Abstract
Purpose of Review:
The reacquisition and preservation of walking ability are highly valued goals in spinal cord injury (SCI) rehabilitation. Recurrent episodes of breathing low oxygen (i.e., acute intermittent hypoxia, AIH) is a potential therapy to promote walking recovery after incomplete SCI via endogenous mechanisms of neuroplasticity. Here, we report on the progress of AIH, alone or paired with other treatments, on walking recovery in persons with incomplete SCI. We evaluate the evidence of AIH as a therapy ready for clinical and home use and the real and perceived challenges that may interfere with this possibility.
Recent Findings:
Repetitive AIH is a safe and an efficacious treatment to enhance strength, walking speed and endurance, as well as, dynamic balance in persons with chronic, incomplete SCI.
Summary:
The potential for AIH as a treatment for SCI remains high, but further research is necessary to understand treatment targets and effectiveness in a large cohort of persons with SCI.
Keywords: low oxygen, acute intermittent hypoxia, walking, plasticity, rehabilitation, spinal cord injury
INTRODUCTION
Walking recovery after SCI: a priority
Persons living with spinal cord injury (SCI) must overcome a broad range of functional barriers that dramatically reduce their quality of life (QOL). Among the various life quality domains that SCI impacts, recovery of overground walking remains a major goal irrespective of injury severity [1–3]. Recovery and maintenance of walking strongly align with long-term recovery goals and significantly impact health. Persons who reacquire walking after injury increase their life expectancy to 90% of able-bodied individuals with self-reported improvement in quality of life [4]; while those who are unable to walk or lose their ability to walk within a year after injury have a life expectancy less than 75% of able-bodied individuals, with self-reported higher incidence of depression and reduction in quality of life [5]. While SCI closely associates with a diverse array of secondary health complications, including Type II diabetes, cardiovascular disease, pulmonary disease, and osteoporosis, studies show recovery of walking also may reduce the risk of developing these secondary conditions [6]. Thus, clinical strategies that prioritize treatments to restore walking after SCI are important not only to improve functional independence but also to reduce the incidence of SCI sequelae.
The recovery of walking remains a high clinical priority to improve functional outcomes in persons with SCI. Treatments provided modalities that ensure walking safety, as well as, improve independence (e.g., negotiating stairs and uneven surfaces, crossing streets, etc.). In many instances, clinicians prescribe orthoses, and hand-held walking aides such as walkers, canes, and forearm crutches to enable walking. Wearable robots or exoskeletons may also be prescribed. However, many of these technologies gravitate toward ways to compensate for as opposed to recover from SCI paralysis. While these technologies may enable new forms of bipedal and quadrupedal (i.e., forearm crutches) walking in people with SCI, they often overlook potential neuromechanical targets that help shape normal bipedal walking. These neural mechanisms offer an abundance of solutions that may offset sensory and motor deficits after injury [7]. Establishing treatments that target physiological mechanisms to overcome not only walking deficits, but also the underlying neuromechanical constraints that give rise to those deficits, is important. Thus, there is an overwhelming need to identify new treatment strategies that focus more on restorative mechanisms known to promote beneficial plasticity and subsequent recovery of walking function. The purpose of this review is to report on the progress of a plasticity-promoting treatment, alone or paired with other treatments, on walking recovery after SCI. We evaluate the evidence of this intervention as a therapy ready for clinical and home use and the real and perceived challenges that may interfere with this possibility.
Acute intermittent hypoxia: a potential adjuvant to SCI rehabilitation
Acute intermittent hypoxia refers to brief (acute), repetitive (intermittent) episodes of breathing oxygen-deprived air (hypoxia) alternating with breathing ambient room air. AIH is an emerging treatment to enhance walking function after SCI. Within the last 23 years, studies show AIH as a promising intervention that triggers rapid mechanisms of neuroplasticity and improves respiratory and non-respiratory motor function in rats with cervical SCI [8, 9]. Recent studies provide foundational support that AIH also induces improvements in breathing capacity, lower limb, and upper limb function in persons with SCI (Table 1), suggesting the translational potential of AIH as a therapeutic strategy in humans with SCI [10]. However, our understanding of the effectiveness of AIH as a treatment to improve walking in a broad range of persons with SCI remains unclear. Thus, the primary focus of this review article is to examine the evidence showing efficacy of AIH as an approach to improve walking after SCI. In particular, we examine AIH dosing regimens on treatment efficacy and the extent to which AIH, alone or paired with other treatments, may augment walking recovery.
Table 1:
Summary of studies examining effects of low oxygen therapy in humans with spinal cord injury. Abbreviations: BL: baseline; AIH: acute intermittent hypoxia: Nx: normoxia: EMG: electromyography: BWST: body weight supported treadmill training: s: second: w: week; L: liter, min: minute: N/m: newton/meter
| Author | AIH Duration | Functional Outcome | Key Results |
|---|---|---|---|
| Tester et al. (2014) [12] | 10 days | Respiratory Function | 29% increase in minute ventilation (L/min) for 30 minutes following AIH + mild hypercapnia. |
| Hayes et al. (2014) [13] | 1 day and 5 days/w for 1w | Overground Walking | 15% increase in walking speed (m/s), 36% increase in walking endurance (m) after 5 days AIH + overground walking training. |
| Trumbower et al. (2012) [10] | 1 day | Ankle Strength | 82% increase in maximum ankle plantar flexion torque (N/m) 30 min after AIH. 43% increase in peak EMG activity. |
| Navarrete-Opazo et al. (2017) [31] | 5 days/w for 1w Followed by 3 days/w, 3w | Overground Walking | 82% greater increase in walking speed (m/s) and 86% greater endurance (m) after 5 days AIH + BWST relative to Nx +BWST. Additional exposures significantly prolonged gains up to 3 weeks. |
| Navarrete-Opazo et al. (2017) [31] | 5 days/w for 1w Followed by 3 days/w. 3w | Walking Balance | AIH + BWST training reduced turning duration (min.) by 53% and reduced turn to sit duration (min.) by 24%. No change in postural sway and Time-Up-and-Go Test. |
| Lynch et al. (2017) [15] | 1 day | Ankle Strength | 18% and 30% increase in ankle plantar flexion torque (N/m) at 30 and 60 min post AIH respectively; No enhancement with ibuprofen. |
| Trumbower et al. (2017) [32] | 5days/w for 1 w | Hand Dexterity and Function | AIH + Hand opening practice improved time to complete Jebsen Taylor Hand Function Test 7.2s relative to BL (11% faster), improved Box-and-Blocks Test (increase of 3 blocks/min). and improved hand opening. |
| Sandhu et al. (2019) [14] | 1 day | Ankle Strength | AIH alone increased maximum planter flexion torque by 29%. AIH + suppression of inflammation with prednisolone increased maximum ankle plantar flexion torque by 41 %. |
In prior human studies, AIH administration involved commercially available generators (e.g., Hypoxico Inc, USA) that rely on pressure-swing absorption (PSA) technology to generate oxygen-deprived air from ambient room air [10–15]. These delivery systems supplied either a continuous concentration of low oxygen air (9–10% oxygen concentration; AIH treatment) or ambient room air (21% oxygen concentration; SHAM treatment). Trained personnel manually connected and disconnected the air supply tubing between the generator and a non-rebreather face mask at prescribed intervals [10]. Manual switching between generator and ambient sources ensured rapid change in oxygen concentration. Figure 1 illustrates a typical AIH protocol that includes temporal and frequency delivery parameters: 1) number of episodes (N), 2) low O2 duration/episode (t0), 3) episode duration (td), 4) duty cycle (t0/td), and 5) treatment duration (T). The episode number corresponds to the number of on-and-off cycles of oxygen-deprived air and room air delivery during a single AIH session, while the time to complete an on-and-off cycle of oxygen-deprived air and room air delivery corresponds to episode duration. Duty cycle is the ratio between the duration of time during an episode of breathing oxygen-deprived air and total episode duration. The treatment duration corresponds to the time to complete a single AIH session and the treatment number corresponds to the number of treatment sessions. The AIH dosage regimen also depends on the percentages of oxygen concentration prescribed during an AIH session.
Figure 1.
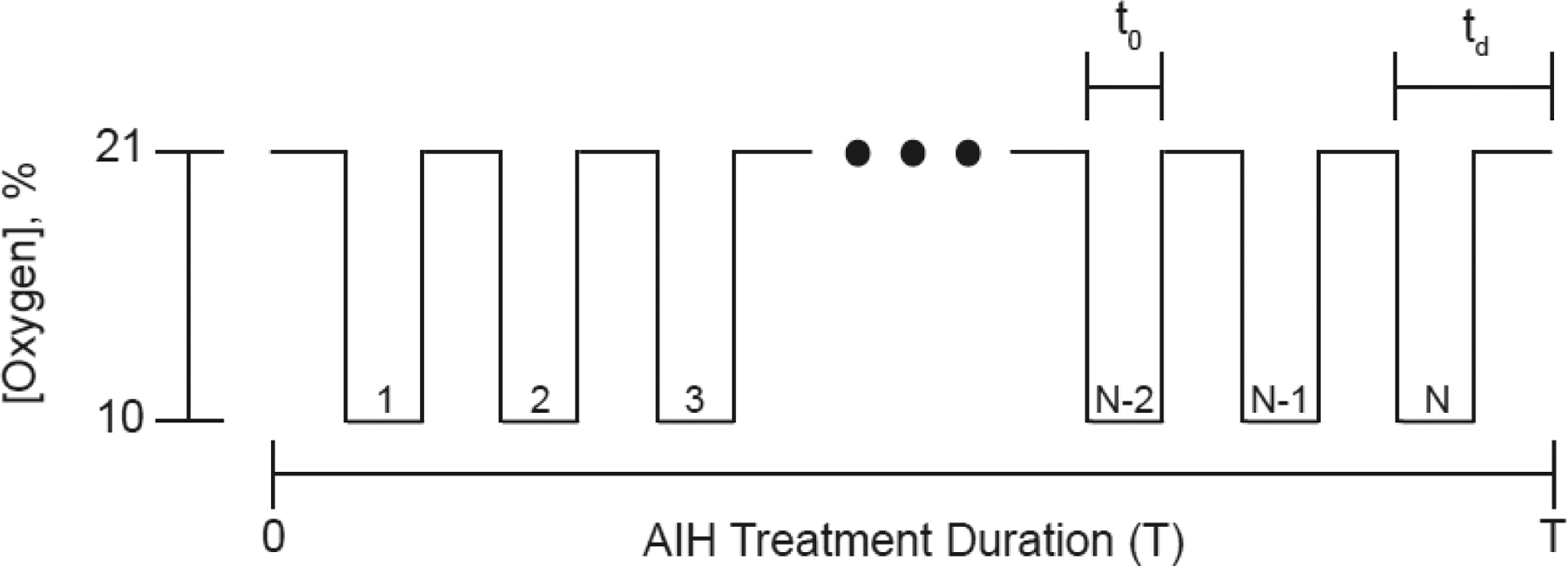
Typical acute intermittent hypoxia protocol. AIH treatment duration (T) corresponds to total treatment time for a single AIH session. A treatment consists of N episodes of breathing low oxygen (10%) and room air (21%) intermittently. The duration, t0, corresponds to the time breathing low oxygen within a single episode duration (td).
Identifying AIH exposure parameters that confer therapeutic benefit without inducing pathology is a critical step toward clinical translation. Safety boundaries for intermittent low oxygen delivery rely on the intensity of the dosage regimen. We refer to intensity as the product of total episode number and low oxygen concentration as discussed previously [16]. More than a dozen reports show low intensity intermittent hypoxia protocols (9–16% oxygen concentration with less than 15 episodes/day) lead to a broad range of beneficial effects without evidence of pathology [8, 10, 17–29]. Clinical trial reports in persons with SCI also demonstrate safe and efficacious AIH treatments with dosing regimens that fall within these safety boundaries: 9–10% oxygen concentration with 0.5–0.6 duty cycle, 120–150s/episode duration, 1–15 episodes/treatment, and up to 14 treatment days. These trials reported no maladaptive changes in muscle function or blood pressure following AIH treatment [10, 12, 15, 30–32]. Navarrete-Opazo et al., also found no evidence of visual or verbal memory impairments following repetitive (14 days) AIH (15 episodes/day; 90s at 10% oxygen concentration with 60s intervals at 21% oxygen concentration) in persons with motor-incomplete SCI [33].
Although considerable progress in both rodent models and human studies elucidate the beneficial effects of mild protocols of AIH on motor function, more severe protocols undoubtedly trigger pathology. Several studies involving animal models and able-bodied humans show intermittent hypoxia protocols that involve less than 9% oxygen concentration and episodes that exceed 48/day result in serious pathologies across multiple physiological systems simultaneously. These parameters correspond to an increase in blood pressure, atherosclerosis, sympathetic activity, and inflammation, as well as, an increase in learning and working memory impairment [33–39]. In some cases, severe exposures may preferentially induce a competing cellular cascade via adenosine 2A receptor activation that inhibits the serotonin dependent pathway [40]. Severe, chronic exposures that approximate patterns observed in persons with obstructive sleep apnea are associated with deficits in cognition and memory function [36]. Thus, moving toward clinical translation will require careful consideration of AIH devices and delivery methods that prevent administration of these more severe protocols.
I. Neural mechanisms of AIH
Our understanding of the molecular and cellular mechanisms of AIH-induced motor enhancement continues to evolve. Rodent studies demonstrated that mild exposure to oxygen-depleted air triggers mechanisms of neural plasticity in the respiratory motor system. Hayashi et al., discovered that rodents breathing low oxygen experience a persistent increase in inspiratory phrenic nerve activity, termed long-term facilitation (LTF) [41]. Other key experiments revealed potential mechanisms of AIH-mediated LTF that include serotonin, dose sensitivity, and brain-derived neurotrophic factor (BDNF) synthesis [42–44]. Excitingly, the neural mechanisms driving AIH-induced plasticity in phrenic motor nuclei appear to promote respiratory motor recovery in rodents with cervical SCI [9, 45, 46]. Serotonin receptor activation leads to downstream synthesis of BDNF resulting in LTF in rats following three 5 min episodes of 11% oxygen concentration [42, 43]. Studies found AIH to elicit LTF in respiratory motor nuclei via spared serotonergic pathways that extend from the raphe nucleus to phrenic motor neurons distal to the C2 hemisection [42, 47]. Specifically, a single treatment of AIH is sufficient to trigger pattern-sensitive release of serotonin onto spinal motor nuclei [44]. Provided ample serotonin projections extend beyond the injury site, the serotonin release activates guanine nucleotide binding protein, q polypeptide (Gq). The Gq protein binds with metabotropic serotonin receptors (5-HT2) prompting de novo synthesis of BDNF, downstream signaling via the high affinity BDNF receptor, tropomyosin receptor kinase (TrkB), and Mitogen-activated protein kinase (MAPK) activation [9, 43, 45, 48]. On this basis, investigators postulate that these signaling events lead to glutamate receptor insertion at the premotor-motor neuron.
Repetitive AIH enhances non-respiratory motor function in rodents with cervical SCI. Lovett-Barr et al. evidence that AIH enhanced not only respiratory, but also non-respiratory motor function in rats with incomplete C2 spinal injuries [8]. Improvements in skilled horizontal ladder walking occurred as early as 3 days after the first session, peaked after seven sessions, and persisted for at least 28 days. Importantly, increased BDNF immunoreactivity after transection alone without AIH was not significantly increased, strongly implicating an AIH-triggered increase in the protein’s expression [8]. In a repeat study, the locomotor gains corresponded to increased protein immunoreactivity in non-respiratory motor nuclei when daily (7 consecutive days) AIH (10, 5-min episodes at 11% oxygen concentration, 5-min intervals at 21% oxygen concentration) paired with ladder walking practice [49]. Further corroborating the enhancement of BDNF expression following AIH, Hassan and associates demonstrated similar results [50]. Such evidence is consistent with the hypothesis that the observed improvements in neuromotor function could be attributed to BDNF induced facilitation of excitatory synaptic transmission to motoneurons.
The underlying mechanisms of AIH may contribute to plasticity of spinal circuitry important for locomotor function. Similar to AIH, physical exercise drives BDNF up-regulation and plays a role in activity-dependent plasticity [51]. Treadmill training in complete spinal transected rats increased BDNF mRNA expression in the lumbar spinal cord motoneurons [52]. Similarly, AIH triggers BDNF expression of somatic motor nuclei and promoted motor recovery in rats with SCI [8]. BDNF function is often associated with promoting adaptive neuromotor plasticity via increasing spinal excitability and activation of interneurons in locomotor circuits [53]. In both spinalized cat and rodent models, delivery of BDNF to the spinal cord lesion site restored stepping patterns and improved kinematic parameters such as step length and height [54, 55].
Alternative mechanisms to AIH motor facilitation are notably present. While the Gq-pathway dominates AIH long-term facilitation in SCI rodent models, chronic injury models of AIH result in the activation of a competing Gs pathway that may interfere with this pathway due, in part, to an increase in intercellular adenosine at or near the motor neurons [56]. The initiation of Gs protein-coupled adenosine or 5-HT7 receptors result in downstream cyclic AMP signaling that leads to crosstalk inhibition between Gq and Gs pathways such that Gs pathway activation results in inhibitory action of the Gq pathway. Applying an adenosine 2a receptor antagonist (i.e., istradefylline) blocked this interference such that the effect of AIH via the Gq pathway nearly doubled [57]. AIH dose regimens and injury acuity may preferentially activate the dominance of one pathway over the other. Important, ongoing clinical trials in humans address whether pretreatment with caffeine, a non-selective adenosine 2a receptor blocker, may facilitate the Gq pathway, leading to enhanced effects on motor recovery. Thus, establishing therapeutic AIH in persons with SCI may require a more detailed characterization of how these two distinct mechanisms interact. While the working hypothesis is that AIH strengthens excitatory transmission onto motor neurons, there is evidence that AIH may also restore inhibitory transmission [58], potentially contributing to spasticity reduction. Although serotonin receptor activation and BDNF associate with spasticity after SCI [59–61], AIH does not appear to increase spasticity in persons with SCI [13]. Prior studies show BDNF upregulation, via exogenous administration or task-specific practice (e.g., walking), also upregulates KCC2 expression and subsequently restores reflex homeostasis in SCI rat models [60, 62]. Based on these concepts, a similar mechanism may lead to AIH-mediated reduction in spasticity, which otherwise begins to emerge only a few months post-injury [63].
II. Benefits of AIH on walking recovery
Studies show that AIH enhances motor behaviors important for functional walking. One of the first known clinical studies, involving persons with chronic SCI, showed a single AIH treatment (1 day, 15 episodes) elicited ~82% increase in ankle torque generation that persisted more than 1 hour after treatment [10]. Since then, groups further corroborate the efficacy of AIH on enhancing ankle strength. Lynch et al., found ankle plantar flexion torque increased ~30% that persisted up to 1 hour following a single AIH treatment [15]. Similarly, Sandhu et al., found ankle plantar flexion torque increased ~22% that persisted 3 hours following a single AIH treatment [14]. Since ankle torque generation accounts for walking energetics during more than half of the gait cycle [64], several investigators suspected the increase in ankle torque production may contribute to an increase in walking performance.
In a multi-site, randomized clinical trial, AIH alone or paired with walking practice increased walking ability in persons with chronic, motor-incomplete SCI [13]. Hayes et al., showed walking speed increased 18% following daily (5 consecutive days) AIH alone, while walking endurance increased ~37% that persisted two weeks following daily AIH combined with 30-min of walking endurance practice [13]; these changes corresponded to more than twice the improvement measured following AIH alone. Despite a modest 5-day treatment, AIH increased walking speed and endurance to levels comparable to those seen with standard clinical gait training protocols typically 4–12 weeks (60 treatments) in duration [65, 66]. More than 70% achieved a clinically meaningful change in walking endurance [65].
More recently, Navarrete-Opazo also showed improvement in walking distance and speed following AIH combined with gait training [31]. They confirmed that persons with motor-incomplete SCI improved walking speed and endurance following repetitive (14 treatment days) AIH combined with body-weight support treadmill (BWST) training. Study participants received an initial 1-week AIH + BWST training treatment followed by thrice weekly treatments for 3 weeks. Improvements in walking speed and endurance persisted up to 6 weeks after treatment, suggesting that the 3-week reminder treatments may prolong the therapeutic benefits of AIH on walking ability. In two pre-clinical studies, researchers found that rats with cervical spinal injuries restored skilled forelimb function in a horizontal ladder walking task following exposure to daily AIH (10 episodes per day, 7 consecutive days) [8, 49]. Collectively, these results confirm that AIH in combination with task-specific training appears to further enhance gains in walking recovery than either alone.
Comprehensive studies on BWST training indicate that relatively high demands of training (weeks to months) are required to elicit modest gains in overground walking performance (Table 1) [67–70]. Intriguingly, the dose of AIH required (Table 2) to promote similar gains in walking recovery are comparatively shorter (~50 min/day, for 1 or 5 days), suggesting a higher functional gain per treatment time. Recent reports indicate that even higher intensities of BWST training are required to promote functional recovery and that BWST training paradigms have not been shown to be more effective than other walking training modalities, including overground walking and functional electrical stimulation, at improving walking performance [71–78]. Given the combinatorial potential of AIH and task specific training, establishing how AIH interacts with more appropriate BWST doses is critical to understanding AIH as an adjuvant for SCI therapy to enhance walking recovery.
Table 2:
Summery of the progression of key findings regarding the effectiveness of body weight supported treadmill training in persons with incomplete spinal cord injury. Abbreviations: BWST: body weight supported treadmill training: EMG: electromyography: FES: functional electrical simulation: MG: medial gastrocnemius: PT: physical therapy; ROM: range of motion; TA: tibialis anterior WISCI II: walking index for spinal cord injury.
| Author | Question | Training Dose | Key Finding |
|---|---|---|---|
| Gorassini et al., 2004 [108] | Do coordinative strategies change after BSWT? | 1–3 months | Reduced step by step foot trajectory variability. |
| Thomas et al., 2005 [91] | Does BWST improve residual corticospinal function? | 10–21 weeks ≤ 5 sessions/week 1 hr/session |
Increases in max MEP of target muscles Improved walking function corresponded to MEP Improved walking ability, walking speed |
| Giangregorio et al., 2006 [70] | Does BWST increase bone mass and reverse muscle atrophy? | 12 months 144 sessions 3 session s/week |
No changes in bone density. Increase in whole body-lean mass. Increase in muscle body cross-sectional area. |
| Gorassini at al., 2008 [76] | Are changes in neuromuscular coordination associated with walking improvements after BSWT? | 10–20 weeks 3day s/week 1h/day |
Functional gait improvements (WISCI II. speed). Decreased co-contraction of proximal muscles. Increases in EMG activation of TA and hamstrings. |
| Field-Fote et al., 2011 [74] | Does BWST achieve similar walking gains as overground training? | 12 weeks 5 sessions/week |
Gains in walking speed equivalent across training. Gains in endurance greater for overground training |
| Yang et al., 2011 [92] | What measures best predicts responders to BWST? | ≥10 weeks 5 days/week 1 hr/day |
Leg strength and muscle activity predict increases in walking speed and functional walking ability |
| Lucareli et al., 2011 [71] | Is BWST more effective than conventional PT in improving gait? | 30 weekly sessions 30 min/ session |
Increases m speed and distance after BWST. No differences in outcomes following conventional PT. |
| Yang etal., 2014 [92] | Is repetitive mass practice or task specific practice lead to better walking performance? | 2 months 5 sessions/week 1 hour/day |
Increased walking distance and endurance after endurance rather than precision walking training |
| Kapadia et al., 2014 [73] | Does FES confer additional benefit when combined with BWST? | 16 weeks 3 sessions/week 45 min session |
Both FE- assisted BWST and aerobic training show similar increases in walking speed, endurance, and balance. |
| Brazg et al., 2017 [78] | Does high intensity locomotor training improve treadmill walking performance and metabolic function? | 4–6 weeks 3–5 sessions/week 20,1 hr. sessions |
High intensity training increased peak treadmill speed and endurance relative to low intensity training. |
| Ardestani et al., 2019 [77] | Does higher intensity BWST change joint and muscle coordination strategies? | 4–6 weeks 20, 1hour sessions | High intensity training increased joint ROM. Intralimb coordination improved in weaker limb. Increase neuromuscular complexity. |
| Covarrubias-Escudero et al., 2019 [75] | Does BWST improve center of mass control? | 6 weeks 3 sessions\week 24 min session |
Improved center of mass control. No changes in gait independence. No significant change in postural sway. |
III. Clinical translation of AIH: challenges and opportunities
Despite growing evidence for AIH as a safe and efficacious treatment to improve motor function in persons with SCI, successful translation of AIH to clinical practice will depend on several factors. Multiple issues modify AIH-induced motor recovery in rodent models, including hypoxia preconditioning, systemic inflammation, injury severity, sleep-disordered breathing (SDB), among others [37, 79–81]. Similar factors such as biomarkers may influence AIH responsiveness in humans, but this possibility has yet to be determined. Identifying factors that facilitate and/or inhibit AIH efficacy will present as major clinical challenges while also as opportunities to ensure the best likelihood of translational success.
Uncertainty in dosing regimens remains a major challenge to clinical translation of AIH. Beneficial responses are variable between individuals and we lack effective biomarkers to determine which individuals will benefit most from treatment. For example, in a study demonstrating increased voluntary ankle plantar flexion torque after a single AIH trial in persons with chronic, incomplete SCI, more than half (6 of 10) did not maintain strength gains four hours post-AIH [10]. Nearly 30% of participants did not improve walking speed or distance after 5 days of AIH; gains in walking distance ranged from 0 to 328m above baseline (131±100m), indicating markedly different responsiveness between individuals [13].
One possible explanation for variations in AIH responsiveness may be concurrent inflammation, which is consistently documented in persons with SCI and limits functional plasticity in preclinical models of SCI SCI [79, 82, 83]. In the acute phase of SCI, Kwon and colleagues showed that elevated inflammatory mediators (i.e., interleukins) correlate inversely with motor recovery 6 months later [84]. In the chronic phase of SCI, investigators identified more than 2000 genes differentially expressed in the circulation of individuals with chronic SCI that included upregulation of the pro-inflammatory Toll-like receptor (TLR) signaling cascade and other pro-inflammatory mediators [82]. Elevated protein levels of the TLR4 ligand HMGB1 also occurred in persons with chronic SCI [85]. In preclinical studies, systemic treatment with the TLR4 ligand LPS provoked spinal inflammation and reduced neuroplasticity induced by AIH [86, 87]. In human studies, pretreatment with prednisolone enhanced AIH-induced ankle strength in persons with SCI [14]. Understanding the extent to and mechanism by which inflammation may limit the neural effects of AIH on enhancing walking recovery after SCI in humans is critical to move the clinical use of AIH forward.
Concurrent SDB may impact AIH responsiveness [80]. Many individuals with SCI exhibit mild to moderate SDB, leading to extended periods of nocturnal “high dose” intermittent hypoxia. Since more severe doses of intermittent hypoxia elicit both neuroplasticity [88] and inflammation [87, 89], SDB following SCI may contribute to between-person variations in response to AIH therapy. SDB involves apnea/hypopnea breathing events during sleep, leading to hypoxia breathing over many years. Chronic hypoxia and other factors associated with SDB ultimately cause pathologies including hypertension, cognitive deficits, hippocampal cell death, peripheral nerve dysfunction, and neuroinflammation [36]. Some of these factors undermine AIH-induced respiratory motor plasticity in rodent models, such as inflammation elicited by a single day of intermittent hypoxia simulating that experienced during moderate sleep apnea and/or sleep fragmentation [90]. Nevertheless, the extent to which SDB enhances or dampens motor responses to AIH remains unknown and poses a tremendous opportunity for further research.
The severity of spinal injury may influence the extent of improvements in motor function following AIH treatment. Identifying the amenability of spared neural connections to AIH treatment in persons with incomplete SCI is important. The notion that spinal cord integrity may serve as a salient predictor of AIH-induced motor recovery draws support from BWST training paradigms where the ability to generate muscle activity predicts training responsiveness [91, 92]. Transcranial magnetic stimulation (TMS) is a supporting paradigm that characterizes gains in excitatory transmission following treatment and may function as a reliable biomarker tool.
The efficacy of AIH-induced plasticity may depend, in part, on the extent of preserved connections in the spinal cord after injury and impact recovery of motor function [93]. Recent evidence indicates AIH induces excitatory changes along the corticospinal tracts (CST) but the association between this effect and functional performance of these residual pathways needs further study [94]. For instance, AIH may enhance corticospinal drive to motoneurons innervating ankle dorsiflexor muscles, which are often weakened after SCI. Strengthening supraspinal input to dorsiflexors is important for restoring functional walking [95, 96] and measures of CST excitability and ankle strength may serve as effective screening tools to predict effectiveness of AIH as a primer for gait-related training in persons with SCI.
Successful translation of AIH requires a greater understanding of mechanisms giving rise to functional improvements after SCI. Although the original concept suggests AIH is a potent tool to strengthen excitatory transmission onto motor neurons, there is more recent evidence in rodents that AIH also may restore inhibitory transmission [48, 60, 97]. Gamma aminobutyric acid (GABA) and glycine are the major inhibitory neurotransmitters acting on motor neurons. Both transmitters require low intracellular chloride concentrations to elicit post-synaptic inhibitory currents. A key factor of GABA/glycine effects is the regulation of the potassium-chloride transporter, KCC2. Whereas high KCC2 expression decreases intracellular chloride concentration and enables inhibitory currents, low KCC2 expression increases intracellular chloride concentration and reduces GABA/glycine-induced currents in post-synaptic cells [98–100]. AIH may drive plasticity in segmental circuits regulating afferent feedback as growing evidence supports the role of BDNF in modulating motor neuron activity via shaping GABAergic inhibitory transmission. In particular, the restoration of spinal inhibitory transmission via the upregulation of BDNF signaling alleviates spasticity and allodynia in rodent models [101]. Tashiro et al., (2015) showed BDNF-dependent enhancement of inhibitory spinal transmission in rats with SCI corresponded to reduction in abnormal co-activation of antagonist limb muscles during locomotion [101]. This opens the possibility that AIH engages a BDNF-dependent cascade affecting balance between inhibitory and excitatory transmission, thereby translating into clinically relevant reductions in abnormal muscle coactivation and spasticity. The reduction and abnormal composition of muscle activation patterns reflect key constraints in the ability to produce appropriate limb mechanics throughout the gait cycle [30]. As such, there is opportunity to identify how voluntary and involuntary lower limb muscle contractions may capture the impact of AIH on spasticity and exaggerated stretch reflex excitability following SCI [102, 103]. AIH provides a compelling paradigm to further clarify how neural plasticity may differentially shape downstream motor output important for walking.
AIH appears most potent when combined with task specific training, but the extent to which the combinatorial gains are specific to the training target or to the nature of the training (endurance vs speed) is not clear [13, 49, 78]. Treatment that combined AIH and BWST training improved dynamic balance parameters such as turn-to-sit duration even though this training did not target balancing skills required during the Timed-Up-and-Go assessment [104]. Furthermore, if even a single sequence of AIH contributes to enhanced somatic motor function, it is not unreasonable to suggest that similar beneficial changes in voluntary muscle strength will extend beyond muscles acting at the ankle joint (e.g., knee, hip), or even the hand [32]. Generalized facilitation may be observed following AIH exposure that is non-specific to a single muscle (i.e, ankle plantar flexors; [10]). This suggests that improvements in walking performance may be partly attributed to increased voluntary drive/gain that spans the entire neuraxis and preferential benefit those pathways spared following the injury.
Finally, determining features of recovery of those who respond favorably to AIH may further guide efforts to optimize patient-specific dosing strategies. One key consideration is the potential role of BDNF genetic variation on AIH-induced functional outcome improvements. A single nucleotide polymorphism (SNP) of the bdnf gene results in a valine (Val) to methionine (Met) substitution at codon 66 (val66met) that associates with changes in cortical and spinal plasticity [105, 106]. Approximately 44% of the population have at least one copy of the bdnf gene variant Met allele, with similar prevalence in SCI. The extent to which the bdnf SNP alters the plasticity promoting effects of AIH is unknown. Similarly, elucidating the biomechanical phenotype of those amenable to AIH may help begin to parse the interaction between AIH induced neural recovery and compensatory strategies used to achieve gains in motor function. Mapping differential changes in kinematic variability, spatiotemporal coordination parameters, and neuromuscular activation after AIH can provide further insight into whether the movement strategies utilized are consistent with neuromotor recovery [30, 76, 107–109].
CONCLUSION
Preliminary findings detailed in this review affirm that breathing mild episodes of oxygen-deprived air appears to be a safe and effective therapeutic primer to enhance motor function (i.e., walking) after SCI. Studies identified AIH as a non-invasive method to treatment paralysis without report of serious adverse side effects. We evaluated the evidence of AIH as an adjuvant for SCI therapy and the potential challenges that may hamper this translation. The potential for AIH as a treatment for SCI remains high, but further research is necessary to understand the treatment’s enduring effects in a large cohort of persons with SCI.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Randy Trumbower reports he has a provisional patent entitled, “Improving method of delivering acute intermittent hypoxia for therapeutic or sports training purposes” pending. Andrew Quesada Tan and Stella Barth declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ditunno PL, et al. , Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord, 2008. 46(7): p. 500–6. [DOI] [PubMed] [Google Scholar]
- 2.Davies H, Hope as a coping strategy for the spinal cord injured individual. Axone, 1993. 15(2): p. 40–6. [PubMed] [Google Scholar]
- 3.Simpson LA, et al. , The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma, 2012. 29(8): p. 1548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shavelle RM, et al. , Mobility, continence, and life expectancy in persons with Asia Impairment Scale Grade D spinal cord injuries. Am J Phys Med Rehabil, 2015. 94(3): p. 180–91. [DOI] [PubMed] [Google Scholar]
- 5.Hiremath SV, et al. , Longitudinal Prediction of Quality-of-Life Scores and Locomotion in Individuals With Traumatic Spinal Cord Injury. Arch Phys Med Rehabil, 2017. 98(12): p. 2385–2392. [DOI] [PubMed] [Google Scholar]
- •6.Hicks AL and Ginis KA, Treadmill training after spinal cord injury: it’s not just about the walking. J Rehabil Res Dev, 2008. 45(2): p. 241–8. [DOI] [PubMed] [Google Scholar]
- 7.Ting LH, et al. , Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron, 2015. 86(1): p. 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •8.Lovett-Barr MR, et al. , Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci, 2012. 32(11): p. 3591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]; Investigators found daily (7 consecutive days) of AIH improed respiratory (breathing capacity) and nonrespiratory (ladder walking) motor function without evidence for associated morbidity in rats with chronic cervical injuries. Functional improvements corresponded to increased neurochemical changes in proteins that contribute to motor plasticity (BDNF and TrkB).
- 9.Golder FJ and Mitchell GS, Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci, 2005. 25(11): p. 2925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••10.Trumbower RD, et al. , Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair, 2012. 26(2): p. 163–72. [DOI] [PubMed] [Google Scholar]; Study reported an 82% increase in maximum ankle plantar flexion torque that persisted up to 90 minutes after AIH in persons with chronic, incomplete spinal cord injury.
- 11.Kotliar IK, Apparatus for hypoxic training and therapy, W.I.P. Organization, Editor. 1996. [Google Scholar]
- •12.Tester NJ, et al. , Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med, 2014. 189(1): p. 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study showed AIH with mild hypercapnia breathing induced a 29% increase in minute ventilation (L/min) in persons with chronic, incomplete spinal cord injury.
- •13.Hayes HB, et al. , Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology, 2014. 82(2): p. 104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study reported 15% increase in walking speed (m/s) and 36% increase in walking endurance (m) after 5 days of AIH combined with overground walking training in persons with chronic, incomplete spinal cord injury.
- •14.Sandhu MS, et al. , Prednisolone Pretreatment Enhances Intermittent Hypoxia-Induced Plasticity in Persons With Chronic Incomplete Spinal Cord Injury. Neurorehabil Neural Repair, 2019. 33(11): p. 911–921. [DOI] [PubMed] [Google Scholar]; Study found AIH alone increased ankle strength 29% in persons with chronic, incomplete SCI. Study also reported 41% increase in ankle strength following AIH combined with oral prednisolone.
- •15.Lynch M, et al. , Effect of acute intermittent hypoxia on motor function in individuals with chronic spinal cord injury following ibuprofen pretreatment: A pilot study. J Spinal Cord Med, 2017. 40(3): p. 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study found a 30% increase in ankle plantar flexion strength following AIH that persisted 60 minutes after treatment in persons with chronic, incomplete SCI. The study reported no improvement in ankle strength following AIH + oral ibuprofen.
- 16.Navarrete-Opazo A and Mitchell GS, Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol, 2014. 307(10): p. R1181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burtscher M, et al. , Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Respir Physiol Neurobiol, 2009. 165(1): p. 97–103. [DOI] [PubMed] [Google Scholar]
- 18.Burtscher M, et al. , Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int J Cardiol, 2004. 96(2): p. 247–54. [DOI] [PubMed] [Google Scholar]
- 19.Casas M, et al. , Intermittent hypobaric hypoxia induces altitude acclimation and improves the lactate threshold. Aviat Space Environ Med, 2000. 71(2): p. 125–30. [PubMed] [Google Scholar]
- 20.Haider T, et al. , Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J Hypertens, 2009. 27(8): p. 1648–54. [DOI] [PubMed] [Google Scholar]
- 21.Knaupp W, et al. , Erythropoietin response to acute normobaric hypoxia in humans. J Appl Physiol (1985), 1992. 73(3): p. 837–40. [DOI] [PubMed] [Google Scholar]
- 22.Lu XJ, et al. , Hippocampal spine-associated Rap-specific GTPase-activating protein induces enhancement of learning and memory in postnatally hypoxia-exposed mice. Neuroscience, 2009. 162(2): p. 404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyamina NP, et al. , Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens, 2011. 29(11): p. 2265–72. [DOI] [PubMed] [Google Scholar]
- 24.Mallet RT, et al. , Beta1-Adrenergic receptor antagonism abrogates cardioprotective effects of intermittent hypoxia. Basic Res Cardiol, 2006. 101(5): p. 436–46. [DOI] [PubMed] [Google Scholar]
- 25.Nichols NL, et al. , Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med, 2013. 187(5): p. 535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez FA, et al. , Intermittent hypobaric hypoxia stimulates erythropoiesis and improves aerobic capacity. Med Sci Sports Exerc, 1999. 31(2): p. 264–8. [DOI] [PubMed] [Google Scholar]
- 27.Serebrovskaya TV, et al. , Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men. High Alt Med Biol, 2011. 12(3): p. 243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkerson JE and Mitchell GS, Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol, 2009. 217(1): p. 116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang J and Zhou Z, Protective effects of intermittent hypoxic adaptation on myocardium and its mechanisms. Biol Signals Recept, 1999. 8(4–5): p. 316–22. [DOI] [PubMed] [Google Scholar]
- 30.Hayes HB, et al. , Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clin Neurophysiol, 2014. 125(10): p. 2024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••31.Navarrete-Opazo A, et al. , Repetitive Intermittent Hypoxia and Locomotor Training Enhances Walking Function in Incomplete Spinal Cord Injury Subjects: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J Neurotrauma, 2017. 34(9): p. 1803–1812. [DOI] [PubMed] [Google Scholar]; Investigators confirmed Hayes et al study that 5 consecutive days of AIH +BWST training induced an 82% increase in walking speed and 86% increase in walking endurance in persons with chronic, incomplete SCI. The study also showed 3 additional weeks (9 treatments) of AIH + BWST enhanced overground walking function that persisted for more than 5 weeks.
- ••32.Trumbower RD, et al. , Effects of acute intermittent hypoxia on hand use after spinal cord trauma: A preliminary study. Neurology, 2017. 89(18): p. 1904–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study found AIH + hand opening practice improved volitional hand opening and dexterity (box-and-blocks test; increase of 3 blocks/min) in persons chronic, incomplete spinal cord injury.
- •33.Navarrete-Opazo A, et al. , Intermittent Hypoxia Does not Elicit Memory Impairment in Spinal Cord Injury Patients. Arch Clin Neuropsychol, 2016. 31(4): p. 332–42. [DOI] [PubMed] [Google Scholar]; This study reports that episodic verbal and visual memory function was not significantly different following a 4 week protocol of repetitive AIH exposure, indicating the repetititve AIH epsosures does not induce deleterious cognitive effects.
- 34.Tamisier R, et al. , 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J, 2011. 37(1): p. 119–28. [DOI] [PubMed] [Google Scholar]
- 35.Lesske J, et al. , Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J Hypertens, 1997. 15(12 Pt 2): p. 1593–603. [DOI] [PubMed] [Google Scholar]
- 36.Gozal D, Daniel JM, and Dohanich GP, Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci, 2001. 21(7): p. 2442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brooks D, et al. , Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest, 1997. 99(1): p. 106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savransky V, et al. , Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med, 2007. 175(12): p. 1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Champod AS, et al. , Effects of acute intermittent hypoxia on working memory in young healthy adults. Am J Respir Crit Care Med, 2013. 187(10): p. 1148–50. [DOI] [PubMed] [Google Scholar]
- 40.Nichols NL, Dale EA, and Mitchell GS, Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985), 2012. 112(10): p. 1678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi F, et al. , Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol, 1993. 265(4 Pt 2): p. R811–9. [DOI] [PubMed] [Google Scholar]
- 42.Bach KB and Mitchell GS, Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol, 1996. 104(2–3): p. 251–60. [DOI] [PubMed] [Google Scholar]
- •43.Baker-Herman TL, et al. , BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci, 2004. 7(1): p. 48–55. [DOI] [PubMed] [Google Scholar]; This study provided evidence that disruptions to BDNF synthesis using RNA interference and blocking of BDNF signaling stops phrenic long-term facilitation. In contrast, intrathecal injections of BDNF elicted phrenic long-term facilitation like effects.
- •44.Baker TL and Mitchell GS, Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol, 2000. 529 Pt 1: p. 215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that continous exposure to hypoxia does not elicit long term facilitation in phrenic nerve activity. Facilitation of phrenic motor output is sensitive to the pattern of hypoxic exposure.
- 45.Fuller DD, et al. , Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci, 2003. 23(7): p. 2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell GS, et al. , Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985), 2001. 90(6): p. 2466–75. [DOI] [PubMed] [Google Scholar]
- 47.Vinit S, Lovett-Barr MR, and Mitchell GS, Intermittent hypoxia induces functional recovery following cervical spinal injury. Respiratory Physiology & Neurobiology, 2009. 169(2): p. 210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strathmann M and Simon MI, G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci U S A, 1990. 87(23): p. 9113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •49.Prosser-Loose EJ, et al. , Delayed Intervention with Intermittent Hypoxia and Task Training Improves Forelimb Function in a Rat Model of Cervical Spinal Injury. J Neurotrauma, 2015. 32(18): p. 1403–12. [DOI] [PubMed] [Google Scholar]; This study demonstrated that pairing 7 consecutive days of AIH with task-specific training improved horizontal ladder walking performance in spinally injured rats. Notably, AIH-treated rats receiving no motor training did not show improvement over SHAM treated rats.
- 50.Hassan A, et al. , Acute intermittent hypoxia and rehabilitative training following cervical spinal injury alters neuronal hypoxia- and plasticity-associated protein expression. PLoS One, 2018. 13(5): p. e0197486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Pinilla F, et al. , Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol, 2002. 88(5): p. 2187–95. [DOI] [PubMed] [Google Scholar]
- 52.Joseph MS, Tillakaratne NJ, and de Leon RD, Treadmill training stimulates brain-derived neurotrophic factor mRNA expression in motor neurons of the lumbar spinal cord in spinally transected rats. Neuroscience, 2012. 224: p. 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyce VS and Mendell LM, Neurotrophins and spinal circuit function. Front Neural Circuits, 2014. 8: p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyce VS, et al. , Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur J Neurosci, 2012. 35(2): p. 221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ollivier-Lanvin K, et al. , Either brain-derived neurotrophic factor or neurotrophin-3 only neurotrophin-producing grafts promote locomotor recovery in untrained spinalized cats. Neurorehabil Neural Repair, 2015. 29(1): p. 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golder FJ, et al. , Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci, 2008. 28(9): p. 2033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffman MS, et al. , Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol, 2010. 588(Pt 1): p. 255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mabrouk B, Vinit S, and Mitchel GS. Intermittent hypoxia restores the KCC2-NKCC1 balance following C2 hemisection. in Society for Neuroscience. 2011. Washington DC. [Google Scholar]
- 59.Bos R, et al. , Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A, 2013. 110(1): p. 348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boulenguez P, et al. , Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med, 2010. 16(3): p. 302–7. [DOI] [PubMed] [Google Scholar]
- 61.Wainberg M, Barbeau H, and Gauthier S, The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiatry, 1990. 53(9): p. 754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cote MP, et al. , Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma, 2011. 28(2): p. 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hiersemenzel LP, Curt A, and Dietz V, From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology, 2000. 54(8): p. 1574–82. [DOI] [PubMed] [Google Scholar]
- 64.Meinders M, Gitter A, and Czerniecki JM, The role of ankle plantar flexor muscle work during walking. Scand J Rehabil Med, 1998. 30(1): p. 39–46. [DOI] [PubMed] [Google Scholar]
- 65.Mehrholz J, Kugler J, and Pohl M, Locomotor training for walking after spinal cord injury. Spine (Phila Pa 1976), 2008. 33(21): p. E768–77. [DOI] [PubMed] [Google Scholar]
- 66.Wirz M, et al. , Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil, 2005. 86(4): p. 672–80. [DOI] [PubMed] [Google Scholar]
- 67.Hicks AL, et al. , Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord, 2005. 43(5): p. 291–8. [DOI] [PubMed] [Google Scholar]
- 68.Cote MP, Murray M, and Lemay MA, Rehabilitation Strategies after Spinal Cord Injury: Inquiry into the Mechanisms of Success and Failure. J Neurotrauma, 2017. 34(10): p. 1841–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ditor DS, et al. , Maintenance of exercise participation in individuals with spinal cord injury: effects on quality of life, stress and pain. Spinal Cord, 2003. 41(8): p. 446–50. [DOI] [PubMed] [Google Scholar]
- 70.Giangregorio LM and McCartney N, Reduced loading due to spinal-cord injury at birth results in “slender” bones: a case study. Osteoporos Int, 2007. 18(1): p. 117–20. [DOI] [PubMed] [Google Scholar]
- 71.Kapadia N, et al. , A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med, 2014. 37(5): p. 511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lucareli PR, et al. , Gait analysis following treadmill training with body weight support versus conventional physical therapy: a prospective randomized controlled single blind study. Spinal Cord, 2011. 49(9): p. 1001–7. [DOI] [PubMed] [Google Scholar]
- 73.Mehrholz J, et al. , Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord, 2017. 55(8): p. 722–729. [DOI] [PubMed] [Google Scholar]
- 74.Field-Fote EC and Roach KE, Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther, 2011. 91(1): p. 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Covarrubias-Escudero F, et al. , Effects of body weight-support treadmill training on postural sway and gait independence in patients with chronic spinal cord injury. J Spinal Cord Med, 2019. 42(1): p. 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gorassini MA, et al. , Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J Neurophysiol, 2009. 101(2): p. 969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ardestani MM, et al. , Kinematic and Neuromuscular Adaptations in Incomplete Spinal Cord Injury after High- versus Low-Intensity Locomotor Training. J Neurotrauma, 2019. 36(12): p. 2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brazg G, et al. , Effects of Training Intensity on Locomotor Performance in Individuals With Chronic Spinal Cord Injury: A Randomized Crossover Study. Neurorehabil Neural Repair, 2017. 31(10–11): p. 944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwab JM, et al. , The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol, 2014. 258: p. 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vivodtzev I, et al. , Mild to moderate sleep apnea is linked to hypoxia-induced motor recovery after spinal cord injury. American Journal Of Respiratory And Critical Care Medicine, 2020. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burns SP, et al. , Sleep apnea syndrome in chronic spinal cord injury: associated factors and treatment. Arch Phys Med Rehabil, 2000. 81(10): p. 1334–9. [DOI] [PubMed] [Google Scholar]
- 82.Herman P, et al. , Persons with Chronic Spinal Cord Injury Have Decreased Natural Killer Cell and Increased Toll-Like Receptor/Inflammatory Gene Expression. J Neurotrauma, 2018. 35(15): p. 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stein A, et al. , Pilot study: elevated circulating levels of the proinflammatory cytokine macrophage migration inhibitory factor in patients with chronic spinal cord injury. Arch Phys Med Rehabil, 2013. 94(8): p. 1498–507. [DOI] [PubMed] [Google Scholar]
- 84.Kwon BK, et al. , Cerebrospinal Fluid Biomarkers To Stratify Injury Severity and Predict Outcome in Human Traumatic Spinal Cord Injury. J Neurotrauma, 2017. 34(3): p. 567–580. [DOI] [PubMed] [Google Scholar]
- 85.Papatheodorou A, et al. , High-Mobility Group Box 1 (HMGB1) Is Elevated Systemically in Persons with Acute or Chronic Traumatic Spinal Cord Injury. J Neurotrauma, 2017. 34(3): p. 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agosto-Marlin IM, Nichols NL, and Mitchell GS, Systemic inflammation inhibits serotonin receptor 2-induced phrenic motor facilitation upstream from BDNF/TrkB signaling. J Neurophysiol, 2018. 119(6): p. 2176–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huxtable AG, et al. , Intermittent Hypoxia-Induced Spinal Inflammation Impairs Respiratory Motor Plasticity by a Spinal p38 MAP Kinase-Dependent Mechanism. J Neurosci, 2015. 35(17): p. 6871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ling L, et al. , Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci, 2001. 21(14): p. 5381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huxtable AG, et al. , Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. J Appl Physiol (1985), 2013. 114(7): p. 879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGuire M, et al. , Sleep fragmentation impairs ventilatory long-term facilitation via adenosine A1 receptors. J Physiol, 2008. 586(21): p. 5215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas SL and Gorassini MA, Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol, 2005. 94(4): p. 2844–55. [DOI] [PubMed] [Google Scholar]
- 92.Yang JF, et al. , Volitional muscle strength in the legs predicts changes in walking speed following locomotor training in people with chronic spinal cord injury. Phys Ther, 2011. 91(6): p. 931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang JF, et al. , Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial. Neurorehabil Neural Repair, 2014. 28(4): p. 314–24. [DOI] [PubMed] [Google Scholar]
- 94.Christiansen L, et al. , Acute intermittent hypoxia enhances corticospinal synaptic plasticity in humans. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hansen NL, et al. , Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol, 2005. 94(2): p. 934–42. [DOI] [PubMed] [Google Scholar]
- 96.Yang JF and Gorassini M, Spinal and brain control of human walking: implications for retraining of walking. Neuroscientist, 2006. 12(5): p. 379–89. [DOI] [PubMed] [Google Scholar]
- 97.Dale-Nagle EA, et al. , Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci, 2010. 1198: p. 252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao BX and Ziskind-Conhaim L, Development of glycine- and GABA-gated currents in rat spinal motoneurons. J Neurophysiol, 1995. 74(1): p. 113–21. [DOI] [PubMed] [Google Scholar]
- 99.Kaila K, Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol, 1994. 42(4): p. 489–537. [DOI] [PubMed] [Google Scholar]
- 100.Yamada J, et al. , Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol, 2004. 557(Pt 3): p. 829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tashiro S, et al. , BDNF Induced by Treadmill Training Contributes to the Suppression of Spasticity and Allodynia After Spinal Cord Injury via Upregulation of KCC2. Neurorehabil Neural Repair, 2015. 29(7): p. 677–89. [DOI] [PubMed] [Google Scholar]
- 102.Nakazawa K, Kawashima N, and Akai M, Enhanced stretch reflex excitability of the soleus muscle in persons with incomplete rather than complete chronic spinal cord injury. Arch Phys Med Rehabil, 2006. 87(1): p. 71–5. [DOI] [PubMed] [Google Scholar]
- 103.Gomez-Soriano J, et al. , Voluntary ankle flexor activity and adaptive coactivation gain is decreased by spasticity during subacute spinal cord injury. Exp Neurol, 2010. 224(2): p. 507–16. [DOI] [PubMed] [Google Scholar]
- •104.Navarrete-Opazo A, et al. , Intermittent Hypoxia and Locomotor Training Enhances Dynamic but Not Standing Balance in Patients With Incomplete Spinal Cord Injury. Arch Phys Med Rehabil, 2017. 98(3): p. 415–424. [DOI] [PubMed] [Google Scholar]; Study found AIH combined with 45 minutes of BWSTT for 5 consecutive days, followed by 3x/week of AIH+BWSTT, for 3 additional weeks. Investigators found improved dynamic balance after AIH+BWSTT. They showed turning duration by 53% (min) and turning to sit duration by 24% in persons with chronic, incomplete SCI. The study did not find significant improvements in postural sway or Timed-Up-and-Go test.
- 105.Kleim JA, et al. , BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci, 2006. 9(6): p. 735–7. [DOI] [PubMed] [Google Scholar]
- 106.Lamy JC and Boakye M, BDNF Val66Met polymorphism alters spinal DC stimulation-induced plasticity in humans. J Neurophysiol, 2013. 110(1): p. 109–16. [DOI] [PubMed] [Google Scholar]
- 107.Sohn WJ, et al. , Variability of Leg Kinematics during Overground Walking in Persons with Chronic Incomplete Spinal Cord Injury. J Neurotrauma, 2018. 35(21): p. 2519–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grasso R, et al. , Distributed plasticity of locomotor pattern generators in spinal cord injured patients. Brain, 2004. 127(Pt 5): p. 1019–34. [DOI] [PubMed] [Google Scholar]
- 109.Thibaudier Y, et al. , Differential deficits in spatial and temporal interlimb coordination during walking in persons with incomplete spinal cord injury. Gait Posture, 2020. 75: p. 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]


