ, Perna L, Brafman D, Lu D, Yao S, Gaasterland T, Carson DA, Willert K. The WNT receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proc Natl Acad Sci USA 2014;111(4):1409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Flanagan DJ, Phesse TJ, Barker N, Schwab RH, Amin N, Malaterre J, Stange DE, Nowell CJ, Currie SA, Saw JT, Beuchert E, Ramsay RG, Sansom OJ, Ernst M, Clevers H, Vincan E. Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5(+) stem cells. Stem Cell Rep. 2015;4(5):759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chakrabarti R, Wei Y, Hwang J, Hang X, Andres Blanco M, Choudhury A, Tiede B, Romano RA, DeCoste C, Mercatali L, Ibrahim T, Amadori D, Kannan N, Eaves CJ, Sinha S, Kang Y. DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat Cell Biol. 2014;16(10):1004–15, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dong B, Vold S, Olvera-Jaramillo C, Chang H. Functional redundancy of frizzled 3 and frizzled 6 in planar cell polarity control of mouse hair follicles. Development 2018;145(19):dev168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ghimire SR, Deans MR. Frizzled3 and Frizzled6 cooperate with Vangl2 to direct cochlear innervation by type II spiral ganglion neurons. J Neurosci. 2019;39(41):8013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reins J, Mossner M, Neumann M, Platzbecker U, Schumann C, Thiel E, Hofmann WK. Transcriptional down-regulation of the Wnt antagonist SFRP1 in haematopoietic cells of patients with different risk types of MDS. Leuk Res. 2010;34(12):1610–6. [DOI] [PubMed] [Google Scholar]
- 69. Li C, Nguyen V, Clark KN, Zahed T, Sharkas S, Filipp FV, Boiko AD. Down-regulation of FZD3 receptor suppresses growth and metastasis of human melanoma independently of canonical WNT signaling. Proc Natl Acad Sci USA 2019;116(10):4548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cantilena S, Pastorino F, Pezzolo A, Chayka O, Pistoia V, Ponzoni M, Sala A. Frizzled receptor 6 marks rare, highly tumourigenic stem-like cells in mouse and human neuroblastomas. Oncotarget 2011;2(12):976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Corda G, Sala G, Lattanzio R, Iezzi M, Sallese M, Fragassi G, Lamolinara A, Mirza H, Barcaroli D, Ermler S, Silva E, Yasaei H, Newbold RF, Vagnarelli P, Mottolese M, Natali PG, Perracchio L, Quist J, Grigoriadis A, Marra P, Tutt AN, Piantelli M, Iacobelli S, De Laurenzi V, Sala A. Functional and prognostic significance of the genomic amplification of frizzled 6 (FZD6) in breast cancer. J Pathol. 2017;241(3):350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6(6):1017–28. [DOI] [PubMed] [Google Scholar]
- 73. Yoshioka S, King ML, Ran S, Okuda H, MacLean JA 2nd, McAsey ME, Sugino N, Brard L, Watabe K, Hayashi K. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/beta-catenin pathway. Mol Cancer Res. 2012;10(3):469–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Steinhart Z, Pavlovic Z, Chandrashekhar M, Hart T, Wang X, Zhang X, Robitaille M, Brown KR, Jaksani S, Overmeer R, Boj SF, Adams J, Pan J, Clevers H, Sidhu S, Moffat J, Angers S. Genome-wide CRISPR screens reveal a Wnt–FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nat Med. 2017;23(1):60–8. [DOI] [PubMed] [Google Scholar]
- 75. Linke F, Zaunig S, Nietert MM, von Bonin F, Lutz S, Dullin C, Janovska P, Beissbarth T, Alves F, Klapper W, Bryja V, Pukrop T, Trumper L, Wilting J, Kube D. WNT5A: A motility-promoting factor in Hodgkin lymphoma. Oncogene 2017;36(1):13–23. [DOI] [PubMed] [Google Scholar]
- 76. Sun S, Liu S, Duan SZ, Zhang L, Zhou H, Hu Y, Zhou X, Shi C, Zhou R, Zhang Z. Targeting the c-Met/FZD8 signaling axis eliminates patient-derived cancer stem-like cells in head and neck squamous carcinomas. Cancer Res. 2014;74(24):7546–59. [DOI] [PubMed] [Google Scholar]
- 77. Yin S, Xu L, Bonfil RD, Banerjee S, Sarkar FH, Sethi S, Reddy KB. Tumor-initiating cells and FZD8 play a major role in drug resistance in triple-negative breast cancer. Mol Cancer Ther. 2013;12(4):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jin X, Jeon HY, Joo KM, Kim JK, Jin J, Kim SH, Kang BG, Beck S, Lee SJ, Kim JK, Park AK, Park WY, Choi YJ, Nam DH, Kim H. Frizzled 4 regulates stemness and invasiveness of migrating glioma cells established by serial intracranial transplantation. Cancer Res. 2011;71(8):3066–75. [DOI] [PubMed] [Google Scholar]
- 79. Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, Rantala J, Alanen K, Nees M, Kallioniemi O. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70(17):6735–45. [DOI] [PubMed] [Google Scholar]
- 80. Gong C, Qu S, Lv XB, Liu B, Tan W, Nie Y, Su F, Liu Q, Yao H, Song E. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat Commun. 2014;5:5406. [DOI] [PubMed] [Google Scholar]
- 81. Fukumoto T, Zhu H, Nacarelli T, Karakashev S, Fatkhutdinov N, Wu S, Liu P, Kossenkov AV, Showe LC, Jean S, Zhang L, Zhang R. N(6)-methylation of adenosine of FZD10 mRNA contributes to PARP inhibitor resistance. Cancer Res. 2019;79(11):2812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tomar T, Alkema NG, Schreuder L, Meersma GJ, de Meyer T, van Criekinge W, Klip HG, Fiegl H, van Nieuwenhuysen E, Vergote I, Widschwendter M, Schuuring E, van der Zee AGJ, de Jong S, Wisman GBA. Methylome analysis of extreme chemoresponsive patients identifies novel markers of platinum sensitivity in high-grade serous ovarian cancer. BMC Med. 2017;15(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ulivieri A, Lavra L, Dominici R, Giacomelli L, Brunetti E, Sciacca L, Trovato M, Barresi G, Foukakis T, Jia-Jing L, Larsson C, Bartolazzi A, Sciacchitano S. Frizzled-1 is down-regulated in follicular thyroid tumours and modulates growth and invasiveness. J Pathol. 2008;215(1):87–96. [DOI] [PubMed] [Google Scholar]
- 84. Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 2005;24(13):2144–54. [DOI] [PubMed] [Google Scholar]
- 85. Yang J, Cusimano A, Monga JK, Preziosi ME, Pullara F, Calero G, Lang R, Yamaguchi TP, Nejak-Bowen KN, Monga SP. WNT5A inhibits hepatocyte proliferation and concludes beta-catenin signaling in liver regeneration. Am J Pathol. 2015;185(8):2194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yuzugullu H, Benhaj K, Ozturk N, Senturk S, Celik E, Toylu A, Tasdemir N, Yilmaz M, Erdal E, Akcali KC, Atabey N, Ozturk M. Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer 2009;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yan J, Liu T, Zhou X, Dang Y, Yin C, Zhang G. FZD6, targeted by miR-21, represses gastric cancer cell proliferation and migration via activating non-canonical wnt pathway. Am J Transl Res. 2016;8(5):2354–64. [PMC free article] [PubMed] [Google Scholar]
- 88. Han K, Lang T, Zhang Z, Zhang Y, Sun Y, Shen Z, Beuerman RW, Zhou L, Min D. Luteolin attenuates Wnt signaling via upregulation of FZD6 to suppress prostate cancer stemness revealed by comparative proteomics. Sci Rep. 2018;8(1):8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Golan T, Yaniv A, Bafico A, Liu G, Gazit A. The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt beta-catenin signaling cascade. J Biol Chem. 2004;279(15):14879–88. [DOI] [PubMed] [Google Scholar]
- 90. Thiele S, Gobel A, Rachner TD, Fuessel S, Froehner M, Muders MH, Baretton GB, Bernhardt R, Jakob F, Gluer CC, Bornhauser M, Rauner M, Hofbauer LC. WNT5A has anti-prostate cancer effects in vitro and reduces tumor growth in the skeleton in vivo. J Bone Miner Res. 2015;30(3):471–80. [DOI] [PubMed] [Google Scholar]
- 91. Thiele S, Zimmer A, Gobel A, Rachner TD, Rother S, Fuessel S, Froehner M, Wirth MP, Muders MH, Baretton GB, Jakob F, Rauner M, Hofbauer LC. Role of WNT5A receptors FZD5 and RYK in prostate cancer cells. Oncotarget 2018;9(43):27293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang MT, Holderfield M, Galeas J, Delrosario R, To MD, Balmain A, McCormick F. K-Ras promotes tumorigenicity through suppression of non-canonical Wnt signaling. Cell 2015;163(5):1237–51. [DOI] [PubMed] [Google Scholar]
- 93. Ohka F, Shinjo K, Deguchi S, Matsui Y, Okuno Y, Katsushima K, Suzuki M, Kato A, Ogiso N, Yamamichi A, Aoki K, Suzuki H, Sato S, Arul Rayan N, Prabhakar S, Goke J, Shimamura T, Maruyama R, Takahashi S, Suzumura A, Kimura H, Wakabayashi T, Zong H, Natsume A, Kondo Y. Pathogenic epigenetic consequences of genetic alterations in IDH-wild-type diffuse astrocytic gliomas. Cancer Res. 2019;79(19):4814–27. [DOI] [PubMed] [Google Scholar]
- 94. Sonnet M, Claus R, Becker N, Zucknick M, Petersen J, Lipka DB, Oakes CC, Andrulis M, Lier A, Milsom MD, Witte T, Gu L, Kim-Wanner SZ, Schirmacher P, Wulfert M, Gattermann N, Lubbert M, Rosenbauer F, Rehli M, Bullinger L, Weichenhan D, Plass C. Early aberrant DNA methylation events in a mouse model of acute myeloid leukemia. Genome Med. 2014;6(4):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu Y, Qiao Y, Zhang H, Li W, Zheng J. Wnt7a, frequently silenced by CpG methylation, inhibits tumor growth and metastasis via suppressing epithelial–mesenchymal transition in gastric cancer. J Cell Biochem. 2019;120(10):18142–51. [DOI] [PubMed] [Google Scholar]
- 96. Lan L, Wang W, Huang Y, Zhao C, Bu X. WNT7A overexpression inhibits growth and migration of hepatocellular carcinoma via the beta-catenin independent pathway. Biomed Res Int. 2019;2019:3605950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, Zeng C, Baron A, Bemis L, Erickson P, Wilder E, Rustgi A, Kitajewski J, Gabrielson E, Bremnes R, Franklin W, Drabkin HA. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci USA 2003;100(18):10429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tennis MA, Van Scoyk MM, Freeman SV, Vandervest KM, Nemenoff RA, Winn RA. Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial–mesenchymal transition, and is regulated by Wnt7A through PPARgamma in non-small cell lung cancer. Mol Cancer Res. 2010;8(6):833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, Bren-Mattison Y, Van Raay TJ, Nemenoff RA, Heasley LE. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280(20):19625–34. [DOI] [PubMed] [Google Scholar]
- 100. Winn RA, Van Scoyk M, Hammond M, Rodriguez K, Crossno JT Jr., Heasley LE, Nemenoff RA. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281(37):26943–50. [DOI] [PubMed] [Google Scholar]
- 101. Jiang Y, Dunbar A, Gondek LP, Mohan S, Rataul M, O’Keefe C, Sekeres M, Saunthararajah Y, Maciejewski JP. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood 2009;113(6):1315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Benevolenskaya EV, Islam AB, Ahsan H, Kibriya MG, Jasmine F, Wolff B, Al-Alem U, Wiley E, Kajdacsy-Balla A, Macias V, Rauscher GH. DNA methylation and hormone receptor status in breast cancer. Clin Epigenetics 2016;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Martinez R, Martin-Subero JI, Rohde V, Kirsch M, Alaminos M, Fernandez AF, Ropero S, Schackert G, Esteller M. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics 2009;4(4):255–64. [DOI] [PubMed] [Google Scholar]
- 104. Saal HM, Prows CA, Guerreiro I, Donlin M, Knudson L, Sund KL, Chang CF, Brugmann SA, Stottmann RW. A mutation in FRIZZLED2 impairs Wnt signaling and causes autosomal dominant omodysplasia. Hum Mol Genet. 2015;24(12):3399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. White JJ, Mazzeu JF, Coban-Akdemir Z, Bayram Y, Bahrambeigi V, Hoischen A, van Bon BWM, Gezdirici A, Gulec EY, Ramond F, Touraine R, Thevenon J, Shinawi M, Beaver E, Heeley J, Hoover-Fong J, Durmaz CD, Karabulut HG, Marzioglu-Ozdemir E, Cayir A, Duz MB, Seven M, Price S, Ferreira BM, Vianna-Morgante AM, Ellard S, Parrish A, Stals K, Flores-Daboub J, Jhangiani SN, Gibbs RA, Baylor-Hopkins Center for Mendelian G, Brunner HG, Sutton VR, Lupski JR, Carvalho CMB. WNT signaling perturbations underlie the genetic heterogeneity of robinow syndrome. Am J Hum Genet. 2018;102(1):27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, Siebert LF, Hoskin-Mott A, Trese MT, Pimstone SN, Shastry BS, Moon RT, Hayden MR, Goldberg YP, Samuels ME. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32(2):326–30. [DOI] [PubMed] [Google Scholar]
- 107. Bethge N, Honne H, Hilden V, Troen G, Eknaes M, Liestol K, Holte H, Delabie J, Smeland EB, Lind GE. Identification of highly methylated genes across various types of B-cell non-hodgkin lymphoma. PLoS One 2013;8(11):e79602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dai W, Teodoridis JM, Zeller C, Graham J, Hersey J, Flanagan JM, Stronach E, Millan DW, Siddiqui N, Paul J, Brown R. Systematic CpG islands methylation profiling of genes in the wnt pathway in epithelial ovarian cancer identifies biomarkers of progression-free survival. Clin Cancer Res. 2011;17(12):4052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li ZT, Zhang X, Wang DW, Xu J, Kou KJ, Wang ZW, Yong G, Liang DS, Sun XY. Overexpressed lncRNA GATA6–AS1 Inhibits LNM and EMT via FZD4 through the Wnt/beta-catenin signaling pathway in GC. Mol Ther Nucleic Acids 2020;19827–40. [DOI] [PMC free article] [PubMed] [Retracted]
- 110. Lin Y, Dong C, Zhou BP. Epigenetic regulation of EMT: The Snail story. Curr Pharm Des. 2014;20(11):1698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res. 2011;17(9):2613–8. [DOI] [PubMed] [Google Scholar]
- 112. Saleh AD, Cheng H, Martin SE, Si H, Ormanoglu P, Carlson S, Clavijo PE, Yang X, Das R, Cornelius S, Couper J, Chepeha D, Danilova L, Harris TM, Prystowsky MB, Childs GJ, Smith RV, Robertson AG, Jones SJM, Cherniack AD, Kim SS, Rait A, Pirollo KF, Chang EH, Chen Z, Van Waes C. Integrated genomic and functional microRNA analysis identifies miR-30-5p as a tumor suppressor and potential therapeutic nanomedicine in head and neck cancer. Clin Cancer Res. 2019;25(9):2860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 2009;137(6):1032–46. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114. Ueno K, Hirata H, Majid S, Yamamura S, Shahryari V, Tabatabai ZL, Hinoda Y, Dahiya R. Tumor suppressor microRNA-493 decreases cell motility and migration ability in human bladder cancer cells by downregulating RhoC and FZD4. Mol Cancer Ther. 2012;11(1):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Listing H, Mardin WA, Wohlfromm S, Mees ST, Haier J. MiR-23a/-24-induced gene silencing results in mesothelial cell integration of pancreatic cancer. Br J Cancer 2015;112(1):131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Krutzfeldt J, Rosch N, Hausser J, Manoharan M, Zavolan M, Stoffel M. MicroRNA-194 is a target of transcription factor 1 (Tcf1, HNF1alpha) in adult liver and controls expression of frizzled-6. Hepatology 2012;55(1):98–107. [DOI] [PubMed] [Google Scholar]
- 117. Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin S, Sun C, Ma M, Huang Y, Xi JJ. Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat Commun. 2011;2:554. [DOI] [PubMed] [Google Scholar]
- 118. Carotenuto P, Hedayat S, Fassan M, Cardinale V, Lampis A, Guzzardo V, Vicentini C, Scarpa A, Cascione L, Costantini D, Carpino G, Alvaro D, Ghidini M, Trevisani F, Te Poele R, Salati M, Ventura S, Vlachogiannis G, Hahne JC, Boulter L, Forbes SJ, Guest R, Cillo U, Said-Huntingford I, Begum R, Smyth E, Michalarea V, Cunningham D, Rimassa L, Santoro A, Roncalli M, Kirnkin V, Clarke P, Workman P, Valeri N, Braconi C. Modulation of biliary cancer chemo-resistance through microRNA-mediated rewiring of the expansion of CD133+ cells. Hepatology 2019;72(3):982–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tennis MA, New ML, McArthur DG, Merrick DT, Dwyer-Nield LD, Keith RL. Prostacyclin reverses the cigarette smoke-induced decrease in pulmonary Frizzled 9 expression through miR-31. Sci Rep. 2016;6:28519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012;488(7413):665–9. [DOI] [PubMed] [Google Scholar]
- 121. Giannakis M, Hodis E, Jasmine Mu X, Yamauchi M, Rosenbluh J, Cibulskis K, Saksena G, Lawrence MS, Qian ZR, Nishihara R, Van Allen EM, Hahn WC, Gabriel SB, Lander ES, Getz G, Ogino S, Fuchs CS, Garraway LA. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet. 2014;46(12):1264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R, Smith TR, Avello M, Charlat O, Xie Y, Porter JA, Pan S, Liu J, McLaughlin ME, Cong F. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci USA 2013;110(31):12649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jiang X, Charlat O, Zamponi R, Yang Y, Cong F. Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Mol Cell 2015;58(3):522–33. [DOI] [PubMed] [Google Scholar]
- 124. Madan B, Walker MP, Young R, Quick L, Orgel KA, Ryan M, Gupta P, Henrich IC, Ferrer M, Marine S, Roberts BS, Arthur WT, Berndt JD, Oliveira AM, Moon RT, Virshup DM, Chou MM, Major MB. USP6 oncogene promotes Wnt signaling by deubiquitylating Frizzleds. Proc Natl Acad Sci USA 2016;113(21):E2945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;6250–60. [DOI] [PMC free article] [PubMed]
- 126. Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013;13(1):11–26. [DOI] [PubMed] [Google Scholar]
- 127. Nambotin SB, Lefrancois L, Sainsily X, Berthillon P, Kim M, Wands JR, Chevallier M, Jalinot P, Scoazec JY, Trepo C, Zoulim F, Merle P. Pharmacological inhibition of Frizzled-7 displays anti-tumor properties in hepatocellular carcinoma. J Hepatol. 2011;54(2):288–99. [DOI] [PubMed] [Google Scholar]
- 128. Fujii N, You L, Xu Z, Uematsu K, Shan J, He B, Mikami I, Edmondson LR, Neale G, Zheng J, Guy RK, Jablons DM. An antagonist of Dishevelled protein–protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res. 2007;67(2):573–9. [DOI] [PubMed] [Google Scholar]
- 129. Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer 2011;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pode-Shakked N, Harari-Steinberg O, Haberman-Ziv Y, Rom-Gross E, Bahar S, Omer D, Metsuyanim S, Buzhor E, Jacob-Hirsch J, Goldstein RS, Mark-Danieli M, Dekel B. Resistance or sensitivity of Wilms’ tumor to anti-FZD7 antibody highlights the Wnt pathway as a possible therapeutic target. Oncogene 2011;30(14):1664–80. [DOI] [PubMed] [Google Scholar]
- 131. Zarei N, Fazeli M, Mohammadi M, Nejatollahi F. Cell growth inhibition and apoptosis in breast cancer cells induced by anti-FZD7 scFvs: Involvement of bioinformatics-based design of novel epitopes. Breast Cancer Res Treat. 2018;169(3):427–36. [DOI] [PubMed] [Google Scholar]
- 132. Fischer MM, Cancilla B, Yeung VP, Cattaruzza F, Chartier C, Murriel CL, Cain J, Tam R, Cheng CY, Evans JW, O’Young G, Song X, Lewicki J, Kapoun AM, Gurney A, Yen WC, Hoey T. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci Adv. 2017;3(6):e1700090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, Stagg R, Kapoun AM, Xu L, Uttamsingh S, Brachmann RK, Smith DC. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. 2017;23(24):7490–7. [DOI] [PubMed] [Google Scholar]
- 134. Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA, Morgan MA, Kapoun AM, Brachmann RK, Stagg R, Farooki A, O’Cearbhaill RE. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;154(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, Kim BG, Fujiwara K, Tewari KS, O’Malley DM, Davidson SA, Rubin SC, DiSilvestro P, Basen-Engquist K, Huang H, Chan JK, Spirtos NM, Ashfaq R, Mannel RS. Bevacizumab and paclitaxel–carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(6):779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J, Nycum LR. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang Y, Morris JPt, Yan W, Schofield HK, Gurney A, Simeone DM, Millar SE, Hoey T, Hebrok M, Pasca di Magliano M. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res. 2013;73(15):4909–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Flanagan DJ, Barker N, Costanzo NSD, Mason EA, Gurney A, Meniel VS, Koushyar S, Austin CR, Ernst M, Pearson HB, Boussioutas A, Clevers H, Phesse TJ, Vincan E. Frizzled-7 is required for Wnt signaling in gastric tumors with and without Apc mutations. Cancer Res. 2019;79(5):970–81. [DOI] [PubMed] [Google Scholar]
- 139. Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA 2012;109(29):11717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Davis SL, Cardin DB, Shahda S, Lenz HJ, Dotan E, O’Neil BH, Kapoun AM, Stagg RJ, Berlin J, Messersmith WA, Cohen SJ. A phase 1b dose escalation study of Wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Invest New Drugs 2020;38(3):821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pavlovic Z, Adams JJ, Blazer LL, Gakhal AK, Jarvik N, Steinhart Z, Robitaille M, Mascall K, Pan J, Angers S, Moffat J, Sidhu SS. A synthetic anti-Frizzled antibody engineered for broadened specificity exhibits enhanced anti-tumor properties. MAbs 2018;10(8):1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nagayama S, Fukukawa C, Katagiri T, Okamoto T, Aoyama T, Oyaizu N, Imamura M, Toguchida J, Nakamura Y. Therapeutic potential of antibodies against FZD 10, a cell-surface protein, for synovial sarcomas. Oncogene 2005;24(41):6201–12. [DOI] [PubMed] [Google Scholar]
- 143. Fukukawa C, Hanaoka H, Nagayama S, Tsunoda T, Toguchida J, Endo K, Nakamura Y, Katagiri T. Radioimmunotherapy of human synovial sarcoma using a monoclonal antibody against FZD10. Cancer Sci. 2008;99(2):432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]