Patients with coronavirus infectious disease 2019 (COVID-19) pneumonia may develop a severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation (ECMO), which is associated with brain injury (i.e., seizures, cerebral infarction or cerebral hemorrhage) in 7% of cases. Moreover, central nervous system manifestations associated with severe COVID-19 have been reported, including acute ischemic strokes and encephalopathies (Ellul et al., 2020). The neurological evaluation of patients under ECMO is challenging, as the clinical examination may be confounded by sedation and neuromuscular blockade. Recent studies conducted in non-COVID-19 patients under ECMO highlighted the usefulness of continuous electroencephalography (cEEG) monitoring for prognostication (Magalhaes et al., 2020, Peluso et al., 2020, Sinnah et al., 2018). In this population, severe encephalopathy (i.e., unreactive EEG background) and burst suppression patterns were reported in adult ECMO patients in 10–20% and 1–3% of cases, respectively (Magalhaes et al., 2020, Peluso et al., 2020). Here we report cEEG analyses in consecutive, confirmed severe COVID-19 patients requiring venovenous (VV)-ECMO (n = 17) or venoarterial (VA)-ECMO (n = 3) support in the intensive care unit (ICU) of Bichat-Claude Bernard Hospital (Paris, France) from March 19th to November 10th, 2020. Patients underwent cEEG monitoring according to a standardized protocol, as described previously (Magalhaes et al., 2020, Sinnah et al., 2018). Data are presented as medians (interquartile range) or numbers (percentages).
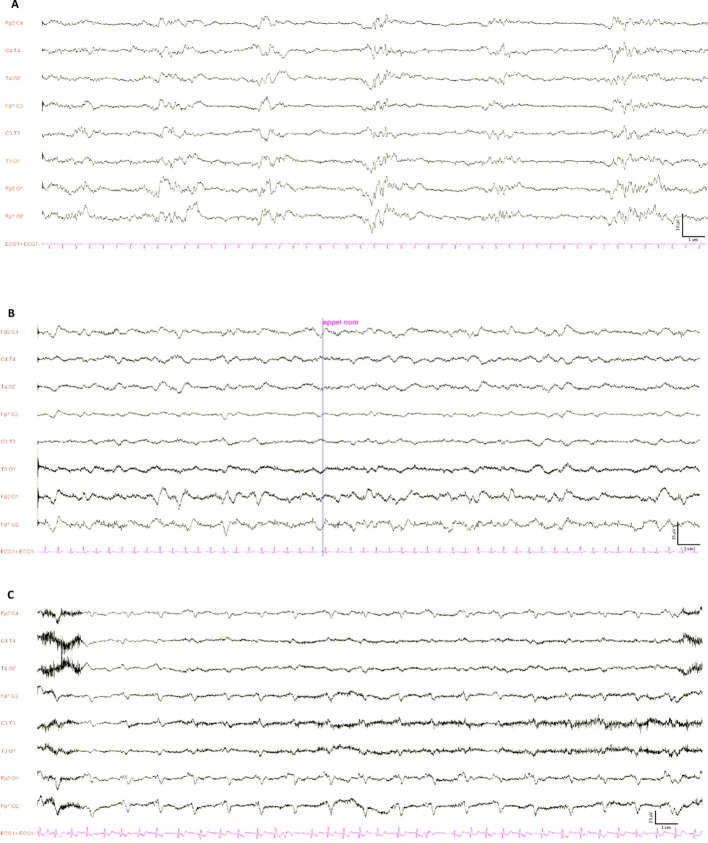
Twenty consecutive patients were studied (Table 1 ). The time between ECMO cannulation and the first cEEG was 2 (1–2) days. At the time of cEEG, all patients were invasively mechanically ventilated, unresponsive to verbal commands, with ongoing continuous infusion of sedative (propofol or midazolam) and opioid (sufentanil) drugs in all patients, and neuromuscular blocking agents in 14 (70%) of them. A total of 26 cEEG were recorded for a duration of 18 (17.7–18.5) hours. Fifteen patients had only one cEEG, and 5 patients underwent repeated cEEG recordings (2 recordings, n = 4; 3 recordings, n = 1). The main findings of the first cEEG recordings are presented in the Table 1. We observed a discontinuous background activity in 10 (50%) cases, with a burst suppression pattern in 3 (15%) cases (Supplementary material , Figure 1A). Discontinuous background activity was associated with hypertension (60% vs. 0%, 2-tail Fisher’s exact test, p = 0.01) and a higher Simplified Acute Physiology Score (SAPS) II score (49 (36–68) vs. 30 (18–36), Wilcoxon test, p = 0.02) at admission, but not with any clinical variable at the time of cannulation (PaO2/FiO2 ratio, cardiovascular or renal failure), or with dose or type (propofol/midazolam) of ongoing sedation during cEEG. Background frequency was mainly recorded in the delta-theta bands, ranging from a minimal frequency of 1 (1–1.5) Hz to a maximal frequency of 4 (2.5–6) Hz (Supplementary material , Figure 1B). The reactivity to auditory and noxious stimuli under sedation was preserved in two-thirds of cases, and sleep transients (spindles, K-complexes) were observed in 11 (55%) cases. Two (10%) patients showed continuous, symmetric, non-reactive, generalized but mainly bifrontal, monomorphic diphasic (or even triphasic), periodic (with short interval 1–2 s) delta slow waves, as previously reported (Supplementary material , Figure 1C) (Vellieux et al., 2020). Notably, no seizures were recorded. The minimal background frequency was the only cEEG parameter associated with ICU mortality, as previously reported in the non-COVID-19 adult ECMO population (Magalhaes et al., 2020). Among patients who received repeated cEEG, the background activity remained discontinuous in 2/5, remained continuous in 1/5, switched from discontinuous to continuous in 1/5, and switched from discontinuous to continuous in 1/5 patients.
Table 1.
Characteristics of the 20 patients and cEEG features.
| Variables | All patients (n = 20) | |
|---|---|---|
| Age, years | 52 (45–60) | |
| Male sex | 19 (95) | |
| BMI, kg/m2 | 32 (26–33) | |
| SAPS II score | 46 (30–61) | |
| Hypertension | 6 (30) | |
| Diabetes | 6 (30) | |
| At time of ECMO cannulation | ||
| Time between intubation and ECMO cannulation, days | 6 (2–8) | |
| PaO2/FiO2 ratio | 75 (65–105) | |
| SOFA score, cardiovascular component > 2 | 12 (60) | |
| SOFA score, renal component > 2 | 6 (30) | |
| Sedation at time of cEEG recording | ||
| Midazolam | 7 (35) | |
| Propofol | 17 (85) | |
| Sufentanil | 20 (100) | |
| cEEG features | ||
| Background activity |
Continuous | 10 (50) |
| Discontinuous without BS | 7 (35) | |
| Discontinuous with BS | 3 (15) | |
| Minimal frequency - Hz | 1 (1–1.5) | |
| Maximal frequency - Hz | 4 (2.5–6) | |
| Symmetrical | 17 (85) | |
| Preserved reactivity | Auditory stimuli | 14 (70) |
| Noxious stimuli | 15 (75) | |
| Periodic discharges | 2 (10) | |
| Seizures | 0 (0) | |
| Sleep transients | 11 (55) | |
| Beta rhythms | 6 (30) | |
| Focal slowing | 2 (10) | |
Data are medians (interquartile range) or numbers (percentages).
Abbreviations: cEEG Continuous Electroencephalography; BMI Body Mass Index; SAPS Simplified Acute Physiology Score; ECMO Extracorporeal membrane oxygenation; SOFA Sepsis Organ Failure Assessment; BS Burst Suppression.
We conclude that early cEEG abnormalities are frequent in patients with severe COVID-19 pneumonia under ECMO, with higher rates of unreactive and/or discontinuous or suppressed background than previously reported in the non-COVID-19 population (Magalhaes et al., 2020, Peluso et al., 2020, Sinnah et al., 2018). The pathophysiology and prognostic significance of monomorphic periodic slow waves, which were observed in 10% of cases of our study, remain to be investigated.
Ethical approval
Ethical approval was obtained from our institutional ethics committee (IRB 00006477, study number 14-050).
Consent for publication
Information was provided to next-of-kin for all participants, followed, whenever possible, by information to the patient.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by a grant from the French Society of Intensive Care Medicine (Société de Réanimation de Langue Française, SRLF).
Authors’ contribution
GV recruited patients, collected and analyzed data, and wrote the manuscript. PJ, AG, and MP recruited patients and collected data. ART analyzed data. RS designed the work, collected and analyzed data, and wrote the manuscript. All authors have read and approved the manuscript.
Competing interests
RS received grants from the French Ministry of Health, the French Society of Intensive Care Medicine (SRLF) and the European Society of Intensive Care Medicine (ESICM), and lecture fees from Baxter. The other authors have no competing interests to disclose.
Acknowledgements
The authors wish to thank the following collaborators: Dr. Etienne de Montmollin, Dr. Paul-Henri Wicky, Dr. Juliette Patrier, Dr. Lucie Le Fevre, Prof. Lila Bouadma, Prof Marie-Pia d’Ortho, Prof. Patrick Nataf, and Prof. Jean-François Timsit, and also the EEG technicians of the neurophysiology unit who contributed to daily EEG recordings.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinph.2021.01.010.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes E., Reuter J., Wanono R., Bouadma L., Jaquet P., Tanaka S., Sinnah F., Ruckly S., Dupuis C., de Montmollin E., Para M., Braham W., Pisani A., d’Ortho M.-P., Rouvel-Tallec A., Timsit J.-F., Sonneville R. Early EEG for prognostication under venoarterial extracorporeal membrane oxygenation. Neurocrit Care. 2020;33(3):688–694. doi: 10.1007/s12028-020-01066-3. [DOI] [PubMed] [Google Scholar]
- Peluso L., Rechichi S., Franchi F., Pozzebon S., Scolletta S., Brasseur A., Legros B., Vincent J.-L., Creteur J., Gaspard N., Taccone F.S. Electroencephalographic features in patients undergoing extracorporeal membrane oxygenation. Crit Care. 2020;24(1) doi: 10.1186/s13054-020-03353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnah F., Dalloz M.-A., Magalhaes E., Wanono R., Neuville M., Smonig R., Radjou A., Mourvillier B., Bouadma L., Timsit J.-F., d’Ortho M.-P., Rouvel-Tallec A., Sonneville R. Early electroencephalography findings in cardiogenic shock patients treated by venoarterial extracorporeal membrane oxygenation. Crit Care Med. 2018;46(5):e389–e394. doi: 10.1097/CCM.0000000000003010. [DOI] [PubMed] [Google Scholar]
- Vellieux G., Rouvel-Tallec A., Jaquet P., Grinea A., Sonneville R., d'Ortho M.-P. COVID-19 associated encephalopathy: is there a specific EEG pattern? Clin Neurophysiol. 2020;131(8):1928–1930. doi: 10.1016/j.clinph.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]