Abstract
Myotonic dystrophy type 1 (DM1) is a multisystem disorder affecting muscle and central nervous system (CNS) function. The cellular mechanisms underlying CNS alterations are poorly understood and no useful treatments exist for the neuropsychological deficits observed in DM1 patients. We investigated the progression of behavioral deficits present in male and female muscleblind-like 2 (Mbnl2) knockout (KO) mice, a rodent model of CNS alterations in DM1, and determined the biochemical and electrophysiological correlates in medial prefrontal cortex (mPFC), striatum and hippocampus (HPC). Male KO exhibited more cognitive impairment and depressive-like behavior than female KO mice. In the mPFC, KO mice showed an overexpression of proinflammatory microglia, increased transcriptional levels of Dat, Drd1, and Drd2, exacerbated dopamine levels, and abnormal neural spiking and oscillatory activities in the mPFC and HPC. Chronic treatment with methylphenidate (MPH) (1 and 3 mg/kg) reversed the behavioral deficits, reduced proinflammatory microglia in the mPFC, normalized prefrontal Dat and Drd2 gene expression, and increased Bdnf and Nrf2 mRNA levels. These findings unravel the mechanisms underlying the beneficial effects of MPH on cognitive deficits and depressive-like behaviors observed in Mbnl2 KO mice, and suggest that MPH could be a potential candidate to treat the CNS deficiencies in DM1 patients.
Keywords: DM1, dopamine, Mbnl2, methylphenidate, microglia
Introduction
Myotonic dystrophy type 1 (DM1) is a particularly complex inherited disease both from the clinical and genetic standpoints. Despite being rare, it is the most common adult-onset form of muscular dystrophy with an estimated worldwide prevalence of 1/8000 people (Baldanzi et al. 2016). DM1 is a multisystem disorder characterized by progressive muscle deterioration, myotonia, endocrine, and central nervous system (CNS) alterations (Harper 2001). It is caused by expanded CTG repeats in the 3′UTR of the dystrophia myotonica protein kinase (DMPK) gene. Transcription of these repeats leads to CUG expansion (CUGexp) RNAs that accumulate within nuclear foci deregulating several RNA-binding factors including the muscleblind-like (MBNL) protein family (Ranum and Cooper 2006). The main 2 proteins are the muscleblind-like 1 (MBNL1), expressed abundantly in skeletal muscle of adult cells, and the muscleblind-like 2 (MBNL2), expressed mostly in the CNS. The resultant sequestration of both MBNL1 and MBNL2 proteins by CUGexp transcripts has been implicated in DM1 pathogenesis (Miller 2000). Converging evidence in experimental animals indicates that MBNL1 loss-of-function plays an important role in muscle impairment (Kanadia et al. 2006), while loss of MBNL2 function leads to extensive changes in postnatal splicing patterns in the brain, some of which are equally dysregulated in the human DM1 brain (Charizanis et al. 2012),
Current research has primarily focused on the muscular issues of DM1, although this disease also presents many debilitating neurological manifestations such as cognitive impairment (Winblad et al. 2016), increased anxiety, depression, and anhedonia (Winblad et al. 2010). Brain abnormalities have been described in imaging studies including atrophy in frontal and temporal lobes, hippocampus (HPC), thalamus, brainstem, cerebral cortex, and striatum (Romeo et al. 2010). In addition, ventricular enlargement, white matter alterations, and microstructural damage have also been observed in corticospinal and limbic pathways of DM1 patients (Minnerop et al. 2011; Wozniak et al. 2014). The loss of white and grey matter in sensori-motor cortical areas, cognitive brain networks, and temporoparietal areas have been associated with neuropsychological deficits in DM1 patients (Caso et al. 2014; Baldanzi et al. 2016), an may result in abnormal functional connectivity in brain areas involved in cognition such as the default network (Serra et al. 2014). Although these data point to relevant brain pathologies in DM1, there is a lack of studies investigating the precise cellular and molecular mechanisms underlying these neurophysiological alterations, and whether or not they are progressive. Moreover, despite the therapeutic advances for improvement of muscular function in DM1 patients (Wheeler et al. 2012; Konieczny et al. 2017) there is a paucity of research strategies for effective treatment of CNS disruptions in DM1. One way to bridge the need for understanding the neurobiological mechanisms underlying this disorder is the use of relevant animal models of DM1 CNS-linked disease models.
Recently, Mbnl2 knockout (KO) mice have been developed displaying several phenotypes consistent with features of DM1 neuropathophysiology, including abnormal REM sleep propensity, and deficits in spatial memory, but no abnormalities in skeletal muscle (Charizanis et al. 2012). However, it is still unknown how the cellular and molecular pathways mediating these neuropathological characteristics are affected in particular brain areas by the loss of MBNL2 protein. In this sense, functional neuroimaging evidence suggests abnormal neural activity in the medial PFC (mPFC) and the HPC of DM1 patients (Romeo et al. 2010), 2 key structures regulating attention, memory, and emotion (Dalley et al. 2004; Vertes 2006; Price and Drevets 2010), functions that are impaired in DM1. Accordingly, neurochemical and neurophysiological dysregulations in these areas have been implicated in several neuropsychiatric disorders involving cognitive deficits that include schizophrenia (Winterer and Weinberger 2004; Uhlhaas and Singer 2015), attention deficit/hyperactivity disorder (ADHD) (Arnsten and Pliszka 2011), and major depression (Mayberg et al. 1999). Moreover, alterations in neurotransmitters that play central roles in these brain areas such as dopamine (DA), and changes in markers of plasticity and oxidative stress have been reported in DM1 patients (Ono et al. 1998; Comim et al. 2015). Notably, a decrease in BDNF serum protein levels has been observed in DM1 patients (Comim et al. 2015), as well as an increase in the blood levels of oxidant agents (Toscano et al. 2005), partially caused by a defective antioxidant isoform of the DMPK gene (Pantic et al. 2013). BDNF controls the translocation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) to the nucleus leading to an increase in its expression (Bouvier et al. 2016). The Nrf2 gene is a key marker of antioxidant response (Perez-Leal et al. 2017). In addition, DM1 patients exhibit increased serum concentrations of IL-6 (Wegner et al. 2013). Although the brain levels of pro- or anti-inflammatory cytokines, microglia, and astroglia have not been evaluated in DM1 patients, microglia activation has been observed in the mPFC and HPC of patients suffering from major depression and cognitive impairment (Rajkowska 2003; Heneka et al. 2015).
Methylphenidate (MPH) is a psychostimulant widely used in the clinic to treat ADHD (Arnsten 2006), and has shown some beneficial effects as an adjuvant in the treatment of depression (Lavretsky et al. 2015). While there is some evidence for MPH improving daytime sleepiness in DM1 patients (Puymirat et al. 2012), no data is available as to its potential effects on other brain-related pathology in this population. The therapeutic effects of MPH for ADHD have been associated with its capacity to primarily block DA transporters (DAT) (Volkow et al. 1998), although more recently it has also been shown that MPH blocks norepinephrine (NE) transporters (NET) in the brain of human subjects (Hannestad et al. 2010). In addition, monoamine uptake inhibitors suppress the toxicity phenotype in a Drosophila model of DM1 with targeted expression of CUG repeats to brain mushroom bodies leading to semilethality (Garcia-Lopez et al. 2008). Therefore, MPH could be a potential candidate to reverse the behavioral alterations observed in Mbnl2 KO mice. However, the exact mechanisms underlying the effects of MPH are still unknown. For instance in mice and rats, the acute administration of low doses of MPH preferentially activates DA and NE levels in the mPFC relative to subcortical regions, while high doses of MPH induce a generalized activation of catecholamine outflow (Berridge et al. 2006; Koda et al. 2010). Although there is a lack of evidence for the effects of MPH on the protein or gene expression levels of monoaminergic receptors, there is data showing that it regulates the expression of DA receptors (Thanos et al. 2007),
The first aim of this study was to investigate the progression of the cognitive and affective deficits in Mbnl2 KO male and female mice. Second, to gain deep insight into the neural substrates of cognitive impairment and affective alterations in Mbnl2 KO mice we studied: 1) the possible alterations in microglia and astrocyte expression, and/or loss of neurons in the mPFC, striatum, and HPC; 2) the changes in gene expression levels related to the DA and NE neurotransmission systems in the mPFC and HPC, and determined the extracellular levels of DA in the mPFC; and 3) the neural activity patterns in the prelimbic cortex and CA1 region of the HPC in freely moving Mbnl2 KO and wild-type (WT) mice. Third, we investigated whether chronic treatment with MPH (1 and 3 mg/kg) could reverse these behavioral deficits, and explored the underlying mechanisms of action. Specifically, we evaluated its effects on the alterations in the expression of genes related to the DA and NE systems and of brain-derived neurotrophic factor (Bdnf), and in the overexpression of microglia observed in the brains of KO mice.
Materials and Methods
Animals
Mbnl2 heterozygous (HE) mice on a mixed C57Bl/129 background (provided by Maurice S. Swanson, Florida, USA) were bred in the animal facilities of the PRBB in order to generate KO and WT littermates. For behavioral studies, mice were grouped-housed except for those that were monitored continuously during 5 days in the circadian activity boxes, and were thus individualized. All mice were maintained in a temperature (21 ± 1°C) and humidity (50 ± 10%) controlled environment in a room with a 12-h light/dark cycle (lights on at 8 am and off at 8 pm) with food and water available ad libitum. All the experiments were performed during the light phase of the light/dark cycle by a trained observer that was blind to experimental conditions. Animal procedures were conducted in accordance with the standard guidelines of the National Institutes of Health 1995; European Communities Directive (86/609 EEC) and approved by the local ethical committee (CEEA-PRBB).
Drugs
Methylphenidate hydrochloride (MPH; Rubio laboratories) was dissolved in saline (0.9% solution of NaCl) and administered intraperitoneally (i.p.). In microdialysis experiments, MPH (1 and 3 mg/kg) was administered acutely. In behavioral assessments, vehicle (VEH) or MPH (1 and 3 mg/kg) was administered chronically once a day for 21 consecutive days. Following surgery, mice were treated during 2 days with meloxicam (Metacam® Boehringer Ingelheim) at the dose of 0.5 mg/kg (microdialysis), and with meloxicam at the dose of 2 mg/kg and buprenorphine (Buprex®) at the dose of 0.1 mg/kg (electrophysiology).
Behavioral Characterization and Drug Treatment
Male and female KO mice and WT littermates were tested at 3 months of age in the novel object recognition task (NOR), the novel open field test (OFT), the elevated plus-maze (EPM), and the forced swim test (FST). When these mice reached 5 months of age, they were tested again in the NOR, the OFT, the elevated zero maze (EZM) and the tail suspension test (TST). A separate group of naïve WT and KO male mice was tested at 3 months of age for circadian activity patterns in the PHECOMP cages (PHECOMP, Panlab, Spain), and at 5 months of age on the active-avoidance paradigm. To determine the optimal doses of MPH to be used for chronic administration, we first determined a dose–response curve in locomotor studies. Male WT mice were treated acutely with vehicle or 1, 3, and 10 mg/kg MPH, and were placed in locomotor cages for 30 min. MPH significantly increased locomotor activity at the dose of 10 mg/kg with respect to vehicle administration, but not at the dose of 1 and 3 mg/kg (data not shown). Thus, to avoid a possible bias from the hyperlocomotor effects of the highest dose of MPH, male WT and KO mice that followed basal behavioral determinations (3 and 5 months of age) were treated with vehicle or MPH (1 and 3 mg/kg) once daily for 21 days, and then assessed in the OFT, the NOR, the TST, and the light-dark box. All animals were habituated to the experimental room 1 h before testing, and handled twice a day for 1 week before the beginning of the experiments (see Supplementary Fig. S1 for a timeline of the experimental procedures).
Memory Assessments
To evaluate long-term recognition memory, the NOR test was performed in the V-maze (15 lux) as described previously (Busquets-Garcia et al. 2013). Briefly, this test consists of 3 phases: the first day, mice were habituated to the empty maze for 9 min. The second day, 2 identical objects were placed in each of the maze’s arms and mice explored during 9 min for the familiarization phase. Twenty-four hours later, one of the familiar objects is replaced randomly with a novel object, and mice explored the maze during 9 min. In this phase, the total time spent exploring each object (novel and familiar) was recorded. Object exploration was defined as the orientation of the nose towards the object and touching it with the nose. A discrimination index (DI) was calculated as the difference in exploration time between novel (Tn) and familiar object (Tf), and divided by the total time exploring both objects (Tn + Tf) (DI = [Tn − Tf]/[Tn + Tf]). A higher DI score reflects greater memory retention for the familiar object.
To assess emotional working memory, mice were trained to associate an aversive foot shock applied to the grid of the floor (unconditioned stimulus [US]) with the presentation of a light as a conditioned stimulus (CS) in a 2-way shuttle box apparatus (Panlab, Barcelona) as previously reported (Martin et al. 2002). A 10 min habituation period was performed before the beginning of each session. Conditioned responses were recorded when the animal actively avoided the US by changing into the other compartment within the 5 s after the onset of CS. If the animal changed compartments due to the presence of the electric stimulus, an unconditioned change was recorded. All mice were subjected to one daily session of 100 trials during 5 consecutive days. To exclude a potential bias by differences in pain thresholds, and ensure that all mice equally detected the aversive stimulus, the nociception response was performed in a separated group of mice using the plantar test as previously reported (Castañé et al. 2006). Briefly, thermal hyperalgesia was assessed by measuring hind paw withdrawal latency in response to radiant heat. Mice were placed into compartment enclosures on a glass surface. The heat source was then positioned under the plantar surface of the hind paw and activated with a light beam intensity of 0.30 mA. A cut-off time of 20 s was imposed to prevent tissue damage in the absence of response. The mean withdrawal latencies for the right and the left hind paws were determined from the average of 3 separate trials, taken at 5 min intervals to prevent thermal sensitization and behavioral disturbances.
Depression-Like Behavioral Assessment
Depressive-like behavior was evaluated in the FST and the TST. Both paradigms are based on the postulation that mice will usually try to escape from an aversive stimulus. Then, enhanced immobility is thought to represent a state of behavioral despair that is characteristic of depression. In the FST, mice were placed in a transparent Plexiglas cylinder filled with water (24 ± 1°C) without being able to touch the bottom and forcing the mouse to swim during 6 min. The total time of immobility, including minimal balanced movements, was measured during the last 4 min of the test (Filliol et al. 2000). In the TST, each mouse was individually suspended by the tail (20 cm from the floor) to a horizontal bar using an adhesive tape (1 cm from tip tail) during 6 min and they could not escape or grip nearby surfaces. The time spent immobile during the last 4 min was recorded for each mouse. In this test, immobility was defined as the total absence of limb movements (Aso et al. 2008). An immobility index (II) was calculate as a total time spent immobile divided by the 240 s period test to facilitate the comparison of the repeated measurements in the same paradigm.
Anxiety-Like Behavioral Assessment
To evaluate anxiety-like behavior, 3 paradigms were used: the EPM (Llorente-Berzal et al. 2011), the EZM (Braun et al. 2011) and the light-dark box (Bura et al. 2010). The EPM consisted of a plastic black maze with 4 arms set in a cross, whereas the EZM comprised a ring-shaped runway. Both mazes have 2 opposite closed arms (delimited by vertical walls), and 2 open arms (with unprotected edges) and are elevated 30 cm above the ground and placed under indirect light (80 lux in open arms, 10 lux in closed arms). During the 5 min trial, the number of entries and the time spent in each arm were recorded. An entry was counted when the mouse moved the 2 front legs into one arm. The light-dark box consisted of 2 compartments subdivided into squares and connected by a tunnel. One compartment is small and dark (<10 lux), while the other compartment is larger and illuminated (500 lux). Mice were placed in the maze during 5 min and the total number of squares crossed and number of entries in each compartment was counted.
Locomotor Activity Assessment
To evaluate locomotor activity, mice were placed in an experimental arena (Plexiglas box, 70 cm wide × 90 cm long × 60 cm high) under bright illumination (100 lux) and divided into 10 cm squares. Each mouse was placed in the center of the arena without previous habituation during 5 min, and the distance traveled was determined by counting the total number of squares crossed (Flores et al. 2014).
To evaluate circadian activity patterns, a separate group of naïve WT and KO male mice were individually and randomly placed in experimental chambers equipped with a food and drink available ad libitum (PHECOMP, Panlab, Barcelona), as previously described (Viñals et al. 2012). Each box has infrared frames (16 × 16 beams, 16 mm spaced), which allow recording resting time, horizontal locomotion and rearing activity. Animals were habituated to the boxes during 5 consecutive days, and monitored during this period. Measurements of circadian locomotor activity were taken on the fifth day.
In Vivo Microdialysis and MPH Administration
Naïve male mice were anesthetized with a mixture of ketamine (100 mg/kg, i.p.) and xylazine (20 mg/kg, i.p.) and placed in a stereotaxic apparatus. Microdialysis surgery was performed as previously described (Viñals et al. 2012). Briefly, a microdialysis probe (CMA7: 2 mm, CMA Microdialysis, Stockholm, Sweden) was implanted in the mPFC, comprising the cingulate, infralimbic, prelimbic, and medial orbital cortices (AP + 1.9 ML ± 0.3 DV −3.4, from bregma) and fixed to the skull with dental cement (Dentalon Plus, Heraeus Kulzer, Germany). Two days after surgery, mice were habituated to the microdialysis environment overnight. The next day, analytical probes were perfused with a Ringer solution (148 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, and 0.8 mM MgCl2; pH 6.0) with a constant flow rate of 1uL/min. Four basal dialysates (20 μL) were collected every 20 min in order to determine the baseline DA efflux. Then, mice were challenged first with vehicle followed by 2 doses of MPH. Three samples were collected after vehicle injection and 6 samples after MPH 1 mg/kg and 3 mg/kg each. Dialysates were injected without any purification into a HPLC system with an automatic injector (Agilent 1100, Palo Alto, USA), a reverse-phase column (Zorbax SB C18, 5 mm, 150 × 4.6 mm2, Agilent Technologies), and a coulometric detector (Coulochem II, ESA Inc., Chelmsford, MA, USA) with a 5011 A analytical cell. DA was quantified as previously reported (Robledo et al. 2007). At the end of the experiment, mice were sacrificed by cervical dislocation and brains were removed and sliced using a cryostat (Leica CM3050 S). Serial coronal sections (20 μm) were stained with Cresyl Violet (Sigma-Aldrich, Madrid, Spain) in order to check and visualize the probe position. Only those mice with correct probe placements were used in the study.
Electrophysiological Activity in Alert Male Mice
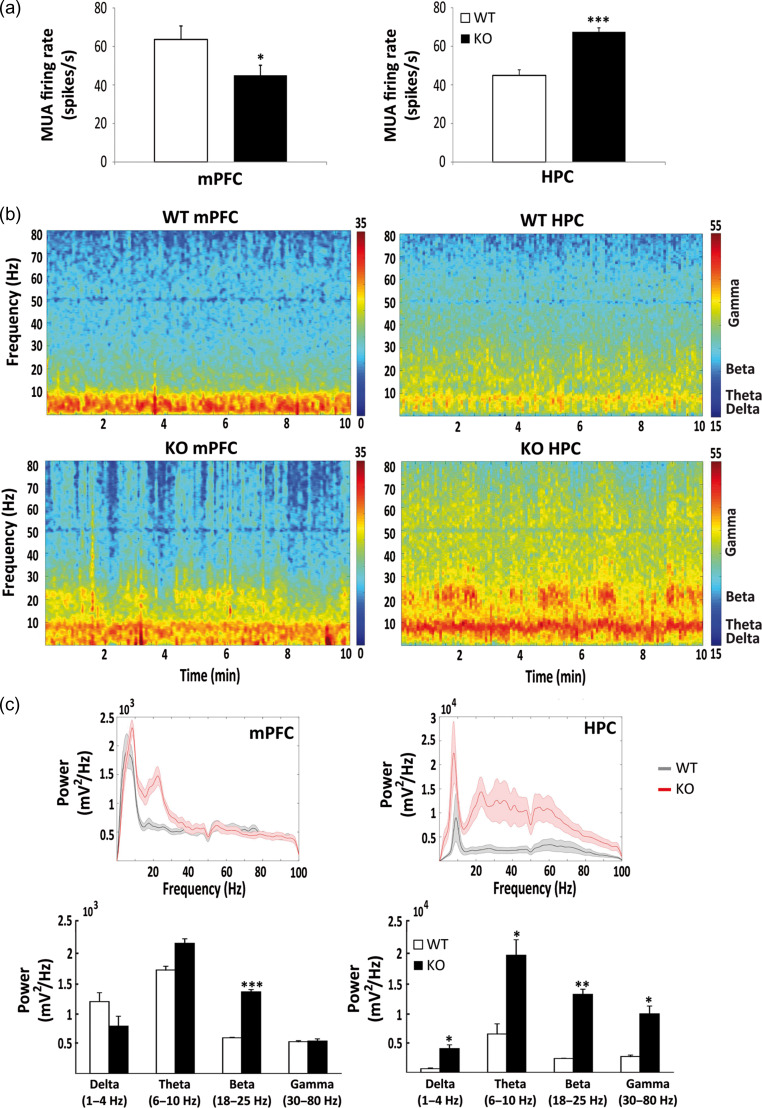
Naïve male mice were anesthetized (induction with ketamine/xylazine and maintenance with isoflurane 0.5–4%), and placed in a stereotaxic apparatus. Two small craniotomies were drilled in the skull, including one above the mPFC and another above the HPC, and several microscrews were screwed to stabilize the implant. Then, tungsten stereotrodes (pairs of electrodes) were lowered down into the prelimbic region of the mPFC (AP: 1.5 mm; ML: ± 0.6 mm; DV: −1.7 mm from bregma) and the CA1 region of the HPC (AP: −1.8 mm; ML: ± 1.3 mm; DV: −1.15 mm) and pinned to an adapter that allowed the connection to the recording system. A screw on top of the cerebellum was used as a general ground. Wire and adapter were implanted with biocompatible dental cement. These miniature implants are well tolerated by mice with only minimal impact on natural behavior. We allowed a recovery period of 1-week after surgery and then electrophysiological recordings were carried out in alert freely moving WT and KO mice. Recordings were implemented with the multichannel Open Ephys system (http://www.open-ephys.org/) at a 30 kHz sampling rate with Intan RHD2132 amplifiers equipped with an accelerometer that allowed monitor whether the animals were sleeping, in quiet alertness, or exploring the environment. After the recordings, animals were euthanized and their brains removed for histological confirmation of electrode placement. Signals were filtered offline (Butterworth filter) to discriminate multiunit activity (MUA; action potentials from multiple neurons recorded simultaneously) and local field potentials (LFPs; rhythmic changes in voltage reflecting ongoing neural oscillations). Continuous signals were first preprocessed in Python: detrended, 50 Hz noise removed with a Notch filter, bandpass filtered between 800 and 2000 Hz to isolate MUA, and bandpass filtered between 0.1 and 450 Hz and down-sampled to 1 kHz to isolate LFPs. Briefly, MUA was detected by thresholding the continuous filtered signals at −3 sigma standard deviation of the average voltage of the signal and artefacts were discarded using principal component analysis (PCA) with Offline Sorter software (Plexon Inc.). We used the accelerator's recorded signals to identify epochs of exploration. Specifically, we quantified the module of acceleration (ACC) in the X, Y, and Z axis. During exploration, ACC exhibited large variability, whereas during quiet alertness or sleep ACC was greatly reduced. Firing rate was calculated as the number of action potentials per second in 1–2 min periods of exploration. Spectrograms and power spectra were built using the multitaper method with the Chronux library in MATLAB using 5–9 tapers. Power was quantified by averaging the absolute power of epochs of exploration (same epochs used to estimate firing rate) at delta (1–4 Hz), theta (6–10 Hz), beta (18–25 Hz), and gamma (30–80 Hz) frequencies across animals.
Biochemical Characterization
Immunostaining Procedure
Tissue preparation: Forty-eight hours after the behavioral assessments (see Supplementary Fig. S1 for timeline), mice were anesthetized by an i.p. injection of a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) were deeply and were transcardially perfused with 4% paraformaldehyde (PFA). Subsequently, the brains were extracted and post fixed in the same fixative solution, and placed in a 30% sucrose–PBS solution at 4 °C. Coronal brain sections (30 μm) were obtained with a cryostat (Leica CM3050 S) and kept in a cryoprotectant solution (30% ethylene glycol, 30% glycerol in 0.1 M PB) at −20 °C until use.
Immunofluorescence: The expression of glial fibrillary acidic protein (GFAP), ionized calcium binding adapter molecule 1 (Iba1), neuron specific nuclear protein (NeuN), interleukin 1 beta (IL-1β) and transforming growth factor beta (TGF-β) was determined in the mPFC, the dorsal striatum and the HPC by immunofluorescence as previously described (Kossatz et al. 2016). Three coronal sections were selected for each brain structure according to anterior-posterior coordinates relative to bregma (Paxinos and Franklin 2001). For the mPFC from +2.71 to +1.70 mm (including cingulate, prelimbic and infralimbic areas); for the striatum from +1.32 to −0.82 mm; and for the HPC from −1.28 to −2.12 mm (including CA1, CA3 and dentate gyrus). Briefly, free-floating slices were rinsed and blocked at room temperature for 2 h and incubated overnight at 4 °C in the same solution with the following primary antibodies: rabbit anti-GFAP (1:500, Dako; CAt. no. #Z0334), rabbit anti-Iba1 (1:500, Wako; Cat. no. 019-19 741), mouse anti-NeuN (1:200; Millipore; Cat. no. #MAB377), mouse anti-TGF-β (1:50; R&D Systems; Cat.no. #MAB240), or goat anti IL-1β (1:500; R&D Systems; Cat.no. #AF-401-NA). For NeuN staining an antigen retrieval step with sodium citrate buffer was required (Jiao et al. 1999). The next day, sections were incubated at room temperature and for 2 h with the secondary antibodies Alexa Fluor® 488 donkey anti-mouse IgG, Alexa Fluor® 594 donkey anti-rabbit IgG or Alexa Fluor® 647 donkey anti-goat IgG (1:500, Jackson ImmunoResearch). After incubation, sections were rinsed, counterstained with DAPI 300 nM and mounted onto gelatine-coated slides with Fluoromount-G (SouthernBiotech) mounting media.
Image analyses: Images were obtained using a Nikon Eclipse Ni-E microscope equipped with an Andor Zyla 4.2 camera at ×10 for the mPFC and the striatum, and at ×20 for the HPC. For each structure, 6 representative images were taken from 3 coronal sections (2 photographs per section). In each image, we defined a region of interest (ROI) that remained constant in all experimental groups studied. Images were then processed by counting positive cells using the ImageJ software (NIH).
Quantitative Real-Time PCR Analysis
In order to evaluate the mechanisms of MPH action, we determined the transcriptional levels of Dat, dopamine receptors D1 (Drd1) and D2 (Drd2), Net, and the NE synthesizing enzyme, dopamine-beta hydroxylase (Dbh) in the mPFC and HPC of male mice. In addition, we measured the mRNA expression of brain-derived neurotrophic factor (Bdnf) and nuclear factor erythroid 2-related factor 2 (Nrf2). This procedure was performed as previously described (Cutando et al. 2013). Briefly, male mice were sacrificed by cervical dislocation 48 h after the behavioral assessments. Brains were quickly removed and dissected to remove the mPFC and HPC areas and stored at −80 °C until use. Total RNA was isolated using an RNeasy Mini Kit (Tissue; QIAGEN) according to the manufacturer’s instructions and the quality and concentration of RNA was quantified with a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). For each animal, 500 ng of total RNA was reverse transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) in a 20-ul reaction. Reverse transcriptase reactions were performed at 25 °C during 10 min, 2 h at 37 °C and followed by 5 min at 85 °C. The following specific primers for mouse were designed using the Primer Express 3.0.1 Software (Applied Biosystems), and its specificity was verified using primer-BLAST search: Dat (forward, 5′-CACACCCGCTGCTGAGTATTT-3′; reverse, 5′-GGTCATCAATGCCACGACTCT-3′), Net (forward, 5′-GCAAAACCGCCGATCTACTA-3′; reverse, 5′-CCACCACCATTCTTGTAGCA-3′), Drd1 (forward, 5′-GTAGCCATTATGATCGTCAC-3′; reverse, 5′- GATCACAGACAGTGTCTTCAG-3′), Drd2 (forward, 5′-TACGTGCCCTTCATCGTCAC-3′; reverse, 5′- GGTGGGTACAGTTGCCCTTG-3′), Bdnf (forward, 5′-AGAGCTGTTGGATGAGGACCAG-3′; reverse, 5′-CAAAGGCACTTGACTACTGAGCA-3′), Nrf2 (forward, 5′-AGCATGATGGACTTGGAGTTG-3′; reverse, 5′-CCTCCAAAGGATGTCAATCAA-3′) and Gapdh (forward, 5′-ATGACTCCACTCACGGCAAAT-3′; reverse, 5′- GGGTCTCGCTCCTGGAAGAT-3′). Real-time PCR (qRT-PCR) was carried out in an Optical 384-well plate with a QuantStudio™ 12 K Sequence Detection System (Applied Biosystems) using the PowerUp SYBR Green Master Mix (Applied Biosystems) according to manufacturer’s protocol. All the samples were tested in triplicate for each quantitative PCR and the mRNA expression of all target genes were normalized by the glyceraldehyde 3-phosphate dehydrogenase (Gapdh) as endogenous housekeeping gene. PCR cycling conditions consisted of 2 activation stops (50 °C for 2 min, then 95 °C for 10 min) followed by 45 cycles of melting (95 °C for 15 s) and annealing (60 °C for 1 min). Samples were analyzed via the comparative cycle threshold (Ct) method to determine gene expression relative quantification (RQ) and the results are reported as fold change compared with the control.
Statistical Analysis
Two-way repeated measures ANOVA was used to analyze the behavioral data with genotype (WT and KO mice) as a between subjects factor, and age (3 and 5 months of age) or day of testing as within subjects factors. The differences in behavioral tests between male and females were also analyzed at 3 and 5 months separately using 2-way ANOVAs. Immunohistochemical and qRT-PCR data at 3 and 5 months were analyzed separately using one-way ANOVAs. Changes in DA extracellular concentrations (pg/sample) and percentage of baseline were analyzed using one-way ANOVA and 2-way ANOVA, respectively. The effects of chronic treatment with MPH were analyzed using 2-way ANOVAs with genotype and dose as between subject factors. The Bonferroni’s post hoc test for multiple comparisons was used when appropriate (SPSS Inc., Chicago, USA). For circadian activity patterns, the nonparametric Mann–Whitney test was applied since the data did not follow a normal distribution according to the Kolmogorov–Smirnov test. Electrophysiological data (basal firing rate, power of each band of interest) were compared only between genotypes using a Student’s t-test.
Results
KO Mice Show Cognitive Impairment and Depressive-Like Behavior
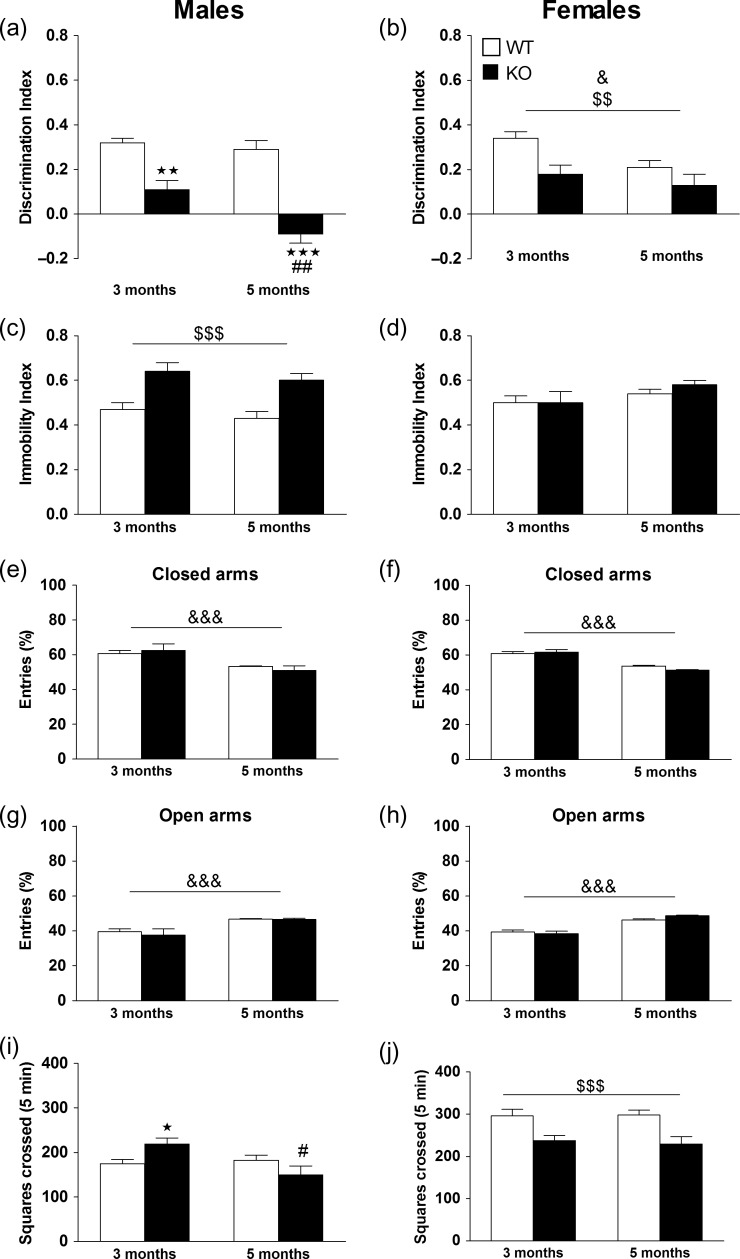
Long-term recognition memory was evaluated in the NOR test in WT (male n = 19; female n = 21) and KO (male n = 13; female n = 16) mice. In males, significant effects of age, genotype, and interaction were found. Subsequent post hoc analysis showed that KO mice displayed significant memory deficits in comparison with WT mice (P < 0.01) at 3 months of age, and that this effect was exacerbated at 5 months (P < 0.001 vs. WT and P < 0.01 vs. KO at 3 months) (Fig. 1a). In females, significant main effects of age and genotype were revealed, but no interaction (Fig. 1b). See Supplementary Table S5 for statistical values. In order to reveal sex differences we also performed statistical analysis between male and female at 3 and 5 months of age. At 3 months of age, no significant effects of genotype, sex, or interaction were observed between male and female in the NOR test. At 5 months of age, a significant effect of genotype and interaction were observed, but no significant main effect of sex. Post hoc analysis showed that KO male mice displayed a higher cognitive impairment than female KO mice (P < 0.01).
Figure 1.
Behavioral performance in male and female WT and KO mice. Long-term recognition memory was assessed in the novel object recognition test by determining the discrimination index in male WT (n = 19) and KO (n = 13) (a) and in female WT (n = 21) and KO (n = 16) (b) mice at 3 and 5 months of age. Values are mean + SEM **P < 0.01, ***P < 0.001 versus WT group; ##P < 0.01 versus KO group at 3 months, &P < 0.05, (age effect), $$P < 0.01 (genotype effect). Depression-like behavior was evaluated by calculating the immobility index in male WT (n = 17) and KO (n = 13) (c) and in female WT (n = 25) and KO (n = 15) (d) mice at 3 and 5 months of age. Values are mean + SEM. $$$P < 0.001 (genotype effect). For anxiety-like behavior, the percentage of entries was evaluated in the closed (e) and open (g) arms in male WT (n = 19) and KO (n = 12) mice, and in the closed (f) and open (h) arms in female WT (n = 24) and KO (n = 15) mice at 3 and 5 months of age. Values are mean + SEM. &&&P < 0.001 (age effect). Locomotor activity was assessed in the novel open field test. Squares crossed in the periphery of the arena are shown for male WT (n = 19) and KO (n = 12) (i) and for female WT (n = 24) and KO (n = 15) (j) mice at 3 and 5 months of age. Values show the mean + SEM. *P < 0.05 versus WT group, #P < 0.05 versus KO at 3 months of age, §§§P < 0.001 (genotype effect) Repeated measures ANOVA followed by Bonferroni’s post hoc analysis. See Supplementary Table S5 for statistical values.
Depressive-like behavior was assessed in WT (male n = 17; female n = 25) and KO (male n = 13; female n = 15) mice. In males, a significant main effect of genotype was observed, but no significant effects of age or interaction. The genotype effect was due to an increase in the immobility index in KO mice at 3 and 5 months of age with respect to WT mice (Fig. 1c). In females, no significant effects of genotype, age or interaction were observed (Fig. 1d). See Supplementary Table S5 for statistical values. Regarding sex differences, at 3 months of age, a significant main effect of genotype and interaction were found, but no significant main effect of sex. Post hoc tests showed that KO male mice presented a higher immobility index in respect to KO female mice (P < 0.05). At 5 months of age, a significant main effect of genotype and interaction were revealed, but no significant main effect of sex. Post hoc tests showed that WT female mice has an increased depressive-like phenotype than WT male mice (P < 0.01). See Supplementary Table S1.
KO Mice do not Show Anxiety-Like Behavior
Anxiety-like behavior was evaluated in WT (male n = 19; female n = 24) and KO (male n = 12; female n = 15) mice. The analysis of the percentage of entries in males and females revealed a significant effect of age in both the closed and open arms, but no significant effects of genotype or interaction (Fig. 1e–h). No significant effects were observed with respect to the time spent in open and closed arms in male or female mice (data not shown). See Supplementary Table S5 for statistical values.
KO Mice Exhibit Mild Alterations in Locomotion
Locomotor activity was determined in the OFT in WT (male n = 19; female n = 24) and KO (male n = 12; female n = 15) mice. In males, analysis for squares crossed revealed significant effects of age and interaction between age × genotype, but no significant main effect of genotype. Post hoc analysis showed that KO mice displayed significantly higher locomotion with respect to WT mice at 3 months (P < 0.05), but this effect disappeared at 5 months of age (Fig. 1i). In females, a significant main effect of genotype was observed, but no significant effects of age or interaction (Fig. 1j). See Supplementary Table S5 for statistical values. The statistical analysis, for squares crossed, between male and female mice at 3 months of age revealed a main effect of sex but no significant effects of genotype or interaction. At 5 months of age, significant main effects of sex and genotype were observed, but no interaction between factors. See Supplementary Table S1.
Since males showed more significant behavioral alterations than females related to cognitive and affective processes, in subsequent behavioral, neurochemical, and electrophysiological experiments we only used male WT and Mbnl2 KO mice.
Circadian Activity and Emotional Working Memory are Altered in KO Mice
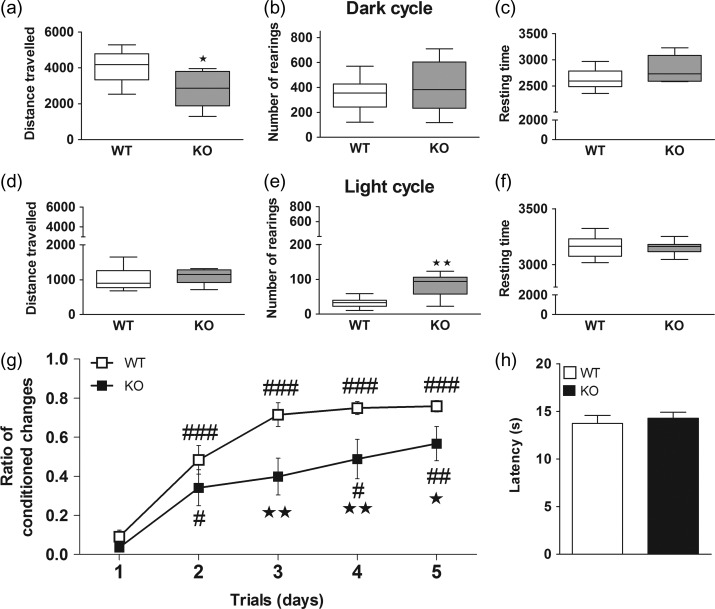
The analysis of circadian activity patterns in WT (n = 13) and KO (n = 9) mice revealed that KO mice traveled significantly less distance during the dark cycle than WT mice (U = 24.0, P < 0.05), and resting time was also slightly increased in these mice. During the light cycle, KO mice showed a significantly higher number of rearings compared with WT mice (U = 11.0, P < 0.01), and a trend to increase the distance traveled (Fig. 2a–f).
Figure 2.
Assessment of circadian activity, emotional working memory and nociception. The circadian activity was evaluated in the PHECOMP cages in male WT (n = 13) and KO (n = 9) mice. Distance traveled, number of rearings and resting time during the dark (a–c) and light (d–f) cycles are shown. Nonparametric data are graphed with box and whiskers plots, where the line indicates the median, the box indicates the 25–75th percentile and the whiskers indicate the 5–95th percentile. *P < 0.05, **P < 0.01 versus WT group (Mann–Whitney test). Emotional memory was evaluated in the active-avoidance test. The ratio of conditioned changes is shown for male WT (n = 13) and KO (n = 8) mice (g) #P < 0.05, ##P < 0.01, ###P < 0.001 versus day 1 of same genotype, *P < 0.05, **P < 0.01 versus WT mice. Nociceptive response was assessed in the plantar test measuring the paw withdrawal latency (h). See Supplementary Table S5 for statistical values.
Male WT (n = 13) and KO (n = 8) mice were trained in the active avoidance paradigm during 5 days, and the ratio between conditioned changes and total number of changes was determined daily. Significant effects of genotype, day of testing and interaction were observed. Post hoc tests revealed that WT mice progressively learned the task during training, reaching more than 70% correct responses on day 5, while KO mice showed worse performance that WT controls on days 3 (P < 0.01), 4 (P < 0.01), and 5 (P < 0.05) of training, and never reached more than 50% correct responding (Fig. 2g). See Supplementary Table S5 for statistical values. In the plantar test, no significant differences in baseline paw withdrawal latencies were found between genotypes, indicating that KO mice perceive and respond normally to nociceptive stimuli (Fig. 2h).
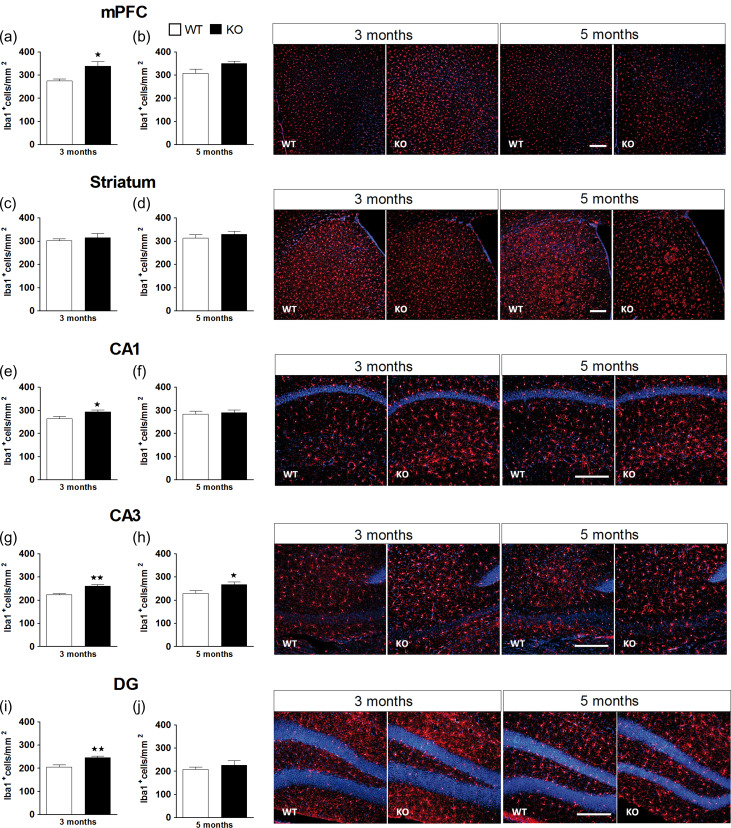
KO Mice Exhibit Increased Microglial Expression in mPFC and HPC
The analysis of microglial expression in mice at 3 months of age showed a significant increase in Iba1 positive cells in KO (n = 6) with respect to WT (n = 6) mice in the mPFC (P < 0.05), CA1 (P < 0.05), CA3 (P < 0.01), and DG (P < 0.01) of the HPC (Fig. 3a,e,g,i), but not in the striatum (Fig. 3c). However, this effect only persisted in time in the CA3 area, where KO mice showed significantly more Iba1 expression than WT mice (P < 0.05) at 5 months of age (Fig. 3h). See Supplementary Table S5 for statistical values. No significant differences in GFAP or NeuN expression were observed in the mPFC, striatum or the HPC of WT (n = 6) and KO (n = 6) mice at 3 or 5 months of age (Supplementary Table S2).
Figure 3.
Microglial expression in the medial prefrontal cortex (mPFC), the striatum and the hippocampus (HPC). Quantification of Iba1 immunofluorescence staining in the mPFC (a, b), the striatum (c, d), and the CA1 (e, f), the CA3 (g, h), and the dentate gyrus (i, j) of the HPC in WT (n = 6) and KO (n = 6) male mice at 3 and 5 months of age. The panels on the right show representative images of Iba1 positive cells (red) and DAPI counterstaining (blue). Scale bar = 200 μm. Images were taken at ×10 in the mPFC and striatum, and at ×20 in the HPC. The data are mean + SEM Iba1+ cells per mm2. *P < 0.05, **P < 0.01 versus WT group (separate one-way ANOVAs for 3 and 5 months). See Supplementary Table S5 for statistical values.
KO Mice Show Dysregulations in the DA System in the mPFC and HPC
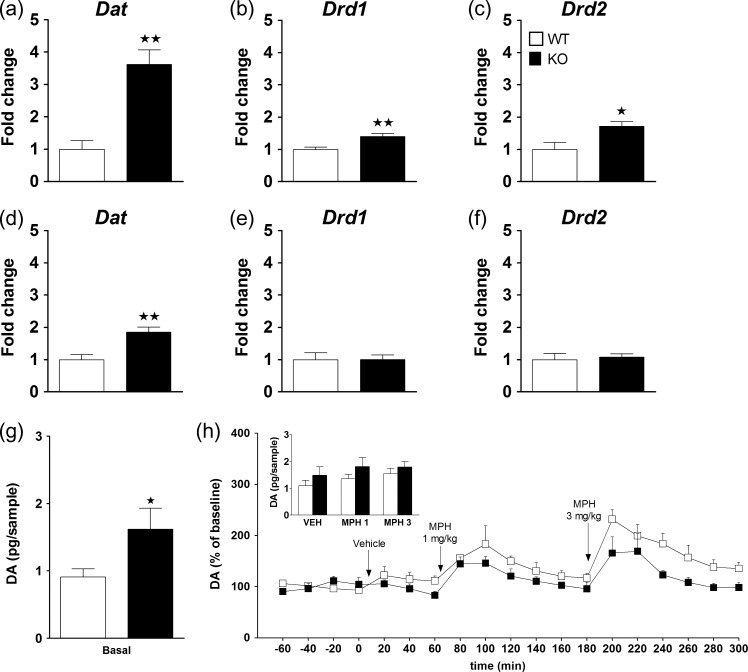
To further examine possible alterations in neurotransmitter systems in KO mice, we determined changes in mRNA levels of Dat, Net, Dbh, Drd1, and Drd2 in the mPFC and HPC of WT and KO mice at 5 months of age. In KO mice, Dat gene expression levels were significantly increased in both the mPFC and HPC with respect to WT controls (Fig. 4a,d). Drd1 and Drd2 gene expression levels were significantly enhanced in the mPFC of KO mice as compared with WT mice (Fig. 4b,c), while they were similar in the HPC of both genotypes (Fig. 4e,f). See Supplementary Table S5 for statistical values. In contrast, Net gene expression levels in the mPFC (Supplementary Fig. S2a) and HPC (Supplementary Fig. S2c) were not significantly different between genotypes. Likewise, Dbh transcriptional levels were not significantly different between genotypes either in mPFC (Supplementary Fig. S2b) or HPC (Supplementary Fig. S2d).
Figure 4.
Changes in the dopaminergic gene expression of KO mice in the medial prefrontal cortex (mPFC) and the hippocampus (HPC) and quantification of extracellular DA levels in mPFC. Real time qPCR was assessed in the mPFC for dopamine transporter (Dat) (a), dopamine receptor D1 (Drd1) (b) and dopamine receptor D2 (Drd2) (c), and in the HPC for Dat (d), Drd1 (e), and Drd2 (f) in WT (n = 7) and KO (n = 7) mice at 5 months of age. The Y-axis shows the mRNA expression as a fold change compared with the WT group. Values show the mean + SEM. *P < 0.05, **P < 0.01 versus WT group (one-way ANOVA). See Supplementary Table S5 for statistical values. The quantification of extracellular levels of dopamine (DA) in the mPFC in WT (n = 6) and KO (n = 6) mice revealed that basal extracellular levels of DA (pg/sample) are increased in KO with respect to WT mice (g) (*P < 0.05, one-way ANOVA). Percent of baseline levels of DA are shown for WT and KO mice challenged with an acute administration of vehicle and methylphenidate (MPH) at the doses of 1 and 3 mg/kg (black arrows) at different time points (h). The inset shows the mean extracellular levels of DA (pg/sample) following acute administration of MPH (1 and 3 mg/kg) or vehicle in WT (white bars) and KO (black bars).
In addition, in vivo microdialysis studies revealed significantly higher basal extracellular levels of DA (pg/sample) in the mPFC of KO (n = 6) as compared with WT (n = 6) mice (P < 0.05) (Fig. 4g). Acute MPH, but not vehicle administration significantly increased the percent of baseline DA outflow in both WT and KO mice at the dose of 1 mg/kg (time effect: F[6,60] = 7.2, P < 0.001) and 3 mg/kg (time effect: F[6,60] = 12.5, P < 0.001) (Fig. 4h). A significant effect of genotype was revealed for the dose of 3 mg/kg (F[1,10] = 5.3, P < 0.05), but not for 1 mg/kg, and no interaction between factors was observed for either dose. Although the percent of baseline increase observed at the dose of 3 mg/kg was significantly lower in KO with respect to WT mice, the net increase in terms of pg/sample was not different between genotypes at either dose (Fig. 4h inset).
KO Mice Show Abnormal Neural Activity in the mPFC and HPC During Alertness
We carried out electrophysiological recordings of the mPFC and HPC in freely moving WT (n = 4) and KO (n = 3) male mice of 6 months of age. The recordings revealed abnormal neural activity in both areas of the KO mice compared with WT controls. Overall spiking activity (MUA) was significantly decreased in the prelimbic mPFC of KO mice (n = 7 electrodes) versus WT mice (n = 9 electrodes) (P < 0.05), but increased in the CA1 region of the HPC in KO mice (n = 6 electrodes) versus WT mice (n = 8 electrodes) (P < 0.001) during exploratory behavior (Fig. 5a). In addition, KO mice exhibited exacerbated beta oscillations (18–25 Hz) in the mPFC (P < 0.001), and the HPC (P < 0.01) with respect to WT mice (Fig. 5b,c). In the HPC, delta (1–4 Hz), theta (6–10 Hz), and gamma (30–80 Hz) oscillations were also significantly exacerbated in KO compared with WT mice (P < 0.05) (Fig. 5c).
Figure 5.
Electrophysiological recordings in male WT and KO mice. Mean firing rate of multiunit activity (MUA) recorded simultaneously in the prelimbic cortex (mPFC) and CA1 (HPC) in freely moving male WT (n = 4) and KO (n = 3) mice (a). MUA was decreased in the mPFC (*P < 0.05) and increased in the HPC (***P < 0.001) of KO with respect to WT mice. Representative examples of spectrograms for WT and KO mice, showing the average power of the different frequency bands during 10 min of recordings (b). Red indicates larger power, which is markedly exacerbated in KO mice at all frequencies analyzed. Average power spectra in WT (black) and KO (red) mice (c). Note the different scale for mPFC and HPC plots. Quantification of power for the different oscillations shows that beta is significantly increased in KO with respect to WT mice in both areas (***P < 0.01, *P < 0.05, respectively), while delta, theta, and gamma are increased in KO versus WT only in the HPC (P < 0.05) (Student’s t-test).
Chronic MPH Administration Reverses Cognitive Impairment and Depressive-Like Behavior Observed in KO Mice
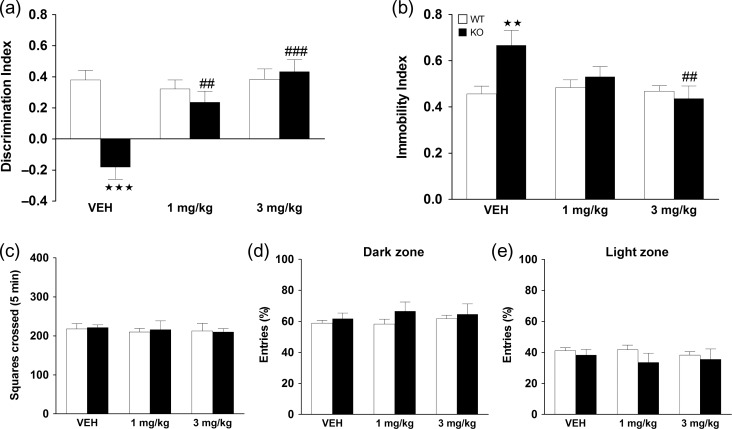
The effects of MPH on cognitive impairments and depressive-like behavior were tested in male mice at 6 months of age. In the NOR test, significant main effects of genotype, treatment, and interaction between factors were revealed. Post hoc tests showed that KO mice treated with vehicle displayed a cognitive impairment in comparison with WT controls (P < 0.001), and treatment with MPH significantly reversed this deficit at the dose of 1 mg/kg (P < 0.01), and 3 mg/kg (P < 0.001) with respect to vehicle treatment (Fig. 6a). Regarding depressive-like behavior, a significant main effect of treatment, and interaction between factors were found, but no significant main effect of genotype. Post hoc tests showed a significant increase in immobility index in KO mice treated with vehicle in comparison with the WT group (P < 0.01). This depressive-like behavior was blunted in KO mice treated with MPH at the dose of 1 and 3 mg/kg, with the effect of 3 mg/kg being significantly different to vehicle administration (P < 0.01) (Fig. 6b). See Supplementary Table S5 for statistical values. Notably, no significant effects of MPH were observed on locomotor activity in the OFT test (Fig. 6c), or in anxiety-like responses in terms of percent of entries (Fig. 6d,e) or percent of time spent in the light and dark compartments in the light-dark test (data not shown).
Figure 6.
Behavioral assessment following chronic methylphenidate (MPH) treatment. WT and KO mice were treated chronically for 21 days with vehicle, 1 and 3 mg/kg of MPH, and behavioral testing was performed one week after. Long-term recognition memory was assessed in the novel object recognition test in WT (VEH n = 14; 1 mg/kg n = 13 and 3 mg/kg n = 15) and KO (VEH n = 5; 1 mg/kg n = 7 and 3 mg/kg n = 7) mice (a). Depressive-like behavior in the tail suspension test was evaluated by the immobility index in WT (VEH n = 14; 1 mg/kg n = 13 and 3 mg/kg n = 14) and KO (VEH n = 5; 1 mg/kg n = 7 and 3 mg/kg n = 7) mice (b). Values show the mean + SEM. **P < 0.01, ***P < 0.001 versus WT group, ##P < 0.01, ###P < 0.001 versus VEH of same genotype (2-way ANOVA followed by Bonferroni’s post hoc analysis). Locomotor activity was determined by measuring the number of squares crossed in the periphery of the arena in the novel open field in WT mice (VEH n = 14; 1 mg/kg n = 14 and 3 mg/kg n = 16) and in KO (VEH n = 5; 1 mg/kg n = 5 and 3 mg/kg n = 8) mice (c). For anxiety-like behavior, the percentage of entries in the dark (d) and light (e) zones was determined in the light-dark box for WT (VEH n = 12; 1 mg/kg n = 13 and 3 mg/kg n = 14) and KO (VEH n = 5; 1 mg/kg n = 7 and 3 mg/kg n = 7) mice. See Supplementary Table S5 for statistical values.
Chronic MPH Treatment Reverses the Increased Microglial Expression Observed in KO Mice in the mPFC and HPC
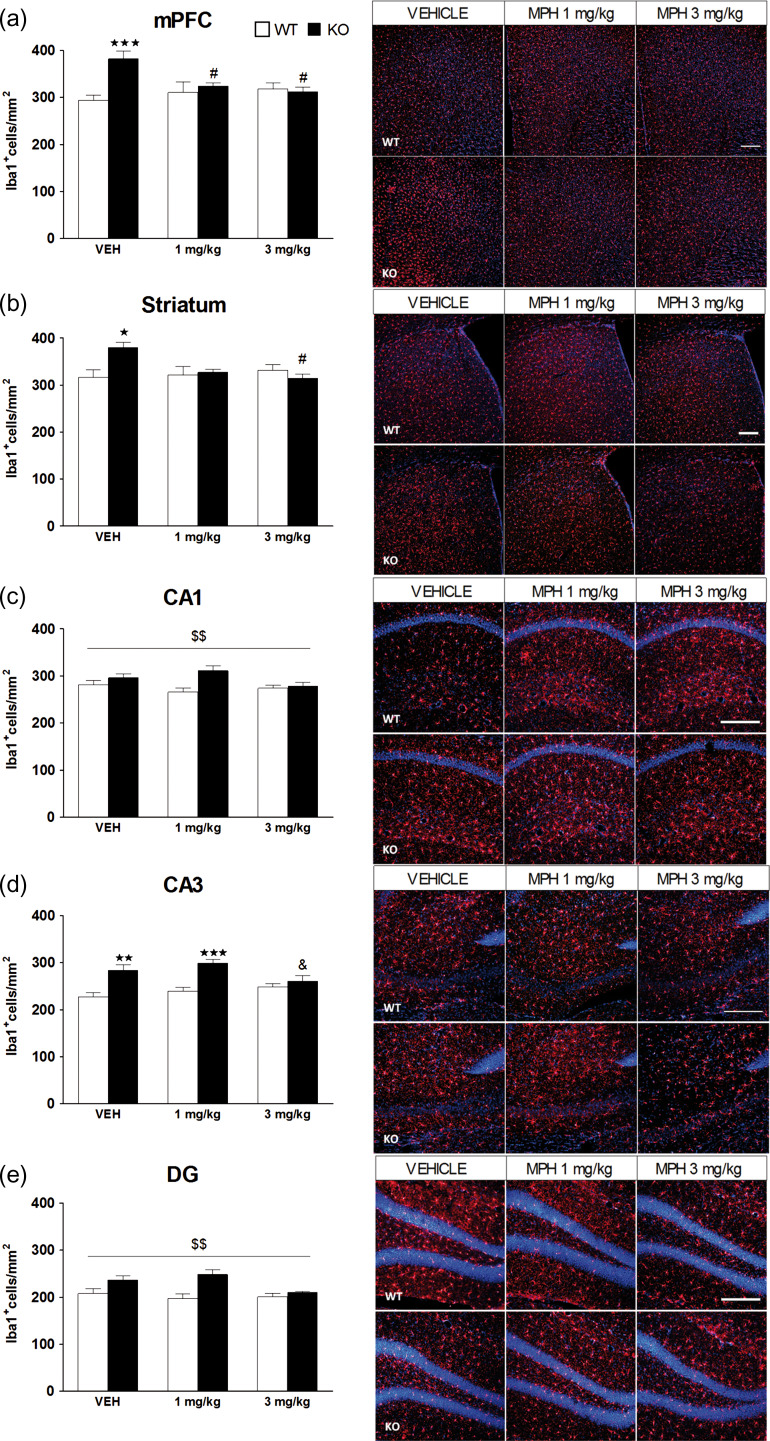
Changes in microglial expression were evaluated in the mPFC and HPC of WT (n = 6) and KO (n = 6) mice 2 weeks after the end of chronic treatment with vehicle, 1 and 3 mg/kg of MPH. In the mPFC, statistical analysis revealed a significant main effect of genotype, no significant effects of treatment, and a significant interaction between factors. Subsequent post hoc analysis revealed a significant increase in Iba1 immunostaining in KO mice treated with vehicle in comparison with the WT group (P < 0.001), and both doses of MPH (1 and 3 mg/kg) reversed this effect (P < 0.05) (Fig. 7a). In the striatum, a significant interaction between factors was revealed, but with no main effect of genotype or treatment (Fig 7b). KO mice treated with vehicle showed significantly more Iba1 expression than WT mice (P < 0.05) and this effect was reversed with MPH at the dose of 3 mg/kg (P < 0.05). In the CA1 area, a significant main effect of genotype was observed, but no significant effect of treatment or interaction between factors (Fig. 7c). For the CA3 area, a significant main effect of genotype and interaction between factors was revealed, but no significant effect of treatment. A significant increase in microglial expression was obtained in KO mice treated with vehicle (P < 0.01) and with MPH 1 mg/kg (P < 0.001) in comparison with the WT group. Notably, KO mice treated with MPH at the dose of 3 mg/kg showed a significant reduction in the number of Iba1-positive cells with respect to KO mice treated with MPH 1 mg/kg (P < 0.05) (Fig. 7d). In the DG, a significant main effect of genotype was found, but no main effect of treatment or interaction between both factors (Fig. 7e). See Supplementary Table S5 for statistical values. No significant differences were observed for GFAP or NeuN expression between groups (Supplementary Table S3).
Figure 7.
Quantification of Iba1 immunostaining in the medial prefrontal cortex (mPFC), striatum, and hippocampus (HPC) following chronic methylphenidate (MPH) treatment. WT and KO mice were treated chronically for 21 days with vehicle, 1 and 3 mg/kg of MPH, and microglial expression was assessed in the mPFC (a), the striatum (b), the CA1 (c), the CA3 (d), and the dentate gyrus (e) of the HPC in WT (n = 6) and KO (n = 6) mice. The panels on the right show representative images of Iba1 positive cells (red) and DAPI counterstaining (blue). Scale bar = 200 μm. Images were taken at ×10 in the mPFC and striatum, and at ×20 in the CA1, CA3, and DG of the HPC. The data are mean + SEM Iba1+ cells per mm2. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT group with same treatment; #P < 0.05 versus same genotype treated with vehicle; &P < 0.05 versus KO treated with MPH 1 mg/kg; $$P < 0.01 (main effect of genotype), (2-way ANOVA followed by Bonferroni’s post hoc analysis). See Supplementary Table S5 for statistical values.
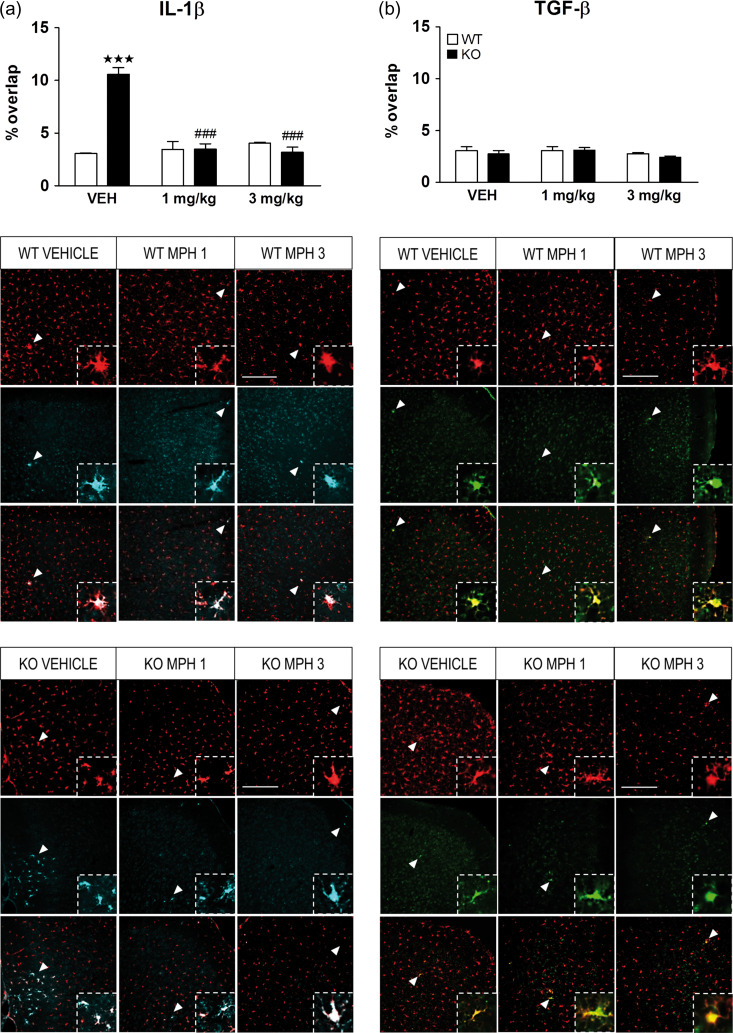
In order to assess whether microglia corresponded to the M1 or M2 states, we performed a double immunofluorescence for Iba1 with IL-1β, a proinflammatory cytokine, or with TGF-β, an anti-inflammatory cytokine. Since previous results revealed main changes in microglial expression after chronic treatment with MPH, we evaluated the coexpression of Iba1 with IL-1β or TGF-β in WT (n = 4) and KO (n = 4) mice treated chronically with MPH. In the mPFC, the statistical analysis of IL-1β exposed a significant main effect of genotype, treatment and interaction between factors. Subsequent post hoc analysis revealed a significant increase in IL-1β colocalization with Iba1 in KO mice treated with vehicle in comparison with the WT group (P < 0.001), and both doses of MPH (1 and 3 mg/kg) reversed this proinflammatory expression (P < 0.001) (Fig. 8a). Surprisingly, the expression of IL-1β in the striatum and the CA3 areas did not show significant differences between groups (Supplementary Table S4). Regarding the TGF-β expression, no statistical differences were observed in the mPFC of KO mice in comparison with WT group (Fig. 8b). See Supplementary Table S5 for statistical values. Moreover, no significant differences were observed in the striatum or CA3 areas (Supplementary Table S4).
Figure 8.
Chronic treatment with methylphenidate (MPH) restores the enhanced microglial phenotype of IL-1β in the medial prefrontal cortex (mPFC). WT (n = 4) and KO (n = 4) mice were treated chronically for 21 days with vehicle, 1 and 3 mg/kg of MPH and the state of microglia was characterized by double label immunofluorescence of Iba1 with interleukin one beta (IL-1β) a proinflammatory marker (a), and Iba1 with transforming growth factor beta (TGF-β) an anti-inflammatory marker (b). The Y-axis shows the coexpression of microglial cells expressing the pro- or anti-inflammatory marker as a percentage of overlap. The panels on the bottom show representative images of Iba1 positive cells (red) with IL-1β (cyan) or TGF-β (green). White arrowheads represent Co-expression of microglia with IL-1β (left panels) or with TGF-β (right panels) (zoomed in on inset). Scale bar = 200 μm. Images were taken at ×20. The data are mean + SEM cells per mm2. ***P < 0.001 versus WT group with same treatment; ###P < 0.001 versus same genotype treated with vehicle (2-way ANOVA followed by Bonferroni’s post hoc analysis). See Supplementary Table S5 for statistical values.
Chronic MPH Administration Reverses Gene Expression Changes in KO Mice
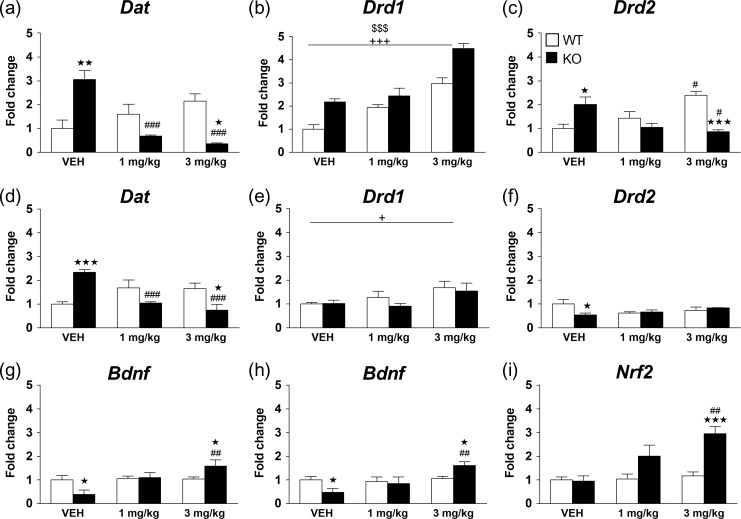
The expression levels of Dat, Drd1, and Drd2 genes were determined in the mPFC and HPC of WT and KO mice treated chronically with vehicle or MPH (1 and 3 mg/kg). In the mPFC, for Dat gene expression, a significant main effect of treatment and a significant interaction between genotype and treatment were observed. Dat levels were significantly increased in KO mice treated with vehicle as compared with WT mice (P < 0.01), and MPH reversed this effect at both doses tested (1 and 3 mg/kg: P < 0.001). In contrast, MPH showed a nonsignificant tendency to increase Dat gene expression in WT mice in this structure (Fig. 9a). For Drd1 gene expression, significant main effects of genotype and treatment were observed, but no interaction between factors (Fig. 9b). For Drd2 gene expression, a significant interaction between genotype and treatment was observed. Post hoc comparisons showed that Drd2 mRNA levels were significantly increased in KO mice treated with vehicle as compared with WT mice (P < 0.05), and MPH reversed this effect the dose of 3 mg/kg (P < 0.001). In contrast, MPH significantly increased Drd2 gene expression in WT mice at the dose of 3 mg/kg with respect to vehicle administration (P < 0.05) (Fig. 9c). See Supplementary Table S5 for statistical values.
Figure 9.
Chronic methylphenidate (MPH) reverses the transcriptional activity of KO mice in the medial prefrontal cortex (mPFC) and the hippocampus (HPC). WT and KO mice were treated chronically for 21 days with vehicle, 1 and 3 mg/kg of MPH and changes in gene expression were determined in the mPFC for dopamine transporter (Dat) (a), dopamine receptor D1 (Drd1) (b), dopamine receptor D2 (Drd2) (c) and in the HPC for Dat (d), Drd1 (e) and Drd2 (f). Brain-derived neurotrophic factor (Bdnf) expression was measured in the mPFC (g), and the Bdnf (h) and the nuclear factor erythroid 2-related factor 2 (Nrf2) (i) expression were determined in the HPC. The Y-axis shows the mRNA expression as a fold change compared with the control group (WT VEH). Data were obtained from WT (VEH n = 5–10; MPH 1 mg/kg n = 4–10 and MPH 3 mg/kg n = 5–12) and KO (VEH n = 5-7; MPH 1 mg/kg n = 4–6 and MPH 3 mg/kg n = 4–7) mice in both structures. Values show the mean + SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT with same treatment; #P < 0.05, ##P < 0.01, ###P < 0.001 versus vehicle of same genotype; $$$P < 0.001 main effect of genotype; +P < 0.05, +++P < 0.001 main effect of treatment (2-way ANOVA followed by Bonferroni’s post hoc analysis). See Supplementary Table S5 for statistical values.
In the HPC, for Dat gene expression, a significant main effect of treatment and a significant interaction between genotype and treatment were observed. Dat levels were significantly increased in KO mice treated with vehicle as compared with WT mice (P < 0.001), and MPH reversed this effect at both doses tested (1 and 3 mg/kg: P < 0.001), while it did not significantly modify DAT gene expression in WT mice (Fig. 9d). With respect to Drd1 gene expression, only a significant main effect of treatment was observed (Fig. 9e). For Drd2 gene expression, a significant interaction was observed between genotype and treatment. Post hoc analysis revealed a significant reduction in Drd2 gene expression in KO mice treated with vehicle with respect to WT mice (P < 0.05) (Fig. 9f). See Supplementary Table S5 for statistical values.
The transcriptional levels of Bdnf were determined in the mPFC and HPC of WT and KO mice. In KO mice treated with vehicle, Bdnf gene expression was significantly downregulated in both the mPFC and HPC with respect to WT controls (Fig. 9g,h). Chronic treatment with MPH at the dose of 1 mg/kg normalized this deregulation, and significantly increased Bdnf transcriptional levels in both brain regions at the dose of 3 mg/kg with respect to vehicle administration in KO mice (P < 0.01), and to WT mice receiving the same treatment (P < 0.05). Nrf2 expression was detected in the HPC, but not the mPFC of both WT and KO mice (Fig. 9i). Statistical analysis for the expression of Nrf2 in the HPC showed a main effect of genotype, treatment and interaction between factors. Post hoc analysis revealed no significant differences between WT and KO mice treated with vehicle. However, Nrf2 expression was increased by MPH at 1 and 3 mg/kg only in KO mice, with the effect at the dose of 3 mg/kg being significantly different from WT mice (P < 0.001) and from KO mice treated with vehicle (P < 0.001). See Supplementary Table S5 for statistical values.
Discussion
In this study, we found that lack of Mbnl2 function induced progressive cognitive and affective alterations in mice that were associated with hyperactivation of the DA system in the mPFC including an increase in DA extracellular levels, increased Dat, Drd1, and Drd2 gene expression, and profound perturbations in neural activity and microgliosis in the mPFC and the HPC. Notably, chronic treatment with MPH reversed the behavioral deficits, and specifically reversed the enhancement in Dat and Drd2 gene expression and the increase in proinflammatory microglia expression in the mPFC of Mbnl2 KO mice.
We first investigated the progression of cognitive, affective and motor alterations in both male and female Mbnl2 KO mice. Our results showed that male KO mice were more affected in terms of memory deficits than female KO mice. Thus, long-term recognition memory seemed to worsen with age in male KO mice, while this effect was not so prominent in female KO mice as compared with WT controls. Additionally, when we further investigated the ability of male KO mice to learn an active-avoidance task, we found that they were severely impaired with respect to WT mice, indicating alterations in emotional working memory processing. Male KO mice also exhibited a persistent depression-like phenotype, while females did not show this behavior. These data suggest that the cognitive and mood disturbances associated with the lack of the Mbnl2 gene are progressive and more prominent in male than female mice. Recent evidence shows that both muscle wasting and cognitive deficits are progressive in DM1 patients (Winblad et al., 2016), while no data is available as to whether the deregulation of affective processes observed in this population also deteriorates with age (Winblad et al. 2010). In addition, there is a lack of clinical studies investigating gender differences with respect to the neuropsychological deficits in DM1. However, Dogan et al. (2016) recently reported that males show higher morbidity and mortality than females. DM1 patients also suffer from apathy, avoidance, moderate anxiety, and sleep disturbances (Phillips et al. 1999; Meola et al. 2003; Gallais et al. 2015). In agreement, we found that circadian activity was perturbed in male KO mice. Locomotor activity was differentially affected in male and female mice, with females of both genotypes showing higher locomotion than males in the novel open field, probably due to their higher sensitivity to new environments (Palanza 2001). Moreover, male KO mice showed inverse effects on locomotor activity between 3 and 5 months with respect to WT mice, while female KO mice exhibited less locomotor activity than WT mice at both ages. These disparities could be attributed to differences in the habituation effect of repeated testing in the novel open field. On the other hand, neither male nor female KO mice exhibited significant persistent anxiety-like alterations with respect to control mice.
We next aimed at gaining deeper insight into the neuropathological substrates that underlie the behavioral alterations observed in Mbnl2 KO mice. We first assessed the expression levels of microglia and astrocytes in the mPFC, striatum and the HPC, and whether or not neuronal death was occurring in these brain areas. Our data showed a persistent increase in microglia expression in the mPFC and the CA3 region, but not in the striatum of KO with respect to WT mice. Interestingly, no significant changes in astrocyte expression or neuronal number were found in the mPFC or HPC of KO mice, consistent with an early pathological process. Therefore, we next investigated the possible alterations in the expression of genes related to the DA and NE systems in the mPFC and the HPC of KO and WT mice. Our findings revealed major changes in the mPFC of KO mice mostly related to the DA system. Thus, Dat, Drd1, and Drd2 gene expression levels were significantly enhanced in the mPFC of KO as compared with WT mice, while no changes in Net or Dbh gene expression were observed in this structure. Overstimulation of both D1 and D2 receptors in the mPFC impairs working memory (Druzin et al. 2000; Vijayraghavan et al. 2007), so excessive activation of these receptors could trigger the cognitive deficits observed in this model. In the HPC, however, only Dat gene expression levels were significantly exacerbated, while Drd1, Drd2, Net, or Dbh levels were not different from those in WT mice. These results suggested that the DA system in the mPFC is particularly affected in KO mice, which lead us to evaluate basal DA extracellular levels in the mPFC and the functionality of DAT in both genotypes using in vivo microdialysis in freely moving mice. Our results showed enhanced DA extracellular levels in KO with respect to WT mice in the mPFC, and a moderate increase in DA outflow in both genotypes following an acute challenge with MPH at the 2 doses tested. Together, these data suggest that the functionality of the DAT is similar in both genotypes albeit the increase in DA output and the changes in Dat gene expression observed in the mPFC of KO mice. The significant alterations in the DA transport system, DA receptors, and increased DA levels in the mPFC could be underlying the behavioral phenotype observed in KO mice, characterized by cognitive deficits and a depressive state. Accordingly, excessive DA release impairs mPFC function (Robbins and Arnsten 2009), and may lead to impaired D1 and/or D2 receptor signaling and to detriments in cognitive and affective processing (Puig et al. 2014; Berk et al. 2007).
KO mice also showed abnormal neural activity in the mPFC and HPC, in agreement with a functional neuroimaging study that reported abnormal activity in similar brain areas of DM1 patients (Romeo et al. 2010). More specifically, we found that spiking activity was significantly reduced in the prelimbic cortex of KO mice. This may be due to excessive stimulation of D1 receptors. Overstimulation of prefrontal D1 receptors suppresses spiking activity of mPFC neurons eroding neural information in a working memory task both in nonhuman primates and rodents (Zahrt et al. 1997; Vijayraghavan et al. 2007). A similar scenario may occur in Mbnl2 KO mice following a chronic increase of mPFC DA levels. Overall oscillatory activity, that reflects neural network synchronization, was intact in KO mice, with the exception of beta oscillations that were greatly exacerbated. The role of beta oscillations in the mPFC is poorly understood. In nonhuman primates, prefrontal beta oscillations play a role in associative learning, memory, and attention and are modulated by the DA system (Benchenane et al. 2011; Puig and Miller 2012; Puig et al. 2014; Antzoulatos and Miller 2016). Although no such studies have been conducted in vivo in mice, increased DA release in mouse mPFC slices enhances the power of beta oscillations (Steullet et al. 2014). Thus, increased DA levels in mPFC may reduce spiking activity and generate aberrant beta oscillations in KO mice interfering with local neural network dynamics having a negative impact on mPFC-dependent cognitive processing. Our electrophysiological studies also showed exacerbated spiking and oscillatory activities in the CA1 region of the HPC that seemed nonspecific and likely originated in aberrant circuit dynamics within the HPC, perhaps following molecular disturbances in CA3 (see below). The dorsal HPC is more closely involved in object recognition memory than the mPFC (Cohen and Stackman 2015; Warburton and Brown 2015). Aberrant oscillatory dynamics in CA1 may have caused severe deficits in object recognition memory in KO mice. On the other hand, exacerbated beta oscillations in the mPFC of Mbnl2 KO mice could participate in the depressive symptoms and emotional learning deficits in this model. Several clinical studies have reported enhanced beta oscillations in the subgenual cingulate cortex of depressive patients (Clark et al. 2016; Merkl et al. 2016). The mouse equivalent of this area is the infralimbic cortex within the mPFC. Although we did not perform electrophysiological recordings in this region, the microdialysis probe covered the cingulate, prelimbic and infralimbic cortices showing a robust increase in DA levels. Thus, it is likely that augmented DA levels in infralimbic cortex generated aberrant beta oscillations (Steullet et al. 2014) that might participate in the depressive symptoms in Mbnl2 KO mice.
We next investigated the possible improvement of the behavioral and neuropathological alterations observed in Mbnl2 KO mice by chronic treatment with MPH. We found that MPH reversed long-term memory deficits and depressive-like behavior in KO mice without affecting locomotor activity or anxiety-like responses. Importantly, MPH induced opposite effects on the levels of Dat and Drd2 gene expression in the mPFC of WT and KO mice: it reversed the enhancement in Dat and Drd2 gene expression in KO mice, while it increased the expression of these genes in WT mice. On the contrary, MPH increased Drd1 gene expression in both genotypes. In the HPC, MPH reversed the changes in Dat gene expression in KO mice, and induced subtle effects in Drd1 and Drd2 in both genotypes. These results indicate that the favorable effects of MPH on CNS-related alterations in this mouse model of DM1 could be attributed to re-establishing an appropriate level of D2 receptors in the mPFC. These receptors are primarily expressed in layer V pyramidal neurons of the mPFC (Lidow et al. 1998; Santana et al. 2009), where they can enhance (Wang and Goldman-Rakic 2004) or suppress excitability (Gulledge and Jaffe 1998). Furthermore, recent data revealed that MPH alters the functional coupling between the PFC and DA neurons through a mechanism involving D2 receptors (dela Peña et al. 2018).
In 6 months old mice, following chronic drug administration, KO mice treated with vehicle continued to exhibit microgliosis in the mPFC and CA3, but also showed increased microglia expression in the striatum, and MPH reduced this overexpression in all 3 areas. Since activated microglia in the brain is associated with either an M1 (proinflammatory) or M2 state (anti-inflammatory), we evaluated whether MPH was specifically affecting each of these phenotypes. Our results revealed an increase in the coexpression of Iba1 and the proinflammatory cytokine, IL-1β, specifically in the mPFC of KO mice, but not in the striatum or the HPC. Interestingly, the presence of this M1 microglia phenotype was observed without the incidence of neuronal damage or astrogliosis. There is a well-known connection between neurodegereration, astrogliosis, and microgliosis (Zhang et al. 2010). However, microglia also respond rapidly to small pathological changes in the CNS, even when no neuronal damage is present, while astrocytes respond later, possibly activated by the secretion of cytokines in microglia (Zhang et al. 2009). Thus, together these data suggest that Mbnl2 KO mice may exhibit the early stages of an inflammatory process appearing first in the mPFC. In DM1 patients, the serum concentrations of IL-6 are increased, and have been associated with diabetes and microvascular complications (Wegner et al. 2013). However, to our knowledge, there are no studies regarding the presence of exacerbated microglia or neuroinflammation in the brain of DM1 patients or in other mouse models of the disease. Microglia activation and proliferation has been reported in Huntington disease, a pathology caused by CAG repeat expansions similar to what occurs in DM1 (Crotti and Glass 2015). Moreover, psychiatric conditions like autistic spectrum disorder also present a marked activation of microglia in young adults (Suzuki et al. 2013). Notably, chronic treatment with MPH at both doses tested specifically reversed the enhancement of M1 proinflammatory microglia expressing IL-1β in the mPFC of KO mice, while it did not affect the levels of M2 anti-inflammatory microglia expressing TGF-β in either genotype. Consistent with these data, repeated administration of MPH decreases serum levels of the proinflammatory markers IL-1β and IL-6 in a rat model of PTSD showing depressive-like behavior and cognitive deficits (Aga-Mizrachi et al. 2014).
Finally, we also found decreased Bdnf gene expression in the mPFC and HPC of KO mice treated with vehicle with respect to WT controls. Dysregulations in BDNF in these brain areas may also be associated with the cognitive alterations and depressive-like phenotype in KO mice since this neurotrophic factor has been implicated in synaptic plasticity and mood disorders (Krishnan and Nestler 2008). MPH reversed the changes observed in Bdnf gene expression in KO mice at the dose of 3 mg/kg without modifying these levels in WT mice. In this respect, a previous study has shown that chronic MPH increased hippocampal BDNF protein expression, and reversed spatial learning memory deficits in a rat model of ADHD (Kim et al. 2011; Jichao et al. 2017). Remarkably, MPH increased Nrf2 gene expression specifically in the HPC of KO mice. BDNF constitutively controls NRF2 translocation to the nucleus leading to an increase in its expression (Bouvier et al. 2016). The Nrf2 gene is a key marker of antioxidant response (Perez-Leal et al. 2017), and altered BDNF-TrkB signaling, and a depressive-like phenotype have been reported in Nrf2 KO mice (Yao et al. 2016). In addition, reactive oxygen species and nitric oxide could lead to cognitive dysfunction and depression in animal models (McAfoose and Baune 2009; Fenn et al. 2014; Iwata et al. 2016), and in humans suffering from psychiatric disorders (Kronfol and Remick 2000). Thus, our data showing an increase of Nrf2 gene expression in the HPC of KO mice following MPH treatment suggests that its beneficial effects on cognition and depression in KO mice may also be due to a reduction in oxidative stress in this structure. This idea is consistent with previous clinical studies showing that MPH diminishes the oxidative index (Guney et al. 2015), and enhances cognitive performance in ADHD patients (Buitelaar and Medori 2010).
In conclusion, using the Mbnl2 KO model of CNS dysfunction in DM1 we discovered that the lack of this gene induced profound behavioral, biochemical and neurophysiological perturbations related to dysregulation of the DA system and to a proinflammatory process in the mPFC. Furthermore, we demonstrated that MPH normalized the cognitive deficits and depressive-like alterations observed, and unraveled the neurobiological mechanisms underlying these beneficial effects consisting in 1) an attenuation of over-activated Dat and Drd2 gene expression in the mPFC, 2) a reduction of proinflammatory microglia in the mPFC, and 3) an augmentation of anti-oxidative stress biomarkers in the HPC. Therefore, MPH could be a novel therapeutic avenue for the treatment of cognitive and mood alterations observed in DM1 patients.
Supplementary Material
Funding
Fundació la Marató de TV3 (231/C/2014), and Instituto de Salud Carlos III (PI14/00210) to P.R.; Agency for Management of University and Research Grants (AGAUR) (ICREA Acadèmia-2015), AGAUR (SGR2009-00731), Ministerio de Economía Industria y Competitividad (MINECO) (SAF2014-59648-P), and Instituto de Salud Carlos III-RETICS/RTA (RD16/0017/0020) to R.M.; MINECO (SAF2016-80726-R) to M.V.P.; and AGAUR (SGR2014-680) to R.T.F.
Notes
We thank Dulce Real for her expert contribution in the microdialysis experiments, Marta Linares for her valuable technical support in histological procedures, Jordi Chanovas and Pau Nebot for assistance with Python, MATLAB coding, and spike sorting. Conflict of Interest: None declared.
References
- Aga-Mizrachi S, Cymerblit-Sabba A, Gurman O, Balan A, Shwam G, Deshe R, Miller L, Gorodetsky N, Heinrich N, Tzezana O, et al. 2014. Methylphenidate and desipramine combined treatment improves PTSD symptomatology in a rat model. Transl Psychiatry. 4:e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzoulatos EG, Miller EK. 2016. Synchronous beta rhythms of frontoparietal networks support only behaviorally relevant representations. Elife. 5:e17822. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. 2006. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 31:2376–2383. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Pliszka SR. 2011. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 99:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso E, Ozaita A, Valdizán EM, Ledent C, Pazos Á, Maldonado R, Valverde O. 2008. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J Neurochem. 105:565–572. [DOI] [PubMed] [Google Scholar]
- Baldanzi S, Cecchi P, Fabbri S, Pesaresi I, Simoncini C, Angelini C, Bonuccelli U, Cosottini M, Siciliano G. 2016. Relationship between neuropsychological impairment and grey and white matter changes in adult-onset myotonic dystrophy type 1. NeuroImage Clin. 12:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. 2011. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol. 21:475–485. [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Sant’Anna M, Malhi GS, Bourin M, Kapczinski F, Norman T. 2007. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand. 116:41–49. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. 2006. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 60:1111–1120. [DOI] [PubMed] [Google Scholar]
- Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal J-H, Cresto N, Doligez N, Rivat C, Do KQ, Bernard C, et al. 2016. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry. 22:1701–1713. [DOI] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. 2011. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol Biochem Behav. 97:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitelaar J, Medori R. 2010. Treating attention-deficit/hyperactivity disorder beyond symptom control alone in children and adolescents: a review of the potential benefits of long-acting stimulants. Eur Child Adolesc Psychiatry. 19:325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bura SA, Burokas A, Martín-García E, Maldonado R. 2010. Effects of chronic nicotine on food intake and anxiety-like behaviour in CB1 knockout mice. Eur Neuropsychopharmacol. 20:369–378. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Gomis-González M, Guegan T, Agustín-Pavón C, Pastor A, Mato S, Pérez-Samartín A, Matute C, de la Torre R, Dierssen M, et al. 2013. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 19:603–607. [DOI] [PubMed] [Google Scholar]
- Caso F, Agosta F, Peric S, Rakočević-Stojanović V, Copetti M, Kostic VS, Filippi M. 2014. Cognitive impairment in myotonic dystrophy type 1 is associated with white matter damage. PLoS One. 9:e104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañé A, Célérier E, Martín M, Ledent C, Parmentier M, Maldonado R, Valverde O. 2006. Development and expression of neuropathic pain in CB1 knockout mice. Neuropharmacology. 50:111–122. [DOI] [PubMed] [Google Scholar]
- Charizanis K, Lee KY, Batra R, Goodwin M, Zhang C, Yuan Y, Shiue L, Cline M, Scotti MM, Xia G, et al. 2012. Muscleblind-like 2-mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron. 75:437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Brown EC, Ramasubbu R, Kiss ZHT. 2016. Intrinsic local beta oscillations in the subgenual cingulate relate to depressive symptoms in treatment-resistant depression. Biol Psychiatry. 80:e93–e94. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW. 2015. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 285:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comim CM, Mathia GB, Hoepers A, Tuon L, Kapczinski F, Dal-Pizzol F, Quevedo J, Rosa MI. 2015. Neurotrophins, cytokines, oxidative parameters and funcionality in progressive muscular dystrophies. An Acad Bras Cienc. 87:1809–1818. [DOI] [PubMed] [Google Scholar]
- Crotti A, Glass CK. 2015. The choreography of neuroinflammation in Huntington’s disease. Trends Immunol. 36:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutando L, Busquets-Garcia A, Puighermanal E, Gomis-González M, Delgado-García JM, Gruart A, Maldonado R, Ozaita A. 2013. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Invest. 123:2816–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. 2004. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 28:771–784. [DOI] [PubMed] [Google Scholar]
- doi: 10.1016/j.neuropharm.2018.01.015. Dela Peña IC, Shen G, Shi WX. 2018. Methylphenidate significantly alters the functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. Neuropharmacology. 131:431–439. [DOI] [PubMed] [Google Scholar]
- Dogan C, De Antonio M, Hamroun D, Varet H, Fabbro M, Rougier F, Amarof K, Bes MCA, Bedat-Millet AL, Behin A, et al. 2016. Gender as a modifying factor influencing myotonic dystrophy type 1 phenotype severity and mortality: a nationwide multiple databases cross-sectional observational study. PLoS One. 11:e0148264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzin MY, Kurzina NP, Malinina EP, Kozlov AP. 2000. The effects of local application of D2 selective dopaminergic drugs into the medial prefrontal cortex of rats in a delayed spatial choice task. Behav Brain Res. 109:99–111. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. 2014. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry. 76:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, et al. 2000. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 25:195–200. [DOI] [PubMed] [Google Scholar]
- Flores Á, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F. 2014. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology. 39:2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallais B, Montreuil M, Gargiulo M, Eymard B, Gagnon C, Laberge L. 2015. Prevalence and correlates of apathy in myotonic dystrophy type 1. BMC Neurol. 15:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lopez A, Monferrer L, Garcia-Alcover I, Vicente-Crespo M, Alvarez-Abril MC, Artero RD. 2008. Genetic and chemical modifiers of a CUG toxicity model in drosophila. PLoS One. 3:e1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. 1998. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 18:9139–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guney E, Cetin FH, Alisik M, Tunca H, Tas Torun Y, Iseri E, Isik Taner Y, Cayci B, Erel O. 2015. Attention deficit hyperactivity disorder and oxidative stress: a short term follow up study. Psychiatry Res. 229:310–317. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, Van Dyck CH, Malison RT, Carson RE, Ding YS. 2010. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 68:854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper PS. 2001. Myotonic dystrophy. 3rd edn. London: WB Saunders. p. 436. [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, et al. 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ishida H, Kaneko K, Shirayama Y. 2016. Learned helplessness activates hippocampal microglia in rats: a potential target for the antidepressant imipramine. Pharmacol Biochem Behav. 150–151:138–146. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A. 1999. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods. 93:149–162. [DOI] [PubMed] [Google Scholar]
- Jichao S, Xinmin H, Xianguo R, Dongqi Y, Rongyi Z, Shuang L, Yue Y, Yuchen S, Jingnan Y. 2017. Saikosaponin a alleviates symptoms of attention deficit hyperactivity disorder through downregulation of DAT and enhancing BDNF expression in spontaneous hypertensive rats. Evid Based Complement Alternat Med. 2017:2695903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanadia RN, Shin J, Yuan Y, Beattie SG, Wheeler TM, Thornton CA, Swanson MS. 2006. Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly(CUG) model for myotonic dystrophy. Proc Natl Acad Sci U S A. 103:11748–11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim SE, Kim BK, Kim TW, Ji ES, Kim JD, et al. 2011. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 504:35–39. [DOI] [PubMed] [Google Scholar]
- Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. 2010. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 114:259–270. [DOI] [PubMed] [Google Scholar]
- Konieczny P, Selma-Soriano E, Rapisarda AS, Fernandez-Costa JM, Perez-Alonso M, Artero R. 2017. Myotonic dystrophy: candidate small molecule therapeutics. Drug Discov Today. 22:1740–1748. [DOI] [PubMed] [Google Scholar]
- Kossatz E, Maldonado R, Robledo P. 2016. CB2 cannabinoid receptors modulate HIF-1α and TIM-3 expression in a hypoxia-ischemia mouse model. Eur Neuropsychopharmacol. 26:1972–1988. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. 2008. The molecular neurobiology of depression. Nature. 455:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfol Z, Remick DG. 2000. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 157:683–694. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Reinlieb M, Cyr NS, Siddarth P, Ercoli LM, Senturk D. 2015. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 172:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Wang F, Cao Y, Goldman-Rakic PS. 1998. Layer V neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse. 28:10–20. [DOI] [PubMed] [Google Scholar]
- Llorente-Berzal A, Fuentes S, Gagliano H, López-Gallardo M, Armario A, Viveros MP, Nadal R. 2011. Sex-dependent effects of maternal deprivation and adolescent cannabinoid treatment on adult rat behaviour. Addict Biol. 16:624–637. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. 2002. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl). 159:379–387. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, et al. 1999. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 156:675–682. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. 2009. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 33:355–366. [DOI] [PubMed] [Google Scholar]
- Meola G, Sansone V, Perani D, Scarone S, Cappa S, Dragoni C, Cattaneo E, Cotelli M, Gobbo C, Fazio F, et al. 2003. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2). Neuromuscul Disord. 13:813–821. [DOI] [PubMed] [Google Scholar]
- Merkl A, Neumann WJ, Huebl J, Aust S, Horn A, Krauss JK, Dziobek I, Kuhn J, Schneider GH, Bajbouj M, et al. 2016. Modulation of beta-band activity in the subgenual anterior cingulate cortex during emotional empathy in treatment-resistant depression. Cereb Cortex. 26:2626–2638. [DOI] [PubMed] [Google Scholar]
- Miller JW. 2000. Recruitment of human muscleblind proteins to (CUG)n expansions associated with myotonic dystrophy. EMBO J. 19:4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnerop M, Weber B, Schoene-Bake JC, Roeske S, Mirbach S, Anspach C, Schneider-Gold C, Betz RC, Helmstaedter C, Tittgemeyer M, et al. 2011. The brain in myotonic dystrophy 1 and 2: evidence for a predominant white matter disease. Brain. 134:3527–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Takahashi K, Jinnai K, Kanda F, Fukuoka Y, Kurisaki H, Mitake S, Inagaki T, Yamano T, Nagao K. 1998. Loss of serotonin-containing neurons in the raphe of patients with myotonic dystrophy: a quantitative immunohistochemical study and relation to hypersomnia. Neurology. 50:535–538. [DOI] [PubMed] [Google Scholar]
- Palanza P. 2001. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 25:219–233. [DOI] [PubMed] [Google Scholar]
- Pantic B, Trevisan E, Citta A, Rigobello MP, Marin O, Bernardi P, Salvatori S, Rasola A. 2013. Myotonic dystrophy protein kinase (DMPK) prevents ROS-induced cell death by assembling a hexokinase II-Src complex on the mitochondrial surface. Cell Death Dis. 4:e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. 2001. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates.
- Perez-Leal O, Barrero CA, Merali S. 2017. Pharmacological stimulation of nuclear factor (erythroidderived 2)-like 2 translation activates antioxidant responses. J Biol Chem. 292:14108–14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MF, Steer HM, Soldan JR, Wiles CM, Harper PS. 1999. Daytime somnolence in myotonic dystrophy. J Neurol. 246:275–282. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. 2010. Neurocircuitry of mood disorders. Neuropsychopharmacology. 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Antzoulatos EG, Miller EK. 2014. Prefrontal dopamine in associative learning and memory. Neuroscience. 282:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Miller EK. 2012. The role of prefrontal dopamine d1 receptors in the neural mechanisms of associative learning. Neuron. 74:874–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puymirat J, Bouchard JP, Mathieu J. 2012. Efficacy and tolerability of a 20-mg dose of methylphenidate for the treatment of daytime sleepiness in adult patients with myotonic dystrophy type 1: a 2-center, randomized, double-blind, placebo-controlled, 3-week crossover trial. Clin Ther. 34:1103–1111. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. 2003. Depression: what we can learn from postmortem studies. Neuroscientist. 9:273–284. [DOI] [PubMed] [Google Scholar]
- Ranum LPW, Cooper TA. 2006. Rna-mediated neuromuscular disorders. Annu Rev Neurosci. 29:259–277. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. 2009. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 32:267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Trigo JM, Panayi F, De La Torre R, Maldonado R. 2007. Behavioural and neurochemical effects of combined MDMA and THC administration in mice. Psychopharmacology (Berl). 195:255–264. [DOI] [PubMed] [Google Scholar]
- Romeo V, Pegoraro E, Squarzanti F, Sorarù G, Ferrati C, Ermani M, Zucchetta P, Chierichetti F, Angelini C. 2010. Retrospective study on PET-SPECT imaging in a large cohort of myotonic dystrophy type 1 patients. Neurol Sci. 31:757–763. [DOI] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. 2009. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 19:849–860. [DOI] [PubMed] [Google Scholar]
- Serra L, Silvestri G, Petrucci A, Basile B, Masciullo M, Makovac E, Torso M, Spanò B, Mastropasqua C, Harrison NA, et al. 2014. Abnormal functional brain connectivity and personality traits in myotonic dystrophy type 1. JAMA Neurol. 71:603–611. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal J-H, Cuénod M, Do KQ. 2014. Fast oscillatory activity in the anterior cingulate cortex: dopaminergic modulation and effect of perineuronal net loss. Front Cell Neurosci. 8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, Yoshihara Y, Omata K, Matsumoto K, Tsuchiya KJ, et al. 2013. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry. 70:49. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. 2007. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 87:426–433. [DOI] [PubMed] [Google Scholar]
- Toscano A, Messina S, Campo GM, Di Leo R, Musumeci O, Rodolico C, Aguennouz M, Annesi G, Messina C, Vita G. 2005. Oxidative stress in myotonic dystrophy type 1. Free Radic Res. 39:771–776. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. 2015. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatry. 77:1001–1009. [DOI] [PubMed] [Google Scholar]
- Vertes RP. 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 142:1–20. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. 2007. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 10:376–384. [DOI] [PubMed] [Google Scholar]
- Viñals X, Molas S, Gallego X, Fernández-Montes RD, Robledo P, Dierssen M, Maldonado R. 2012. Overexpression of α3/α5/β4 nicotinic receptor subunits modifies impulsive-like behavior. Drug Alcohol Depend. 122:247–252. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. 1998. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 155:1325–1331. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW. 2015. Neural circuitry for rat recognition memory. Behav Brain Res. 285:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, Araszkiewicz A, Piorunska-Stolzmann M, Wierusz-Wysocka B, Zozulinska-Ziolkiewicz D. 2013. Association between IL-6 concentration and diabetes-related variables in DM1 patients with and without microvascular complications. Inflammation. 36:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler TM, Leger AJ, Pandey SK, MacLeod AR, Nakamori M, Cheng SH, Wentworth BM, Bennett CF, Thornton CA. 2012. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature. 488:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad S, Jensen C, Månsson J-E, Samuelsson L, Lindberg C. 2010. Depression in myotonic dystrophy type 1: clinical and neuronal correlates. Behav Brain Funct. 6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad S, Samuelsson L, Lindberg C, Meola G. 2016. Cognition in myotonic dystrophy type 1: a 5-year follow-up study. Eur J Neurol. 23:1471–1476. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. 2004. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 27:683–690. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Lim KO, Hemmy LS, Day JW. 2014. Tractography reveals diffuse white matter abnormalities in myotonic dystrophy type 1. J Neurol Sci. 341:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Zhang JC, Ishima T, Ren Q, Yang C, Dong C, Ma M, Saito A, Honda T, Hashimoto K. 2016. Antidepressant effects of TBE-31 and MCE-1, the novel Nrf2 activators, in an inflammation model of depression. Eur J Pharmacol. 793:21–27. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. 1997. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 17:8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hu X, Qian L, O’Callaghan JP, Hong JS. 2010. Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol. 41:232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hu X, Qian L, Wilson B, Lee C, Flood P, Langenbach R, Hong JS. 2009. Prostaglandin E2 released from activated microglia enhances astrocyte proliferation in vitro. Toxicol Appl Pharmacol. 238:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.