Abstract
Background
Rabies is a viral zoonosis that imposes a substantial disease and economic burden in many developing countries. Dogs are the primary source of rabies transmission; eliminating dog rabies reduces the risk of exposure in humans significantly. Through mass annual dog rabies vaccination campaigns, the national program of rabies control in Mexico progressively reduced rabies cases in dogs and humans since 1990. In 2019, the World Health Organization validated Mexico for eliminating rabies as a public health problem. Using a governmental perspective, we retrospectively assessed the economic costs, effectiveness, and cost-effectiveness of the national program of rabies control in Mexico, 1990–2015.
Methodology
Combining various data sources, including administrative records, national statistics, and scientific literature, we retrospectively compared the current scenario of annual dog vaccination campaigns and post-exposure prophylaxis (PEP) with a counterfactual scenario without an annual dog vaccination campaign but including PEP. The counterfactual scenario was estimated using a mathematical model of dog rabies transmission (RabiesEcon). We performed a thorough sensitivity analysis of the main results.
Principal findings
Results suggest that in 1990 through 2015, the national dog rabies vaccination program in Mexico prevented about 13,000 human rabies deaths, at an incremental cost (MXN 2015) of $4,700 million (USD 300 million). We estimated an average cost of $360,000 (USD 23,000) per human rabies death averted, $6,500 (USD 410) per additional year-of-life, and $3,000 (USD 190) per dog rabies death averted. Results were robust to several counterfactual scenarios, including high and low rabies transmission scenarios and various assumptions about potential costs without mass dog rabies vaccination campaigns.
Conclusions
Annual dog rabies vaccination campaigns have eliminated the transmission of dog-to-dog rabies and dog-mediated human rabies deaths in Mexico. According to World Health Organization standards, our results show that the national program of rabies control in Mexico has been highly cost-effective.
Author summary
Rabies is a virus that affects wild and domestic animals that can affect humans. Rabies infection is almost certainly fatal once clinical symptoms appear but can be prevented with timely post-exposure prophylaxis administration. Dogs are the primary source of rabies transmission; eliminating dog rabies reduces the risk of exposure in humans significantly. In Mexico, the national program of dog rabies vaccination has accomplished a progressive reduction of rabies episodes since 1990. The impact of these campaigns has not been systematically assessed, and that evaluation is critical to inform public health decisions. Using a mathematical model of rabies transmission, we estimated the costs and effectiveness of the dog rabies vaccination program in Mexico, compared to a scenario with no vaccination. Our results suggest that from 1990 through 2015, the national dog rabies vaccination program in Mexico prevented about 13,000 human rabies deaths, at an additional cost (MXN 2015) of $4,700 million (USD 300 million). We estimated an average cost of $360,000 (USD 23,000) per human rabies death averted, $6,500 (USD 410) per additional year-of-life, and $3,000 (USD 190) per dog rabies death averted. According to World Health Organization standards, the annual dog vaccination campaign in Mexico is highly cost-effective.
Introduction
Rabies is a viral zoonosis that imposes a high disease burden in many developing countries [1,2]. Rabies affects the host’s central nervous system [3,4] and is almost always fatal once clinical symptoms appear [1–3]. Dogs are the main rabies reservoirs in urban areas; about 20,000 to 60,000 people die each year from rabies transmitted through dog bites globally [2,5,6]. Global prevention efforts have focused on reducing the incidence of dog rabies using mass vaccination strategies for dogs living in urban and rural areas, significantly reducing the risk of human exposure to the virus [1–3,7–11].
In Mexico, the national strategy for the control and elimination of rabies, including mass dog rabies vaccination, accomplished a progressive reduction in dog rabies cases and dog-mediated human rabies deaths since the program was implemented in 1990 [8,12,13]. This elimination of rabies cases was mostly achieved through annual dog rabies vaccination campaigns. In 2019, the World Health Organization validated Mexico as the first country to eliminate rabies as a public health program [14]. Wildlife is currently considered the primary reservoir of rabies. Annual campaigns have expanded from 7.1 million dog vaccine doses administered in 1990 to 18.4 million dog vaccine doses administered in 2015, achieving estimated immunization levels of more than 80% of the dog population in most states [8,15,16]. The absence of dog-mediated human rabies deaths has been one of the critical criteria to keep funding dog rabies vaccination programs. However, no cost-effectiveness evaluation of annual dog rabies vaccination campaigns in Mexico has been performed to date.
The systematic evaluation of public health programs and interventions is essential to evaluate their continuity, redefine priorities, characterize and understand population health progress, and inform public health decision-making [17–19]. This article aims to evaluate the economic costs, effectiveness, and cost-effectiveness of the national program of rabies control in Mexico through the mass annual dog rabies vaccination campaigns, 1990–2015, from the government’s perspective.
Methods
Ethics statement
CENAPRECE prepared the datasets requested for the analysis. Administrative data were free of personal identifiers; all other data were publicly available in government open data repositories or published in scientific journals. The work team did not have access to sensitive information that could result in the identification of individuals.
Methods overview
We retrospectively evaluated the national program of rabies control in Mexico, including mass annual dog rabies vaccination campaigns and post-exposure prophylaxis (PEP) for dog bite victims, from 1990 through 2015. We drew from several data sources, including administrative records from national annual dog rabies vaccination campaigns, national health and zoonosis statistics, and scientific literature. We compared the current scenario of a national program of rabies control to a counterfactual scenario of what would have happened if the Ministry of Health in Mexico had not implemented mass dog-vaccination campaigns but had instead offered PEP to dog bite victims. We used RabiesEcon, a mathematical modeling tool developed by the US Centers for Disease Control and Prevention (CDC) [20,21], to estimate the counterfactual scenario without mass dog-vaccinations. We used epidemiological data of dog rabies in Mexico corresponding to the year of initiation of the mass vaccination campaigns of dogs against rabies, 1990, as our baseline estimate, and estimated the total cases of human and dog rabies from 1990 through 2015. We provide further details below.
Transmission model: RabiesEcon
We estimated the counterfactual scenario using RabiesEcon [20,21]. This tool is an adaptation of the deterministic mathematical model developed by Zinsstag et al.[22] on dynamics of rabies transmission in dogs and humans. RabiesEcon can estimate cases of rabies transmission and the cost of death averted and years of life gained from vaccination programs. This tool has been adapted and used in several low and middle-income countries with a potential risk of transmission of canine rabies [20], including Mexico, Tanzania, and Zambia, showing reliable results. RabiesEcon can estimate the potential cases of rabies in dogs and humans in different vaccination scenarios [20,21]. The main assumptions of our evaluation include (a) canine rabies is endemic in the no vaccination scenario (i.e., it is stable); (b) mass vaccination programs are implemented in a 10-week time interval; (c) the dog population can only increase up to 120% beyond the size of the population entered initially into the model; (d) the design and implementation of vaccination and control programs for animals with rabies are applied in the same way in urban and peri-urban areas (a complete list of assumptions of our evaluation is included in the Supporting Information S1 Text; also see Borse et al.[20] and Jeon et al.[21] for other applications).
Considering that the counterfactual scenario of no annual mass dog rabies vaccination campaign results in a higher proportion of rabid dogs in the country, we assumed that the proportion of dog bites that would have resulted in a bite investigation would remain equivalent to 1990 (the first year of mass vaccination campaign). We used the total number of bite victims from suspected rabid dogs to estimate the number of dogs in isolation and quarantine. We assumed that all such rabies exposures would have resulted in a laboratory investigation. We examined the validity of these assumptions using an extensive sensitivity analysis.
Epidemiology and demography
Table 1 shows the main parameters used to estimate the counterfactual scenario without mass dog rabies vaccination, using RabiesEcon [20].
Table 1. Primary demographic and epidemiological data used to define the counterfactual scenario without a national dog rabies vaccination campaign, 1990, using RabiesEcon [20].
| Input | Model values | Source |
|---|---|---|
| Epidemiology and demography | ||
| Area of implementationa (km2) | 171,817 | SEDATU |
| Human populationb | 58,407,633 | INEGI |
| Human population density (per km2) | 339.9 | Calculated |
| Human birth rate (per 1000 population)b | 27.9 | INEGI |
| Human life expectancyb | 70.4 | INEGI |
| Number of humans-per-dog | 6.5 | Wallace et al.[7] |
| Dog population | 8,917,713 | Calculated |
| Dog population density (per km2) | 51.9 | Calculated |
| Dog birth rate (per 1000 population)c | 350 | Tlaxcala |
| Dog life expectancyd | 3.20 | Tlaxcala |
| Dog-to-dog bites from suspected rabid dogse | 2.35 | CENAPRECE |
| Rabies Ro dog-to-dog | 1.14 | Calculated |
| Annual deaths in the program | 276 | Calculated |
| National program of rabies control | ||
| Proportion of the dog population that is vaccinatedf | 0.0% | SINAIS |
| Vaccination frequency | Anual | Model assumption |
| Proportion of spayed or neutered dogs | 0.0% | Model assumption |
| Probability of receiving PEPg | 30.1% | SINAIS |
| Epidemiology | ||
| PEP efficacyh | 0.90 | CENAPRECE |
| Dog rabies vaccine efficacyi | 0.95 | Manning et al.[23] |
| Probability of acquiring rabies if exposed with no PEP | 0.19 | Shim et al.[24] |
| Share of bite victims who do not seek medical care | 20.5% | CENAPRECE |
| Share of bite victimos who receive PEP without dog vaccination program | 30.1% | SINAIS |
| Costs (MXN pesos 2015) | ||
| Isolation and/or quarantine j | 350.3 | CENAPRECE |
| Laboratory testing of dogs | 251.5 | CENAPRECE |
| Bite investigation | 36.7 | CENAPRECE |
| Cost / vaccinek | variable | Calculated |
| Cost / PEPk | variable | Calculated |
Notes.
a Territorial extension of urban and peri-urban areas as reported by the Secretaría de Desarrollo Agrario, Territorial y Urbano (SEDATU).
b Reported by the Instituto Nacional de Información y Estadística (INEGI) [25].
c Estimated using 2014–2015 data from rabies control in the State of Tlaxcala for dogs younger than one year of age.
d Estimated using 2014–2015 data from rabies control in the State of Tlaxcala for dogs older or equal to one year of age.
e Estimated average 2007–2015 (supporting information S1). CENAPRECE: Centro Nacional de Programas Preventivos y Control de Enfermedades
f The Dirección General de Información en Salud, Sistema Nacional de Información en Salud SINAIS [26] estimates vaccination rate is 69.4% based on state health services. We used 0% vaccination for our counterfactual scenarios with no vaccination program.
g Estimated from cases with a high risk of exposure to rabies reported to SINAIS [26]. Data are an estimate of state health services.
h The proportion of dog bite victims who do not finish PEP was estimated from a retrospective review of clinical records of people with suspected rabies cases.
i Vaccine efficacy is estimated at 95%, provided that bite victims follow the recommended dosage schedule [23].
j Estimated value of the quarantine, considering four site visits per dog, with an estimated average time of four hours per visit.
k Cost per vaccine and complete PEP regimen varies annually, as estimated by CENAPRECE (S1 Text).
We combined several data sources to characterize the national program of rabies control in urban areas of Mexico (1990–2015). We used national registries from mass rabies vaccination campaigns from 1990 to 2015; data on metropolitan areas and population reported by the Secretaría de Desarrollo Agrario, Territorial y Urbano and the Instituto Nacional de Información y Estadística (INEGI) [25,26]. Dog birth rates were estimated by the Centro Nacional de Programas Preventivos y Control de Enfermedades (CENAPRECE), and defined as the number of births per year per 1,000 dogs. To estimate dog life expectancy and project dog population, we used age ranges registered in the dog rabies vaccination certificates from a convenience sample of six municipalities in the State of Tlaxcala in 2013–2016. A more detailed description of the epidemiological and demographic data used for the program’s retrospective evaluation is provided in S1 Text.
We estimated years of potential life lost (YLL) due to premature death from rabies infection using life expectancy in Mexico [26] and the distribution of suspected human exposures to rabies by age [27] (Table C in S1 Text). We obtained the number of rabid dogs from data reported by CENAPRECE. We analyzed 97 epidemiological studies carried out by state health services to estimate the average number of dogs attacked by a rabid dog, complemented with a study of the distribution of dog rabies cases in Hermosillo, México [27]. The number of people exposed to rabies from dog bites and the number of people who received PEP were estimated from national records provided by the CENAPRECE and an epidemiological study of dog bites in Hermosillo, México [26,27]. To estimate human rabies exposures, we used the probability of acquiring rabies if the bite victim was exposed to rabies but did not receive PEP [24] and the proportion of bite victims in Mexico who seek healthcare (from the Ministry of Health). To estimate dog rabies’ cases, we used the average number of attacks on humans by a rabid dog, as reported by the Ministry of Health in Mexico. A more detailed description of data sources, calculations, and main assumptions is shown in the S1 Text.
Programmatic and economic variables
We used administrative records to estimate programmatic variables: annual dog vaccination coverage, cost per dog vaccinated, post-exposure prophylaxis (PEP), bite investigations, laboratory tests, and the number of dogs quarantined or put in isolation [20,23,24,28,29]. We also estimated the costs and benefits of the mass dog vaccination campaign in urban areas from the government’s perspective [30]. Specifically, we estimated the number of dog-mediated human rabies deaths averted, dog rabies averted, and the national vaccination program’s total costs. All monetary values presented are in 2015 Mexican pesos (MXN).
Sensitivity analysis
We carried out a sensitivity analysis, focusing on two fundamental aspects: the variables with more significant uncertainty and the variables that could change the model’s final results or affect a health policy recommendation. The principal sensitivity analysis considered two counterfactual scenarios: low and high dog-to-dog rabies transmission [28]. We also defined two additional scenarios with more conservative and less conservative assumptions, compared to our reference scenario, about the number of investigations per dog bite, laboratory diagnoses, and people receiving PEP (further details in S1 Text).
Results
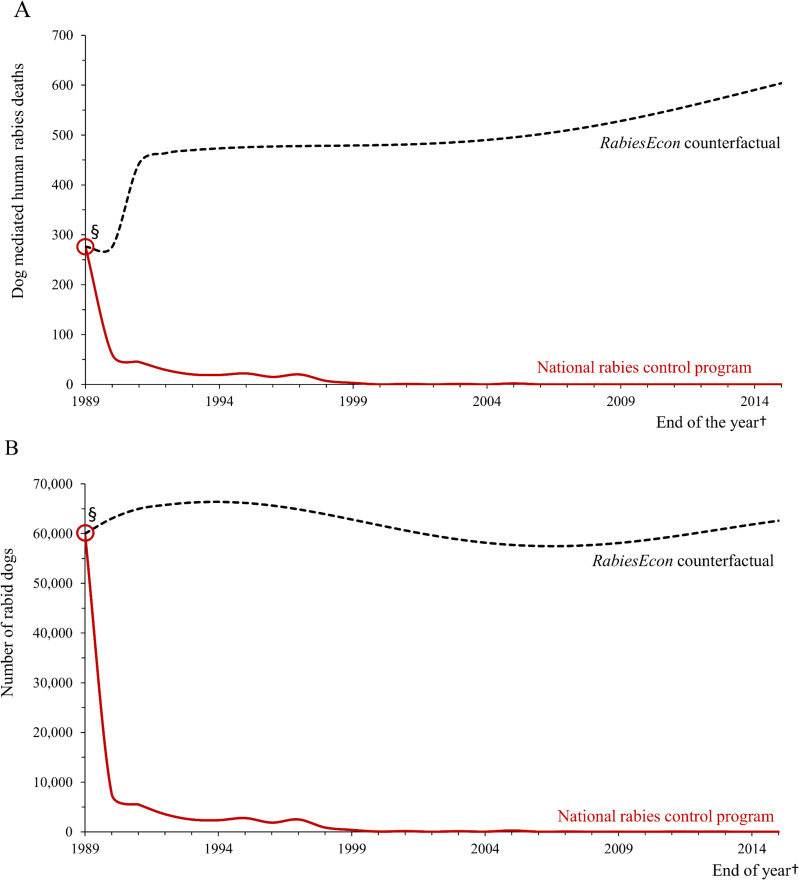
Fig 1 and Table 2 show dog mediated human deaths from dog rabies and the estimated number of rabid dogs for the two comparison scenarios: current scenario based on observed data and the counterfactual scenario estimated with RabiesEcon (estimates for a third scenario, with no mass dog rabies vaccination campaigns and without the availability of PEP, are shown in S1 Text). The first national dog vaccination campaign as part of the national program of rabies control in Mexico occurred in 1990. Reported data correspond to the end-of-the-year estimates; thus, the estimated annual rabies cases in 1990 are after vaccination. Table 2 also shows health results as estimated years of life lost due to premature death. Both results suggest a robust difference in the scenarios with and without an annual mass dog rabies vaccination program.
Fig 1.
Estimated annual results of the national program of rabies control in Mexico 1990–2015: Dog mediated human rabies deaths with the program in place, as reported by CENAPRECE, and without the program, as estimated by RabiesEcon (A); and estimated number of rabid dogs with the program, as reported by CENAPRECE, and without the program, as estimated by RabiesEcon (counterfactual) (B). § For the counterfactual scenario (no dog vaccination), we estimated the annual number of dog-mediated human deaths and dog rabies cases using the population and rabies transmission parameters reported by CENAPRECE and modeled using RabiesEcon.
Table 2. Main estimated health indicators for the national program of rabies control in Mexico 1990–2015: dog-mediated human rabies deaths, years of life lost (YLL), and number of rabid dogs.
| Year | Rabies deaths without annual mass dog rabies vaccination campaigns (RabiesEcon) | Rabies deaths with annual mass dog rabies vaccination campaigns (current scenario) | ||||
|---|---|---|---|---|---|---|
| Human | YLLa | Rabid dogs | Human | YLLa | Rabid dogs | |
| 1990 | 276 | 14 403 | 63 026 | 60 | 3 131 | 7 652 |
| 1991 | 448 | 23 555 | 64 965 | 45 | 2 367 | 5 529 |
| 1992 | 476 | 25 210 | 65 766 | 29 | 1 537 | 3 588 |
| 1993 | 488 | 26 049 | 66 246 | 20 | 1 067 | 2 458 |
| 1994 | 497 | 26 687 | 66 380 | 19 | 1 019 | 2 341 |
| 1995 | 504 | 27 176 | 66 174 | 22 | 1 187 | 2 748 |
| 1996 | 508 | 27 525 | 65 656 | 15 | 813 | 1 869 |
| 1997 | 510 | 27 742 | 64 881 | 20 | 1 089 | 2 542 |
| 1998 | 510 | 27 850 | 63 917 | 7 | 382 | 906 |
| 1999 | 508 | 27 879 | 62 839 | 3 | 164 | 429 |
| 2000 | 507 | 27 862 | 61 726 | 0 | 0 | 48 |
| 2001 | 504 | 27 829 | 60 647 | 1 | 55 | 146 |
| 2002 | 503 | 27 813 | 59 664 | 0 | 0 | 49 |
| 2003 | 502 | 27 840 | 58 827 | 1 | 55 | 133 |
| 2004 | 503 | 27 934 | 58 172 | 0 | 0 | 5 |
| 2005 | 505 | 28 116 | 57 726 | 2 | 111 | 251 |
| 2006 | 509 | 28 399 | 57 499 | 0 | 0 | 26 |
| 2007 | 515 | 28 792 | 57 494 | 0 | 0 | 0 |
| 2008 | 523 | 29 198 | 57 702 | 0 | 0 | 1 |
| 2009 | 533 | 29 730 | 58 105 | 0 | 0 | 3 |
| 2010 | 545 | 30 399 | 58 676 | 0 | 0 | 1 |
| 2011 | 559 | 31 248 | 59 379 | 0 | 0 | 52 |
| 2012 | 574 | 32 164 | 60 172 | 0 | 0 | 38 |
| 2013 | 590 | 33 194 | 61 007 | 0 | 0 | 48 |
| 2014 | 606 | 34 251 | 61 833 | 0 | 0 | 9 |
| 2015 | 623 | 35 304 | 62 598 | 0 | 0 | 4 |
| Total | 13 327 | 734 149 | 1 601 076 | 244 | 12 978 | 30 874 |
Notes. Results were derived by combining CENAPRECE data and modeling using RabiesEcon [20], adapted to the annual dog vaccination campaign in Mexico by the CENAPRECE team. On average, there were 58 reported annual deaths before 1990, with substantial annual variation. While there was no national concerted and coordinated dog vaccination campaigns before 1990, some dogs were vaccinated against rabies, and about 30% of humans exposed to rabies received PEP. We used 58 deaths to initiate RabiesEcon, as a benchmark for circulating rabies virus in Mexico. Based on the factors that affect rabies virus transmission (Table 1), we estimated 276 human deaths in the counter factual scenario of what would have occurred without any dog vaccinations. We estimated human rabies exposures based on the probability of acquiring rabies if the bite victim was exposed to rabies but did not receive PEP [24] and the proportion of bite victims in Mexico who seek healthcare (also see [31]).
a YLL: years of life lost from premature death. YLLs were estimated based on the life expectancy in Mexico, and the estimate of dogs with rabies was obtained from data reported by CENAPRECE. Table J in the S1 Text shows additional results for a scenario with no mass dog rabies vaccination campaigns and without the availability of PEP for bite victims.
The costs associated with rabies included costs of the vaccination campaign, PEP for bite victims, community bite investigations, quarantine and isolation of suspected rabid dogs, and costs of laboratory investigations. The estimated average cost per dose of dog rabies vaccine was approximately $8.44, and the average cost per vaccinated dog was $14.16 (Tables E and F, respectively, in S1 Text). This estimate considered the costs of health supplies used in the implementation of the campaign, such as syringes, vaccines, ice, biological transport thermos, vaccination certificates, and soap, personnel costs, such as salaries for vaccinators, brigade supervisors, coordinators, and non-medical costs, such as gasoline, and workers’ per diem. We estimated that about 80% of people who had been bitten by a dog seek healthcare treatment; of those, approximately 24% receive PEP, of whom about 11% do not finish the treatment (Table D in S1 Text). The average cost of PEP was estimated at approximately $643.00. Details of vaccination campaign costs, PEP for bite victims, and cost estimates are shown in more detail in S1 Text.
Table 3 shows the main results for the average cost-effectiveness of the national program of rabies control in Mexico between 1990 and 2015, from the government’s perspective. The results suggest that the program has prevented approximately a total of 13,000 human deaths from rabies, equivalent to 721,000 years of life lost due to premature death averted. The economic costs associated with the program amount to approximately $ 4,700 million Mexican pesos (USD 300 million). The program has saved about $200 million in bite research, dog quarantine and isolation, laboratory, and PEP from a decrease in rabies incidence. The average cost for averted dog-mediated human rabies death was approximately $360,000 (USD 23,000), and $6,500 (USD 410) per year of life gained. Finally, the cost per averted dog rabies case was approximately $3,000 (USD 190).
Table 3. Main results for the average cost-effectiveness evaluation of the national program of rabies control in Mexico, 1990–2015 (MXN 2015), compared with an estimated counterfactual scenario without mass dog rabies vaccination program, from the government’s perspective.
| Indicator | Without dog vaccination program (counterfactual) | With dog vaccination program (current situation) | Difference |
|---|---|---|---|
| Epidemiologic | |||
| Total dog rabies cases | 1 601 076 | 30 854 | 1 570 089 |
| Dog mediated human rabies deaths | 13 327 | 244 | 13 083 |
| Years of life lost | 734 149 | 12 978 | 721 171 |
| Costs (MXN peso) | |||
| Dog vaccination campaign | - | 4 836 123 729 | 4 836 123 729 |
| Dog bite investigationsa | 41 928 504 | 35 295 083 | -6 633 421 |
| Dog isolation and quarantinesa | 529 622 122 | 497 852 480 | -31 769 642 |
| Laboratory investigationsa | 238 669 607 | 215 863 562 | -22 806 045 |
| PEPb | 600 695 835 | 494 560 020 | -106 135 815 |
| Total | 1 410 916 067 | 6 079 694 874 | 4 668 778 80 |
| Average cost-effectiveness 1990–2015c | |||
| Cost per dog rabies case averted | - | 2 973 | 2 973 |
| Cost per human death averted | - | 356 865 | 356 865 |
| Cost per life-year gained | - | 6 474 | 6 474 |
Notes. The evaluation only considered dog rabies transmission and one annual dog vaccination campaign with no reinforcement. We did not consider sterilization activities. The methods and main assumptions are explained further in the discussion section and in S1 Text.
a To estimate the number of dogs in isolation and quarantine and the number of laboratory investigations, the additional number of rabies exposures were estimated using RabiesEcon [20]. We assumed that all rabid dog bites to humans would have been investigated in a setting without dogs’ mass vaccination (see sensitivity analysis for further details).
b The proportion of people bitten by a suspected rabid dog who begin PEP is higher with more endemic rabies transmission. In contrast, in scenarios with lower rabies transmission, the proportion of people receiving PEP would also decrease. For the scenario without mass vaccination, we used PEP treatment initiation rates of 1990 (i.e., at the beginning of the mass vaccination campaigns).
c Average cost-effectiveness included vaccination, PEP, dog bite investigation, dog isolation and quarantines, and laboratory investigations. Using the 2015 exchange rate of USD 1 = 15.70 MXN, the average cost per dog rabies cases averted was USD189, cost per human death averted was USD 22 737, and the cost per life-year gained was USD 412. The gross domestic product per capita in Mexico in 2015 was USD 12 041 [32]. Because death is the only clinical outcome in most rabies cases, a disability-adjusted life year saved is equivalent to a year of life gained.
The results from the evaluation of the national program of rabies control in Mexico depends on the definition of the counterfactual scenario, that is, what would have happened without a mass dog vaccination program in Mexico. Table 4 shows the main results for two additional counterfactual scenarios: a low rate of dog-to-dog rabies transmission and a high rate of dog-to-dog rabies transmission (specific parameters for each scenario are shown in more detail in S1 Text). Table 4 shows that our cost-effectiveness evaluation’s main results are robust to different assumptions about dog rabies’ epidemiology in Mexico. The dog vaccination program shows better cost-effectiveness indicators in a hypothetical counterfactual scenario of high dog-to-dog rabies transmission (R0 = 1.75), compared to the low dog-to-dog rabies transmission scenario (R0 = 1.07). In the high transmission scenario, a mass dog rabies vaccination program results in a greater number of dog-mediated human rabies death and dog rabies deaths averted. We show two additional scenarios in S1 Text. We further vary our assumptions to estimate the number of investigations per bite, laboratory tests, and people receiving PEP in a counterfactual setting. The main results shown in Table 3 were also robust to these changes; that is, they would not change any of our conclusions or public policy implications.
Table 4. Sensitivity analysis.
Main results for the average cost-effectiveness evaluation of the national program of rabies control in Mexico, 1990–2015 (MXN 2015), in two hypothetical scenarios of (i) low dog-to-dog rabies transmission (R0 = 1.07), and (ii) high dog-to-dog rabies transmission (R0 = 1.75)a.
| Indicator | Without dog vaccination program (counterfactual) | With dog vaccination program (current situation) | Difference |
|---|---|---|---|
| i. Low rabies transmission (R0 = 1.07) | |||
| Epidemiologic | |||
| Total dog rabies cases | 1 270 486 | 25 432 | 1 245 054 |
| Dog mediated human rabies deaths | 12 912 | 244 | 12 668 |
| Years of life lost | 711 408 | 12 978 | 698 430 |
| Costs (MXN peso) | |||
| Dog vaccination campaign | - | 4 836 123 729 | 4 836 123 729 |
| Dog bite investigations | 41 928 504 | 35 295 083 | -6 633 421 |
| Dog isolation and quarantines | 528 623 285 | 497 852 480 | -30 770 805 |
| Laboratory investigations | 237 952 585 | 215 863 562 | -22 089 023 |
| PEP | 600 695 835 | 494 560 020 | -106 135 815 |
| Total | 1 409 200 209 | 6 079 694 874 | 4 670 494 666 |
| Average cost-effectiveness 1990–2015 | |||
| Cost per dog rabies case averted | 3 751 | 3 751 | |
| Cost per human death averted | 368 671 | 368 671 | |
| Cost per life-year gained | 6 687 | 6 687 | |
| ii. High rabies transmission (R0 = 1.75) | |||
| Epidemiologic | |||
| Total dog rabies cases | 2 419 314 | 39 898 | 2 379 417 |
| Dog mediated human rabies deaths | 15 805 | 244 | 15 561 |
| Years of life lost | 871 696 | 12 978 | 858 717 |
| Costs (MXN peso) | |||
| Dog vaccination campaign | - | 4 836 123 729 | 4 836 123 729 |
| Dog bite investigations | 41 928 504 | 35 295 083 | -6 633 421 |
| Dog isolation and quarantines | 535 499 167 | 497 852 480 | -37 646 687 |
| Laboratory investigations | 242 888 482 | 215 863 562 | -27 024 920 |
| PEP | 600 695 835 | 494 560 020 | -106 135 815 |
| Total | 1 421 011 988 | 6 079 694 874 | 4 658 682 887 |
| Average cost-effectiveness 1990–2015 | |||
| Cost per dog rabies case averted | - | 1 958 | 1 958 |
| Cost per human death averted | - | 299 373 | 299 373 |
| Cost per life-year gained | - | 5 425 | 5 425 |
Discussion
Mexico has continuously included human rabies prevention into its federal public health programs and has invested substantial resources to prevent and treat possible dog rabies exposures [5,12,13,33]. The National Rabies Control Program has virtually eliminated dog mediated human rabies deaths and dog rabies cases in the past 25 years. From 2006 through 2012, no dog mediated human rabies cases were reported, and reported rabid dogs decreased from 42 in 2007 to 12 in 2012 [5,26,33]. Consistent with evidence from vaccination studies in other countries [5,20,22], this reduction was achieved primarily through annual mass dog rabies vaccination campaigns, with more than 80% of the dog population immunized in most states [8,15].
We comprehensively analyzed the cost-effectiveness of mass vaccination campaigns for dogs in Mexico. Our study is aligned with global health agencies’ recommendations to evaluate the impact of health programs and interventions to support decision-making [17,18]. Although annual mass dog vaccination campaigns are expensive, the cost per additional year of life gained is well below the threshold for interventions considered highly cost-effective according to the recommendations of the World Health Organization’s Commission on Macroeconomics and Health [34]. These thresholds classify interventions as cost-effective if the costs to avert a disability-adjusted life-year is less than one (very cost-effective) or three (cost-effective) times the country’s gross domestic product per capita. The thresholds are intended as a reference only and should not be used as a unique decision criterion [35–37]. Mexico’s gross domestic product per capita in 2015 was $188,955 (USD12,041) [32], so a highly cost-effective intervention would cost less than $188,955 per life-year gained. The average cost per life-year gained in rabies was $6500 (USD 412) and $357,000 per life saved (USD 22,737). This estimate represents a higher cost per life saved compared to a similar program (with only 50% of dogs vaccinated) in East Africa (USD 385–451) [20], and higher than a similar program in Chad (USD 596 per human death averted) [22]. Mindekem et al. reported a lower cost of USD 121 per life-year gained in Chad [38], lower than our estimated average of USD 412 for Mexico. These differences probably reflect the relatively higher costs of implementing rabies control and prevention interventions in a middle high-income country.
The study’s main results are robust to changes in our main assumptions to generate the counterfactual comparison scenario (estimated with RabiesEcon [20]). The conclusions and implications for public health did not change when using counterfactual scenarios of low or high transmission of rabies between dogs, nor using more and less conservative assumptions for the number of investigations of dog bites, quarantine and isolation of dogs, the number of laboratory tests, and the percentage of people receiving PEP (S1 Text). We also included a scenario with no public health interventions (i.e., no mass dog rabies vaccination or PEP for dog bite victims) in S1 Text.
The results shown suggest that PEP treatment is highly effective in saving lives in people who have been attacked by rabid dogs. Consistent with studies in countries with endemic rabies transmission, our results show that PEP treatment combined with mass rabies vaccination campaigns considerably reduces human mortality from rabies, years of life lost due to premature death, and the costs of dealing with dog bite wounds [24,29,39,40]. Some authors have suggested strategies to lower PEP implementation costs, for example, by reducing the indiscriminate application of PEP treatment to people with very low or no risk of contracting rabies, based on the health of the attacking dog and the specific conditions of the incident [2,24,31].
The Ministry of Health has implemented mass dog rabies vaccination campaigns continuously since 1990. The government has kept a reliable administrative record of reported rabies cases and deaths, with a uniform reporting protocol, which allows for a robust estimate of the epidemiological situation of dog rabies in Mexico. As in other countries [19,41–44], some cases of human and dog rabies may not have been recognized or reported to health authorities, especially in the first years of the vaccination campaign. However, incomplete information about rabies becomes more unlikely as transmission decreases. An essential element to consider in reducing dog rabies is the biological used in vaccination campaigns. Mexico uses biologicals based on cell culture, which have shown a higher quality, especially in their potency in international units (IU) [45], more than double what is recommended by the Expert Committee on Rabies of the World Health Organization [2].
One of the strengths of this study is the estimation of the economic costs, effectiveness, and cost-effectiveness of the vaccination program compared to a counterfactual scenario that describes a plausible scenario of what could have happened without vaccination (but with PEP), using RabiesEcon. In Mexico, RabiesEcon was adapted and standardized for use in a joint CDC effort with workers responsible for the federal and state zoonosis programs in CENAPRECE. This tool has also been used in other contexts, to estimate the cost-effectiveness of mass rabies vaccination campaigns in Africa [20] and of preventing dog rabies’ reintroduction through dog vaccination [21].
In 2019, the World Health Organization validated Mexico as the first country to eliminate rabies as a public health program [14]. Following rabies elimination, there is still a risk that rabies could be reintroduced to the country from importation of a rabid animal, incursion from an endemic area, or host shift from an enzootic rabies variant [21,46]. For example, in Mexico’s southern border, Guatemala is still considered a rabies-endemic country, and there are sporadic dog rabies cases in Belize [47]. In the United States, where dogs are no longer considered a rabies reservoir, there are about 60 annual reports of rabid dogs who have become infected from wildlife species such as bats, and dog vaccination is still required [46,48]. Dog rabies introductions or reintroductions could potentially result in endemic transmission [49,50]. Preventive dog rabies vaccination is one strategy to prevent and control rabies following elimination. The necessary dog vaccination coverage depends on the risk of rabies reintroduction, but estimates suggest it is a cost-effective intervention [21] considering World Health Organization guidelines [34]. Other complementary strategies include border control and surveillance.
This study has some limitations. First, our estimate of the counterfactual scenario was limited to Mexico’s urban and peri-urban areas, where the vast majority of rabies cases occur. RabiesEcon allows distinguishing between urban and rural areas and different vaccination regimes. Because our evaluation is at the national level, there is vast variation in population density, connectivity, dog ownership practices, interactions with wildlife, among other factors that may affect rabies transmission between states. To describe such variation is theoretically possible but would require many assumptions and more disaggregated administrative and survey data, which is lacking at the moment. The objective of RabiesEcon is to assist decision-making; therefore, we opted for a parsimonious model that reflects policy decisions and typical constraints faced by policymakers [51]. Second, available administrative data were not always representative of the country. For example, the analysis focuses on PEP treatment implementation and costs provided by the Ministry of Health, excluding data from other social security institutions. However, rabies mortality records encompass the entire health sector. Third, we had to make several assumptions about rabies’ epidemiology and government interventions to generate a plausible counterfactual scenario. It is impossible to know what would have happened without a mass dog rabies vaccination program. To verify how much these assumptions influenced our estimates, we did an extensive sensitivity analysis that suggests that the results and main conclusions were robust (Table 4, and in Tables J, K, and L in S1 Text). We did not include stochastic variations, which may be necessary for the context of a low number of rabies infections [6]. Last, our estimates are also limited by modeling decisions. RabiesEcon is a modeling tool for the economic evaluation of dog rabies control programs and interventions [20]. As such, it does not provide a detailed representation of virus transmission between dogs and between dogs and humans, but rather on the factors that are most likely to affect health-policy decisions. RabiesEcon does not consider the dog population’s spatial distribution, variations in dog ownership preferences, or human behavior, potentially affecting the estimated number of rabies cases in dogs and humans [22,52,53].
In conclusion, this systematic evaluation shows that mass rabies vaccination campaigns for dogs in Mexico have been the primary way to progressively reduce the transmission of dog-to-dog rabies and dog mediated human rabies deaths. In 2019, the World Health Organization validated Mexico for eliminating rabies as a public health problem. The program has resulted in thousands of years of life gained from averted premature deaths since 1990. PEP treatment is an effective tool for preventing human deaths in all scenarios analyzed and combined with dog vaccination are a highly cost-effective strategy to prevent deaths from rabies. Following rabies elimination, there is still a risk that rabies could be reintroduced to the country from importation, incursion, or host shift. Mass dog vaccination has been a successful strategy to progressively reduce and finally eliminate rabies transmission since the program began in 1990.
Supporting information
1. Additional background; 2. Transmission model: RabiesEcon; 3. Model inputs; 4. Additional results: health indicators of the rabies control program; 5. Additional sensitivity analysis. Supporting figure legends: Figure A. Sensitivity analysis of cost-effectiveness indicators: incremental cost (with and without the dog vaccination program) for (i) dog rabies cases averted, (ii) per human death averted (in MXN 100s), (iii) per year of life gained. Supporting table legends: Table A. Main epidemiological and demographic variables used in the evaluation of the national rabies vaccination campaign in urban areas, Mexico 1990–2015; Table B. Vaccination certificates of the State of Tlaxcala, 2013–2016; Table C. Distribution of suspected human exposures to rabies by age; Table D. Epidemiological description of the compared scenarios: National Rabies Control Program and counterfactual scenario without annual vaccination (RabiesEcon); Table E. Costs associated with the dog rabies vaccine (MXN 2015); Table F. Estimated unit costs of the dog vaccine per year (MXN 2015); Table G. Treatment costs for post-exposure prophylaxis (PEP) in cases with probable dog rabies exposures (MXN 2015); Table H. Control measures for suspected rabid dogs (quarantine and isolation, lab tests, and bite investigations); Table I. Summary of costs and coverage of dog vaccination campaigns in Mexico, 1990–2015; Table J. Main health indicators of the rabies control program evaluation: human deaths from rabies, the equivalent in years of life potentially lost due to premature death (YLL), and the estimated number of rabid dogs México, 1990–2015; Table K. Sensitivity analysis: most conservative scenario. Main results for the average cost-effectiveness evaluation of the national program of rabies control in Mexico, 1990–2015 (MXN 2015), compared with an estimated counterfactual scenario without mass dog rabies vaccination program, from the government’s perspective; Table L. Sensitivity analysis: least conservative scenario. Main results for the average cost-effectiveness evaluation of the national program of rabies control in Mexico, 1990–2015 (MXN 2015), compared with an estimated counterfactual scenario without mass dog rabies vaccination program, from the government’s perspective; Table M. Parameters for the sensitivity analysis in scenarios of low and high rabies transmission, to estimate the counterfactual scenario without annual mass dog vaccination campaigns, Mexico 1990–2015.
(PDF)
Acknowledgments
We appreciate the contribution of workers from the federal and state zoonosis programs of Centro Nacional de Programas Preventivos y Control de Enfermedades (CENAPRECE), who participated in the workshop “Análisis de costo-efectividad del Programa Nacional de Vacunación Antirrábica de Perros” in CENAPRECE, Ciudad de México, during the week of May 15–20, 2016. We also want to highlight the support received from the Division of Global Migration and Quarantine (DGMQ) of the US Centers for Disease Control and Prevention (CDC), especially Margarita Elsa Villarino, and also to Jesse Blanton and Ryan Wallace of the Poxvirus and Rabies Branch, CDC, and Seonghye Jeon from the Health Economics and Modeling UNIT, CDC, for their technical support and suggestions in the development of this evaluation. Finally, we want to thank Nancy Armenta and Óscar Beltrán PhD’s excellent work in the development of this analysis, and Mariana Arellano, Daniela Islas, and many other people from CENAPRECE that were key to the development of this research.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention (CDC).
Data Availability
The data underlying the results presented in the study are available in the supplementary material.
Funding Statement
EU’s work was partially funded by the ANID Millennium Science Initiative [grant NCN17_081], https://www.iniciativamilenio.cl/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Other author(s) received no specific funding for this work.
References
- 1.World Health Organization. Rabies vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85(32):309–20. 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Expert Consultation on Rabies. Third Report.Geneva: World Health Organization Department of Control of Neglected Tropical. Diseases. 2018. [Google Scholar]
- 3.Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2(6):327–43. 10.1016/s1473-3099(02)00287-6 [DOI] [PubMed] [Google Scholar]
- 4.Hankins DG, Rosekrans JA, editors. Overview, prevention, and treatment of rabies. Mayo Clin Proc; 2004: Elsevier. [DOI] [PubMed] [Google Scholar]
- 5.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9(4):e0003709. 10.1371/journal.pntd.0003709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace RM, Undurraga EA, Blanton JD, Cleaton J, Franka R. Elimination of Dog-Mediated Human Rabies Deaths by 2030: Needs Assessment and Alternatives for Progress Based on Dog Vaccination. Front Vet Sci. 2017;4(9). 10.3389/fvets.2017.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan American Health Organization. Eliminación de la rabia humana transmitida por perros en América Latina. Análisis de la situación. Washington, DC: World Health Organization, 2005. [Google Scholar]
- 9.Pan American Health Organization. Rabies. Washington, DC: World Health Organization, 2015. [Google Scholar]
- 10.Wallace RM, Undurraga EA, Gibson A, Boone J, Pieracci EG, Gamble L, et al. Estimating the effectiveness of vaccine programs in dog populations. Epidemiol Infect. 2019;147:e247. Epub 2019/07/29. 10.1017/S0950268819001158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undurraga EA, Millien MF, Allel K, Etheart MD, Cleaton J, Ross Y, et al. Costs and effectiveness of alternative dog vaccination strategies to improve dog population coverage in rural and urban settings during a rabies outbreak. Vaccine. 2020;38:6162–73. 10.1016/j.vaccine.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 12.Vigilato MA, Cosivi O, Knöbl T, Clavijo A, Silva HM. Rabies update for Latin America and the Caribbean. Emerg Infect Dis. 2013;19(4):678. 10.3201/eid1904.121482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigilato MAN, Clavijo A, Knobl T, Silva HMT, Cosivi O, Schneider MC, et al. Progress towards eliminating canine rabies: policies and perspectives from Latin America and the Caribbean. Philosophical Transactions of the Royal Society of London B. Biological Sciences. 2013;368(1623):20120143. 10.1098/rstb.2012.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Mexico is free from human rabies transmitted by dogs Geneva: Pan American Health Organization, World Health Organization,; 2020 [cited 2020 November 1]. Available from: https://bit.ly/2J9ZkrY.
- 15.Secretaría de Salud México. Lineamientos generales primera semana nacional de vacunación antirrábica canina y felina 2015. Del 22 al 28 de Marzo. Mexico DF: Centro Nacional de Programas Preventivos y Control de Enfermedades, Subsecretaría de Prevención y Promoción de la Salud, 2015 August 26. Report No.
- 16.Pan American Health Organization Reunión de los directores del programa de control de la rabia en Las Américas(REDIPRA). Washington CD: Panamerican Health Organization, 2010. [Google Scholar]
- 17.Chan M, Kazatchkine M, Lob-Levyt J, Obaid T, Schweizer J, Sidibe M, et al. Meeting the demand for results and accountability: A call for action on health data from eight global health agencies. PLoS Med. 2010;7(1):e1000223. 10.1371/journal.pmed.1000223 WOS:000274162800017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frieden TR. The future of public health. N Engl J Med. 2015;373(18):1748–54. 10.1056/NEJMsa1511248 [DOI] [PubMed] [Google Scholar]
- 19.Lembo T, Hampson K, Kaare MT, Ernest E, Knobel D, Kazwala RR, et al. The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS Negl Trop Dis. 2010;4(2):e626. 10.1371/journal.pntd.0000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borse RH, Atkins CY, Gambhir M, Undurraga EA, Blanton JD, Kahn EB, et al. Cost-effectiveness of dog rabies vaccination programs in East Africa. PLoS Negl Trop Dis. 2018;12(5):e0006490. 10.1371/journal.pntd.0006490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon S, Cleaton J, Meltzer MI, Kahn EB, Pieracci EG, Blanton JD, et al. Determining the post-elimination level of vaccination needed to prevent re-establishment of dog rabies. PLoS Negl Trop Dis. 2019;13(12):e0007869. 10.1371/journal.pntd.0007869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinsstag J, Dürr S, Penny M, Mindekem R, Roth F, Gonzalez SM, et al. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proc Natl Acad Sci. 2009;106(35):14996–5001. 10.1073/pnas.0904740106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, et al. Human Rabies Prevention—United States. 2008 MMWR Recomm Rep. 2008;57:1–28. [PubMed] [Google Scholar]
- 24.Shim E, Hampson K, Cleaveland S, Galvani AP. Evaluating the cost-effectiveness of rabies post-exposure prophylaxis: a case study in Tanzania. Vaccine. 2009;27(51):7167–72. 10.1016/j.vaccine.2009.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Instituto Nacional de Estadística y Geografía. Anuario Estadístico y Geográfico por Entidad Federativa 2015 Mexico City: Instituto Nacional de Estadística y Geografía(INEGI),; 2015 [cited 2016 May 18]. Available from: http://internet.contenidos.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/AEGPEF_2015/702825077297.pdf.
- 26.Secretaría de Salud México. Sistema nacional de información en salud Mexico DF: Secretaría de Salud; 2016 [cited 2016 May 18]. Available from: http://www.sinais.salud.gob.mx.
- 27.Eng T, Fishbein D, Talamante H, Hall D, Chavez G, Dobbins J, et al. Urban epizootic of rabies in Mexico: epidemiology and impact of animal bite injuries. Bull World Health Organ. 1993;71(5):615–24. [PMC free article] [PubMed] [Google Scholar]
- 28.Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7(3):e1000053. 10.1371/journal.pbio.1000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meltzer MI. Rupprecht CE. A review of the economics of the prevention and control of rabies. PharmacoEconomics. 1998;14(4):365–83. 10.2165/00019053-199814040-00004 [DOI] [PubMed] [Google Scholar]
- 30.Drummond MF, Sculpher MJ, Torrance G, W., O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Third ed. New York: Oxford University Press; 2005. [Google Scholar]
- 31.Undurraga EA, Meltzer MI, Tran CH, Atkins CY, Etheart MD, Millien MF, et al. Cost-Effectiveness Evaluation of a Novel Integrated Bite Case Management Program for the Control of Human Rabies, Haiti 2014–2015. Am J Trop Med Hyg. 2017;96(6):1307–17. 10.4269/ajtmh.16-0785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Bank. GDP per capita 2020 [cited 2020 10 november]. Available from: https://data.worldbank.org/.
- 33.Secretaría de Salud México. Prevención y Control de la Rabia Humana 2013–2018. Programa de Acción Específico. Mexico DF: D.R. Secretaría de Salud; 2015. Available from: https://www.gob.mx/cms/uploads/attachment/file/38496/PAE_PrevencionControlRabiaHumana2013_2018.pdf.
- 34.World Health Organization. Choosing interventions that are cost effective(WHO—CHOICE). Cost effectiveness and strategic planning Geneva2017 [cited 2020 March]. Available from: http://www.who.int/choice/costs/en/.
- 35.Hutubessy R, Chisholm D, TT-T E. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):8. 10.1186/1478-7547-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shillcutt SD, Walker DG, Goodman CA, Mills AJ. Cost effectiveness in low-and middle-income countries. PharmacoEconomics. 2009;27(11):903–17. 10.2165/10899580-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newall A, Jit M, Hutubessy R. Are current cost-effectiveness thresholds for low-and middle-income countries useful? Examples from the world of vaccines. PharmacoEconomics. 2014;32(6):525–31. 10.1007/s40273-014-0162-x [DOI] [PubMed] [Google Scholar]
- 38.Mindekem R, Lechenne MS, Oussiguéré A, Kebkiba B, Moto DD, Alfaroukh IO, et al. Cost description and comparative cost efficiency of post-exposure prophylaxis and canine mass vaccination against rabies in N’Djamena. Chad Front Vet Sci. 2017;4:38. 10.3389/fvets.2017.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bögel K, Meslin F. Economics of human and canine rabies elimination: guidelines for programme orientation. Bull World Health Organ. 1990;68(3):281–91. [PMC free article] [PubMed] [Google Scholar]
- 40.Elser J, Hatch B, Taylor L, Nel L, Shwiff S. Towards canine rabies elimination: Economic comparisons of three project sites. Transbound Emerg Dis. 2018;65(1):135–45. 10.1111/tbed.12637 [DOI] [PubMed] [Google Scholar]
- 41.Cleaveland S, Fevre EM, Kaare M, Coleman PG. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull World Health Organ. 2002;80(4):304–10. [PMC free article] [PubMed] [Google Scholar]
- 42.Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer MI, Miranda MEG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83(5):360–8. doi: /S0042-96862005000500012 [PMC free article] [PubMed] [Google Scholar]
- 43.Hampson K, Dobson A, Kaare M, Dushoff J, Magoto M, Sindoya E, et al. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl Trop Dis. 2008;2(11):e339. 10.1371/journal.pntd.0000339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatch B, Anderson A, Sambo M, Maziku M, Mchau G, Mbunda E, et al. Towards Canine Rabies Elimination in South-Eastern Tanzania: Assessment of Health Economic Data. Transbound Emerg Dis. 2017;64(3):951–8. 10.1111/tbed.12463 [DOI] [PubMed] [Google Scholar]
- 45.Lucas C, Pino FV, Baer G, Morales PK, Cedillo V, Blanco M, et al. Rabies control in Mexico. Dev Biol. 2008;131:167. [PubMed] [Google Scholar]
- 46.Pieracci EG, Pearson CM, Wallace RM, Blanton JD, Whitehouse ER, Ma X, et al. Vital signs: trends in human rabies deaths and exposures—United States, 1938–2018. Morb Mortal Wkly Rep. 2019;68(23):524. 10.15585/mmwr.mm6823e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. Human rabies: 2016 updates and call for data. Wkly Epidemiol Rec. 2017;92(7):77–86. [PubMed] [Google Scholar]
- 48.Wallace RM, Gilbert A, Slate D, Chipman R, Singh A, Cassie W, et al. Right Place, Wrong Species: A 20-Year Review of Rabies Virus Cross Species Transmission among Terrestrial Mammals in the United States. PLoS One. 2014;9(10):e107539. 10.1371/journal.pone.0107539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Townsend SE, Lembo T, Cleaveland S, Meslin FX, Miranda ME, Putra AAG, et al. Surveillance guidelines for disease elimination: a case study of canine rabies. Comp Immunol Microbiol Infect Dis. 2013;36(3):249–61. 10.1016/j.cimid.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castillo-Neyra R, Zegarra E, Monroy Y, Bernedo R, Cornejo-Rosello I, Paz-Soldan V, et al. Spatial Association of Canine Rabies Outbreak and Ecological Urban Corridors, Arequipa, Peru. Tropical Medicine and Infectious Disease. 2017;2(3):38. 10.3390/tropicalmed2030038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee BY, Bartsch SM. How to determine if a model is right for neglected tropical disease decision making. PLoS Negl Trop Dis. 2017;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajeev M, Metcalf CJE, Hampson K. Modeling canine rabies virus transmission dynamics. In: Fooks A, Jackson A, editors. Rabies Scientific Basis of the Disease and Its Management. 4th edition ed: Academic Press; 2020. [Google Scholar]
- 53.Funk S, Salathé M, Jansen VA. Modelling the influence of human behaviour on the spread of infectious diseases: a review. J R Soc Interface. 2010;7(50):1247–56. 10.1098/rsif.2010.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Additional background; 2. Transmission model: RabiesEcon; 3. Model inputs; 4. Additional results: health indicators of the rabies control program; 5. Additional sensitivity analysis. Supporting figure legends: Figure A. Sensitivity analysis of cost-effectiveness indicators: incremental cost (with and without the dog vaccination program) for (i) dog rabies cases averted, (ii) per human death averted (in MXN 100s), (iii) per year of life gained. Supporting table legends: Table A. Main epidemiological and demographic variables used in the evaluation of the national rabies vaccination campaign in urban areas, Mexico 1990–2015; Table B. Vaccination certificates of the State of Tlaxcala, 2013–2016; Table C. Distribution of suspected human exposures to rabies by age; Table D. Epidemiological description of the compared scenarios: National Rabies Control Program and counterfactual scenario without annual vaccination (RabiesEcon); Table E. Costs associated with the dog rabies vaccine (MXN 2015); Table F. Estimated unit costs of the dog vaccine per year (MXN 2015); Table G. Treatment costs for post-exposure prophylaxis (PEP) in cases with probable dog rabies exposures (MXN 2015); Table H. Control measures for suspected rabid dogs (quarantine and isolation, lab tests, and bite investigations); Table I. Summary of costs and coverage of dog vaccination campaigns in Mexico, 1990–2015; Table J. Main health indicators of the rabies control program evaluation: human deaths from rabies, the equivalent in years of life potentially lost due to premature death (YLL), and the estimated number of rabid dogs México, 1990–2015; Table K. Sensitivity analysis: most conservative scenario. Main results for the average cost-effectiveness evaluation of the national program of rabies control in Mexico, 1990–2015 (MXN 2015), compared with an estimated counterfactual scenario without mass dog rabies vaccination program, from the government’s perspective; Table L. Sensitivity analysis: least conservative scenario. Main results for the average cost-effectiveness evaluation of the national program of rabies control in Mexico, 1990–2015 (MXN 2015), compared with an estimated counterfactual scenario without mass dog rabies vaccination program, from the government’s perspective; Table M. Parameters for the sensitivity analysis in scenarios of low and high rabies transmission, to estimate the counterfactual scenario without annual mass dog vaccination campaigns, Mexico 1990–2015.
(PDF)
Data Availability Statement
The data underlying the results presented in the study are available in the supplementary material.