Abstract
We aimed to determine the effects of feeding a high-oleic peanut (HOPN) diet to egg-producing laying hens on egg quality, digestibility, and feed conversion. Three isonitrogenous and isocaloric dietary treatments were formulated with 1) Control diet (CON)—a corn-soybean meal conventional diet with 7.8 % added poultry fat, 2) HOPN diet—dietary inclusion of ~20% coarse-ground whole HOPN, and 3) oleic acid (CON-OA) diet—a control diet supplemented with 2.6% oleic fatty acid oil. Ninety-nine 57-wk-old brown Leghorn laying hens were randomly assigned to 33 animals per treatment. Animals were housed individually for 8 wk. Body and feed weights were recorded weekly and feed conversation ratio was calculated. Bi-weekly, shell eggs were analyzed for quality (yolk color, albumen height, and Haugh unit [HU]). Jejunum samples were collected at week 8 for histomorphometric analysis. Analysis of variance was performed on all variables using a general linear mixed model. Laying hens fed the CON-OA diet produced greater number of eggs relative to those fed the HOPN and control diets (P < 0.05). The roche yolk color value was higher (P < 0.001) in eggs from hens fed the HOPN diet. There were no differences in laying hen performance, eggshell color, eggshell strength, eggshell elasticity and egg albumen height, or egg HU, ileal fat digestibility, or villi surface among treatment groups. However, the apparent metabolizable energy (P < 0.01) and ileal protein digestibility (P = 0.02) were greater in laying hens fed the HOPN diet relative to the CON diet. This study suggests that whole unblanched high-oleic peanuts may be an acceptable alternative feed ingredient for laying hens.
Keywords: laying hens, alternative feed ingredients, high-oleic peanuts, feed ingredients, feed digestibility
INTRODUCTION
The poultry industry is one of the fastest growing sectors in the world and has met the growing global demand for food in the form of animal protein utilizing high-quality feed ingredients. Yet, over the last decade, animal feed ingredients have become more expensive with increased demand for biofuel, as well as crop shortages in different regions of the world (Seppelt et al., 2014). The poultry industries are of great economic importance and represent a significant portion of the agricultural products sold in the southeastern United States (APHIS–USDA, 2015). However, the need for poultry feed ingredients such as corn and soybean meal far exceed the ability to produce these ingredients within this region. Hence, large quantities of grains and high-protein oilseeds are imported from South America and the U.S. Midwest (ERS–USDA, 2017) for animal feed and food production.
Shapira et al. (2008) demonstrated that an extruded linseed-supplemented diet fed to laying hens increased the omega-3 polyunsaturated fatty acid content in table eggs compared with conventional eggs. Moreover, laying hens fed diets with varying levels of fish meal (0% to 20%) had increased docosahexaenoic acid in egg yolk relative to increased dietary inclusion of fish meal in comparison to the controls with the exception of 15% and 10% fish meal (Howe et al., 2002). Nonetheless, although other laying hen feeding trials utilizing oilseed supplemented diets (sesame meal, sunflower meal) reported no adverse effects on growth performance or egg quality, the lipid profile of the eggs produced from these feeding treatments was not different from conventional eggs (Mamputu and Buhr, 1995; Laudadio et al., 2014).
Over the last two decades, agricultural and scientific research interests have focused on the whole peanut and peanut skins as an alternative feed ingredient for poultry (Toomer et al., 2019). The poultry industries (broilers, eggs, turkeys) predominate within the U.S. southeast with Georgia being the top U.S. producer (National Agricultural Statistics Service, USDA, 2020). However, within this region, the need for poultry feed components such as corn and soybean meal exceed the ability to produce these ingredients locally. Therefore, these grains are imported from South America (Crop Prophet, 2019) and U.S. Midwest (Agweek, 2009). High-oleic and normal-oleic peanuts are grown abundantly within the U.S. Southeast with Georgia being the #1 U.S. producer. Hence, the use of high-oleic peanuts, as a protein and energy-rich feed ingredient for poultry, utilizes regionally abundant commodities, such as peanuts to support animal production within the U.S. Southeast, without the associated cost of importation of much needed corn and soybean meal feed stock rations.
Previous feeding trials defined peanut meal made from normal-oleic peanuts (52% oleic acid and 27% linoleic acid) as a suitable laying hen feed ingredient at 21%, 28%, and 35% inclusion of the diet (Pesti et al., 2003), and 35% inclusion of broiler diets (Costa et al., 2001). Toomer et al. (2019) demonstrated that eggs produced from laying hens fed an unblanched high-oleic peanut (20%) and corn diet had greater yolk color scores, oleic fatty acid, and β-carotene levels relative to the controls. However, no studies to date have examined the use of whole high-oleic peanut cultivars (80% oleic acid and 2% linoleic acid) as a feed ingredient to improve digestibility and feed conversion in laying hens. Thus, we aim to determine the effects of feeding a 20% high-oleic peanut diet to egg-producing laying hens on egg quality, ileal digestibility, and feed conversion of laying hens.
MATERIALS AND METHODS
The procedures used in these studies were approved by the North Carolina State University Institutional Animal Care and Use Committee (IACUC #19–761).
Experimental Design, Animal Husbandry, and Dietary Treatments
Three experimental diets were formulated to be isonitrogenous (18% crude protein) and isocaloric (3,080 kcal/kg) with an estimated particle size between 800 and 1,000 µm (Table 1). The diets were prepared 1 wk prior to the onset of the study and maintained at the feed mill (North Carolina State University) in a cool dry location. A basal control diet (CON, treatment 1) was formulated using yellow corn, conventional defatted soybean meal, corn gluten meal, wheat bran, limestone, and poultry fat as the six major feed ingredients containing 10% total fat. A whole unblanched high-oleic peanut (HOPN) experimental diet (treatment 2) was formulated utilizing peanuts to replace conventional defatted soybean meal (dietary protein source) and poultry fat (dietary lipids) feed ingredients in the basal control diet with 12.7% total fat in the finished feed, while keeping the remaining four major feed ingredient components the same as the basal control diet (yellow corn, corn gluten meal, wheat bran, and limestone). As a consequence of limiting amino acid lysine, threonine, and methionine in peanuts, it was necessary to supplement the high-oleic peanut experimental diet with l-lysine, l-tryptophan, and l-threonine (combined total <1%) to meet the amino acid requirements for egg-producing hens (NRC, 1994). A third experimental diet (treatment 3) was formulated using oleic fatty acid oil to replace 98% of the poultry fat feed ingredient in the basal control diet, while maintaining similar levels of the five major feed ingredients found in the basal diet (yellow corn, conventional defatted soybean meal, corn gluten meal, wheat bran, and limestone). The oleic acid (CON-OA) used was food grade quality purchased from Millipore Sigma (Burlington, MA) with the finished feed containing 6.2% total fat. Experimental finished diets were analyzed and determined to be free of aflatoxin and microbiological contaminants by the North Carolina Department of Agriculture and Consumer Services, Food and Drug Protection Division Laboratory (Raleigh, NC).
Table 1.
Formulated Dietary Treatments and Composition of Experimental Diets in Percent by Weight
| Ingredient, % | CON1 | HOPN2 | CON-OA3 |
|---|---|---|---|
| Corn yellow | 46.44 | 39.00 | 52.25 |
| Soybean meal | 21.44 | 0.00 | 20.45 |
| High oleic peanut, raw | 0.00 | 20.00 | 0.00 |
| Corn gluten | 5.00 | 10.45 | 4.95 |
| Wheat bran | 6.00 | 16.75 | 6.00 |
| Limestone | 10.81 | 10.75 | 11.27 |
| Poultry fat | 7.80 | 0.00 | 0.05 |
| Oleic acid oil | 0.00 | 0.00 | 2.60 |
| Di-calcium phosphorous | 1.58 | 1.41 | 1.51 |
| Salt, plain | 0.25 | 0.25 | 0.25 |
| dl-Methionine | 0.11 | 0.08 | 0.10 |
| l-Lysine | 0.00 | 0.49 | 0.00 |
| l-Tryptophan | 0.00 | 0.06 | 0.00 |
| l-Threonine | 0.00 | 0.12 | 0.01 |
| Propionic acid | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.17 | 0.24 | 0.16 |
| Trace mineral mix4 | 0.20 | 0.20 | 0.20 |
| Vitamin mix5 | 0.10 | 0.10 | 0.10 |
| Selenium6 | 0.05 | 0.05 | 0.05 |
1CON = conventional corn and soybean diet.
2HOPN = high-oleic peanut + corn.
3CON-OA = control spiked with 2.6% oleic fatty acid oil.
4NC State University mineral premix supplied the following per kg of diet: manganese, 120 mg manganese, 120 mg zinc, 80 mg iron, 10 mg copper, 2.5 mg iodine, and 1 mg cobalt.
5NC State University vitamin premix supplied the following per kg of diet: 13,200 IU vitamin A, 4,000 IU vitamin D3, 33 IU vitamin E, 0.02 mg vitamin B12, 0.13 mg biotin, 2 mg menadione (K3), 2 mg thiamine, 6.6 mg riboflavin, 11 mg d-pantothenic acid, 4 mg vitamin B6, 55 mg niacin, and 1.1 mg folic acid.
6NC State University selenium premix provided 0.2 mg Se (as Na2SeO3) per kg of diet.
Ninety-nine 57-wk brown leghorn laying hens (North Carolina State University Flock) were randomly assigned to one of three treatment groups. Each treatment was replicated three times with 11 laying hens per replicate with 33 laying hens and one laying hen per replicate cage at the Chicken Educational Unit (North Carolina State University). Laying hens were provided feed and water ad libitum throughout the 8-wk study. Body and feed weights were recorded weekly. Shell eggs were collected, enumerated, and weighed daily and recorded from each hen. Bi-weekly, shell eggs were analyzed for quality and USDA grading in the Egg Quality Lab (North Carolina State University).
Laying Hen Feed Analysis
Total nitrogen was determined in homogenized samples by combustion using an Elementar N cube analyzer (Elementar Americas, Mt. Laurel, PA) according to the method 990.03 (AOAC, 2006). A Kjeldahl conversion factor of 6.25 (mixed food) was used to calculate total protein in the samples. Total fat as triglycerides was determined in the samples gravimetrically after Soxhlet extraction. Samples were extracted for 6 h using continuous extraction with hexane (Method 920.39; AOAC, 1990).
Egg Quality and Grading
Albumen height was measured and Haugh unit (HU; Haugh, 1937) was calculated and recorded using the TSS QCD system (Technical Services and Supplies, Dunnington, York, UK) to determine egg albumen quality. Yolk color was determined by using TSS QCD System yolk color scan, which was calibrated to the DSM Yolk Color Fan (a color index 1 to 15 to distinguish the yolk color density from lightest to darkest color intensity; Vuilleumier, 1969). Eggs were evaluated for exterior and interior grade standards in accordance with the USDA Standards (USDA, 2010). Eggs were sized weekly by treatment (USDA, 2010). Egg sizing was classified by a minimum net weight per egg: peewee (<42.6 g), small (42.6 < 49.7 g), medium (49.7 < 56.8 g), large (56.8 < 63.9 g), extra-large (63.9 < 70.9 g), and jumbo (>70.9 g). Eggshell strength was tested using a TA-HD plus texture analyzer and elasticity for all treatment groups (Texture Technologies Corp. and Stable Micro Systems Ltd., Hamilton, MA).
Lipid and Fatty Acid Analysis
Lipid and fatty acid analysis (total fat, total cholesterol, total palmitic acid, total stearic acid, total oleic fatty acid, n9 elaidic acid, total linolenic acid, and total linoleic fatty acid) of feed samples from the three treatment groups (Table 2) were analyzed using modified direct methylation methods as described by Wang et al. (2000) in the Food Science and Market Quality and Handling Research Unit.
Table 2.
Chemical Lipid and Fatty Acid Analysis of Dietary Treatments1
| Chemical component | CON2 | HOPN3 | CON-OA4 | SEM | P-value |
|---|---|---|---|---|---|
| Percentage of total lipid content, % | |||||
| Crude fat | 10.06b | 12.71a | 6.18c | 0.037 | <0.001 |
| Palmitic acid (16:0) | 22.58a | 7.51c | 14.64b | 0.005 | <0.001 |
| Palmitoleic acid (16:1cis) | 5.46a | 0.510c | 1.27b | 0.001 | <0.001 |
| Oleic acid (18:1) | 38.42c | 74.19a | 43.8b | 0.003 | <0.001 |
| Elaidic acid (trans-9 C18:1) | 2.78b | 0.94c | 5.84a | 0.012 | <0.001 |
| Linoleic acid (18:2) | 21.69a | 8.77c | 20.67b | 0.001 | <0.001 |
| Linolenic acid (18:3cis) | 1.04b | 0.42c | 1.66a | 0.001 | <0.001 |
| Stearic acid (18:0) | 5.37a | 1.92c | 3.81b | 0.002 | <0.001 |
| Omega 6 | 21.69a | 8.77b | 1.28c | 0.001 | <0.001 |
| Total cholesterol | 50.40a | 8.40b | 3.59c | 0.007 | <0.001 |
| Gross energy, kcal/kg feed | 4,129b | 4,205a | 3,802c | 0.045 | <0.001 |
1Three replicate samples were collected from each dietary treatment and were analyzed for lipid and fatty acid content. Fatty acid content defined as the percentage of the total lipid content of 100-g sample.
2CON = conventional corn and soybean diet.
3HOPN= high-oleic peanut + corn.
4CON-OA = control spiked with 2.6% oleic fatty acid oil.
a,bMeans within the same row lacking a common superscript differ significantly (P < 0.05).
Ileal Digestibility, Metabolize Energy, and Intestinal Morphology Analysis
To determine the digestibility of the feed ingredients, the feed that was fed to the birds during the last 5 d contained 2% of CELITE (Diatomaceous Earth; Celite Corp, Lompoc, CA) as an insoluble ash marker in the diet. This was done to evaluate the digestibility of nutrients with a partial excreta collection, according to Huang et al. (2006).
At termination of the experimental period, nine laying hens per treatment (three per replicate) were selected for sampling of the jejunum, ileum content, and excreta. Samples of the jejunum were collected for histomorphometric analysis using standard light histological H&E staining procedures. Briefly, a 1-cm segment of the midpoint of the duodenum and the distal end of the lower ileum were removed and fixed in 10% buffered formalin for 72 h. Each segment was then embedded in paraffin, and a 2-μm section of each sample was placed on a glass slide and stained with hematoxylin and eosin for examination with a light microscope (Sakamoto et al., 2000). Jejunal villus height was measured from the villus tip to the villus crypt junction and crypt depth was identified as the depth between two adjacent villi, according the methodology of Solis de los Santos et al. (2005). The parameters evaluated were villus height, villus base, villus surface area, lamina propria thickness, and crypt depth. Morphological parameters were measured using the Image Pro Plus v 4.5 (Media Cybernetics, Rockville, MD).
To collect the ileum contents after separation of the whole intestine tract, from Meckel’s diverticulum to the ileal–cecal–colon junction, the digesta contents were gently squeezed (using fingers and small amounts of distilled water from a wash bottle) directly into 250-mL specimen cups. Ileum digesta samples were held on ice and then frozen (−15 °C) and stored for future analysis. Excreta from each hen was collected for 48 hr in aluminum pans directly placed under each cage and hen 2 d prior to termination of the experiment. Subsequently, the excreta from each cage was keep in separate plastic bags and stored at −18 °C, until further analyzes.
Excreta, ileal contents, and feed samples were oven dried at 70 °C for 48 h and ground through a 1-mm screen prior to analysis. Gross energy (GE) of feed and dried digesta was determined by adiabatic oxygen bomb calorimeter (IKA model C5003 connected to compressed oxygen with NESLAB Refrigerated Re-circulator CFT-25). Digesta and feed samples were placed in a pellet press and compacted. Compacted samples were weighed and recorded. Samples were placed into a metal thimble and IKA brand 50J cotton twist for combustion in the decomposition vessel or combustion canister. Samples were placed within the decomposition vessel, sealed, and the calibrated samples were combusted. Acid-insoluble ash determination of feed and excreta samples were determined using ash residue (Vogtmann et al., 1975). Samples were boiled in 25 mL of 4N hydrochloric acid, washed, and filtered using ash-less paper. Subsequently, the filter paper and residue were placed in a muffle furnace at 600 °C for 10 h, cooled, and weighed. Digesta collected from the ileum and feed samples were dried over a 2-d period in an industrial Blue-M Drying oven at 70 °C. Dried samples were finely ground. Total fat content was determined gravimetrically after Soxhlet extraction. Samples were filtered and placed within Whatman Cellulose extraction thimbles (26 mm × 60 mm) and loaded within the Soxhtec System HT 6 1043 extraction unit Foss Tecator. Clean and labeled 100-mL metal canisters were weighed by the same scale and then filled with 50 mL of diethyl ether. Weights of the samples and the canisters were recorded. The metal canisters were placed into metal loaders and sealed within the extraction unit underneath the cellulose thimbles and heated to 60 °C. Loaded samples were allowed to remain in diethyl ether for 40 min at 60 °C. Extracted fat present within the metal canister was weighed and recorded to determine ratio of fat of each sample. Additionally, feed, ileum, and fecal samples were shipped to ATC Scientific (Little Rock, AR) for proximate analysis of crude protein. Total nitrogen levels were determined through combustion using an Elementar N cube analyzer (Elementar Americas, Mt. Laurel, PA) on homogenized samples according to method 990.03 (AOAC, 2006) methods. The total protein in each sample was calculated using a Kjeldahl conversion factor of 6.25. Apparent metabolizable energy, corrected by nitrogen calculated, was calculated using the following formula (Titus, 1956; Li et al., 2013):
Statistical Analysis
Individual dependent variables (with each cage per hen serving as an experimental unit) were analyzed as a one-way analysis of variance (ANOVA) using the Proc Mixed procedure of JMP (2013) using a general linear mixed model to evaluate differences between the control and treatments. Means were separated at P < 0.05 using least squares means with Tukey–Kramer adjustment for multiple comparisons. Data are expressed as means and SEM. Means were considered significantly different among treatments when P < 0.05.
RESULTS
Feed Analysis
The experimental diets were formulated to be isocaloric (18% crude protein) and isonitrogenous (3080 kcal/kg feed); however, upon analysis, the fatty acid profiles for the diets were different. The analyzed crude protein content of the experimental diets was the following: CON 16.84% crude protein, HOPN diet 17.68% crude protein, and CON-OA diet 19.18% crude protein (data not shown). Upon chemical analyzation, the HOPN diet had greater (P < 0.001) crude total fat relative to the CON and CON-OA diets (Table 2). The CON diet had a greater content (P < 0.001) of saturated fatty acids, palmitic acid, and stearic acid, relative to the HOPN and CON-OA diets. Additionally, the CON-OA diet was greater in both palmitic acid (P < 0.001) and steric acid (P < 0.001) compared with the HOPN diet.
The HOPN diet had a greater (P < 0.001) amount of the monounsaturated oleic fatty acid relative to the control diet. In addition, the CON-OA had a greater (P < 0.001) content of OA relative to the control diet. However, the monounsaturated fatty acid palmitoleic acid was greater (P < 0.001) in the control diet compared with the HOPN and CON-OA diets. Additionally, the CON-OA diet had a greater (P < 0.001) palmitoleic acid content compared with the HOPN diet. The CON-OA diet had a greater (P < 0.001) amount of trans-fatty acid elaidic acid compared with the HOPN and control diets. Yet, the CON diet had a greater (P < 0.001) amount of elaidic acid compared with the HOPN diet.
The control and CON-OA experimental diets had greater (P < 0.001) amounts of the polyunsaturated fatty acid linoleic acid in comparison to the control diet. Additionally, the CON-OA diet had a greater (P < 0.001) linoleic acid (18:2) content compared with the HOPN diet. Also, the CON and CON-OA diets had greater (P < 0.001) amount of the linolenic acid (18:3) relative to the HOPN diet. The CON diet had greater (P < 0.001) omega 6 and total cholesterol levels in comparison to the HOPN and CON-OA diets. However, the HOPN diet had a greater (P < 0.001) amount of omega 6 and cholesterol relative to the CON-OA diet. Lastly, the HOPN diet had more (P < 0.001) gross energy in comparison to the CON and CON-OA diets, while the CON diet had more (P < 0.001) gross energy relative to the CON-OA diet (Figure 1).
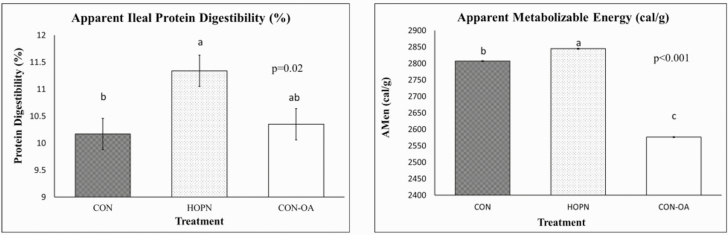
Figure 1.
Effect of a high-oleic peanut diet on apparent protein ileal digestibility and metabolizable energy in laying hens. Three isonitrogenous experimental diets (approximately 18% crude protein) were fed to ninety-nine 57-wk-old laying hens with 33 laying hens per treatment with three replicates of 11 laying hens per replicate for 8 wk: Conventional corn and soybean diet (CON), high-oleic peanut + corn (HOPN), or control spiked with 2.6% oleic fatty acid oil (CON-OA). Each bar graph represents the average ± SEM. a,bBar graphs with differing superscript are significantly different (P < 0.05).
Egg Production Parameters
Laying hens fed the CON-OA diet had higher number of eggs/hens at time points week 2 (P = 0.005) and week 6 (P = 0.03) as compared with hens fed HO PN diet (Table 3). Yet, there were no treatment differences in the total number of eggs produced between treatments groups at week 4 and week 8. Also, there were no differences in egg weight between experimental groups at weeks 4 and 8, while there were differences at week 2 (P = 0.005) and week 6 (P = 0.005) in egg weights, with smaller egg weights produced from hens fed the HOPN diet relative to the CON diets. There were no differences (P < 0.05) in the egg HU between the treatment groups at any of the time points measured (weeks 2, 4 6, and 8). Laying hens fed the CON diet had a higher feed conversion ratio (FCR; kg feed/dozen eggs) at week 2 (P = 0.01) and week 4 (P = 0.0002) relative to the CON-OA and HOPN diets, whereas there were no differences in FCR between treatments at week 6 and week 8. There were no significant differences between treatment blocks and replicates within each treatment.
Table 3.
Laying hen egg production parameters and quality of eggs produced by hens fed diets containing high-oleic peanuts (HO PN), oleic acid (OA), or a conventional soybean meal
| Dietary treatment | |||||
|---|---|---|---|---|---|
| Item | CON1 | HOPN2 | CON-OA3 | SEM4 | P-value |
| Number of eggs/hen5 | |||||
| Week 2 | 12.9ab | 12.5b | 13.6a | 0.22 | 0.005 |
| Week 4 | 12.6 | 12.4 | 13.1 | 0.30 | 0.18 |
| Week 6 | 19.1ab | 18.6b | 20.0a | 0.40 | 0.03 |
| Week 8 | 10.72 | 10.12 | 10.6 | 0.40 | 0.5 |
| Egg weight, g | |||||
| Week 2 | 67.25a | 62.36b | 64.65ab | 1.02 | 0.005 |
| Week 4 | 66.59 | 65.57 | 66.26 | 1.20 | 0.82 |
| Week 6 | 66.56a | 62.01b | 65.25ab | 0.97 | 0.005 |
| Week 8 | 68.02 | 65.70 | 64.87 | 1.22 | 0.18 |
| Haugh unit, HU | |||||
| Week 2 | 90.05 | 92.50 | 92.72 | 2.26 | 0.65 |
| Week 4 | 95.16 | 94.14 | 92.85 | 1.56 | 0.58 |
| Week 6 | 89.74 | 94.93 | 94.85 | 1.80 | 0.07 |
| Week 8 | 91.52 | 91.31 | 91.13 | 1.64 | 0.99 |
| FCR, kg feed/dozen eggs6 | |||||
| Week 2 | 3.46a | 2.98ab | 2.63b | 0.20 | 0.01 |
| Week 4 | 2.28a | 1.79b | 1.72b | 0.10 | < 0.001 |
| Week 6 | 1.91 | 1.81 | 1.80 | 0.09 | 0.68 |
| Week 8 | 2.01 | 2.13 | 2.22 | 0.16 | 0.63 |
1CON = conventiosnal corn and soybean diet.
2HOPN = high-oleic peanut + corn.
3CON-OA = control spiked with 2.6% oleic fatty acid oil.
4SEM = standard error of mean.
5Number of eggs/hen at 2-wk intervals.
6FCR = feed conversion ratio at 2-wk intervals, kg total feed consumed/total dozen eggs produced.
a,bMeans within the same row lacking a common superscript differ significantly (P < 0.05).
Egg Quality
All eggs produced at all-time points (week 1 to week 8) between the experimental treatments were graded as USDA Grade AA of superior quality. The shells were clean, without defects and there were minimal number of blood spots or meats pots. The yolk color roche score was examined bi-weekly to determine the yolk color intensity produced from the dietary treatments (Table 4). Interestingly, the yolk color roche value was greater (P < 0.001) in eggs produced from laying hens fed the HOPN diets relative to the other treatment at all-time points measured. At weeks 2, 4, and 8, there were no differences in egg yolk color intensity between laying hens fed the CON and CON-OA diets. At week 6, egg yolk color intensity was different between all treatment groups, with eggs produced from hens fed the HOPN diet having the greatest yolk color intensity, and eggs produced from hens fed the CON diet having the lowest yolk color intensity. There were no differences in shell color, shell strength, shell elasticity, and albumen height between the treatment groups at any of the time points measured (weeks 2, 4, 6, and 8). There were no significant differences between treatment blocks and replicates within each treatment.
Table 4.
Egg quality of eggs produced from laying hens fed a diet with high-oleic peanuts (HO PN), oleic acid (OA), or a conventional diet with soybean meal
| Dietary treatment | |||||
|---|---|---|---|---|---|
| Item | CON1 | HOPN2 | CON-OA3 | SEM4 | P-value |
| Yolk color (1 to 15)5 | |||||
| Week 2 | 5.28b | 6.17a | 5.17b | 1.64 | <0.0001 |
| Week 4 | 4.67b | 7.44a | 5.22b | 0.19 | <0.0001 |
| Week 6 | 5.00c | 6.83a | 5.94b | 0.20 | <0.0001 |
| Week 8 | 4.78b | 6.22a | 5.33b | 0.22 | <0.0001 |
| Shell color6 | |||||
| Week 2 | 26.89 | 29.02 | 25.89 | 1.41 | 0.28 |
| Week 4 | 26.95 | 27.88 | 24.82 | 1.17 | 0.17 |
| Week 6 | 23.41 | 24.44 | 24.55 | 1.02 | 0.68 |
| Week 8 | 27.14 | 26.01 | 24.38 | 1.08 | 0.20 |
| Shell strength, g | |||||
| Week 2 | 5,147.5 | 4597.5 | 4738.0 | 179.2 | 0.08 |
| Week 4 | 4,890.8 | 5406.8 | 5240.4 | 208.3 | 0.21 |
| Week 6 | 4,969.3 | 5366.3 | 5048.0 | 158.5 | 0.18 |
| Week 8 | 4,305.1 | 4712.6 | 4629.5 | 190.8 | 0.28 |
| Shell elasticity, mm | |||||
| Week 2 | 0.286 | 0.229 | 0.243 | 0.006 | 0.17 |
| Week 4 | 0.231 | 0.234 | 0.243 | 0.007 | 0.44 |
| Week 6 | 0.240 | 0.250 | 0.240 | 0.008 | 0.57 |
| Week 8 | 0.215 | 0.233 | 0.227 | 0.006 | 0.16 |
| Albumen height, mm7 | |||||
| Week 2 | 8.77 | 8.80 | 8.89 | 0.35 | 0.96 |
| Week 4 | 9.63 | 9.2 | 8.98 | 0.37 | 0.45 |
| Week 6 | 8.59 | 9.20 | 9.35 | 0.32 | 0.22 |
| Week 8 | 8.84 | 8.73 | 8.64 | 0.31 | 0.90 |
1CON = conventional corn and soybean diet.
2HOPN = high-oleic peanut + corn.
3CON-OA = control spiked with 2.6% oleic fatty acid oil.
4SEM = standard error of mean.
5Yolk color was determined using the Roche Color Fan color index 1 to 15 to distinguish lightest to darkest, respectively.
6Shell color is based on the reflectance with the lower the number the whiter the shell.
7Egg albumen height (mm was calculated to determine egg albumen quality).
a,bMeans within the same row lacking a common superscript differ significantly (P < 0.05).
Apparent Ileal Digestibility, Apparent Metabolizable Energy, and Intestinal Morphology
There were no differences in apparent ileal fat digestibility (P = 0.14; CON 95.7 ± 5.5 vs. HOPN 80.4 ± 5.5 vs. CON-OA 92.6 ± 5.5 %) or villi surface area (P = 0.58; CON 2,6797.9 ± 5,359 vs. HOPN 18,594.6 ± 5,620 vs. CON-OA 22,547.4 ± 22,547 µm2) between treatment groups after 8 wk of feeding the experimental diets in laying hens. However, the corrected apparent metabolizable energy (P < 0.001; Figure 1) was greater in laying hens fed the HOPN diet compared with hens fed the CON and CON-OA diets. Ileal protein digestibility was greater in hens fed the HOPN diet relative to the controls (P = 0.02; Figure 1), whereas the ileal protein digestibility was similar between hens fed the control and CON-OA diets. There were no significant differences between treatment blocks and replicates within each treatment.
DISCUSSION
Although the experimental dietary treatments were formulated to be isocaloric and isonitrogenous, the HOPN diet had a greater amount of crude fat and gross energy relative to the CON and CON-OA diets. Dietary energy is acquired from dietary carbohydrates and lipids, found in the yellow corn and poultry fat feed ingredients in the CON diet. Oleic fatty acid oil replaced 98% of the poultry fat in the CON diet formulation, thus yellow corn, and oleic fatty acid along with minor amounts of poultry fat were sources of dietary energy in the CON-OA feed treatment. High-oleic peanuts are rich in (≈50%) lipids and (≈30%) proteins (Settaluri et al., 2012; Zhao et al., 2012), thus formulation with HOPN served to replace two of the six major feed ingredient components of the basal control diet (soybean meal and poultry fat) and provided both dietary energy and protein in combination. Additionally, the HOPN feeding treatment was formulated with yellow corn, providing a major source of dietary energy, which explains increased levels of total gross energy relative to the other experimental diets and increased apparent metabolizable energy. In general peanuts (normal oleic and high oleic) and peanut oil contain three major fatty acids that are present as acylglycerols esters formed from glycerol and fatty acids palmitic, oleic acid, and trace levels of linoleic acid (Carrin and Carelli, 2010). Studies have shown that linoleic essential fatty acid deficiencies in the diets of laying hens adversely affect egg production, whereas dietary fats are required for the absorption of fat-soluble vitamins (A, D, E, and K) needed for egg production (Jacob et al., 1998). Thus, the poultry fat (control diet), high-oleic peanuts (HOPN diet), and oleic fatty acid oil (CON-OA diet) in the experimental diets provided the dietary fats needed for absorption of fat-soluble vitamins, while providing ample dietary linoleic essential fatty acid for egg production.
Laying hens fed the CON-OA diet produced a greater number of eggs relative to the number of eggs produced by hens fed the HOPN diet, with exception of the last week of the study. However, there were no differences in the total number of eggs produced upon comparison between the CON-OA and CON and upon comparison of the HOPN and CON, suggesting that egg production is not influenced by source of dietary lipids (98% oleic fatty acid + 2% poultry fat in CON-OA diet vs. 100% poultry fat in CON diet). Also, these results suggest that egg production is not influence by the source of dietary protein/amino acids upon comparison of egg production between HOPN fed hens and CON fed hens (high-oleic peanuts providing a combined source of protein and lipids in the HOPN diet versus soybean meal providing primary source of protein in the control diet). Research has shown that laying hen egg production is adversely affected by inadequate dietary energy, protein, or calcium and requires a nutritionally balanced diet for optimal egg production (Jacob et al., 1998).
Within the first 4 wk of the study, hens fed the CON-OA diet had an improved FCR compared with hens fed the CON diet. Additionally, hens fed the HOPN experimental diet had improved FCR in comparison to hens fed the conventional control diet at the 4-week time point. Hence, suggesting that HOPN fed birds consumed less of the energetically dense HOPN experimental diet to meet the metabolic needs of similar bi-weekly egg production comparative to the other treatment groups while utilizing less total feed (kilogram) and improved FCR.
At weeks 2 and 6, eggs produced from hens fed the HOPN diet had reduced egg weights relative to the CON, which parallel results by Van Elswyk et al. (1994) demonstrating that hens fed a diet rich in unsaturated fatty acids from fish oil produced eggs with lower weights relative to conventional eggs. Thus, it could be assumed that feeding a diet rich in unsaturated fatty acids in laying hens as a potential feeding regimen to reduce oversized eggs produced in older laying hens.
In this study, there were no differences in the egg HU, between laying hens fed the control, HOPN and CON-OA diets across all experimental time points (weeks 2, 4, 6, and 8). In parallel, Krawczyk et al. (2015) evaluated the effects of various dietary inclusion levels of raw yellow lupine seed meal on egg quality and reported that no differences were found between the conventional controls and experimental treatment groups. Also, Toomer et al. (2019) reported that no differences in egg quality were found between laying hens feed a HOPN supplemented diet and CON diet after 10 weeks. But other studies by Yuan et al. (2019) demonstrated that dietary supplementation with rapeseed oil to egg-producing hens improved albumen quality in the eggs produced.
The yolk color (DSM Yolk Color Fan) score was examined bi-weekly to determine the yolk color intensity of eggs produced from the three experimental diets. Yolk color was greater in eggs produced from hens fed the HOPN diets at all-time points when compared with eggs produced from hens fed the CON-OA and CON. Similarly, Toomer et al. (2019) reported that eggs produced from laying hens fed a HOPN diet had greater yolk color scores relative to hens fed a conventional soybean meal and corn control diet. Although Sangkaew et al. (2019) demonstrated no significant differences in yolk color scores between eggs produced from layers fed a conventional diet versus a diet of high-oleic sunflower oil (HOSO) diet, yolk color scores were observably darker in eggs produced from hens fed the HOSO diet relative to the controls.
Yolk color depends on the consumption of pigmented substances (i.e., carotenoids) found in the feed (Lessire et al., 2017). Sangkaew et al. (2019) suggested that the color intensity increase may be due to the high level of pigment (β-carotene) in the diets in which sunflower oil contains the natural pigment β-carotene similar to that found in the unblanched high-oleic peanut diet and peanut oil. A possible explanation for the increase in yolk color intensity may be due to the carotenoid and/or polyphenolic compounds found present in unblanched high-oleic peanuts (Toomer et al., 2019) in addition to the source and level of natural pigments precursors in the diet (An et al., 2010). In this study, the HOPN diet contained slightly higher corn gluten meal, which may have contributed to a minor influence on yolk color intensity. Jiang et al. (2013) and Swiatkiewicz and Koreleski (2006) demonstrated that increasing the concentration of corn distillers dried grains with solubles (DDGS) fed to laying hens increases the yellow color intensity in the yolk. As the DDGS level increased in the diet xanthophyll pigment concentration also increases, resulting in the increase in yolk color intensity (Sun et al., 2013). In addition, the xanthophyll content in DDGS is three times as concentrated due to the removal of starch in the fermentation process during preparation (Jiang et al., 2013). Abd El-Hack et al. (2019) also demonstrated that increasing the concentrations of DDGS (0%, 6%, 12%, and 18%) increased yolk color density. However, eggs produced from laying hens fed diets supplemented with rapeseed oil (Yuan et al., 2019) and/or canola oil at concentrations 2%, 4%, and 6% (Gul et al., 2012) had reduced yolk color intensity scores relative eggs produced by laying hens fed the control diet.
Eggshell quality, color, and strength can be affected by many factors such as strain, age, and nutrition (Mu et al., 2019). In this study, there were no differences in shell color, shell strength, shell elasticity, and albumen height between dietary treatment groups at weeks 2, 4, 6, and 8. In parallel, other studies demonstrated that eggs produced from laying hens fed diets supplemented with various feed ingredients such as myoinositol (Zyla et al., 2012), full fat soybeans at inclusion levels 10% to 22% (Senkoylu et al., 2005), glycerol (Cufadar et al., 2016), yellow lupine (Krawczyk et al., 2015), and hemp seed and hemp seed oil (Gakhar et al., 2012; Neijat et al. (2014) had no effect on egg shell strength, eggshell weight, eggshell thickness, and albumen height. However, Jiang et al. (2013) and Shalash et al. (2010) reported that including DDGS increased eggshell thickness while not affecting eggshell strength.
In this study, there were no differences in fat digestibility or villi surface area between dietary treatment groups (data not shown). However, the apparent metabolizable energy and ileal protein digestibility were higher in laying hens fed the HOPN diet relative to the CON diet, implying that the HOPN diet supplied a greater amount of digestible dietary energy relative to the other treatment groups. Dietary energy is obtained from carbohydrate and lipid sources within the diet. Yellow corn and poultry fat were the primary sources of dietary energy in the conventional control diet, whereas the lipids in the high-oleic peanuts would have provided the primary source of dietary energy in the HOPN diet. Nonetheless, the utilization of a grain or oilseed-based experimental diets complicates the interpretation of the effects of these feed ingredients on the desired variables due to the presence of a combination of various nutrients found within grains and oilseeds. Protein ileal digestibility was enhanced in hens fed the HOPN diet relative to CON fed hens, implying that protein within the high-oleic peanuts (predominate source of protein) and the minor amount of free amino acids supplemented to the HOPN diet (<1% total) provided greater digestible protein relative to soybean meal in the control diet. Although grains and oilseeds may provide a primary source of nutrients (yellow corn-carbohydrates, peanuts-protein, soybean meal-protein), they also provide other nutrients in combination (carbohydrates, fiber, minerals, vitamins). Thus, we aim to perform future studies utilizing purified experimental diets for improved dietary comparisons and interpretation of the dietary effects between feed ingredients.
In summary, feeding a HOPN diet to laying hens increased egg yolk color, apparent metabolizable energy, and ileal protein digestibility relative to a conventional soybean meal and corn laying hen diet. Thus, suggesting that whole unblanched high-oleic peanuts may be an acceptable protein and energy-rich feed ingredient for laying hens. However, additional studies are needed to further explore the effects of a HOPN diet on the nutrient digestibility in laying hens and to determine the true economic feasibility of whole unblanched high-oleic peanuts as a poultry feed ingredient.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Prestage Department of Poultry Science, the NC State University Feed Mill, Birdsong Peanuts, and the Food Science and Market Quality and Handling Research Unit-ARS for their contributions to this study. This work was supported by funds from the North Carolina Peanut Growers Association (Funding Source 572099-87361) and the Food Science & Market Quality & Handling Research Unit – ARS – United States Department of Agriculture (CRIS Project Number 6070-43440-011-00D).
Conflict of interest statement. The authors have no conflicts of interest to declare.
LITERATURE CITED
- Abd El-Hack, M. E., Mahrose K. M., Attia F. A. M., Swelum A. A., Taha A. E., Shewita R. S., and Alowaimer A. N.. . 2019. Laying performance, physical, and internal egg quality criteria of hens fed distillers dried grains with solubles and exogenous enzyme mixture. Animals 9:1–17. doi: 10.3390/ani9040150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agweek. 2009. Top 10 corn and soybean producing states. Available from http://www.agweek.com/news/3787583-top-10-corn-and-soybean-producing-states. (Accessed 13 January 2021).
- An, S. Y., Guo Y. M., Ma S. D., Yuan J. M., and Liu G. Z.. . 2010. Effects of different oil sources and vitamin E in breeder diet on egg quality, hatchability and development of the neonatal offspring. Asian-Australas J. Anim. Sci. 23:234–239. doi: 10.5713/ajas.2010.90140 [DOI] [Google Scholar]
- AOAC 920.39. 1990. Fat (crude) or ether extract in animal feed. In: K. Helrich, editor. Official methods of analysis of the association of official analytical chemists, 15th edn. Arlington (VA): AOAC Inc. P. 79.
- AOAC. 2006. Protein (crude) in animal feed, combustion method. In: Horwitz W. H. and Latimer G. W., editors. Official methods of analysis of the association of official analytical chemists, 18th edn. Gaithersburg (MD): AOAC International. p. 30–31. [Google Scholar]
- APHIS–USDA. 2015. Overview of U.S. livestock, poultry, and aquaculture production. Available from https://www.aphis.usda.gov/animal_health/nahms/downloads/Demographics2015.pdf. (Accessed September 2019). doi: 10.1079/WPS200565 [DOI]
- Carrin, M. E., and Carelli A. A.. . 2010. Peanut oil: compositional data. Eur. J. Lipid Sci. Tech. 112:697–707. doi: 10.1002/ejlt.200900176 [DOI] [Google Scholar]
- Costa, E. F., Miller B. R., Pesti G. M., Bakalli R. I., and Ewing H. P.. . 2001. Studies on feeding peanut meal as a protein source for broiler chickens. Poult. Sci. 80:306–313. doi: 10.1093/ps/80.3.306 [DOI] [PubMed] [Google Scholar]
- Crop Prophet. 2019. Top 10 soybean producing countries. Available from https://www.cropprophet.com/top-10-global-soybean-producers/ (accessed 13 January 2021).
- Cufadar, Y., Göçmen R., and Kanbur G.. . 2016. The effect of replacing soya bean oil with glycerol in diets on performance, egg quality and egg fatty acid composition in laying hens. Animal 10:19–24. doi: 10.1017/S1751731115001950 [DOI] [PubMed] [Google Scholar]
- Economic Research Service (ERS)–USDA. 2017. Soybeans and oil crops: trade-major foreign soybean exporters and importers. Available from https://www.ers.usda.gov/webdocs/outlooks/84268/ocs-17g.pdf?v=8986.1. (Accessed August 2019).
- Gakhar, N., Goldberg E., Jing M., Gibson R., and House J. D.. . 2012. Effect of feeding hemp seed and hemp seed oil on laying hen performance and egg yolk fatty acid content: evidence of their safety and efficacy for laying hen diets. Poult. Sci. 91:701–711. doi: 10.3382/ps.2011-01825 [DOI] [PubMed] [Google Scholar]
- Gul, M., Yoruk M. A., Aksu T., Kaya A., and Kaynar O.. . 2012. The effect of different levels of canola oil on performance, eggshell quality and fatty acid composition of laying hens. Int. J. Poult. Sci. 11:769–776. doi: 10.3923/ijps.2012.769.776 [DOI] [Google Scholar]
- Haugh, R. R. 1937. The Haugh unit for measuring egg quality. US Poult. Mag. 43:552–573. [Google Scholar]
- Howe, P. R., Downing J. A., Grenyer B. F., Grigonis-Deane E. M., and Bryden W. L.. . 2002. Tuna fishmeal as a source of DHA for n-3 PUFA enrichment of pork, chicken, and eggs. Lipids 37:1067–1076. doi: 10.1007/s11745-002-1002-3 [DOI] [PubMed] [Google Scholar]
- Huang, K. H., Li X., Ravindran V., and Bryden W. L.. . 2006. Comparison of apparent ileal amino acid digestibility of feed ingredients measured with broilers, layers, and roosters. Poult. Sci. 85:625–634. doi: 10.1093/ps/85.4.625 [DOI] [PubMed] [Google Scholar]
- Jacob, J. P., Wilson H. R., Miles R. D., Butcher G. D., and Mather F. B.. . 1998. Factors affecting egg production in backyard chicken flocks.University of Florida IFAS Extension. Available from https://edis.ifas.ufl.edu/pdffiles/PS/PS02900.pdf. [Google Scholar]
- Jiang, W., Zhang L., and Shan A.. . 2013. The effect of vitamin E on laying performance and egg quality in laying hens fed corn dried distillers grains with solubles. Poult. Sci. 92:2956–2964. doi: 10.3382/ps.2013-03228 [DOI] [PubMed] [Google Scholar]
- Krawczyk, M., Przywitowski M., and Mikulski D.. . 2015. Effect of yellow lupine (L. luteus) on the egg yolk fatty acid profile, the physicochemical and sensory properties of eggs, and laying hen performance. Poult. Sci. 94:1360–1367. doi: 10.3382/ps/pev092 [DOI] [PubMed] [Google Scholar]
- Laudadio, V., Ceci E., Nahashon S. N., Introna M., Lastella N. M., and Tufarelli V.. . 2014. Influence of substituting dietary soybean for air-classified sunflower (Helianthus annuus L.) meal on egg production and steroid hormones in early-phase laying hens. Reprod. Domest. Anim. 49:158–163. doi: 10.1111/rda.12245 [DOI] [PubMed] [Google Scholar]
- Lessire, M., Gallo V., Prato M., Akide-Ndunge O., Mandili G., Marget P., Arese P., and Duc G.. . 2017. Effects of faba beans with different concentrations of vicine and convicine on egg production, egg quality and red blood cells in laying hens. Animal 11:1270–1278. doi: 10.1017/S1751731116002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., Liu Y., Yin R. Q., Yang X. J., Yao J. H., Sun F. F., Li G. J., Liu Y. R., and Sun Y. J.. . 2013. Nitrogen-corrected true Metabolizable energy and amino acid digestibility of Chinese corn distillers dried grains with Solubles in adult cecectomized roosters. Asian-Australas. J. Anim. Sci. 26:838–844. doi: 10.5713/ajas.2012.12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamputu, M., and Buhr R. J.. . 1995. Effect of substituting sesame meal for soybean meal on layer and broiler performance. Poult. Sci. 74:672–684. doi: 10.3382/ps.0740672 [DOI] [PubMed] [Google Scholar]
- Mu, Y., Zhang K., Bai S., Wang J. P., Zeng Q., and Ding X.. . 2019. Effects of vitamin E supplementation on performance, serum biochemical parameters and fatty acid composition of egg yolk in laying hens fed a diet containing ageing corn. J. Anim. Physiol. Anim. Nutr. (Berl). 103:135–145. doi: 10.1111/jpn.13017 [DOI] [PubMed] [Google Scholar]
- National Agricultural Statistics Service, USDA. 2020. Charts and maps. Available from https://www.nass.usda.gov/Charts_and_Maps/Poultry/index.php (Accessed 13 January 2021).
- Neijat, M., Gakhar N., Neufeld J., and House J. D.. . 2014. Performance, egg quality, and blood plasma chemistry of laying hens fed hempseed and hempseed oil. Poult. Sci. 93:2827–2840. doi: 10.3382/ps.2014-03936 [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 1994. Nutrient requirements for poultry. In: Chapter 2. nutrient requirements of chickens, 9th. Rev. ed. Washington (DC): National Academy Press. [Google Scholar]
- Pesti, G. M., Bakalli R. I., Driver J. P., Sterling K. G., Hall L. E., and Bell E. M.. . 2003. Comparison of peanut meal and soybean meal as protein supplements for laying hens. Poult. Sci. 82:1274–1280. doi: 10.1093/ps/82.8.1274 [DOI] [PubMed] [Google Scholar]
- Sakamoto, K., Hirose H., Onizuka A., Hayashi M., Futamura N., Kawamura Y., and Ezaki T.. . 2000. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 94:99–106. doi: 10.1006/jsre.2000.5937 [DOI] [PubMed] [Google Scholar]
- Sangkaew, M., Rahman M., and Koh K.. . 2019. Effects of high-oleic acid sunflower oil on egg quality and fatty acid composition of egg yolk in laying hens. Journal of Advanced Agric. Tech. 4:180–184. doi: 10.18178/joaat.4.2.180-184 [DOI] [Google Scholar]
- Senkoylu, N., Samli H. E., Akyurek H., Agma A., and asar S.. . 2005. Use of high levels of full-fat soybeans in laying hen diets. J. App. Poult. Res. 14:32–37. doi: 10.1093/japr/14.1.32 [DOI] [Google Scholar]
- Seppelt, R. A., Manceur M., Liu J., Fenichel E. P., and Klotz S.. . 2014. Synchronized peak-rate years of global resources use. Ecol. Soc.19:50–64. doi: 10.5751/ES-07039-190450 [DOI] [Google Scholar]
- Settaluri, V. S., Kandala C.V., Puppala N., and Sundaram J.. . 2012. Peanuts and their nutritional aspects-a review. Food Nutr. Sci. 3:1644–1650. doi: 10.4236/fns.2012.312215 [DOI] [Google Scholar]
- Shalash, S. M., Abou El-Wafa S., Hassan R. A., Ramadam N. A., Mohamed M. S., and El-Gabry H. E.. . 2010. Evaluation of distillers dried grains with solubles as feed ingredient in laying hen diets. Inter. J. Poult. Sci. 9:537–545. doi: 10.3923/ijps.2010.537.545 [DOI] [Google Scholar]
- Shapira, N., Weill P., and Loewenbach R.. . 2008. Egg fortification with n-3 polyunsaturated fatty acids (PUFA): nutritional benefits versus high n-6 PUFA western diets, and consumer acceptance. Isr. Med. Assoc. J. 10:262–265. [PubMed] [Google Scholar]
- Solis de Santos, F., Farnell M. B., Tellez G., Balog J. M., Anthony N. B., Torres-Rodriguez A., Higgins S., Hargis B. M., and Donoghue A. M.. . 2005. Effect of prebiotic on gut development and ascites incidence of broilers reared in a hypoxic environment. Poult. Sci. 84:1092–1100. doi: 10.1093/ps/84.7.1092 [DOI] [PubMed] [Google Scholar]
- Sun, H., Lee E. J., Samaraweera H., Persia M., and Ahn D. U.. . 2013. Effects of increasing concentrations of corn distillers dried grains with solubles on chemical composition and nutrient content of egg. Poult. Sci. 92:233–242. doi: 10.3382/ps.2012-02346 [DOI] [PubMed] [Google Scholar]
- Swiatkiewicz, S., and Koreleski J.. . 2006. Effect of maize distillers dried grains with soluble and dietary enzyme supplementation on the performance of laying hens. J. Anim. Feed Sci. 5:252–260. doi: 10.22358/jafs/66897/2006 [DOI] [Google Scholar]
- Titus, H. W. 1956. Energy values of feedstuffs for poultry. Pages 10–14 in Proc. Semi-Annu. Mtg., Nutr. Council. Am. Feed Manufact. Assoc., St. Louis, MO. [Google Scholar]
- Toomer, O. T., Hulse-Kemp A. M., Dean L. L., Boykin D. L., Malheiros R., and Anderson K. E.. . 2019. Feeding high-oleic peanuts to laying hens enhances egg yolk color and oleic fatty acid content in shell eggs. Poult. Sci. 98:1732–1748. doi: 10.3382/ps/pey531 [DOI] [PubMed] [Google Scholar]
- USDA. 2017. Food composition database. Accessed 26 July 2019.
- Van Elswyk, M. E., Hargis B. M., Williams J. D., and Hargis P. S.. . 1994. Dietary menhaden oil contributes to hepatic lipidosis in laying hens. Poult. Sci. 73:653–662. doi: 10.3382/ps.0730653 [DOI] [PubMed] [Google Scholar]
- Vogtmann, H., Pfirter H. P., and Prabucki A. L.. . 1975. A new method of determining metabolizality of energy and digestibility of fatty acids in broiler diets. Br. Poult. Sci. 16:531–534. doi: 10.1080/00071667508416222 [DOI] [PubMed] [Google Scholar]
- Vuilleumier, J. P. 1969. The ‘Roche yolk color fan’ – an instrument for measuring yolk color. Poult. Sci. 48:767–779. [Google Scholar]
- Wang, Y., Sunwoo H., Cherian G., and Sim J. S.. . 2000. Fatty acid determination in chicken egg yolk: a comparison of different methods. Poult. Sci. 79:1168–1171. doi: 10.1093/ps/79.8.1168 [DOI] [PubMed] [Google Scholar]
- Yuan, N., Wang J. P., Ding X. M., Bai S. P., Zeng Q. F., Su Z. W., Xuan Y., Peng H. W., Fraley G. S., and Zhang K. Y.. . 2019. Effects of supplementation with different rapeseed oil sources and levels on production performance, egg quality, and serum parameters in laying hens. Poult. Sci. 98:1697–1705. doi: 10.3382/ps/pey494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Chen J., and Du F.. . 2012. Potential use of peanut by-products in food processing: a review. J. Food Sci. Technol. 49:521–529. doi: 10.1007/s13197-011-0449-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyla, K., Mika M., Duliński R., Swiatkiewicz S., Koreleski J., Pustkowiak H., and Piironen J.. . 2012. Effects of inositol, inositol-generating phytase B applied alone, and in combination with 6-phytase A to phosphorus-deficient diets on laying performance, eggshell quality, yolk cholesterol, and fatty acid deposition in laying hens. Poult. Sci. 91:1915–1927. doi: 10.3382/ps.2012-02198 [DOI] [PubMed] [Google Scholar]